Abstract
Resting energy expenditure (REE) is partially dependent on the sympathetic nervous system as evidenced by the fact REE decreases during systemic beta-adrenergic blockade. It is not known how gastric bypass affects the sympathetically mediated component of REE or muscle sympathetic nerve activity (MSNA). We measured REE before and after beta-blockade in female subjects approximately three years post-gastric bypass surgery and in female obese individuals for comparison. We also measured MSNA in a subset of these subjects. The gastric bypass subjects had no change in REE after systemic beta-blockade, reflecting a lack of sympathetic support of REE, in contrast to obese subjects where REE was reduced by beta-blockade by approximately 5% (P<0.05). The gastric bypass subjects, while still overweight (BMI = 29.3 vs 38.0 kg/m2 for obese subjects, P<0.05), also had significantly lower MSNA compared to obese subjects (10.9 ± 2.3 vs. 21.9 ± 4.1 bursts/min, P<0.05). The reasons for low MSNA and a lack of sympathetically mediated support of REE after gastric bypass are likely multifactorial and may be related to changes in insulin sensitivity, body composition, and leptin, among other factors. These findings may have important consequences for the maintenance of weight loss after gastric bypass. Longitudinal studies are needed to further explore the changes in sympathetic support of REE and if changes in MSNA or tissue responsiveness are related to the sympathetic support of REE.
Keywords: Obesity, weight loss, beta-adrenergic antagonists
Introduction
It has been demonstrated that a component of resting energy expenditure (REE) is dependent on the sympathetic nervous system 1. Specifically, systemic administration of the non-specific beta-adrenergic receptor antagonist propranolol reduces REE between 3–5% 2. In the context of obesity, it has been hypothesized that the increase in resting sympathetic neural activity (tone) that occurs in response to weight gain is an adaptive mechanism to increase REE and promote restoration of weight back to a given set-point 3. However, there is evidence that an increase in sympathetic tone is not always associated with an increase in the sympathetic component of REE. For example, aging is associated with increased baseline sympathetic activity 4 but a smaller sympathetically mediated component of REE 5 that appears to be due to a decrease in tissue adrenergic responsiveness induced by chronic sympathetic activation 6. Thus, the role of sympathetic activation in maintaining weight balance is not clear. Importantly, sympathetic control of REE after bariatric surgery has not been studied.
Bariatric surgery is an increasingly common treatment for patients with medically complicated obesity who have difficulty losing weight by other means. The success of this surgery is related to both the sustained weight loss and the improvements in cardiovascular and metabolic function that occur relatively rapidly after the surgery. In this context, while indirect indices of sympathetic activity have been shown to be lower after various types of bariatric surgery 7–9, similar to the effects of weight loss induced by dieting and exercise 10, sympathetic activity after gastric bypass has not been directly measured.
The goals of the present study were to compare the contribution of the sympathetic nervous system to REE between individuals who had undergone gastric bypass surgery and obese subjects/individuals. The sympathetic component of REE was determined by measuring REE before and after systemic beta-adrenergic receptor blockade. A second goal of this study was to compare levels of sympathetic neural activity between these same groups of individuals and determine the relationship between sympathetic activity and the sympathetic support of REE. Microneurography was used to measure multi-unit muscle sympathetic nerve activity (MSNA) as a marker of baseline sympathetic activity. We hypothesized that gastric bypass surgery would be associated with an increase in the sympathetic component of REE compared to that of obese individuals. We further hypothesized that the increase in sympathetically mediated REE would be associated with lower levels of MSNA compared with obese subjects, reflecting upregulation of adrenergic responsiveness.
Materials and Methods
The Mayo Institutional Review Board (IRB) approved the study and the subjects gave written informed consent. The Clinical Research Unit (CRU) of the Mayo Clinic Center for Translational Science Activities (CTSA) was used for this study.
Subjects
Subjects between the ages of 18 and 45 years were included in this study. As we were only able to recruit women for the gastric bypass group, consistent with the fact that approximately 85% of the individuals who undergo bariatric surgery are women11, we only studied female subjects. Seventeen subjects who had undergone gastric bypass (GB) at least twelve months prior were recruited from a database of patients who had open or laparoscopic proximal Roux-en-Y (limb length ~150 cm) gastric bypass operations at Mayo Clinic for medically complicated obesity refractory to behavioral modification. The average BMI of the gastric bypass subjects prior to their surgery was 45.7 ± 1.2 kg/m2. The average time from surgery to their study day was 39±5 months and their weight at the time of study was 36 ± 2% less than their pre-surgical weight. We also recruited nineteen healthy obese subjects (BMI > 35 kg/m2). Five of the obese control subjects had previously undergone either abdominal and/or gynecological surgical procedures that were not related to weight loss surgery and none had procedures done within nine months of the study.
Individuals allergic to the drugs used in this study or known to have metabolic, neurological, pulmonary, renal, or cardiovascular diseases were ineligible for the study. Subjects who smoked or were taking any drugs known to affect metabolic, cardiovascular, or neurological function were excluded. Female subjects had a confirmed negative pregnancy test within 48 hrs of any experiment and were studied during the early follicular phase of the menstrual cycle or days 3–7 of the placebo phase of oral contraceptive therapy. Each subject underwent a medical history and a physical exam by a study physician and had a fasting lipid panel, glucose, and creatinine measured and a resting electrocardiogram performed. Persons taking part in an endurance training program (aerobic exercise > 60 min/day and > 5 days/week for > 1 month) were also excluded.
Body composition measurements were performed prior to the study day. Whole-body and regional fat mass, and fat-free mass were measured with dual-energy X-ray absorptiometry (DEXA) (DPX-IQ, Lunar Radiation, Madison, WI). Single-slice CT scans were performed at abdomen (second lumbar vertebrae level) and quadriceps (mid-thigh) levels to measure visceral, abdominal subcutaneous, and thigh fat content 12.
Sympathetic support of REE was measured in all subjects as described below. Resting MSNA was measured as described below in 25 of the subjects (obese = 14, gastric bypass = 11) on the same day prior to measuring the sympathetic support of REE. For three days preceding these measurements, subjects received weight-maintenance meals provided by the CRU metabolic kitchen with a macronutrient distribution of 50% carbohydrate, 20% protein, and 30% fat. Total meal energy was calculated as the sum of basal energy expenditure (estimated from the Harris-Benedict equation) and estimated energy expenditure from activity.
Studies were conducted at the same time each day (starting at 0700 hrs). Subjects were studied fasting except for water after midnight. An intravenous catheter was placed into a hand vein for drug administration. In subjects undergoing measurements of MSNA, a 20-gauge, 5-cm catheter was placed into the brachial artery under aseptic conditions using ultrasound guidance and local anesthesia (2% lidocaine). Subjects were studied in a supine or semi-recumbent position at rest with care taken to minimize distractions. Throughout the study, heart rate (HR) was measured from a 3-lead ECG. Blood pressure (BP) was measured using automated sphygmomanometry every 5 min (Cardiocap/5, Datex-Ohmeda, Louisville, CO) or from the arterial catheter, if present. Blood samples were drawn for the measurement of plasma renin activity and leptin, insulin, aldosterone, epinephrine, and norepinephrine concentrations. Standard assays were performed by the Immunochemistry Core Laboratory of the Clinical Research Unit of the Mayo Clinic CTSA and the Endocrine Laboratory of the Mayo Clinic Department of Laboratory Medicine and Pathology.
Measurement of the sympathetic support of REE
After a rest period, an intravenous bolus of saline was given followed by a continuous intravenous infusion of saline for 30 min during which HR and BP were measured and measurements of REE were performed by trained respiratory therapists using indirect calorimetry (Deltatrack, Sensormedics, Yorba Linda, CA). After the control measurements were complete, a bolus of propranolol (non-selective beta-adrenergic antagonist) was given at the dose of 0.25 mg/kg followed by a continuous intravenous infusion of 0.004 mg/kg/min for 30 min during which measurements of REE, HR, and BP were repeated. This dose of propranolol has been used in previous studies of the sympathetic support of REE and has been shown to result in complete beta-adrenergic blockade based on both plasma concentrations of propranolol and a lack of a response to exogenous adrenergic agonists 2. The volumes of the saline bolus and infusion administered were equivolume to propranolol. The order of infusions was not randomized due to the long duration of action of propranolol. The sympathetic support of energy expenditure was calculated as the difference in REE measured between saline and propranolol infusions.
Measurements of MSNA
Multiunit MSNA was recorded with a tungsten microelectrode in the peroneal nerve, posterior to the fibular head, as previously described 13. Real-time, 2-dimensional ultrasound guidance (M-Turbo, SonoSite Inc., Bothell, WA) was used in some subjects to place the microelectrode into the peroneal nerve under direct visualization 14. The signal was amplified 80,000-fold, band-pass filtered (700 to 2000 Hz), rectified and integrated (resistance-capacitance integrator circuit, time constant 0.1 sec) by a nerve-traffic analyzer. Data were recorded at 250 Hz using a computer data acquisition system (WinDaq, DATAQ Instruments, Akron, OH) and stored for off-line analysis. Sympathetic bursts in the integrated neurogram were identified using a custom-manufactured automated analysis program 15. A single observer then corrected burst identification to eliminate artifacts. The program then compensated for baroreflex latency and associated each sympathetic burst with the appropriate cardiac cycle. MSNA was quantified as bursts per minute (burst frequency) and bursts per 100 heartbeats (burst incidence).
All data are presented as mean ± SEM. Paired t-tests were used to compare energy expenditure during saline and propranolol infusions. Tukey tests and t-tests were used for pair-wise comparisons. To access the relationships between sympathetic support of REE and MSNA and other variables, linear regression analysis was performed and Pearson correlation coefficients calculated. P values < 0.05 were considered significant.
Results
Subject characteristics and baseline laboratory values are shown in Table 1. At baseline, there was no difference between the gastric bypass and obese groups in resting mean arterial pressure. Systemic administration of propranolol resulted in a significant decrease in heart rate of 8 ± 1 beats/min (P < 0.0001) but no change in mean arterial blood pressure. There was no difference in the change in heart rate during propranolol infusion between the groups.
Table 1.
Baseline characteristics of subjects and fasting laboratory values.
| Obese | GBS | |
|---|---|---|
| Age (yrs) | 31.5±2.3 | 37.1±1.4* |
| Height (cm) | 168.8±1.7 | 167.3±1.4 |
| Weight (kg) | 108.4±3.7 | 81.8±3.1* |
| BMI (kg/m2) | 38.0±0.9 | 29.3±1.2* |
| Body Fat (%) | 55.0±1.5 | 42.8±2.2* |
| Fat Mass (kg) | 57.6±2.9 | 34.2±3* |
| Lean Body Mass (kg) | 27.9±1.3 | 24.8±0.8 |
| Abdominal Visceral Fat (cm2) | 124.7±11.8 | 54.1±8.9* |
| Total Abdominal Fat (cm2) | 614.8±36.3 | 355.7±37.0* |
| Subcutaneous Abdominal Fat (cm2) | 486.6±33.0 | 301.6±30.3* |
| HR (beats/min) | 69.8±1.9 | 65.5±1.9 |
| MAP (mmHg) | 85.3±3.5 | 80.3±3.0 |
| Glucose (mmol/L) | 86.5±2.7 | 87.4±2.6 |
| Cholesterol (mg/dL) | 179±7 | 166±5 |
| Triglycerides (mg/dL) | 93±11 | 84±7 |
| Insulin (pmol/L) | 11.5±1.0 | 3.9±0.4* |
| HDL (mg/dL) | 48±4 | 66±3* |
| LDL (mg/dL) | 113±5 | 83±5* |
| Renin (ng/mL) | 1.4±0.3 | 1.2±0.4 |
| Leptin (ng/mL) | 48.1±4.4 | 22.4±3.7* |
| Aldosterone (ng/mL) | 7.8±1.7 | 7.2±1.8 |
| Epinephrine (ng/mL) | 20.2±4.6 | 26±2.4 |
| Norepinephrine (ng/mL) | 155.2±19.1 | 101.6±12.9* |
Data are mean ± SEM. BMI = body mass index, HR = heart rate, MAP = mean arterial pressure, HDL = high density lipoprotein, LDL = low density lipoprotein.
= P < 0.05 vs. obese
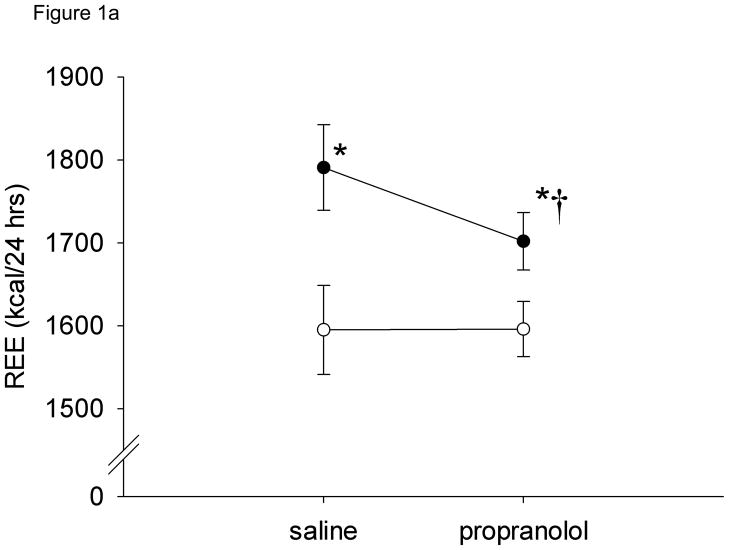
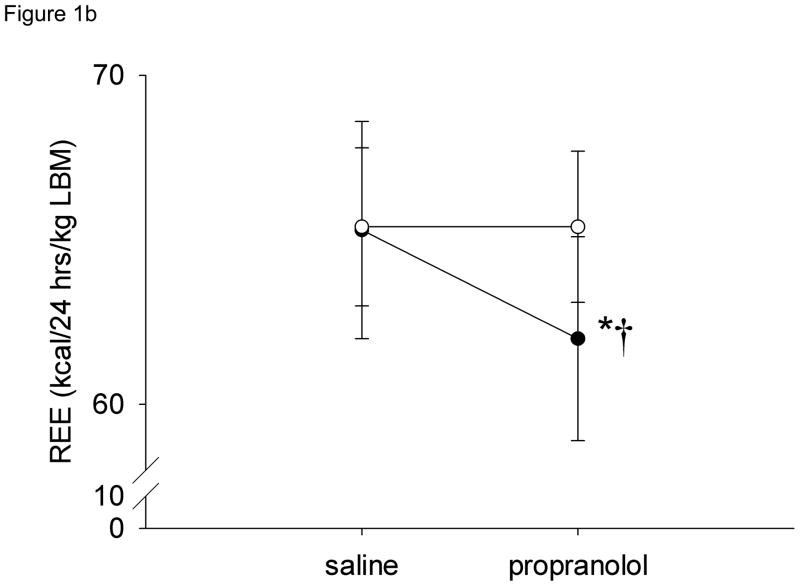
Resting energy expenditure (kcal/24 hrs) during saline (control) infusions was significantly greater in obese subjects (1816.8 ± 53.5) compared to gastric bypass subjects (1595 ± 48.4) (P < 0.05) (Figure 1a) but when expressed per kg of lean body mass, REE was similar between the groups (Figure 1b). Resting energy expenditure significantly decreased 5.0 ± 0.8 % after systemic beta-adrenergic blockade in obese subjects (−89 ± 14.3 kcal/24 hrs) but there was no change in REE in the gastric bypass subjects (Figure 1a). Similar results were found when the data were adjusted for lean body mass (Figure 1b).
Figure 1.
Resting energy expenditure (REE) during saline and propranolol infusions by subject group. a) absolute REE, b) REE normalized to lean body mass. * = P < 0.05 vs. obese, † = P < 0.05 vs. saline. Open circles = gastric bypass, closed circles = obese
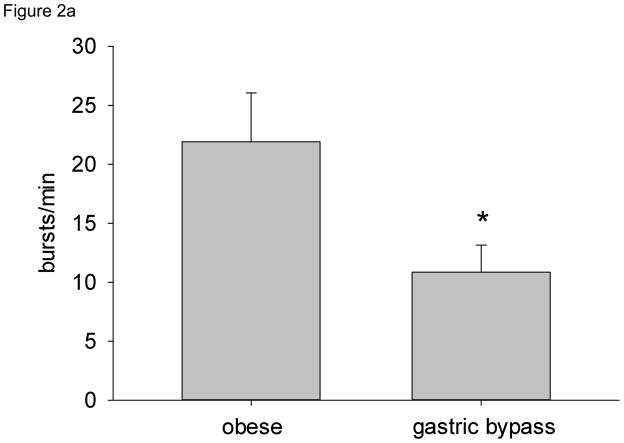
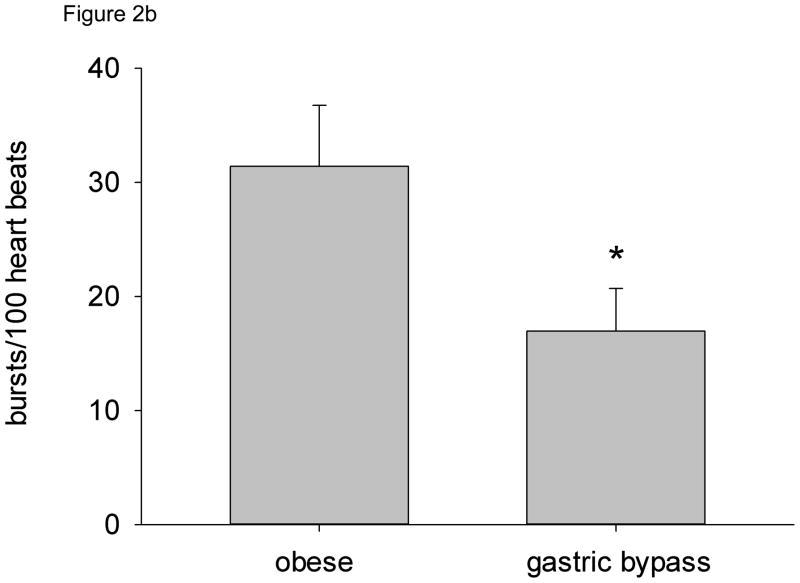
MSNA (both burst frequency and bursts/100 heart beats) was significantly lower in gastric bypass subjects compared with obese individuals (Figure 2a and 2b). Plasma norepinephrine concentrations were also significantly lower in gastric bypass subjects compared with obese subjects (Table 1). There was no relationship between MSNA and age. When all subjects were considered, resting MSNA showed strong and significant relationships to indices of fatness, including BMI (R2 = 0.32, P < 0.01), % body fat (R2 = 0.38, P < 0.01), total fat mass (R2 = 0.37, P < 0.01), and abdominal fat subcutaneous fat (R2 = 0.38, P < 0.01). We also found a significant association between plasma leptin and insulin concentrations and MSNA (bursts/min) (R2 = 0.31, P < 0.05 and R2 = 0.30, P < 0.05, respectively).
Figure 2.
Resting MSNA by subject group. a) Burst frequency (bursts/min), b) Burst incidence (bursts/100 heart beats). * = P < 0.05 vs. obese.
Since age is know to be associated with a decrease in the sympathetic support of energy expenditure 5 as well as an increase in muscle sympathetic nerve activity 16 and there was a small but significant difference in the age between the obese and gastric bypass subjects in this study, we repeated all group comparisons using multivariable methods with age included as a covariate. Using this approach, we again found significant differences between the obese and gastric bypass subjects in the change in energy expenditure during systemic beta-adrenergic blockade (P<0.01) and in resting MSNA (bursts/min and bursts/100 heart beats, P<0.05, both).
Overall, REE was not related to resting MSNA. Similarly, there was no overall relationship between MSNA and the change in REE after beta-blockade. However, when the data were analyzed by subject group, we did find a strong and significant inverse relationship between MSNA and the change in REE after beta-blockade in the obese (R2 = 0.48, P < 0.05) but not gastric bypass subjects.
Discussion
This is the first study to have measured the sympathetic component of REE in individuals who have undergone gastric bypass and the first to use microneurography to directly measure sympathetic activity in this population. The main finding of this study was that, unlike obese subjects, subjects who had undergone gastric bypass had no sympathetic component of REE (i.e., no change in REE with beta blockade). A second important finding was that resting sympathetic activity (measured as MSNA) was lower in gastric bypass subjects compared with obese subjects. Finally, we found that there was a relationship between the sympathetic support of REE and MSNA in obese subjects, but this relationship was absent in the gastric bypass subjects.
As expected, the sympathetic component of REE was approximately 5% of the total REE in the obese subject groups, similar to previous studies 6. However, we were surprised to find that that there was a complete lack of sympathetic support of REE in the gastric bypass subject group. It has been shown that high levels of MSNA in older (lean) individuals are paradoxically associated with a very low sympathetic support of REE 5 and that older individuals have a lower increase in energy expenditure in response to exogenous adrenergic agonists 17. This suggests that the low sympathetic support of REE in older individuals is due to a decreased responsiveness in the tissues to sympathetic stimulation as a result of chronic elevation in sympathetic tone. Based on these data from older lean subjects and the fact that gastric bypass surgery has been shown to increase the skin vasoconstrictor response to deep inspiration 18 (indicating that the response to sympathetic activation is increased after gastric bypass), we had hypothesized that lower levels of MSNA might result in greater sympathetic support of REE. Instead, we found that the low levels of resting MSNA in this group seemed to translate directly into a lower sympathetic support of REE. The combination of lower sympathetic activity and reduced sympathetic component of REE is similar to that seen during energy restriction, low activity levels, and low energy flux (low caloric intake accompanied by low energy expenditure) 5, 19. Preliminary data from our lab suggests that the gastric bypass subjects have a low level of daily physical activity 20. This indicates that low energy flux and low activity levels may be responsible for the low sympathetic support of metabolism. Further studies are needed to determine if following gastric bypass, individuals have the ability to increase their energy expenditure in response to sympathetic stimulation.
In our study, the subjects who had undergone gastric bypass surgery had lower levels of MSNA than the obese subjects and it has been shown that body fat is a major determinant of MSNA 21. While the gastric bypass subjects had a large percentage of body fat for their body mass, our data supports the idea that the relative distribution of body fat is important. Central obesity is particularly associated with sympathetic activation 22 and abdominal fat was highly correlated with MSNA in the current study. The gastric bypass subjects, despite the fact that they still had a relatively large amount of body fat, had approximately half the abdominal fat compared with the obese subjects and an even lower proportion of visceral fat. One possible explanation for the increased MSNA in abdominal obesity that has been proposed is an increase in leptin secretion from this adipose tissue 23, 24. We also found a significant relationship between leptin and MSNA in this study, consistent with this theory.
We also found that both plasma insulin and leptin levels were lower in the gastric bypass subjects compared with the obese subjects and were related to MSNA levels. We have previously shown in a similar group of subjects who had undergone gastric bypass surgery that insulin sensitivity, particularly the insulin sensitivity of lipolysis, and fasting insulin levels are almost equal to that of young lean persons 25. Insulin stimulates sympathetic activity 26, 27 and improved insulin sensitivity (and the resulting decrease in plasma insulin levels) after weight loss has been associated with a decrease in MSNA 28. Free fatty acids (FFA) have been shown to increase MSNA 29 and greater suppression of lipolysis by insulin may have contributed to the lower levels of MSNA in this group.
This is also the first study to provide data regarding the relationship of MSNA with the sympathetic support of REE. The strong inverse relationship between MSNA and the sympathetic support of REE in the obese subjects indicates that obesity-associated increases in sympathetic nerve activity resulted in lower sympathetic support of REE. Similar results of an inverse relationship between plasma norepinephrine concentration and the sympathetic support of REE have been reported before 2. While it has been proposed that the increased sympathetic tone associated with obesity is an adaptive response that increases REE in order to maintain a normal weight 30, our results support the idea that the sustained sympathetic activation that occurs during chronic obesity results instead in a reduced sympathetic responsiveness 28, 31. The lack of a relationship between MSNA and the sympathetic support of REE in the gastric bypass group suggests an uncoupling of sympathetic activity and its support of REE by massive and sustained weight loss.
Although the sympathetic component of REE seems small compared with total REE, the “extra” 90 kcal/day that the obese group expends via sympathetically mediated pathways translates into approximately 4.3 kg of body weight per year. The importance (for body weight) of the sympathetically mediated component of REE is demonstrated by the fact that individuals who start chronic beta-adrenergic antagonist therapy demonstrate a 1 kg weight gain after only five months 32. Weight reaches its nadir approximately two years after gastric bypass surgery at which time some weight gain is commonly seen 33. It may be that the low level of sympathetic support of REE we have shown is partially responsible for long-term weight regain after gastric bypass surgery. Alternatively, it may be that individuals who require gastric bypass to lose weight have a fundamental inability to increase energy expenditure via sympathetic stimulation.
There are some limitations to our study: First, it was not a prospective study and therefore the time course of the effects of gastric bypass surgery on MSNA and sympathetic support of REE cannot be determined. Thus, we cannot determine from the present data whether a lack of a sympathetic component of REE in this group contributed to their extreme obesity and need for gastric bypass surgery, or was the result of their weight loss. Further studies are needed to further evaluate this and related questions. Second, although we attempted to match for age, there was a small but significant difference in age between the obese and post-gastric bypass subjects due to difficulties recruiting healthy obese subjects and younger gastric bypass subjects. Although age is generally associated with increased MSNA and age > 60 has been shown to be associated with a decreased sympathetic support of REE 5, the gastric bypass subjects were relatively close in age to the obese subjects and also had lower (not greater) levels of MSNA. The results of performing group comparisons using multivariable methods with age included as a covariate suggest that the small difference in age did not affect our results. Finally, we did not match subjects by body composition, activity levels, or leptin and insulin concentrations. Unfortunately, the relatively small numbers of subjects in each study group do not allow meaningful further multivariable modeling to be being performed. Further studies are needed to determine what specific factors are responsible for the differences we observed in our study.
In conclusion, gastric bypass is associated with an almost complete absence of sympathetic contribution to REE and lower levels of resting sympathetic activity. Just as the increase in MSNA in obesity is likely multifactorial, the effect of gastric bypass on MSNA probably is as well. The physiological and/or pathophysiological consequences of the low sympathetic nervous system activity after gastric bypass are not known. The lack of sympathetic contribution to REE may be related to lower sympathetic activity or tissue responsiveness, and may have implications for weight gain after gastric bypass surgery. Similarly, whether or not weight loss achieved via behavior modifications such as caloric restriction and/or exercise has similar effects on the sympathetic support of energy expenditure needs to be explored.
Acknowledgments
Special thanks to Dr. J. Eisenach, Dr. N. Nicholson, and D. Schroeder of Mayo Clinic for support in designing and conducting the studies. We also thank S. Roberts, J. Knutson, K. Krucker, K. Edens, C. Johnson, N. Strom, H. Tonyan, B. Walker, L. Matzek, B. Kluck, P. Engrav, N. Meyer, and D. Vlazny of Mayo Clinic for their assistance in conducting the studies and/or preparing the manuscript.
The project described was supported by the Mayo Foundation for Medical Education and Research and NIH Grant Numbers K23 DK82424, R01 HL67933, R01 HL083947, and UL1 RR024150. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of Mayo Foundation or the NIH.
References
- 1.Shibao C, Gamboa A, Diedrich A, et al. Autonomic contribution to blood pressure and metabolism in obesity. Hypertension. 2007;49:27–33. doi: 10.1161/01.HYP.0000251679.87348.05. [DOI] [PubMed] [Google Scholar]
- 2.Monroe MB, Seals DR, Shapiro LF, Bell C, Johnson D, Parker Jones P. Direct evidence for tonic sympathetic support of resting metabolic rate in healthy adult humans. Am J Physiol Endocrinol Metab. 2001;280:E740–4. doi: 10.1152/ajpendo.2001.280.5.E740. [DOI] [PubMed] [Google Scholar]
- 3.Landsberg L. Diet, obesity and hypertension: an hypothesis involving insulin, the sympathetic nervous system, and adaptive thermogenesis. Q J Med. 1986;61:1081–90. [PubMed] [Google Scholar]
- 4.Fagius J, Wallin BG. Long-term variability and reproducibility of resting human muscle nerve sympathetic activity at rest, as reassessed after a decade. Clin Auton Res. 1993;13(3):245–9. 201–5. doi: 10.1007/BF01826234. [DOI] [PubMed] [Google Scholar]
- 5.Bell C, Seals DR, Monroe MB, et al. Tonic sympathetic support of metabolic rate is attenuated with age, sedentary lifestyle, and female sex in healthy adults. J Clin Endocrinol Metab. 2001;86:4440–4. doi: 10.1210/jcem.86.9.7855. [DOI] [PubMed] [Google Scholar]
- 6.Seals DR, Bell C. Chronic sympathetic activation: consequence and cause of age-associated obesity? Diabetes. 2004;53:276–84. doi: 10.2337/diabetes.53.2.276. [DOI] [PubMed] [Google Scholar]
- 7.Karason K, Molgaard H, Wikstrand J, Sjostrom L. Heart rate variability in obesity and the effect of weight loss. Am J Cardiol. 1999;83:1242–7. doi: 10.1016/s0002-9149(99)00066-1. [DOI] [PubMed] [Google Scholar]
- 8.Machado MB, Velasco IT, Scalabrini-Neto A. Gastric bypass and cardiac autonomic activity: influence of gender and age. Obes Surg. 2009;19:332–8. doi: 10.1007/s11695-008-9665-x. [DOI] [PubMed] [Google Scholar]
- 9.Pontiroli AE, Pizzocri P, Paroni R, Folli F. Sympathetic overactivity, endothelial dysfunction, inflammation, and metabolic abnormalities cluster in grade III (World Health Organization) obesity: reversal through sustained weight loss obtained with laparoscopic adjustable gastric banding. Diabetes Care. 2006;29:2735–8. doi: 10.2337/dc06-1417. [DOI] [PubMed] [Google Scholar]
- 10.Straznicky NE, Grima MT, Eikelis N, et al. The effects of weight loss versus weight loss maintenance on sympathetic nervous system activity and metabolic syndrome components. J Clin Endocrinol Metab. 2011;96:E503–8. doi: 10.1210/jc.2010-2204. [DOI] [PubMed] [Google Scholar]
- 11.Samuel I, Mason EE, Renquist KE, Huang YH, Zimmerman MB, Jamal M. Bariatric surgery trends: an 18-year report from the International Bariatric Surgery Registry. Am J Surg. 2006;192:657–62. doi: 10.1016/j.amjsurg.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Jensen MD, Kanaley JA, Reed JE, Sheedy PF. Measurement of abdominal and visceral fat with computed tomography and dual-energy x-ray absorptiometry. Am J Clin Nutr. 1995;61:274–8. doi: 10.1093/ajcn/61.2.274. [DOI] [PubMed] [Google Scholar]
- 13.Sundlof G, Wallin BG. Muscle-nerve sympathetic activity in man. Relationship to blood pressure in resting normo- and hyper-tensive subjects. Clin Sci Mol Med Suppl. 1978;4:387s–9s. doi: 10.1042/cs055387s. [DOI] [PubMed] [Google Scholar]
- 14.Curry TB, Charkoudian N. The use of real-time ultrasound in microneurography. Autonomic neuroscience : basic & clinical. 2011;162:89–93. doi: 10.1016/j.autneu.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kienbaum P, Karlssonn T, Sverrisdottir YB, Elam M, Wallin BG. Two sites for modulation of human sympathetic activity by arterial baroreceptors? J Physiol (Lond) 2001;531:861–9. doi: 10.1111/j.1469-7793.2001.0861h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ng AV, Callister R, Johnson DG, Seals DR. Age and gender influence muscle sympathetic nerve activity at rest in healthy humans. Hypertension. 1993;21:498–503. doi: 10.1161/01.hyp.21.4.498. [DOI] [PubMed] [Google Scholar]
- 17.Kerckhoffs DA, Blaak EE, Van Baak MA, Saris WH. Effect of aging on beta-adrenergically mediated thermogenesis in men. The American journal of physiology. 1998;274:E1075–9. doi: 10.1152/ajpendo.1998.274.6.E1075. [DOI] [PubMed] [Google Scholar]
- 18.Bobbioni-Harsch E, Bongard O, Habicht F, et al. Relationship between sympathetic reactivity and body weight loss in morbidly obese subjects. Int J Obes Relat Metab Disord. 2004;28:906–11. doi: 10.1038/sj.ijo.0802620. [DOI] [PubMed] [Google Scholar]
- 19.Bell C, Day DS, Jones PP, et al. High energy flux mediates the tonically augmented beta-adrenergic support of resting metabolic rate in habitually exercising older adults. J Clin Endocrinol Metab. 2004;89:3573–8. doi: 10.1210/jc.2003-032146. [DOI] [PubMed] [Google Scholar]
- 20.Ramirez-Marrero F, Somaraju M, Vaa B, Joyner M, Curry T. Self-reported and accelerometer determined physical activity in patients after gastric bypass, obese, and non-obese controls [Abstract] Med Sci Sports Exerc. 2009;41:126. [Google Scholar]
- 21.Scherrer U, Randin D, Tappy L, Vollenweider P, Jequier E, Nicod P. Body fat and sympathetic nerve activity in healthy subjects. Circulation. 1994;89:2634–40. doi: 10.1161/01.cir.89.6.2634. [DOI] [PubMed] [Google Scholar]
- 22.Grassi G, Dell’Oro R, Facchini A, Quarti Trevano F, Bolla GB, Mancia G. Effect of central and peripheral body fat distribution on sympathetic and baroreflex function in obese normotensives. J Hypertens. 2004;22:2363–9. doi: 10.1097/00004872-200412000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Monroe MB, Van Pelt RE, Schiller BC, Seals DR, Jones PP. Relation of leptin and insulin to adiposity-associated elevations in sympathetic activity with age in humans. Int J Obes Relat Metab Disord. 2000;24:1183–7. doi: 10.1038/sj.ijo.0801364. [DOI] [PubMed] [Google Scholar]
- 24.Snitker S, Pratley RE, Nicolson M, Tataranni PA, Ravussin E. Relationship between muscle sympathetic nerve activity and plasma leptin concentration. Obes Res. 1997;5:338–40. doi: 10.1002/j.1550-8528.1997.tb00561.x. [DOI] [PubMed] [Google Scholar]
- 25.Curry TB, Roberts SK, Basu R, et al. Gastric bypass surgery is associated with near-normal insulin suppression of lipolysis in nondiabetic individuals. Am J Physiol Endocrinol Metab. 2011;300:E746–51. doi: 10.1152/ajpendo.00596.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez GE, Ballard TP, Beske SD, Davy KP. Subcutaneous obesity is not associated with sympathetic neural activation. Am J Physiol Heart Circ Physiol. 2004;287:H414–8. doi: 10.1152/ajpheart.01046.2003. [DOI] [PubMed] [Google Scholar]
- 27.Scherrer U, Sartori C. Insulin as a vascular and sympathoexcitatory hormone: implications for blood pressure regulation, insulin sensitivity, and cardiovascular morbidity. Circulation. 1997;96:4104–13. doi: 10.1161/01.cir.96.11.4104. [DOI] [PubMed] [Google Scholar]
- 28.Straznicky NE, Lambert EA, Lambert GW, Masuo K, Esler MD, Nestel PJ. Effects of dietary weight loss on sympathetic activity and cardiac risk factors associated with the metabolic syndrome. J Clin Endocrinol Metab. 2005;90:5998–6005. doi: 10.1210/jc.2005-0961. [DOI] [PubMed] [Google Scholar]
- 29.Florian JP, Pawelczyk JA. Non-esterified fatty acids increase arterial pressure via central sympathetic activation in humans. Clin Sci (Colch) 2009;118:61–9. doi: 10.1042/CS20090063. [DOI] [PubMed] [Google Scholar]
- 30.Landsberg L. Insulin-mediated sympathetic stimulation: role in the pathogenesis of obesity-related hypertension. J Hypertens. 2001;19:523–8. doi: 10.1097/00004872-200103001-00001. [DOI] [PubMed] [Google Scholar]
- 31.Valentini M, Julius S, Palatini P, et al. Attenuation of haemodynamic, metabolic and energy expenditure responses to isoproterenol in patients with hypertension. J Hypertens. 2004;22:1999–2006. doi: 10.1097/00004872-200410000-00024. [DOI] [PubMed] [Google Scholar]
- 32.Messerli FH, Bell DS, Fonseca V, et al. Body weight changes with beta-blocker use: results from GEMINI. Am J Med. 2007;120:610–5. doi: 10.1016/j.amjmed.2006.10.017. [DOI] [PubMed] [Google Scholar]
- 33.Christou NV, Look D, Maclean LD. Weight gain after short- and long-limb gastric bypass in patients followed for longer than 10 years. Ann Surg. 2006;244:734–40. doi: 10.1097/01.sla.0000217592.04061.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]