Abstract
Background
Cardiovascular disease is a leading cause of death worldwide. Arrhythmias are associated with significant morbidity and mortality related to cardiovascular disease. Recent work illustrates that many cardiac arrhythmias are initiated by a pathologic imbalance between kinase and phosphatase activities in excitable cardiomyocytes.
Objective
We tested the relationship between myocyte kinase/phosphatase imbalance and cellular and whole animal arrhythmia phenotypes associated with ankyrin-B cardiac syndrome.
Methods
Using a combination of biochemical, electrophysiological, and in vivo approaches, we tested the ability of CaMKII inhibition to rescue imbalance in kinase/phosphatase pathways associated with human ankyrin-B-associated cardiac arrhythmia.
Results
The cardiac ryanodine receptor (RyR2), a validated target of kinase/phosphatase regulation in myocytes, displays abnormal CaMKII-dependent phosphorylation (pS2814 hyperphosphorylation) in ankyrin-B+/− heart. Notably, RyR2 dysregulation is rescued in myocytes from ankyrin-B+/− mice overexpressing a potent CaMKII-inhibitory peptide (AC3I) and aberrant RyR2 open probability observed in ankyrin-B+/− hearts is normalized by treatment with the CaMKII inhibitor KN-93. CaMKII-inhibition is sufficient to rescue abnormalities in ankyrin-B+/− myocyte electrical dysfunction including cellular afterdepolarizations, and significantly blunts whole animal cardiac arrhythmias and sudden death in response to elevated sympathetic tone.
Conclusions
These findings illustrate the complexity of the molecular components involved in human arrhythmia and define regulatory elements of the ankyrin-B pathway in pathophysiology. Furthermore, the findings illustrate the potential impact of CaMKII-inhibition in the treatment of a congenital form of human cardiac arrhythmia.
Keywords: ankyrin, CaMKII, ryanodine receptor, spectrin, arrhythmia
Introduction
Cardiovascular disease is the leading cause of death in the United States, resulting in more than a third of all annual deaths.1 Moreover, cardiovascular complications account for an estimated $40 billion in direct and indirect healthcare costs in the United States.1 Over half of heart disease fatalities are due to cardiac electrical and/or structural defects; therefore understanding the mechanisms underlying potentially fatal cardiac arrhythmias is paramount for uncovering new therapies.
Ankyrins play key roles in cardiac structural and electrical regulation. Three ankyrin genes encode ankyrin polypeptides (ankyrin-R, -B, and –G) with specific functions in cardiac physiology. Ankyrin-R isoforms are linked with organization and regulation of the sarcoplasmic reticulum (SR) and structural proteins obscurin and titin.2, 3 Ankyrin-G polypeptides target voltage-gated Na+ channels to myocyte intercalated disc membranes and dysfunction in ankyrin-G pathways is linked with the Brugada Syndrome.4-7 Finally, ankyrin-B is found at cardiac transverse-tubule/SR junctions and regulates the local organization of multiple structural and electrical proteins.8 Ankyrin-B dysfunction is linked to acquired and congenital forms of human arrhythmia including sinus node disease, atrial fibrillation, ventricular tachycardia, and sudden cardiac death.9-13
Individuals harboring ankyrin-B loss-of-function mutations and ankyrin-B deficient mice display catecholamine-induced ventricular arrhythmia (CPVT).12, 13 However, the multifunctional nature of ankyrin-B complicates the process of linking ankyrin-B dysfunction to specific molecular targets in disease. Previously, we identified defects in ankyrin-B dependent targeting of Na/K ATPase and Na/Ca exchanger as important in providing a pro-arrhythmic substrate in vivo.14-16 However, strategies to define additional molecular events that support ankyrin-B-dependent arrhythmia in response to increased sympathetic tone have been unsuccessful. In this study, we utilized a combination of biochemical, electrophysiological, and in vivo approaches to identify a role for kinase/phosphatase imbalance in ankyrin-based arrhythmia at the levels of the myocyte and whole animal. We show that ankyrin-B deficiency results in ryanodine receptor hyperphosphorylation, and link this altered post-translational modification state with the calcium/calmodulin-dependent kinase (CaMKII). Utilizing AC3I mice that overexpress a potent CaMKII inhibitory peptide17, we demonstrate that CaMKII inhibition is sufficient to rescue biochemical, cellular, and whole animal defects and is potent at preventing ankyrin-based cellular afterdepolarizations and fatal cardiac arrhythmias. In summary, these findings define new regulatory roles for ankyrin-B in the cardiac myocyte in the regulation of local CaMKII function in the cardiac dyad.
Methods
Electrophysiology
Current recordings were measured by conventional whole-cell patch-clamp technique with an Axon 200B patch-clamp amplifier controlled by a computer using a Digidata 1320A acquisition board driven by pClamp 8.0 software (Axon Instruments, Foster City, CA). Action potentials (APs) were evoked by brief current pulses 1.5–4 pA, 0.5–1 ms. AP duration (APD) was assessed as the time from the AP upstroke to 90% (or 20 and 50%) repolarization to baseline (APD90).18 APs were recorded using the perforated (amphotericin B) patch-clamp technique in Tyrode’s solution (bath) with the pipette filled with (mmol/L): 130 potassium aspartate, 10 NaCl, 10 HEPES, 0.04 CaCl2, 2.0 MgATP, 7.0 phosphocreatine, 0.1 NaGTP, and amphotericin B 240 μg/mL, with the pH adjusted to 7.2 with KOH.19 APs were measured at physiological temperature ±1 μM isoproterenol.20
Statistics
Statistical significance was determined with a paired Student’s t test (2-tailed) or ANOVA with the Bonferroni post-hoc test, when appropriate, for continuous data. The null hypothesis was rejected for p<0.05. For experiments in Figure 5B, contingency tables were generated and statistical significance was determined by Fisher’s exact test. Data for Figure 5C were analyzed via the Mantel-Cox (logrank) test. Statistical analyses were conducted using GraphPad Prism V4 (GraphPad Software Inc., La Jolla, CA) or SigmaPlot.
Figure 5. CaMKII inhibition normalizes ankyrin-B+/− arrhythmia phenotypes.
A) Representative ECG recordings from wild-type, ankyrin-B+/−, AC3I, and ankyrin-B+/− X AC3I mice at baseline and following exercise and treatment with adrenergic agonist. Center recording for ankyrin-B+/− mouse illustrates typical example of arrhythmia lasting minutes from this mouse model. B) Incidence of sustained ventricular arrhythmia in wild-type, ankyrin-B+/−, AC3I, and ankyrin-B+/− X AC3I mice following exercise and treatment with β-adrenergic agonist. In panel B, # denotes p<0.05 for ankyrin-B+/− mice vs. wild-type mice and Δ denotes p<0.05 for ankyrin-B+/− mice vs. ankyrin-B+/− X AC3I mice. C) Survival of wild-type, ankyrin-B+/−, AC3I, and ankyrin-B+/− X AC3I mice following exercise and treatment with adrenergic agonist. In panel C, * denotes p<0.05 of ankyrin-B+/− vs. wild-type mice. For B-C, N values are listed in panels.
Animal models
Mouse models included wild-type C57/Bl6 mice, ankyrin-B+/− mice, AC3I mice, and ankyrin-B+/− mice crossed with AC3I mice.
Single channel recordings
Single channel recordings were performed as described.21 See Supplemental Information for additional details.
Immunoblotting
Immunoblots were done as described.16 See Supplemental Information for additional details.
Antibodies
Custom polyclonal anti-RyR2 pS2808 (1:1000) and anti-RyR2 pS2814 (1:1000) phosphorylated epitope-specific antibodies were generated using the peptide C-RTRRI-(pS)-QTSQV corresponding to the PKA phosphorylation site at RyR2 S2808 and peptide CSQTSQV-(pS)-VD corresponding to CaMKII phosphorylation site at RyR2 S2814, respectively. These custom antibodies have been used extensively and have been well characterized in previous work.21 A monoclonal anti-GAPDH (1:5000, Fitzgerald) and a polyclonal anti-RyR2 antibody (1:1000, Millipore) were also used.
Conscious ECG experiments
ECG recordings of ambulatory animals were obtained using radiotelemetry (DSI) with transmitters implanted subcutaneously and superficial to the peritoneum seven days before recordings. Recording were obtained from mice both at resting conditions, post-exercise and epinephrine injection. For stress tests, animals were run on a treadmill for 1 hour and then injected IP with epinephrine (2 mg/kg). Non-sustained and sustained arrhythmias were identified using standard ECG analysis guidelines.22 Based on prior data linking significant arrhythmia and sudden cardiac death in the ankyrin-B+/− mouse model13, we limited experiments to a minimal number necessary to obtain statistical power. Arrhythmia was defined as sustained if >2 sec.13
Adult cardiomyocyte preparations
Adult cardiomyocytes were prepared as previously described.23 Murine hearts were obtained after animals were euthanized by acute CO2 asphyxiation followed by cervical dislocation in accordance with the Guide for the Care and Use of Laboratory Animals published by the NIH and IACUC approved protocols (Ohio State University).
Results
Ankyrin-B deficient mice display RyR2 hyperphosphorylation
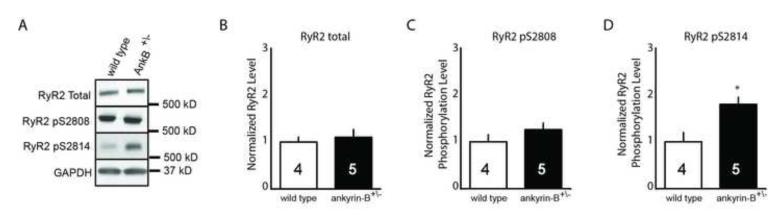
Based on the role of ankyrin-B in regulation of protein phosphatase 2A targeting and regulation in the myocyte transverse-tubule/sarcoplasmic reticulum dyad,24, 25 we hypothesized that aberrant control of local phosphorylation events contributes to ankyrin-B cardiac disease. The cardiac ryanodine receptor (RyR2) is critical for sarcoplasmic reticulum (SR) calcium release, and RyR2 dysfunction is linked with multiple forms of congenital and acquired cardiac pathologies including exercise- and stress-induced ventricular arrhythmias, atrial fibrillation, and heart failure.26-28 Importantly, defects in RyR2 phosphorylation status are linked with abnormal diastolic SR calcium release and arrhythmia.27-29 We first tested whether total RyR2 expression levels were altered in ankyrin-B+/− (display ~50% reduction in ankyrin-B) mouse hearts by immunoblot. As shown in Figure 1, we observed no statistical difference in total RyR2 expression in heart lysates prepared from age- and sex-matched wild-type and ankyrin-B+/− mice (Figure 1; 1.0 vs. 1.04; p=N.S.). We next evaluated the phosphorylation status of the cardiac RyR2 by immunoblot using antibodies directed against two identified RyR2 phosphorylation sites: S2808, previously linked with protein kinase A (PKA) regulation of RyR2 open probability (Po) and function,30 and S2814, a regulatory site for the calcium/calmodulin-dependent kinase II (CaMKII31). We did not observe statistical differences in RyR2 S2808 phosphorylation between wild-type and ankyrin-B+/− cardiac lysates (Figure 1; 1.0 vs. 1.25; p=N.S.). In contrast, RyR2 S2814, was hyperphosphorylated in ankyrin-B+/− cardiac lysates compared with wild-type mouse heart lysates (Figure 1; 1.0 vs. 1.79; p<0.05.). These data suggest that ankyrin-B+/− hearts display aberrant CaMKII-dependent regulation of cardiac RyR2.
Figure 1. Altered RyR2 phosphorylation in ankyrin-B+/− hearts.
A) Representative immunoblots indicating expression of Total RyR2, RyR2 pS2808, and RyR2 pS2814 in wild-type and ankyrin-B+/− hearts. For all experiments, protein values were normalized against internal loading control (GAPDH). B-D) Densitometry analysis indicating the relative levels of total RyR2 (B), RyR2 pS2808 (C), and RyR2 +/− pS2814 (D) expression in WT and ankyrin-B hearts (N values are noted in each panel). In panel D, * denotes p<0.05.
CaMKII is directly linked with abnormal RyR2 phospho-regulation in ankyrin-deficient hearts
CaMKII-dependent phosphorylation of RyR2 is directly linked with catecholamine-triggered arrhythmias.29 Therefore, we evaluated whether RyR2 hyperphosphorylation in ankyrin-B+/− hearts could be directly linked to CaMKII-dependent activity. We assessed the direct role of CaMKII by utilizing AC3-I mice, a transgenic mouse model that overexpresses a potent inhibitor of the CaMKII enzyme in cardiac myocytes.17 We compared the phosphorylation status of RyR2 in wild-type mice, ankyrin-B+/− mice, and ankyrin-B+/− 2 crossed with AC3I mice (ankyrin-B+/− X AC3I). We observed a striking reduction in RyR2 pS2814 phosphorylation in ankyrin-B+/− X AC3I mouse hearts compared with hearts from ankyrin-B+/− littermates (Figure 2, p<0.05). In fact, RyR2 pS2814 levels in ankyrin-B+/− x AC3I mouse hearts were not significantly different from wild-type mouse hearts (Figure 2, p=N.S.). We observed no difference in total RyR2 levels between the three genotypes (Figure 2, p=N.S.). These data support a direct link between RyR2 S2814 hyperphosphorylation and CaMKII in ankyrin-B+/− mice.
Figure 2. CaMKII inhibition normalizes RyR2 hyperphosphorylation in ankyrin-B+/− hearts.
A) Representative immunoblots showing levels of RyR2 pS2814 in wild-type, ankyrin-B+/−, and ankyrin-B+/− X AC3I hearts. Total RyR2 levels were unchanged between the three genotypes studies (p=N.S.). For all experiments, protein values were normalized against internal loading control (GAPDH). Line between lanes in A denotes that data collected from non-contiguous lanes of same gel. B) Densitometric analysis of RyR2 pS2814 in wild-type, ankyrin-B+/−, and AC3I x ankyrin-B+/− animals after treatment with isoproterenol. N values are listed in panel and * represents p<0.05 for wild-type vs. ankyrin-B+/− and ankyrin-B+/− vs. ankyrin-B+/− X AC3I.
CaMKII inhibition rescues ankyrin-based cardiac electrical dysfunction
As noted above, ankyrin-B+/− mice display electrical dysfunction and patients with ankyrin-B loss-of-function mutations display QTc abnormalities and arrhythmia susceptibility.14-16 Moreover, ankyrin-B+/− myocytes display severe defects in myocyte electrical function and afterdepolarizations. 13, 14 Based on our biochemical data, we tested whether CaMKII inhibition was sufficient to prevent electrical dysfunction in primary ankyrin-B+/− adult ventricular cardiomyocytes. We observed mild prolongation of APD90 in ankyrin-B+/− myocytes compared with wild-type myocytes, consistent with QTc interval changes in specific kindreds harboring ANK2 loss-of-function variants13 (Figure 3A-B; 0.5 Hz, p<0.05). Changes in APD90 in ankyrin-B+/− myocytes were exaggerated in response to adrenergic stimulation (Figure 3A-B, p<0.05) and as expected13 we observed significant afterdepolarizations in ankyrin-B+/− myocytes (Figure 3C, p<0.05). In contrast, we observed normalized APD90 in ankyrin-B+/− x AC3I mouse myocytes at baseline compared with myocytes from ankyrin-B+/− littermates (Figure 3A-B, p<0.05). Similar trends were observed for APD20 and APD50 for ankyrin-B+/− X AC3I mice compared with ankyrin-B+/− mice (Supplemental Figure 1). Furthermore, ankyrin-B+/− X AC3I mouse myocytes were resistant to isoproterenol-induced APD prolongation seen in wild-type or ankyrin-B+/− myocytes (Figure 3A-B, p<0.05). Most notably, we observed complete inhibition of cellular afterdepolarizations in isoproterenol treated ankyrin-B+/− X AC3I mice compared with ankyrin-B+/− myocytes (Figure 3C, p<0.05). In summary, our findings demonstrate that CaMKII inhibition rescues both RyR2 pS2814 hyperphosphorylation and pro-arrhythmic cellular phenotypes in ankyrin-B deficient mice.
Figure 3. CaMKII inhibition normalizes ankyrin-B+/− myocyte electrical phenotypes.
A) Action potential morphology in wild-type, ankyrin-B+/−, AC3I, and ankyrin-B+/− X AC3I myocytes ± isoproterenol (1 μM). Red arrow indicates after-depolarization. Note difference in time scales for each genotype. B) Action potential duration (APD90) of wild-type, ankyrin-B+/−, AC3I, and ankyrin-B+/− X AC3I cardiomyocytes paced at 0.5 Hz at baseline (black bars) or in the presence of beta-adrenergic agonist (red bars). In B, * denotes p<0.05 vs. each respective β-AR stimulation group; # denotes p<0.05 vs. each respective wild-type group; and Δ denotes p<0.05 vs. each respective ankyrin-B+/− group. C) CaMKII inhibition normalizes ankyrin-B+/− myocyte afterdepolarizations. Bars denote incidence of myocyte afterdepolarizations ±isoproterenol treatment. In C, * denotes p<0.05 vs. each respective β-AR stimulation group and # denotes p<0.05 vs. each respective wild-type group. For B and C, N values are noted in each panel.
Increased ankyrin-B+/− RyR2 open probability is rescued by KN-93 treatment
Based on altered RyR2 phosphorylation status in ankyrin-B+/− mice, we next tested whether ankyrin-B+/− mouse hearts displayed abnormal RyR2 open probability compared to wild-type mouse heart. Consistent with our biochemistry findings, RyR2 channels from ankyrin-B+/− cardiac SR membrane vesicles21 displayed a significant increase in open probability (Po) compared with wild-type mice (Figure 4A-B; 0.154±0.043 vs. 0.007±0.004, p<0.05). Notably, application of the CaMKII inhibitor KN-93 (10 μM) significantly reduced RyR2 Po in ankyrin-B+/− preparations from 0.176±0.039 to 0.099 ±0.027 (Figure 4C, 4 channels from 3 mice, p<0.05). In contrast, KN-93 did not alter RyR2 Po in wild-type mice (Figure 4C, 0.011±0.008 vs. 0.006±0.005, 3 channels from 3 mice; p=N.S.). Collectively, these data support the role of CaMKII-dependent RyR2 phosphorylation in ankyrin-B+/− electrical phenotypes.
Figure 4. Ankyrin-B+/− mouse hearts display increased RyR2 open probability (Po).
A) Representative RyR2 single channel recordings from lipid bilayers prepared from wild-type and ankyrin-B+/− mouse hearts. B) Average Po of RyR2 from wild-type and ankyrin-B+/− mice. Po was increased in ankyrin-B+/− group (7 channels from 3 mice) compared to wild-type group (6 channels from 3 mice). C) Effect of KN-93 on Po of RyR2 channels. Application of CaMKII inhibitor KN-93 reduced Po of RyR2 from ankyrin-B+/− hearts, but did not alter Po of RyR2 from WT mouse hearts. Numbers in each bar represent number of channels (number of mice) in the bar graph (* denotes p<0.05).
CaMKII inhibition protects against ankyrin-based fatal cardiac arrhythmias
Individuals harboring ankyrin-B loss-of-function mutations display a host of cardiac phenotypes often linked with adrenergic dysregulation.11-13, 32 Ankyrin-B+/− mice are haploinsufficient and display similar catecholamine-based arrhythmia.13 Therefore, we tested whether CaMKII inhibition could protect the in vivo ankyrin-B+/− human disease model from life threatening arrhythmia. To assess arrhythmia susceptibility, we compared ECGs from conscious mice following surgical implantation of radiotelemetry devices. This protocol provides the ability to monitor cardiac activity at physiological heart rates (compared with anesthetized mice) as well as compare ECG parameters following physiological (exercise) or pharmacological intervention. Compared with wild-type mice, ankyrin-B+/− mice subjected to a catecholamine stress protocol (exercise plus intraperitoneal injection of an adrenergic receptor agonist) displayed consistent and severe polymorphic ventricular arrhythmia including ventricular tachycardia/torsade de pointes followed by severe bradycardia and ultimately death (Figure 5A-C). In contrast, we observed no episodes of sustained arrhythmia (Figure 5A-B, 0/6) and no death (Figure 5C, 0/6;). Ankyrin-B+/− mice crossed with AC3I mice subjected to the identical protocol, while showing short intermittent (<2 sec) non-sustained arrhythmia events, lacked significant sustained arrhythmia phenotypes compared with ankyrin-B+/− mice (Figure 5A-B, p<0.05). Similarly, in vivo CaMKII inhibition rescued survival following the cardiac stress protocol (Figure 5C, p<0.05 vs. ankyrin-B+/−) in wild-type or AC3I animals. In conclusion, our data link ankyrin-deficiency with cardiac RyR2 hyperphosphorylation and abnormal electrical dysfunction at the level of the single cell and whole animal. Moreover, our studies demonstrate that CaMKII inhibition blocks RyR2 hyperphosphorylation, and rescues single cell afterdepolarizations and arrhythmias.
Discussion
Protein kinase-dependent regulation of cardiac signaling is essential for normal physiology and excitation contraction coupling. Both congenital and acquired forms of human cardiovascular disease are now clearly linked with an imbalance in the kinase/phosphatase axis and cellular dysfunction. In fact, catecholaminergic polymorphic ventricular tachycardia, a disease directly linked with elevated sympathetic tone, exemplifies the striking requirement of precise adrenergic regulation for control of cardiac automaticity.33, 34 Beta-adrenergic receptor blockers remain the mainstay for treatment of CPVT patients. However, emerging work from both human and animal models suggests that the protection provided by beta-blockers against potentially lethal arrhythmias is incomplete, demonstrating the need to identify new molecular therapeutic targets for this disease.34, 35 Our data link human ankyrin-B-based arrhythmia phenotypes with alterations in the CaMKII signaling pathway. We identify aberrant RyR2 pS2814 levels in animal models of cardiac ankyrin-B syndrome. Moreover, our data demonstrate that biochemical, cellular, and whole animal phenotypes that are linked with a pro-arrhythmic trigger are either reduced or eliminated in the presence of an in vivo cellular CaMKII inhibitory peptide. These studies not only provide important new data regarding the molecular components involved in human arrhythmia, but also define important new regulatory elements of the ankyrin-B pathway for normal physiology.
One interesting finding in this story is the apparent link between ankyrin-B and the kinase/phosphatase axis in the cardiac myocyte. Protein phosphatase 2A (PP2A) is an important negative regulatory factor for both PKA and CaMKII-dependent phosphorylation in heart. The PP2A holoenzyme contains three subunits (A, B, and C), that serve phosphatase targeting, catalytic, and regulatory functions. Notably, we previously demonstrated that ankyrin-B directly associates with B56α, a PP2A regulatory subunit that is highly expressed in heart at the cardiac transverse-tubule. 24, 25 Moreover, ankyrin-B deficient myocytes display lack of normal B56α targeting presumably that is at least partially responsible for the alterations in RyR2 phosphorylation observed in this study. Notably, work from Terentyev and colleagues previously showed that miR-1-targeted loss of B56α in myocytes results in CaMKII-dependent hyperphosphorylation of RyR2 resulting in altered calcium handling and pro-arrhythmic electrical function.36 Together, these findings support a model in which ankyrin-B supports a key role in maintaining local kinase/phosphatase balance in the cardiac dyad by strategically positioning populations of PP2A near important targets including RyR2 (Figure 6). Thus loss of ankyrin-B-dependent targeting of dyadic PP2A results in increased RyR2 phosphorylation. It will be important in future experiments to determine whether PP2A activity is reduced and define additional cardiac targets of ankyrin-B-associated PP2A.
Figure 6. Model for link between ankyrin-B and local sympathetic regulation.
In myocytes, ankyrin-B targets local PP2A activity through an interaction with B56α, one regulatory subunit of the PP2A holoenzyme. When ankyrin-B function is altered, this targeting mechanism is disrupted allowing CaMKII to function unopposed on myocyte targets (including RyR2) resulting in electrical dysfunction.
CaMKII inhibition has been widely reported as a potential therapeutic strategy for the prevention of heart failure and arrhythmia.37 In fact, relevant for this study, Priori and colleagues recently demonstrated the ability of CaMKII inhibition for protection against arrhythmia in an animal model of CPVT associated with human RyR2 mutation.38 While our data link CaMKII inhibition to a reduction in RyR2 hyperphosphorylation, inhibition of afterdepolarizations, and protection from cardiac arrhythmia the direct mechanisms underlying these phenotypes is clearly complex and likely due to both direct and indirect phosphorylation events. While CaMKII targets RyR2 regulation, this multifunctional enzyme tunes the activity of a host of regulatory pathways including membrane proteins, transcriptional and metabolic pathways, as well as key SR proteins.37 Thus, additional investigations beyond the scope of this study will be required to uncover the detailed calcium-based regulatory mechanisms altered in this incredibly complex human disease.
Limitations
There are a number of important limitations to our study. First, while our data implicate the CaMKII regulatory pathway in ankyrin-B cardiomyocyte function, we acknowledge that dysfunction in other key molecular components are involved in ankyrin-B cardiac phenotypes. For example, work from our group and others have demonstrated that ankyrin-B targets and regulates a number of key ion channels and transporters in the heart including the Na/Ca exchanger, Na/K ATPase, and Kir6.2-all contributing important roles in cardiac regulation at baseline and in disease.13, 16, 39-41 In fact, we predict that loss of specific transverse-tubule populations of Na/Ca exchanger and Na/K ATPase contribute to the pro-arrhythmic substrate by altering local Na+ and Ca2+ concentration gradients similar to the actions of cardiac glycosides. In fact, we predict that these cellular phenotypes, which ultimately raise SR calcium load, provide the substrate for the arrhythmias triggered by altered adrenergic and CaMKII balance observed in our current study and others.
A second key limitation is the relative role of the cardiac RyR2 for arrhythmia susceptibility related to CaMKII function in our study. As observed for the cardiac RyR2 and voltage-gated Na+ channel, multiple post-translational regulatory sites likely create an integrated “tuning” rheostat to control ion channel function and thus response to the immediate cellular environment. While our work has assessed RyR2 S2808 and S2814 sites, these sites are not used to define the critical target for regulation, but instead provide a physiologically relevant surrogate of CaMKII function in heart. We predict that other CaMKII downstream proteins will also play critical roles in the ankyrin-B phenotype in response to altered sympathetic tone.
Supplementary Material
Acknowledgements
This work was supported in part by the Saving Tiny Hearts Society (PJM), by the National Institutes of Health [HL084583, HL083422 to PJM]; [HL079031, HL62494, HL70250 to MEA]; [HL089598, HL091947 to XHW]; Gilead Sciences Research Scholars Program (TJH), Fondation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease (PJM, XHW, MEA, TJH), and American Heart Association (PJM).
This work was supported in part by the Saving Tiny Hearts Society (PJM), by the National Institutes of Health [HL084583, HL083422 to PJM]; [HL079031, HL62494, HL70250 to MEA]; [HL089598, HL091947 to XHW]; Gilead Sciences Research Scholars Program (TJH), Fondation Leducq Award to the Alliance for Calmodulin Kinase Signaling in Heart Disease (PJM, XHW, MEA, TJH), and American Heart Association (PJM).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest/Disclosure: MEA is a cofounder of Allosteros Therapeutics and an inventor on patents claiming to treat heart failure and arrhythmias by CaMKII inhibition. Other authors report no potential conflicts.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010 Feb 23;121:948–954. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 2.Kontrogianni-Konstantopoulos A, Jones EM, Van Rossum DB, Bloch RJ. Obscurin is a ligand for small ankyrin 1 in skeletal muscle. Mol Biol Cell. 2003 Mar;14:1138–1148. doi: 10.1091/mbc.E02-07-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhou D, Birkenmeier CS, Williams MW, Sharp JJ, Barker JE, Bloch RJ. Small, membrane-bound, alternatively spliced forms of ankyrin 1 associated with the sarcoplasmic reticulum of mammalian skeletal muscle. J Cell Biol. 1997;136:621–631. doi: 10.1083/jcb.136.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sato PY, Coombs W, Lin X, et al. Interactions between ankyrin-G, Plakophilin-2, and Connexin43 at the cardiac intercalated disc. Circulation research. 2011 Jul 8;109:193–201. doi: 10.1161/CIRCRESAHA.111.247023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lowe JS, Palygin O, Bhasin N, et al. Voltage-gated Nav channel targeting in the heart requires an ankyrin-G dependent cellular pathway. J Cell Biol. 2008 Jan 14;180:173–186. doi: 10.1083/jcb.200710107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mohler PJ, Rivolta I, Napolitano C, et al. Nav1.5 E1053K mutation causing Brugada syndrome blocks binding to ankyrin-G and expression of Nav1.5 on the surface of cardiomyocytes. Proc Natl Acad Sci U S A. 2004 Dec 14;101:17533–17538. doi: 10.1073/pnas.0403711101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hund TJ, Koval OM, Li J, et al. A beta(IV)-spectrin/CaMKII signaling complex is essential for membrane excitability in mice. The Journal of clinical investigation. 2010 Oct 1;120:3508–3519. doi: 10.1172/JCI43621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smith S, Curran J, Hund TJ, Mohler PJ. Defects in cytoskeletal signaling pathways, arrhythmia, and sudden cardiac death. Front Physiol. 2012;3:122. doi: 10.3389/fphys.2012.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cunha SR, Hund TJ, Hashemi S, et al. Defects in ankyrin-based membrane protein targeting pathways underlie atrial fibrillation. Circulation. 2011 Sep 13;124:1212–1222. doi: 10.1161/CIRCULATIONAHA.111.023986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Le Scouarnec S, Bhasin N, Vieyres C, et al. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008 Oct 7;105:15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohler PJ, Le Scouarnec S, Denjoy I, et al. Defining the cellular phenotype of “ankyrin-B syndrome” variants: human ANK2 variants associated with clinical phenotypes display a spectrum of activities in cardiomyocytes. Circulation. 2007 Jan 30;115:432–441. doi: 10.1161/CIRCULATIONAHA.106.656512. [DOI] [PubMed] [Google Scholar]
- 12.Mohler PJ, Splawski I, Napolitano C, et al. A cardiac arrhythmia syndrome caused by loss of ankyrin-B function. Proc Natl Acad Sci U S A. 2004 Jun 15;101:9137–9142. doi: 10.1073/pnas.0402546101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohler PJ, Schott JJ, Gramolini AO, et al. Ankyrin-B mutation causes type 4 long-QT cardiac arrhythmia and sudden cardiac death. Nature. 2003 Feb 6;421:634–639. doi: 10.1038/nature01335. [DOI] [PubMed] [Google Scholar]
- 14.Camors E, Mohler PJ, Bers DM, Despa S. Ankyrin-B reduction enhances Ca spark-mediated SR Ca release promoting cardiac myocyte arrhythmic activity. J Mol Cell Cardiol. 2012 Jun;52:1240–1248. doi: 10.1016/j.yjmcc.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cunha SR, Bhasin N, Mohler PJ. Targeting and stability of Na/Ca exchanger 1 in cardiomyocytes requires direct interaction with the membrane adaptor ankyrin-B. J Biol Chem. 2007 Feb 16;282:4875–4883. doi: 10.1074/jbc.M607096200. [DOI] [PubMed] [Google Scholar]
- 16.Mohler PJ, Davis JQ, Bennett V. Ankyrin-B coordinates the Na/K ATPase, Na/Ca exchanger, and InsP3 receptor in a cardiac T-tubule/SR microdomain. PLoS Biol. 2005 Dec;3:e423. doi: 10.1371/journal.pbio.0030423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang R, Khoo MS, Wu Y, et al. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005 Apr;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 18.Koval OM, Guan X, Wu Y, et al. CaV1.2 beta-subunit coordinates CaMKII-triggered cardiomyocyte death and afterdepolarizations. Proc Natl Acad Sci U S A. 2010 Mar 16;107:4996–5000. doi: 10.1073/pnas.0913760107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y, Gao Z, Chen B, et al. Calmodulin kinase II is required for fight or flight sinoatrial node physiology. Proc Natl Acad Sci U S A. 2009 Apr 7;106:5972–5977. doi: 10.1073/pnas.0806422106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gao Z, Singh MV, Hall DD, et al. Catecholamine-independent heart rate increases require Ca2+/calmodulin-dependent protein kinase II. Circulation Arrhythmia and electrophysiology. 2011 Jun 1;4:379–387. doi: 10.1161/CIRCEP.110.961771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Oort RJ, McCauley MD, Dixit SS, et al. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010 Dec 21;122:2669–2679. doi: 10.1161/CIRCULATIONAHA.110.982298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mitchell GF, Jeron A, Koren G. Measurement of heart rate and Q-T interval in the conscious mouse. Am J Physiol. 1998;274:H747–751. doi: 10.1152/ajpheart.1998.274.3.H747. [DOI] [PubMed] [Google Scholar]
- 23.Hund TJ, Wright PJ, Dun W, Snyder JS, Boyden PA, Mohler PJ. Regulation of the ankyrin-B-based targeting pathway following myocardial infarction. Cardiovasc Res. 2009 Mar 1;81:742–749. doi: 10.1093/cvr/cvn348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bhasin N, Cunha SR, Mudannayake M, Gigena MS, Rogers TB, Mohler PJ. Molecular basis for PP2A regulatory subunit B56alpha targeting in cardiomyocytes. American journal of physiology Heart and circulatory physiology. 2007 Jul;293:H109–119. doi: 10.1152/ajpheart.00059.2007. [DOI] [PubMed] [Google Scholar]
- 25.Cunha SR, Mohler PJ. Obscurin Targets Ankyrin-B and Protein Phosphatase 2A to the Cardiac M-line. J Biol Chem. 2008 Nov 14;283:31968–31980. doi: 10.1074/jbc.M806050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Go LO, Moschella MC, Watras J, Handa KK, Fyfe BS, Marks AR. Differential regulation of two types of intracellular calcium release channels during end-stage heart failure. J Clin Invest. 1995;95:888–894. doi: 10.1172/JCI117739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chelu MG, Sarma S, Sood S, et al. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009 Jul;119:1940–1951. doi: 10.1172/JCI37059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dobrev D, Wehrens XH. Calmodulin kinase II, sarcoplasmic reticulum Ca2+ leak, and atrial fibrillation. Trends Cardiovas Med. 2010 Jan;20:30–34. doi: 10.1016/j.tcm.2010.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McCauley MD, Wehrens XH. Ryanodine receptor phosphorylation, calcium/calmodulin-dependent protein kinase II, and life-threatening ventricular arrhythmias. Trends Cardiovasc Med. 2011 Feb;21:48–51. doi: 10.1016/j.tcm.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wehrens XH, Lehnart SE, Reiken S, Vest JA, Wronska A, Marks AR. Ryanodine receptor/calcium release channel PKA phosphorylation: a critical mediator of heart failure progression. Proc Natl Acad Sci U S A. 2006 Jan 17;103:511–518. doi: 10.1073/pnas.0510113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wehrens XH, Lehnart SE, Reiken SR, Marks AR. Ca2+/calmodulin-dependent protein kinase II phosphorylation regulates the cardiac ryanodine receptor. Circ Res. 2004 Apr 2;94:e61–70. doi: 10.1161/01.RES.0000125626.33738.E2. [DOI] [PubMed] [Google Scholar]
- 32.Mohler PJ, Healy JA, Xue H, et al. Ankyrin-B syndrome: enhanced cardiac function balanced by risk of cardiac death and premature senescence. PLoS ONE. 2007;2:e1051. doi: 10.1371/journal.pone.0001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Leenhardt A, Lucet V, Denjoy I, Grau F, Ngoc DD, Coumel P. Catecholaminergic polymorphic ventricular tachycardia in children. A 7-year follow-up of 21 patients. Circulation. 1995;91:1512–1519. doi: 10.1161/01.cir.91.5.1512. [DOI] [PubMed] [Google Scholar]
- 34.Priori SG, Napolitano C, Memmi M, et al. Clinical and molecular characterization of patients with catecholaminergic polymorphic ventricular tachycardia. Circulation. 2002 Jul 2;106:69–74. doi: 10.1161/01.cir.0000020013.73106.d8. [DOI] [PubMed] [Google Scholar]
- 35.Cerrone M, Colombi B, Santoro M, et al. Bidirectional ventricular tachycardia and fibrillation elicited in a knock-in mouse model carrier of a mutation in the cardiac ryanodine receptor. Circ Res. 2005 May 27;96:e77–82. doi: 10.1161/01.RES.0000169067.51055.72. [DOI] [PubMed] [Google Scholar]
- 36.Terentyev D, Belevych AE, Terentyeva R, et al. miR-1 overexpression enhances Ca(2+) release and promotes cardiac arrhythmogenesis by targeting PP2A regulatory subunit B56alpha and causing CaMKII-dependent hyperphosphorylation of RyR2. Circ Res. 2009 Feb 27 104;:514–521. doi: 10.1161/CIRCRESAHA.108.181651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swaminathan PD, Purohit A, Hund TJ, Anderson ME. Calmodulin-Dependent Protein Kinase II: Linking Heart Failure and Arrhythmias. Circ Res. 2012 Jun 8;110:1661–1677. doi: 10.1161/CIRCRESAHA.111.243956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu N, Ruan Y, Denegri M, et al. Calmodulin kinase II inhibition prevents arrhythmias in RyR2(R4496C+/−) mice with catecholaminergic polymorphic ventricular tachycardia. Journal of molecular and cellular cardiology. 2011 Jan;50:214–222. doi: 10.1016/j.yjmcc.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 39.Kline CF, Kurata HT, Hund TJ, et al. Dual role of K ATP channel C-terminal motif in membrane targeting and metabolic regulation. Proc Natl Acad Sci U S A. 2009 Sep 29;106:16669–16674. doi: 10.1073/pnas.0907138106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li J, Kline CF, Hund TJ, Anderson ME, Mohler PJ. Ankyrin-B regulates Kir6.2 membrane expression and function in heart. J Biol Chem. 2010 Sep 10;285:28723–28730. doi: 10.1074/jbc.M110.147868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li ZP, Burke EP, Frank JS, Bennett V, Philipson KD. The cardiac Na+-Ca2+ exchanger binds to the cytoskeletal protein ankyrin. J Biol Chem. 1993;268:11489–11491. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.