Abstract
Objective
Atherosclerosis is an inflammatory process resulting from the interaction between genetic and environmental factors. Leukotrienes are inflammatory mediators generated from arachidonic acid, and genetic polymorphisms involved in leukotriene metabolism are implicated in atherosclerosis. The objectives of this study are to examine whether genetic variants in key leukotriene enzymes are associated with atherosclerosis, and whether dietary intake of competing leukotriene substrates modifies the effect of leukotriene variants on atherosclerosis.
Methods
Atherosclerosis was assessed by common carotid intima-media thickness (IMT) using ultrasound. Sequence variants within arachidonate 5-lipoxygenase activating protein (ALOX5AP) and leukotriene A4 hydrolase (LTA4H) genes were analyzed with 32 single nucleotide polymorphisms (SNPs) in 169 Caucasian twin pairs from the Vietnam Era Twin Registry. The associations between genetic polymorphisms and carotid atherosclerosis, and gene × diet interactions were examined by generalized estimating equation controlling for potential confounders.
Results
A six-SNP haplotype in LTA4H, designated HapE, was significantly associated with carotid IMT after adjusting for known coronary risk factors. Twins carrying HapE had a much lower IMT compared to twins not carrying (695 μm vs. 750 μm, p=0.0007). Moreover, dietary intake of polyunsaturated fatty acids strongly augmented the cardioprotective effect of HapE among those with this haplotype but not those without, suggesting a haplotype × diet interaction (Interaction PHapE × n-3 = 0.03, PHapE × n-6 = 0.015).
Conclusion
We identified a novel leukotriene haplotype that appears to be protective towards subclinical atherosclerosis. This association is modified by dietary intake of polyunsaturated fatty acids.
Keywords: leukotriene haplotype, gene × diet interaction, carotid atherosclerosis, twin study
Introduction
Atherosclerosis is a complex process resulting from the interplay between genetic and environmental factors. Leukotrienes are a class of biologically active lipid mediators that have been implicated in atherosclerosis [1]. The rate of biosynthesis of leukotrienes from arachidonic acid is limited by several key enzymes, such as arachidonate 5-lipoxygenase (ALOX5), arachidonate 5-lipoxygenase activating protein (ALOX5AP) and leukotriene A4 hydrolase (LTA4H). Allelic variants in genes encoding these leukotriene enzymes have been implicated in atherosclerotic cardiovascular disease (CVD). For example, two haplotypes (called HapA and B) in ALOX5AP confer risk for myocardial infarction (MI) and stroke by increasing leukotriene production and inflammation in the arterial wall [2]. Another haplotype in LTA4H (called HapK) was also associated with an increased risk for MI [3], though the association was not confirmed except for the finding of a weak association with atherosclerosis [4]. A genetic variant in ALOX5 promoter was also associated with carotid IMT, and this association was modified by dietary intake of omega-3 (n-3) and omega-6 (n-6) fatty acids, such that higher dietary intake of n-6 fatty acids exacerbated the atherogenic effect of the ALOX5 variant alleles, whereas higher intake of n-3 fatty acids blunted this effect [5]. A significant gene × diet interaction was also observed in another study, in which ALOX5 variants increased MI risk in high arachidonic acid (AA) intake, a common dietary n-6 fatty acid, but decreased MI risk in low AA intake [6].
Polyunsaturated fatty acids (PUFA), mainly n-3 and n-6 fatty acids, are important precursors for leukotriene production [7]. Eicosapentaenoic acid (EPA; C20: 5, n-3) and docosahexaenoic acid (DHA; C22:6, n-3) are two common n-3 fatty acids, whereas linoleic acid (LA, C18: 2, n-6) and arachidonic acid (AA; C20:4, n-6) belong to the n-6 fatty acid class. Dietary intake of n-3 and n-6 fatty acids is associated with reduced CVD risk [8, 9], though the cardioprotective effect of n-6 fatty acids remains controversial [10]. The impact of n-3 and n-6 polyunsaturated fatty acids on CVD may be mediated by their inflammatory properties [7, 11] through the leukotriene enzymatic pathway [12].
The purpose of this study was to examine whether genetic polymorphisms in ALOX5AP and LTA4H are associated with carotid atherosclerosis, and whether dietary intake of n-3 and n-6 fatty acids modifies this association. We used a well-characterized twin sample to test these hypotheses because twins are well-matched on many measured and unmeasured risk factors. Subclinical atherosclerosis was assessed by carotid IMT, an established surrogate marker for early atherosclerosis. Our previous studies have shown that carotid IMT [13] is strongly influenced by genetic factors. Thus, searching for genes using this quantitative measure of atherosclerosis is particularly useful.
Subjects and Methods
Study population
The Twins Heart Study (THS) is an investigation of psychological, behavioral and biological risk factors for subclinical CVD in twins, and the methods were previously described [14]. Briefly, the THS included 180 twin pairs drawn from the Vietnam Era Twin Registry [15]. This registry is composed of 7,369 middle-aged male-male twin pairs both of whom served in the United States military during the time of the Vietnam War.
As part of the THS, we studied 180 twin pairs who were born between 1946 and 1956 and were without a history of symptomatic CVD and major depression as of 1990, based on pre-existing survey data [16]. All twin pairs were examined at the Emory University General Clinical Research Center between March 2002 and March 2006, where their medical history was updated. Among the 180 twin pairs (102 monozygotic and 78 dizygotic), 169 pairs were Caucasians, 6 pairs were African Americans, 3 pairs were American Indians, 1 pair was Hispanic and 1 pair was Asian/Pacific. To avoid population stratification, the present analyses only include the 169 Caucasian pairs (95 monozygotic and 74 dizygotic). Zygosity information was determined by DNA analysis.
Measurement for carotid intima-media thickness (IMT)
Common carotid artery IMT was measured using high resolution B-mode ultrasonography with standard techniques [17]. Briefly, IMT was quantified both on the near and far wall at the distal 1.0 cm of the left and right common carotid arteries proximal to the bifurcation. For each segment, the sonographer used multiple different scanning angles to identify the longitudinal image of IMT showing the maximum IMT. At least 10 pictures for each segment were stored digitally, and measurements were made off-line by one observer blinded to other twin data using semi-automated computerized analytical software (Carotid Tools, MIA Inc., Iowa City, Iowa). Of the stored images, the one with maximum thickness was selected, and IMT measured, for each segment. Average values of the IMT of each of the four segments (right near and far walls, and left near and far walls) were used as the IMT values for each twin in the analysis (total mean of maximum IMT). In order to minimize error, the same investigator did IMT measurements throughout the study, and the same equipment and analytical software was used to measure IMT for all the twin participants. In our lab, the mean absolute difference in IMT measured in 7 subjects on whom 2 carotid artery examinations were performed 3 days apart, was 0.03 (±0.02) mm. The mean difference in 2 successive readings of the same 10 segments of common carotid IMT was 0.02 (±0.02) mm with a Pearson correlation coefficient of 0.93.
Dietary assessment
We used the Willett self-administered semiquantitative food frequency questionnaire [18], which collected dietary data for the 12 months prior to testing. The questionnaire classifies average food intake according to 9 frequency categories ranging from “almost never or less than once per month” to “ ≥ 6 times/day” using standardized portion sizes for each dietary item, including beverages and nutritional supplements. Questionnaires were scored by the Nutrition Questionnaire Service Center, Channing Laboratory, Harvard University, and nutrient intake data were derived following the nutrient database of the US Department of Agriculture. Daily food intake in grams was calculated from food intake frequency and portion sizes.
Risk factor measurements
All measurements were performed in the morning after an overnight fast, and both twins in a pair were tested at the same time. A medical history and a physical exam were obtained from all twins. Weight and height were used to calculate body mass index (BMI) as weight in kilograms divided by height in meters squared. Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured by mercury sphygmomanometer on the right arm with the subject in sitting position after 10 minutes of rest. The average of two measurements 5-minute apart was used in the statistical analyses. Physical activity was assessed by means of a modified version of the Baecke Questionnaire of Habitual Physical Activity used in the Atherosclerosis Risk in Communities (ARIC) Study [19], a 16-question instrument documenting level of physical activity at work, during sports and non-sports activities. The total physical activity score was used in the analysis. Cigarette smoking was classified into current smoker (any number of cigarettes) versus never or past smoker. Information on alcohol consumption was collected by asking about the number of alcoholic drinks (beer, wine or liquor) consumed in a typical week. Diabetes was defined as a fasting glucose ≥ 126 mg/dL, or current treatment with insulin or oral hypoglycemic agents. Hypertension was defined as SBP ≥ 140 mm Hg and/or DBP ≥ 90 mm Hg, or current pharmacological treatment for hypertension. Current use of medications was also recorded.
SNPs genotyping
A total of thirty-two tagging SNPs, including nineteen in the ALOX5AP gene and thirteen in the LTA4H gene, were genotyped in all twins. Of these, 16 out of the 19 SNPs in ALOX5AP and 9 out of the 13 SNPs in LTA4H were tag SNPs chosen by the computer program Haploview 4.2 with minor allele frequency (MAF) ≥ 5% based on pair-wise linkage disequilibrium (r2 ≥ 0.80)[20] and Illumina design scores (quantifying how likely a SNP can be genotyped). To replicate the associations of leukotriene haplotypes (HapA, B and K) with atherogenesis reported by Helgadottir et al [3, 21], we also genotyped three additional SNPs in ALOX5AP (rs17216473, rs17222814 and rs17222342) and four additional SNPs in LTA4H (rs1978331, rs2660899, rs17677715 and rs61937881). Information for the thirty-two studied SNPs is shown in Table 1. SNP genotyping was done using the Illumina VeraCode technology (Illumina, Inc., San Diego, CA) at the Emory Genetics Laboratory. The average genotyping call rates are > 98% for all SNPs and concordance rates with controls or blinded data for the nuclear genome are > 99%.
Table 1.
Information for the studied SNPs
| Gene/SNP name | Major/minor allele a | MAF |
|---|---|---|
| ALOX5AP | ||
| rs3935645 | A/G | 0.41 |
| rs4075131 | T/C | 0.21 |
| rs4468448 | C/T | 0.32 |
| rs4597169 | A/G | 0.05 |
| rs4769055 | C/A | 0.30 |
| rs4769874 | G/A | 0.05 |
| rs9315045 | T/C | 0.22 |
| rs9315050 | A/G | 0.06 |
| rs9551963 | C/A | 0.49 |
| rs9578196 | C/T | 0.09 |
| rs9579645 | A/C | 0.13 |
| rs9579648 | G/C | 0.18 |
| rs9579649 | C/T | 0.12 |
| rs10162089 | A/G | 0.49 |
| rs10507391 | T/A | 0.32 |
| rs11617473 | G/A | 0.27 |
| rs17216473 | G/A | 0.12 |
| rs17222814 | G/A | 0.10 |
| rs17222342 | G/A | 0.08 |
| LTA4H | ||
| rs2247330 | A/G | 0.34 |
| rs2247570 | A/G | 0.32 |
| rs2540475 | C/T | 0.21 |
| rs2540482 | A/G | 0.27 |
| rs2660845 | A/G | 0.32 |
| rs2660880 | G/A | 0.44 |
| rs2660898 | A/C | 0.35 |
| rs2660899 | G/T | 0.21 |
| rs4762659 | T/C | 0.22 |
| rs6538697 | A/G | 0.09 |
| rs1978331 | A/G | 0.42 |
| rs17677715 | A/G | 0.19 |
| rs61937881 | C/A | 0.25 |
Minor allele is defined as the least prevalent base for a given SNP.
Statistical analyses
Hardy-Weinberg equilibrium (HWE) was tested by a χ2 test with one degree of freedom (df) in one twin of each pair chosen at random to prevent inflated significance. Log-transformed IMT values were used in statistical analyses.
a) Genetic association analysis
Association analyses were performed using generalized estimating equation (GEE) to account for within twin pair correlations by including twin pair as a clustering variable. Analyses were done separately for each SNP and followed up by haplotype analyses. For individual SNP association analyses, we first tested a 2-df additive genetic model (major allele homozygotes vs. heterozygotes vs. minor allele homozygotes). In the presence of a significant association, a dominant model (combined heterozygotes and minor allele homozygotes vs. major allele homozygotes) and a recessive model (minor allele homozygotes vs. all other individuals) were further tested to find the best mode of inheritance. For haplotype association analyses, we first inferred haplotypes from genotype data, separately for each gene, using the computer program PHASE 2.1 [22]. Due to the large number of haplotype combinations, we only analyzed two-SNP, three-SNP, and up to six-SNP haplotypes from adjacent SNPs in each gene. Haplotypes with frequencies < 1% were ignored. In this study, we defined twins carrying at least one copy of a specific haplotype as haplotype carriers, and those not carrying the specific haplotype under study as noncarriers. A binary variable for the carrier status of a specific haplotype (i.e., carrier vs. noncarrier) was used in the haplotype association analysis.
To examine the effects of covariates on the association between haplotype and subclinical atherosclerosis, and to examine whether the association was independent of known coronary risk factors, we fitted three hierarchical models. Model 1 only adjusted for age; Model 2 further adjusted for behavioral/lifestyle factors, including current smoking (current vs. never or past), alcohol consumption, level of physical activity, dietary intake of saturated fat (g/d), and total daily caloric intake (Kcal/d). Model 3 further adjusted for biological factors (BMI and Framingham risk score) and preventive treatment (use of statins). The Framingham risk score is a commonly used summary index of coronary risk which incorporates information on presence and severity of the following cardiovascular risk factors: age, current smoking, LDL-cholesterol, HDL-cholesterol, blood pressure, and history of diabetes mellitus. It was used here to reduce the number of variables in the model in order to avoid model overfitting.
b) Gene × diet interaction
To examine whether dietary intake modifies the association between genetic variants and carotid atherosclerosis, we tested for interactions of haplotype (carrier vs. noncarrier) with dietary intake (continuous) by assessing the statistical significance of the interaction term in GEE models that also included the main effects, controlling for the same covariates listed in model 3. Because the relationship between dietary intake of n-3 fatty acids and inflammation may depend on the intake of n-6 fatty acids or vice versa [23], the interactions for n-3 or n-6 fatty acids with haplotypes were adjusted for one another.
In the present study, we examined whether dietary n-3 PUFAs (EPA and DHA) and n-6 PUFAs (LA and AA) modulate the association between haplotype and IMT. Owing to the significant correlation between dietary intakes of EPA and DHA (γ = 0.90, p<0.0001), and the high correlation between linoleic acid and arachidonic acid (γ =0.63, p<0.0001), we summed dietary EPA and DHA as the total intake of n-3 fatty acids and summed dietary LA and AA as the total intake of n-6 fatty acids in the statistical analyses.
c) Sensitivity analyses
Although EPA and DHA are highly correlated, they may affect carotid IMT differently [24, 25]. Similarly, LA and AA may also present differential effects on inflammation or atherosclerosis. We therefore conducted sensitivity analyses to examine whether the nutrigenetic interaction is mainly driven by a specific nutrient. This was done by testing the interaction of HapE with each individual nutrient in n-3 fatty acids (i.e., EPA and DHA) or n-6 fatty acids (i.e., LA and AA), separately, controlling for the same covariates in model 3. In addition, as participants may have changed their diet after a diagnosis of disease, we performed additional analyses to test for interaction between diet and disease, such as diabetes or hypertension.
To adjust for multiple testing, we used the Benjamini-Hochberg false discovery rate (FDR) procedure [26] to correct for the number of SNPs or haplotypes evaluated and used an FDR-adjusted P value (q value) threshold of 0.05 to determine statistical significance. The PROC MULTTEST procedure in SAS 9.2 was used to calculate the adjusted-FDR (q value).
Results
The age of the twins ranged from 47 to 59 years with a mean of 55. There was no significant difference between monozygotic (MZ) and dizygotic (DZ) twins in any of the risk factors examined. Carotid IMT was negatively correlated with dietary intake of n-3 (r = −0.01) or n-6 fatty acids (r = −0.05), but the correlations were not statistically significant.
1) Association between a novel leukotriene haplotype and atherosclerosis
All SNPs were in Hardy-Weinberg equilibrium. None of the SNPs was individually associated with carotid IMT after accounting for multiple comparisons. The ALOX5AP haplotypes (HapA and HapB) and LAT4H haplotype (HapK) which were previously found to be associated with coronary artery disease [2, 3] did not show an association with IMT in this study. However, a 6-SNP haplotype in the LTA4H gene was significantly associated with carotid IMT. This haplotype spans the markers rs61937881 (C), rs1978331 (G), rs17677715 (T), rs2660899 (G), rs2540482 (T) and rs2660845 (A). For simplicity, we designated this haplotype as “HapE”. The prevalence of this leukotriene haplotype was 21% in our sample.
Compared to twins without HapE, those carrying HapE had significantly lower carotid IMT (742 μm vs. 701 μm, p = 0.007), suggesting a potential protective effect of HapE on atherosclerosis. This association remained statistically significant after adjusting for age (p=0.006), further adjusting for behavioral/lifestyle risk factors (p = 0.002), biological risk factors and preventive treatment (p = 0.0007). Additional adjustments for dietary intake of PUFAs (n-3 and n-6 fatty acids) and total energy intake did not attenuate this association (p=0.0008). The distribution of major cardiovascular risk factors according to HapE carrier status is listed in Table 2, which shows that HapE carriers consumed significantly less n-6 fatty acids 6.4 vs 8.0 g/d, p=0.006), saturated fat (19.2 vs. 22.9 g/d, p = 0.03) and total daily energy intake (1320 vs. 1562 Kcal/d, p=0.01) than HapE noncarriers. Table 3 shows the association between HapE and carotid atherosclerosis. Results for multivariate GEE analysis is presented in Table 4.
Table 2.
Distribution of major cardiovascular risk factors by HapE carrier status
| Variables | HapE (−)
|
HapE (+)
|
P* |
|---|---|---|---|
| Mean ± SD or % | Mean ± SD or % | ||
| Age (years) | 54.5±2.9 | 54.4±2.6 | 0.75 |
| Current smoking (%) | 19.6 | 22.2 | 0.47 |
| Type 2 diabetes (%) | 7.1 | 13.3 | 0.18 |
| Hypertension (%) | 35.7 | 34.1 | 0.84 |
| Physical activity score | 7.5±1.5 | 7.3±1.7 | 0.57 |
| Dietary intake of n-3 fatty acids (g/d) | 0.17±0.23 | 0.14±0.17 | 0.29 |
| Dietary intake of n-6 fatty acids (g/d) | 8.01±5.56 | 6.42±2.58 | 0.006 |
| Dietary intake of saturated fat (g/d) | 22.9±13.6 | 19.2±8.2 | 0.03 |
| Total daily energy intake (Kcal) | 1562±805 | 1319±494 | 0.01 |
| Total cholesterol(mg/dL) | 187.2±40.9 | 180.5±33.5 | 0.28 |
| Total triglyceride (mg/dL) | 182.5±99.6 | 169.2±98.8 | 0.43 |
| High-density lipoprotein(mg/dL) | 39.1±9.1 | 38.1±12.5 | 0.62 |
| Low-density lipoprotein (mg/dL) | 121.7±35.8 | 116.3±31.3 | 0.36 |
| Systolic blood pressure (mmHg) | 129.8±15.4 | 126.8±16.8 | 0.25 |
| Diastolic blood pressure (mmHg) | 80.7±9.6 | 78.9±12.4 | 0.36 |
| Body mass index (kg/m2) | 29.2±4.2 | 29.9±5.9 | 0.41 |
| Use of statins (%) | 22.8 | 31.1 | 0.25 |
Adjusted for within twin pair correlations by GEE
Table 3.
Association between HapE and carotid atherosclerosis
| Variable | IMT (μm)
|
P* | |
|---|---|---|---|
| HapE noncarriers | HapE carriers | ||
| No covariates | |||
| Mean ± SD | 742±108 | 701±94 | 0.007 |
| Median | 725 | 687 | 0.03 |
| Minimum | 565 | 555 | |
| Maximum | 1185 | 960 | |
| Multivariate analysis‡ | |||
| Model 1 (mean ± SE) | 744±8 | 702±12 | 0.006 |
| Model 2 (mean ± SE) | 748±9 | 701±12 | 0.002 |
| Model 3 (mean ± SE) | 749±8 | 695±12 | 0.0007 |
Model 1 adjusted for age; Model 2 additional adjusted for current smoking, alcohol consumption, physical activity, dietary intake of saturated fat and total caloric intake; Model 3 further adjusted for BMI, use of statins and Framingham score, which is a composite variable containing age, smoking, blood pressure, LDL, HDL and history of type 2 diabetes.
P values for differences between means were computed by GEE, and were adjusted for multiple testing by adjusted-FDR (q value). The p value for the difference in medians was calculated using the Kruskal-Wallis test.
Table 4.
Association between HapE and IMT by multivariate GEE analysis
| Variables | Regression coefficient | Multivariate-adjusted P* |
|---|---|---|
| HapE | −0.07 | 0.0008 |
| BMI | 0.003 | 0.10 |
| Alcohol consumption | 0.02 | 0.32 |
| Physical activity | −0.001 | 0.89 |
| Framingham risk score‡ | 0.01 | 0.005 |
| Use of statins | 0.03 | 0.37 |
| Dietary saturated fat | 0.001 | 0.41 |
| Daily energy intake | −0.000 | 0.11 |
Adjusted for body mass index, alcohol consumption, physical activity level, dietary intake of saturated fat, total daily energy intake, use of statins and Framingham risk score.
Framingham risk score is a composite variable including age, current smoking, LDL, HDL, blood pressure and diabetes mellitus.
2) HapE × diet interaction
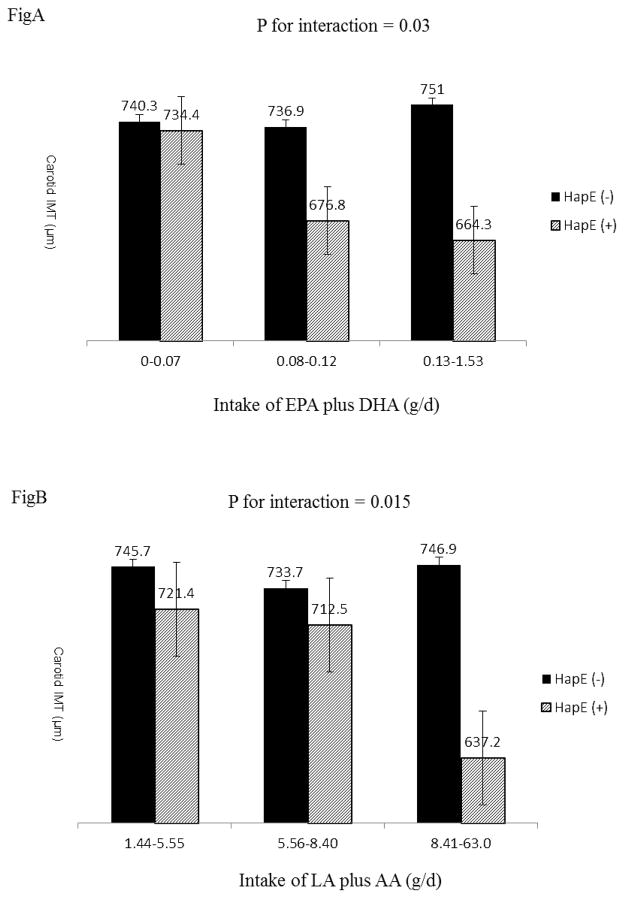
In the statistical modeling for HapE × diet interaction by multivariate GEE, neither HapE nor PUFA (i.e., n-3 or n-6 fatty acids) exhibited a main effect on IMT. However, their interactions contributed significantly to the interindividual variability for carotid IMT (p=0.03 for HapE × n-3 fatty acids, p= 0.015 for HapE × n-6 fatty acids). These interactions were independent of multiple risk factors, including lifestyles (smoking, alcohol drink, and physical activity), biological factors (BMI and Framingham risk score), dietary intake of saturated fat and total daily caloric intake as well as use of statins. Normalization of n-3 and n-6 PUFAs to the total caloric intake (expressed g/Kcal) did not change the results. Results for HapE × diet interactions are shown in Table 5. A schematic illustration of the gene × diet interaction is also presented in Figure 1. It shows that an increased intake of n-3 and n-6 fatty acids was associated with a decrease in IMT among HapE carriers, but not noncarriers, suggesting a nutrigenetic interaction effect on atherosclerosis. In addition, it appears that there exists a dose-response relationship between dietary intake of PUFAs and IMT among twins carrying HapE but not those not carrying this haplotype. The difference in IMT between HapE carriers and noncarriers was statistically significant in the highest tertile of both n-3 fatty acids (p=0.02) and n-6 fatty acids (p=0.01), but no difference was observed for the other two groups (lowest and middle tertiles).
Table 5.
Interaction between HapE and diet on carotid IMT by multivariate GEE analysis
| Variables | HapE × n-3 PUFA
|
HapE × n-6 PUFA
|
||
|---|---|---|---|---|
| Coefficient | Multivariate-P* | Coefficient | Multivariate-P* | |
| HapE | −0.03 | 0.14 | −0.06 | 0.14 |
| n-3 fatty acids | −0.22 | 0.11 | −0.01 | 0.75 |
| n-6 fatty acids | 0.001 | 0.59 | −0.02 | 0.06 |
| n-3 × HapE | −0.25 | 0.03 | - | - |
| n-6 × HapE | - | - | −0.02 | 0.015 |
| Body mass index | 0.003 | 0.14 | 0.003 | 0.09 |
| Alcohol consumption | −0.02 | 0.47 | 0.02 | 0.41 |
| Physical activity | −0.002 | 0.67 | −0.003 | 0.54 |
| Framingham risk score | 0.01 | 0.001 | 0.01 | 0.001 |
| Use of statins | 0.03 | 0.37 | 0.02 | 0.58 |
| Dietary saturated fat | 0.001 | 0.44 | 0.001 | 0.54 |
| Daily energy intake | −0.0001 | 0.12 | −0.000 | 0.20 |
Adjusted for body mass index, alcohol consumption, physical activity level, dietary intake of saturated fat, total daily energy intake, use of statins and Framingham risk score, which is a composite variable including age, current smoking, LDL-cholesterol, HDL-cholesterol, blood pressure and diabetes mellitus.
Figure 1.
Interactions between HapE and dietary intake of n-3 fatty acids (Fig A) or n-6 (Fig B) fatty acids on carotid IMT. It shows that an increased intake of n-3 and n-6 fatty acids was associated with a decrease in IMT among HapE carriers, but not noncarriers. In addition, there seems to be a dose-response relationship between dietary intake and IMT among HapE carriers, but not noncarriers. The difference in IMT between HapE carriers and noncarriers was statistically significant in the highest tertile of both n-3 fatty acids (p=0.02) and n-6 fatty acids (p=0.01), but the difference was nonsignificant in the lowest and middle tertiles. All interactions were adjusted for alcohol consumption, physical activity, total caloric intake, BMI, use of statins and Framingham risk score. The interactions shown in Fig A and Fig B were adjusted for each other.
3) Sensitivity analysis
This analysis demonstrates that both EPA and DHA significantly interacted with HapE on carotid atherosclerosis (both P for interaction = 0.026), while only LA significantly interacted with HapE on IMT (P HapE × LA = 0.01), but not AA (P HapE × AA = 0.10). Because the results obtained by using the sum of EPA and DHA, or the sum of LA and AA, are almost identical to that by using a specific fatty acid, we chose to present the results from the sum of fatty acids. We did not find an interaction of dietary intake of PUFAs with diabetes or hypertension on carotid IMT.
Discussion
We found that a novel haplotype in the gene encoding LTA4H (named HapE) was significantly associated with carotid IMT. Twins with at least one copy of HapE had significantly lower IMT compared to twins without this haplotype, suggesting a potential cardioprotective effect of this haplotype. In addition, habitual dietary intake of n-3 and n-6 fatty acids clearly modulates the association between HapE and carotid IMT. The genetic association between HapE and atherosclerosis, and the HapE × diet interaction persisted after controlling for a detailed set of coronary risk factors and other potential confounders, indicating that this novel haplotype may influence the susceptibility of atherosclerosis through biological pathways that are independent of established risk factors.
LTA4H catalyzes the final step in the biosynthesis of the proinflammatory compound leukotriene B4 from leukotriene A4. Genetic variants could potentially upregulate or downregulate the expression of LTA4H, thereby influencing leukotriene production and contributing to atherosclerosis. A previous study reported an association of a ten-SNP haplotype in LTA4H, named HapK, with MI [3] and early onset coronary artery disease [4]. HapE defined in this study, however, is distinct from HapK. Though both haplotypes were constructed using SNPs in the LTA4H gene and shared four SNPs (rs1978331, rs17677715, rs2540482 and rs2660845), the susceptible alleles in three of them (rs1978331, rs2540482 and rs2660845) are different (i.e., alternative alleles), which may provide an intuitive explanation for the opposite effects of these two haplotypes on CVD. Moreover, the observed main haplotypic or nutrigenetic effect on carotid IMT is unlikely to be driven by HapK or by the three shared SNPs because haplotypes constructed using the three shared SNPs were not associated with IMT in our study sample. Furthermore, there was no interaction between haplotypes inferred from the shared SNPs and dietary intake of PUFAs. It is interesting that HapE was also associated with a lower risk of depression [27], an independent risk factor for CVD. The anti-atherogenic effect of HapE is in agreement with recent research demonstrating an anti-inflammatory role of LTA4H through degrading neutrophil chemoattractant Pro-Gly-Pro (PGP) [28, 29].
Dietary intake of PUFAs has been reported to be beneficial on CVD [9, 30], probably through their anti-inflammatory effects on the cardiovascular system [9, 23]. In support of this, we found that dietary intake of n-3 and n-6 fatty acids is inversely correlated with plasma levels of high-sensitivity C-reaction protein (r =−0.10, p=0.08) and fibrinogen (r = −0.14, p=0.01), respectively. However, subjects who consume same amount of PUFAs may have different risk for CVD, and this interindividual variability in response to diet might be attributed to the different genetic background between individuals. Our analysis demonstrates that habitual dietary intake of PUFAs could influence atherosclerosis susceptibility through interacting with leukotriene genes. Given that both LTA4H and ALOX5 are key enzymes involved in the leukotriene metabolic pathway, our finding lends further support to previous studies demonstrating a nutrigenetic interaction effect of the leukotriene genetic variants on CVD [5, 6].
Although the dietary intakes of EPA and DHA are highly correlated, they may affect carotid IMT differently. For example, a recent study shows that plasma EPA but not DHA was inversely associated with IMT [25], while DHA showed a more potent anti-atherogenic effect than EPA in another study [24]. Similarly, LA and AA may also affect inflammation or atherosclerosis differently. Our sensitivity analyses demonstrate that the interaction between HapE and n-3 fatty acids (sum of EPA and DHA) may not be driven by a specific fatty acid. In contrast, only LA but not AA interacts significantly with HapE on carotid IMT, suggesting that the observed interaction between HapE and n-6 fatty acids is likely to be driven by LA. This finding may imply that LA and AA could function differently in modifying the association between HapE and carotid atherosclerosis. However, inclusion or exclusion of AA did not affect the interaction between HapE and n-6 fatty acids. Therefore, we present the results by using the sum of LA and AA.
Two ALOX5AP haplotypes (called HapA and B) have been reported to confer risk of myocardial infarction in Caucasians [2]. These associations were replicated in some [31–33] but not other studies [34–36]. In our analysis, we did not find an association between ALOX5AP SNPs, either singly or in combination, and carotid atherosclerosis. The discrepancy could be due to the different genetic background between populations and/or the smaller sample size in our analyses. We also did not observe an interaction between diet and HapA or B on carotid atherosclerosis.
Our results should be interpreted in light of the following limitations. First, the use of FFQ to assess fatty acid intake is not as accurate as the direct measure of these fatty acids in blood, and thus it could possibly lead to measurement error. In addition, the FFQ is not validated in our twin sample. Second, the gene × diet interaction reported in this study is statistical but not biological. The extent to which the statistical interactions found imply biological or functional interaction is unclear. Third, though the precise mechanism underlying the nutrigenetic interaction effect on IMT is unclear, it is possible that habitual dietary PUFA intake influences atherogenesis by changing leukotriene levels, which could not be determined in this study. Fourth, though we were able to control for many known lifestyle, behavioral and biological risk factors, we cannot entirely rule out the possibility of confounding by other unknown or unmeasured factors. In addition, though we cannot exclude recall bias as an explanation for our results, recall bias seems unlikely given that we focused on a subclinical measure of CVD. In addition, in sensitivity analyses the overall dietary associations did not differ by known risk factors such as diabetes or hypertension (i.e., diet × disease interaction), which would shed some light on this potential issue of recall bias. Finally, our sample is derived from a twin registry of relatively healthy, middle-aged male military veterans, and therefore may not be generalized to other populations, such as women and younger subjects or populations with clinically manifested CVD.
In summary, in a matched twin sample, we demonstrate for the first time that a novel LTA4H haplotype is associated with a reduced risk for atherosclerosis. This seemingly cardioprotective effect is augmented by dietary intake of n-3 and n-6 fatty acids. If replicated, these findings could potentially inform future individualized dietary and molecular strategies for the prevention or management of CVD.
Research highlights.
Polymorphisms in leukotriene genes have been associated with atherosclerosis.
We examined whether diet modifies the association of leukotriene variants with subclinical atherosclerosis.
Dietary intake of polyunsaturated fatty acids significantly modifies the association of a novel leukotriene haplotype with subclinical atherosclerosis.
Acknowledgments
This study was supported by grants 0730100N from the American Heart Association and NIH grants K01AG034259, R01DK091369, R21HL092363-01A2, R01 HL68630, R01AG026255, and K24HL077506. The United States Department of Veterans Affairs has provided financial support for the development and maintenance of the Vietnam Era Twin (VET) Registry. Numerous organizations have provided invaluable assistance, including: VA Cooperative Study Program; Department of Defense; National Personnel Records Center, National Archives and Records Administration; the Internal Revenue Service; NIH; National Opinion Research Center; National Research Council, National Academy of Sciences; and the Institute for Survey Research, Temple University. The authors gratefully acknowledge the continued cooperation and participation of the members of the VET Registry and their families. Without their contribution this research would not have been possible.
Footnotes
Author contributions: JZ designed research, collected data, performed data analysis and wrote the manuscript; JG contributed to discussion and edited/reviewed the manuscript; VV collected data, contributed to discussion and edited/reviewed the manuscript. JZ had primary responsibility for final content. All authors read and approved the final manuscript.
Conflict of interest: None of the authors disclosed any possible conflicts of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jala VR, Haribabu B. Leukotrienes and atherosclerosis: new roles for old mediators. Trends Immunol. 2004;25(6):315–22. doi: 10.1016/j.it.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 2.Helgadottir A, Manolescu A, Thorleifsson G, et al. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nat Genet. 2004;36(3):233–9. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 3.Helgadottir A, Manolescu A, Helgason A, et al. A variant of the gene encoding leukotriene A4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nat Genet. 2006;38(1):68–74. doi: 10.1038/ng1692. [DOI] [PubMed] [Google Scholar]
- 4.Crosslin DR, Shah SH, Nelson SC, et al. Genetic effects in the leukotriene biosynthesis pathway and association with atherosclerosis. Hum Genet. 2009;125(2):217–29. doi: 10.1007/s00439-008-0619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dwyer JH, Allayee H, Dwyer KM, et al. Arachidonate 5-lipoxygenase promoter genotype, dietary arachidonic acid, and atherosclerosis. N Engl J Med. 2004;350(1):29–37. doi: 10.1056/NEJMoa025079. [DOI] [PubMed] [Google Scholar]
- 6.Allayee H, Baylin A, Hartiala J, et al. Nutrigenetic association of the 5-lipoxygenase gene with myocardial infarction. Am J Clin Nutr. 2008;88(4):934–40. doi: 10.1093/ajcn/88.4.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.James MJ, Gibson RA, Cleland LG. Dietary polyunsaturated fatty acids and inflammatory mediator production. Am J Clin Nutr. 2000;71(1 Suppl):343S–8S. doi: 10.1093/ajcn/71.1.343s. [DOI] [PubMed] [Google Scholar]
- 8.Kris-Etherton PM, Harris WS, Appel LJ. Fish consumption, fish oil, omega-3 fatty acids, and cardiovascular disease. Circulation. 2002;106(21):2747–57. doi: 10.1161/01.cir.0000038493.65177.94. [DOI] [PubMed] [Google Scholar]
- 9.Harris WS, Mozaffarian D, Rimm E, et al. Omega-6 fatty acids and risk for cardiovascular disease: a science advisory from the American Heart Association Nutrition Subcommittee of the Council on Nutrition, Physical Activity, and Metabolism; Council on Cardiovascular Nursing; and Council on Epidemiology and Prevention. Circulation. 2009;119(6):902–7. doi: 10.1161/CIRCULATIONAHA.108.191627. [DOI] [PubMed] [Google Scholar]
- 10.Simopoulos AP. The importance of the omega-6/omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp Biol Med (Maywood) 2008;233(6):674–88. doi: 10.3181/0711-MR-311. [DOI] [PubMed] [Google Scholar]
- 11.Sacks FM, Campos H. Polyunsaturated fatty acids, inflammation, and cardiovascular disease: time to widen our view of the mechanisms. J Clin Endocrinol Metab. 2006;91(2):398–400. doi: 10.1210/jc.2005-2459. [DOI] [PubMed] [Google Scholar]
- 12.Henderson WR., Jr The role of leukotrienes in inflammation. Ann Intern Med. 1994;121(9):684–97. doi: 10.7326/0003-4819-121-9-199411010-00010. [DOI] [PubMed] [Google Scholar]
- 13.Zhao J, Cheema FA, Bremner JD, et al. Heritability of carotid intima-media thickness: a twin study. Atherosclerosis. 2008;197(2):814–20. doi: 10.1016/j.atherosclerosis.2007.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vaccarino V, Brennan ML, Miller AH, et al. Association of major depressive disorder with serum myeloperoxidase and other markers of inflammation: a twin study. Biol Psychiatry. 2008;64(6):476–83. doi: 10.1016/j.biopsych.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldberg J, Curran B, Vitek ME, et al. The Vietnam Era Twin Registry. Twin Res. 2002;5(5):476–81. doi: 10.1375/136905202320906318. [DOI] [PubMed] [Google Scholar]
- 16.Scherrer JF, Xian H, Bucholz KK, et al. A twin study of depression symptoms, hypertension, and heart disease in middle-aged men. Psychosom Med. 2003;65(4):548–57. doi: 10.1097/01.psy.0000077507.29863.cb. [DOI] [PubMed] [Google Scholar]
- 17.Simon A, Gariepy J, Chironi G, et al. Intima-media thickness: a new tool for diagnosis and treatment of cardiovascular risk. J Hypertens. 2002;20(2):159–69. doi: 10.1097/00004872-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 18.Willett W. Nutritional Epidemiology. Oxford University Press; New York, NY: 1998. Food frequency methods; pp. 74–91. [Google Scholar]
- 19.Richardson MT, Ainsworth BE, Wu HC, et al. Ability of the Atherosclerosis Risk in Communities (ARIC)/Baecke Questionnaire to assess leisure-time physical activity. Int J Epidemiol. 1995;24(4):685–93. doi: 10.1093/ije/24.4.685. [DOI] [PubMed] [Google Scholar]
- 20.Consortium TIH. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helgadottir A, Gretarsdottir S, St Clair D, et al. Association between the gene encoding 5-lipoxygenase-activating protein and stroke replicated in a Scottish population. Am J Hum Genet. 2005;76(3):505–9. doi: 10.1086/428066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. Am J Hum Genet. 2001;68(4):978–89. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pischon T, Hankinson SE, Hotamisligil GS, et al. Habitual dietary intake of n-3 and n-6 fatty acids in relation to inflammatory markers among US men and women. Circulation. 2003;108(2):155–60. doi: 10.1161/01.CIR.0000079224.46084.C2. [DOI] [PubMed] [Google Scholar]
- 24.Sekikawa A, Kadowaki T, El-Saed A, et al. Differential association of docosahexaenoic and eicosapentaenoic acids with carotid intima-media thickness. Stroke. 2011;42(9):2538–43. doi: 10.1161/STROKEAHA.110.613042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindqvist HM, Sandberg AS, Fagerberg B, et al. Plasma phospholipid EPA and DHA in relation to atherosclerosis in 61-year-old men. Atherosclerosis. 2009;205(2):574–8. doi: 10.1016/j.atherosclerosis.2008.12.032. [DOI] [PubMed] [Google Scholar]
- 26.Benjamin Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 1995;57(1):289–300. [Google Scholar]
- 27.Zhao J, Quyyumi AA, Patel R, et al. Sex-specific association of depression and a haplotype in leukotriene A4 hydrolase gene. Psychosom Med. 2009;71(7):691–6. doi: 10.1097/PSY.0b013e3181b05c57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Snelgrove RJ. Leukotriene A4 hydrolase: an anti-inflammatory role for a proinflammatory enzyme. Thorax. 2011;66(6):550–1. doi: 10.1136/thoraxjnl-2011-200234. [DOI] [PubMed] [Google Scholar]
- 29.Snelgrove RJ, Jackson PL, Hardison MT, et al. A critical role for LTA4H in limiting chronic pulmonary neutrophilic inflammation. Science. 2010;330(6000):90–4. doi: 10.1126/science.1190594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Harris WS, O’Keefe JH., Jr Preventive use of N-3 fatty acids. Circulation. 2003;108(19):e139. doi: 10.1161/01.CIR.0000099902.61378.96. author reply e139. [DOI] [PubMed] [Google Scholar]
- 31.Linsel-Nitschke P, Gotz A, Medack A, et al. Genetic variation in the arachidonate 5-lipoxygenase-activating protein (ALOX5AP) is associated with myocardial infarction in the German population. Clin Sci (Lond) 2008;115(10):309–15. doi: 10.1042/CS20070468. [DOI] [PubMed] [Google Scholar]
- 32.Tsai AK, Li N, Hanson NQ, et al. Associations of genetic polymorphisms of arachidonate 5-lipoxygenase-activating protein with risk of coronary artery disease in a European-American population. Atherosclerosis. 2009;207(2):487–91. doi: 10.1016/j.atherosclerosis.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 33.Girelli D, Martinelli N, Trabetti E, et al. ALOX5AP gene variants and risk of coronary artery disease: an angiography-based study. Eur J Hum Genet. 2007;15(9):959–66. doi: 10.1038/sj.ejhg.5201854. [DOI] [PubMed] [Google Scholar]
- 34.Lohmussaar E, Gschwendtner A, Mueller JC, et al. ALOX5AP gene and the PDE4D gene in a central European population of stroke patients. Stroke. 2005;36(4):731–6. doi: 10.1161/01.STR.0000157587.59821.87. [DOI] [PubMed] [Google Scholar]
- 35.Zee RY, Cheng S, Hegener HH, et al. Genetic variants of arachidonate 5-lipoxygenase-activating protein, and risk of incident myocardial infarction and ischemic stroke: a nested case-control approach. Stroke. 2006;37(8):2007–11. doi: 10.1161/01.STR.0000229905.25080.01. [DOI] [PubMed] [Google Scholar]
- 36.Meschia JF, Brott TG, Brown RD, Jr, et al. Phosphodiesterase 4D and 5-lipoxygenase activating protein in ischemic stroke. Ann Neurol. 2005;58(3):351–61. doi: 10.1002/ana.20585. [DOI] [PMC free article] [PubMed] [Google Scholar]