Abstract
Research models that replicate the diverse genetic and molecular landscape of breast cancer are critical for developing the next generation therapeutic entities that can target specific cancer subtypes. Patient-derived tumorgrafts, generated by transplanting primary human tumor samples into immune-compromised mice, are a valuable method to model the clinical diversity of breast cancer in mice, and are a potential resource in personalized medicine. Primary tumorgrafts also enable in vivo testing of therapeutics and make possible the use of patient cancer tissue for in vitro screens. Described in this unit are a variety of protocols including tissue collection, biospecimen tracking, tissue processing, transplantation, and 3-dimensional culturing of xenografted tissue, that enable use of bona fide uncultured human tissue in designing and validating cancer therapies.
Keywords: Breast cancer, Tumorgraft, Organoid, 3D Culture, Matrigel, EHS matrix, Biospecimen, Tissue repository, Tissue collection, Xenograft
INTRODUCTION
Development of patient-derived orthotopic tumorgrafts and three-dimensional organoid models for human breast cancer
Model systems are essential for discovery, development, and testing of new therapies for breast cancer. Generating the appropriate models for particular applications is challenging, as all models have both advantages and disadvantages.(Gupta and Kuperwasser, 2004; Voskoglou-Nomikos et al., 2003) Immortalized cell lines are used routinely to study breast cancer, are easy to grow and manipulate, and are ideal for examining the molecular mechanisms of tumor cell biology.(Holliday and Speirs, 2011; Burdall et al., 2003) Likewise, implantation of breast cancer cell lines into mice for in vivo studies is a useful way to study mechanisms of tumor growth, progression, metastasis and drug response.(Cespedes et al., 2006; Kerbel, 2003; Clarke, 1996) A caveat in the use of cell lines, however, is their prolonged adaptation to tissue culture conditions that has lead to genetic and epigenetic alterations over time, (Tsuji et al., 2010; Kao et al., 2009; Neve et al., 2006) and the necessity to grow xenografts in immune-compromised mice in vivo where the tumor environment is different from that of native tumors.(Cespedes et al., 2006; Kerbel, 2003; Clarke, 1996)
Genetically engineered mouse models, on the other hand, allow the study of tumor development and progression in immune-competent mice(Caligiuri et al., 2012; Borowsky, 2011) However, most mouse genetic models rely on the expression of a single, potent oncogene to drive the tumor, and frequently do not recapitulate the full phenotypic repertoire of the human tumor nor metastatic patterns seen in breast cancer patients. A review of various cancer models and their utility for drug development can be found in UNIT 14.22 and also as a summary in Table 1.
Table 1.
Comparison of different breast cancer models.
| Breast Cancer Model | Strengths | Weakness |
|---|---|---|
| Carcinogen-induced mouse model |
Immune-comptetent mouse and diverse tumor subtypes are generated |
Tumor is not human, may not reflect the genetic variations that occur in humans, short tumor evolution may not replicate events that occur during the longer evolution of human tumors, intra-tumor heterogeneity often limited, requires genetic tools, laborious to generate, expensive, and limited number of cancer types are modeled |
| Genetically engineered mouse models (GEMM) |
Defined oncogenic driver, limited variablity between tumors, inducible modeling possible, targeted oncogensis in specific cell types, immune competent mouse, and ability to model early events in oncogenesis |
Tumor is not human, may not reflect the genetic variations that occur in humans, short tumor evolution may not replicate events that occur during the longer evolution of human tumors, laborious to generate, expensive, limited number of cancer types currently modeled |
| Human cell line xenograft |
Human tissue, cell of origin and oncogenic drivers are directly associated human cancer, cells are easily grown and manipulated in culture, many cells lines well characterized in the literature, and can be genetically modified and selected |
Genetic drift, selective pressure from cell culture, and requires immune-compromised mice |
| Patient-derived tumorgrafts |
Human tissue, cell of origin and oncogenic drivers directly associated with human disease, can be genetically modified, natural evolution in patients, both chemo-naïve and standard-of-care chemo-resistant tissue, no selective pressure from in vitro culturing, molecular and genetic markers highly representative of primary tumors, potential for personalized medicine |
Not all subtypes grow well in mice, requires immune- compromised mice, laborious, expensive, establishment of bio- bank requires significant coordination between physicians and departments, quality control of specimen processing, IBC regulatory approval for tissue collection, HIPPA, PHI, and intellectual property issues |
We have recently reported that directly implanting breast tumor tissues derived from patients into mouse mammary fat pads results in a remarkable recapitulation of human breast cancer. These “tumorgrafts” closely resemble the original tumors from the patient by: i) maintaining their clinical markers and histopathologies ii) maintaining hormone dependence or independence; iii) maintaining their gene expression and DNA copy number variations, and iv) in recapitulating clinically relevant metastasis.(DeRose et al., 2011 and Figure 1) Although the techniques to generate these tumorgrafts can be challenging, the effort is worthwhile as they are the best currently available model of human breast cancer. Such a resource is likely to become a valuable asset for drug discovery efforts, particularly for preclinical animal studies to assess the effectiveness of lead compounds on specific cancer subtypes. In this way, tumorgrafts could help distinguish cancer subtypes that are either responsive or unresponsive to a compound, and enable clinical trials to be designed around the most appropriate patient population. While a significant investment must be made to establish a bank of transplantable and subtype-diverse tumorgrafts, better prediction of a drug’s effectiveness should lead to more successful clinical trials, which would appreciably outweigh the initial investment. Still, the validity and practicality of primary tumorgrafts remains to be verified in a drug development program.
Figure 1.
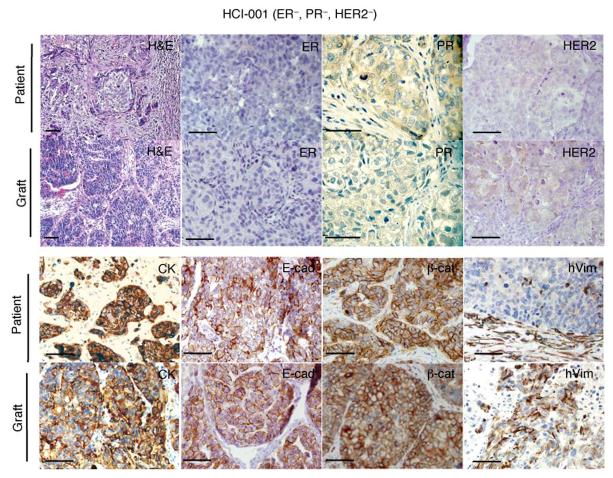
Tumorgrafts resembled the original tumors from which they were derived. A representative ER−PR−HER2− tumor graft (HCI—001) is shown in comparison to the original patient sample. The tumor ID and the original clinical diagnosis for ER, PR, and HER2 are shown at the top. Sections from the patient’s primary breast tumor (patient), and from representative tumor grafts from the same patient (graft). Stains shown are hematoxylin and eosin (H&E) as well as antibody stains for ER, PR, HER2, cytokeratin (CK), E—cadherin (E—cad), β—catenin (β—cat), and human specific vimentin (hVim). Positive antibody signals are brown in color, with hematoxylin (blue) counterstain. Some images are shown at higher magnification to visualize nuclear staining. All scale bars correspond to 100 μm. This figure was reprinted from DeRose et al., 2011 with permission from Nature Medicine.
Tumors removed from patients can be processed in many different ways (Table 2). Thus, it is important to identify the most appropriate processing technique for a research purpose. Here, we describe three types of tissue processing: tumor fragments, organoids, and single cell suspensions. The processing occurs sequentially. Tumors are first removed from patients and manually dissected into tissue fragments that can be used for tissue transplantation. Processing of tumor fragments by mincing and enzymatic digestion with collagenase and hyaluronidase leads to tumor organoids, which can be used for transplantation or 3D culturing. Finally, tumor organoids can be digested with trypsin/EDTA to generate a single cell suspension for use in transplantation, cell culturing, virus infection, and FACS. Thus, tumor tissue is serially processed through stages as needed for specific experimental applications.
Table 2.
Processing methods for patient-derived primary tumor tissue.
| Tumor Fractionate |
Processing Technique |
Processing Method |
Composition of Fractionate | Usage |
|---|---|---|---|---|
| Fragment | Manual | Tumor is manually cut into fragments with scalpel or razor |
>1mm diameter tumor fragments, cell contacts are maintained, may contain blood vessels, stroma, immune cells, extracellular matrix |
Transplantation |
| Organoid | Manual and Enzymatic |
Tumor fragments are chopped and then digested with collagenase and hyaluronidase |
50-200μm tumor fragment, cell contacts are maintained, some stroma may be present |
Transplantation and/or 3D culturing |
| Single Cells | Manual and Enzymatic |
Organoids are isolated as above, then digested with trypsin/EDTA |
Isolated tumor cells, cell contacts are lost, diverse tumor and stromal cell populations present but dispersed in suspension |
Transplantation, 2D culturing, viral infection, FACS |
Outlined in the present unit are procedures for processing human breast cancer tissue and implanting it into mice (Basic Protocol 1); processing human body fluid (e.g., pleural effusions) for transplantation or storage (Basic Protocol 2); characterizing single cells isolated from human breast tissue or pleural effusions by cell surface markers (Basic Protocol 3); implanting estrogen pellets subcutaneously into mice (Basic Protocol 4); implanting breast tissue into cleared mammary fat pads (Basic Protocol 5); and, implanting breast cancer cells with Matrigel into cleared mammary fat pads (Basic Protocol 6). Details are also provided for the isolation of tumor cells from tumor grafts using enzymatic digestion (Basic Protocol 7), producing and transducing the cells with lentiviruses (Basic Protocol 8), and growing tumorgraft or primary patient organoids in three-dimensional (3D) cultures for drug discovery and validation (Basic Protocol 9). Support protocols include describing the isolation and storage of human breast tumor tissue (Support Protocol 1); preparation of estrogen pellets (Support Protocol 2); and preparation of growth factor-reduced Engelbreth-Holm-Swarm (EHS) tumor matrix (Support Protocol 3).
NOTE: All human patient tissue samples must be collected from informed, consenting patients under an approved Institutional Review Board for Human Participants (IRB) protocol. Tissue collection at the Huntsman Cancer Institute is coordinated by the Tissue Resource and Applications Core shared resource facility using protocols described in Supporting Protocol #1. Specific training is provided to all staff members involved with obtaining patient consent, and in the collection and processing of tissue to ensure the acquisition of high quality biospecimens and compliance with the Health Insurance Portability and Accountability Act (HIPAA) and the protection of Personal Health Information.
Basic Protocol #1: Processing human breast tumors and tumorgrafts into fragments, organoids, and single cells
We prefer to store primary patient and xenograft tumor tissue as fragments since they have a higher transplantation success rate than either organoids or single cell suspensions (DeRose et al., 2011 and unpublished observations). All fresh tumor tissue samples must be kept on ice and processed in sterile conditions on the day of collection. All tissue processing is performed in a biosafety cabinet (hood) using Biosafety Level 2 (BSL2) techniques. See Supporting Protocol #1 for information on the collection and cataloging of primary human tissue.
Materials
Human breast primary tumor tissue, metastasis tissue, effusion samples (fresh; the quantity usually varies) or fresh tumorgrafts derived from mice
Ice
Petri dish, sterile
Disposable scalpels (#10 blades), razor blades, sterile
Digestion buffer (see REAGENTS AND SOLUTIONS)
Collagenase enzyme stock (10×) (see REAGENTS AND SOLUTIONS)
Hyaluronidase enzyme stock (100×) (see REAGENTS AND SOLUTIONS)
37°C shaker/incubator
Disposable cell lifter (Fisher #08-100-240), sterile
Cell strainer (100μm, BD Falcon #352360), sterile
24-well ultra low adhesion plates (Corning Costar #3473)
24-well standard tissue culture plate
70% ethanol
1× phosphate-buffered saline (PBS), sterile
Hyclone Hank’s Balanced Salt Solution (HBSS; Thermo Scientific #SH30268.02)
Characterized fetal bovine serum (FBS), heat inactivated (Thermo Scientific #SH30071.03)
HBEC medium (see REAGENTS AND SOLUTIONS)
Nalgene Cryo 1 °C freezing container filled with isopropanol (Nalgene #5100-0001)
50 mL conical tubes, sterile
1 – 1.5 mL cryovial tubes, sterile
1.5 mL eppendorf tubes, sterile
TAC buffer (see REAGENTS AND SOLUTIONS)
HBEC freezing medium (see REAGENTS AND SOLUTIONS)
Parafilm
0.05% Trypsin-EDTA (Invitrogen #25300-054)
Trypan Blue Solution, 0.4% (Sigma #T8154)
Procedures
A. Processing solid tissue into fragments
Place the solid tumor tissue (size can vary) in a 10 cm petri dish, pouring sterile DMEM medium on the tissue to keep it moist.
-
Cut the tumor into pieces using a scalpel or razor blade, removing necrotic tissue if present.
Nectrotic human tissue is typically whiter and softer compared to the surrounding tumor tissue since it is less vascularized. Necrotic tumorgraft tissue from mice is normally darker and softer compared to the surrounding tumor.
Proceed to transplantation (Basic Protocol #5) or cryopreservation (Basic Protocol #1, Step 4), as needed.
-
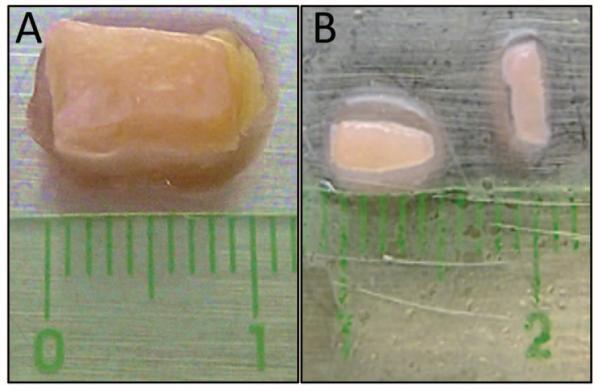
For cryopreservation: Cut the tumor tissue into 4mm × 2mm fragments (Figure 2). Place 5 fragments in a cryovial and fill with Tissue Freezing Medium (do not confuse with HBEC freezing medium). Freeze the cells by slowly decreasing the temperature 1°C/min. This can be done by placing the vials in a Nalgene Cryo 1 °C cell-freezing container filled with room temperature isopropanol. Place the container at −80°C overnight before transferring the vials to liquid nitrogen cryotanks.
We freeze 5 fragments per vial so that each vial is sufficient for implantation into 5 mice when thawed. Do not re-freeze tissue fragments.
Figure 2.
Example of breast tumor tissue isolated from a patient. A. Surgical sample containing tumor tissue (white-pink color) and fat (yellow color). B. Fragments of the same tissue sample following removal of the fat and dissection into approximately 4 mm × 2 mm pieces, ready for implantation into mammary fat pads. The halo surrounding the tissue is due to the fluid used to keep the samples moist during processing.
B. Processing solid tissue into organoids
Weigh the tissue.
The quantity of tissue will depend on the amount obtained from surgery and the amount reserved as fragments. Mince the tissue using a crisscross motion with two disposable scalpels until finely chopped, then transfer the minced tissue to a 50 mL conical vial using a cell lifter.
- Add digestion buffer to a final volume of 10 mL per gram of tissue.
- For primary human specimens: Add 10× Collagenase Solution and 100× Hyaluronidase Solution (see REAGENTS AND SOLUTIONS) to a final concentration of 1× each.
- For tumor graft tissue: Add only 10× Collagenase Solution to a final concentration of 1×.
Close the tube with a cap and wrap the cap with parafilm (parafilm helps to prevent leakage and potential bacteria/yeast contamination of the area around the cap). Place the tube on its side in a 37°C shaker at low to moderate speed (e.g. 200 rpm).
- The incubation time varies between primary human tissue and tumorgraft tissue.
- For primary human samples from patients: Due to the extensive stromal component, human tumor samples require more time in digestion media. Shake/incubate human primary tissue overnight (~18 hr) in digestion media.
-
For tumorgraft tissue: Tumorgrafts (derived from mice) contain less stroma and digest easier than primary human samples. Shake for 40 min and determine if tissue is well digested. If not, incubate longer, checking every 5-10 min.Monitor the digestion by gross observation and light microscopy of the digestion media. Grossly, large undigested material can sometimes be seen in the digestion media. The large undigested tissue may contain cell debris, DNA from lysed cells, fat, and extracellular matrix. This material typically forms large strands rather than chunks, and will not digest or produce many organoids. Organoids are best visualized by microscopy. Take a 25ul sample of the digestion media and dilute 1:10-1:100 in PBS and place 25ul of the dilution in an empty well of a 24 well standard tissue culture dish. Look for large refractory clusters of epithelial cells, called organoids. More digestion may be appropriate if most of the organoids are >200um diameter. If the tissue does not digest well, remove all undigested fragments and debris by straining the digested tissue into a new tube using a 100um cell strainer. Keep the digestion media that passes through the strainer and visualize under a microscope as described above. If organoids are present, continue protocol with this solution at step 6. Place the strained material in a 50 mL conical tube, add fresh digestion buffer, then shake for 1-3 more hours to recover more digested tissue. Continue with step 6 when most organoids are <200um diameter.
Centrifuge at 530 × g at room temperature for 5 min to pellet the cells.
-
Aspirate the supernatant, which contains fat. If the pellet contains red blood cells (observed as a red layer on top of the pellet), resuspend the pellet in 5 – 10 mL of TAC buffer and incubate 3 – 10 min in a 37°C water bath. Centrifuge at 530 × g at room temperature for 5 min. Repeat this step until the red blood cells are no longer visible.
Steps 8-9 in this protocol function to separate organoids, which contain most of the tumor epithelium, from stromal cells and debri using a process called differential centrifugation. The two key principles are: 1) the collagenase/hyaluronidase digestion preferentially disrupts cell contacts between stromal cells causing their release as single cells, while tumor epithelium remains mostly adherent, resulting in large 100-200um diameter clusters of tumor cells (organoids); and 2) the difference in weight between single cells/debri and organoids enables fractionation by centrifugation. This process must be optimized with different centrifuges since acceleration and deceleration kinetics varies with each instrument.
Resuspend the pellet in 10 mL of DMEM/F12 medium (with no supplements) and centrifuge at 530 × g at room temperature for 10-30 sec. Collect both the supernatant (suspension fraction) and pellet (organoid fraction). Resuspend each fraction in 1ml DMEM/F12 medium (with no supplements). Take a 10-25ul sample of the fraction media and dilute 1:10-1:100 in PBS and place 25ul of the dilution in an empty well of a 24 well standard tissue culture dish. Examine the quality of the fraction under a light microscope. The organoid fraction should be more enriched for refractile, solid clusters of tumor cells, termed “organoids,” and few single cells (Figure 3). The suspension fraction should be enriched in single cells and be relatively free of organoids. Continue fractionating by differential centrifugation as described in step 9.
-
For the organoid fraction: resuspend the pellet in 9 mL of DMEM/F12 medium (with no supplements) and centrifuge at 530 × g at room temperature for 10-30 sec to pellet the cells. For the suspension fraction: centrifuge at 530 × g at room temperature for 5 min to pellet the suspended cells. Analyze both samples as described in step 8. To further enrich organoids from single cells, perform differential centrifugation by repeating this step 3-4 times until the desired organoid enrichment is achieved.
The fractionation procedure is significantly impacted by the type/brand of centrifuge used due to differences in time-to-full-speed and time-to-stop. Thus, this step requires optimization of the centrifugation times for different centrifuges. The investigator must also determine a desired level of organoid enrichment for each experiment. For example, more highly enriched organoids have fewer stromal and immune cells in the preparation. Thus, it is beneficial to perform more differential centrifugations for applications such as culturing organoids in 3D, processing organoids into single cells for FACS analysis, and viral transduction. In contrast, it is not necessary to have highly enriched organoids for transplantation.
-
Estimate the number of organoids in the final sample and calculate the approximate total number of organoids recovered from the tissue. The 24 well plate containing the 25 μl samples used to assess the fractionation procedure (step 9) can be cultured overnight in 0.5 mL of HBEC medium/well to determine whether or not the sample is contaminated with bacteria or fungus.
Contamination can be a problem because samples are not handled using sterile technique in most gross pathology labs. At our institution, we eliminated the problem of contamination by providing the lab with a sterile breast sample collection kit to be used for collection of viable specimens (see Support Protocol #1).
Proceed to cryopreservation (Step 12), transplantation (Basic Protocol #6), single cell processing (Basic Protocol #1, Step C), or 3D cell culture (Basic Protocol #9), if applicable.
For cryopreservation: Resuspend the organoids in freezing medium in aliquots of 1.5 ml containing up to 10,000 organoids per vial. Freeze the cells by slowly decreasing the temperature 1°C/min. This can be done by placing the vials in a Nalgene Cryo 1 °C cell-freezing container filled with room temperature isopropanol. Place the container at −80°C overnight before transferring the vials to liquid nitrogen cryotanks. If there is a problem with the overnight culture (e.g. contamination with fungus or bacteria), discard the frozen organoids.
Figure 3.
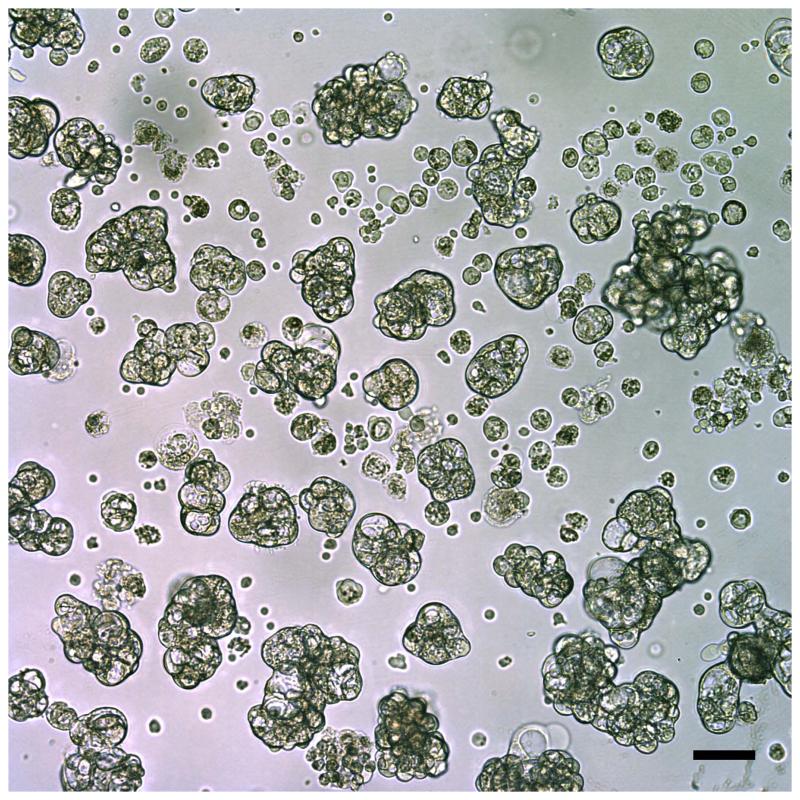
Metastatic tumor cells collected from a pleural effusion and viewed using light microscopy. These breast tumor cells aggregated into organoid-like structures, which were easily separated from single cells such as leukocytes that were present in the original specimen. Scale bar is 100 μm.
C: Processing organoids into single cells
Centrifuge the solution containing organoids or large clumps of cells for 5 min at 400 × g at 4 °C.
Aspirate the supernatant and carefully resuspend the pellet with 10 ml of PBS by pipetting up and down on ice.
Centrifuge the cells for 5 min at 400 × g at 4 °C.
-
Aspirate the supernatant and carefully resuspend the pellet with 1-5 ml of trypsin. Place the conical tube in a 37 °C shaker/incubator at 150-300 rpm. Every 5 min, remove a 10 μl aliquot of the suspension and observe under a microscope. Continue the incubation if organoids are still present.
Some tumor organoids can take >20 min to dissociate into single cells and may require addition of more trypsin. However, longer incubation times with trypsin will reduce cell viability. To increase viability when long trypsin incubations are required, it may be necessary to separate single cells from clusters every 10 min (using differential centrifugation or cell straining). Single cells could be washed (as in step 5) and stored on ice while the clusters are digested for a longer.
Once single cells are obtained, add 10 ml of HBSS containing 2% FBS and centrifuge the cells for 5 min at 400 × g at 4 °C.
Aspirate the supernatant and carefully resuspend the pellet with 5 ml of HBSS containing 2% FBS by pipetting up and down. Perform a trypan blue exclusion test using a hemocytometer to determine the number of viable cells per ml (Strober, 2001)
For cryopreservation: Resuspend the pellet in HBEC Freezing Medium (do not confuse with Tissue Freezing Medium) at a concentration of approximately 5-10 million cells per ml and freeze in 1 ml aliquots as described above.
Proceed to transplantation (Basic Protocol #6), FACS (Basic Protocol #3), lentiviral infection (Basic Protocol #8) or 3D culture (Basic Protocol #9).
Basic Protocol #2: Processing human body fluid (e.g. pleural effusion, ascites etc.)
In addition to solid tumors, patients with metastatic breast cancer can present with a pleural effusion, which is a buildup of fluid and tumor cells in the pleural cavity. It is not unusual for 500 mL to 1.5 L of effusion fluid to be collected from a single patient, which can contain large amounts of tumor cells. This fluid can be a very rich source of metastatic breast cancer cells, but can contain other contaminating cell types, such as lymphocytes, macrophages and fibroblasts. Therefore, cells isolated from pleural effusions or ascites fluid, should be characterized by cell surface markers, as described in Basic Protocol #3.
Materials
Fresh human effusion samples (handle under BSL2 conditions)
Ice
500 ml centrifuge bottles (Nalgene #3122-0500)
50 mL conical tubes, sterile
Cell Strainer (100μm, BD Falcon #352360), sterile
70% ethanol (EtOH)
1× phosphate-buffered saline (PBS), sterilized
Hyclone Hank’s Balanced Salt Solution (HBSS; Thermo Scientific #SH30268.02)
HBEC medium (see REAGENTS AND SOLUTIONS)
Nalgene Cryo 1 °C freezing container filled with isopropanol (Nalgene #5100-0001)
1 – 1.5 mL cryovial tubes, sterilized
1.5 mL eppendorf tubes, sterilized
TAC solution (see REAGENTS AND SOLUTIONS)
HBEC freezing medium (see REAGENTS AND SOLUTIONS)
Transfer the freshly acquired effusion fluid to a 50 ml conical tube on ice. For large volume samples, use sterile 500 ml Nalagene centrifuge bottles.
-
Centrifuge the fluid for 5 min at 530 × g at 4 °C and collect the pellet.
If using a rotor that is also used to centrifuge bacterial medium, be sure to disinfect the outside of the bottles thoroughly with ethanol prior to performing the next steps to prevent contamination.
If the pellet contains red blood cells (observed in the top layer of the cell pellet), resuspend the pellet in 5 – 10 ml of TAC buffer and incubate the sample for 3 – 10 min in a 37°C water bath. Centrifuge at 530 × g for 5 min at room temperature. Repeat this step until the blood cells are no longer visible.
Resuspend the pellet in 5 – 10 ml of HBSS or 1× PBS.
Centrifuge at 530 × g for 5 min at room temperature.
-
Resuspend the pellet in 5 – 10 ml HBSS or 1× PBS and assess its content by plating 25 μl into a dish and examine the cellular organization under a light microscope (organoids and single cells) (Table 2 and Figure 3).
The organization of tumor cells in pleural effusions/ascites varies significantly, ranging from organoid-like aggregates to fully dispersed single cells. In addition, the number of tumor and immune cells is highly variable (Figure 4). Both the cellular organization (based on microscopy) and cell content (based on FACS, Basic Protocol #3) should be recorded for each effusion.
Centrifuge at 530 × g at room temperatre for 5 min.
Proceed to transplantation (Basic Protocol #6), FACS (Basic Protocol #3), lentiviral infection (Basic Protocol #8) or 3D culture (Basic Protocol #9).
For cryopreservation: Resuspend the pellet in HBEC Freezing Medium (do not confuse with Tissue Freezing Medium) to a concentration of approximately 1,000,000 organoids or 5-10 million cells per ml and freeze in 1 ml aliquots as described in Basic Protocol 1.
Figure 4.
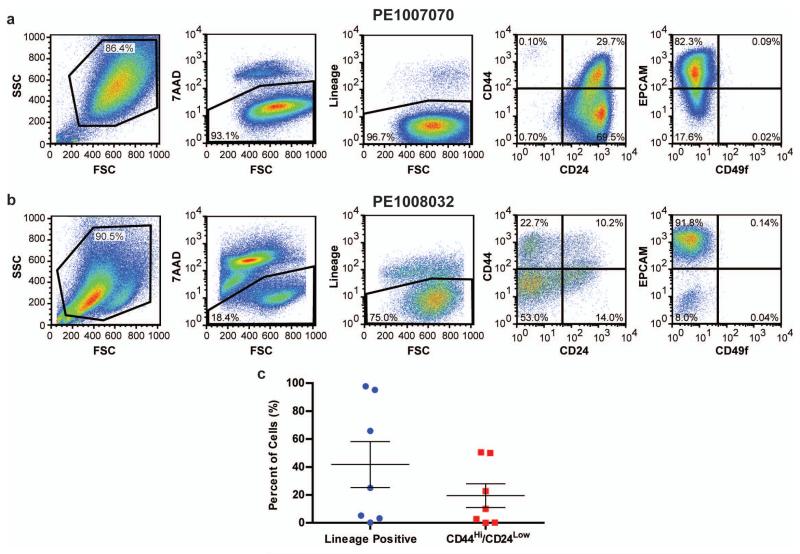
(A) PE1007070 and (B) PE1008032, which are primary cells isolated from pleural effusions from two separate patients were analyzed by FACS for 7-AAD and Lineage markers in combination with either CD44/CD24 or EPCAM/CD49f. (C) Primary cells isolated from pleural effusions from seven patients were analyzed by FACS for Lineage markers and CD44/CD24.
Basic Protocol #3: Characterization of single cells isolated from human tumors, body fluid or from tumor graft tissue using fluorescence activated cell sorting (FACS)
Primary human tumors typically contain heterogeneous cell populations, including stromal and immune cells in addition to tumor cells with diverse tumorigenic capacity. It has been shown that these different populations can be characterized using cell surface markers such as epithelial cell adhesion molecule (EPCAM) and CD49f (α6 integrin), which can differentiate luminal (EPCAMHi/CD49fLow) versus basal/myoepithelial cells (EPCAMlow/CD49fHi) in normal breast tissue, and exhibit distinct expression in breast cancer subtypes (Keller et al., 2010). In addition, the cell surface markers CD44Hi and CD24Low have been used to identify a population of breast cancer cells with enhanced tumor initiating capabilities in mouse transplantation assays (Al-Hajj et al., 2003). Since every patient sample may contain different populations of tumor cells as well as contaminating non-epithelial cells, such as lymphocytes, macrophages and fibroblasts, it is important to characterize single cells isolated from human breast tumors (Basic Protocol #1), human body fluids (Basic Protocol #2) or tumorgraft tissue (Basic Protocol #8).
The protocol involves staining single cell suspensions derived from tumors with a variety of antibodies that differentially mark specific cell populations. For example, 7-AAD is used to exclude dead cells and a cocktail of antibodies (CD2, CD3, CD10, CD16, CD18, CD31, CD64 and CD140b) is used to exclude non-epithelial cells (lineage positive), such as lymphocytes, macrophages and fibroblasts. Simultaneously the cells are stained with combinations of antibodies that recognize tumor markers, such as EPCAM, CD49f, CD44, CD24, that are expressed on diverse tumor cell populations. The cell suspension is then analyzed or sorted into enriched cell fractions by flow cytometry. An example of a typical FACS analysis of primary cells isolated from pleural effusions from two patients is illustrated in Figure 4 A and B. Additionally, the percentage of lineage positive (non-epithelial) and CD44Hi/CD24Low (tumor initiating) cells from seven different primary patients samples is presented in Figure 4 C. These data demonstrate that every patient sample contains different percentages of lineage positive and CD44Hi/CD24Low populations. Typically, we do not utilize primary patient cells that contain high levels (>30-40%) of lineage positive for the three-dimensional primary tumor cultures described in Basic Protocol #9.
Materials
Characterized fetal bovine serum (FBS), heat inactivated (Thermo Scientific #SH30071.03)
Hyclone Hank’s Balanced Salt Solution (HBSS; Thermo Scientific #SH30268.02)
Modified M87 medium (see REAGENTS AND SOLUTIONS)
0.05% Trypsin-EDTA (Invitrogen #25300-054
Trypan Blue Solution, 0.4% (Sigma #T8154)
DNaseI (AMRESCO #0649)
10 cm tissue culture treated polystyrene cell culture dish (BD Falcon # 353003)
1× phosphate-buffered saline (PBS), sterile
15 mL conical tubes, sterile
50 mL conical tubes, sterile
100 μm cell strainer (BD Falcon #352360)
12 × 75 mm round bottom polystyrene FACS tube (BD Falcon # 352008)
37°C 5% CO2 Incubator
37°C Shaker/Incubator
All of the reagents/antibodies listed in Table 3
4 color FACS machine capable of detecting FITC, PE, 7-AAD, and APC Fluorochromes
Swinging bucket centrifuge
Table 3.
Antibody and reagent concentrations for FACS
| Reagent | Source | Catalog # | Concentration |
|---|---|---|---|
| PE mouse anti-human CD2 | BD Biosciences | 555327 | 1:100 vol/vola |
| PE mouse anti-human CD3 | BD Biosciences | 555333 | 1:100 vol/vola |
| PE mouse anti-human CD10 | BD Biosciences | 555375 | 1:100 vol/vola |
| PE mouse anti-human CD16 | BD Biosciences | 555407 | 1:100 vol/vola |
| PE mouse anti-human CD18 | BD Biosciences | 555924 | 1:100 vol/vola |
| PE mouse anti-human CD31 | BD Biosciences | 555446 | 1:100 vol/vola |
| PE mouse anti-human CD64 | BD Biosciences | 558592 | 1:100 vol/vola |
| PE mouse anti-human CD140b | BD Biosciences | 558821 | 1:100 vol/vola |
| FITC mouse anti-human CD24 | BD Biosciences | 555427 | 1:50 vol/vol |
| APC mouse anti-human CD44 | BD Biosciences | 559942 | 1:50 vol/vol |
| FITC rat anti-human CD49f | BD Biosciences | 555735 | 1:50 vol/vol |
| APC mouse anti-human EPCAM | BD Biosciences | 347200 | 1:100 vol/vol |
| 7-AAD staining solution | BD Biosciences | 559925 | 0.25 μg/106 cells |
All of the PE conjugated antibodies are mixed together to generate the linage cocktail.
Procedure
A. Culturing freshly prepared tumor cells overnight
The cells are cultured overnight in order to allow them to recover from the tissue processing procedure (Basic Protocols #1and #8) before performing FACS staining. Alternatively, cells can be used directly after processing, but they may be more susceptible to dying during the staining procedure
For freshly isolated tumor cells: Prepare fresh tumor cells from body fluids as described in Basic Protocol # 1 and #2, then continue this protocol at step 5.
For cryopreserved cells: Defrost a vial of cryopreserved human primary tumor cells by submerging it in a 37°C water bath for 2-3 min. As soon as the vial is thawed, transfer the contents to a 15 ml conical tube containing 10 ml of the modified M87 media that has been pre-warmed to 37°C.
Centrifuge the cells for 5 min at 400 × g at room temperature.
Aspirate the supernatant and carefully resuspend the pellet into 1 ml of modified M87 media that has been pre-warmed to 37°C by pipetting up and down.
Perform a trypan blue exclusion test using a hemocytometer to determine the number of viable cells per ml (Strober, 2001).
Adjust the concentration of cells to 4-6 × 106 cells in 10 ml by addition of modified M87 media that has been pre-warmed to 37°C.
Add 10 ml of the cell suspension to a 10 cm tissue culture treated dish. Incubate the cells at 37°C in 5% CO2 overnight.
B. Collecting cells after overnight incubation
-
Remove the plate from the incubator and collect the media, which may contain non-adherent tumor cells, and transfer it to a 50 ml conical tube on ice.
Some pleural effusions contain non-adherent tumor cells that do not need to be trypsinized prior to FACS. However, if the non-adherent tumor cells are aggregated in clusters or organoids, they should be processed into single cells as described in Basic Protocol #1 Step C. The presence or absence of adherent cells does not necessarily correlate with the percentage of lineage positive (non-epithelial) cells.
To remove adherent cells, immediately wash the plate with 10 ml of PBS and transfer the solution to the 50 ml conical tube containing the cell suspension on ice. Add 1 ml of trypsin to the plate.
Incubate the cells with trypsin for 1-5 min at 37 °C until the adherent cells detach. Gently pipette up and down to mix the trypsin solution and transfer it to the 50 ml conical tube containing the suspension cells.
Wash the plate with 10 ml of HBSS containing 2% FBS and transfer the solution to the 50 ml conical tube containing the cell suspension on ice.
Centrifuge the cells for 5 min at 400 × g at 4 °C.
Aspirate the supernatant and carefully resuspend the pellet with 5 ml of HBSS containing 2% FBS by pipetting up and down. Perform a trypan blue exclusion test using a hemocytometer to determine the number of viable cells per ml (Strober, 2001)
-
Adjust the concentration to 1.0 × 106 cells/ml by adding HBSS containing 2% FBS on ice and proceed to the staining, Step C.
If large adherent clusters of cells are present, they will need to be further processed into single cells. Centrifuge the cell suspension for 5 min at 400 × g at 4 °C and resuspend the pellet in 1ml of trypsin. Repeat steps 3-7 until the cells are disaggregated. DNA released from cell lysis during the trypsinization step may cause the media to become viscous and difficult to handle, and may cause cells to clump together. If this occurs, add DNaseI (10ug/ml final concentration DNaseI) to the media and incubate for ~5 min at room temperature, then repeat steps 5-7.
C. Preparing cells for FACS analysis of CD44/CD24 and EPCAM/CD49f markers
- Adjust the concentration of single cells to 1.0 × 106 cells/ml by adding HBSS containing 2% FBS on ice. Add 1 ml of the single cell suspension at 1.0 × 106 cells/ml to nine 15 ml conical tubes (1.0 × 106 cells/sample) for each staining condition listed below. For the remainder of the staining procedure, keep the cells on ice and in the dark. Also, keep the HBSS containing 2% FBS on ice.
- No antibody
- CD24-FITC (FL1 setup)
- Lineage-PE Cocktail, which is a mixture of PE conjugated CD3, CD10, CD16, CD 18, CD31, CD64 and CD140b antibodies (FL2 setup)
- 7-AAD (FL3 setup)
- CD44-APC (FL4 setup)
- CD44/CD24 Analysis (7-AAD, Lineage PE cocktail, CD24-FITC and CD44-APC)
- CD49f-FITC (FL1 setup)
- EPCAM-APC (FL4 setup)
- EPCAM/CD49f Analysis (7-AAD, Lineage PE cocktail, CD49f-FITC and EPCAM-APC)
Centrifuge the cells for 5 min at 400 × g at 4 °C.
Aspirate the supernatant and carefully resuspend the pellet with 200 μl of HBSS containing 2% FBS.
-
Add the appropriate antibodies to tubes b, c and e-i in step 1 according to the dilutions listed in Table 3
Alternatively, use a stock solution of all eight PE-conjugated antibodies to resuspend the samples requiring the lineage cocktail in step 3.
Incubate the cells for 30 min at 4 °C.
Add 1 ml of HBSS containing 2% FBS to each tube
Centrifuge the cells for 5 min at 400 × g at 4 °C. Wash the cells by aspirating the supernatant and carefully resuspending the pellet with 1 ml of HBSS containing 2% FBS.
Centrifuge the cells for 5 min at 400 × g at 4 °C. Aspirate the supernatant and carefully resuspend the pellet with 300 μl of HBSS containing 2% FBS.
Add 5 μl of 7-AAD (0.25 μg/1.0 × 106 cells) to samples d, f, and i.
Pass the cell suspension through a 100 μm strainer and transfer to a FACS tube
Incubate for 15 min at 4 °C.
D. FACS Analysis and gating procedure
Analyze tubes a-e, g and h to setup the gates, detector voltages and compensation.
- Analyze samples f and i and use the sequential gates described below. Examples of the FACS plots and gating procedure are illustrated in Figure 4 A and B.
- SSC vs. FSC: eliminate debris and cell clusters
- FL3 (7-AAD) vs. FSC: gate only live cells (7-AAD negative)
- FL2 (PE) vs. FSC: gate only lineage negative cells (PE negative)
- FL4 (APC) vs. FL1 (FITC): determine either the percent of CD44Hi/CD24Low for sample f or EPCAMHi/CD49fLow for sample i.
Basic Protocol #4: Subcutaneous implantation of estrogen pellets in mice receiving estrogen receptor-positive tumors
In the clinic, breast cancer is subtyped by pathological and molecular analyses into estrogen receptor (ER) positive and negative tumors. ER status provides prognostic information and guides therapeutic options for patients. Importantly, ER+ breast cancers often require systemic estrogen for growth in vivo. It is important to provide supplemental estrogen to mice receiving ER+ tumors (supplementation is not necessary for ER-breast cancer tissue). The ER status of breast cancer samples can be obtained from the pathology report of the patient. If a pathology report is not available, a small portion of the patient’s tumor specimen should be analyzed for ER status (by paraffin embedding, sectioning and immunohistochemistry against ER (DeRose et al., 2011)) while the remaining tissue can be cryopreserved (preferably as fragments). Once the ER status is verified, the cryopreserved tissue can be transplanted into mice. It is best to use high-dose estrogen pellets that provide adequate estrogen supplementation for up to 1 year (Rudali et al., 1975), although we typically replace these pellets at 6 months.
Materials
Estrogen pellets, stored on ice (see Supporting Protocol #3 for preparation of pellets)
3-4 week old NOD/SCID female mice from Jackson Labs (stock #1303)
Heating pad (water circulating)
Label tape
Lidocaine-Bupivacaine mixture (see REAGENTS AND SOLUTIONS)
Depilatory cream (such as Nair)
Cotton swabs, sterile
Povidone-Iodine swabsticks (widely available)
Isoflurane (Baxter #1001936040)
Anesthetic vaporizer (VetEquip #911103)
Compressed oxygen
Anesthesia induction chamber (VetEquip #941444)
Surgical platform, such as a foam board
Dissecting scissors, sterile
Forceps, sterile
CLIDOX-S®
Hot bead sterilizer (Fine Science Tools, item #18000-45)
Sterile 7 mm wound clips (#12031-07), wound clip applicator (#12032-07), and would clip remover (#12033-00; all from Fine Science Tools)
NOTE: All protocols using live animals must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) regarding the care and use of laboratory animals.
Surgical tools should be autoclaved prior to the procedure and disinfected in between animals using a hot bead sterilizer. Disinfect the surgical bench area by wiping with a disinfectant such as CLIDOX-S®. Turn on the heating pad and set the water temperature to 38°C.
Using an induction chamber, anesthetize the mouse by exposure to 2-2.5% vaporized isoflurane in oxygen, then transfer the animal to the surgical platform and place it in a prone position. Stabilize the mouse by gently fixing its legs to the platform with tape, making sure that the nose cone is secured for continuous delivery of vaporized isoflurane. Remove the fur from the surgical area between the shoulder blades (approximately 6mm × 6mm) by applying a depilatory cream and gently removing the hair with a cotton swab.
Pinch the footpad to make certain the mouse is in the proper plane of anesthesia before surgery. If the animal responds to the pinch by twitching, allow more time for the anesthesia to take effect.
When the mouse is fully anesthetized, disinfect the skin with 70% ethanol and wipe three times with betadine using a swab stick.
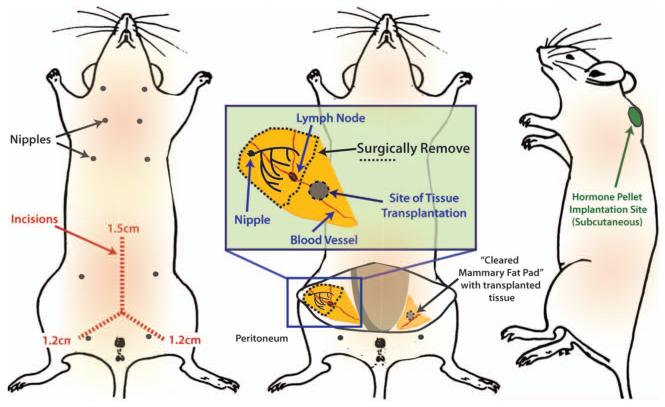
Make a small (3mm) incision between the shoulder blades (see Figure 5), being careful not to cut any muscle. Make a subcutaneous pocket for the estrogen pellet using blunt dissection technique.
Insert one pellet into the pocket.
Close the incision with a wound clip. 8. Proceed to breast tumor transplantation (Basic Protocol 5).
Figure 5.
Schematic showing location of mouse mammary fat pads, surgical incisions, and sites for tissue/cell transplants and the estrogen pellet implant. Mouse diagram was adapted from Hummel et al., 1966 with permission of The Jackson Laboratory.
Basic Protocol #5: Implantation of breast tumor tissue fragments into mice
This is a subcutaneous procedure that does not require laparotomy. We perform this procedure on mice that are 3-4 weeks of age to facilitate simultaneous clearing of the immature endogenous mouse mammary tissue. (A video demonstrating clearance of mouse mammary fat pads for tissue transplantation can be found at: http://www.jove.com/video/1849/mammary-epithelial-transplant-procedure.) It is also possible to pre-clear mammary tissue at 3-4 weeks, followed by a second surgery to implant tumor cells at a later date. All tumors are implanted into the inguinal (4th) mammary fat pad (Hummel et al., 1966 and Figure 5). We occasionally implant tumors into both contralateral fat pads if the goal is to amplify tumor tissue. We use the same procedure for NOD/SCID (Jackson Laboratories stock #1303) and NOD/SCID/IL2Rγ−/− (NSG) mice (Jackson Laboratories stock #5557). Surgery should not be performed on the same day the mice are transported from another facility. If mice are shipped from a vendor, we allow them to acclimatize for 4-7 days prior to surgery.
Materials
NOD/SCID (Jackson Laboratories stock #1303) (Alternatively, the more immune-compromised NOD/SCID/IL2Rγ−/− (NSG) mice (Jackson Laboratories stock #5557) can be used)
Samples for implantation, on ice (see Basic Protocol #1 and #2)
50 mL conical tubes, sterile
Heating pad (water circulating)
Label tape
Lidocaine-Bupivacaine mixture (see REAGENTS AND SOLUTIONS)
Depilatory cream (such as Nair)
Cotton swabs, sterile
Povidone-Iodine swabsticks (widely available)
Isoflurane (Baxter #1001936040)
Anesthetic vaporizer (VetEquip #911103)
Anesthesia induction chamber (VetEquip #941444)
Surgical platform, such as a foam board
Tweezers (ROBOZ #N5, cross action superfine points), sterile
27G × ½” syringes (Terumo U-100 insulin syringe), sterile
Dissecting scissors, sterile
Forceps, sterile
Sterile 7 mm wound clips (#12031-07), wound clip applicator (#12032-07), and would clip remover (#12033-00; all from Fine Science Tools)
Batter operated small vessel cauterizer (Fine Science Tools, item #18000-00)
Calipers for measurement of tumor size
NOTE: All protocols using live animals must be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) regarding the care and use of laboratory animals.
Procedure
Prepare 4mm × 2mm tissue fragments as described in Basic Protocol #1. Place fresh tissue fragments in a sample tube with about 0.5 ml sterile HBEC medium (alternatively, 1× PBS, DMEM F/12 or RPMI can be used) to keep the tissue moist. Place the tube on ice until implantation.
For cryopreserved tissue: Thaw frozen tissue fragments by placing the vial in a 37°C water bath and removing before the medium completely melts. Spray the outside of the vial with 70% ethanol, then move the vial into a biosafety hood and place the contents in a 50 ml conical tube. Add 10 mL of HBEC medium, then aspirate to remove the DMSO-containing freezing medium. Repeat three times. Add fresh HBEC medium, close the lid and place the tube on ice until the implantation.
Set up surgical suite. Turn on the heating pad and set the water temperature to 38°C. Surgical tools should be autoclaved at the beginning of the procedure and are disinfected in between animals using a hot bead sterilizer. Disinfect the surgical bench area by wiping with a disinfectant such as CLIDOX-S®.
Anesthetize the mouse by exposure to 2-2.5% vaporized isoflurane in oxygen using an induction chamber, then transfer it to the surgical platform and place it in a supine position. Stabilize the mouse by gently fixing its legs to the platform with tape, making sure the nose cone is in place for continuous delivery of vaporized isoflurane. Denude the surgical area by applying a depilatory cream and gently removing the hair with a cotton swab.
Pinch the footpad to make certain the mouse is in the proper plane of anesthesia. If the animal responds to the pinch by twitching, allow more time for the anesthesia to take effect. When the mouse is fully anesthetized, disinfect the skin with 70% ethanol and wipe three times with betadine using a swab stick.
-
Make two small subcutaneous incisions in the skin to expose the fat pad: one along the midline (approximately 1.5 cm) and one a short distance down the leg (about 1.2 cm) (Figure 5). Apply lidocaine/bupivicaine anesthetic mixture to the incision using a swab stick.
Ensure the incisions are not too close to the genitals since the wound clips may impede their function. Take care not to puncture the peritoneal membrane or to cut the vessels in the leg. There should be little or no bleeding with this procedure.
Expose the mammary fat pad using a sterile cotton swab, and pin the skin down using a small needle. Locate the inguinal lymph node in the middle of the fat pad (it is paler than the surrounding fat pad, has a kidney bean shape and is located at the junction of blood vessels). Using the lymph node as a reference for the center of the gland, the site of transplantation is in the center of the half of the fat pad that is proximal to the peritoneum (see Figure 5).
Insert the tip of a cross-action tweezer into the center of the transplantation site and make a pocket about 3mm long. Place a tumor fragment into the pocket. Carefully remove the tweezers once the tissue has been placed.
-
Clear the mammary fat pad by using forceps and scissors. Grasp the portion of fat pad closest to the nipple and cut under the fat pad with the scissors, keeping the scissors parallel and adjacent to the skin. Continue cutting the fat pad along the skin until reaching the lymph node. Cut across the width of the fat pad immediately adjacent (on the peritoneal side) to the lymph node, and remove this fat pad tissue to clear the gland. Be careful not to disrupt the pocket containing the tumor fragment.
Some bleeding may occur as the fat pad is cleared. It is advisable to have a battery-operated cautery unit on hand to stop bleeding if necessary. If the #5 mammary fat pad is attached or in close proximity to the cleared #4 fat pad, the endogenous mammary epithelium from the #5 gland will invade the cleared #4 fat pad. Removal of any #5 mammary tissue that is close or attached to the cleared fat pad will prevent this from occurring.
Close the incision with wound clips. Place the mouse on the warm pad until it awakens. Return the mouse to its home cage.
Monitor the mice according to the approved IACUC protocol. We monitor the mice daily for 3-5 days post-surgery and remove wound clips within 7 – 10 days. Check tumor growth weekly and determine and record its size with the caliper. If the mice show any sign of discomfort or illness, a veterinarian should be consulted. If the tumor reaches 2 cm in diameter or ulcerates, the mice should be humanely euthanized.
Basic Protocol #6: Transplanting tumor cells and organoids into mice
Organoids and cells isolated from digestion of solid tissue, or separated from body fluid (using procedures described in Basic Protocols #1 and #2), can be injected into cleared mouse fat pads to generate tumor grafts. We typically inject cells and organoids with Matrigel to facilitate their growth.
Materials
Organoids or cells from Basic Protocol #1 or #2
NOD/SCID (Jackson Laboratories stock #1303) Alternatively, the more immunocompromised NOD/SCID/IL2Rγ−/− (NSG) mice (Jackson Laboratories stock #5557) can be used.
Growth factor-reduced Matrigel (BD# 354230; or see Support Protocol 3 for preparation of EHS matrix)
Breast tumor cells resuspended in Matrigel, on ice
Heating pad (water circulating)
Label tape
Lidocaine-Bupivacaine mixture (see REAGENTS AND SOLUTIONS)
Depilatory cream (such as Nair)
Cotton swabs, sterile
Povidone-Iodine swabsticks (widely available)
Isoflurane (Baxter #1001936040)
Anesthetic vaporizer (VetEquip #911103)
Anesthesia induction chamber (VetEquip #941444)
Surgical platform, such as a foam board
Tweezers (ROBOZ #N5, cross action superfine points), sterile
27G × ½” syringes (Terumo U-100 insulin syringe), sterile
Dissecting scissors, sterile
Forceps, sterile
Sterile 7 mm wound clips (#12031-07), wound clip applicator (#12032-07), and would clip remover (#12033-00; all from Fine Science Tools)
Thaw the Matrigel at 4°C, and keep it cold at all times to prevent solidification. Resuspend the organoids or cells in Matrigel. Adjust the concentration to 1-2 million cells in 20 μl Matrigel for each intended injection. Keep tube with Matrigel/cell mixture on ice at all times. When not using the syringe, keep it on ice to prevent the Matrigel from solidifying prior to injection. If 10 fat pads are to be injected, prepare sufficient suspension for 11 injections. For this number, resuspend 11-22 million cells in 220 μl of Matrigel to compensate for material loss on the tube and syringe.
The cell/Matrigel mixture is injected into mammary fat pads using a similar procedure described in Basic Protocol #5: Breast tumor tissue implantation with the exception that a tissue pocket is not used. Instead, inject 20 μl Matrigel/cell mixture directly into the mammary fat pad using a 27G × ½” syringe. The mammary gland is then cleared, and the mice are closed and monitored as described in Basic Protocol #5: Breast tumor tissue implantation.
Basic Protocol #7: Harvesting tumorgrafts from mice
If the tumor cells are not fluorescent or otherwise labeled, necropsy must be performed very carefully to identify potential sites of metastasis. Tumor and metastasis samples must be kept on ice to help maintain viability as well as prevent degradation of protein, DNA, or RNA. Store the tissue samples in 1× PBS to prevent drying while harvesting other tissues. Keep the 4% PFA on ice with the cap closed, or wrap the opening with parafilm to prevent PFA vapor from escaping. Separate the tools used for fixatives from those used for live tissue to prevent cross contamination.
Materials
Tumor-bearing mouse
Ice
1× phosphate-buffered saline (PBS)
4% paraformaldehyde (PFA)
15 mL conical tubes, sterile
50 mL conical tubes, sterile
1 – 1.5 mL cryovial tubes, sterilized
Plastic petri dish (Fisher Scientific #08-757-14)
Liquid nitrogen
Tissue Freezing medium (see REAGENTS AND SOLUTIONS)
Hyclone DMEM/F12 (1:1) with HEPES (Thermo Scientific # SH30023.01)
Procedure
Clean the workbench and all equipment with 70% ethanol.
Euthanize the mouse with CO2 following IACUC approved procedures.
Lay the mouse in a supine position on the surgical platform and clean the incision area with 70% ethanol.
Make an incision to expose the tumor. Carefully detach the skin from the tumor using cotton swabs and scissors.
Measure the tumor (length × width × height) and record. It is easier to measure the tumor before isolating it from the skin. Carefully remove the tumor.
Record the wet weight of the tumor.
When harvesting the tumor: Put the tumor sample in a petri dish and place it on ice. Divide the tumor into portions for different applications, such as histology, nucleic acid and protein isolation, and generation of viable organoids or cells. Portions do not need to be equally divided, but should be a size most appropriate for the subsequent application. For example, histology can performed on small pieces dissected from several different regions of the tumor, while protein and viable cell preparations need significant amounts of starting tissue. Process the tissue as described in Steps 9-12.
For metastases: Look for metastases by carefully examining the axillary lymph nodes, lungs, intestines, spleen, liver, kidneys, ovaries, uterus, and other lymph nodes. Look for abnormal nodules in soft tissue, enlarged organs, and enlarged lymph nodes (2 mm or larger) as potential indicators of metastasis. However, micrometastases and bone metastases are usually not grossly observable. Thus, more sensitive methods, such as histology, must be used to determine if micrometastases are present. Place organs with potential metastasis in a petri dish on ice. If the metastasis is large enough, samples can be processed for histological analysis (to confirm the metastasis) and any remaining tissue can be used for transplantation as fragments. Photograph the samples to record the gross abnormality suspected to be a metastasis. Process the tissue as described in Steps 9-12.
For histological analysis: Fix tissue samples in 2-5 ml of cold 4% PFA in a 15 ml conical tube. Keep the sample tubes on ice until they are ready to be stored in the refrigerator. Fixation is typically overnight at 4°C. Move the samples to 70% ethanol and store at 4°C no longer than 1 week prior to embedding in paraffin. Section and stain the tissue as desired.
For nucleic acid and protein isolation: Place the samples in a cryovial or wrap in aluminum foil and submerse in liquid nitrogen to flash freeze. Transfer the tissue to a −80°C freezer. Isolate nucleic acids or protein using any of several standard methods.
For transplantation: Cut the tumor tissue into approximately 4 mm × 2 mm size fragments and freeze in Tissue Freezing medium (do not confuse with HBEC freezing medium) in cryovials. Freeze at −80°C overnight as described in Basic Protocol #1B and then store in liquid nitrogen. Transplant the tissue as described in Basic Protocol #5.
For processing into viable cells: Place the solid tumor tissue (size can vary) in a 50 ml conical tube containing sterile DMEM/F12 medium. Keep the tissue on ice until processing can be performed as described in Basic Protocol #1.
Basic protocol #8: Producing and utilization of lentiviruses for transduction of tumor cells
Bioluminescent and/or fluorescent imaging techniques are useful for monitoring tumor growth in live animals and to locate metastases. However, tumor cells derived from patients and tumorgrafts can have low transfection or transduction rates when standard procedures are employed. Therefore, concentrated lentiviruses are utilized since they are able to effectively transduce primary cells. This protocol describes the production and titering of a concentrated lentivirus, which co-expresses both ZsGreen and luciferase (pHIV-Luc-ZsGreen), for the purpose of labeling cells to identify micrometastases in tumorgrafts. In addition, a suspension infection method adapted from Welm et al., 2008, which is used to transduce primary cells including mixed adherent/suspension cultures is described below.
Materials
HEK 293T Cells (ATCC #CRL-11268)
DMEM media with high glucose and L-glutamine (Gibco # 11965-092)
Characterized fetal bovine serum (FBS), heat inactivated (Thermo Scientific #SH30071.03)
10 cm tissue culture treated polystyrene cell culture dish (BD Falcon # 353003)
37°C 5% CO2 Incubator
0.05% Trypsin (Invitrogen #25300-054)
Hemocytometer
1× phosphate-buffered saline (PBS), sterilized
OPTI-MEM reduced serum media with L-glutamine (Gibco # 31985-070)
Polyethylenimine (PEI), branched, average MW ~25,000 by LS (Sigma #408727-100ml)
0.5 to 1 μg/μl pHIV-Luc-ZsGreen (Addgene #39196)
0.5 to 1 μg/μl solution of pMDLg/pRRE 3rd generation packaging plasmid (Addgene #12251)
0.5 to 1 μg/μl solution of pRSV-Rev 3rd generation packaging plasmid (Addgene #12253)
0.5 to 1 μg/μl solution of pCMV-VSV-G envelope plasmid (Addgene #8454)
0.45 μm cellulose acetate filter membrane and 150 ml bottle (Corning #431155)
Swinging bucket centrifuge
Ultra clear 38.5 ml, 25 × 89 mm thin wall centrifuge tube (Beckman Coulter #344058)
Balance
Ultra centrifuge (Beckman Coulter Optima LE80k with swinging bucket SW28 rotor)
Hyclone DMEM-F12 medium with HEPES (Thermo Scientific #SH30023.01)
Hyclone Hank’s Balanced Salt Solution (HBSS; Thermo Scientific #SH30268.02)
DNaseI (AMRESCO #0649)
6 well tissue culture treated polystyrene cell culture plate (BD Falcon # 353224)
12 × 75 mm round bottom polystyrene FACS tube (BD Falcon # 352008)
100 μm cell strainer (BD Falcon #352360)
FACS machine capable of detecting ZsGreen (488 nm) fluorescence
DuPont ProShield NexGen Sleeves, 18 in long (Fisher Scientific # 19-088-2165)
Three-ply facemask with polyfiltration medium (Fisher Scientific #01-361-92)
Standard bleach
4% paraformaldehyde (PFA)
80% Ethanol in water
15 ml conical tubes, sterile
50 ml conical tubes, sterile
1.5 ml eppendorf tubes, sterile
500 μl eppendorf tubes, sterile
Disassociated primary breast tumor samples or xenografted tumor cells (organoids)
HBEC medium (see REAGENTS AND SOLUTIONS)
Six-well ultra low attachment plates (Corning #3471)
10× Polybrene (hexadimethrine bromide, Sigma #H9268) solution: 10mg/ml in 1× PBS, sterile filtered and stored in aliquots at −20°C.
Procedure
A. Preparation of Concentrated Lentiviral Particles
NOTE: All protocols using lentiviruses must be reviewed and approved by an Institutional Biosafety Committee. BSL2 techniques are employed when generating and utilizing lentiviruses.
-
Expand HEK 293T cells in DMEM media supplemented with 10% FBS (heat inactivated) in 10 cm tissue culture dishes. The cells are cultured at 37 °C in 5% CO2.
For unknown reasons, high passage HEK 293T cells do not produce high titer lentivirus. It is best to obtain a fresh stock of HEK 293T from the ATCC (#CRL-11268) rather than acquire stocks from other laboratories. Also, cryopreserve early passage cells and thaw a fresh stock for the generation of high titer lentivirus.
Aspirate the media and wash each plate with 5 ml of PBS.
Add 1 ml/plate of trypsin and incubate for 2-5 min. Gently pipette up and down to mix the trypsin solution.
Add 10 ml/plate of DMEM media supplemented with 10% FBS (heat inactivated) and determine the number of cells per ml using a hemocytometer.
-
Plate 4-7 × 106 cells into each 10 cm tissue culture dish and adjust the volume of each plate to 10 ml with DMEM media supplemented with 10% FBS (heat inactivated). Culture the cells overnight at 37 °C in 5% CO2.
Alternatively a confluent 10cm plate of HEK293T cells can be re-plated at a 1:10 dilution and cultured for 2-5 days until they are approximately 90% confluent. A typical procedure utilizing 24 10cm plates yields approximately 0.9-1.2 ml of a solution containing between 5.0 × 107 to 5.0 × 108 infectious units/ml (IU/ml).
Aspirate the media and add 8 ml/plate of DMEM media supplemented with 10% FBS (heat inactivated) that has been pre-warmed to 37°C and culture for 2 h.
-
Prepare PEI at 1 mg/ml (w/v) in deionized sterile water.
The transfection procedure described in this protocol is optimized for PEI (Huh et al., 2007). The PEI solutions can be aliquoted into 1.5 ml eppendorf tubes and stored at −20 °C for up to a year. If a different transfection reagent will be used, optimization will be necessary.
-
Add 720 μl of the 1 mg/ml (w/v) PEI solution to 11.28 ml of OPTI-MEM media in a 50 ml conical tube, which yields a solution sufficient to transfect 24 plates (30 μg of PEI per plate in 500 μl of OPTI-MEM).
The volume can be adjusted depending on the number of plates utilized.
-
Prepare a solution of plasmids in a separate 15 ml conical tube, which is enough to transfect 24 plates, by adding 120 μg of pHIV-Luc-ZsGreen (5 μg/plate), 40.8 μg of pMDLg/pRRE (1.7 μg/plate), 40.8 μg of pRSV-Rev (1.7 μg/plate), and 40.8 μg of pCMV-VSV-G (1.7 μg/plate) to a final volume of 12 ml of OPTI-MEM media (500 μl/plate).
It is important to utilize plasmid solutions at >0.5 μg/μl since lower concentrations can dilute the media/DNA solution and decrease the transfection efficiency. In addition, to achieve high viral titers, it is necessary to mix all four plasmids together before mixing with the PEI solution.
Add 12 ml of the plasmids/OPTI-MEM to the 12 ml of PEI/OPTI-MEM and mix. Incubate the solution for 30 min at room temperature.
Add 1 ml/plate dropwise of the PEI and plasmid OPTI-MEM solution, gently swirl the plates and culture cells with solution for 24 h at 37 °C in 5% CO2.
-
From this point forward, utilize BSL2 procedures according to your institution’s biosafety protocol. Every time before working with solutions containing virus, place a large beaker containing >50 ml of bleach in a biosafety cabinet to disinfect any media and pipettes (make sure the final concentration of bleach is >10% before removing it from the biosaftey cabinet).
The use of personal protection equipment, such as double gloves, arm sleeves, face masks, eye protection, laboratory coats, etc. are required. Tubes containing virus can only be opened in a biosafety cabinet. Sealed tubes must be sprayed with an 80% ethanol solution before removal from the biosafety cabinet.
Remove the media from each plate and transfer it to the beaker containing bleach. Add 8 ml/plate of DMEM media containing 10% FBS (heat inactivated) that has been pre-warmed to 37°C and incubate overnight at 37 °C in 5% CO2.
-
The following day (48 h post transfection), remove the media from each plate and transfer it to a 50 ml conical tube on ice. Immediately add 8 ml/plate of fresh pre-warmed DMEM media containing 10% FBS (heat inactivated) to each plate and culture overnight at 37 °C in 5% CO2.
The transfection efficiency can be estimated at this stage by using a tissue culture microscope with fluorescence capability and observing the percent of cells fluorescent for Zsgreen. It is advisable that this is performed on an extra plate of cells that are fixed with 4% paraformaldehyde, to inactivate the lentivirus, prior to microscopy. Continue with the concentration procedure only if >50% of the cells are fluorescent. If <50% of the cells are transfected, optimize the transfection procedure before proceeding.
Centrifuge the 50 ml conical tubes containing the media for 10 min at 2,500 × g at 4 °C to pellet any large debris.
Pass the supernatant through a 0.45 μm cellulose acetate filter and transfer 32 ml (about 4 plates worth) to each of the six ultracentrifuge tubes. Place tubes in titanium buckets on ice. Balance bucket pairs by adjusting the weight with DMEM media (this is done in the biosafety cabinet until the buckets are sealed).
To pellet the virus, ultra centrifuge the solution for 1 h 45 min at 112,000 × g at 4 °C using a swinging bucket rotor.
Carefully remove the supernatant and transfer it to a beaker containing bleach. Immediately (do not let the pellet dry) add 150 μl of DMEM/F12 media to each centrifuge tube on ice. Gently pipette up and down without creating bubbles to dissolve the pellet. Place the ultracentrifuge tubes in a 50 ml conical tube and cap the conical tube to prevent evaporation of the solution. Store the conical tubes at 4 °C overnight.
-
The following day, collect the second round of media from the plates (72 h post transfection) and process it in the same manner as steps 14-17.
Add bleach at a final concentration of 10% to the plates, incubate at room temperature for 20 minutes in the biosafety cabinet, and discard plates and media.
After the ultracentrifugation step 17, remove the supernatant. Resuspend the pellets by transferring the DMEM/F12 solution containing the concentrated virus that was collected 48 h post transfection to each tube. Gently pipette up and down to dissolve the pellet. Place the ultracentrifuge tubes in a 50 ml conical vial to prevent evaporation and incubate at 4 °C for 2 h to ensure the virus is completely dissolved.
-
Pipette up and down to resuspend the pellets, being careful to limit bubbles. Combine all of the resuspended pellets into one tube and gently pipet to mix. Aliquot 50-100 μl of the concentrated virus into 500 μl eppendorf tubes and store at −80 °C.
The virus solutions are aliquoted to minimize freeze/thaws, which can significantly reduce the number of IU/ml. The virus solutions can be stored for up to 1 year at −80 °C.
B. Determining Infectious Units (IU)/ml by FACS
Expand HEK 293T cells in DMEM media supplemented with 10% FBS (heat inactivated) in 10 cm tissue culture dishes.
Aspirate the media and wash each plate with 5 ml of PBS.
Add 1 ml/plate of trypsin and incubate for 2-5 min at 37 °C in 5% CO2. Gently pipette up and down to mix the trypsin solution.
Add 10 ml/plate of DMEM media supplemented with 10% FBS (heat inactivated) and determine the number of cells per ml using a hemocytometer.
Plate 2.0 × 105 cells in each well of a 6 well tissue culture plate and adjust the volume to 3 ml/well with DMEM media supplemented with 10% FBS (heat inactivated). Culture the cells overnight at 37 °C in 5% CO2.
Aspirate the media from four wells of the 6 well plate and add 1 ml/well of DMEM media supplemented with 10% FBS (heat inactivated) that has been pre-warmed to 37°C.
Aspirate the media from the remaining two wells of the 6 well plate and carefully wash with 2 ml/well of PBS so the cells do not detach.
Add 0.5 ml of trypsin to each of the two wells and incubate for 2-5 min at 37 °C in 5% CO2. Gently pipette up and down to mix the trypsin solution.
Add 0.5 ml/well of DMEM media supplemented with 10% FBS and determine the number of cells present in each well independently using a hemocytometer. Calculate the average number of cells present in each of the two wells. This number will be used to determine the IU/ml.
From this point forward, utilize BSL2 procedures according to your institution’s biosafety protocol. Thaw an aliquot of concentrated virus in the biosafety cabinet.
Dilute the virus solution 1:10 in DMEM medium supplemented with 10% FBS (heat inactivated). For example, add 8 μl of virus solution to 72 μl of media.
-
Add different amounts of the diluted virus to each of the remaining four wells as described below, gently mix and culture overnight.
- No virus control
- 1 μl of diluted virus (1:10,000 final dilution)
- 10 μl of diluted virus (1:1,000 final dilution)
- 50 μl of diluted virus (1:200 final dilution)
The final dilution values are based upon the use of a 1:10 initial dilution of the virus and 1 ml of media in each well. Different dilutions are required since a sample containing <15% ZsGreen positive cells determined by FACS is required to more accurately calculate the IU/ml.
Remove the media 24 h post infection and put into bleach. Add 3 ml/well of DMEM media supplemented with 10% FBS that has been pre-warmed to 37°C and culture for 2 additional days.
Remove the media 72 h post infection and put into bleach. Carefully wash the wells with 2 ml/well of PBS so the cells do no detach.
Add 0.5 ml/well of trypsin and incubate for 2-5 min. Gently pipette up and down to mix the trypsin solution.
Transfer the cell suspension to a 15 ml conical tube on ice. Wash each well with 1 ml of HBSS containing 2% FBS and transfer this solution to the respective 15 ml conical vial.
Add paraformaldehyde to a final concentration of 2% to each tube and incubate at 4 °C for 20 minutes to inactivate any residual virus that may be present.
Centrifuge the cells for 5 min at 400 × g at 4 °C. 19. Aspirate the supernatant and carefully resuspend the pellet with 1 ml of HBSS containing 2% FBS
Centrifuge the cells for 5 min at 400 × g at 4 °C. 21. Aspirate the supernatant and carefully resuspend the pellet with 250 μl of HBSS containing 2% FBS
Pass the cell suspension through a 100 μm cell strainer and transfer to a FACS tube
- Setup the gates and detector voltages, and analyze samples using the sequential gates described below.
- SSC vs. FSC: eliminate debris and gate only single cells
- FL1 (ZsGreen) histogram: determine percent ZsGreen positive cells
-
Calculate the IU/ml using the formula below. IU/ml = (average # of cells/well) * (% ZsGreen positive cells/100) *(virus dilution)
The average # of cells/well was determined above in step 9. We typically use a dilution where the cells are <15% ZsGreen positive. Titers ranging from 5 × 107 to 5 × 108 IU/ml are commonly achieved using this protocol.
C. Transduction of primary cells
Prepare organoids as described in Basic Protocol #1.
-
Resuspend the organoids in a 1:1 trypsin:PBS solution, and place the sample into one well of a 6-well plate to facilitate viewing under the microscope.
We typically use 1 ml of diluted trypsin solution for each 0.5 g of dissected tumor tissue (measured prior to collagenase digestion).
Trypsinize the tissue at room temperature, and carefully monitor the organoid dissociation process every few minutes under low magnification using a light microscope. Trypsinize for 1 min, then very gently pipette the cells up and down with a 1 ml pipet to help dissociate the organoids into single cells. Repeat every minute until most of the organoids are dissociated.
-
Once the organoids are dissociated, immediately add 5 ml HBEC medium (or DMEM-F12 containing 10% FBS) to neutralize the trypsin, and centrifuge the cells at 530 × g at room temperature for 5 min. Wash cells with 5 ml medium two additional times. Resuspend the pellet in HBEC medium and count the cells using a hemocytometer.
If the pellet is difficult to resuspend because of viscosity, it is likely due to the presence of DNA from cells that lysed. In this case, incubate the cells with 10ug/ml DNaseI (final concentration) in HBEC media for ~5 min at room temperature.
-
Seed 250,000 cells per well in a 6-well plate in 0.5 ml of HBEC medium.
For suspension infections, use ultra-low attachment plates, and for monolayer infections, use regular tissue culture-treated plates. The best method of infection must be determined empirically for each tumor. For monolayer infections, allow the cells to attach for 24-48 hr prior to adding virus.
-
Add polybrene to a final concentration of 1 – 4 μg/ml. The actual concentration should be empirically determined using the target cells. Add concentrated lentivirus using a multiplicity of infection (MOI) of 5-20 viral particles/target cell. Add additional HBEC medium to bring the final volume to 1 ml per well.
The multiplicity of infection may also have to be optimized for different tumors.
-
Centrifuge the plate for 1 hr at 700 × g at room temperature. Alternatively, you can proceed directly to step #9 without performing the centrifugation, replacing the virus-containing medium with fresh HBEC medium after overnight incubation.
Some cells are more efficiently infected using a combination of the spin method and overnight suspension culturing with virus. This can only be determined empirically.
Remove the virus medium and add 2 ml of fresh HBEC medium.
Incubate at 37°C with 5% CO2. Expression of virus proteins (e.g. ZsGreen) should be detectable by fluorescence microscopy after 72 hours. Feed the cells every day by exchanging the medium.
-
Expand the cells until a sufficient number is obtained to transplant the desired number of mouse mammary fat pads (See Basic Protocol #6).
During culture some of the tumors attach to tissue culture-treated dishes, some grow in suspension, and some are mixed. To propagate suspension or mixed culture, centrifuge the solution containing cells at 530 × g at room temperature for 5 min, aspirate the media, suspended the cells in 1 ml of HBEC media and add them with fresh HBEC media to the adherent population. Adherent cells can be collected by first washing the plate with 5 ml of 1× PBS followed by addition of 1 ml of trypsin. Once the cells detach, the resulting suspension can be mixed with the suspended cells, if applicable. Feed the cells every day by aspirating the media and adding fresh media for adherent cells. For suspension cells, follow the procedure described above for propagation. We have only attempted to maintain primary tumor cells in culture for up to 2 weeks using these conditions.
Basic Protocol #9: Three-dimensional primary tumor cultures
The physical organization of breast cancer in 3-dimensions (3D) modulates signaling pathways that affect the survival, growth, and sensitivity of tumor cells to therapeutics. Primary tumor organoids can be grown in 3D culture to study the complex cell-cell and cell-matrix interactions that occur in vivo. Every primary human or xenograft tumor may require different media supplements for optimal growth. A standardized media is typically used to simplify in vitro culturing conditions. The media that we use is a modified version of the M87 media developed by the Stampfer laboratory, that supports the growth of human primary mammary epithelial cells.(Garbe et al., 2009) We have cultured several primary and xenograft tumor cells successfully for up to two weeks using this media, although additional optimization may be possible.
Materials
15 mL conical tubes, sterile
Trypan Blue Solution, 0.4% (Sigma #T8154)
Modified M87 medium (see REAGENTS AND SOLUTIONS)
Growth Factor-Reduced Matrigel (see REAGENTS AND SOLUTIONS)
55 mL disposable reagent reservoir (Bioexpress #B-0812-7)
24 well Ultra Low Cluster (Adhesion) Flat Bottom Plates (Corning Costar #3473)
96 well black cell star clear flat bottom plate (Greiner Bio-one #655090)
96 well untreated clear U-bottom plates (BD Falcon #351177)
96 well clear flat bottom plates (BD Falcon #353915)
Cell Titer 96 Aqueous non-radioactive cell proliferation assay (MTS Assay) (Promega #G5421)
Live/dead Viability/cytotoxicity kit for mammalian cells (Invitrogen #L-3224)
50-mL conical tubes, sterile
Plate reader capable of reading absorbance at 490 nm
Procedures
A. Defrosting cryopreserved tumor cells (if applicable)
Defrost a vial of cryopreserved human primary tumor cells by submerging it in a 37°C water bath for 2-3 min. As soon as the vial is thawed, transfer the contents to a 15 ml conical tube containing 10 ml of the modified M87 media that has been pre-warmed to 37°C.
Centrifuge the cells for 5 min at 700 × g at room temperature.
Aspirate the supernatant and carefully resuspend the pellet into 1 ml of modified M87 media by pipetting up and down. Perform a trypan blue exclusion test using a hemocytometer to determine the number of viable cells per ml. (Strober, 2001)
B. Aggregation of single tumor cells (if applicable)
Typically, organoids are used for the 3D in vitro assay. Organoids prepared as described in Basic Protocol #1 can be embedded directly in Matrigel. Dissociated tumor cells and pleural effusion cells (Basic Protocol #2) should be aggregated prior to embedding in Matrigel. However, some pleural effusion cells do not aggregate under these conditions.
Suspend the single cells in modified M87 media to achieve a concentration of 1-4 × 106 cells/ml.
Add 1 ml of tumor cell suspension to each well of a 24 well ultra low adhesion plate. Incubate the cells at 37°C in 5% CO2 overnight.
Tumor aggregates are separated from single cells by differential centrifugation. Place aggregates in a 15 ml conical tube with 5 ml of modified M87 media and centrifuge for 30 sec to 1 min at 450 × g at room temperature. Single cells will remain in suspension while cellular aggregates pellet. Remove the supernatant and resuspend the pellet in 5 ml of modified M87 media. Monitor the organoid enrichment procedure by analyzing 25μl samples of the resuspended pellet by light microscopy. Repeat centrifugation steps 2-5 times as needed to enrich for aggregated tumor cells.
C. Embedding tumor organoids or aggregates in Matrigel
Defrost a bottle or aliquot of frozen growth factor reduced Matrigel at 4°C. This process can take several hours to overnight. It is very important to keep the Matrigel at 4°C at all times to prevent it from solidifying.
Carefully transfer the organoids/aggregates to a 15 ml conical tube containing 10 ml of the modified M87 media that has been pre-warmed to 37°C. Centrifuge the cells for 30 sec at 400 × g at room temperature. Aspirate the supernatant to remove the single cells. Resuspend the pellet by slowly adding 1 ml of modified M87, taking care to prevent breaking up the organoids. Perform a trypan blue exclusion test using a hemocytometer to determine the number of viable organoids/aggregates per ml. (Strober, 2001)
-
Centrifuge the organoids/aggregates at 400 × g for 5 min at room temperature. Remove the supernatant and place the tube on ice. Determine the number of organoids and the volume of Matrigel to be added to each well of a 96-well plate.
For a viability assay using MTS as the endpoint, 300-500 organoids are typically embedded in 30-40 μl of Matrigel per well of a 96 well U bottom clear, non-tissue culture treated plate. U-bottom plates are used to minimize the area of matrigel touching the plastic to reduce the number of tumor organoids adhering to the plate. To acquire high-resolution images using confocal microscopy with a live/dead readout, 100-300 organoids are usually embedded in 50-100 μl of Matrigel per well of a 96 well bottom clear bottom, black wall plate.
Based upon the experiment (MTS or Live/Dead), determine the total number of wells and matrigel required to achieve the proper concentration of organoids/aggregates per ml. Carefully suspend the pellet of organoids/aggregates in Matrigel on ice.
Transfer the Matrigel cell suspension to a 50 ml disposable reservoir on ice. Using a 12-channel pipette, carefully mix the suspension by gently pipetting up and down. If performing an MTS endpoint assay, add 30-40 μl of the cell suspension/Matrigel mixture to each well of the 96-well plate. If performing a Live/Dead assay, add 50-100 μl of the Matrigel/cell suspension to each well of the 96-well plate. Care should be taken to avoid the formation of bubbles by pipetting slowly.
To reduce the settling of organoids onto the bottom of the plate while the Matrigel solidifies, carefully invert the 96-well plate (surface tension will maintain the Matrigel/cell suspension in the well) and place it in a 37°C, 5% CO2 incubator for 30 min.
Remove the plate from the incubator and add 75-100 μl of modified M87 media to each well with a 12-channel pipette. Incubate the cells overnight at 37°C in a 5% CO2 incubator.
D. Treatment of 3-dimensional cultures with test compounds
Primary human tumor cells typically proliferate significantly less compared to established cell lines. Therefore, longer treatment times with test compounds may be required to assess their effect on proliferation.
-
Dilute compounds in modified M87 media to 2× the desired final concentration. Be certain to include proper vehicle (e.g DMSO) and positive controls (e.g. cytotoxic compound for viability).
We have successfully used 20 μM Doxorubicin or 1 μM Staurosporine as a positive cytotoxic control in these assays.
-
Remove the plate from the incubator and add 75-100 μl of modified M87 media containing 2× compound or controls to each well with a 12-channel pipette to yield a final compound concentration of 1×.
Alternatively, the media can be removed (discarded) using a 12 channel pipette by tilting the plate 45°, making sure not to disturb the Matrigel. Fresh media containing 1× concentration of the compounds or controls is then added.
-
Incubate the cells for 2-5 days at 37 °C in a 5% CO2 incubator.
The majority of primary cells can be cultured without a media change for several days. Since every tumor is different, optimization of media changes and test compound concentrations may be required. Monitor the cultures carefully. Evaporation of the outer wells of a 96 well plate may become an issue when culturing cells for extended periods of time. When possible, we culture cells in the center wells of the plate and fill unused wells with sterile water to increase the internal humidity within the plate.
E. Performing an MTS endpoint viability assay
Defrost and combine the MTS and PMS solutions according to the manufacturers instructions. Transfer the MTS reagent to a 50 ml multi-channel pipetting reservoir.
Remove the plate from the incubator and, using a 12-channel pipette, add 15-25 μl of the MTS reagent to each well (10% of the media volume).
Incubate the cells for 1-5 hr at 37°C in a 5% CO2 incubator until the vehicle control wells develop a dark red color.
-
Remove the plate from the incubator. Using a 12-channel pipette, transfer 100μl of the medium containing the MTS reagent to a new, clear, flat bottom 96 well plate. Read the absorbance at 490 nm using a plate reader, and normalize the absorbance values to the vehicle controls.
Care should be taken to not disturb the Matrigel, and to prevent the formation of bubbles in the culture. Alternatively, the absorbance can be read directly through the Matrigel without transferring the media, but the Matrigel and organoids can cause light scattering, increasing the variability of the results.
F. Performing a live/dead assay
Thaw live/dead reagents (calcein AM/ethidium bromide homodimer-1) and dilute them in either PBS or DMEM/F12 (no serum) to a final concentration of 1-5 μM.
Remove the plate from the incubator. Using a 12-channel pipette, carefully aspirate and discard the media by tilting the plate 45°, making sure not to disturb the Matrigel.
Transfer the diluted live/dead reagent to a 50 ml reservoir. Using a 12-channel pipette, add 50-100 μl of the live/dead reagent to each well. Incubate the cells for 15-30 min at room temperature.
Transfer the plate to a confocal microscope, which can be utilized to capture images of calcein AM (em/ex 494/517 nm) fluorescence, which represents the live cells. In addition, images of ethidium bromide (em/ex 528/617 nm) should also be acquired, which identifies the dead cells. Subsequently merging the fluorescent images will yield a compiled picture of the relative number live and dead cells in each field of view. Comparison of the relative amount of live and dead cells from organoids treated with test compounds and the controls can be used to determine the efficacy of the test compound.
For example, organoids from a patient were treated with increasing concentrations of valproic acid (Figure 6 A) (Cohen et al., 2011). A significant increase in relative number of dead (red) cells and simultaneous decrease in live cells (green) was observed at higher concentrations of valproic acid. These results suggest that valproic acid is inducing a cytotoxic effect on this patient’s cells. In another example, organoids from a patient were treated with DMSO or 800 nM Taxol (additional concentrations can also be used). The taxol treated cells had significantly more dead (red) cells compared to the vehicle control, which suggests a cytotoxic effect on these cells. This assay can be performed on numerous primary patient cells, such as aggregated pleural effusions, to determine if some patient samples are more resistant to a test compound than others.
Figure 6.
Analysis of the viability of patient-derived breast cancer organoids in 3D culture following drug treatment. Primary tumor organoids were embedded in EHS matrix and grown in 3D culture. Organoids were treated with (A) DMSO or valproic acid for 4 days or (B) DMSO or taxol for 7 days and imaged using both DIC (bottom rows) and fluorescence microscopy (top rows). Live/dead discrimination was performed using calcein AM (green) to mark viable cells, and ethidium bromide homodimer-1 (red) to identify dead cells. Scale bars represents 20 μm. Panel A was adapted from Cohen, et al., 2011 with permission of Nature Publishing Company.
SUPPORTING PROTOCOLS
Support Protocol #1: Obtaining fresh breast tumor (e.g. surgical specimens, body fluids, and metastases) from patients
Fresh tumor tissue is used for tissue transplantation and for nucleic acid isolation. For high quality RNA isolation, it is imperative that the tissue be flash frozen within 10-20 min after removal from the body to prevent degradation. For tissue transplantation, time is less critical, but the tissue should remain on ice and in medium until transplantation. We have successfully transplanted tissue after overnight storage on ice.
Materials
- Collection kit: (all reagents and supplies must be sterile)
- Latex gloves
- Disposable bibs
- Sterile Gauze
- Swabs
- Long, straight trimming blades
- Sterile Scalpels
- Tissue culture dishes
- Sterile Forceps
- 50ml conical vials
- Sterile blue, green and black ink
RPMI 1640 (Fisher Scientific #SH30255FS)
RNase/DNase-free cryovials
Bleach
70% ethanol
RNAlater RNA Stabilization Reagent (Applied Biosystems #AM7021)
Dewar of liquid nitrogen
Procedures
A. Coordinating tissue collection
Tissue collection staff checks the surgery schedule daily to identify participants and assign a “collection event number” (de-identified number) that is not directly associated with patient information as required by Health Insurance Portability and Accountability Act (HIPAA) regulations. This ensures that researchers will not be able to identify the patient with this number. The tissue collection staff also reviews and notes the patient history and the clinical diagnosis.
A tissue collection coordinator alerts the surgeon(s), pathologist(s), investigator(s) and tissue repository staff by e-mail 24 hr prior to surgery.
Informed consent must be obtained from the patient prior to the tissue collection. This is typically handled by either a nurse or other qualified hospital staff member.
Prior to the surgical procedure, the repository technician communicates with the operating room staff that a specimen for research has been planned.
The operating room staff notifies the tissue repository technician 10 min prior to when the tissue is removed from the patient. This is to ensure the repository technician can get to the operating room before the tissue is removed from the patient in order to minimize the amount of time prior to processing (see D. Tissue Sample Quality Control).
When the breast tissue is removed, the technician notes the time of day, and places the tissue into a container on ice for immediate transport to the pathology laboratory. The specimen is potentially infectious and is handled with blood-borne pathogen considerations.
The pathologist verifies the patient identification, and removes surplus tumor and adjacent uninvolved (normal) tissue for research.
Cryovials are labeled with the pre-assigned, de-identified number. The tissue type (cancerous or adjacent uninvolved), location within the breast from where the tissue was removed, and procurement time are noted on a specimen worksheet.
Three 40 mg pieces from each tissue are placed into separate cryovials, and immediately flash frozen in liquid nitrogen (record the time of day). The frozen tissues are stored in vapor phase liquid nitrogen (-196°C).
Three additional 20 mg pieces from each tissue type are placed into separate cryovials containing 400 μl of RNAlater. These tubes are stored at 4°C overnight. Remove the RNAlater as per manufacturer’s directions and store the specimens at −80°C.
If the remaining tumor specimen is a minimum of 100 mg (300 mg minimum for grossly uninvolved normal tissue), the tissues are placed into separate 50 mL conical tubes containing RPMI for immediate tissue implantation or disassociation to organoids. If the specimens are smaller, the tissue is generally not processed into viable cells due to low yields of viable cells. If the minimum amount of tissue is not available for viable cell processing, the remaining tissue is processed as described in Steps 9 and 10, as necessary.
Patient identification, specimen collection information, and storage information are entered into a biospecimen tracking database such as the itBioPath Biospecimen Management System (see C. itBioPath Biospecimen Managment System).
The itBio Path Biospecimen Managment system subsequently generates a new de-identified number for each specimen type (RNA, organoids, tissue, etc) associated with that specific patient/collection. The second number is utilized to separately track each specimen type. Barcode labels are generated with the de-identified number and are placed on each cryovial. This number is linked in a de-identified manner to subsequent processing and/or clinical and pathological follow-up information.
C. itBioPath Biospecimen Managment System
itBioPath is a proprietary system created by the Research Informatics Shared Resource at Huntsman Cancer Institute that manages, annotates and tracks storage locations, consent information, protocols, and all associated clinical diagnoses and surgical pathology reports. Contact the Research Informatics Shared Resource at HCI (www.huntsmancancer.org) for more information about the software suite.
The itBioPath System complements and enables high quality patient-derived research with the following capabilities:
Tracks specimen collections, processing and storage information, banked specimen availability for originating specimens, succeeding xenograft transformations, and related specimen products.
Provides multigenerational bidirectional linkage of specimen transformations, including the relationship to the originating patient specimen, annotations and consents.
Provides separation of patient specimen and xenograft specimen pathology annotations.
Integrates with genomic experimentation data in the HCI Research Informatics’ GNomEx product suite.
Integrates with the University of Utah Health Sciences Electronic Data Warehouse for access to clinical information.
Integrates with a patient-centric system for Cancer Clinical Research (CCR) for capturing information on patients being treated in the multidisciplinary teams, and others.
Captures chronological and audit documentation of specimen management and disbursement activities.
Allows for batch data entry capabilities to ensure high quality data collection.
Controls security of data access for each user dependent on protocol requirements and the role of the user interacting with the data.
Offers user designed Ad Hoc queries for full data retrieval.
D. Tissue Sample Quality Control
The quality of nucleic acid or cell preparations from tissue depends greatly on the quality of the tissue. In particular, the time required for tissue procurement after removal from the patient should be minimized to limit hypoxic effects that can cause loss of tissue integrity. We have found that if the key personnel are aware that collection times are being annotated, the collection becomes faster.
Always record the procurement time from excision of tissue to freezing or fixation. It is strongly recommended that processing be completed within 20-30 min.
Before utilizing the RNA, always determine the quality using an instrument such as the Agilent 2100 Bioanalyzer. Typically, only RNA with an integrity number (RIN) >7 is utilized in downstream applications such as RT-PCR, microarray, etc.
Record refrigerator and freezer temperatures daily. Automated alarm systems are recommended, such as a 24 hr Sensaphone surveillance system.
Review and update Standard Operating Procedures at least annually, with input from investigators and repository staff.
Support Protocol #2: Preparation of estrogen pellets
Estrogen pellets are made in-house and are a less expensive reliable alternative to purchasing them commercially. These pellets are designed to last up to 1 year in mice, however, we typically replace after 6 months.
Materials
50 mg Estradiol (1,3,5{10}-Estratriene-3, 17 β-diol, Sigma #E8875)
1.95 g Beeswax (Sigma #243221)
22 mL glass sample vial
Magnetic stir bar, sterile
Hot plate
Aluminum foil
Dry ice
70% ethanol
Glass Pasteur pipet and bunsen burner
- Eppendorf tubes, sterile
- Weigh 1.95 g of beeswax and place it into a 22 ml glass sample vial.
- Begin melting on a 60°C hot plate.
- Place a sheet of aluminum foil on top of dry ice. Wipe a weigh boat with 70% ethanol, and place it on top of the aluminum foil.
- As soon as the wax melts, add the estradiol powder to it. Add a small, sterile magnetic stir bar to aid the dissolving process. Typically, it takes 10-15 min to dissolve the estrogen at 60°C.
- Using a glass Pasteur pipet, drop the wax mixture onto the cooled weigh boat to create a pellet consisting of three drops of the melted beeswax solution. Flame the pipet to prevent the wax from solidifying in it. Each drop will weigh approximately 10 mg, resulting in approximately 30 mg pellets with each pellet containing ~1 mg estrogen. Collect the solidified pellets into 1.5 ml eppendorf tubes and store for up to 6 months at 4°C.
Support Protocol #3: Preparation of growth factor-reduced EHS matrix
Growth factor-reduced Matrigel is available from BD Biosciences (catalog #354230). Matrigel (EHS matrix) is composed of extracellular matrix derived from Englebreth-Holm-Swarm (EHS) tumors. The following protocol, which is modified from a published procedure, (Kibbey, 1994) details a method to generate EHS matrix. The EHS matrix is obtained by xenografting the EHS cell line (ATCC catalog #CRL-2108) according to the manufacturer’s instructions. Xenografted EHS tumors can be flash frozen and stored at −80°C until needed.
It is important that every step of the protocol is conducted at 4 °C or the solutions will solidify and the entire batch will be unusable.
Materials
10 g Englebreth-Holm-Swarm (EHS) sarcoma tumor tissue isolated from mice (ATCC #CRL-2108).
3.4 M NaCl buffer (see REAGENTS AND SOLUTIONS)
50 ml centrifuge bottle (Nalgene #3119-0050)
250 ml centrifuge bottle (Nalgene #3120-0250)
Electric tissue homogenizer (Fisher Scientific PowerGen 125)
1.5 inch magnetic stir bar
Stir plate (Thermolyne Nuova II)
Dialysis tubing (8000 molecular weight cut-off; Spectrum Labs #132114)
1 L glass beaker
Ammonium sulfate powder (Sigma# A4418)
Chloroform (VWR# BDH1109)
DMEM media with high glucose and L-glutamine (Gibco # 11965-092)
Scissors and hemostat, sterilized
50 ml Conical tubes, sterile
1.5 ml eppendorf tubes, sterile
Procedure: (Perform all steps on ice or at 4°C)
Thaw 10 g of EHS tumor on ice in a 50 ml Nalgene centrifuge tube (takes approximately 3 hr).
Add 30 ml (3 ml buffer/g tumor) of cold 3.4 M NaCl buffer to the centrifuge tube. Use an electric tissue homogenizer (PowerGen 125), to homogenize the tumor tissue at high speed (setting 6) until the tissue dissolves into a slurry.
Place the Nalgene tubes containing the tumor homogenate in a JA-25.50 rotor (Beckman), balance, and centrifuge at 8000 × g at 4 °C for 15 min. Discard the supernatant.
Add 30 ml of the 3.4 M NaCl buffer to the tube. Electronically homogenize the tumor pellets again at high speed (setting 6) until the pellet dissolves into a slurry.
Balance the tube and centrifuge at 8000 × g in the JA-25.50 rotor at 4 °C for 15 min. Discard the supernatant.
Repeat steps 4 and 5 again.
Add 1 ml/g of original tissue of cold 2M urea buffer to the tube. Electronically homogenize the tumor pellets at high speed (setting 6) until the pellet dissolves into a slurry.
Transfer the mixture to a 250 ml Nalgene centrifuge tube. Add a 1.5 inch stir bar and stir at a medium speed (5 out of 10) overnight at 4°C.
Centrifuge mixture at 23,708 × g at 4 °C for 20 min. Save supernatant.
Add 0.5 ml/g of original tissue of cold 2M urea buffer to the tube. Electronically homogenize the tumor pellets at high speed (setting 6) until the pellet dissolves into a slurry.
Centrifuge the mixture at 23,708 × g at 4 °C for 20 min. Save supernatant.
Combine supernatants from steps 9 and 11 (14 ml) and place the supernatants in dialysis tubing. Seal the dialysis tube with a snap closure.
Add at least 10 volumes of cold TBS to a 1 L glass beaker (see REAGENTS AND SOLUTIONS). Place the dialysis tubing in the beaker with a 1.5 inch stir bar. Subsequently dialyze by stirring the TBS solution at medium speed (5 out of 10) for 2 h. Repeat at least 2 additional times with fresh buffer, for a total of at least 6 hr of dialysis with at least 3 buffer changes.
Empty the dialysis bags into a 250 ml Nalgene centrifuge tube with a stir bar and add 20% (w/v) ammonium sulfate powder (about 16.4g/100ml, 2.296g in this case) slowly while stirring at a medium speed (setting 5 out of 10) until all the (NH4)2SO4 is dissolved, then stir at medium speed for 1 hr.
Collect the precipitate by centrifugation at 7000 × g at 4 °C for 15mim. Discard the supernatant.
Add 14 ml of cold TBS and homogenize the pellet at high speed (setting 6) until the pellet breaks up into a slurry. Add a stir bar and stir at medium speed (5 out of 10) until the slurry dissolves.
Transfer the solution to dialysis tubing and seal the tube with a snap closure. Add at least 10 volumes of cold TBS to a 1 l glass beaker. Place the dialysis tubing in the beaker with a 1.5 inch stir bar. Subsequently dialyze by stirring the TBS solution at medium speed (5 out of 10) for 2 h. Repeat the dialysis with fresh TBS for at least 2 more hr.
Repeat steps 14 and 15.
Add 7 ml of cold TBS and homogenize the pellet at high speed (setting 6) until the pellet breaks up into a slurry. Add a stir bar and stir at medium speed (5 out of 10) until the slurry dissolves.
Transfer the solution to dialysis tubing and seal the tube with a snap closure. Add at least 10 volumes of cold TBS containing 5 ml chloroform per liter to a 1 l glass beaker. Place the dialysis tubing in the beaker with a 1.5 inch stir bar. Subsequently dialyze by stirring the TBS chloroform solution at medium speed (5 out of 10) until the mixture becomes clear, which typically can take between 2 – 10 hours. A glass container or cylinder must be used since the solution contains chloroform, which is utilized to sterilize the mixture.
After the mixture is no longer cloudy, remove the dialysis bag, clean the container thoroughly to remove the chloroform and continually dialyze for at least 2 hr against at least 10 vol of cold TBS with stirring at a medium speed (5 out of 10). Repeat the dialysis at least 3 more times with fresh buffer, for a total of at least 8 hours of dialysis with at least 4 buffer changes.
Perform the final dialysis against at least 10 vol of DMEM media in a 1 L glass beaker with medium stirring (5 out of 10) overnight at 4°C.
In a biosafety cabinet, spray the outside of the dialysis bag with 70% ethanol then open and hold the bag with sterile scissors and hemostats.
Remove the EHS matrix with a sterile pipet and/or pour it into a sterile 15 ml conical tube on ice. Aliquot the matrix into sterile 1ml Eppendoff tubes on ice. This protocol typically generates a final EHS matrix protein concentration of 6-7 mg/ml. The aliquots can be stored at 4 °C for up to a month or at −20 °C for up to a year. Make sure to thaw frozen aliquots at 4 °C.
Reagents and Solutions
The source of each reagent is listed only once, and remains the same unless otherwise specified. Reagents and solutions are listed alphabetically.
70% Ethanol (v/v)
700 ml ethanol (absolute)
300 ml deionized water
Store at room temperature. Use within 1 month.
Human Breast Epithelial Cell (HBEC) medium
Hyclone DMEM/F12 (1:1) with HEPES (Thermo Scientific # SH30023.01)
supplemented with:
10 mM HEPES (Sigma #H3537)
5% fetal bovine serum (FBS, Sigma #F6178)
1 mg/ml bovine serum albumin (BSA, Sigma #A7906)
1 μg/ml insulin (with transferrin/selenium, Invitrogen #51500-056)
0.5 μg/ml hydrocortisone (Sigma #H0888)
50 μg/ml gentamycin (HyClone #3V30080.01)
2.5 μg/ml Fungizone (Amphotericin B, HyClone #SV30078.01)
Sterile filter and store at 4°C. Use within 30 days.
HBEC Freezing Medium
HBEC medium (above) supplemented with:
5% FBS
10 % DMSO (Sigma #D2650)
Sterile filter and store at 4°C. Use within 30 days.
Do not confuse with Tissue Freezing Medium.
Lidocaine-Bupivacaine mixture
5 mg/ml Lidocaine (Hospira #0409-2066-05)
2.5 mg/ml Bupivacaine (University or other pharmacy)
Dilute in HBSS
Sterile filter, aliquot in 1.5 ml eppendorf tube and store at room temperature until the expiration date listed on the lidocaine and bupivacaine samples.
Modified M87 medium
Prepare fresh stocks of the different media components described in Table 4 using sterile techniques. The stock solutions can be stored for up to 1 month at −20°C, except cholera toxin and T3, which are stored at 4°C.
Table 4.
Preparation of 500 ml of modified M87 media for culturing primary or xenografted human tumor cells.
| Component | Stock Concentration |
Final Concentration |
Volume of Stock for 500 ml of Media |
|---|---|---|---|
| DMEM/F12 | 479 ml | ||
| Fetal Bovine Serum (FBS) | 2% | 10 ml | |
| Insulin-Transferrin-Selenium-X | 100× | 1× | 5 ml |
| Penicillin-Streptomycin-Glutamine | 100× | 1× | 5 ml |
| Human Epidermal Growth Factora | 100 μg/ml | 5 ng/ml | 25 μl |
| Hydrocortisoneb | 10 mg/ml | 0.3 μg/ml | 15 μl |
| Cholera Toxinc | 10 μg/ml | 0.5 ng/ml | 25 μl |
| 3,3′,5-Triiodo-L-thyronine (T3)d | 200 μM | 5 nM | 12.5 μl |
| β-Estradiol (E2)b | 20 μM | 0.5 nM | 12.5 μl |
| (±)-Isoproterenol Hydrochloridee | 5 mM | 5 μM | 500 μl |
| Ethanolaminec,f | 100 mM | 50 nM | 250 μl |
| O-Phosphorylethanolaminec,f | 100 mM | 50 nM | 250 μl |
Stock prepared in PBS.
Stock prepared in absolute ethanol.
Stock prepared in deionized water.
T3 was dissolved in deionized water and a drop of 0.1 M NaOH was added and was passed through a 0.22 μm filter.
Stock was prepared in 95% ethanol in a fume hood.
Solution was passed through a 0.22 μm filter.
Hyclone DMEM/F12 (1:1) (Thermo Scientific #SH30023.01)
Hyclone Characterized Fetal Bovine Serum (Thermo Scientific #SH30071.03)
Insulin-Transferrin-Selenium-X Supplement (100×) (Invitrogen #51500-056)
Penicillin-Streptomycin-Glutamine (100×), liquid (Invitrogen #10378-016)
Human recombinant Epidermal Growth Factor (EGF) (BD Biosciences #354052)
Hydrocortisone (Sigma #H0135)
Cholera Toxin (Sigma #C8052)
3,3′,5-Triiodo-L-thyronine (T3) (Sigma #T0281)
β-Estradiol (E2) (Sigma #E2758)
(±)-Isoproterenol Hydrochloride (Sigma #I6504)
Ethanolamine (Sigma #E9508)
O-Phosphorylethanolamine (Sigma #P0503)
Add the specified volume of media component stocks to DMEM/F12 to achieve the appropriate final concentration of each supplement (Table 4). Pass the modified M87 media through a 0.22μm filter. The media can be stored up to 1 week at 4°C.
NaCl buffer (3.4 M), pH 7.4
397 g NaCl
25 ml 2 M Tris buffer (see below)
1.8 liter deionized water
3.0 g EDTA (ethylenediaminetetraacetic acid) (Sigma #E9884)
0.5 g NEM (N-ethyl-maleimide) (Sigma #692905)
Adjust pH to 7.4
Store at room temperature for up to 6 months.
TAC solution (1×)
Mix 1 part of 170 mM Tris, pH 7.4 with
9 parts of 150 mM NH4Cl, pH 7.4)
Sterile filter and store at room temperature for up to 6 months.
Tissue Digestion reagents
For human tumors
Digestion buffer
DMEM-F/12 supplemented with:
10 mM HEPES
2% BSA
5 μg/ml insulin
0.5 μg/ml hydrocortisone
50 μg/mL gentamycin
Sterile filter and store at 4°C. Use within 1 month.
Collagenase enzyme stock (10×)
20 mg/ml Type 3 Collagenase (Worthington #LS004182) in 1× PBS
Sterile filter and store aliquots at −20°C for up to 1 year.
Hyaluronidase enzyme stock (100×)
Hyaluronidase (Sigma #H3884) 10,000 U/ml in 1× PBS
Sterile filter and store at −20°C for up to 1 year.
For xenografted or murine tumors
Digestion buffer
Hyclone RPMI 1640 (Thermo Scientific #SH30255.01) supplemented with:
2.5% FBS
10 mM HEPES
1× Pen-Strep (HyClone #SV30010)
Sterile filter and store at 4°C. Use within 30 days.
Collagenase enzyme stock (10×)
10 mg/ml Type IV Collagenase (Sigma #C5138) in digestion buffer.
Sterile filter and store aliquots at −20°C for up to one year.
Tissue Freezing medium
95% FBS
5% DMSO
Sterile filter and store at 4°C. Use within 1 month.
Do not confuse with HBEC Freezing Medium
2 M Tris buffer, pH 7.4
242.28 g Tris base
800 ml deionized water
Adjust pH to 7.4 with HCl
Add deionized water to 1 liter
Store at room temperature for up to 6 months.
Tris-buffered Saline (TBS)
12.1 g Tris base
18 g NaCl 1.8 liter deionized water
Adjust pH to 7.4 with HCl
Add deionized water to 2 liters
Sterile filter and store at room temperature for up to 6 months.
Urea buffer (2 M), pH 7.4
240 g urea
12.1 g Tris base
18 g NaCl
1.8 liter deionized water
Adjust pH to 7.4 with HCl
Add deionized water to 2 liters
Store at room temperature for up to 2 months.
COMMENTARY
Background information
Patient-derived tumorgrafts growing in mice can recapitulate clinical manifestations and pathological features of a patient’s original tumor, including its histology, molecular subtype, genomic organization, and metastatic dissemination (DeRose et al., 2011). Thus, mice carrying primary tumorgrafts are ideal experimental models for studying the in vivo events of human tumorigenesis, and for assessing the efficacy of investigational compounds in diverse cancer subtypes. Tumorgrafting also provides a method to propagate patient-derived tissue for more extensive in vivo and in vitro studies. While the complicated methodology, cost, variability and time requirements of tumorgrafting makes this technique impractical for widespread adoption into the clinic, there are potential opportunities to apply tumorgrafts in future clinical practice. For example, tumorgrafts can provide diagnostic information on the aggressiveness of a patient’s tumor (successful engraftment is associated with worse patient outcome (DeRose et al., 2011)), and for functionally evaluating the sensitivity of tumors to specific drugs prior to treating patients. While tumorgrafts are getting traction in early translational research programs, significant barriers must still be overcome before they are practical for clinical applications.
A tumorgraft bank is an effective tool for many research purposes. Currently, the rate-limiting step is the initial investment, both cost and time, to establish a tumorgraft bank that not only spans the breadth of cancer subtypes, but also contains replicates of each tumor subtype. Once a diverse bank of transplantable tissue is categorized into molecular and genomic subtypes, incorporation of patient-derived tissue into basic research and drug discovery platforms becomes significantly more practical. Ideally, a centralized repository would be established to consolidate and disseminate tissue samples, which would eliminate the most significant barriers associated with generating individual banks and increase the diversity of tumor subtypes available for research. Until then, the protocols described in this unit should facilitate in-house development of tumorgrafts banks.
One of the most suitable applications for tumorgrafts in drug discovery is pre-clinical evaluation of select compounds in a “pseudo-clinical study” or “Phase 2 mouse study.” New chemical entities in early development can be tested against cohorts of tumors representing specific molecular subtypes to determine differential activity across a broad spectrum of tumors. An understanding of a compound’s range of activity in diverse tumor subtypes has become increasingly important when designing clinical trials, particularly with targeted therapies that are often effective only in tumors with specific oncogenic drivers. Thus, tumorgrafts can fill a critical void between conventional pre-clinical cancer models and clinical trials in patients.
While tumorgrafts provide distinct advantages over cell lines or xenografted cell lines, they are still not ideal for all basic research purposes due to their many limitations. The critical hurdles of generating tumorgrafts include regulatory processes, such as Institutional Review Board and HIPPA approvals; establishing the infrastructure and collaborations necessary to obtain surgical specimens from patients; cost to obtain, cryopreserve, transplant and characterize; long tumor propagation times in mice; necessity for growth in an immune-compromised hosts; and challenges with performing in vitro experimental manipulations. Thus, tumorgrafts are best employed in focused studies to more closely establish relevance of an experimental observation to the human disease. Similarly, tumorgrafts would not be efficient in high-throughput drug screening; rather, they would be more effectively employed to validate specific lead compounds as a step toward bringing a drug to the clinic.
Critical Parameters
When establishing a tumorgraft bank, the most critical parameter is the quality of the surgical specimen. Clearly, tumor samples with low viability are inadequate for tumor grafting. Thus, a reliable research team with defined roles in obtaining and processing surgical specimens is absolutely critical. All post-collection procedures described in this unit can be optimized and customized as necessary for experiments, but these efforts are in vain without quality specimens. To begin, an investigator should establish a repertoire with all members of the clinical team involved in collecting the tissue, including administrative staff, nurses, surgeons, pathologists, and researchers. The team must collect and distribute the surgical specimens to laboratory personnel within a defined time, and understand that tissue quality is affected by several variables associated with collection and processing, such as time-to-transplant and sterility of the sample. Quarterly meetings with the entire team for general discussions and presentations on the quality of tissue specimens, updates on research studies utilizing tissue sample, and reviewing banking procedures is one effective method to keep the team informed and motivated. It is also important to recognize the efforts of everyone involved by including individuals critical to the collections on manuscripts, including them as key personnel on grant applications, and acknowledging them in presentations.
Once a tissue bank is generated, it is important to assess the molecular and histological diversity of the samples and to compare the tumorgrafts with originating tumor. For this, it is important to obtain de-identified medical reports that provide diagnoses, treatments, medications, tumor pathology, and if possible, paraffin blocks of the patient’s tumor. Molecular and histological analyses, as described in DeRose et al., 2011, can then be performed to ensure that the tumor graft faithfully replicates the patient’s tumor. While in the majority of cases a tumorgraft closely replicates the patient’s tumor, we have observed one case in which a human lymphoma arose following transplantation of a breast cancer surgical specimen from an 80-year old individual. Thus, tumor grafts should be carefully monitored for their ability to replicate the molecular, genomic, and histologic phenotypes of the patient’s primary tumor.
Each patient-derived tumor sample is unique. As such, the method used for processing tissues is dynamic and requires careful monitoring and alterations as needed. While this unit provides a standard protocol that works well for many tumorgrafts, changes to the method may be necessary to optimize processing of different samples. As such, the educated judgment of an experienced researcher is often required. For example, the abundance and composition of stroma and extracellular matrix may differ between tumor tissue, which directly affects the time needed for enzymatic digestion by collagenase and hyaluronidase. Other variables include the size of the surgical specimen, level of necrosis, quantity of red blood cells and inflammatory cells, and contamination with bacteria or yeast, all of which affect tissue processing and future applications. During the procedure, the tissue sample should be carefully monitored both visually and microscopically for the degree of enzymatic digestion, cellular morphology (signs of necrosis/apoptosis) and viscosity of the media (release of DNA from dead cells) to assess the quality of the preparation. In addition, enrichment of tumor organoids from debris, stroma, and inflammatory cells, should be monitored microscopically, with adjustments made to differential centrifugation steps as described in the unit. These observations, and any other information about the quality or viability of the preparation, should be noted for each sample and uploaded to the tumor bank’s database. Careful observation and procedural adjustments during tissue processing should improve the quality of the preparation.
Time Consideration
Growth rates of patient-derived tumor samples in mice are highly variable. To date, our fastest growing tumorgraft became palpable around two weeks after transplantation, and was harvested approximately two months following engraftment. It is common for tumor grafts to take several months to become palpable and 7-8 months to be ready to harvest for the first time. Careful palpation is critical to avoid termination of tumorgraft lines that otherwise might be successful. When re-transplanting characterized tissue, an additional month should be added to the expected growth time if the tumor fragment is frozen prior to implantation. Often, the re-transplanted tissue will have reduced latency compared to the original patient-derived sample. When cohorts of mice are transplanted with similar size fragments or cell numbers from the same tumorgraft sample, tumors typically grow with similar latency.
Troubleshooting
a. Lack of growth
Once a transplantable tumor graft line has been established, the successful re-transplantation of the line is high (>90%). The most common cause for lack of growth of an established tumor graft is that the tumor fragment that was implanted into the host was of low quality (e.g. containing more stroma than tumor or extensive necrosis) or because of a failure to supplement mice carrying ER+ tumors with estrogen. If necrosis limits the growth of tumorgrafts, co-engraftment of human mesenchymal stem cells can stimulate tumor angiogenesis and promote growth (DeRose et al., 2011). If primary, patient-derived tumor tissue fails to engraft upon first implantation, it may be among the 70-74% of breast tumors that do not engraft using the methods in this unit. It is more efficient to first establish a bank of established tumorgraft lines than rely on primary surgical specimens for experiments.
b. Genetic or phenotypic drift
Serially-transplanted tumorgrafts sometimes tend to grow faster than earlier passages (DeRose et al., 2011). Careful observation of the tumor characteristics and behavior is important, as “drift” can be a problem. To retain the features of the original tumor as much as possible, we strongly suggest maintaining many frozen vials of early passage tumorgraft fragments for future implantation.
Anticipated Results
Using these protocols, the origin of the breast tumor (primary or metastatic site), ER/PR status, or HER2 status did not reliably predict successful engraftment of the original tumors into mice in our study using approximately 50 patient samples (DeRose et al., 2011). However, positive engraftment correlated with poor patient outcome, independent of the aforementioned factors (DeRose et al., 2011). In patients, triple negative tumors (those testing negative for estrogen receptor, progesterone receptor and HER2) are typically aggressive, and correspondingly, once they are engrafted they tend to grow faster than other subtypes of breast cancer (DeRose et al., 2011). Primary tumor tissues from patients who have been treated with neoadjuvant chemotherapy have not successfully engrafted, at least in our experience so far. By contrast, metastases do engraft even after chemotherapy or radiation therapy. We generally allow six months after the first engraftment for a tumor to grow. If growth is not detected by six months the engraftment is considered unsuccessful. Based on attempted engraftment of approximately 50 different patient tumors into NOD/SCID mice, the engraftment rate from surgical specimens was 30-36% (DeRose et al., 2011). Although the overall engraftment rate for surgical specimens was relatively low, when one tissue fragment from a patient grows, all transplanted fragments from that patient generally grow. Likewise, when a tumor does not successfully engraft, none of the fragments grow. Once a tumor has successfully engrafted, approximately 100% of subsequent transplants from that line can be expected upon serial passage without intervening culture steps. It’s important to note that engraftment data discussed in this unit were obtained from tissue/cells that were not cultured, and it is unknown whether culturing, in either monolayer or 3D, will have an effect on the phenotype of subsequent tumorgrafts.
Acknowledgements
The work described in this article was funded by grants from the National Institute of Health (CA143815 and CA140296), the Department of Defense (W81XWH-09-1-0431 and W81XWH-08-1-0109), and the American Association for Cancer Research with the Breast Cancer Research Foundation (07-60-26-WELM), as well as with funds from the Huntsman Cancer Foundation/Institute.
Literature Cited
- Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. 2003;100:3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky AD. Choosing a mouse model: Experimental biology in context—the utility and limitations of mouse models of breast cancer. Cold Spring Harbor Perspectives in Biology. 2011;3:a009670. doi: 10.1101/cshperspect.a009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdall S, Hanby A, Lansdown M, Speirs V. Breast cancer cell lines: Friend or foe? Breast Cancer Res. 2003;5:89–95. doi: 10.1186/bcr577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caligiuri I, Rizzolio F, Boffo S, Giordano A, Toffoli G. Critical choices for modeling breast cancer in transgenic mouse models. J. Cell. Physiol. 2012;227:2988–2991. doi: 10.1002/jcp.24031. [DOI] [PubMed] [Google Scholar]
- Cespedes MV, Casanova I, Parreno M, Mangues R. Mouse models in oncogenesis and cancer therapy. Clin. Transl. Oncol. 2006;8:318–329. doi: 10.1007/s12094-006-0177-7. [DOI] [PubMed] [Google Scholar]
- Clarke R. Human breast cancer cell line xenografts as models of breast cancer. The immunobiologies of recipient mice and the characteristics of several tumorigenic cell lines. Breast Cancer Res. Treat. 1996;39:69–86. doi: 10.1007/BF01806079. [DOI] [PubMed] [Google Scholar]
- Cohen AL, Soldi R, Zhang H, Gustafson AM, Wilcox R, Welm BE, Chang JT, Johnson E, Spira A, Jeffrey SS, Bild AH. A pharmacogenomic method for individualized prediction of drug sensitivity. Mol. Syst. Biol. 2011;7:513. doi: 10.1038/msb.2011.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeRose YS, Wang G, Lin Y-C, Bernard PS, Buys SS, Ebbert MTW, Factor R, Matsen C, Milash BA, Nelson E, Neumayer L, Randall RL, Stijleman IJ, Welm BE, Welm AL. Tumor grafts derived from women with breast cancer authentically reflect tumor pathology, growth, metastasis and disease outcomes. Nat. Med. 2011;17:1514–1520. doi: 10.1038/nm.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garbe JC, Bhattacharya S, Merchant B, Bassett E, Swisshelm K, Feiler HS, Wyrobek AJ, Stampfer MR. Molecular distinctions between stasis and telomere attrition senescence barriers shown by long-term culture of normal human mammary epithelial cells. Cancer Res. 2009;69:7557–7568. doi: 10.1158/0008-5472.CAN-09-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta PB, Kuperwasser C. Disease models of breast cancer. Drug Discovery Today: Disease Models. 2004;1:9–16. [Google Scholar]
- Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13:215. doi: 10.1186/bcr2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh SH, Do HJ, Lim HY, Kim DK, Choi SJ, Song H, Kim NH, Park JK, Chang WK, Chung HM, Kim JH. Optimization of 25 kDa linear polyethylenimine for efficient gene delivery. Biologicals. 2007;35:165–171. doi: 10.1016/j.biologicals.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Hummel KP, Richardson FL, Fekete E. Anatomy. In: Green EL, editor. Biology of the Laboratory Mouse. Dover Publications Inc.; New York: 1966. Chapter 13. http://www.informatics.jax.org/greenbook/ [Google Scholar]
- Kao J, Salari K, Bocanegra M, Choi YL, Girard L, Gandhi J, Kwei KA, Hernandez-Boussard T, Wang P, Gazdar AF, Minna JD, Pollack JR. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS One. 2009;4:e6146. doi: 10.1371/journal.pone.0006146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller P, Lin A, Arendt L, Klebba I, Jones A, Rudnick J, DiMeo T, Gilmore H, Jefferson D, Graham R, Naber S, Schnitt S, Kuperwasser C. Mapping the cellular and molecular heterogeneity of normal and malignant breast tissues and cultured cell lines. Breast Cancer Res. 2010;12:R87. doi: 10.1186/bcr2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: Better than commonly perceived-but they can be improved. Cancer Biol. Ther. 2003;2:S134–139. [PubMed] [Google Scholar]
- Kibbey MC. Maintenance of the EHS sarcoma and matrigel preparation. Methods Cell Sci. 1994;16:227–230. [Google Scholar]
- Neve RM, Chin K, Fridlyand J, Yeh J, Baehner FL, Fevr T, Clark L, Bayani N, Coppe JP, Tong F, Speed T, Spellman PT, DeVries S, Lapuk A, Wang NJ, Kuo WL, Stilwell JL, Pinkel D, Albertson DG, Waldman FM, McCormick F, Dickson RB, Johnson MD, Lippman M, Ethier S, Gazdar A, Gray JW. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell. 2006;10:515–527. doi: 10.1016/j.ccr.2006.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudali G, Apiou F, Muel B. Mammary cancer produced in mice with estriol. Eur. J. Cancer. 1975;11:39–41. doi: 10.1016/0014-2964(75)90035-3. [DOI] [PubMed] [Google Scholar]
- Strober W. Current protocols in immunology. John Wiley & Sons, Inc.; Hoboken, N.J.: 2001. Trypan blue exclusion test of cell viability; pp. A.3B.1–A.3B.2. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Kawauchi S, Saito S, Furuya T, Ikemoto K, Nakao M, Yamamoto S, Oka M, Hirano T, Sasaki K. Breast cancer cell lines carry cell line-specific genomic alterations that are distinct from aberrations in breast cancer tissues: comparison of the cgh profiles between cancer cell lines and primary cancer tissues. BMC Cancer. 2010;10:15. doi: 10.1186/1471-2407-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voskoglou-Nomikos T, Pater JL, Seymour L. Clinical predictive value of the in vitro cell line, human xenograft, and mouse allograft preclinical cancer models. Clin. Cancer Res. 2003;9:4227–4239. [PubMed] [Google Scholar]
- Welm BE, Dijkgraaf GJP, Bledau AS, Welm AL, Werb Z. Lentiviral transduction of mammary stem cells for analysis of gene function during development and cancer. Cell Stem Cell. 2008;2:90–102. doi: 10.1016/j.stem.2007.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]