Abstract
We synthesized 30 lipophilic bisphosphonates and tested them in malaria parasite killing (targeting parasite geranylgeranyl diphosphate synthase, GGPPS) and human γδ T cell activation (targeting human farnesyl diphosphate synthase, FPPS). Similar patterns of activity were seen in inhibiting human FPPS and Plasmodium GGPPS, with short to medium chain-length species having most activity. In cells, shorter chain-length species had low activity, due to poor membrane permeability, and longer chain length species were poor enzyme inhibitors. Optimal activity was thus seen with ∼C10 side-chains, which have the best combination of enzyme inhibition and cell penetration. We also solved the crystal structure of one potent inhibitor, bound to FPPS. The results are of interest since they suggest the possibility of a combined chemo/immuno-therapeutic approach to antimalarial development in which both direct parasite killing and γδ T cell activation can be achieved with a single compound.
Keywords: Bisphosphonates, immunology, inhibitors, malaria
Malaria is a major cause of mortality and morbidity from parasitic protozoan diseases worldwide1 and drug resistance is of concern.2,3 There is thus interest in new drugs and new drug targets, as well as unconventional approaches involving host innate immunity.4 Activated γδ T cells are of interest in this context since they can not only kill tumor cells,5 bacteria6 and influenza virus-infected cells,7 γδ T cells can also produce TNF-α on activation, and TNF-α is known to prevent the development of pre-erythrocytic stage parasites.8 One class of drug molecules called bisphosphonates are known to activate γδ T cells (containing the Vγ2 Vδ2 T cell receptor), so these molecules might be used as immunomodulators.9 Most bisphosphonates are, however, poorly taken up into cells and bind tightly to bone mineral.10 Recently, we showed that lipophilic bisphosphonates11 did not bind to bone mineral10 and, in addition, were more active in γδ T cell activation12 than were the current bisphosphonate drugs used to treat bone resorption diseases and cancer. We also discovered that lipophilic bisphosphonates were active in killing liver stage malaria parasites13 and that a lipophilic analogue of the bisphosphate zoledronate was a potent inhibitor of intraerythrocytic Plasmodium both in vitro and in vivo in mice.14
Bisphosphonates such as zoledronate (1) activate γδ T cells by inhibiting the enzyme farnesyl diphosphate synthase (FPPS). This results in accumulation of the FPPS substrates, isopentenyl diphosphate (IPP), and dimethylallyl diphosphate (DMAPP), both of which are phosphoantigens that activate γδ T cells.12,15 However, zoledronate has essentially no effect on the intraerythrocytic form of the malaria parasites since it is poorly membrane permeable. In contrast, lipophilic bisphosphonates (containing N-alkyl side-chains) do kill the parasites.14 Here, the target is the Plasmodium geranylgeranyl diphosphate synthase (GGPPS). This enzyme is unusual in that it is structurally more similar to human FPPS than human GGPPS and, unlike human GGPPS, is potently inhibited by bisphosphonates. Inhibiting GGPPS in the parasite blocks formation of protein prenylation16 as well as carotenoid,17 menaquinone,18 and vitamin E formation,19 Figure 1, and results in direct parasite killing. Plus, this killing effect is blocked by the addition of geranylgeraniol,14 confirming a GGPPS target.
Figure 1.
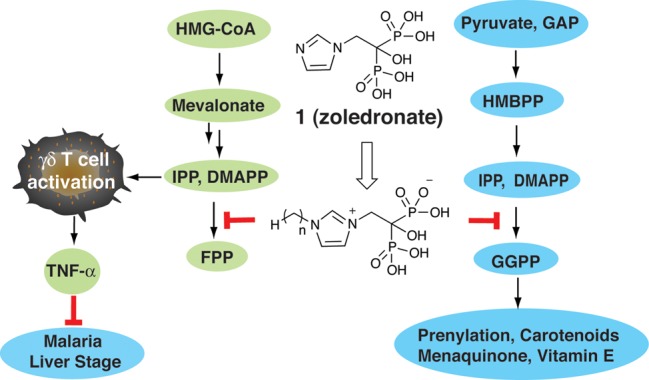
Schematic illustration of pathways involved in zoledronate–analogue activity in γδ T cells and in malaria parasites. Green = human cell; cyan = malaria parasite. HMG-CoA = hydroxymethylglutaryl coenzyme A; IPP = isopentenyl diphosphate; DMAPP = dimethylallyl diphosphate; FPP = farnseyl diphosphate; GAP = glyceraldehyl-3-phosphate; HMBPP = 4-hydroxyl-3-methyl-but-2-enyl diphosphate; GGPP = geranylgeranyl diphosphate; TNF-α = tumor necrosis factor α.
Here, we sought to find a lipophilic bisphosphonate that would kill malaria parasites as well as activate γδ T cells, a possible new route to malaria chemo-immunotherapy.
We synthesized the 16 pairs of zoledronate (1) species (1–32) shown in Figure 2a in which we varied the length of the alkyl chain (n = 0 through n = 15 carbons) and the presence or absence of the 1-OH group that is involved in bone-binding and that has been proposed (with zoledronate, 1) to be important in γδ T cell activation.10,20 Synthesis and characterization details are provided in the Supporting Information. We then tested all 32 compounds for human FPPS inhibition activity. The most potent FPPS inhibitors were those with medium length side-chains, and these were ∼3–10× more potent than zoledronate itself, Figure 2b and Table 1. As the N-alkyl chain length increases beyond C10, FPPS inhibition decreases, due presumably to the onset of steric repulsion with the highly conserved Phe 98 and 99 residues in the FPPS active site that limit chain elongation.21
Figure 2.
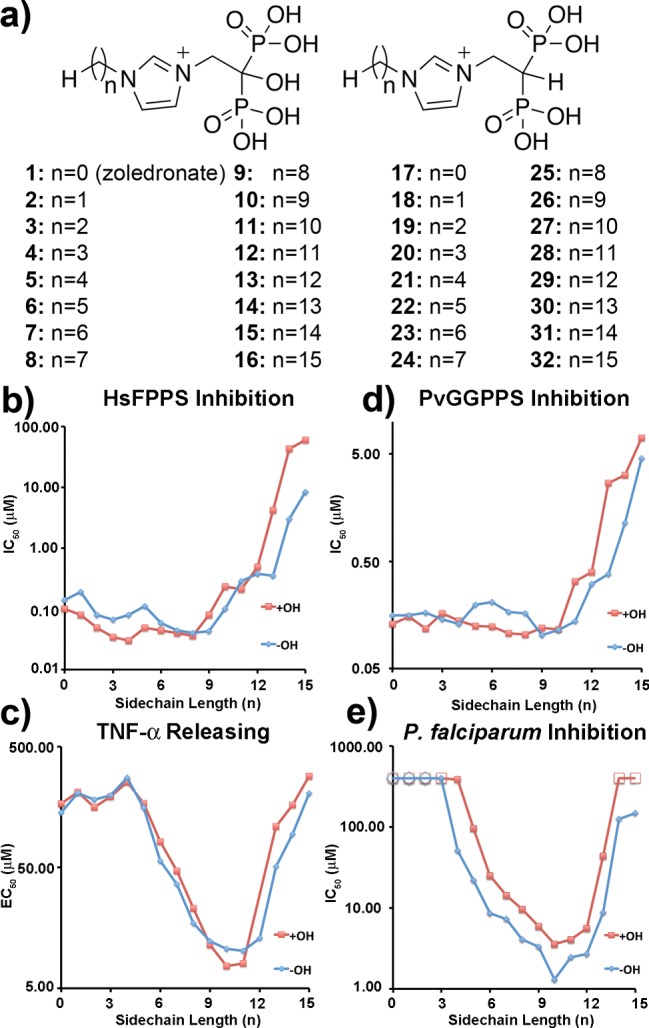
Chain length dependence of enzyme and cell growth inhibition/activation and effects of the 1-OH group. (a) Structures of compounds investigated; (b) HsFPPS; (c) γδ T cell activation/TNF-α release; (d) PvGGPPS inhibition; (e) intraerythrocytic P. falciparum cell growth inhibition.
Table 1. Enzyme Inhibition Together with γδ T Cell Activation and P. falciparum Cell Growth Inhibition.
| ID | side-chain length (n, OH/H) | HsFPPS IC50 (μM) | TNF-α IC50 (μM) | PvGGPPS IC50 (μM) | P. falciparum IC50 (μM) |
|---|---|---|---|---|---|
| 1 | 0, OH | 0.10 | 170 | 0.13 | >400 |
| 2 | 1, OH | 0.080 | 210 | 0.15 | >400 |
| 3 | 2, OH | 0.049 | 160 | 0.12 | >400 |
| 4 | 3, OH | 0.034 | 190 | 0.16 | >400 |
| 5 | 4, OH | 0.030 | 250 | 0.14 | 390 |
| 6 | 5, OH | 0.049 | 170 | 0.12 | 97 |
| 7 | 6, OH | 0.044 | 81 | 0.12 | 25 |
| 8 | 7, OH | 0.040 | 47 | 0.11 | 14 |
| 9 | 8, OH | 0.036 | 23 | 0.10 | 9.6 |
| 10 | 9, OH | 0.080 | 11 | 0.12 | 5.9 |
| 11 | 10, OH | 0.23 | 7.6 | 0.12 | 3.6 |
| 12 | 11, OH | 0.21 | 8.0 | 0.32 | 4.1 |
| 13 | 12, OH | 0.49 | 8.8 | 0.40 | 5.6 |
| 14 | 13, OH | 4.2 | 110 | 2.7 | 44 |
| 15 | 14, OH | 42 | 160 | 3.2 | >400 |
| 16 | 15, OH | 60 | 280 | 7.1 | >400 |
| 17 | 0, H | 0.14 | 140 | 0.16 | >400 |
| 18 | 1, H | 0.19 | 210 | 0.16 | >400 |
| 19 | 2, H | 0.08 | 180 | 0.17 | >400 |
| 20 | 3, H | 0.066 | 200 | 0.14 | >400 |
| 21 | 4, H | 0.079 | 280 | 0.13 | 51 |
| 22 | 5, H | 0.11 | 160 | 0.20 | 22 |
| 23 | 6, H | 0.058 | 56 | 0.21 | 8.6 |
| 24 | 7, H | 0.044 | 36 | 0.17 | 7.2 |
| 25 | 8, H | 0.040 | 17 | 0.16 | 4.1 |
| 26 | 9, H | 0.043 | 12 | 0.10 | 3.3 |
| 27 | 10, H | 0.10 | 11 | 0.11 | 1.3 |
| 28 | 11, H | 0.28 | 10 | 0.14 | 2.5 |
| 29 | 12, H | 0.37 | 13 | 0.31 | 2.7 |
| 30 | 13, H | 0.35 | 51 | 0.38 | 8.8 |
| 31 | 14, H | 3.0 | 94 | 1.1 | 120 |
| 32 | 15, H | 8.3 | 210 | 4.5 | 150 |
We next investigated the effects of chain-length and the presence/absence of the 1-OH group on γδ T cell activation, as determined in a TNF-α release assay.22 As can be seen in Table 1 and Figure 2c, there is a monotonic increase in activity beyond C4 with both series of compounds with increasing chain length up to n ≈ 11, then activity rapidly decreases with n > 12. The decrease in activity with the longer chain species occurs at a longer chain length in cells than in FPPS inhibition due, we believe, to the importance of hydrophobicity with the more lipophilic species, which facilitates cell entry. These results also clearly show, at least with the γδ T cell lines we have used, that there is no major difference in activity due to the presence or absence of the 1-OH group.
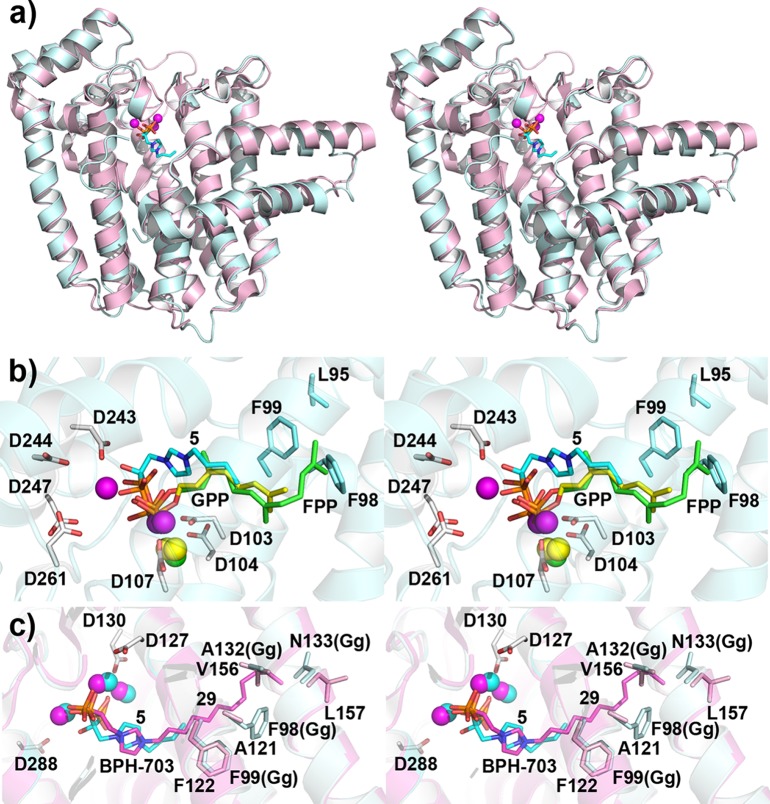
To see how these lipophilic zoledronate derivatives bound to FPPS, we obtained the X-ray crystallographic structure of 5 (IC50 ≈ 30 nM) bound to human FPPS (HsFPPS), as shown in Figure 3a (in cyan; PDB ID code 4GA3). Full crystallographic data acquisition and structure refinement details are given in Supporting Information Table S1. Compound 5 binds into the same site as does zoledronate23,24 with its two phosphonate groups bound to the [Mg2+]3 cluster, and there is a 0.7 Å rmsd between the [Mg2+]3, bisphosphonate, and imidazole rings in the two structures. The alkyl chain extends into the GPP/FPP side-chain site, Figure 3b (FPPS structures, PDB ID code 1UBX and 1UBW). The origin of the more potent FPPS inhibition by the N-alkyl bisphosphonates over that seen with the unsubstituted species is likely due to an enhanced hydrophobic interaction as opposed to a purely Coulombic interaction since the results of a solid-state NMR and quantum chemical investigation25 show that the imidazole nitrogen in zoledronate also carries a +1 charge (due there to protonation), when bound to FPPS.
Figure 3.
Structural results in stereo representation. (a) X-ray structure of HsFPPS/5 complex (cyan, PDB ID code 4GA3) superimposed on PvGGPPS structure (purple, PDB ID code 3RBM). The Cα rmsd over 331 residues in 1.44 Å. (b) Comparison between the X-ray structures of 5 bound to HsFPPS, GPP (yellow), and FPP (green) bound to avian FPPS (PDB ID codes, 1UBX and 1UBW). The bisphosphonate 5 binds to the allylic (GPP) site. Chain elogation in FPP is blocked by F98 and F99, corresponding to decreased HsFPPS inhibition by bisphosphonate inhibitors with N-alkyl chains longer than ∼C10. (c) Structures of HsPPPS/5 (cyan) overlaid on 29 (BPH-703; pink) bound to PvGGPPS (PDB ID code 3RBM). The bisphosphonate, imidazolium, and N-alkyl side-chain structures are quite similar. Optimum activity in PvGGPPS is at ∼C11, then steric repulsion ensures.
We next investigated the inhibition of Plasmodium vivax GGPPS (PvGGPPS) and the direct killing of intraerythrocytic parasites by 1–32. As can be seen in Figure 2d,e and Table 1, several of the compounds most effective in inhibiting PvGGPPS were also very effective in inhibiting P. falciparum growth. The correlation between PvGGPPS and cell growth inhibition was poor (R = 0.26) but improved to R = 0.80 on addition of the log P and solvation energy descriptors reported previously.26 The ability to inhibit FPPS and GGPPS with the same chain length compounds is likely due to their mimicking the FPP product (in human FPPS) or the FPP substrate (in Plasmodium GGPPS), together with the presence of the third Asp in PvGGPPS that is essential for bisphosphonate binding to [Mg2+]3 with, and as can be seen in Figure 3c, the structures of 5 bound to HsFPPS and 29 bound to PvGGPPS being very similar (rmsd = 0.9 Å).
In conclusion, the results we have presented here are of interest for a number of reasons. First, we constructed a library of 31 N-alkyl analogues of the bisphosphonate drug, zoledronate, with and without 1-OH groups, and tested them (and zoledronate) for activity in inhibiting human FPPS. The results show that medium chain length species inhibit human FPPS most potently, while longer chain species are inactive, due, we propose, to a steric clash with the FPPS chain-length-determining residues Phe 98 and 99. Second, we investigated the activity of all 32 compounds in γδ T cell activation: the most active species had 10 ± 1 carbons in the N-alkyl side-chain. We propose that the increased activity of these lipophilic zoledronate–analogue bisphosphonates in cells compared with zoledronate itself is due to the improved cell uptake of the more lipophilic compounds. Third, we determined the X-ray crystallographic structure of one potent inhibitor of human FPPS bound to the enzyme, finding that the bisphosphonate and imidazole groups occupied the same position as in zoledronate bound to FPPS, as well as 29 bound to GGPPS. Fourth, we find that the most potent Vγ2 Vδ2 T cell activators also kill malaria parasites in vitro (and in vivo14). This opens up the intriguing possibility of a combined chemo-immunotherapeutic approach to the development of new antimalarials in which both host innate immunity (host FPPS inhibition/γδ T cell activation/TNF-α-mediated killing) and direct killing (via parasite GGPPS inhibition, carotenoid, menaquinone, and vitamin E biosynthesis inhibition) are targeted by a single molecule.
Acknowledgments
We thank R. Hui for providing the PvGGPPS expression system and M.-J. Ku for providing the P. falciparum parasites.
Glossary
Abbreviations
- GGPPS
geranylgeranyl diphosphate synthase
- FPPS
farnesyl diphosphate synthase
- TNF-α
tumor necrosis factor α
- IPP
isopentenyl diphosphate
- DMAPP
dimethylallyl diphosphate
- GPP
geranyl diphosphate
- FPP
farnesyl diphosphate
Supporting Information Available
Experimental details of inhibitor syntheses, HsFPPS and PvGGPPS expression, purification, and inhibition, γδ T cell activation, P. falciparum growth inhibition, and HsFPPS X-ray crystallography. This material is available free of charge via the Internet at http://pubs.acs.org.
Author Contributions
¶ These authors contributed equally.
This work was supported by grants from the by the United States Public Health Service (NIH grants GM065307, AI074233, AR045504, CA158191, and CA097274) and the Department of Veterans Affairs (BX000972). Use of the Life Science Collaborative Access Team Sector 21 was supported by the Michigan Economic Development Corporation and the Michigan Technology Tri-Corridor (Grant 085P1000817).
C.T.M. is a co-inventor of US Patent 8,012,466 on the development of live bacterial vaccines for activating γδ T cells. The other authors declare no conflict of interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Wells T. N.; Alonso P. L.; Gutteridge W. E. New medicines to improve control and contribute to the eradication of malaria. Nat. Rev. Drug Discov. 2009, 8, 879–891. [DOI] [PubMed] [Google Scholar]
- WHO. Global report on antimalarial efficacy and drug resistance: 2000–2010. http://www.who.int/malaria/publications/atoz/9789241500470/en/index.html.
- Dondorp A. M.; Fairhurst R. M.; Slutsker L.; Macarthur J. R.; Breman J. G.; Guerin P. J.; Wellems T. E.; Ringwald P.; Newman R. D.; Plowe C. V. The threat of artemisinin-resistant malaria. N. Engl. J. Med. 2011, 365, 1073–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bottger E.; Multhoff G.; Kun J. F.; Esen M. Plasmodium falciparum-infected erythrocytes induce granzyme B by NK cells through expression of host-Hsp70. PLoS One 2012, 7, e33774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunzmann V.; Bauer E.; Feurle J.; Weissinger F.; Tony H. P.; Wilhelm M. Stimulation of γδ T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood 2000, 96, 384–392. [PubMed] [Google Scholar]
- Wang L.; Kamath A.; Das H.; Li L.; Bukowski J. F. Antibacterial effect of human Vγ2Vδ2 T cells in vivo. J. Clin. Invest. 2001, 108, 1349–1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu W.; Zheng J.; Liu Y.; Sia S. F.; Liu M.; Qin G.; Ng I. H.; Xiang Z.; Lam K. T.; Peiris J. S.; Lau Y. L. The aminobisphosphonate pamidronate controls influenza pathogenesis by expanding a γδ T cell population in humanized mice. J. Exp. Med. 2011, 208, 1511–1522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Depinay N.; Franetich J. F.; Gruner A. C.; Mauduit M.; Chavatte J. M.; Luty A. J.; van Gemert G. J.; Sauerwein R. W.; Siksik J. M.; Hannoun L.; Mazier D.; Snounou G.; Renia L. Inhibitory effect of TNF-α on malaria pre-erythrocytic stage development: influence of host hepatocyte/parasite combinations. PLoS One 2011, 6, e17464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das H.; Wang L.; Kamath A.; Bukowski J. F. Vγ2Vδ2 T-cell receptor-mediated recognition of aminobisphosphonates. Blood 2001, 98, 1616–1618. [DOI] [PubMed] [Google Scholar]
- Mukherjee S.; Huang C.; Guerra F.; Wang K.; Oldfield E. Thermodynamics of bisphosphonates binding to human bone: a two-site model. J. Am. Chem. Soc. 2009, 131, 8374–8375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Cao R.; Yin F.; Hudock M. P.; Guo R. T.; Krysiak K.; Mukherjee S.; Gao Y. G.; Robinson H.; Song Y.; No J. H.; Bergan K.; Leon A.; Cass L.; Goddard A.; Chang T. K.; Lin F. Y.; Van Beek E.; Papapoulos S.; Wang A. H.; Kubo T.; Ochi M.; Mukkamala D.; Oldfield E. Lipophilic bisphosphonates as dual farnesyl/geranylgeranyl diphosphate synthase inhibitors: an X-ray and NMR investigation. J. Am. Chem. Soc. 2009, 131, 5153–5162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y.; Cao R.; Yin F.; Lin F. Y.; Wang H.; Krysiak K.; No J. H.; Mukkamala D.; Houlihan K.; Li J.; Morita C. T.; Oldfield E. Lipophilic pyridinium bisphosphonates: potent γδ T cell stimulators. Angew. Chem., Int. Ed. 2010, 49, 1136–1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh A. P.; Zhang Y.; No J. H.; Docampo R.; Nussenzweig V.; Oldfield E. Lipophilic bisphosphonates are potent inhibitors of Plasmodium liver-stage growth. Antimicrob. Agents Chemother. 2010, 54, 2987–2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- No J. H.; de Macedo Dossin F.; Zhang Y.; Liu Y. L.; Zhu W.; Feng X.; Yoo J. A.; Lee E.; Wang K.; Hui R.; Freitas-Junior L. H.; Oldfield E. Lipophilic analogs of zoledronate and risedronate inhibit Plasmodium geranylgeranyl diphosphate synthase (GGPPS) and exhibit potent antimalarial activity. Proc. Natl. Acad. Sci. U.S.A. 2012, 109, 4058–4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzaid I.; Monkkonen H.; Stresing V.; Bonnelye E.; Green J.; Monkkonen J.; Touraine J. L.; Clezardin P. High phosphoantigen levels in bisphosphonate-treated human breast tumors promote Vγ9Vδ2 T-cell chemotaxis and cytotoxicity in vivo. Cancer Res. 2011, 71, 4562–4572. [DOI] [PubMed] [Google Scholar]
- Buckner F. S.; Eastman R. T.; Yokoyama K.; Gelb M. H.; Van Voorhis W. C. Protein farnesyl transferase inhibitors for the treatment of malaria and African trypanosomiasis. Curr. Opin. Invest. Drugs 2005, 6, 791–797. [PubMed] [Google Scholar]
- Tonhosolo R.; D’Alexandri F. L.; de Rosso V. V.; Gazarini M. L.; Matsumura M. Y.; Peres V. J.; Merino E. F.; Carlton J. M.; Wunderlich G.; Mercadante A. Z.; Kimura E. A.; Katzin A. M. Carotenoid biosynthesis in intraerythrocytic stages of Plasmodium falciparum. J. Biol. Chem. 2009, 284, 9974–9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonhosolo R.; Gabriel H. B.; Matsumura M. Y.; Cabral F. J.; Yamamoto M. M.; D’Alexandri F. L.; Sussmann R. A.; Belmonte R.; Peres V. J.; Crick D. C.; Wunderlich G.; Kimura E. A.; Katzin A. M. Intraerythrocytic stages of Plasmodium falciparum biosynthesize menaquinone. FEBS Lett. 2010, 584, 4761–4768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Meer J. Y.; Hirsch A. K. The isoprenoid-precursor dependence of Plasmodium spp. Nat. Prod. Rep. 2012, 29, 721–728. [DOI] [PubMed] [Google Scholar]
- Simoni D.; Gebbia N.; Invidiata F. P.; Eleopra M.; Marchetti P.; Rondanin R.; Baruchello R.; Provera S.; Marchioro C.; Tolomeo M.; Marinelli L.; Limongelli V.; Novellino E.; Kwaasi A.; Dunford J.; Buccheri S.; Caccamo N.; Dieli F. Design, synthesis, and biological evaluation of novel aminobisphosphonates possessing an in vivo antitumor activity through a γδ-T lymphocytes-mediated activation mechanism. J. Med. Chem. 2008, 51, 6800–6807. [DOI] [PubMed] [Google Scholar]
- Tarshis L. C.; Proteau P. J.; Kellogg B. A.; Sacchettini J. C.; Poulter C. D. Regulation of product chain length by isoprenyl diphosphate synthases. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 15018–15023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H.; Sarikonda G.; Puan K. J.; Tanaka Y.; Feng J.; Giner J. L.; Cao R.; Monkkonen J.; Oldfield E.; Morita C. T. Indirect stimulation of human Vγ2Vδ2 T cells through alterations in isoprenoid metabolism. J. Immunol. 2011, 187, 5099–5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondeau J. M.; Bitsch F.; Bourgier E.; Geiser M.; Hemmig R.; Kroemer M.; Lehmann S.; Ramage P.; Rieffel S.; Strauss A.; Green J. R.; Jahnke W. Structural basis for the exceptional in vivo efficacy of bisphosphonate drugs. ChemMedChem 2006, 1, 267–273. [DOI] [PubMed] [Google Scholar]
- Kavanagh K. L.; Guo K.; Dunford J. E.; Wu X.; Knapp S.; Ebetino F. H.; Rogers M. J.; Russell R. G.; Oppermann U. The molecular mechanism of nitrogen-containing bisphosphonates as antiosteoporosis drugs. Proc. Natl. Acad. Sci. U.S.A. 2006, 103, 7829–7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J.; Mukherjee S.; Zhang Y.; Cao R.; Sanders J. M.; Song Y.; Meints G. A.; Gao Y. G.; Mukkamala D.; Hudock M. P.; Oldfield E. Solid-state NMR, crystallographic, and computational investigation of bisphosphonates and farnesyl diphosphate synthase-bisphosphonate complexes. J. Am. Chem. Soc. 2006, 128, 14485–14497. [DOI] [PubMed] [Google Scholar]
- Mukkamala D.; No J. H.; Cass L. M.; Chang T. K.; Oldfield E. Bisphosphonate inhibition of a Plasmodium farnesyl diphosphate synthase and a general method for predicting cell-based activity from enzyme data. J. Med. Chem. 2008, 51, 7827–7833. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.