Abstract
We report a localized surface plasmon enhanced upconversion luminescence in Au/SiO2/Y2O3:Yb3+,Er3+ nanoparticles when excited at 980 nm. By adjusting the silica spacer's thickness, a maximum 9.59-fold enhancement of the green emission was obtained. Effect of the spacer distance on the Au-Y2O3:Yb3+, Er3+ green upconversion mechanism was numerically simulated and experimentally demonstrated. In theory for radiative decay and excitation rates, they can be largely enhanced at the spacer thicknesses of less than 70 and 75 nm, respectively, and the quenching can be caused by the non-radiative energy transferring at the distance of less than 55 nm.
Keywords: Plasmon coupling, Core/spacer/shell, Rare earth, Luminescence, Decay trace.
Introduction
Fluorescence imaging using nanoparticles could be a powerful tool for both biological studies and clinical medical applications due to its potential of convenience, high resolution and high sensitivity 1. Conventional biolabels for imaging mainly include organic dyes and quantum dots (QD). Unfortunately, organic dyes are normally vulnerable to chemical and metabolic degradation, limiting the long-term cell tracking in experiments. On the other hand, possible toxicity of residual QDs has been a wide and persistent concern, even though much work has been underway to synthesize less harmful QDs or to enhance their biocompatibility 2. Furthermore, excitation of these traditional biolabels usually requires the use of high energy photons such as UV radiation, which actually results in a series of drawbacks: (i) low signal-to-noise ratio due to the significant auto-fluorescence from biological samples under UV irradiation, (ii) low penetration depth due to the considerable absorption and scattering effects for short wavelength photons, and (iii) possible severe photo-damage on cells and even the cell death further caused by the long-term irradiation of such high energy photons 3. Therefore, it is desirable to obtain new fluorescent biolabels that can be excited by lower energy infrared (IR) light, which will be much safer to human body and can penetrate into tissues as far as several inches. Upconversion luminescent nanoparticles, which can convert long wavelength radiation into short wavelength fluorescence via a two- or multi-photon mechanism, are emerging as a new class of fluorescent biolabels 4-7. Unfortunately, this upconversion process in general associates with low efficiency.
Past researches have been extensively focused on enhancing upconversion luminescence by using metallic nanoparticles or nanostructures 8-12. In 2009, Yan et al obtained 2.3 and 3.7-fold enhancement for green and red emissions in NaYF4:Yb3+, Er3+ nanocrystals by attaching them to Ag nanowires 13. In 2010, Benson et al. demonstrated a 3.8-fold upconversion enhancement by catching a single NaYF4:Yb3+, Er3+ nanocrystal adjacent to an Au nanoparticle 14. Zhang et al. showed that the enhancement factor of approximately 2.6 can be achieved by attaching NaYF4: Yb3+,Tm3+ hexaplate nanocrystals to gold nanoparticles 15, and a 4-fold upconversion enhancement was also found in Ag/Y2O3:Er3+ nanoparticles 16. Most impressively, a five-fold overall enhancement of upconversion emission was demonstrated in NaYF4: Yb3+, Er3+ nanocrystals when coupled them with gold island films 17. However, all previously reported enhancement factors were less than 5-fold, and in many cases, quenching was unavoidable 18, 19, mainly due to the following reasons: (1) frequency mismatching between the localized plasmon resonance (usually determined by the used metal, their shape, size, the dielectric environment, and the spacer distance) and the used emission/excitation light, (2) a competition of a few processes including a increase of the excitation rate by the local field enhancement (LFE) 20, an enhancement of radiative decay rate by the surface plasmon-coupled emission (SPCE) and quenching that reduces the efficiency caused by the non-radiative energy transferring (NRET) from the upconversion material to the metal surfaces 21,22, all of which will be greatly dependent on the spacing distance between the upconversion material and the metal 23-26.
In this research, we have adopted a series of strategies with a goal to further enhance the upconversion luminescence: 1) instead of using Ag in many previous researches, we selected Au nanoparticle, considering a better overlapping between Au's intrinsic plasmon resonance and the visible emission; 2) the most widely used sensitizer Yb3+ was co-doped with the activator Er3+, first-timely in Y2O3 as shell to cap onto the SiO2 spacer and the Au core in the Au/SiO2/Y2O3:Yb3+,Er3+ (core/spacer/shell) nanostructure to be synthesized in this work. This was intended to increase the absorption cross section of Er3+ ions around 980nm; 3) the SiO2 spacer thickness between Au and Y2O3:Yb3+, Er3+ was intentionally changed with a goal to optimize the core/spacer/shell structure, and also to compare with the previous results 16. In our results, a maximum 9.59-fold enhancement of green emission was obtained in ~30 nm Au/SiO2/Y2O3:Yb3+,Er3+ nanoparticles with an optimized distance, and the distance's effect on the excitation rate, the radiative decay rate and the NRET were also simulated using a 3D-comsol in details, which would help us to further understand the enhancement process.
Experimental and characterizations
Synthesis of gold nanoparticles
Gold Nanoparticles were prepared using the method introduced by Frens 27. A mean diameter of 30 nm Au nanoparticles were prepared by rapidly injecting a sodium citrate solution (1.3 mL, 38.8mM) into a boiling HAuCl4 aqueous solution (100 mL, 0.3 mM) under vigorous stirring. After refluxing for 15 min., product was cooled to room temperature.
Synthesis of Au/SiO2 nanoparticles
By a modified Stöber method 28, the Au/SiO2 nanoparticles were synthesized. The freshly prepared gold colloid solution (10 mL) was added to isopropanol (40 mL) in a 100 mL conical flask. Under vigorous stirring, ammonia (2 mL, 28~30%) and tetraethoxysilane (TEOS) were subsequently added until a homogeneous solution was formed. After stirring for 8h, the product was separated by centrifugation at 6000 rpm for 15 min. and washed with water until pH=7.
Synthesis of Au/SiO2/Y2O3:20at%Yb3+,2at%Er3+ (20/2%) nanoparticles
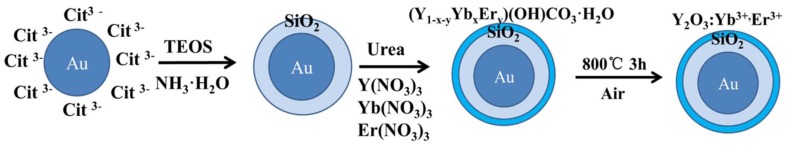
The final product was synthesized via homogeneous precipitation and calcination 29. The as-prepared 0.05g Au/SiO2 nanoparticle was dissolved in 100 mL deionized water. After ultrasoniced for 30 min., 1.78 mL (0.1M) Y(NO3)3, 0.20 mL (0.1M) Yb(NO3)3 , 0.02 mL (0.1M) Er(NO3)3 solutions and 2.7g urea were subsequently added to the solution, then stirring 30 min. until uniform dispersion. The solution was heated to 80℃ for 5h with continuous stirring, then isolated by centrifugation and washed three times with water and ethanol. The final product was obtained after dried under vacuum at 80℃ for 12h, and calcined for 3h at 800℃. Figure 1 shows the detailed synthesis procedure of the Au/SiO2/Y2O3:Yb3+, Er3+ nanoparticles.
Figure 1.
Synthesis procedure of the Au/SiO2/Y2O3:Yb3+,Er3+ nanoparticles.
Synthesis of Y2O3:Yb3+, Er3+ (20/2%) hollow nanoparticles
The Y2O3:Yb3+, Er3+ hollow nanoshells were synthesized by using NaOH solution (5M) to etch silica in the SiO2/Y2O3: Yb3+, Er3+ nanoparticles.
Characterization of physical properties
The sizes and morphologies of all samples were observed by JEOL JEM-2011 transmission electron microscope (TEM) under a working voltage of 200 kV, as well as the EDX spectrum. The phase composition and crystallinity of the samples were characterized by XRD (Philips x'pert diffractometer employing Cu Kα radiation (λ=1.5405Å). Ultraviolet-visible-near infrared (UV-Visible-NIR) absorption spectra were recorded with a Shimadzu SolidSpec-3700.
The upconversion emission spectra were recorded by use of Zeiss CLSM 710 confocal microscopy with a 100x plan-Apochromat objective (1.4 oil) equipped with a 980nm laser. All Au/SiO2/Y2O3:Yb3+, Er3+ samples with different spacer thicknesses were prepared in the same concentration in ethanol solution. We then spun 20μL from the bulk solution on a cover glass substrate, in this effort we could suggest all the samples have the same concentration. The upconversion luminescence data of such nanoparticles was analyzed as follows: Firstly, we randomly choose a luminescent area of 84.85μm×84.85μm from the full image of total upconversion luminescence. Secondly, we calculated the emission intensity per μm2 from the selected area. This process was repeated a few times on different selected area positions, and then we average the emission intensity with the repeated times. Finally, this averaged emission intensity per μm2 was multiplied with the superficial area of a single core/spacer/shell nanoparticle, and then we obtained the approximate upconversion emission from a single nanoparticle. This entire averaging process is statistically meaningful, and is necessary in our work because the setup we used cannot distinguish a single nanoparticle.
Results and discussion
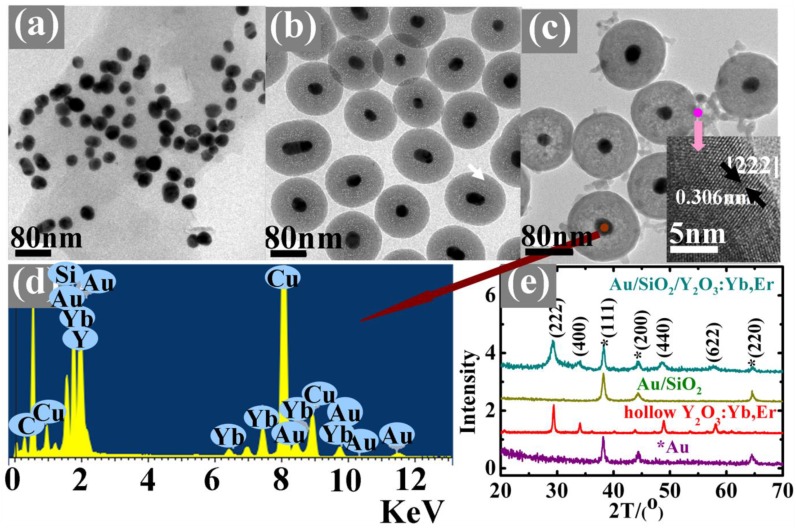
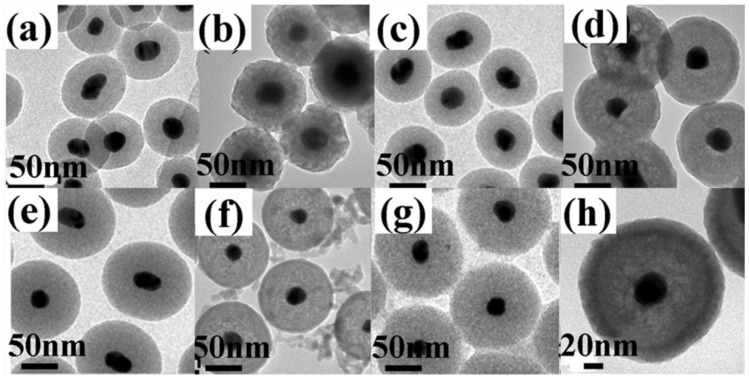
The nanoparticles were characterized by TEM, EDX, XRD and HRTEM, as shown in Figure 2. Figure 2(a) shows a typical TEM image of the samples in which well-dispersed Au nanoparticles with an average size of 30 nm can be clearly seen. After the surface modification with a ~40nm thick SiO2 dielectric spacer, the Au/SiO2 becomes more uniform than before and highly mono-dispersed (Figure 2(b)). Then, a layer of ~12nm Y2O3: Yb3+,Er3+was coated on the Au/SiO2 surface (Figure 2(c)), which can be demonstrated by the energy-dispersive X-ray microanalysis (EDX, Figure 2(d)) and X-ray diffraction (XRD, Figure 2(e)) patterns. A HRTEM image of Y2O3:Yb3+,Er3+ layer indicates the adjacent lattice fringes distance of 0.306nm, which can be assigned to the {222} crystal plane of the Y2O3 bcc phase (the insert of Figure 2(c)). The XRD patterns show well-defined peaks, indicating the high crystallinity of the as-prepared nanoparticles. Except the hollow Y2O3:Yb3+, Er3+ (Figure 4(a)), all of the patterns show Au characteristic peaks with a fcc structure according to the PDF 04-0784 [Fm-3m (225)]. Because of the amorphous nature of the SiO2 layer, there are no corresponding diffraction peaks. The Au/SiO2/Y2O3:Yb3+, Er3+ shows Y2O3 characteristic diffraction peaks indicating a cubic structure with a space group Ia-3(206) (PDF 01-0831), and the similar diffraction peaks also exist in the hollow Y2O3:Yb3+, Er3+ patterns. Besides, TEM images of all the ~30nm Au/SiO2 and ~30nm Au/SiO2/Y2O3:Yb3+, Er3+ nanostructures with different silica thicknesses were shown in Figure 3, which indicated that the thickness of Y2O3 shells are about 12 nm in all the samples.
Figure 2.
TEM images of (a) ~30nm Au (b) ~30nm Au/~40nmSiO2 (c) ~40nmAu/~40nmSiO2/~10nmY2O3:Yb3+,Er3+ (the insert is HRTEM image of lattice fringes of the Y2O3:Yb3+, Er3+ layer) (d) EDX in a selected area of Au core and (e) XRD patterns of discussed samples (* represents Au diffraction peaks).
Figure 4.
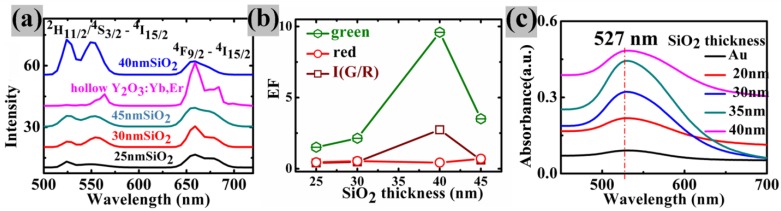
(a) The upconversion spectra in Au/SiO2/Y2O3:Yb3+,Er3+ nanoparticles with different SiO2 thickness and the hollow Y2O3:Yb, Er (b) the plots of EFgreen , EFred and I(G/R) verse silica thickness (the integral of green emission intensity from 500nm to 560nm, the integral of red emission intensity from 650nm to 750nm); (c) UV-VIS absorption spectra of all the discussed samples.
Figure 3.
TEM images of (a) ~30nmAu/~25nmSiO2; (b) ~30nmAu/~25nmSiO2/~12nmY2O3:Yb3+, Er3+; (c) ~30nmAu/~30nmSiO2; (d) ~30nmAu/~30nmSiO2/~12nmY2O3:Yb3+,Er3+; (e) ~30nmAu/~40nmSiO2; (f) ~30nmAu/~40nmSiO2/~12nmY2O3:Yb3+,Er3+; (g) ~30nmAu/~45nmSiO2; and (h) ~30nmAu/~45nmSiO2/~12nmY2O3:Yb3+,Er3+.
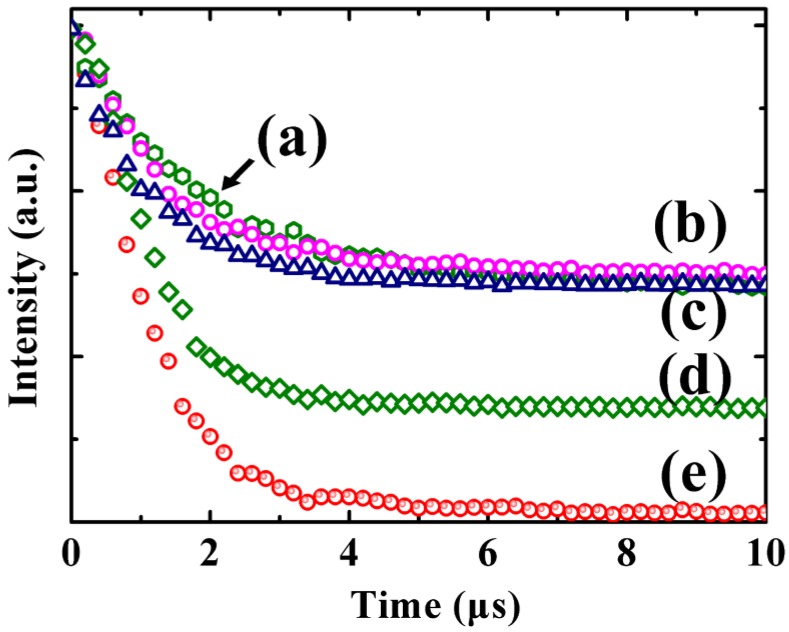
In order to investigate the distance-dependence of metal-enhanced upconversion luminescence in Au/SiO2/Y2O3:Yb3+,Er3+nanoparticles with different SiO2 thicknesses. The samples were measured by the Zeiss LCSM 710 confocal microscopy with a 100× plan-Apochromat objective (1.4oil) equipped with a 980nm laser. There are two main characteristic emission bands observed in Figure 4(a), corresponding to 2H11/2/4S3/2-4I15/2 (green emission at ~524nm, ~549 nm) and 4F9/2 -4I15/2 (red emission at ~665 nm) radiative transitions, respectively. Here, we defined a parameter EFgreen (enhancement factor for green emission) and EFred (enhancement factor for red emission) by dividing integrated fluorescence intensities of Au/SiO2/Y2O3:Yb3+, Er3+ nanoparticles by those of hollow Y2O3:Yb3+, Er3+ nanoshells in the range of 500-560nm and 650-750 nm, respectively. In contrast to hollow Y2O3:Yb3+, Er3+nanoparticles, the green emission intensity of all core/spacer/shell nanoparticles is enhanced (EFGreen>1) (Figure 4(b)) and the corresponding red emissions of all samples are quenched (EFred<1), which is attributed to the surface plasmon resonance frequency of Au nanoparticles having strong overlap with green emission band (Figure 4(c)), that is, an increase in emission rate by surface plasmon coupled emission (SPCE). SPCE is characterized by the increase of upconversion intensity accompanied by a decrease in the lifetime of upconversion nanoparticles located in the proximity of the metallic nanostructures. Therefore, decay curves of samples with and without Au core (Y2O3:Yb3+, Er3+ hollow shell) have been also carried out. As shown in Figure 5, Au/SiO2/Y2O3:Yb3+, Er3+ nanoparticles exhibit shorter lifetime compared with the corresponding Y2O3:Yb3+, Er3+ hollow shell, which supply direct proof for the conclusion that the enhancement originates from the increased emission rate. With the increase of SiO2 thickness, EFGreen increased and reached the maximum 9.59 when SiO2 thickness is ~40 nm, and the ratio of green to red emission I(G/R) tracks well with the EFGreen across the range of SiO2 spacers, demonstrating a preferential enhancement of the green emission band. As mentioned in the introduction, the metal-enhanced/quenching fluorescence resulted from a competition of processes including an increase of excitation rate by LFE 20, an enhancement of radiative decay rate by surface plasmon-coupled emission (SPCE) and the quenching caused by NRET from upconversion materials to metal surfaces 21, 22. Near the metal surface, the upconversion intensity fall rapidly as the NRET from the emitters to the metal occurred. With the increase of the distance, SPCE and LFE became dominant and the EF reached the maximum at about 40 nm. Because the enhancement of emission and excitation rate cause by SPCE and LFE is proportion to the square of localized electric field, and the electric field decreased with the distance increase, the upconversion luminescence became weaker beyond the 40 nm distance.
Figure 5.
Decay curves for (a) Y2O3:Yb3+,Er3+ hollow shells; (b)Au/~45nmSiO2/Y2O3:Yb3+,Er3+; (c)Au/~40nmSiO2/Y2O3:Yb3+,Er3+; (d)Au/~30nmSiO2/Y2O3:Yb3+,Er3+ and (e)Au/~25nmSiO2/Y2O3:Yb3+,Er3+ nanoparticles.
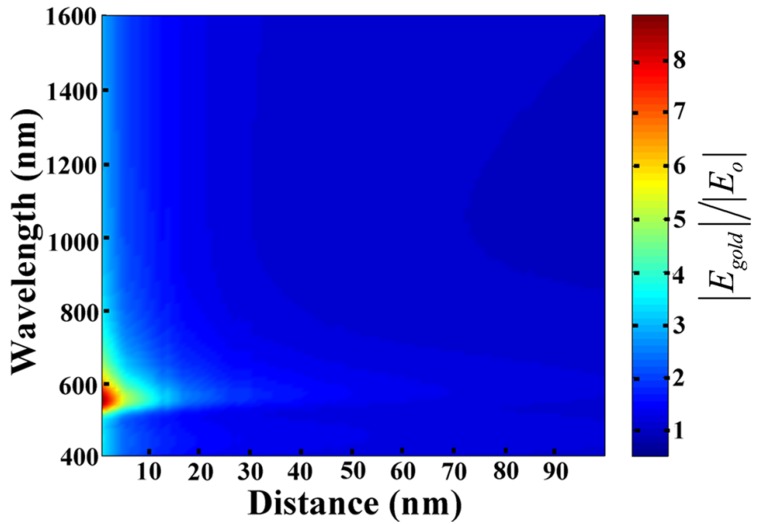
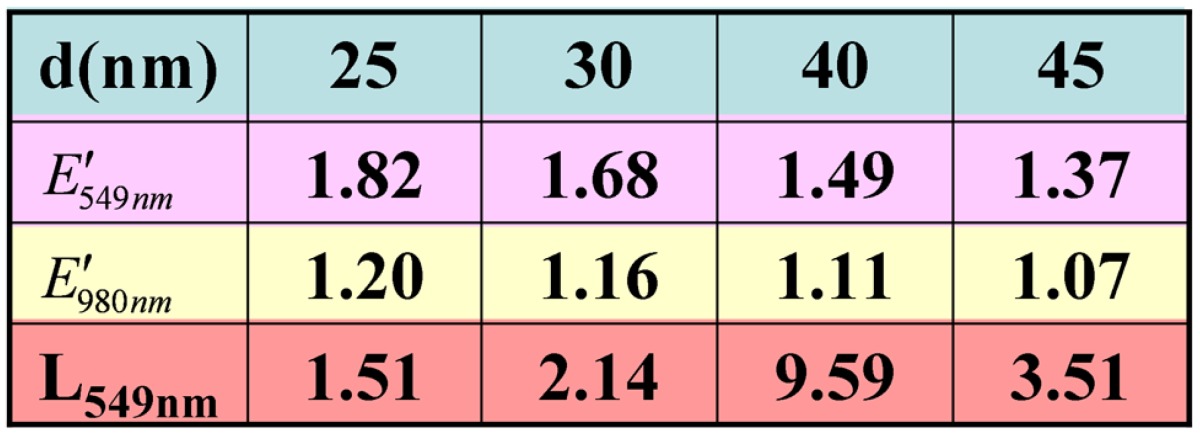
To understand deeply the distance-dependence of upconversion luminescence in Au/SiO2/Y2O3:Yb3+, Er3+ nanoparticles, the electric field enhancement factor E′=|Egold|/|Eo|, where Egold and Eo were the electric field with and without gold, was calculated by 3D-comsol, as presented in Figure 6. We studied a very general case by 3D-comsol: the Au nanoparticles were imbedded in a homogeneous, lossless SiO2 medium, supposing the Au nanosphere is small as compared to the light wavelength. In Figure 6, d is the distance to the surface of Au nanospheres. The wavelength-dependence of electric field is similar with the measured absorption spectra of Au nanoparticles (see Figure 4(c)), and the greatest electric field strength was found to 8.9 at 525 nm. With the increase of d, the electric field falls rapidly, and near to unity at d=90 nm. The detailed unit-less parameters: the electric field enhancement factor at 549 nm and 980 nm (E′549nm and E′980nm), and the overall enhancement factor of emission intensity at 549 nm, L549nm, are listed in Table 1.
Figure 6.
Electric field enhancement (E) dependence as a function of the wavelength and distance to a ~30nm diameter Au nanoparticle surface.
Table 1.
The detailed list of the electric filed enhancement factor at 549 nm and 980 nm (E549nm and E980nm) and enhancement factor at 549 nm (L549nm) for the Au/SiO2/Y2O3:Yb,Er nanoparticle.
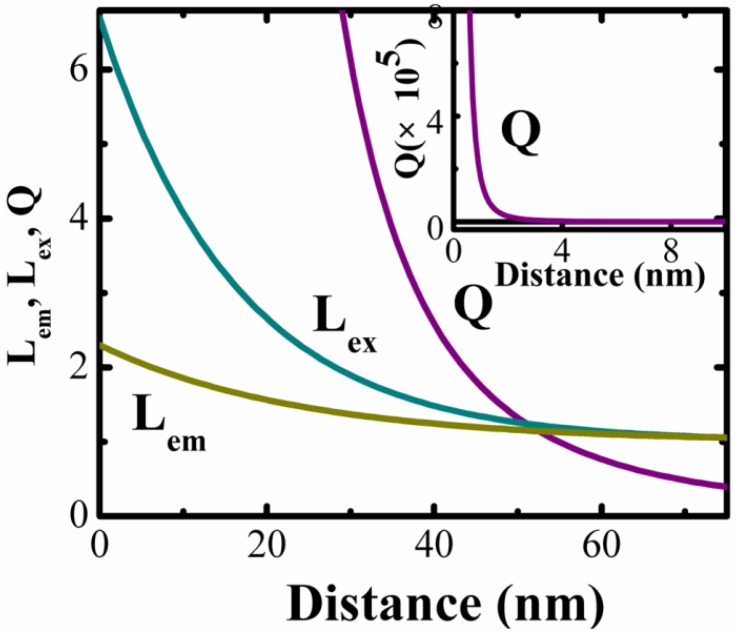
The enhancement factor in the excitation (Lex) and emission (Lem) rates as a funtion of the distance between Au and Y2O3:Yb3+,Er3+ (d) could be expressed as 30:
| Lex(d) = Lex(d=0) exp(-d/Rex) + 1 | (1) |
| Lem(d) = Lem(d=0) exp(-d/Rem) + 1 | (2) |
Rem and Rex is the characteristic distance over which Lem(d=0) and Lex(d=0) decrease to 1/e exponentially, respectively.
The enhancement factor of excitation and emission rate by surface-plasmon-coupled emission is also proportional to the square of corresponding electric field enhancement 26. Hereby, we analyzed our experimental upconversion intensity at 549 nm at various distance from the plasoninc metal-nanostructured surface. By fitting the experimental data with equation 1 and equation 2, the values of the free parameters Lem(d=0)=5.7, Lex(d=0)=1.3, Rem=16.2 nm and Rex=23.9 nm were obtained. Near the metal surface, the fluorescence intensity fall rapidly due to non-radiative energy transfer from the fluorescence materials to the metal surface. A complete, classical theory of NRET has been published by Campion et al.. When d≤λ, the expression of the unitless parameter quenching factor (Q) caused by NRET is as follows 31:
| Q = ωET/ωR = {ωR×Ιm[(ε2-ε1)/(ε2+ε1)]×λ3} / [8π3×(ε1)3/2×4d3×ωR] = {Ιm[(ε2-ε1)/(ε2+ε1)]×λ3} / [32π3×(ε1)3/2×d3] | (3) |
Where ωET is nonradiative rate, ωR is the radiative rate constant, λ is the emission wavelength; ε1(549nm)=1.46, is the dielectric constant of the spacer layer, and ε2(549nm)=0.306+2.88i, is the complex dielectric constant of the metal 32.
The enhancement of excitation and radiative decay rate (Lex, Lem) and the quenching factor by NRET (Q) are plotted as a function of Au-Y2O3 nanoparticle distance, as shown in Figure 7. It is apparent from distance-dependent plot that the radiative decay rate could be enhanced even at a distance of ~0 nm, and the enhanced excitation takes place below 75 nm. The quenching factor by NRET (Q) decreased rapidly as the distance increased, and near to Lemand Lex at a distance of 55 nm.
Figure 7.
Changing of excitation rate Lex(S-1) and radiative decay rate Lem(S-1), and the quenching factor by NRET (Q), as a function of the spacing distance from the metal surface.
Conclusions
In conclusion, plasmon-enhanced upconversion luminescence was observed in Au/SiO2/Y2O3: Yb3+,Er3+ nanoparticles. With the increase of silica dielectric thickness, the green emission intensity increased and reached a maximum 9.59-fold enhancement when silica thickness is ~40 nm, which attributed to the competition of non-radiative energy transfer (NRET) from Y2O3:Yb3+, Er3+ to Au core, enhancement of radiative decay rate by surface plasmon-coupled emission (SPCE) and an increase of excitation rate by local field enhancement (LFE). The distance's effect on the enhancement factor of radiative decay and excitation rate and quenching factor by NRET was simulated. The radiative decay rate could be enhanced even at a distance of 70 nm, and the enhanced excitation rare takes place below 75 nm. The quenching factor by NRET (Q) decreased rapidly with the distance increase, and is near to Lem and Lexat a distance of 55 nm.
Acknowledgments
This work was supported by the National Basic Research Program of China (973 Program, 2012CB922003), the Natural Science Foundation of China (51102224), the Fundamental Research Fund for the Central Universities (WK2060140006, WK2060140005), and China Postdoctoral Science Foundation (BH2060140010).
References
- 1.Grunwald D, Singer R. In vivo imaging of labelled endogenous beta-actin mRNA during nucleocytoplasmic transport. Nature. 2010;467:604–607. doi: 10.1038/nature09438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Derfus A, Chan W, Bhatia S. Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 2004;4:11–18. doi: 10.1021/nl0347334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jalil R, Zhang Y. Biocompatibility of silica coated NaYF4 upconversion fluorescent nanocrystals. Biomaterials. 2008;29:4122–8. doi: 10.1016/j.biomaterials.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Han Y, Lim CS, Lu Y, Wang J, Xu J, Chen H, Zhang C, Hong M, Liu X. Simultaneous phase and size control of upconversion nanocrystals through lanthanide doping. Nature. 2010;463:1061–5. doi: 10.1038/nature08777. [DOI] [PubMed] [Google Scholar]
- 5.Chen GY, Ohulchanskyy TY, Liu S, Law W, Wu F, Swihart MT. Core/shell NaGdF4: Nd3+/NaGdF4 nanocrystals with efficient near-infrared to near-infrared downconversion photoluminescence for bioimaging applications. ACS Nano. 2012;6:2969–77. doi: 10.1021/nn2042362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen GY, Ohulchanskyy TY, Kachynski A, Agren H, Prasad PN. Intense visible and near-infrared upconversion photoluminescence in colloidal LiYF4:Er3+ nanocrystals under excitation at 1490 nm. ACS Nano. 2011;5:4981–6. doi: 10.1021/nn201083j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang C, Cheng L, Xu H, Liu Z. Towards whole-body imaging at the single cell level using ultra-sensitive stem cell labeling with oligo-arginine modified upconversion nanoparticles. Biomaterials. 2012;33:4872–81. doi: 10.1016/j.biomaterials.2012.03.047. [DOI] [PubMed] [Google Scholar]
- 8.Aisaka T, Fujii M, Hayashi S. Enhancement of upconversion luminescence of Er doped Al2O3 films by Ag island films. Appl. Phys. Lett. 2008;92:132105–7. [Google Scholar]
- 9.Esteban R, Laroche M, Greffet JJ. Influence of metallic nanoparticles on upconversion processes. J.Appl. Phys. 2009;105:033107–16. [Google Scholar]
- 10.Ming T, Zhao L, Yang Z, Chen H, Sun LD, Wang J, Yan CH. Strong polarization dependence of plasmon-enhanced fluorescence on single gold nanorods. Nano Lett. 2009;9:3896–3903. doi: 10.1021/nl902095q. [DOI] [PubMed] [Google Scholar]
- 11.Priyam A, Idris NM, Zhang Y. Gold nanoshell coated NaYF4 nanoparticles for simultaneously enhanced upconversion fluorescence and darkfield imaging. J. Mater. Chem. 2012;22:960–5. [Google Scholar]
- 12.Lu Y, Chen X. Plasmon-enhanced luminescence in Yb3+: Y2O3 thin film and the potential for solar cell photon harvesting. Appl. Phys. Lett. 2009;94:193110–2. [Google Scholar]
- 13.Feng W, Sun LD, Yan CH. Ag nanowires enhanced upconversion emission of NaYF4:Yb,Er nanocrystals via a direct assembly method. Chem.Commun. 2009;29:4393–5. doi: 10.1039/b909164e. [DOI] [PubMed] [Google Scholar]
- 14.Schietinger S, Aichele T, Wang H, Nann T, Benson O. Plasmon-enhanced upconversion in single NaYF4:Yb3+/Er3+ Codoped Nanocrystals. Nano Lett. 2010;10:134–8. doi: 10.1021/nl903046r. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H, Li Y, Ivanov IA, Qu Y, Huang Y, Duan X. Plasmonic modulation of the upconversion fluorescence inNaYF4:Yb/Tm hexaplate nanocrystals using gold nanoparticles or nanoshells. Angew. Chem. Int. Ed. 2010;49:2865–8. doi: 10.1002/anie.200905805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang F, Braun GB, Shi Y, Zhang Y, Sun X, Reich NO, Zhao D, Stucky G. Fabrication of Ag@SiO2@Y2O3:Er nanostructures for bioimaging: tuning of the upconversion fluorescence with silver nanoparticles. J. Am. Chem. Soc. 2010;132:2850–1. doi: 10.1021/ja909108x. [DOI] [PubMed] [Google Scholar]
- 17.Zhang H, Xu D, Huang Y, Duan X. Highly spectral dependent enhancement of upconversion emission with sputtered gold island films. Chem. Commun. 2011;47:979–81. doi: 10.1039/c0cc03566a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Boyer JC, Cuccia LA, Capobianco JA. Synthesis of colloidal upconverting NaYF4: Er3+/Yb3+ and Tm3+/Yb3+ monodisperse nanocrystals. Nano. Lett. 2007;7:847–52. doi: 10.1021/nl070235+. [DOI] [PubMed] [Google Scholar]
- 19.Vetrone F, Naccache R, Juarranz A, Fuente A, Rodriguez FS, Maestro LM, Rodriguez EM, Jaque D, Sole JG. Capobianco JA, Temperature sensing using fluorescent nanothermometers. ACS Nano. 2010;4:3254–8. doi: 10.1021/nn100244a. [DOI] [PubMed] [Google Scholar]
- 20.Lakowicz JR. Radiative decay engineering: biophysical and biomedical applications. Anal. BioChem. 2001;298:1–24. doi: 10.1006/abio.2001.5377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gryczynski I, Malicka J, Gryczyski Z, Lakowicz JR. Radiative decay engineering 4: experimental studies of surface plasmon-coupled directional emission. Anal. BioChem. 2004;324:170–182. doi: 10.1016/j.ab.2003.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Campion A, Gallo AR, Harris CB, Robota HJ, Whitmore PM. Electronic-energy transfer to metal-surfaces-a test of classical image dipole theory at short distances. Chem. Phys. Lett. 1980;73:447–50. [Google Scholar]
- 23.Guillermo PA, Martina B, Ingo HS, Christian S, Anton K, Phil H, Robert S, Alexander M, Fernando DS, Tim L, Friedrich CS, Philip T. Distance dependence of single-fluorophore quenching by gold nanoparticles studied on DNA origami. ACS Nano. 2012;6:3189–95. doi: 10.1021/nn2050483. [DOI] [PubMed] [Google Scholar]
- 24.Schneider G, Decher G. Distance-dependent fluorescence quenching on gold nanoparticles ensheathed with layer-by-layer assembled polyelectrolytes. Nano Lett. 2006;6:530–6. doi: 10.1021/nl052441s. [DOI] [PubMed] [Google Scholar]
- 25.Akbay N, Lakowicz JR, Ray K. Distance-dependent metal-enhanced intrinsic fluorescence of proteins using polyelectrolyte layer-by-layer assembly and aluminum nanoparticles. J. Phys. Chem. C. 2012;116:10766–73. doi: 10.1021/jp2122714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kümmerlen J, Leitner A, Brunner H, Aussenegg FR, Wokaun A. Enhanced dye fluorescence over silver island films: analysis of the distance dependence. Mol. Phys. 1993;80:1031–46. [Google Scholar]
- 27.Frens G. Controlled nucleation for regulation pf particle-size in monodisperse gold suspensions. Nature. 1973;241:20–22. [Google Scholar]
- 28.Lu Y, Yin YD, Li ZY, Xia YA. Synthesis and self-assembly of Au@SiO2 core-shell colloids. Nano Lett. 2002;2:785–788. [Google Scholar]
- 29.Rosa ILV, Oliveira LH, Suzuki CK, Varela JA, Leite ER, Longo E. SiO2-GeO2 soot preform as a core for Eu2O3 nanocoating: synthesis and photophysical study. J Fluoresc. 2008;18:541–545. doi: 10.1007/s10895-007-0297-7. [DOI] [PubMed] [Google Scholar]
- 30.Akbay N, Lakowicz JR, Ray K. Distance-dependent metal-enhanced intrinsic fluorescence of proteins using polyelectrolyte layer-by-layer assembly and aluminum nanoparticles. J. Phys. Chem. C. 2012;116:10766–73. doi: 10.1021/jp2122714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Campion A, Gallo AR, Harris CB, Robota HJ, Whitemore PM. Electronic energy transfer to metal surfaces: a test of classical image dipole theory at short distances. Chem. Phys. Lett. 1980;73:447–9. [Google Scholar]
- 32.Lynch DW, Hunter WR. Handbook of Optical Constants of Solids. New York: Academic Press; 1985. pp. 274–760. [Google Scholar]