Abstract
Coatomer is required for the retrieval of proteins from an early Golgi compartment back to the endoplasmic reticulum. The WD40 domain of α-COP is required for the recruitment of KKTN-tagged proteins into coatomer-coated vesicles. However, lack of the domain has only minor effects on growth in yeast. Here, we show that the WD40 domain of β′-COP is required for the recycling of the KTKLL-tagged Golgi protein Emp47p. The protein is degraded more rapidly in cells with a point mutation in the WD40 domain of β′-COP (sec27-95) or in cells lacking the domain altogether, whereas a point mutation in the Clathrin Heavy Chain Repeat (sec27-1) does not affect the turnover of Emp47p. Lack of the WD40 domain of β′-COP has only minor effects on growth of yeast cells; however, absence of both WD40 domains of α- and β′-COP is lethal. Two hybrid studies together with our analysis of the maturation of KKTN-tagged invertase and the turnover of Emp47p in α- and β′-COP mutants suggest that the two WD40 domains of α- and β′-COP bind distinct but overlapping sets of di-lysine signals and hence both contribute to recycling of proteins with di-lysine signals.
INTRODUCTION
COP I coat proteins mediate an essential and conserved retrieval pathway that continually recycles several classes of proteins, and lipids, from the cis-Golgi back to the endoplasmic reticulum (ER) (Letourneur et al., 1994; Gaynor et al., 1998; Lanoix et al., 1999). Additionally, the COP I coat functions in retrograde transport and perhaps also in anterograde transport, within the Golgi complex (Orci et al., 1997; Pelham and Rothman, 2000). The minimal machinery for the budding of COP I-coated vesicles consists of coatomer, a stable cytosolic complex comprising seven equimolar subunits, α-, β-, β′-, γ-, δ-, ε-, and ζ-COP, and the small GTPase ARF in its GTP-bound form (Rothman and Wieland, 1996; Spang et al., 1998). Binding of ARF-GTP to Golgi membranes leads to recruitment of coatomer, deformation of the membrane, and budding of COP I-coated vesicles. GTP hydrolysis by ARF is a prerequisite for vesicle uncoating (Rothman and Wieland, 1996). The GTP/GDP cycle of ARF proteins is regulated by guanine nucleotide exchange factors and GTPase-activating proteins (GAPs) (Donaldson and Jackson, 2000).
Cargo transported in COP I vesicles in mammalian cells and yeast includes type I membrane proteins harboring a di-lysine trafficking signal, which functions only when the lysines are either in -3 and -4, or -3 and -5 positions from the carboxy terminus. This di-lysine motif is necessary and sufficient to target reporter proteins into the retrograde pathway in vivo, and coatomer can directly interact with such motifs in vitro (Jackson et al., 1993; Gaynor et al., 1994; Cosson and Letourneur, 1994; Schröder-Kohne et al., 1998). Using reporter proteins tagged with variants of di-lysine motifs, it has been previously demonstrated clearly that small changes in the local sequence context of the lysines can dramatically alter the signal strength of the di-lysine motif and thus generate a broad spectrum of trafficking phenotypes (Zerangue et al., 2001).
In this study, we analyze in detail interactions of coatomer with variants of the di-lysine motifs as present on two well characterized proteins in yeast: Wbp1p, a subunit of the ER-localized oligosaccharyl transferase complex (Gaynor et al., 1994); and Emp47p, a protein that achieves its steadystate Golgi localization by continuous recycling between the endoplasmic reticulum (ER) and the Golgi (Schröder et al., 1995; Schröder-Kohne et al., 1998). Emp47 and its close homologue Emp46p have been proposed to function as cargo receptors at the ER exit site (Sato and Nakano, 2002, 2003). Wbp1p harbors a classical di-lysine motif, KKTN, and Emp47p presents a variant di-lysine motif, KTKLL (Gaynor et al., 1994; Schröder et al., 1995). A genetic selection for yeast mutants with specific defects in trafficking dependent on the di-lysine KKTN motif, as present on Wbp1p, yielded mutations in α-, γ-, δ-, and ζ-COP, which provided strong evidence for a function of COP I in Golgi-to-ER retrieval (Letourneur et al., 1994). In particular, ret1-1, an α-COP mutant, displays a strong in vivo KKTN-retrieval defect even at permissive temperature, and coatomer from ret1-1 cells has lost the ability to interact with the KKTN-motif in vitro. Anterograde traffic is virtually unaffected in this mutant even upon shift to restrictive temperature (Letourneur et al., 1994). Significantly, the point mutations in four different alleles of ret1 identified in the selection, including ret1-1, cluster within 85 residues in or close to an amino-terminal domain containing WD40 repeats, providing strong genetic evidence for a role of this domain of α-COP in KKTN-binding (Letourneur et al., 1994; Schröder-Kohne et al., 1998). In vitro, an α-, β′-, ε-COP subcomplex strongly binds to the KKTN motif (Lowe and Kreis, 1995). Consistent with these data an artificial, very strong di-lysine signal, KKYL, isolated in a systematic screen for ER trafficking signals in mammalian cells was shown to bind to α-COP in the yeast two-hybrid assay (Zerangue et al., 2001).
These data suggested that the α-COP subunit of coatomer is the receptor for di-lysine motifs in both mammals and yeast. Intriguingly, however, it was found that the recycling of Emp47p, which harbors the variant motif KTKLL, is unaffected in all α-COP mutants that strongly mislocalize KKTN-tagged proteins. Thus, α-COP cannot be the only receptor for di-lysine tagged COP I cargo proteins in yeast (Schröder et al., 1995; Schröder-Kohne et al., 1998). Both photo-cross-linking data (Harter et al., 1996; Harter and Wieland, 1998) and in vitro binding data (Wu et al., 2000) have suggested that a binding site for di-lysine motifs is present on mammalian γ-COP. An equivalent binding site has so far not been characterized in the yeast γ-COP orthologue. Furthermore, it is unknown which subunit in coatomer is responsible for binding variant di-lysine motifs, e.g., KTKLL. Thus, the exact nature of the molecular interactions that lead to sorting of cargo proteins harboring di-lysine type motifs into COP I vesicles is still controversial.
Both α- and β′-COP harbor conserved WD40 domains of ∼285 residues in their amino-terminal regions. WD40 repeats are conserved sequence motifs of 44–60 residues with a GH dipeptide at the N terminus and a WD dipeptide near the C terminus of the repeat (Smith et al., 1999). WD40 domains are widespread recognition modules thought to link partner proteins in intracellular networks of signaling and sorting. X-ray crystallographic data on WD40 domain-containing proteins, e.g., the β-subunit of heterotrimeric G proteins, and several other proteins (Wall et al., 1995; Sondek et al., 1996; Beisel et al., 1999), suggest that WD40 domains generally fold into a β-propeller, a highly regular structure composed of β-strands and turns, capable of accommodating multiple ligands (Neer and Smith, 2000; ter Haar et al., 2000; Holm et al., 2001). The WD40 domains of α- and β′-COP comprise six and five WD40 repeats, respectively, and display high levels of sequence conservation across evolutionarily distant species (Duden et al., 1994). Furthermore, the sequences of α- and β′-COP WD40 domains are highly related to each other, suggesting a possible overlap in their functions. In this study, we revisited the issue of the di-lysine signal-binding sites on coatomer. Our data suggest that the WD40 domains of α- and β′-COP bind distinct but overlapping sets of di-lysine signals, and that both domains contribute to target cargo proteins harboring di-lysine motifs into COP I vesicles in vivo.
MATERIALS AND METHODS
Yeast Strains and Media
Yeast strains used are listed in Table 1. Yeast media were either rich medium (YP), consisting of 1% Bacto Yeast extract, 2% Bacto Peptone, and either 2% glucose (YP-Glu) or 2% galactose/2% glycerol (YP-Gal/Gly), or minimal medium, containing 0.7% yeast nitrogen base (Difco, Detroit, MI) supplemented with appropriate amino acids (Sigma-Aldrich, St. Louis, MO) and the same carbon sources.
Table 1.
Yeast strains used
| Strain | Genotype | Reference |
|---|---|---|
| EGY 48 | MATα, his3, trp1, ura3, leu2::pLEU2-lexAop3 | Gyuris et al., 1993 |
| EGY191 | MATα, his3, trp1, ura3, leu2::LEU2-LexAop1 | Gyuris et al., 1993 |
| PC70 | MATα, ret1-1, ura3, trp1, leu2 | Letourneur et al., 1994 |
| WPY153 | MATα, sec27-95, ura3, his3, leu2 | Prinz et al., 2000 |
| RDY146 | MATα, sec27-1, ura3, trp1, leu2 | Duden et al., 1994 |
| YPH500 | MATα, ura3, lys2, ade2, trp1, his3, leu2 | Sikorski and Hieter, 1989 |
| YAE2-1c | MATa, ura3, lys2, ade2, trp1, his3, leu2, ret1Δ::HIS3 + pCEN::RET1(CEN, URA3) | Eugster et al., 2000 |
| YAE6 | MATa, ura3, lys2, ade2, trp1, his3, leu2, ret1Δ::HIS3 + pCEN::ret1Δ1-285 (CEN, LEU2) | Eugster et al., 2000 |
| YAE7 | MATa, ura3, lys2, ade2, trp1, his3, leu2, ret1Δ::HIS3 + pCEN::RET1 (CEN, LEU2) | Eugster et al., 2000 |
| YAE8-13c | MATα, ura3, lys2, ade2, trp1, his3, leu2, sec27Δ::LEU2 + pCEN::SEC27 (CEN, URA3) | This study |
| YAE9-3c | MATα, ura3, lys2, ade2, trp1, his3, leu2, ret1Δ::HI3, sec27Δ::LEU2 + pCEN::ret1Δ1-285 (CEN, LEU2) + pCEN::SEC27 (CEN, URA3) | This study |
| YAE10 | MATα, ura3, lys2, ade2, trp1, his3, leu2, sec27Δ::LEU2 + pCEN::sec27Δ1-285 (CEN, TRP1) | This study |
| YAE11 | MATα, ura3, lys2, ade2, trp1, his3, leu2, sec27Δ::LEU2 + pCEN::SEC27 (CEN, TRP1) | This study |
| YAE13 | MATa/α, ura3, lys2, ade2, trp1, his3, leu2, RET1/ret1Δ::HI3, SEC27/sec27Δ::LEU2 + pCEN::ret1Δ1-285 (CEN, LEU2) + pCEN::sec27Δ1-285 (CEN, TRP1) | This study |
Fusion Constructs; Two-Hybrid Assays
The LexA two-hybrid system (Estojak et al., 1995; Golemis et al., 1996) was used as described previously (Eugster et al., 2000). Yeast reporter strains EGY48 (Table 1), as well as the bait plasmids pEG202 and the prey plasmids pJG4-5 were used (Golemis et al., 1996). Prey fusions were under the control of the GAL1 promoter. Fusion constructs were created by ligating PCR products made using custom primers into vectors mentioned above, generating in frame fusions with LexA (in the bait vector) or the “acid blob B42′ (in the prey vector).” Reporter yeast strain EGY48 (harboring URA3 plasmid pSH18-34; Golemis et al., 1996), was cotransformed with a bait and a prey fusion construct and transformants were selected on -HIS, -TRP, -URA plates. To assess growth on media lacking leucine, transformants were streaked onto plates lacking histidine, tryptophane, uracil, and leucine, containing 2% galactose/1% raffinose or 2% glucose, and incubated at 24 or 30°C for 3 or 4 d, respectively.
Immunoblotting, Radiolabeling, and Immunoprecipitation Antibodies
SDS-PAGE, immunoblot analysis by using enhanced chemiluminescence radiolabeling of cells, immunoprecipitation, and PhosphorImager quantitation were as described previously (Duden et al., 1994, 1998; Eugster et al., 2000). Rabbit antisera used were anti-coatomer, anti-α-COP, anti-β′-COP (Duden et al., 1994); anti-γ-COP (Hosobuchi et al., 1992); anti-ε-COP (Eugster et al., 2000); anti-Sed5p (this study); and anti-Emp47p (Schröder-Kohne et al., 1998). The α-COP fragment was specifically detected using an antiserum against a COOH-terminal α-COP peptide (residues 1155–1171 of Ret1p). Anti-carboxypeptidase Y (CPY), anti-invertase, anti-α-COP, and anti-Emp47p antisera were kindly provided by Drs. Randy Schekman (University of California, Berkeley, CA), Scott Emr (University of California, San Diego, CA), Pierre Cosson (University of Geneva, Switzerland), Francois Letourneur (Institut de Biologie et Chimie des Proteines, Lyon, France), and Stephan Schröder-Kohne (BioMedTec, Franken, Germany), respectively.
DNA Cloning, Sequencing, Computer Analysis, and Plasmids
Escherichia coli strain DH5α was used for plasmid isolation, and polymerase chain reaction (PCR) by using Vent DNA polymerase, restriction enzyme digests, and ligations were performed by standard methods. Database searches were performed using the BLAST and Ψ BLAST servers at National Institutes of Health. Multiple alignments were done with the program MEGALIGN by using the CLUSTAL method. Plasmids pEG1-KK and pEG1-QK encoding Inv.-Wbp1p fusion proteins harboring a KKTN or a nonfunctional QKTN motif (Gaynor et al., 1994) were used in pulse-chase experiments (Duden et al., 1998). For in vitro binding studies pGST-WBP1-KK and pGST-WBP1-SS (plasmids pFL67 and pFL68) encoding GST-fusions with the cytoplasmic tail of Wbp1p harboring either a KKTN or SSTN motif were used (Cosson and Letourneur, 1994), or KTKLL- or STSLL-GST fusions [i.e., harboring the wild-type Emp47p-tail (LIRQEIIKTKLL) or the corresponding double-serine mutant] (Schröder-Kohne et al., 1998). pCEN::sec27Δ1-285 was created by introducing two PCR-amplified fragments: a BstBI-BamHI fragment containing the SEC27 promoter and an artificial ATG, ending at the start ATG, and a BamHI-ApaI fragment starting at residue 285 into BstBI-ApaI cut pCEN::SEC27 (pRS314, TRP).
Strain Construction
A diploid SEC27/sec27Δ::LEU2 strain (Duden et al., 1994) was transformed with the pRS316::SEC27, (URA3, CEN) plasmid and subsequently dissected, to yield the haploid strain YAE8-13c. To construct the strain expressing a truncated Sec27p lacking the WD40 domain as the only form of β′-COP at near endogenous level, YAE8-13c was transformed with pCEN::sec27Δ1-285 and pCEN::SEC27 expressing the SEC27 protein under the control of the authentic SEC27 promoter from a TRP, CEN plasmid. Ura-, Trp+, Leu+ colonies were selected on SD 5-fluoroorotic acid (5-FOA) plates, yielding the strains YAE10 and YAE11. In an attempt to construct a strain harboring deletions of the WD40 domains of both α- and β′-COP, the haploid strain YAE6 [ret1Δ::HIS3 + pCEN::ret1Δ 1-285, (CEN, LEU2)] was crossed with the haploid strain YAE8-13c [sec27Δ::LEU2 + pRS316::SEC27, (CEN, URA3)]. The diploid strain YAE9 a/α was sporulated and tetrads were dissected to yield YAE9-3c. YAE9-3c was transformed with pCEN::sec27Δ1-285 (CEN, TRP1) YAE9-3c and tested for viable Ura-, Trp+, Leu+, His+ colonies on SD 5-FOA plates. Alternatively, YAE6 [ret1Δ::HIS3 +pCEN::ret1Δ 1-285 (CEN, LEU2)] was crossed with the haploid strain YAE10 (sec27Δ::LEU2 + pCEN::sec27Δ 1-285 (CEN, TRP1)], the diploid strain YAE13 a/α was sporulated, 50 tetrads dissected, and screened for Leu+, His+, Trp+ colonies.
Cytosol Preparation; Gel Filtration; In Vitro Binding Experiments
Preparation of cytosol and Superose 6 gel filtration were as described previously (Duden et al., 1998). GST-fusion proteins of Wbp1p-derived tails carrying a KKTN or SSTN motif, or of Emp47p-derived tails carrying a KTKLL or STSLL motif as described above, were expressed in E. coli and purified on glutathione-Sepharose 4B beads (Amersham Biosciences, Piscataway, NJ). For binding experiments, yeast spheroplasts were lysed at 100 OD600/ml in HEPES-Triton buffer [50 mM HEPES, pH 7.3, 90 mM KCl, 0.5% Triton X-100, 1 mM 4-(2-aminoethyl)benzenesulfonyl fluoride]. Cell lysates were centrifuged for 15 min at 20,000 × g. Supernatants, or in some experiments yeast cytosol prepared as described previously (Duden et al., 1998) were incubated twice for 1 h at 4°C with beads containing GST only, followed by 2 h with beads harboring the fusion proteins. Beads were washed once in HEPES-Triton buffer and five times in 50 mM HEPES, pH 7.3, and bound proteins eluted in SDS sample buffer and analyzed by immunoblot.
RESULTS
A novel β′-COP Mutant Lacking the WD40 Domain Is Viable, but Temperature-sensitive for Growth
α- and β′-COP across distant species harbor conserved N-terminal domains composed of WD40 repeats (Figure 1A, schematic representation). We previously demonstrated, by using a truncation mutant lacking this domain, that the WD40 domain of α-COP is not required for yeast cell viability but is essential for KKTN-dependent trafficking and binding to the KKTN peptide in vitro (Eugster et al., 2000). Here, we investigate the function of the corresponding WD40 domain present on β′-COP. β′-COP in yeast is encoded by an essential gene, SEC27 (Duden et al., 1994). To test whether the WD40 domain on β′-COP is essential, we constructed a mutant, sec27Δ1-285, in which the only SEC27 protein present in the cells lacks this domain. The sec27Δ1-285 mutant is viable, but is temperature-sensitive (Figure 1B). Cells grow like wild-type cells at 24°C, but display severely reduced growth at 30°C. In comparison, the corresponding α-COP WD40 domain truncation mutant, ret1Δ1-285, grows well up to 35°C (Figure 1B; Eugster et al., 2000). Thus, neither the α-COP nor the β′-COP WD40 domain is essential for yeast cell viability.
Figure 1.
(A) Schematic of the domain structure of α- and β′-COP. In this study, we show that the WD40 domain of β′-COP (residues 1–285) is nonessential but required for selective interactions with a KTKLL motif, whereas the α-COP WD40 domain interacts with the “classical” KKTN-motif (Eugster et al., 2000). A carboxy-terminal region of β′-COP, defined by sec27-1 (a G688D mutation) is involved in maintaining α-COP stability and coatomer integrity (see text for details). (B) Growth phenotype of α- and β′-COP mutants harboring a deletion of the respective WD40 domain. Cells were streaked onto YEPD plates and grown for 3 d at the temperatures indicated. Strains were as indicated: wild-type control strains CEN::RET1, CEN::SEC27 and mutant strains CEN::ret1Δ1-285 and CEN::sec27Δ1-285. Note that sec27Δ1-285 cells are strongly temperature sensitive.
At 24°C all coatomer subunits are present in this β′-COP mutant at levels comparable to ret1Δ1-285 cells or congenic wild-type control cells (Figure 2). Coatomer from sec27Δ1-285 cells grown at 24°C fractionated identically to coatomer from wild-type cells upon gel filtration, and even after a shift for 3 h to restrictive temperature, 34°C, no differences were found between coatomer from sec27Δ1-285 cells and wild-type cells (our unpublished data). Thus, the temperature-sensitive growth defect displayed by sec27Δ1-285 cells is not due to a gross defect in coatomer composition or structure.
Figure 2.
Levels of coatomer subunits in sec27Δ1-285 cells are normal. Whole cell extracts of ret1Δ1-285, sec27Δ1-285, control cells (CEN::RET1 and CEN::SEC27), and wild-type cells (YPH500) grown at 24°C were analyzed by immunoblot on equal OD600 equivalents of cells. Note the difference in molecular weight between wild-type and truncated α-COP or β′-COP. Further note that all other subunits are present in comparable amounts in all strains.
At Least One WD40 Domain, on Either α- or β′-COP, Is Required for Yeast Cell Viability
Neither the α-COP WD40 domain nor the β′-COP WD40 domain is required for yeast viability individually. Next, we asked whether yeast cells that lack both WD40 domains are viable. Our data show that the inviability of a Δsec27::LEU2/Δret1::HIS3 double mutant cannot be rescued by simultaneous expression of truncated versions of α- and β′-COP that lack their WD40 domains (Figure 3). Expression of the truncated proteins was achieved using plasmids pCEN::sec27Δ1-285 and pCEN::ret1Δ1-285, which can rescue the single deletion of the SEC27 or RET1 gene (Eugster et al., 2000). We also tried to construct a double truncation mutant strain by genetic crosses, and again no viable double mutants were obtained (see MATERIALS AND METHODS). Thus, the presence of at least one WD40 domain in coatomer is essential for yeast cell viability. The synthetic lethality resulting from a simultaneous deletion of both WD40 domains prompted us to test whether the domains may overlap in their function.
Figure 3.
A strain lacking the WD40 domains of both α-COP and β′-COP is inviable. The mutant strains ret1Δ1-285 + pCEN::RET1 (URA3), sec27Δ1-285 +pCEN::SEC27 (URA3), ret1Δ::HIS3/sec27Δ::LEU2 +pCEN::ret1Δ1–285 +pCEN::sec27Δ1-285 +pCEN::SEC27 (URA3) and congenic control strains CEN::RET1 +pCEN::RET1 (URA3), CEN::SEC27 +pCEN::SEC27 (URA3), and ret1Δ::HIS3/sec27Δ::LEU2 +pCEN::ret1Δ1–285 +pCEN:: SEC27 +pCEN::SEC27 (URA3) were streaked out onto 5-FOA plates to induce loss of the URA3 plasmids, and incubated at 24°C for 4 d. Note that a ret1Δ1-285/sec27Δ1-285 strain is not viable without the pCEN::SEC27 plasmid.
Yeast Cells Harboring Point Mutations in β′-COP
To date only two β′-COP yeast mutants have been described, sec27-1 (Duden et al., 1994) and sec27-95 (Prinz et al., 2000). The sec27-1 mutant was originally isolated in a screen for mutants with defects in mitotic spindle formation (Duden et al., 1994). On shift to a restrictive temperature, sec27-1 cells display a modest defect in KKTN-dependent trafficking and also mislocalize Emp47p, a KTKLL-motif bearing protein, to the vacuole (Letourneur et al., 1994; Schröder-Kohne et al., 1998). Coatomer in cytosol from temperature-shifted sec27-1 cells shows a reduced ability to bind to KKTN-peptide in vitro (Letourneur et al., 1994), but unlike with coatomer from ret1-1 cells, binding to the KKTN-motif is not abolished completely. On shift to restrictive temperature the sec27-1 mutant cells accumulate ER precursor forms of carboxypeptidase Y (p1-CPY) and α-factor, thus displaying also a modest anterograde transport defect (Duden et al., 1994). The sec27-95 mutant was identified in a visual screen for mutants that show defects in the structure of the cortical endoplasmic reticulum at restrictive temperature (Prinz et al., 2000). Coatomer in cytosol from sec27-95 mutant cells grown at a permissive temperature, 24°C, fractionates like wild-type coatomer upon gel filtration, and levels of all coatomer subunits are comparable to wild-type cells, suggesting that coatomer biosynthesis and stability are normal (our unpublished data). The trafficking phenotype in the sec27-95 mutant has not been characterized so far and is described in this study.
In the hope that a molecular characterization of the mutations in sec27-1 and sec27-95 cells might help us to better understand domains on β′-COP, we undertook DNA sequencing of the sec27 genomic regions in these mutants after PCR amplification. sec27-1 harbors a point mutation in a carboxy-terminal region, changing glycine at position 688 to aspartic acid. The sec27-95 mutation affects a residue in the amino-terminal WD40 domain, changing serine at position 114 to tyrosine. In this study, we have used the two point mutants, as well as sec27Δ1-285 cells, to analyze the functions of the amino- and carboxy-terminal domains of β′-COP.
α-COP Is Destabilized in the sec27-1 Mutant
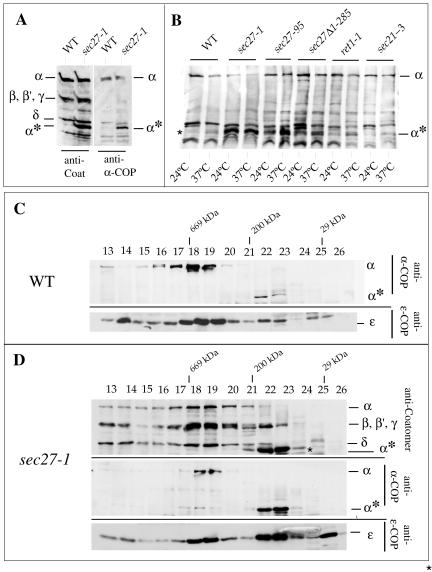
Intriguingly, sec27-1 cells display defects for both KKTN- and KTKLL-tagged proteins, but only upon a shift to restrictive temperature (Letourneur et al., 1994; Schröder-Kohne et al., 1998). Neither of these defects is apparent at permissive temperature. Therefore, to learn more about the nature of the defect in coatomer in sec27-1 cells we shifted sec27-1 cells, and wild-type cells as a control, for 2 h to 37°C and prepared cytosol. When immunoblotting cytosolic proteins with an antiserum against coatomer that recognizes all seven COPs (Duden et al., 1994), we noticed a prominent additional band of ∼65 kDa from sec27-1 cells that was undetectable in wild-type extracts at the same exposure (Figure 4A). To identify this band, we immunoblotted the extracts with antisera against individual coatomer subunits. No difference between wild-type and sec27-1 extracts was found with antisera against β′-, β-, γ-, and δ-COP (our unpublished data) but the ∼65-kDa band was recognized by an antiserum specific to a carboxy-terminal peptide of yeast α-COP (residues 1155–1171 of Ret1p) (Figure 4A). The additional band is thus an α-COP proteolytic fragment (“α*” in Figure 4) that roughly corresponds to the carboxy-terminal half of α-COP.
Figure 4.
α-COP is destabilized in sec27-1 cells. (A and B) Identification of a ∼65-kDa α-COP fragment (α*) in sec27-1 cells and other coatomer mutants as indicated, and in wild-type cells (WT). For details, see text. (C) Superose 6 gel filtration of cytosolic proteins from temperature-shifted wild-type cells (WT). α-COP species were identified with the α-COP peptide antiserum, and ε-COP with a specific rabbit antiserum. (D) Superose 6 gel filtration of cytosolic proteins from sec27-1 cells. SDS-PAGE separated proteins of column fractions were probed with anti-coatomer antiserum to identify α-, β′-, β-, and γ-COP and the α-COP fragment (α*) (top). The antiserum against the carboxy-terminal α-COP peptide (“anti-α-COP”) was used (middle); the bottom panel visualizes ε-COP. Note the reduced signal for intact α-COP in fractions 18 + 19 from sec27-1 cells compared with wild type. Further note that a large fraction of α-COP immunoreactivity is present in fractions 22 + 23, corresponding to a partial complex of ∼200 kDa in which ε-COP cofractionates. An additional peak of monomeric ε-COP is seen in fraction 26. Fraction numbers and positions of marker proteins are indicated: thyroglobulin (669,000), β-amylase (200,000), and carboanhydrase (29,000).
We next tested whether this α-COP fragment still associates with coatomer. On gel filtration of cytosol from temperature-shifted wild-type cells full-length α-COP cofractionated with all other COPs in fractions 18 + 19 that correspond to intact coatomer complex, as expected (Figure 4C). The ∼65-kDa α-COP fragment was exclusively present in a partial complex of ∼200 kDa, which also contained a peak of ε-COP (Figure 4, C and D). Although the ∼65-kDa α-COP fragment was detectable from wild-type cells (Figure 4C), its abundance was dramatically increased in temperature-shifted sec27-1 cells, concomitant with a decrease in full-length α-COP (Figure 4D). In wild-type cells, ε-COP was predominantly present in fractions 18 + 19, but in sec27-1 cells ε-COP was mostly present in fractions 22 + 23 (i.e., in the partial ∼200-kDa complex) and in fraction 26, as monomeric ε-COP (Figure 4D). Additionally, also β′-COP was detected in a peak in fractions 22 + 23 in sec27-1 cells (Figure 4D; our unpublished results). Clearly, the structural integrity of coatomer is severely compromised in sec27-1 cells upon shift to restrictive temperature, a phenomenon not observed in most other temperature-shifted coatomer mutant strains, including sec27Δ1-285 cells (our unpublished data). However, this behavior is somewhat reminiscent of ret1-3, a strain harboring a point mutation (S1188F) in the carboxy-terminal region of α-COP, in which the stability of both α- and ε-COP, and thus of coatomer, is compromised at high temperature (Duden et al., 1998; Eugster et al., 2000).
Next, we analyzed whether the presence of the ∼65-kDa α-COP fragment was specific to sec27-1 cells. We prepared total cell lysates from wild-type cells and several coatomer mutants, sec21-3 (γ), ret1-1 (α), ret1-3 (α), sec27-1 (β′), sec27-95 (β′), ret2-1 (δ), sec28Δ (a strain lacking ε-COP), and ret3-1 (ζ), either grown at 24°C or after a 2-h shift to 37°C. Total cell extracts were probed for presence of the ∼65-kDa α-COP fragment by immunoblotting. At 37°C, the α-COP fragment, although most prominent in sec27-1 cells, was detectable in all coatomer mutants to varying degrees, and a small amount even in wild-type cells (Figure 4B). This suggests that this α-COP fragment represents an intermediate in the normal cellular turnover of α-COP. Previously, we had noticed a peak of ε-COP in an identically sized partial complex of coatomer in yeast cells overexpressing α-COP (Eugster et al., 2000). Using the anti-α-COP peptide antiserum, we report here that this partial coatomer complex contains clipped α-COP in addition to ε-COP (Figure 4D).
Significantly, in sec27-1 cells the α-COP fragment was prominent even at 24°C. Thus, in this β′-COP mutant α-COP is more proteolytically susceptible, and thus coatomer less stable, than in all other coatomer mutants tested (Figure 5B; our unpublished data). This observation provides a straightforward molecular explanation for the previously reported data (Letourneur et al., 1994; Schröder-Kohne et al., 1998) that shifting sec27-1 cells to a semirestrictive temperature induces a retrograde transport defect for both a KKTN- and a KTKLL-tagged protein, and defects for the binding to the KKTN motif in vitro. Our data suggest that the sec27-1 mutation adversely affects interactions of subunits within the coatomer complex to some degree even at permissive temperature.
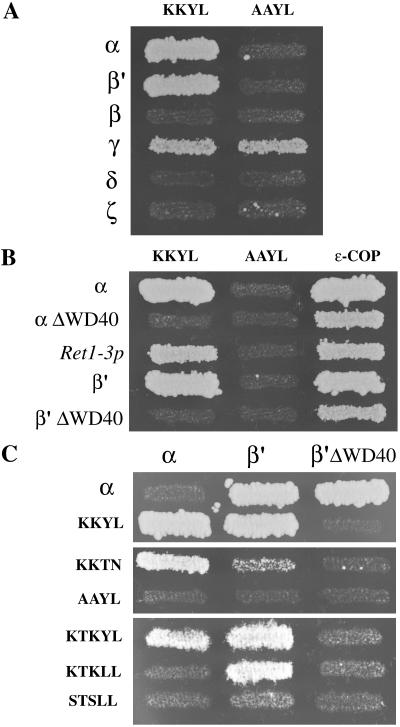
Figure 5.
α- and β′-COP directly interact with variants of di-lysine motifs. (A) Coatomer subunits, denoted with Greek symbols, in the bait vector were tested against the KKYL or AAYL motifs in the prey vector. Note that both α- and β′-COP interact with the KKYL motif, in a manner that depends on the presence of the lysines. γ-COP displayed background and thus could not be analyzed. (B) The interaction of α- and β′-COP with the KKYL motif depends on the presence of their WD40 domains. As expected, the interaction of α- and β′-COP with ε-COP is unaffected by truncation of the WD40 domains. “αΔWD40” and “β'ΔWD40” correspond to ret1Δ1-285 and sec27Δ1-285 in the text. (C) α-COP, or the different di-lysine motifs, in the bait vector were tested against α-COP, β′-COP, or the β′ΔWD40 mutant in the prey vector. Note that the KKTN motif interacts with α-COP and the KTKLL motif with β′-COP. The presence of the WD40 domain on β′-COP is required for the interaction with KTKLL, but not with α-COP. Results shown are from the growth assay on minimal plates lacking leucine.
The WD40 Domains of α- and β′-COP Are Required for Direct Binding to Di-lysine Motifs in the Two-Hybrid System
The yeast two-hybrid system has been successfully used to dissect the specificity of the interaction of the μsubunit of the adaptor complexes AP-1, AP-2, and AP-3 with tyrosinebased trafficking motifs (see e.g., Ohno et al., 1995). In a first attempt to use the two-hybrid system to understand interactions of COP I subunits with di-lysine motifs, we tested the yeast coatomer subunits in the prey vector against several cytoplasmic tails of proteins containing KKTN, or similar motifs, in our standard LexA two-hybrid assay. We observed no specific signals by using this approach (our unpublished data), consistent with the negative results reported by others by using mammalian coatomer subunits in a standard GAL4 two-hybrid system (Zerangue et al., 2001). Interestingly, however, it has been shown previously that fusing an oligomerization domain in frame to short peptide tails containing di-lysine motifs is a technical improvement that can result in specific, positive signals by using such short-peptide two-hybrid constructs (Zerangue et al., 2001). Using this modified two-hybrid system and a strong binding variant of the di-lysine motif, KKYL (which does not occur naturally in cells), a specific interaction with mammalian α-COP has been demonstrated by Zerangue et al. (2001). The signal in the modified system is thought to be enhanced by an increased effective local concentration of tail sequences when presented as an oligomeric array (Zerangue et al., 2001).
We used this approach in our LexA two-hybrid system (Eugster et al., 2000) to test interactions of yeast coatomer subunits with the artificially strong di-lysine motif, KKYL, as well as with two naturally occurring di-lysine motifs, KKTN as present on Wbp1p, and KTKLL as present on the cycling Golgi protein Emp47p (Figure 5). We find that the KKYL motif interacts with both α- and β′-COP, whereas no interaction was seen with a mutated motif, AAYL (Figure 5A). No interaction with KKYL was detected with β-, δ-, or ζ-COP, whereas the γ-COP construct showed nonspecific background and thus could not be analyzed (Figure 5A). We next tested whether mutations in α- or β′-COP affect the interaction with the KKYL motif (Figure 5B). A truncated α-COP lacking its WD40 domain (ret1Δ1-285) was unable to interact with the KKYL motif, but as expected still interacted with ε-COP used as a control. The ret1-3 mutation, which resides in the carboxy terminus of α-COP and affects the interaction with ε-COP at restrictive temperature (Eugster et al., 2000), affected neither the interaction with the KKYL motif nor with ε-COP at the permissive temperature (24°C) tested (Figure 5B, ret1-3). The two-hybrid results with α-COP are consistent with our previous study in which we demonstrated the importance of the α-COP WD40 domain for interactions with the KKTN motif in vivo and in vitro (Eugster et al., 2000). Similar to α-COP, a truncated β′-COP lacking its WD40 domain (sec27Δ1-285) was unable to interact with the KKYL motif, but still interacted with ε-COP used as a control. Therefore, we demonstrate here for the first time the ability of β′-COP to specifically interact with a di-lysine type motif, in a manner that requires the presence of the β′-COP WD40 domain. The fact that the artificial, strong motif KKYL can interact with both α- and β′-COP demonstrates an overlap in the binding activities of α-and β′-COP. However, for naturally occurring and thus weaker signals we find a clear selectivity (Figure 5, B and C). The KKTN motif (as present on Wbp1p) interacts predominantly with α-COP and the KTKLL motif (as present on Emp47p) interacts only with β′-COP, but not α-COP (Figure 5C). Again, as expected these interactions required the presence of the two lysines, because mutating them to alanine or serine abolished them. To test whether the two-hybrid signal for KTKLL motif is, like the KKTN motif, dependent on the local sequence context we also tested the KTKYL motif, which does not occur naturally. This motif could, like the strong KKYL motif, interact with both α-COP and β′-COP. This again highlights some degree of overlap in the binding activities of the WD40 domains. In summary, the WD40 domains of α- and β′-COP can both directly interact with di-lysine motifs but display a preferential interaction with two naturally occurring variants of the di-lysine motif, KKTN and KTKLL, respectively.
The WD40 Domain of β′-COP Is Required for the Correct Localization of the KTKLL-tagged Protein Emp47p
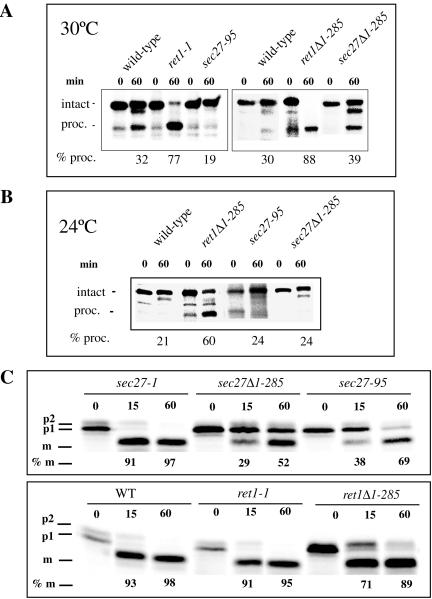
To understand the effect of mutations affecting the WD40 domain of β′-COP on trafficking, we had to extend our analysis to the Golgi protein Emp47p. This type I integral membrane protein maintains its steady-state localization by continuous recycling between the ER and the Golgi complex. This recycling is dependent on its carboxy-terminal KTKLL signal (Schröder et al., 1995; Schröder-Kohne et al., 1998; Sato and Nakano, 2002, 2003). We tested the intracellular fate of Emp47p in α- and β′-COP mutants at a permissive temperature for growth of all mutants tested, to score primary trafficking defects caused by the mutations, rather than secondary defects that may arise upon temperature shift. We reasoned that if the KTKLL motif is recognized by the β′-COP WD40 domain, as indicated by our two-hybrid data, then mutants affecting this domain may be compromised for Emp47p stability already at a permissive temperature, whereas other coatomer mutants may behave like wild-type cells. Only upon shift to a restrictive temperature would such mutants mislocalize Emp47p. To test this, we first measured the degradation of Emp47p occurring in different mutant cells by using immunoblotting as an assay, as described by Lewis and Pelham (1996). Briefly, wild-type and mutant strains were grown at 24°C. Protein synthesis was inhibited by adding 20 μg/ml cycloheximide to the culture, cultures shifted to 30°C, and then one sample taken immediately and two others after further incubation for 1 and 2 h. Emp47p levels in total cell extracts were analyzed by immunoblot with a rabbit antiserum against Emp47p (Schröder-Kohne et al., 1998) and an antiserum against Sed5p as a control (Figure 6A). In this experiment, a reduction in the level of Emp47p is indicative of its mislocalization to the vacuole where degradation occurs. We tested wild-type cells, the β′-COP mutants sec27-95, sec27Δ1-285, and sec27-1, and the α-COP mutants ret1-1 and ret1Δ1-285 (Figure 6A). Little difference in Emp47p levels between the 0-, 1-, and 2-h time points was found in wild-type and the α-COP mutant cells, consistent with previously published data (Schröder et al., 1995; Schröder-Kohne et al., 1998). Furthermore, the sec27-1 mutant also behaved similar to wild-type cells. However, in cells of the β′-COP WD40 domain point mutant sec27-95 and, to a somewhat lesser extent also in the sec27Δ1-285 WD40 domain truncation mutant, the level of Emp47p was greatly reduced at the 2-h time point (Figure 6A).
Figure 6.
Emp47p is destabilized in β′-COP WD40 domain mutants. (A) Degradation of Emp47p measured by immunoblotting in the strains as indicated; for details, see text. Note a significant reduction of Emp47p levels in cells of the β′-COP WD40 domain mutants sec27-95 and sec27Δ1-285, but not in sec27-1 cells or ret1-1 and ret1Δ1-285 cells. (B) wild-type (wt), sec27-1 and sec27-95 cells were pulsed for 20 min at 24°C and chased for the times indicated, and Emp47p levels were quantitated. Note a dramatic reduction of Emp47p levels in sec27-95 cells but not sec27-1 cells at 120 min. (C) Turnover of Emp47p in α- and β′-COP WD40 domain mutants. The experiment was performed as in B, by using wild-type cells and the sec27Δ1-285, sec27-95, ret1Δ1-285, ret1-1 mutants. Curves represent PhosphorImager quantitation of a representative experiment. Note the reduction of Emp47p levels in sec27Δ1-285 and sec27-95 cells but not in the α-COP mutants.
In temperature-shift experiments with yeast, temperature-sensitive mutants secondary defects frequently arise. In previous studies, Emp47p levels had been analyzed only after a preshift to the restrictive temperature for the mutants tested (Schröder et al., 1995; Lewis and Pelham, 1996; Schröder-Kohne et al., 1998). In fact, in cells shifted to restrictive temperature Emp47p has been reported previously to be largely mislocalized to the vacuole in a broad variety of coatomer mutants (sec27-1 [β′], sec21-1 [γ], ret2-1 [δ], and ret3-1 [ζ]) as well as in other retrograde transport mutants, e.g., sec20-1 or ufe1-1 (Schröder-Kohne et al., 1998; Lewis and Pelham, 1996). However, the important exception are the ret1-1 (α) and similar α-COP mutants in which Emp47p is stable even at restrictive temperature (Schröder-Kohne et al., 1998). Considering these data in the literature together, we hypothesized that the Emp47p mislocalization previously observed in coatomer mutants at high temperature is a consequence of a general coatomer inactivation with the resulting overall shut-down of Golgi-to-ER retrieval. Therefore, such results do not reveal exactly how Emp47p interacts with coatomer.
To obtain quantitative data on Emp47p degradation, pulse-chase experiments were performed with cells grown at 24°C, as described by Schröder-Kohne et al. (1998). Briefly, cells were radiolabeled for 20 min, chased for 0, 30, 60 and 120 min and then lysed. Emp47p was recovered with an Emp47p antiserum, resolved by SDS-PAGE, and analyzed using a PhosphorImager. In a first set of experiments, we compared Emp47p stability in sec27-1, sec27-95, and wild-type cells grown at 24°C (Figure 6B), which was interesting to us because at restrictive temperature sec27-1 cells display a strong defect for Emp47p (Schröder-Kohne et al., 1998). We find that at 24°C, sec27-1 cells behaved similar to wild-type, displaying a reduction to 72% of the initial Emp47p level at 120-min chase, compared with 78% in wild-type cells (Figure 6B). A similar result was obtained when testing the γ-COP mutant sec21-1 at 24°C (our unpublished data). sec27-95 cells displayed a strong defect even at this permissive temperature, resulting in a dramatic reduction of the Emp47p level to 22% at 120-min chase (Figure 6B).
Next, we compared Emp47p stability in our α- and β′-COP WD40 domain mutants, i.e., ret1-1, ret1Δ1-285, sec27-95, and sec27Δ1-285 cells, as well as wild-type cells. The data are shown graphically in Figure 6C. Both β′-COP WD40 domain mutants displayed a defect for Emp47p stability, namely, a reduction of Emp47p in sec27-95 and sec27Δ1-285 to 24 and 47%, respectively, after 2-h chase. The α-COP WD40 domain mutants behaved similar to wild-type, with values for Emp47p of 95, 90, and 77% for wild-type, ret1Δ1-285, and ret1-1 cells, respectively, after 2-h chase (Figure 6C). On the basis of our data, we conclude that the trafficking of the protein Emp47p, which harbors the variant di-lysine motif KTKLL, is dependent on the WD40 domain of β′-COP rather than α-COP, and therefore β′-COP is required for recruitment of some cargo proteins with a di-lysine type signal at their carboxy terminus.
Mutations in the β′-COP WD40 Domain Do Not Affect KKTN-dependent Trafficking
We next analyzed the ability of the β′-COP WD40 domain mutants sec27Δ1-285 and sec27-95 to retrieve a KKTN-tagged protein from the Golgi to the ER in vivo at permissive temperature, 24°C. As a reporter we used a well characterized reporter, Inv-Wbp1p, consisting of invertase fused to the transmembrane and cytoplasmic domains of Wbp1p, a KKTN-tagged protein resident in the ER (Gaynor et al., 1994; Letourneur et al., 1994). In wild-type cells this chimera continuously cycles between the Golgi and the ER, but in coatomer mutants defective for KKTN-dependent retrieval Inv-Wbp1p escapes to the vacuole where it is cleaved, thus enabling a quantitative evaluation of the retrieval defect. It has been previously shown that both the WD40 domain mutants ret1-1 and ret1Δ1-285 display a strong KKTN-retrieval defect in vivo, even at permissive temperature (Letourneur et al., 1994; Eugster et al., 2000).
Wild-type cells, and cells of the mutants sec27Δ1-285, sec27-95 cells, sec27-1, and ret1Δ1-285, all expressing the Inv-Wbp1 fusion, were radiolabeled for 20 min and chased for 0 and 60 min at 24°C. The Inv-Wbp1 fusion was recovered by immunoprecipitation, treated with endoglycosidase H, and resolved by SDS-PAGE (Figure 7A). Sixty percent of the protein was found to be cleaved in ret1Δ1-285 cells (Figure 7B). In sec27Δ1-285 cells and sec27-95 cells only 24% of the protein was processed, very similar to the 21% in wild-type cells (Figure 7B). sec27-1 cells also behaved like wild-type cells at 24°C (our unpublished data). Even upon shift to 30°C for 3 h, a semipermissive temperature for sec27Δ1-285, this mutant did not display a strong defect in KKTN-dependent trafficking. At 30°C only 39% of the protein was processed in sec27Δ1-285 cells, compared with 30% in wild-type cells (Figure 7A). Similar values were obtained for sec27-95 (Figure 7A, left) and sec27-1 cells (our unpublished data). Under the same conditions, 77 and 88% of the fusion protein was cleaved in ret1-1 and ret1Δ1-285 cells, respectively (Figure 7A).
Figure 7.
(A) The β′-COP WD40 domain mutants sec27Δ1-285 and sec27-95 behave like wild-type for KKTN trafficking in vivo. sec27Δ1-285 and sec27-95 cells and ret1Δ1-285 and wild-type (CEN::SEC27) cells as controls, all expressing the Wbp1-Inv.-KKTN fusion construct, were grown at 24°C. Before the experiment cells were preshifted to 30°C for 3 h and then pulse labeled at 30°C, and chased for 0 and 1 h at 30°C. Intact and PEP4 processed bands migrate at 70 and 56 kDa, respectively. The percentage of processing after 1 h was quantified using a PhosphorImager. (B) Same as in A, except that ret1Δ1-285, sec27Δ1-285, and control (CEN::SEC27) cells were grown at 24°C, pulse labeled for 20 min at 24°C, and chased for 0 and 1 h at 24°C. Note that ret1Δ1-285 cells show a strong KKTN retrieval defect at both 24 and 30°C, whereas sec27Δ1-285 cells behave like wild-type. (C) sec27Δ1-285 and sec27-95 cells display a kinetic delay in CPY transport at permissive temperature. Analysis of CPY transport in sec27Δ1-285, sec27-95, ret1Δ1-285, ret1-1, and wild-type (WT) cells. Cells were radiolabeled for 10 min at 24°C and chased for 0, 15, and 60 min. p1, p2, and mature forms of CPY are indicated, and processing to the mature form is quantified.
Our attempts to directly compare the two naturally occurring di-lysine signals, KKTN and KTKLL, with regard to their effect on the maturation of the reporter protein invertase failed due to the general weakness of the KTKLL signal noticed in earlier studies (Schröder-Kohne et al., 1998). Specifically, we generated KTKLL-/STSLL-tagged invertase reporter proteins in an attempt to compare them with the KKTN invertase reporter (Gaynor et al., 1994) used here. However, even in wild-type cells the KTKLL-tagged invertase reporter was rapidly transported to the vacuole and degraded, indistinguishable from untagged invertase reporter (our unpublished data), and therefore failed to provide an assay for KTKLL-mediated recycling.
To estimate the degree to which the β′-COP WD40 domain mutants can support normal anterograde transport of proteins in the secretory pathway at permissive temperature, 24°C, we analyzed the transport of the vacuolar protease CPY from the ER to the vacuole. We find that the β′-COP WD40 domain mutants sec27-95 and sec27Δ1-285 display a modest kinetic delay in the maturation of CPY, whereas ret1-1, ret1Δ1-285, and the sec27-1 mutants behaved like wild-type cells (Figure 7C). This difference between the two β′-COP mutants in their ability to perform forward transport (i.e., with sec27Δ1-285 cells affected more strongly with regard to CPY transport than sec27-95 cells) explains in a straightforward manner why Emp47p is less strongly mislocalized in sec27Δ1-285 cells compared with sec27-95 cells, because forward transport of Emp47p is a prerequisite for its degradation in the vacuole.
In summary, in contrast to ret1Δ1-285 and ret1-1 cells, neither of the three β′-COP mutants display a defect in the retrieval of a KKTN-tagged protein either at permissive temperature or upon shift to the semipermissive temperature, 30°C. The trafficking phenotypes of the α- and β′-COP mutants with regard to proteins harboring KKTN- or KTKLL-signals are summarized in Table 2.
Table 2.
Summary of phenotypes of β′- and α-COP mutants
| Alleles | Changes | Defects in vivo/in vitro | References |
|---|---|---|---|
| sec27-1 (β′-COP) | G688D | ts; no KKTN transport defect and no defect for Emp47p (KTKLL) stability at permissive temperature; after ts-shift α-COP instability and both KKTN and KTKLL transport defects | This study; Duden et al., 1994; Letourneur et al., 1994; Schröder-Kohne et al., 1998 |
| sec27-95 (β′-COP) | S114Y | ts; strong defect for Emp47p (KTKLL) stability; no KKTN transport defect | This study; Prinz et al., 2000 |
| sec27Δ1-285 (β′-COP) | residues 1-285 deleted | ts; strong defect for Emp47p (KTKLL) stability; no KKTN transport defect; defective for binding KKTN and KTKLL motifs in vitro | This study |
| ret1-1 (α-COP) | G227D | weak ts; no defect for Emp47p (KTKLL) stability, strong KKTN transport defect; defective for binding to KKTN, but able to bind KTKLL motifs in vitro | Letourneur et al., 1994; Schröder-Kohne et al., 1998 |
| ret1Δ1-285 (α-COP) | residues 1-285 deleted | ts; no defect for Emp47p (KTKLL) stability, strong KKTN transport defect; defective for binding KKTN and KTKLL motifs in vitro | This study; Eugster et al., 2000 |
ts, temperature sensitive.
Binding of Coatomer from α- and β′-COP Mutants to Di-lysine Motifs In Vitro
To directly assess the ability of coatomer from β′-COP mutant cells to interact with di-lysine motifs, in vitro binding experiments were performed using GST-fusion proteins harboring the cytoplasmic tail of Wbp1p, which terminates in a “classical” di-lysine motif, KKTN, and the cytosolic tail of Emp47p, which terminates in a variant di-lysine motif, KTKLL. GST fusions in which the lysines were mutated to serines were used as controls. These experiments used whole cell extracts from cells grown at 24°C, and the high salt/0.5% Triton X-100 binding conditions described by Cosson and Letourneur (1994). We analyzed extracts from the mutants sec27-95, sec27Δ1-285, ret1-1, and ret1Δ1-285, and from wild-type cells. We find that wild-type coatomer bound to both KKTN- and KTKLL-GST and ret1-1 coatomer only to KTKLL-GST, as reported previously (Schröder-Kohne et al., 1998). Coatomer from the truncation mutants sec27Δ1-285 and ret1Δ1-285 was unable to bind to either motif. Coatomer from sec27-95 cells showed only weak binding to KTKLL-GST and no binding to the KKTN motif (Figure 8). We speculate that coatomer lacking one of its WD40 domains or from sec27-95 cells failed to withstand the rather harsh incubation/washing conditions required to reveal binding of wild-type or ret1-1 coatomer to the di-lysine tagged GST fusions in a significant manner (Schröder-Kohne et al., 1998). On the other hand, in vivo the correct targeting of a KKTN-dependent reporter and of the endogenous KTKLL-tagged protein Emp47p clearly depends on one of two WD40 domains of coatomer in a mutually independent manner, as shown above.
Figure 8.
Coatomer from WD40 domain truncation mutants is unable to bind to either the KKTN- or KTKLL motifs in vitro. Whole cell lysate of wild-type cells (WT), or mutants (ret1Δ1-285 cells, ret1-1 cells, sec27-95, sec27-1, sec27Δ1-285) was incubated for 2 h at 4°C with immobilized GST fusion proteins harboring the KKTN- or KTKLL motifs, or mutated motifs in which the lysines were changed to serine, SSTN or STSLL. Bound proteins eluted from the beads were analyzed by immunoblot. Note the expected binding pattern of coatomer from wild-type and ret1-1 mutant cells, and the absence of binding for coatomer from the α- and β′-COP WD40 domain truncation mutants. Coatomer from sec27-95 cells bound only weakly to KTKLL and not at all to the KKTN motif.
DISCUSSION
We wish to better understand the interaction between vesicle cargo proteins and the COP I/coatomer coat. The WD40 domain of α-COP has been previously shown to be required for the binding and trafficking of proteins tagged with the classical di-lysine retrieval motif KKTN (Schröder-Kohne et al., 1998; Eugster et al., 2000). Here, we studied the role of the WD40 domain of β′-COP in the recognition of the variant di-lysine motif, KTKLL. To this end we analyzed two known point mutations (sec27-1 and sec27-95) and a truncated version (sec27Δ1-285) of β′-COP with regard to their effects on the binding and trafficking of proteins tagged with these two di-lysine motifs. Our data demonstrate that the WD40 domain of β′-COP can directly bind the variant di-lysine signal KTKLL and thus is involved in cargo selection and that a carboxy-terminal domain on β′-COP contributes to the structural integrity of the coatomer complex.
A Carboxy-Terminal Region of β′-COP Is Involved in Maintaining Coatomer Integrity
Sequencing of the β′-COP mutant sec27-1 revealed a G688D mutation in the Clathrin Heavy Chain Repeat of β′-COP (Table 2 and Figure 1). The clathrin heavy chain protein consists of three distinct domains. A terminal domain is responsible for interaction with adaptor complexes, whereas the proximal and distal domains provide the clathrin leg that forms the characteristic outer layer of clathrin-coated vesicles (Kirchhausen, 2000). The clathrin leg consists of seven copies of the Clathrin Heavy Chain Repeat (Ybe et al., 1999). Single copies of this repeat have been found in a number of proteins involved in membrane traffic, including α-COP (Conibear and Stevens, 1998) and β′-COP (Eugster et al., 2000) (Figure 1). Biochemical characterization of sec27-1 revealed a reduced stability of the α-COP subunit, to some degree even at permissive temperature, and a loss of the α-, β′-, ε-COP subcomplex from the coatomer complex. This explains the rather general nature of coatomer-related trafficking phenotypes found in previous studies by using this mutant, which were performed upon shift to semipermissive or restrictive temperatures (Letourneur et al., 1994; Schröder-Kohne et al., 1998). Further analysis of the sec27-1 mutant may help to understand the role of the clathrin heavy chain repeat in a nonclathrin complex in molecular detail.
β′-COP WD40 Domain Mutants Mislocalize the KTKLL-tagged Protein Emp47p
α- and β′-COP share a close sequence similarity extending from residues 1–780 (Eugster et al., 2000). Both proteins harbor highly conserved WD40 domains in their aminoterminal regions, and unique regions with no sequence relationship are found at their carboxy termini (Figure 1). To understand the role of the WD40 domain of β′-COP in COP I-mediated trafficking, we had to extend our analysis to the Golgi protein Emp47p. This type I integral membrane protein maintains its steady-state localization by continuous recycling between the endoplasmic reticulum and the Golgi complex (Schröder et al., 1995; Schröder-Kohne et al., 1998; Sato and Nakano, 2002, 2003). This recycling is dependent on its carboxy-terminal variant di-lysine signal, KTKLL (Schröder et al., 1995; Schröder-Kohne et al., 1998). We show here that deletion of the β′-COP WD40 domain or its inactivation by the point mutation of sec27-95 cells results in loss of Emp47p from the Golgi complex. The recycling of a KKTN-tagged reporter protein, on the other hand, is unaffected in these cells (Table 2). Our findings thus provide an explanation for earlier observations that the KTKLL-tagged protein Emp47 is not mislocalized in α-COP WD40 domain mutants (Schröder et al., 1995; Schröder-Kohne et al., 1998). Apparently, this variant di-lysine motif is dependent on the WD40 domain on β′-COP rather than on α-COP, and therefore β′-COP is required for recruitment of some cargo proteins with a di-lysine–related signal at their carboxy terminus into COP I vesicles.
WD40 Domains as Recognition Modules for Variants of the Di-lysine Motif
The WD40 repeat was originally identified in the β-subunit of heterotrimeric G proteins (Gβ) (Fong et al., 1986). Later, it was found in >160 functionally diverse proteins by sequence analysis, including the COP II coat proteins Sec13p and Sec31p (Shaywitz et al., 1997) and the COP I proteins α- and β′-COP (Letourneur et al., 1994; Duden et al., 1994; Gerich et al., 1995). The crystal structure of Gβ revealed a propeller-like fold for the WD40 domain (Wall et al., 1995). A WD40 repeat consists of four β-strands arranged in an antiparallel β-sheet, providing one of the blades to the overall propeller-like structure. This β-propeller derives rigidity from hydrophobic interactions between the β-sheets, whereas solvent-exposed residues between β strands mediate interactions with other proteins (Smith et al., 1999).
Using a modified version of the two-hybrid system, we show that the WD40 domains of α- and β′-COP are capable of direct interactions with two naturally occurring variants of the di-lysine motif, namely, KKTN and KTKLL (Figure 5). Interestingly, this direct and specific interaction of a short peptide motif is dependent on the presence of a tetramerization domain in the construct presenting the di-lysine signal, as described earlier by Zerangue et al. (2001). We find that the classic di-lysine signal KKTN derived from the oligosaccharyl transferase subunit Wbp1p binds preferentially to the WD40 domain of α-COP. The variant di-lysine signal KTKLL found on the Golgi protein Emp47p binds exclusively to the WD40 domain of β′-COP. The strong artificial di-lysine signal KKYL, however, which has been identified in a screen for functional di-lysine signals (Zerangue et al., 2001), is able to interact with both WD40 domains with high affinity. Another artificial di-lysine signal, KTKYL, can also interact with both WD40 domains, albeit with lower affinity. These data highlight some degree of overlap in the binding activities of the two WD40 domains. However, our two-hybrid data also clearly show that the two WD40 domains of α- and β′-COP are able to discriminate between two naturally occurring di-lysine signals. The trafficking phenotypes observed in a panel of α- and β′-COP WD40 domain mutants are consistent with this notion. Another interaction site in coatomer for di-lysine type motifs has been indicated in mammalian γ-COP by using a photo-cross-linking approach (Harter et al., 1996; Harter and Wieland, 1998) and by in vitro binding data (Wu et al., 2000). However, this binding site must be structurally different from the binding sites on α- and β′-COP reported here, because γ-COP does not harbor WD40 repeats.
An increasing number of WD40 domain-containing proteins have been found to interact with short sequences or small structural motifs with high specificity (Beisel et al., 1999; Holm et al., 2001). For example, a regulatory protein from Arabidopsis thaliana with a seven bladed β-propeller formed by WD40 repeats has been reported to bind a variety of ligands presenting the binding motif V-P-E/D-Φ-G; (with Φ being a bulky hydrophobic residue) in conjunction with four to five negatively charged residues upstream of the motif (Holm et al., 2001). X-ray crystallography on the lectin Tachylectin-2 in a complex with its ligand revealed one N-acetyl carbohydrate-binding site per blade, i.e., five binding sites in a five bladed β-propeller (Beisel et al., 1999). Structural studies will have to show whether there are multiple binding sites for di-lysine motifs on each of the WD40 domains in coatomer.
In summary, we have demonstrated that the α-COP WD40 domain directly interacts with the classical KKTN-motif, whereas the β′-COP WD40 domain directly interacts with a variant di-lysine motif, KTKLL. Our data suggest that the WD40 domains of α- and β′-COP bind distinct but overlapping sets of di-lysine signals and that both domains contribute to recruitment of cargo proteins with di-lysine motifs into COP I vesicles. Similarly, a variety of ER export signals are recognized by subunits of the COP II coat in a manner that also involves multiple, distinct cargo recognition sites (for review, see Barlowe, 2003). It will be interesting to analyze further COP I cargo proteins with regard to their direct interactions with α- or β′-COP: Rer1p, a recycling receptor for the transmembrane protein Sec12p that is required for COP II vesicle budding (Sato et al., 2001), Golgi-resident type II integral membrane proteins such as Och1p (Harris and Waters, 1996), or the HDEL/KDEL-receptor Erd2p (Townsley et al., 1993). Our WD40 domain mutants of α- and β′-COP should enable us to study the trafficking of COP I cargo proteins with regard to their binding site on coatomer. Analysis of the exact ligand specificity of the WD40 domains will require the use of recombinant, soluble WD40 domains for in vitro binding assays. This approach may allow characterization of the interaction of these domains with di-lysine-type motifs in structural detail.
Acknowledgments
We are indebted to Drs. Pierre Cosson, Francois Letourneur, Tom Rapoport, Randy Schekman, Hans-Dieter Schmitt, Stephan Schröder, Blanche Schwappach, and Gerry Waters for generously sharing plasmids, strains and antibodies. We thank Drs. Margaret “Scottie” Robinson and Karin Römisch for helpful comments on the manuscript. This work was supported by The Wellcome Trust (Senior Research Fellowship; grant 047578 to R.D.).
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–10–0724. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-10-0724.
References
- Barlowe, C. (2003). Molecular recognition of cargo by the COP II complex: a most accommodating coat. Cell 114, 395-399. [DOI] [PubMed] [Google Scholar]
- Beisel, H.G., Kawabata, S., Iwanaga, S., Huber, R., and Bode, W. (1999). Tachylectin- 2, crystal structure of a specific GlcNAc/GalNAc-binding lectin involved in the innate immunity host defense of the Japanese horseshoe crab Tachypleus tridentatus. EMBO J. 18, 2313-2322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conibear, E., and Stevens, T.H. (1998). Multiple sorting pathways between the late Golgi and the vacuole in yeast. Biochim. Biophys. Acta 1404, 211-230. [DOI] [PubMed] [Google Scholar]
- Cosson, P., and Letourneur, F. (1994). Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science 263, 1629-1631. [DOI] [PubMed] [Google Scholar]
- Donaldson, J.G., and Jackson, C.L. (2000). Regulators and effectors of the ARF GTPases. Curr. Opin. Cell Biol. 12, 475-482. [DOI] [PubMed] [Google Scholar]
- Duden, R., Hosobuchi, M., Hamamoto, S., Winey, M., Byers, B., and Schekman, R. (1994). Yeast β- and β′-coat proteins (COP): two coatomer subunits essential for ER-to-Golgi traffic. J. Biol. Chem. 269, 24486-24495. [PubMed] [Google Scholar]
- Duden, R., Kajikawa, L., Wuestehube, L., and Schekman, R. (1998). ε-COP is a structural component of coatomer that functions to stabilize α-COP. EMBO J. 17, 985-995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estojak, J., Brent, R., and Golemis, E.P. (1995). Correlation of two-hybrid affinity data with in vitro measurements. Mol. Cell Biol. 15, 5820-5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eugster, A., Frigerio, G., Dale, M., and Duden, R. (2000). COP I domains required for coatomer integrity, and novel interactions with ARF and ARF-GAP. EMBO J. 19, 3905-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong, H.K., Hurley, J.B., Hopkins, R.S., Miake-Lye, R., Johnson, M.S., Doolittle, R.F., and Simon, M.I. (1986). Repetitive segmental structure of the transducin beta subunit: homology with the CDC4 gene and identification of related mRNAs. Proc. Natl. Acad. Sci. USA 83, 2162-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynor, E.C., Graham, T.R., and Emr, S.D. (1998). COPI in ER/Golgi and intra-Golgi transport: do yeast COPI mutants point the way? Biochim. Biophys. Acta, 1404, 33-51. [DOI] [PubMed] [Google Scholar]
- Gaynor, E.C., Te Heesen, S., Graham, T.R., Aebi, M., and Emr, S.D. (1994). Signal-mediated retrieval of a membrane protein from the Golgi to the ER in yeast. J. Cell Biol. 127, 653-665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerich, B., Orci, L., Tschochner, H., Lottspeich, F., Ravazzola, M., Amherdt, M., Wieland, F., and Harter, C. (1995). Non-clathrin-coat protein alpha is a conserved subunit of coatomer and in Saccharomyces cerevisiae is essential for growth. Proc. Natl. Acad. Sci. USA 92, 3229-3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golemis, E.A., Gyuris, J., and Brent, R. (1996). Interaction trap/two hybrid system to identify interacting proteins. In: Current Protocols in Molecular Biology. New York: John Wiley & Sons, Inc., 20.1.1.-20.1.28
- Harris, S.L., and Waters, M.G. (1996). Localization of a yeast early Golgi mannosyltransferase, Och1p, involves retrograde transport. J. Cell Biol. 132, 985-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter, C., Pavel, J., Coccia, F., Draken, E., Wegehingel, S., Tschochner, H., and Wieland, F. (1996). Nonclathrin coat protein gamma, a subunit of coatomer, binds to the cytoplasmic di-lysine motif of membrane proteins of the early secretory pathway. Proc. Natl. Acad. Sci. USA 93, 1902-1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harter, C., and Wieland, F.T. (1998). A single binding site for di-lysine retrieval motifs and p23 within the γ-subunit of coatomer. Proc. Natl. Acad. Sci. USA 95, 11649-11654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm, M., Hardtke, C.S., Gaudet, R., and Deng, X.W. (2001). Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J. 20, 118-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosobuchi, M., Kreis, T., and Schekman, R. (1992). SEC21 is a gene required for ER to Golgi protein transport that encodes a subunit of a yeast coatomer. Nature 360, 603-605. [DOI] [PubMed] [Google Scholar]
- Jackson, M.R., Nilsson, T., and Peterson, P.A. (1993). Retrieval of transmembrane proteins to the endoplasmic reticulum. J. Cell Biol. 121, 317-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchhausen, T. (2000). Three ways to make a vesicle. Nat. Rev. Mol. Cell. Biol. 1, 187-198. [DOI] [PubMed] [Google Scholar]
- Lanoix, J., Ouwendijk, J., Lin, C.C., Stark, A., Love, H.D., Ostermann, J., and Nilsson, T. (1999). GTP hydrolysis by arf-1 mediates sorting and concentration of Golgi resident enzymes into functional COP I vesicles. EMBO J. 18, 4935-4948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur, F., Gaynor, E., Hennecke, S., Demolliere, C., Duden, R., Emr, S., Riezman, H., and Cosson, P. (1994). Coatomer is essential for retrieval of di-lysine-tagged proteins to the ER. Cell 79, 1199-1207. [DOI] [PubMed] [Google Scholar]
- Lewis, M.J., and Pelham, H.R.B. (1996). SNARE-mediated retrograde traffic from the Golgi complex to the endoplasmic reticulum. Cell 85, 205-215. [DOI] [PubMed] [Google Scholar]
- Lowe, M., and Kreis, T.E. (1995). In vitro assembly and disassembly of coatomer. J. Biol. Chem. 270, 31364-31371. [DOI] [PubMed] [Google Scholar]
- Neer, E.J., and Smith, T.F. (2000). A groovy new structure. Proc. Natl. Acad. Sci. USA 97, 960-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno, H., Stewart, J., Fournier, M.C., Bosshart, H., Rhee, I., Miyatake, S., Saito, T., Gallusser, A., Kirchhausen, T., and Bonifacino, J.S. (1995). Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269, 1872-1875. [DOI] [PubMed] [Google Scholar]
- Orci, L., Stamnes, M., Ravazzola, M., Amherdt, M., Perrelet, A., Sollner, T.H., and Rothman, J.E. (1997). Bidirectional transport by distinct populations of COPI-coated vesicles. Cell 90, 335-349. [DOI] [PubMed] [Google Scholar]
- Pelham, H.R., and Rothman, J.E. (2000). The debate about transport in the Golgi - two sides of the same coin? Cell 102, 713-719. [DOI] [PubMed] [Google Scholar]
- Prinz, W.A., Grzyb, L., Veenhuis, M., Kahana, J.A., Silver, P.A., and Rapoport, T.A. (2000). Mutants affecting the structure of the cortical endoplasmic reticulum in Saccharomyces cerevisiae. J. Cell Biol. 150, 461-474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman, J.E., and Wieland, F.T. (1996). Protein sorting by transport vesicles. Science 272, 227-234. [DOI] [PubMed] [Google Scholar]
- Sato, K., and Nakano, A. (2002). Emp47p and its close homolog Emp46p have a tyrosine-containing endoplasmic reticulum exit signal and function in glycoprotein secretion in Saccharomyces cerevisiae. Mol. Biol. Cell 13, 2518-2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., and Nakano, A. (2003). Oligomerization of a cargo receptor directs protein sorting into COPII-coated transport vesicles. Mol. Biol. Cell 14, 3055-3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato, K., Sato, M., and Nakano, A. (2001). Rer1p, a retrieval receptor for endoplasmic reticulum membrane proteins, is dynamically localized to the Golgi apparatus by coatomer. J. Cell Biol. 152, 935-944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder, S., Schimmöller, F., Singer-Krüger, B., and Riezman, H. (1995). The Golgi-localization of yeast Emp47p depends on its di-lysine motif but is not affected by the ret1–1 mutation in α-COP. J. Cell Biol. 131, 895-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schröder-Kohne, S., Letourneur, F., and Riezman, H. (1998). α-COP can discriminate between distinct, functional di-lysine signals in vitro and regulates access into retrograde transport. J. Cell Sci. 111, 3459-3470. [DOI] [PubMed] [Google Scholar]
- Shaywitz, D.A., Espenshade, P.J., Gimeno, R.E., and Kaiser, C.A. (1997). COPII subunit interactions in the assembly of the vesicle coat. J. Biol. Chem. 272, 25413-2546. [DOI] [PubMed] [Google Scholar]
- Smith, T.F., Gaitatzes, C., Saxena, K., and Neer, E.J. (1999). The WD repeat: a common architecture for diverse functions. Trends Biochem. Sci. 24, 181-185. [DOI] [PubMed] [Google Scholar]
- Sondek, J., Bohm, A., Lambright, D.G., Hamm, H.E., and Sigler, P.B. (1996). Crystal structure of a G-protein β γ dimer at 2.1A resolution. Nature 379, 369-374. [DOI] [PubMed] [Google Scholar]
- Spang, A., Matsuoka, K., Hamamoto, S., Schekman, R., and Orci, L. (1998). Coatomer, Arf1p, and nucleotide are required to bud coat protein complex I-coated vesicles from large synthetic liposomes. Proc. Natl. Acad. Sci. USA 95, 11199-11204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ter Haar, E., Harrison, S.C., and Kirchhausen, T. (2000). Peptide-in-groove interactions link target proteins to the β-propeller of clathrin. Proc. Natl. Acad. Sci. USA 97, 1096-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsley, F.M., Wilson, D.W., and Pelham, H.R. (1993). Mutational analysis of the human KDEL receptor: distinct structural requirements for Golgi retention, ligand binding and retrograde transport. EMBO J. 12, 2821-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wall, M.A., Coleman, D.E., Lee, E., Iniguez-Lluhi, J.A., Posner, B.A., Gilman, A.G., and Sprang, S.R. (1995). The structure of the G protein heterotrimer Giα1β1γ2. Cell 83, 1047-1058. [DOI] [PubMed] [Google Scholar]
- Wu, W.J., Erickson, J.W., Lin, R., and Cerione, R.A. (2000). The gamma-subunit of the coatomer complex binds Cdc42 to mediate transformation. Nature 405, 800-804. [DOI] [PubMed] [Google Scholar]
- Ybe, J.A., Brodsky, F.M., Hofmann, K., Lin, K., Liu, S.H., Chen, L., Earnest, T.N., Fletterick, R.J., and Hwang, P.K. (1999). Clathrin self-assembly is mediated by a tandemly repeated superhelix. Nature 399, 371-375. [DOI] [PubMed] [Google Scholar]
- Zerangue, N., Malan, M.J., Fried, S.R., Dazin, P.F., Jan, Y.N., Jan, L.Y., and Schwappach, B. (2001). Analysis of endoplasmic reticulum trafficking signals by combinatorial screening in mammalian cells. Proc. Natl. Acad. Sci. USA 98, 2431-2436. [DOI] [PMC free article] [PubMed] [Google Scholar]