Abstract
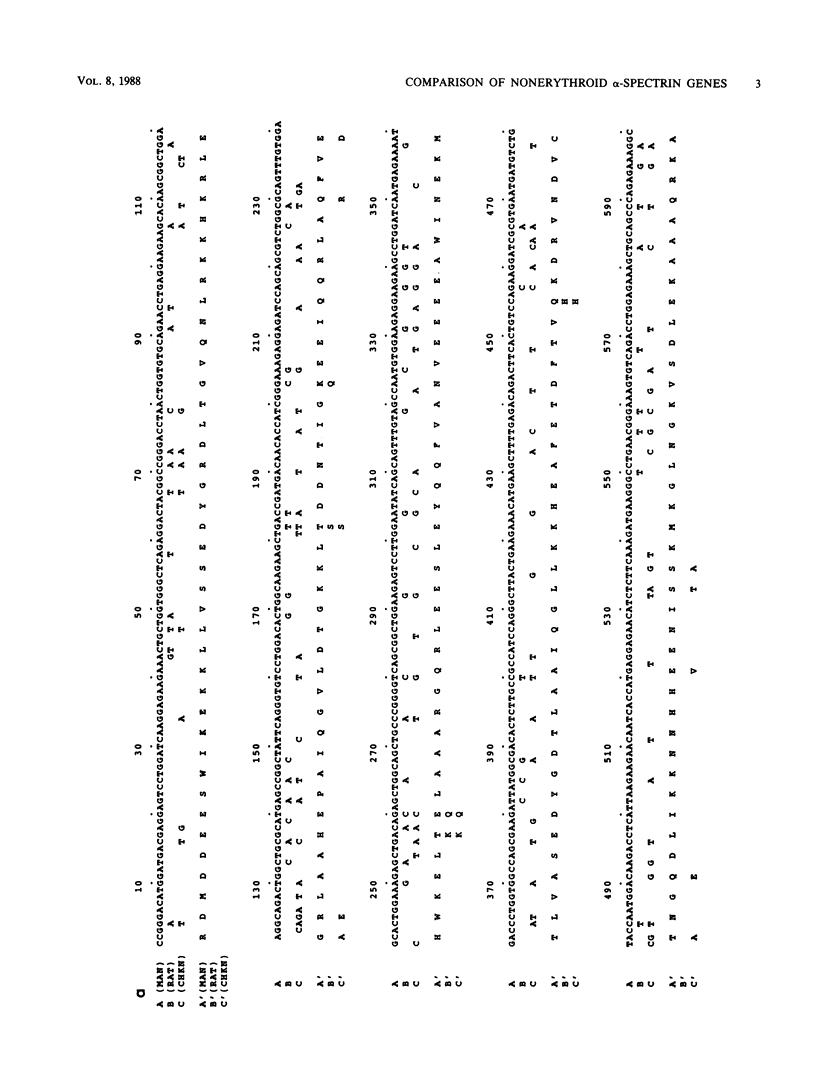
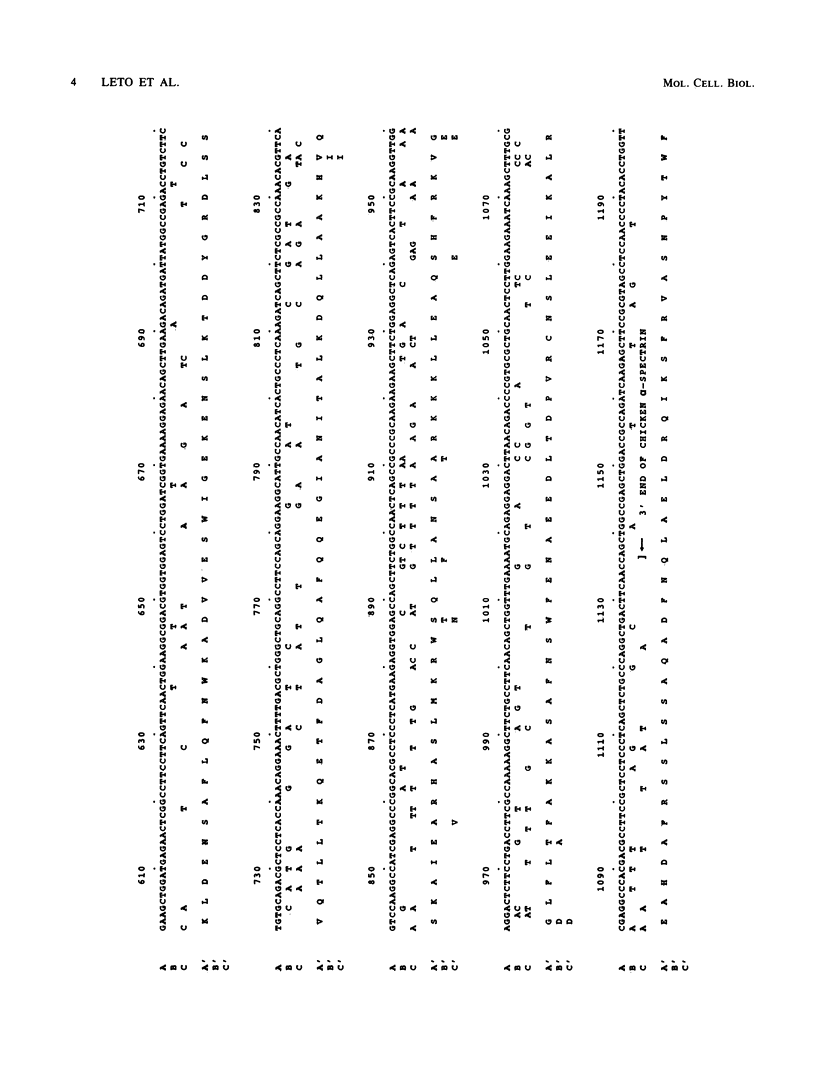
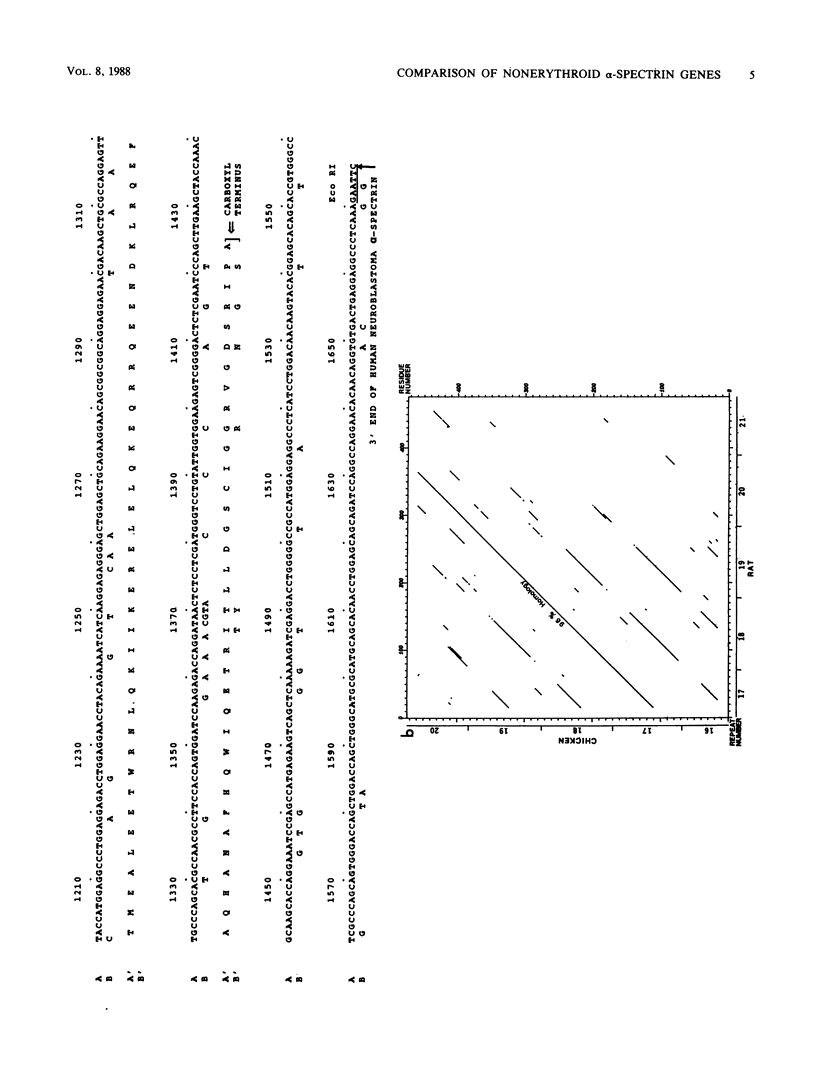
The spectrins are a family of widely distributed filamentous proteins. In association with actin, spectrins form a supporting and organizing scaffold for cell membranes. Using antibodies specific for human brain alpha-spectrin (alpha-fodrin), we have cloned a rat brain alpha-spectrin cDNA from an expression library. Several closely related human clones were also isolated by hybridization. Comparison of sequences of these and other overlapping nonerythroid and erythroid alpha-spectrin genes demonstrated that the nonerythroid genes are strictly conserved across species, while the mammalian erythroid genes have diverged rapidly. Peptide sequences deduced from these cDNAs revealed that the nonerythroid alpha-spectrin chain, like the erythroid spectrin, is composed of multiple 106-amino-acid repeating units, with the characteristic invariant tryptophan as well as other charged and hydrophobic residues in conserved locations. However, the carboxy-terminal sequence varies markedly from this internal repeat pattern and may represent a specialized functional site. The nonerythroid alpha-spectrin gene was mapped to human chromosome 9, in contrast to the erythroid alpha-spectrin gene, which has previously been assigned to a locus on chromosome 1.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton D. E., Yang-Feng T. L., Francke U. The human tyrosine aminotransferase gene mapped to the long arm of chromosome 16 (region 16q22----q24) by somatic cell hybrid analysis and in situ hybridization. Hum Genet. 1986 Mar;72(3):221–224. doi: 10.1007/BF00291881. [DOI] [PubMed] [Google Scholar]
- Bennett V., Davis J., Fowler W. E. Brain spectrin, a membrane-associated protein related in structure and function to erythrocyte spectrin. Nature. 1982 Sep 9;299(5879):126–131. doi: 10.1038/299126a0. [DOI] [PubMed] [Google Scholar]
- Bennett V. The membrane skeleton of human erythrocytes and its implications for more complex cells. Annu Rev Biochem. 1985;54:273–304. doi: 10.1146/annurev.bi.54.070185.001421. [DOI] [PubMed] [Google Scholar]
- Birkenmeier C. S., Bodine D. M., Repasky E. A., Helfman D. M., Hughes S. H., Barker J. E. Remarkable homology among the internal repeats of erythroid and nonerythroid spectrin. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5671–5675. doi: 10.1073/pnas.82.17.5671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis P. J., Palumbo A., Ming J., Fraser P., Cioe L., Meo P., Shane S., Rovera G. Sequence comparison of human and murine erythrocyte alpha-spectrin cDNA. Gene. 1985;36(3):357–362. doi: 10.1016/0378-1119(85)90191-x. [DOI] [PubMed] [Google Scholar]
- Dale R. M., McClure B. A., Houchins J. P. A rapid single-stranded cloning strategy for producing a sequential series of overlapping clones for use in DNA sequencing: application to sequencing the corn mitochondrial 18 S rDNA. Plasmid. 1985 Jan;13(1):31–40. doi: 10.1016/0147-619x(85)90053-8. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Ferguson-Smith M. A., Aitken D. A., Turleau C., de Grouchy J. Localisation of the human ABO: Np-1: AK-1 linkage group by regional assignment of AK-1 to 9q34. Hum Genet. 1976 Sep 10;34(1):35–43. doi: 10.1007/BF00284432. [DOI] [PubMed] [Google Scholar]
- Francke U., Busby N., Shaw D., Hansen S., Brown M. G. Intrachromosomal gene mapping in man: assignment of nucleoside phosphorylase to region 14cen leads to 14q21 by interspecific hybridization of cells with a t(X;14) (p22;q21) translocation. Somatic Cell Genet. 1976 Jan;2(1):27–40. doi: 10.1007/BF01539240. [DOI] [PubMed] [Google Scholar]
- Francke U., Francke B. Requirement of the human chromosome 11 long arm for replication of herpes simplex virus type 1 in nonpermissive Chinese hamster x human diploid fibroblast hybrids. Somatic Cell Genet. 1981 Mar;7(2):171–191. doi: 10.1007/BF01567656. [DOI] [PubMed] [Google Scholar]
- Francke U., Pellegrino M. A. Assignment of the major histocompatibility complex to a region of the short arm of human chromosome 6. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1147–1151. doi: 10.1073/pnas.74.3.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francke U. Random X inactivation resulting in mosaic nullisomy of region Xp21.1----p21.3 associated with heterozygosity for ornithine transcarbamylase deficiency and for chronic granulomatous disease. Cytogenet Cell Genet. 1984;38(4):298–307. doi: 10.1159/000132078. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Osborn M., Weber K. An F-actin- and calmodulin-binding protein from isolated intestinal brush borders has a morphology related to spectrin. Cell. 1982 Apr;28(4):843–854. doi: 10.1016/0092-8674(82)90063-0. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Glenney P., Weber K. Erythroid spectrin, brain fodrin, and intestinal brush border proteins (TW-260/240) are related molecules containing a common calmodulin-binding subunit bound to a variant cell type-specific subunit. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4002–4005. doi: 10.1073/pnas.79.13.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper M. E., Saunders G. F. Localization of single copy DNA sequences of G-banded human chromosomes by in situ hybridization. Chromosoma. 1981;83(3):431–439. doi: 10.1007/BF00327364. [DOI] [PubMed] [Google Scholar]
- Harris A. S., Green L. A., Ainger K. J., Morrow J. S. Mechanism of cytoskeletal regulation (I): functional differences correlate with antigenic dissimilarity in human brain and erythrocyte spectrin. Biochim Biophys Acta. 1985 Aug 8;830(2):147–158. doi: 10.1016/0167-4838(85)90022-6. [DOI] [PubMed] [Google Scholar]
- Heisterkamp N., Groffen J., Stephenson J. R., Spurr N. K., Goodfellow P. N., Solomon E., Carritt B., Bodmer W. F. Chromosomal localization of human cellular homologues of two viral oncogenes. Nature. 1982 Oct 21;299(5885):747–749. doi: 10.1038/299747a0. [DOI] [PubMed] [Google Scholar]
- Huebner K., Palumbo A. P., Isobe M., Kozak C. A., Monaco S., Rovera G., Croce C. M., Curtis P. J. The alpha-spectrin gene is on chromosome 1 in mouse and man. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3790–3793. doi: 10.1073/pnas.82.11.3790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohl N. E., Gee C. E., Alt F. W. Activated expression of the N-myc gene in human neuroblastomas and related tumors. Science. 1984 Dec 14;226(4680):1335–1337. doi: 10.1126/science.6505694. [DOI] [PubMed] [Google Scholar]
- Levine J., Willard M. Redistribution of fodrin (a component of the cortical cytoplasm) accompanying capping of cell surface molecules. Proc Natl Acad Sci U S A. 1983 Jan;80(1):191–195. doi: 10.1073/pnas.80.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Maizel J. V., Jr, Lenk R. P. Enhanced graphic matrix analysis of nucleic acid and protein sequences. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7665–7669. doi: 10.1073/pnas.78.12.7665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi V. T. Stabilizing infrastructure of cell membranes. Annu Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- McMahon A. P., Giebelhaus D. H., Champion J. E., Bailes J. A., Lacey S., Carritt B., Henchman S. K., Moon R. T. cDNA cloning, sequencing and chromosome mapping of a non-erythroid spectrin, human alpha-fodrin. Differentiation. 1987;34(1):68–78. doi: 10.1111/j.1432-0436.1987.tb00052.x. [DOI] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Morrow J. S., Marchesi V. T. Self-assembly of spectrin oligomers in vitro: a basis for a dynamic cytoskeleton. J Cell Biol. 1981 Feb;88(2):463–468. doi: 10.1083/jcb.88.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson W. J., Colaço C. A., Lazarides E. Involvement of spectrin in cell-surface receptor capping in lymphocytes. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1626–1630. doi: 10.1073/pnas.80.6.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Repasky E. A., Granger B. L., Lazarides E. Widespread occurrence of avian spectrin in nonerythroid cells. Cell. 1982 Jul;29(3):821–833. doi: 10.1016/0092-8674(82)90444-5. [DOI] [PubMed] [Google Scholar]
- Repasky E. A., Symer D. E., Bankert R. B. Spectrin immunofluorescence distinguishes a population of naturally capped lymphocytes in situ. J Cell Biol. 1984 Jul;99(1 Pt 1):350–355. doi: 10.1083/jcb.99.1.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sears D. E., Marchesi V. T., Morrow J. S. A calmodulin and alpha-subunit binding domain in human erythrocyte spectrin. Biochim Biophys Acta. 1986 Apr 22;870(3):432–442. doi: 10.1016/0167-4838(86)90251-7. [DOI] [PubMed] [Google Scholar]
- Speicher D. W., Marchesi V. T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984 Sep 13;311(5982):177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Speicher D. W. The present status of erythrocyte spectrin structure: the 106-residue repetitive structure is a basic feature of an entire class of proteins. J Cell Biochem. 1986;30(3):245–258. doi: 10.1002/jcb.240300306. [DOI] [PubMed] [Google Scholar]
- Wasenius V. M., Saraste M., Knowles J., Virtanen I., Lehto V. P. Sequencing of the chicken non-erythroid spectrin cDNA reveals an internal repetitive structure homologous to the human erythrocyte spectrin. EMBO J. 1985 Jun;4(6):1425–1430. doi: 10.1002/j.1460-2075.1985.tb03797.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]