Abstract
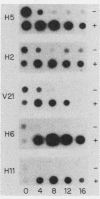
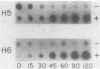
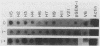
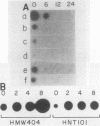
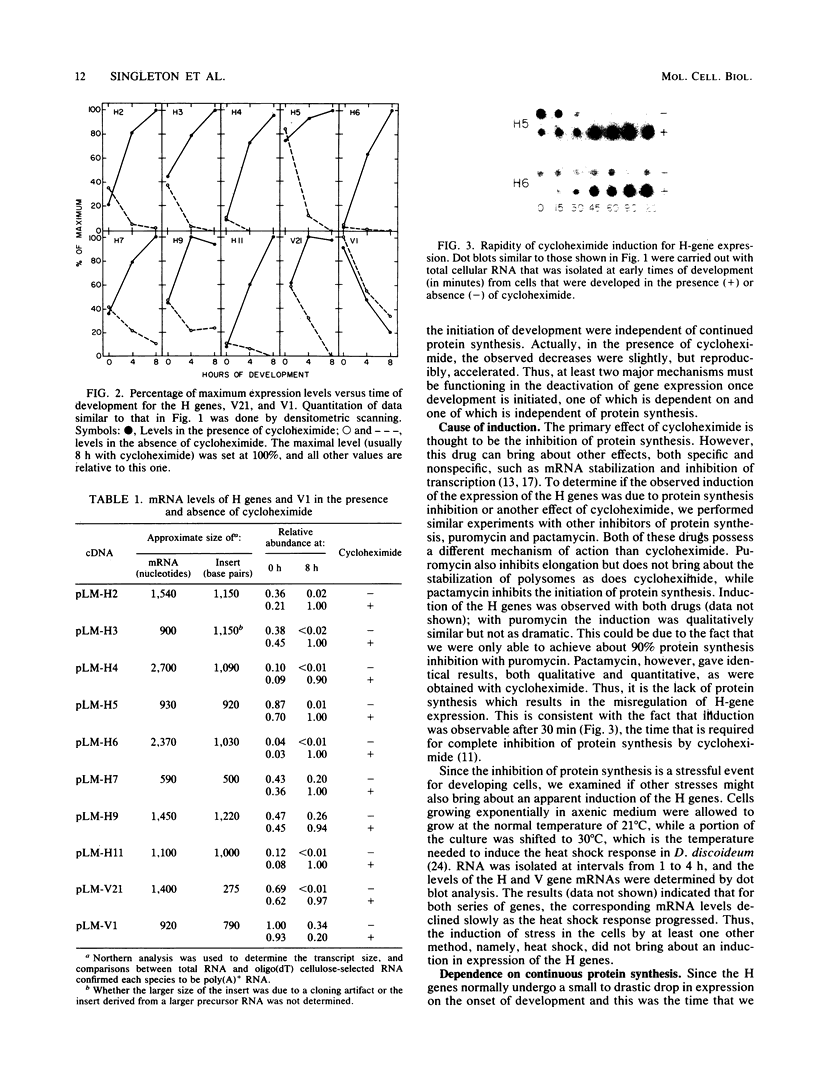
Several genes which are deactivated on the initiation of development of Dictyostelium discoideum were identified by differential screening of various cDNA libraries. These genes have in common a decrease in the steady-state levels of their corresponding mRNAs on the onset of development and as development proceeds. When development was carried out in the absence of protein synthesis by inhibition with cycloheximide, the decrease in mRNA levels for most genes (V genes) was normal or slightly accelerated. For about 5% of the genes (H genes), however, cycloheximide caused an apparent induction of expression, as revealed by a slight or dramatic increase in mRNA levels, instead of the normal decrease. This effect was due to inhibition of protein synthesis and not to cycloheximide per se. The induction was found to be due to an enhancement of the transcription rate; normal rates of transcription for the H genes were dependent on continued protein synthesis during vegetative growth and development. Thus, two general regulatory classes exist for deactivation of gene expression on initiation of development, one of which is dependent on and one of which is independent of protein synthesis. Analysis of expression of these genes in mutant strains which are aggregation deficient allowed the classes to be subdivided further. Taken together, these characterizations allow several distinct regulatory mechanisms to be identified that are involved in the deactivation of gene expression on the onset of development in D. discoideum.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alton T. H., Lodish H. F. Developmental changes in messenger RNAs and protein synthesis in Dictyostelium discoideum. Dev Biol. 1977 Oct 1;60(1):180–206. doi: 10.1016/0012-1606(77)90118-x. [DOI] [PubMed] [Google Scholar]
- Barklis E., Lodish H. F. Regulation of dictyostelium discoideum mRNAs specific for prespore or prestalk cells. Cell. 1983 Apr;32(4):1139–1148. doi: 10.1016/0092-8674(83)90297-0. [DOI] [PubMed] [Google Scholar]
- Cardelli J. A., Knecht D. A., Wunderlich R., Dimond R. L. Major changes in gene expression occur during at least four stages of development of Dictyostelium discoideum. Dev Biol. 1985 Jul;110(1):147–156. doi: 10.1016/0012-1606(85)90072-7. [DOI] [PubMed] [Google Scholar]
- Chisholm R. L., Barklis E., Lodish H. F. Mechanism of sequential induction of cell-type specific mRNAs in Dictyostelium differentiation. Nature. 1984 Jul 5;310(5972):67–69. doi: 10.1038/310067a0. [DOI] [PubMed] [Google Scholar]
- Crowley T. E., Nellen W., Gomer R. H., Firtel R. A. Phenocopy of discoidin I-minus mutants by antisense transformation in Dictyostelium. Cell. 1985 Dec;43(3 Pt 2):633–641. doi: 10.1016/0092-8674(85)90235-1. [DOI] [PubMed] [Google Scholar]
- Elder P. K., Schmidt L. J., Ono T., Getz M. J. Specific stimulation of actin gene transcription by epidermal growth factor and cycloheximide. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7476–7480. doi: 10.1073/pnas.81.23.7476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo T., Nadal-Ginard B. Transcriptional and posttranscriptional control of c-myc during myogenesis: its mRNA remains inducible in differentiated cells and does not suppress the differentiated phenotype. Mol Cell Biol. 1986 May;6(5):1412–1421. doi: 10.1128/mcb.6.5.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finney R. E., Langtimm C. J., Soll D. R. A characterization of the preaggregative period of Dictyostelium discoideum. Dev Biol. 1985 Jul;110(1):157–170. doi: 10.1016/0012-1606(85)90073-9. [DOI] [PubMed] [Google Scholar]
- Finney R. E., Langtimm C. J., Soll D. R. Regulation of protein synthesis during the preaggregative period of Dictyostelium discoideum development: involvement of close cell associations and cAMP. Dev Biol. 1985 Jul;110(1):171–191. doi: 10.1016/0012-1606(85)90074-0. [DOI] [PubMed] [Google Scholar]
- Finney R., Varnum B., Soll D. R. "Erasure" in Dictyostelium: a dedifferentiation involving the programmed loss of chemotactic functions. Dev Biol. 1979 Dec;73(2):290–303. doi: 10.1016/0012-1606(79)90068-x. [DOI] [PubMed] [Google Scholar]
- Gomer R. H., Datta S., Firtel R. A. Cellular and subcellular distribution of a cAMP-regulated prestalk protein and prespore protein in Dictyostelium discoideum: a study on the ontogeny of prestalk and prespore cells. J Cell Biol. 1986 Nov;103(5):1999–2015. doi: 10.1083/jcb.103.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg M. E., Hermanowski A. L., Ziff E. B. Effect of protein synthesis inhibitors on growth factor activation of c-fos, c-myc, and actin gene transcription. Mol Cell Biol. 1986 Apr;6(4):1050–1057. doi: 10.1128/mcb.6.4.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby D. B., Engle J. A., Towle H. C. Induction of a rapidly responsive hepatic gene product by thyroid hormone requires ongoing protein synthesis. Mol Cell Biol. 1987 Apr;7(4):1352–1357. doi: 10.1128/mcb.7.4.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R., Shaw D. R., Ennis H. L. Role of protein synthesis in decay and accumulation of mRNA during spore germination in the cellular slime mold Dictyostelium discoideum. Mol Cell Biol. 1987 Feb;7(2):799–805. doi: 10.1128/mcb.7.2.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Identification of a set of genes expressed during the G0/G1 transition of cultured mouse cells. EMBO J. 1985 Dec 1;4(12):3145–3151. doi: 10.1002/j.1460-2075.1985.tb04057.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linial M., Gunderson N., Groudine M. Enhanced transcription of c-myc in bursal lymphoma cells requires continuous protein synthesis. Science. 1985 Dec 6;230(4730):1126–1132. doi: 10.1126/science.2999973. [DOI] [PubMed] [Google Scholar]
- Livi G. P., Cardelli J. A., Dimond R. L. alpha-Mannosidase-1 mutants of Dictyostelium discoideum: early aggregation-essential genes regulate enzyme precursor synthesis, modification, and processing. Differentiation. 1985;29(3):207–215. doi: 10.1111/j.1432-0436.1985.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Loomis W. F., Wheeler S. Heat shock response of Dictyostelium. Dev Biol. 1980 Oct;79(2):399–408. doi: 10.1016/0012-1606(80)90125-6. [DOI] [PubMed] [Google Scholar]
- Mehdy M. C., Ratner D., Firtel R. A. Induction and modulation of cell-type-specific gene expression in Dictyostelium. Cell. 1983 Mar;32(3):763–771. doi: 10.1016/0092-8674(83)90062-4. [DOI] [PubMed] [Google Scholar]
- Oyama M., Blumberg D. D. Changes during differentiation in requirements for cAMP for expression of cell-type-specific mRNAs in the cellular slime mold, Dictyostelium discoideum. Dev Biol. 1986 Oct;117(2):550–556. doi: 10.1016/0012-1606(86)90323-4. [DOI] [PubMed] [Google Scholar]
- Poole S. J., Firtel R. A. Genomic instability and mobile genetic elements in regions surrounding two discoidin I genes of Dictyostelium discoideum. Mol Cell Biol. 1984 Apr;4(4):671–680. doi: 10.1128/mcb.4.4.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringold G. M., Dieckmann B., Vannice J. L., Trahey M., McCormick F. Inhibition of protein synthesis stimulates the transcription of human beta-interferon genes in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1984 Jul;81(13):3964–3968. doi: 10.1073/pnas.81.13.3964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Baltimore D. Inducibility of kappa immunoglobulin enhancer-binding protein Nf-kappa B by a posttranslational mechanism. Cell. 1986 Dec 26;47(6):921–928. doi: 10.1016/0092-8674(86)90807-x. [DOI] [PubMed] [Google Scholar]
- Singleton C. K., Delude R. L., McPherson C. E. Characterization of genes which are deactivated upon the onset of development in Dictyostelium discoideum. Dev Biol. 1987 Feb;119(2):433–441. doi: 10.1016/0012-1606(87)90047-9. [DOI] [PubMed] [Google Scholar]
- Soll D. R., Mitchell L., Kraft B., Alexander S., Finney R., Varnum-Finney B. Characterization of a timing mutant of Dictyostelium discoideum which exhibits "high frequency switching". Dev Biol. 1987 Mar;120(1):25–37. doi: 10.1016/0012-1606(87)90100-x. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Lee K. L., Kenney F. T. Differential degradation of messenger RNAs in mammalian cells. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2634–2638. doi: 10.1073/pnas.73.8.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weijer C. J., Duschl G., David C. N. A revision of the Dictyostelium discoideum cell cycle. J Cell Sci. 1984 Aug;70:111–131. doi: 10.1242/jcs.70.1.111. [DOI] [PubMed] [Google Scholar]
- Williams J. G., Ceccarelli A., McRobbie S., Mahbubani H., Kay R. R., Early A., Berks M., Jermyn K. A. Direct induction of Dictyostelium prestalk gene expression by DIF provides evidence that DIF is a morphogen. Cell. 1987 Apr 24;49(2):185–192. doi: 10.1016/0092-8674(87)90559-9. [DOI] [PubMed] [Google Scholar]