Abstract
PURPOSE:
The purpose of this study was to describe an approach to surgical management of bronchogenic cysts based on the natural course observed from the time of antenatal screening to surgical resection in patients treated at our institution and reported in the literature.
MATERIALS AND METHODS:
We retrospectively reviewed the clinical features of all children presenting bronchogenic cyst diagnosed antenatally from 2007 to 2010. A total of six children were included.
RESULTS:
Antenatal diagnosis was accurate in 62.5% of cases. In the first year of life, the size of the cyst remained stable in four patients, doubled in one, and increased 30% within six months in one. The indication for surgery was emphysema of the left bronchus in two patients and rapid growth in two patients. One patient is still awaiting surgery.
CONCLUSION:
Bronchogenic cysts grow slowly in the first months of life, but growth is exponential even in the absence of complications. We recommend complete resection before the age of two years to prevent infectious complications and facilitate surgery.
Keywords: Adenomatoid malformation, antenatal diagnosis, bronchogenic cyst, fetus, growth, thoracoscopy
INTRODUCTION
Bronchogenic cyst is a benign congenital malformation usually located in the mediastinum. During childhood, these lesions may remain totally asymptomatic but they frequently lead to symptoms and complications including infection or compression of the airways and/or heart. Complications can also occur in adulthood. Before the advent of antenatal diagnosis, most bronchogenic cysts were identified late in childhood either as an incidental finding or following complications. Antenatal diagnosis has altered the treatment paradigm by enabling early intervention, but the optimal strategy has not been defined. The purpose of this study was to describe an approach to surgical management of bronchogenic cysts based on the natural course observed from the time of antenatal screening to surgical resection in patients treated at our institution and reported in the literature.
MATERIALS AND METHODS
This retrospective study included all bronchogenic cysts diagnosed antenatally at the Timone University Children's Hospital from January 2007 to May 2010. The clinical history of these children was reviewed including the following items: Date of diagnosis, cyst location, size and growth evolution, eventual complications, and indication/date of surgery. Cyst size was defined as the maximal diameter along a single axis. For radiological diagnosis, bronchogenic cyst was defined as a single, homogeneous, well-limited, fluid-filled mass located in the mediastinum close to the tracheobronchial tree or esophagus.
RESULTS
During the study period, a total of twelve children were treated for bronchogenic cyst[1] including sixin whom diagnosis was achieved antenatally. Four children that were treated surgically were excluded because diagnosis was not achieved antenatally and two because subsequent post-natal evaluation demonstrated cystic adenomatoid malformation. One infant in whom antenatal diagnosis was cystic adenomatoid malformation but postnatal diagnosis demonstrated bronchogenic cyst could not be included because complete data was not available. Table 1 shows clinical findings and management techniques used in these 6 children.
Table 1.
Clinical data and management in the six infants included in the study
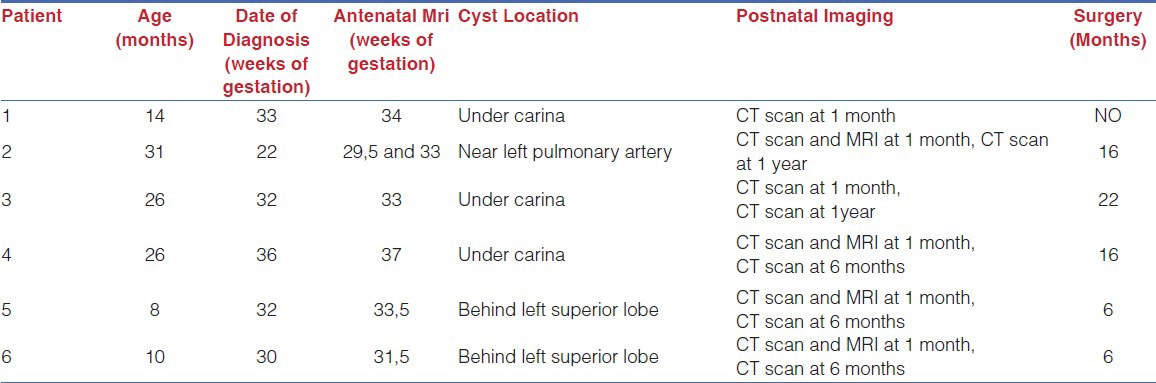
There were five boys and one girl ranging in age from five to thirty-one months. In one patient, antenatal diagnosis was achieved by morphological ultrasound during the second trimester of pregnancy. In the remaining five patients, diagnosis was made during the third trimester. In all cases, antenatal magnetic resonance imaging (MRI) was performed at the beginning of the third trimester. After birth, computed tomography (CT) scan was carried out at one month in all cases and repeated at one year in cases 1, 2 and 3 and at six months in cases 4, 5 and 6. In cases 2, 4, 5 and 6, MRI was performed one month after birth to confirm diagnosis. Overall, antenatal screening for pulmonary malformations led to a correct diagnosis in 62.5% (5/8) of cases (patients 1, 2, 4, 5 and 6), false-positive diagnosis in two (patients 7 and 8), and false-negative diagnosis in one (patient 3).
The bronchogenic cyst was located under the carina in three cases (patients 1, 3 and 4), near the left pulmonary artery in one (patient 2) and behind the left superior lobe in two (patients 5 and 6). Cyst diameter at one month ranged from five to twenty-two millimeters. For four children (patients 1, 2, 4 and 5), imaging depicted no change in cyst size between the time of antenatal detection and CT scan at one month of life. Conversely, cyst size increased 40% in patient 3 and 150% in patient 6. During the first year of life, cyst size remained stable in four (patients 1, 3, 5 and 6), doubled in one (patient 2), and increased by 30% within six months in one (patient 4) [Figure 1].
Figure 1.
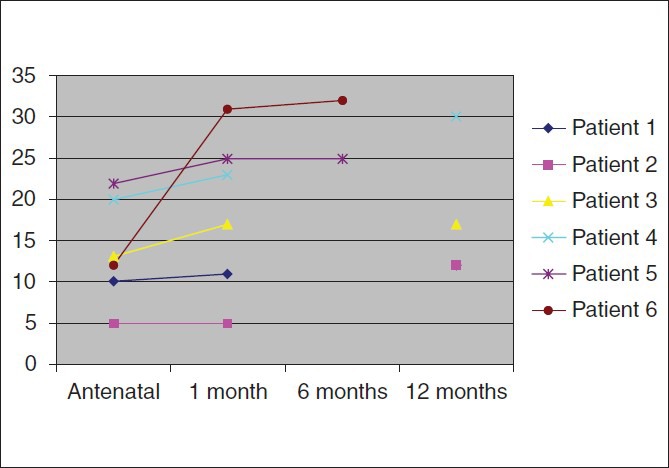
Growth of bronchogenic cysts (in mm) from antennal diagnosis to the time of surgery
The only adverse event observed was pulmonary infection that occurred in only one case (patient 3) at eleven months and was treated at another hospital. No other adverse events occurred in this child, but follow-up examination with pulmonary x-ray at one year depicted stretching of the left bronchus and persistent tracheobronchial tree compression on the CT scan. Elective resection of the stable cyst was carried out at twenty two months. In two cases (patients 5 and 6), surgical resection was indicated at six months for emphysema of the left superior lobe and rapid cyst growth. In two cases (patients 2 and 4), resection was performed at 16 months for emphysema of the left superior lobe and rapid cyst growth. Patient 1 is awaiting surgery at fourteen months.
Histological examination of surgical specimens confirmed diagnosis of bronchogenic cyst in all five cases. All specimens exhibited ciliated epithelium associated, in some cases, with a cartilaginous component inside the cyst.
DISCUSSION
The purpose of this study was to describe the natural history of bronchogenic cysts between antenatal diagnosis and postnatal management, as a basis for planning therapeutic strategy. The first finding was a high rate of error in antenatal diagnosis with wrong conclusions being drawn in three out of eight children (37.5%). Davenport et al. reported accurate antenatal ultrasound diagnosis in 77 to 100% of cases with better performance of equipment and interpretation of images.[2] Antenatal MRI provides better identification of pulmonary malformations including congenital cystic adenomatoid malformation (CCAM), pulmonary sequestration, bronchogenic cyst, but reported error rates still range from 6 to 16%.[3–6] Bronchogenic cysts appear as well delineated, round, homogeneous, fluid-filled, unilocular masses. In 85% of cases, the cyst is located close to tracheobronchial tree or esophagus. Differential diagnosis is difficult with several entities including CCAM, esophageal duplication, emphysema, and neurenteric cyst. In accordance with previous reports, our series confirms the need for postnatal confirmation by CT-scan that allows detailed study of blood supply and connections with tracheobronchial tree. If necessary to confirm diagnosis, MRI can also be performed.[2,3,7] If respiratory status allows, postnatal imaging to confirm etiology and determine anatomic relations with the tracheal-bronchial tree and adjacent structures can be carried out at one month of life without anesthesia. Our approach consists of performing simple pulmonary x-ray at one month followed by CT-scan at one year of life or if complications occur. We agree with previous authors[2] that yearly CT scan in small children is contraindicated due to risks associated with repeated radiation exposure. This position also explains the short follow-up in this series.
Another interesting finding of this study involved cyst growth during the first year of life. The cyst increased 1.3 to 2 fold in two patients and remained stable in four. Growth was probably due to increased mucus secretion. Considering a cyst as a sphere (volume = 4/3πr3), a 0.5 fold increase in size would correspond to a 14-fold increase in volume. Two patientsunderwent resection due to rapid cyst growth from one and two centimeters antenatally to about threecentimeters at the time of resection. In patient 2, cyst size increased (five to twelve centimeters) but the indication for surgery was presence of emphysema. To our knowledge, no previous studies have provided data on cyst growth. In their series including twenty two children operated on for bronchogenic cyst at ages ranging from one month to nine years, J.L Michel et al.[8] only indicated cyst size at the time of the surgery: Three to four centimeters at four years, of age, three centimeters at six years and fivecentimeters at nine years. In studies including adults, mean cyst size was about ten to fifteen centimeters.[9] Based on these findings, it can be speculated that cysts have an exponential growth from about one to two centimeters at birth to ten centimeters in adulthood. This suggests that early surgery is indicated and that the best time is during the second year of life. This timing is supported by the facts that early intervention is possible since no parenchymal resection is required and that larger thoracic size in the second year of life greatly facilitates surgery. In this regard, thoracoscopic resection is often possible because larger cyst size decreases the risk of damaging the bronchus.[10] Early surgery may reduce the risk of wide resection that led to lobectomy in nine of the thirteen patients in the series of Takeda et al.[11]
All children in our series were asymptomatic at birth. Only one developed infectious symptoms at one month old. In the 13-patient series of Takeda et al.,[11] only one child who presented mediastinal deviation on the pulmonary x-ray at birth developed symptoms, i.e., dyspnea, in the first year. The other twelve children who underwent resection at later times ranging from two to ten years did not present repeated infectious symptoms until the last months before surgery. In their comparison of bronchogenic cysts in childhood and adulthood,[3,9] Ribet et al. reported that occurrence of symptoms and compressive complications was more common in children and more strongly correlated with location than size. No case of malignant transformation has been reported in the literature and the dysplasia rate in adult cases has ranged from 15 to 30%.[3,9]
Our series is too small to draw any conclusions about the most appropriate follow-up strategy in children that undergo resection for bronchogenic cysts. However, perusal of previous reports on congenital pulmonary malformations highlights a consensus for yearly pulmonary CT-scan.[1] Our approach after antenatal diagnosis calls for CT-scan without anesthesia at one month after birth to confirm the malformation followed by chest x-ray every three months to rule out parenchymal complications. Repeat CT-scan is performed at one year before surgery.
CONCLUSION
Based on our experience and perusal of the literature, we have concluded that the growth rate of bronchogenic cysts is slow in the first months of life, but that, even in the absence of complications, size increases constantly at an exponential rate. We recommend complete resection before the age of two in order to prevent complications and facilitate surgery. Patients should be kept under regularly surveillance even though most bronchogenic cysts are now diagnosed antenatally.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Zeidan S, Gorincour G, Potier A, Ughetto F, Dubus JC, Chrestian MA, et al. Congenital lung malformation: Evaluation of prenatal and postnatal radiological findings. Respirology. 2009;14:1005–11. doi: 10.1111/j.1440-1843.2009.01591.x. [DOI] [PubMed] [Google Scholar]
- 2.Davenport M, Warne SA, Cacciaguerra S, Patel S, Greenough A, Nicolaides K. Current outcome of antenally diagnosed cystic lung disease. J Pediatr Surg. 2004;39:549–56. doi: 10.1016/j.jpedsurg.2003.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Ribet ME, Copin MC, Gosselin B. Bronchogenic cysts of the mediastinum. J Thorac Cardiovasc Surg. 109:1003–10. doi: 10.1016/S0022-5223(95)70327-6. [DOI] [PubMed] [Google Scholar]
- 4.Pumberger W, Hormann M, Deutinger J, Bernaschek G, Bistricky E, Horcher E. Longitudinal observation of antenatally detected congenital lung malformations (CLM): Natural history, clinical outcome and long-term follow-up. Eur J Cardiothorac Surg. 2003;24:703–11. doi: 10.1016/j.ejcts.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugam G, Mac Arthur K, Pollock JC. Congenital lung malformations - antenatal and postnatal evaluation and management. Eur J Cardiothorac Surg. 27:45–52. doi: 10.1016/j.ejcts.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 6.Roggin KK, Breuer CK, Carr SR, Hansen K, Kurkchubasche AG, Wesselhoeft CW, Jr, et al. The unpredictable character of congenital cystic lung lesions. J Pediatr Surg. 2000;35:801–5. doi: 10.1053/jpsu.2000.6088. [DOI] [PubMed] [Google Scholar]
- 7.Eber E. Antenatal diagnosis of congenital thoracic malformations: Early surgery, late surgery or no surgery? Semin Respir Crit Care Med. 2007;28:355–66. doi: 10.1055/s-2007-981656. [DOI] [PubMed] [Google Scholar]
- 8.Michel JL, Revillon Y, Montupet P, Sauvat F, Sarnacki S, Sayegh N, et al. Thoracoscopic treatment of mediastinal cysts in children. J Pediatr Surg. 1998;33:1745–8. doi: 10.1016/s0022-3468(98)90276-7. [DOI] [PubMed] [Google Scholar]
- 9.Ribet ME, Copin MC, Gosselin BH. Bronchogenic cysts of the lung. Ann Thorac Surg. 1996;61:1636–40. doi: 10.1016/0003-4975(96)00172-5. [DOI] [PubMed] [Google Scholar]
- 10.Tolg C, Abelin K, Laudenbach V, de Heaulme O, Dorgeret S, Lipsyc ES, et al. Open vsthorascopic surgical management of bronchogenic cysts. Surg Endosc. 2005;19:77–80. doi: 10.1007/s00464-003-9328-x. [DOI] [PubMed] [Google Scholar]
- 11.Takeda S, Miyoshi S, Inoue M, Omori K, Okumura M, Yoon HE. Clinical spectrum of congenital cystic disease of the lung in chldren. Eur J Cardiothorac Surg. 1999;15:11–7. doi: 10.1016/s1010-7940(98)00262-0. [DOI] [PubMed] [Google Scholar]


