Abstract
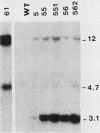
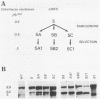
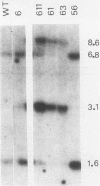
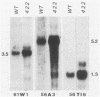
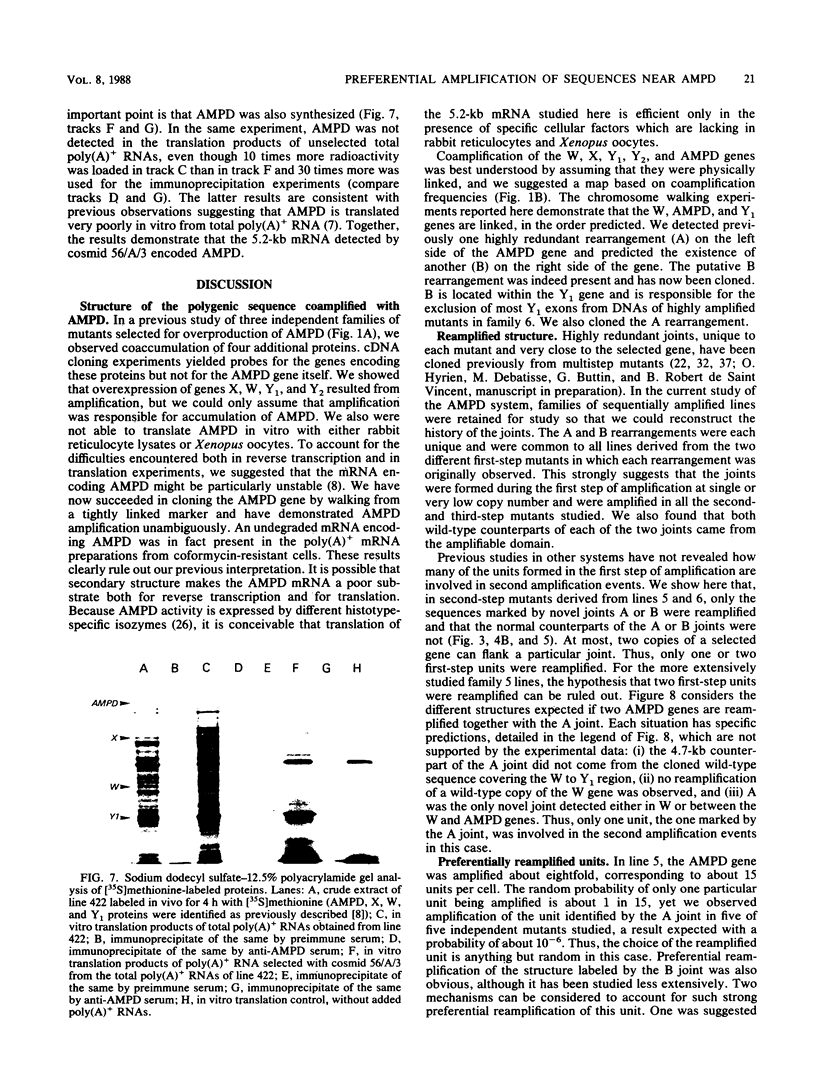
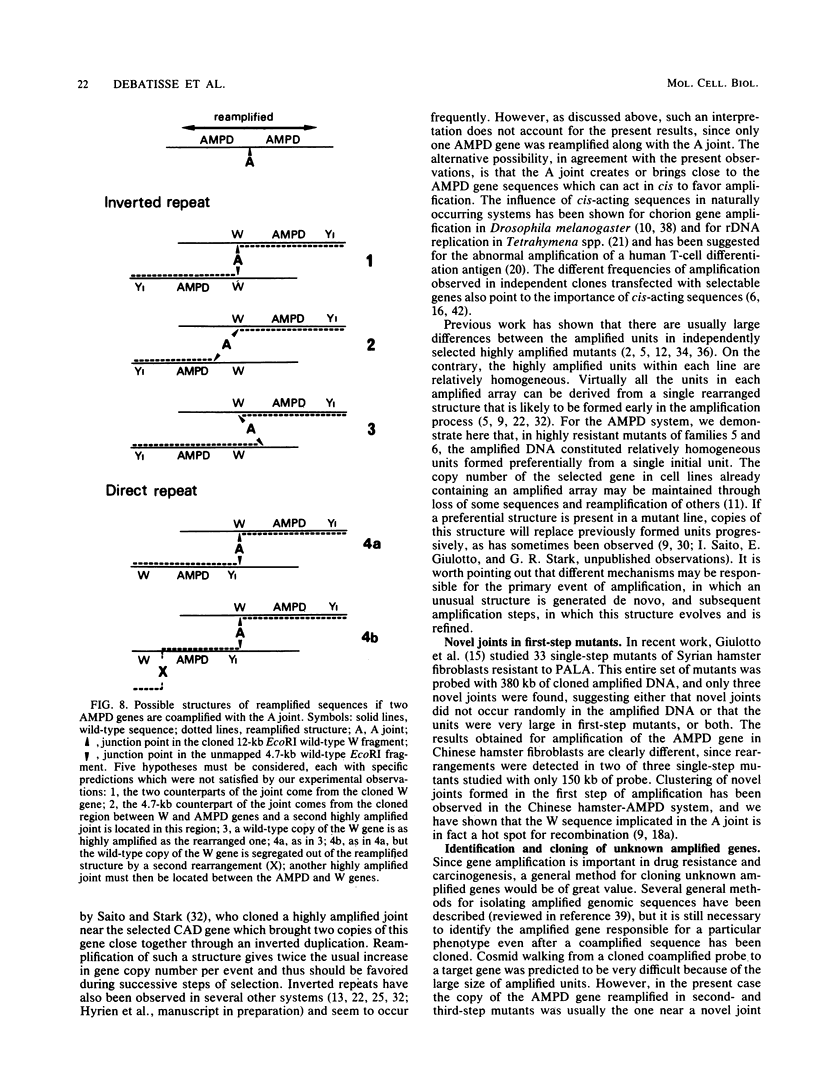
In a previous study of three independent families of mutants selected for overproduction of adenylate deaminase (AMPD), we were not able to isolate a cDNA probe for the gene and so could not demonstrate its amplification directly. In addition to overproduction of AMPD, four proteins of unknown function, designated W, X, Y1, and Y2, accumulated, and by using the corresponding cDNA probes, we demonstrated amplification of all four genes. In independent mutant clones, sometimes all and sometimes only a subset of these genes were amplified. Assuming that all five genes are linked, the pattern of their coamplification suggested a genetic map in which AMPD lies between W and Y1. We show here that a two-step chromosome walk joins the W and Y1 genes, that the AMPD gene is the only expressed sequence between them, and that its amplification is indeed responsible for overproduction of the AMPD protein. In the course of this work, we cloned and studied two novel joints which mark rearrangements on either side of the AMPD gene. Each joint was generated independently in a single first-step mutant at single or low copy number. Remarkably, each joint was amplified preferentially in every second- and third-step mutant derived from the first-step line in which it was originally present, suggesting that the two independent rearrangements each generated amplification-prone structures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agarwal R. P., Parks R. E. Potent inhibition of muscle 5'-AMP deaminase by the nucleoside antibiotics coformycin and deoxycoformycin. Biochem Pharmacol. 1977 Apr 1;26(7):663–666. doi: 10.1016/0006-2952(77)90046-6. [DOI] [PubMed] [Google Scholar]
- Ardeshir F., Giulotto E., Zieg J., Brison O., Liao W. S., Stark G. R. Structure of amplified DNA in different Syrian hamster cell lines resistant to N-(phosphonacetyl)-L-aspartate. Mol Cell Biol. 1983 Nov;3(11):2076–2088. doi: 10.1128/mcb.3.11.2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brezin C., Cazenave P. A. La réaction croisee entre le motif allotypique Aa1 des immunoglobulines du lapin et les anticorps dirigés contre le motif allotypique Aa3: participation des variantes de la spécificité Aa1 à cette réaction croiśee. Immunochemistry. 1975 Mar;12(3):241–247. doi: 10.1016/0019-2791(75)90238-4. [DOI] [PubMed] [Google Scholar]
- Brison O., Ardeshir F., Stark G. R. General method for cloning amplified DNA by differential screening with genomic probes. Mol Cell Biol. 1982 May;2(5):578–587. doi: 10.1128/mcb.2.5.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caizzi R., Bostock C. J. Gene amplification in methotrexate-resistant mouse cells. IV. Different DNA sequences are amplified in different resistant lines. Nucleic Acids Res. 1982 Nov 11;10(21):6597–6618. doi: 10.1093/nar/10.21.6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll S. M., Gaudray P., De Rose M. L., Emery J. F., Meinkoth J. L., Nakkim E., Subler M., Von Hoff D. D., Wahl G. M. Characterization of an episome produced in hamster cells that amplify a transfected CAD gene at high frequency: functional evidence for a mammalian replication origin. Mol Cell Biol. 1987 May;7(5):1740–1750. doi: 10.1128/mcb.7.5.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
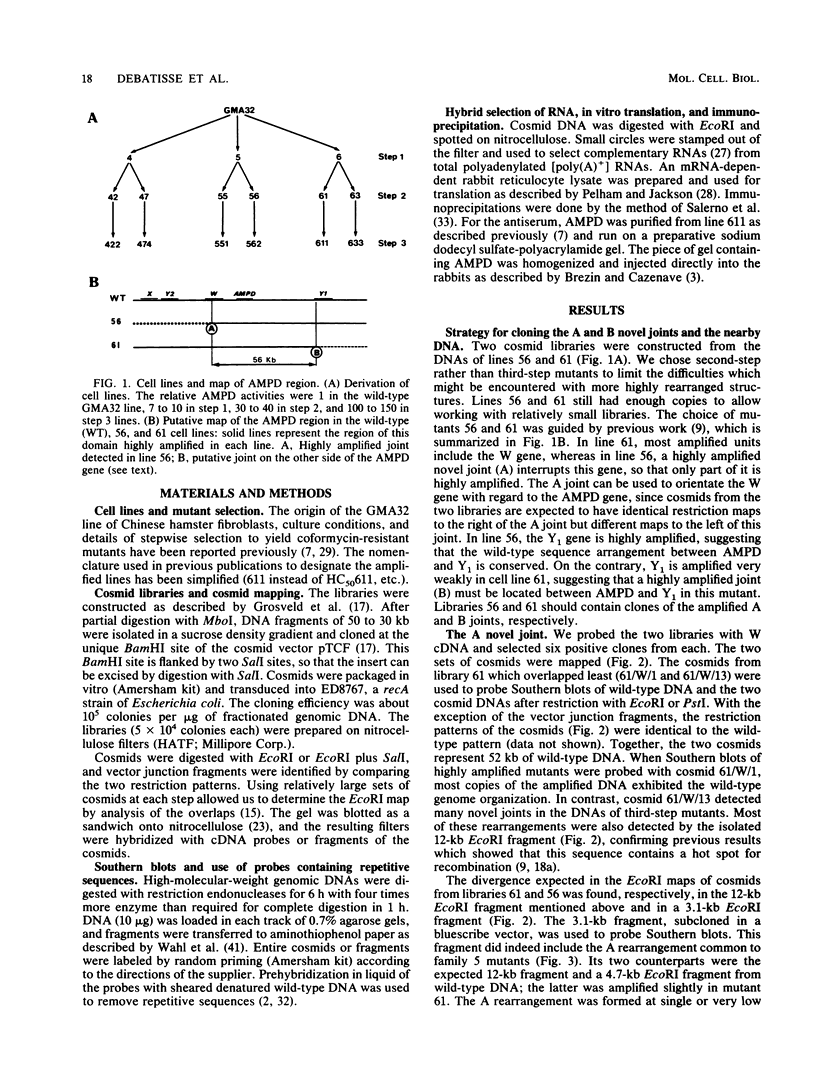
- Debatisse M., Berry M., Buttin G. Stepwise isolation and properties of unstable Chinese hamster cell variants that overproduce adenylate deaminase. Mol Cell Biol. 1982 Nov;2(11):1346–1353. doi: 10.1128/mcb.2.11.1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debatisse M., Hyrien O., Petit-Koskas E., de Saint-Vincent B. R., Buttin G. Segregation and rearrangement of coamplified genes in different lineages of mutant cells that overproduce adenylate deaminase. Mol Cell Biol. 1986 May;6(5):1776–1781. doi: 10.1128/mcb.6.5.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debatisse M., de Saint Vincent B. R., Buttin G. Expression of several amplified genes in an adenylate-deaminase overproducing variant of Chinese hamster fibroblasts. EMBO J. 1984 Dec 20;3(13):3123–3127. doi: 10.1002/j.1460-2075.1984.tb02268.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federspiel N. A., Beverley S. M., Schilling J. W., Schimke R. T. Novel DNA rearrangements are associated with dihydrofolate reductase gene amplification. J Biol Chem. 1984 Jul 25;259(14):9127–9140. [PubMed] [Google Scholar]
- Fojo A. T., Whang-Peng J., Gottesman M. M., Pastan I. Amplification of DNA sequences in human multidrug-resistant KB carcinoma cells. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7661–7665. doi: 10.1073/pnas.82.22.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford M., Fried M. Large inverted duplications are associated with gene amplification. Cell. 1986 May 9;45(3):425–430. doi: 10.1016/0092-8674(86)90328-4. [DOI] [PubMed] [Google Scholar]
- George D. L., Powers V. E. Cloning of DNA from double minutes of Y1 mouse adrenocortical tumor cells: evidence for gene amplification. Cell. 1981 Apr;24(1):117–123. doi: 10.1016/0092-8674(81)90507-9. [DOI] [PubMed] [Google Scholar]
- Giulotto E., Saito I., Stark G. R. Structure of DNA formed in the first step of CAD gene amplification. EMBO J. 1986 Sep;5(9):2115–2121. doi: 10.1002/j.1460-2075.1986.tb04474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glanville N. Unstable expression and amplification of a transfected oncogene in confluent and subconfluent cells. Mol Cell Biol. 1985 Jun;5(6):1456–1464. doi: 10.1128/mcb.5.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamlin J. L., Milbrandt J. D., Heintz N. H., Azizkhan J. C. DNA sequence amplification in mammalian cells. Int Rev Cytol. 1984;90:31–82. doi: 10.1016/s0074-7696(08)61487-4. [DOI] [PubMed] [Google Scholar]
- Hyrien O., Debatisse M., Buttin G., de Saint Vincent B. R. A hotspot for novel amplification joints in a mosaic of Alu-like repeats and palindromic A + T-rich DNA. EMBO J. 1987 Aug;6(8):2401–2408. doi: 10.1002/j.1460-2075.1987.tb02518.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanda N., Schreck R., Alt F., Bruns G., Baltimore D., Latt S. Isolation of amplified DNA sequences from IMR-32 human neuroblastoma cells: facilitation by fluorescence-activated flow sorting of metaphase chromosomes. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4069–4073. doi: 10.1073/pnas.80.13.4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavathas P., Herzenberg L. A. Amplification of a gene coding for human T-cell differentiation antigen. Nature. 1983 Nov 24;306(5941):385–387. doi: 10.1038/306385a0. [DOI] [PubMed] [Google Scholar]
- Larson D. D., Blackburn E. H., Yaeger P. C., Orias E. Control of rDNA replication in Tetrahymena involves a cis-acting upstream repeat of a promoter element. Cell. 1986 Oct 24;47(2):229–240. doi: 10.1016/0092-8674(86)90445-9. [DOI] [PubMed] [Google Scholar]
- Looney J. E., Hamlin J. L. Isolation of the amplified dihydrofolate reductase domain from methotrexate-resistant Chinese hamster ovary cells. Mol Cell Biol. 1987 Feb;7(2):569–577. doi: 10.1128/mcb.7.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milbrandt J. D., Heintz N. H., White W. C., Rothman S. M., Hamlin J. L. Methotrexate-resistant Chinese hamster ovary cells have amplified a 135-kilobase-pair region that includes the dihydrofolate reductase gene. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6043–6047. doi: 10.1073/pnas.78.10.6043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbantoglu J., Meuth M. DNA amplification--deletion in a spontaneous mutation of the hamster aprt locus: structure and sequence of the novel joint. Nucleic Acids Res. 1986 Nov 11;14(21):8361–8371. doi: 10.1093/nar/14.21.8361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara N., Goto H., Watanabe T. Isozymes of rat AMP deaminase. Biochim Biophys Acta. 1975 Oct 22;403(2):530–537. doi: 10.1016/0005-2744(75)90081-9. [DOI] [PubMed] [Google Scholar]
- Parnes J. R., Velan B., Felsenfeld A., Ramanathan L., Ferrini U., Appella E., Seidman J. G. Mouse beta 2-microglobulin cDNA clones: a screening procedure for cDNA clones corresponding to rare mRNAs. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2253–2257. doi: 10.1073/pnas.78.4.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Roberts J. M., Axel R. Gene amplification and gene correction in somatic cells. Cell. 1982 May;29(1):109–119. doi: 10.1016/0092-8674(82)90095-2. [DOI] [PubMed] [Google Scholar]
- Roninson I. B. Detection and mapping of homologous, repeated and amplified DNA sequences by DNA renaturation in agarose gels. Nucleic Acids Res. 1983 Aug 25;11(16):5413–5431. doi: 10.1093/nar/11.16.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito I., Stark G. R. Charomids: cosmid vectors for efficient cloning and mapping of large or small restriction fragments. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8664–8668. doi: 10.1073/pnas.83.22.8664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salerno G., Verde P., Nolli M. L., Corti A., Szöts H., Meo T., Johnson J., Bullock S., Cassani G., Blasi F. Monoclonal antibodies to human urokinase identify the single-chain pro-urokinase precursor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):110–114. doi: 10.1073/pnas.81.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Shiloh Y., Korf B., Kohl N. E., Sakai K., Brodeur G. M., Harris P., Kanda N., Seeger R. C., Alt F., Latt S. A. Amplification and rearrangement of DNA sequences from the chromosomal region 2p24 in human neuroblastomas. Cancer Res. 1986 Oct;46(10):5297–5301. [PubMed] [Google Scholar]
- Shiloh Y., Shipley J., Brodeur G. M., Bruns G., Korf B., Donlon T., Schreck R. R., Seeger R., Sakai K., Latt S. A. Differential amplification, assembly, and relocation of multiple DNA sequences in human neuroblastomas and neuroblastoma cell lines. Proc Natl Acad Sci U S A. 1985 Jun;82(11):3761–3765. doi: 10.1073/pnas.82.11.3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spradling A. C., de Cicco D. V., Wakimoto B. T., Levine J. F., Kalfayan L. J., Cooley L. Amplification of the X-linked Drosophila chorion gene cluster requires a region upstream from the s38 chorion gene. EMBO J. 1987 Apr;6(4):1045–1053. doi: 10.1002/j.1460-2075.1987.tb04857.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark G. R. DNA amplification in drug resistant cells and in tumours. Cancer Surv. 1986;5(1):1–23. [PubMed] [Google Scholar]
- Tyler-Smith C., Bostock C. J. Gene amplification in methotrexate-resistant mouse cells. III. Interrelationships between chromosome changes and DNA sequence amplification or loss. J Mol Biol. 1981 Dec 5;153(2):237–256. doi: 10.1016/0022-2836(81)90276-x. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Robert de Saint Vincent B., DeRose M. L. Effect of chromosomal position on amplification of transfected genes in animal cells. Nature. 1984 Feb 9;307(5951):516–520. doi: 10.1038/307516a0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weith A., Winking H., Brackmann B., Boldyreff B., Traut W. Microclones from a mouse germ line HSR detect amplification and complex rearrangements of DNA sequences. EMBO J. 1987 May;6(5):1295–1300. doi: 10.1002/j.1460-2075.1987.tb02367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cicco D. V., Spradling A. C. Localization of a cis-acting element responsible for the developmentally regulated amplification of Drosophila chorion genes. Cell. 1984 Aug;38(1):45–54. doi: 10.1016/0092-8674(84)90525-7. [DOI] [PubMed] [Google Scholar]
- de Saint-Vincent B. R., Buttin G. Studies on 1-beta-D-arabinofuranosyl-cytosine-resistant mutants of Chinese-hamster fibroblasts. A mitochondrial deoxycytidine kinase devoid of activity on arabino-cytosine. Eur J Biochem. 1973 Sep 3;37(3):481–488. doi: 10.1111/j.1432-1033.1973.tb03009.x. [DOI] [PubMed] [Google Scholar]