Abstract
AIM
To construct a population pharmacokinetic (popPK) model for ketamine (Ket), norketamine (norKet), dehydronorketamine (DHNK), hydroxynorketamine (2S,6S;2R,6R)-HNK) and hydroxyketamine (HK) in patients with treatment-resistant bipolar depression.
METHOD
Plasma samples were collected at 40, 80, 110, 230 min on day 1, 2 and 3 in nine patients following a 40 min infusion of (R,S)-Ket (0.5 mg kg−1) and analyzed for Ket, norKet and DHNK enantiomers and (2S,6S;2R,6R)-HNK, (2S,6S;2R,6R)-HK and (2S,6R;2R,6S)-HK. A compartmental popPK model was constructed that included all quantified analytes, and unknown parameters were estimated with an iterative two-stage algorithm in ADAPT5.
RESULTS
Ket, norKet, DHNK and (2S,6S;2R,6R)-HNK were present during the first 230 min post infusion and significant concentrations (>5 ng ml−1) were observed on day 1. Plasma concentrations of (2S,6S;2R,6R)-HK and (2S,6R;2R,6S)-HK were below the limit of quantification. The average (S) : (R) plasma concentrations for Ket and DHNK were <1.0 while no significant enantioselectivity was observed for norKet. There were large inter-patient variations in terminal half-lives and relative metabolite concentrations; at 230 min (R,S)-DHNK was the major metabolite in four out of nine patients, (R,S)-norKet in three out of nine patients and (2S,6S;2R,6R)-HNK in two out of nine patients. The final PK model included three compartments for (R,S)-Ket, two compartments for (R,S)-norKet and single compartments for DHNK and HNK. All PK profiles were well described, and parameters for (R,S)-Ket and (R,S)-norKet were in agreement with prior estimates.
CONCLUSION
This represents the first PK analysis of (2S,6S;2R,6R)-HNK and (R,S)-DHNK. The results demonstrate that while norKet is the initial metabolite, it is not the main metabolite suggesting that future Ket studies should include the analysis of the major metabolites.
Keywords: (2S, 6S;2R, 6R)-hydroxynorketamine; bipolar depression; (R, S)-dehydronorketamine; (R, S)-norketamine; stereoselectivity
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
(R,S)-ketamine is a phencyclidine derivative that was initially developed as an anaesthetic agent and which is currently being studied in the treatment of pain and depression. After administration, the drug is extensively N-demethylated to (R,S)-norketamine. The pharmacokinetics of ketamine and norketamine have been extensively studied in volunteers and patients after the administration of anaesthetic and sub-anaesthetic doses. However, ketamine and norketamine are extensively transformed into a series of diastereomeric hydroxyketamines and hydroxynorketamines and (R,S)-dehydronorketamine metabolites. The plasma kinetics of these metabolites have not been elucidated.
WHAT THIS STUDY ADDS
The current study expands the characterization of the disposition kinetics of (R,S)-ketamine and (R,S)-norketamine and presents a population pharmacokinetic analysis of (R)-ketamine, (S)-ketamine, (R)-norketamine, (S)-norketamine, (R)-dehydronorketamine, (S)- dehydronorketamine and (2S,6S;2R,6R)-hydroxynorketamine and the serum concentration–time profiles of multiple ketamine metabolites observed in the plasma of patients after a single 40 min infusion of a sub-anaesthetic dose of the drug. The data demonstrate that while norketamine is an initial metabolite, it is not the major circulating metabolite and suggest that the determination of the downstream metabolites of ketamine may play a role in the pharmacological effects of the drug.
Introduction
(R,S)-ketamine ((R,S)-Ket, Figure 1), is a chiral phencyclidine derivative that was initially developed as an anaesthetic agent and which is currently being studied in the treatment of pain and depression [1, 2]. Sub-anaesthetic doses of (R,S)-Ket have been successfully used in the treatment of patients with complex regional pain syndrome (CRPS) [3, 4], postoperative pain in opioid tolerant patients [5], in emergency room treatments [6] and in patients suffering from major depressive disorder and treatment-resistant bipolar depression [7–9]. In the USA, Ket is used as a racemic (50:50) mixture of (R)-Ket and (S)-Ket. However, the administration of the single isomer, (S)-Ket, is effective in the treatment of CRPS [10–12].
Figure 1.
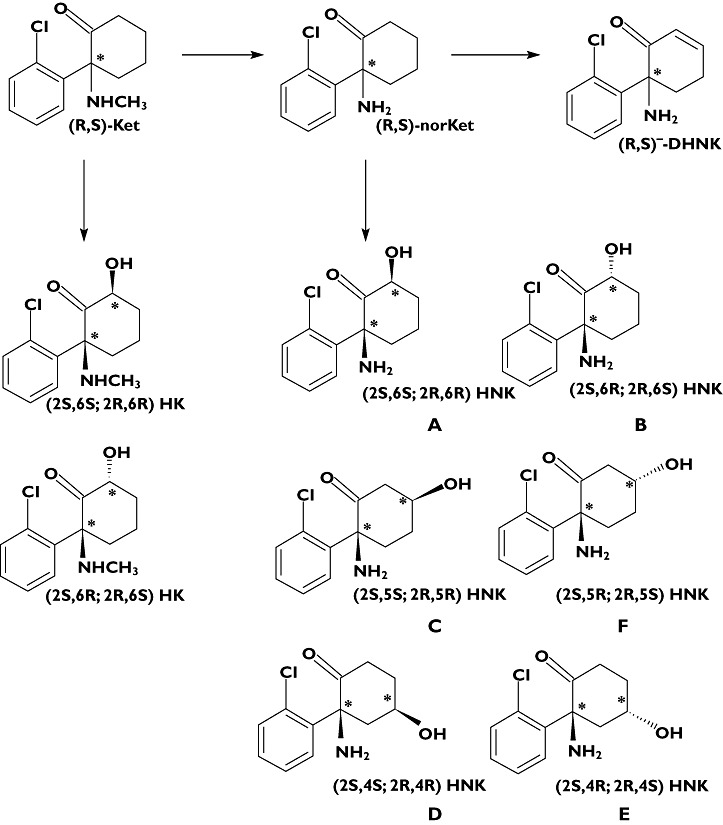
The metabolism of ketamine
After administration (R,S)-Ket is extensively N-demethylated to norketamine ((R,S)-norKet, Figure 1), which has anaesthetic [13] and antinociceptive properties [14, 15] and is considered as the ‘active’ metabolite. (R,S)-Ket and (R,S)-norKet are further transformed into two diasteromeric hydroxyKet and six diastereomeric hydroxynorKet metabolites (HK and HNK, respectively, Figure 1) and dehydronorketamine (DHNK, Figure 1) [16, 17]. An early study of the anaesthetic activity of a key HNK metabolite, (2S,6S;2R,6R)-HNK, indicated that this compound did not produce anaesthesia in the rat [13], and there has been no subsequent characterization of the pharmacological activity of this metabolite or any of the other hydroxylated metabolites of Ket and norKet. However, recent work in our laboratories has demonstrated that (R,S)-DHNK is a potent inhibitor of the α7-nicotinic acetylcholine receptor and the data suggest that this metabolite may play a role in the clinical effects of Ket (unpublished data).
The pharmacokinetics of (R,S)-Ket, (R)-Ket and (S)-Ket have been extensively studied in volunteers and patients after the administration of anaesthetic and sub-anaesthetic doses of (R,S)-Ket and sub-anaesthetic doses of (S)-Ket [11, 18–22]. Since (R,S)-norKet was identified as the ‘active’ metabolite, these studies have often included the determination of the pharmacokinetic profiles of (R,S)-norKet or the separate enantiomers, but not the HK, HNK or DHNK metabolites. However, significant plasma concentrations of (2S,6S;2R,6R)-HNK were detected in the rat following administration of (R,S)-Ket or (R,S)-norKet [13] and (R)- and (S)-DHNK were identified in plasma samples obtained from patients [23] and rats [24] receiving (R,S)-Ket and in Shetland ponies after administration of (R,S)-Ket or (S)-Ket [25, 26]. All of the major metabolites of (R,S)-Ket, with the exception of (2S,6R;2R,6S)-HK, have been identified in urine samples obtained from volunteers who received a single 50 mg oral dose of (R,S)-Ket [27] and in the plasma and urine of CRPS patients receiving a continuous 5 day infusion of (R,S)-Ket [28].
Although significant plasma and urine concentrations of (2S,6S;2R,6R)-HK, HNK and DHNK metabolites have been observed after Ket administration, their plasma concentration–time profiles have not been determined. Thus, the aim of the current study was to apply a chiral-archiral LC-MS/MS assay validated for the determination of the plasma concentrations of (2S,6S;2R,6R)-HK, (2S,6S;2R,6R)-HNK and (R,S)-DHNK [28] to the analysis of plasma samples obtained from patients receiving (R,S)-Ket for the treatment of treatment-resistant bipolar depression and to use these profiles to perform a population PK (popPK) analysis of (R)-Ket, (S)-Ket, (R)-norKet, (S)-norKet, (R)-DHNK, (S)-DHNK, (2S,6S;2R,6R)-HNK and (2S,6S;2R,6R)-HK.
Methods
Patient samples
The plasma samples analyzed in this study were obtained during a previously reported study of the effect of (R,S)-Ket in the treatment of treatment-resistant bipolar depression [8]. In brief, after obtaining informed consent, the patients were entered into a randomized double-blind crossover study in which they received a 40 min infusion of 50 ml of either 0.9% saline solution or a 0.5 mg kg−1 dose of (R,S)-Ket hydrochloride. There was a 2 week period between infusions. Plasma samples were collected prior to the initiation of the infusion, at 40 min (end of the infusion), 80 min, 110 min, 230 min and on days 1, 2 and 3 post-infusion and frozen at −80°C until analysis. During the course of the study the patients were required to take either lithium or valproate within a specified range and no other psychotropic medications were allowed.
Bioanlaytical methods
The plasma concentrations of (R)-Ket, (S)-Ket, (R)-norKet, (S)-norKet, (R)-DHNK, (S)-DHNK, (2S,6S;2R,6R)-HNK, (2S,6S;2R,6R)-HK and (2S,6R;2R,6S)-HK were determined using a previously described achiral-chiral liquid chromatography–mass spectrometry method [28] that was revalidated for the lower plasma concentrations observed in this study. The chromatographic experiments were carried out on a Shimadzu Prominence HPLC system (Shimadzu, Columbia, MD, USA) and total analyte concentrations were determined using an Eclipse XDB-C18 guard column and a Varian Pursuit XRs 5 C18 analytical column (Varian, Inc., Palo Alto, CA, USA) and the relative enantiomeric concentrations of (R)- and (S)-Ket, (R)- and (S)-norKet and (R)- and (S)-DHNK were determined using Chiral-AGP guard and analytical columns (Advanced Separation Technologies, Whippany, NJ, USA). The MS/MS analysis was performed using a triple quadrupole mass spectrometer model API 4000 system from Applied Biosystems/MDS Sciex equipped with Turbo Ion Spray® (TIS) (Applied Biosystems, Foster City, CA, USA). The data were acquired and analyzed using Analyst version 1.4.2 (Applied Biosystems). Positive electrospray ionization data were acquired using multiple reaction monitoring (MRM) and quantification was accomplished using area ratios calculated using D4-(R,S)-Ket as the internal standard, where the concentration of the internal standard was set at 50 ng ml−1.
Calibration curves and quality control standards were prepared using the racemic mixtures of the analytes. The eight point calibration curves used to measure (R,S)-Ket and (R,S)-DHNK ranged from 3.9 to 500 ng ml−1 and the calibration curves used to measure (R,S)-norKet, (2R,6R;2S,6S)-HNK, (2S,6S;2R,6R)-HK and (2S,6R;2R,6S)-HK were from 1.9 to 250 ng ml−1. The calibration curves were linear for all of the analytes with r2 values ranging from 0.98 to 0.99. Quality control (QC) standards (lower, middle, upper) for (R,S)-Ket and (R,S)-DHNK were 7.8, 31.25 and 250 ng ml−1, respectively and for (R,S)-norKet, (2R,6R;2S,6S)-HNK, (2S,6S;2R,6R)-HK and (2S,6R;2R,6S)-HK the QCs (lower, middle, upper) were 20, 40 and 80 ng ml−1, respectively. The coefficient of variation was <6% for the high QC standards and <14% for the low QC standards. The LOQ for (R,S)-Ket and (R,S)-DHNK was 3.9 ng ml−1 and the LOQ for (R,S)-norKet, (2S,6S;2R,6R)-HNK, (2S,6S;2R,6R)-HK and (2S,6R;2R,6S)-HK was 1.9 ng ml−1.
Pharmacokinetic model
(R,S)-Ket was administered as a constant rate infusion (K0). The model structure for each enantiomer was identical, and a schematic of the final pharmacokinetic model is shown in Figure 2. The distribution of (S)-Ket was described by a central volume (V1s) and two peripheeral compartments (A2s, A3s) and defined by the following system of equations:
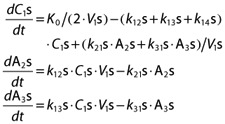 |
where C1s is the plasma concentration of (S)-Ket and drug distribution is regulated by first order rate constants k12s, k21s, k13s and k31s. Metabolism of (S)-Ket to (S)-norKet was described by the first order elimination rate constant k14s. Individual infusions were set equal to 50% of the total infusion to account for the racemic mixture. A two compartment model (central, C4s and peripheral, A5s) was used to describe the rate of change of (S)-norKet plasma concentrations (C4s):
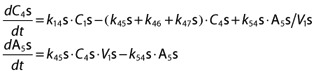 |
with first order distribution rate constants k45s and k54s. The central volume of norKet was assumed to be equal to the (S)-Ket central compartment volume. NorKet undergoes further hydroxylation and dehydrogenation to yield corresponding HNK and DHNK. The rate of change of plasma (S)-DHNK was described by a one compartment model (C6s):
where conversion of (S)-norKet to (S)-DHNK and the elimination of (S)-DHNK are governed by first order rate constants k46s and k60s. A one compartment model was also used to characterize (2S,6S)-/(2R,6R)-HNK plasma concentrations (C7). However, separate enantiomers were not measured for diastereomeric HNK, and both (R)- and (S)-norKet contribute to the formation of total (2S,6S)-/(2R,6R)-HNK concentrations:
with first order rate constants k47 and k70 defining the formation and elimination of (2S,6S)-/(2R,6R)-HNK. Only the equations for (S)-enantiomers are shown, as the system is identical for the (R)-enantiomers.
Figure 2.
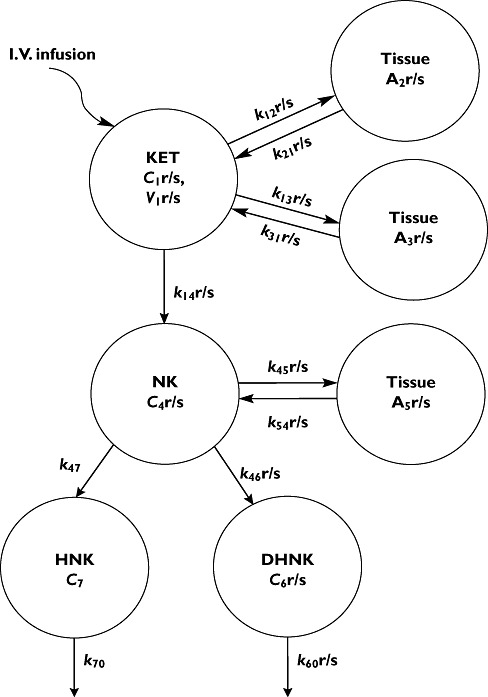
Schematic of the final pharmacokinetic model of (R)- and (S)-ketamine and corresponding metabolites. Abbreviations and system equations are defined under Pharmacokinetic model in Methods
Data analysis
All measurements of (R)- and (S)-enantiomers for parent drug and metabolites were modelled simultaneously using an iterative two stage (ITS) algorithm as implemented in ADAPT5 (Biomedical Simulations Resource, USC, Los Angeles, CA). Parameters were assumed to be log-normally distributed within the population and up to 1000 iterations were used to obtain parameter distributions. Model selection during the development process was based on the objective function, goodness of fit plots and distribution of residuals. Residual variability was modelled using the additive plus proportional variance model:
where Vari is the variance of the ith data point, σ1 and σ2 are the estimated variance model parameters and Ypred is the ith model predicted value. Final estimated parameters were reported as population mean estimates and inter-individual variability (IIV; CV%).
Results
Subject characteristics
The subject population in this study included six females and three males whose age ranged from 23 to 62 years (mean 46 ± 15 years), weight from 51 to 118 kg (mean 90 ± 19 kg), and body mass index from 19 to 39 kg m2 (mean 31 ± 6 kg m2). All subjects were diagnosed with treatment-resistant bipolar depression, and additional study details have been previously published [8].
Plasma concentrations of the stereosiomers of Ket and its metabolites
The achiral-chiral analytical method used in this study detected measurable quantities of (R)-Ket, (S)-Ket, (R)-norKet, (S)-norKet, (R)-DHNK, (S)-DHNK and (2S,6S;2R,6R)-HNK in the samples assessed in this study. The plasma concentrations of (2S,6S;2R,6R)-HK were ≤3.0 ng ml−1 in the 40–230 min samples and (2S,6R;2R,6S)-HK was not detected in any of the samples. Representative chromatograms from the achiral analyses of plasma samples obtained from a patient at 230 min and day 1 are presented in Figures 3 and 4, respectively. The chromatogram obtained from the 230 min plasma sample (Figure 3) also contained peaks corresponding to five additional hydroxynorketamine metabolites, identified as B-F in Figure 1, but these compounds were not quantified due to a lack of analytical standards. The plasma sample obtained from this patient at 24 h after the administration of (R,S)-Ket also contained (R)- and (S)-Ket, (R)- and (S)-norKet, (R)- and (S)-DHNK, (2S,6S;2R,6R)-HNK and (2S,5R;2R,5S)-HNK, Compound F (Figure 3).
Figure 3.
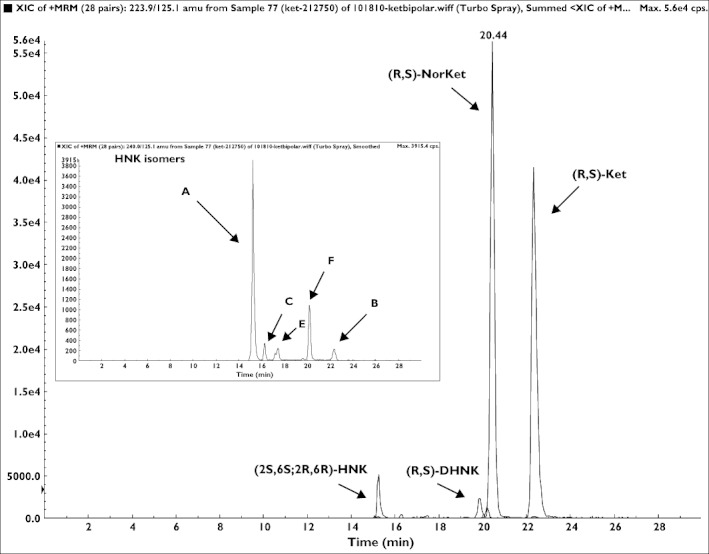
The chromatographic trace from the achiral analysis of a plasma sample obtained 230 min after administration of a 0.5 mg kg−1 dose of (R,S)-Ket where: A = (2S,6S;2R,6R)-HNK; B = (2S,6R;2R,6S)-HNK; C = (2S,5S;2R,5R)-HNK; E = (2S,4R;2R,4S)-HNK; F = (2S,5R;2R,5S)-HNK
Figure 4.
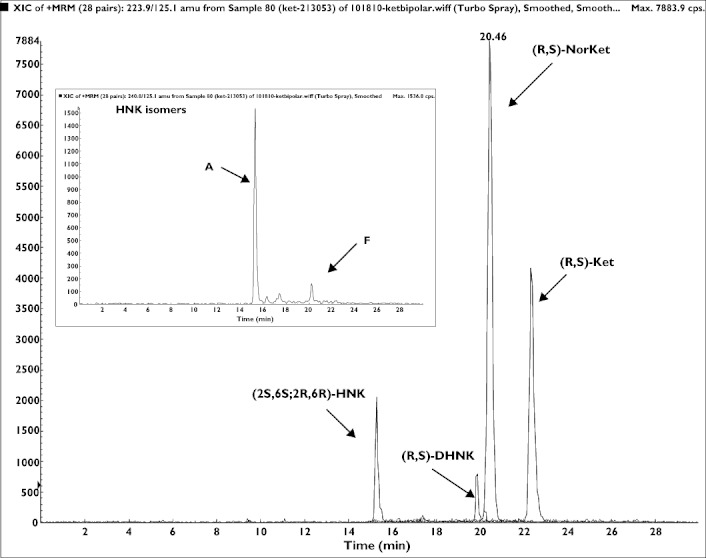
The chromatographic trace from the achiral analysis of a plasma sample obtained 1 day (day 1) after administration of a 0.5 mg kg−1 dose of (R,S)-Ket where: A = (2S,6S;2R,6R)-HNK; F = (2S,5R;2R,5S)-HNK
There was a wide inter-patient variation in the plasma concentrations of the target analytes, which is reflected in the average plasma concentrations presented in Figure 5. Significant concentrations (>8 ng ml−1) of all of the metabolites were observed in the day 1 plasma and (R,S)-DHNK and (2S,6S;2R,6R)-HNK were still quantifiable in the day 2 and day 3 plasma samples. There were also large variations in the relative total plasma concentrations, i.e. the combined concentrations of the enantiomers, of the N-demethylated metabolites. For example, in the samples obtained 230 min post dose (R,S)-DHNK was the major metabolite in four out of nine patients, followed by (R,S)-norKET (three out of nine) and (2S,6S;2R,6R)-HNK (two out of nine). In this study, the concentrations of (R,S)-DHNK and (2S,6S;2R,6R)-HNK tended to increase over time relative to (R,S)-norKet.
Figure 5.
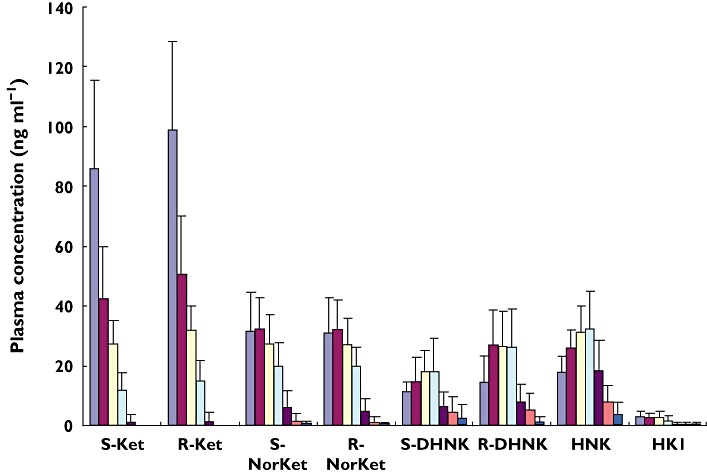
The average plasma concentrations, presented as ng ml−1, of the stereoisomers of (R,S)-Ket and its major metabolites in nine patients treated with a 0.5 mg kg−1 dose of (R,S)-Ket. 40 min ( ); 80 min (
); 80 min ( ); 110 min (
); 110 min ( ); 230 min (
); 230 min ( ); Day 1 (
); Day 1 ( ); Day 2 (
); Day 2 ( ); Day 3 (
); Day 3 ( )
)
When the plasma samples were analyzed on the chiral liquid chromatography system, the average enantiomeric ratio, expressed as (S)-enantiomer : (R)-enantiomer, for the 40–230 min samples was 0.84 ± 0.04 for Ket, 0.67 ± 0.09 for DHNK and 1.00 for norKet. This enantioselectivity is consistent with the previously reported average (S)- and (R)-Ket plasma concentrations after the administration of (R,S)-Ket [19, 20, 22] and the (S)- : (R)-DHNK plasma ratios of 0.71 determined in both Shetland ponies 120 min after a 2 h infusion of (R,S)-Ket [25] and in a CRPS patient on day 3 of a 5 day continuous infusion of (R,S)-Ket [28].
Pharmacokinetic analysis
Both (R)- and (S)-Ket showed a rapid initial decline in plasma concentrations after termination of the 40 min infusion (Figure 6). On the other hand, the average times to peak plasma concentrations of the metabolites were about 1.33 h for both (R)- and (S)-norKet and 3.83 h for (R)-DHNK, (S)-DHNK and (2S,6S;2R,6R)-HNK (Figure 6). All plasma metabolite concentrations gradually decreased, but with variable terminal elimination half-lives.
Figure 6.
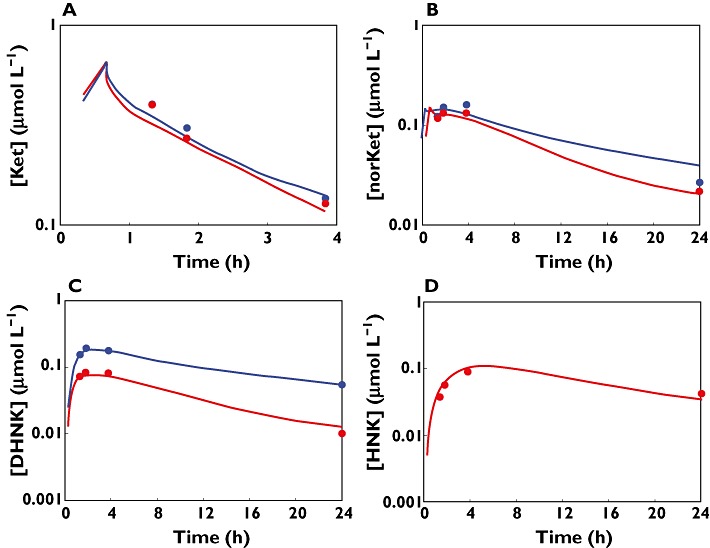
Pharmacokinetic profiles for ketamine and corresponding metabolites in a representative subject; (A) ketamine, (B) norketamine, (C) dehydronorketamine, and (D) (2S,6S)-/(2R,6R)-HNK. Red circles are measured (S)-enantiomer concentrations, blue circles are measured (R)-enantiomer concentrations and continuous lines are model fitted profiles
The pharmacokinetics of Ket have been characterized previously with one, two or three compartment models [12, 14, 19, 22, 29]. The one compartment model failed to capture the elimination phase of (R)- and (S)-Ket concentration profiles (data not shown). Although improvements in model fitting were obtained with a two compartment model, a slight bias was evident in diagnostic plots (e.g. residuals vs. predicted concentrations, data not shown). The ultimate three compartment model (Figure 2) provided the best description of (R)- and (S)-Ket profiles and objective criteria, such as diagnostic plots and a lower Akaike's information criterion (AIC). The number of compartments for norKet and the remaining metabolites were similarly optimized. The disposition of norKet has been previously described using one or two compartment models [11, 14]. Inclusion of a peripheral norKet compartment resulted in a decreased AIC value of 160 points and significant improvements in diagnostic plots (data not shown). Some pharmacokinetic analyses have included transit compartments to confer a time delay in the formation of norKet [11, 14]. However, this model structure introduced bias in the terminal phase of norKet (data not shown). The final pharmacokinetic model, consisting of three (R)- and (S)-Ket, two (R)- and (S)-norKet, one (R)- and (S)-DHNK and one (2S,6S;2R,6R)-HNK compartment is depicted in Figure 2.
Additional first order elimination rate constants of (R)- and (S)-Ket and a corresponding norKet compartment, representing alternate elimination pathways, were also tested. However, values were estimated to be relatively small and were set to zero to avoid overparameterization. Final model parameter estimates were obtained by simultaneously fitting the model to all available concentration−time profiles. Mean population parameter estimates are listed in Table 1. (R)- and (S)-Ket distributed very rapidly from the central compartment to the peripheral compartment with distribution rate constants k13r of 56.5 h−1 and k13s of 64.3 h−1. The central volume is 14 l for (R)-Ket and 9.15 l for (S)-Ket. Interestingly, the first order elimination rate constants for (R)- and (S)-DHNK are 1.31 and 1.1 h−1, or 1.5 to 2-fold greater than that of (2S,6S;2R,6R)-HNK (0.629 h−1), which might indicate that (R)-DHNK is a major metabolite in urine. This is consistent with the observation that (R)-DHNK was the predominate urinary metabolite in samples obtained from CRPS patients treated with a 5 day infusion of (R,S)-Ket [28].
Table 1.
Population pharmacokinetic parameter estimates for (R)- and (S)-ketamine and corresponding metabolites
| Parameter (units) | Definition | (S)-enantiomer | (R)-enantiomer | ||
|---|---|---|---|---|---|
| Mean | CV%a | Mean | CV%a | ||
| k 12 (h−1) | Distribution rate constant of Ket | 12.1 | 46.2 | 7.30 | 51.6 |
| k 21 (h−1) | 0.040 | 80.2 | 0.16 | 12.3 | |
| k 13 (h−1) | Distribution rate constant of Ket | 64.3 | 4.74 | 56.5 | 4.23 |
| k 31 (h−1) | 3.19 | 59.7 | 6.18 | 59.7 | |
| k 14 (h−1) | Formation rate constant of NorKet | 2.37 | 33.0 | 2.65 | 34.2 |
| k 46 (h−1) | Formation rate constant of DHNK | 0.94 | 37.6 | 1.63 | 44.5 |
| k 60 (h−1) | Elimination rate constant of DHNK | 1.10 | 61.7 | 1.31 | 58.0 |
| k 45 (h−1) | Distribution rate constant of NorKet | 4.45 | 9.47 | 8.95 | 3.31 |
| k 54 (h−1) | 0.75 | 5.30 | 1.37 | 79.0 | |
| k47 (h−1) | Formation rate constant of HNK | 0.60 | 48.6 | –b | –b |
| k 70 (h−1) | Elimination rate constant of HNK | 0.63 | 44.2 | –b | –b |
| V 1(l) | Central compartment volume of Ket | 9.15 | 11.1 | 14.0 | 8.35 |
% coefficient of variation representing inter-individual variability.
b Shared parameter between enantiomers. Ket, ketamine; NorKet, norketamine; DHNK, dehydronorketamine; HNK (2S,6S;2R,6R)-hydroxynorketamine.
The time course of (R)- and (S)-Ket and corresponding metabolite concentrations in a representative subject are shown in Figure 6, and individual model predicted profiles are in good agreement with experimental data. Model diagnostic plots are shown as supplemental figures, and observed vs. individual fitted drug concentrations were reasonable for parent drug and metabolites with no systematic trend or bias (Figure S1). Individual standardized weighted residuals as a function of time for (R)- and (4S)-ketamine and corresponding metabolites are reasonably distributed about zero, with only a slight bias for norKet and HNK, likely due to the limited number of subjects and blood samples (Figure S2).
Discussion
This study reports the determination of the plasma concentration–time profiles of the stereoisomers of Ket, norKet, DHNK and (2S,6S;2R,6R)-HNK after administration of a sub-anesthetic dose of (R,S)-Ket (0.5 mg kg−1 as a single 40 min infusion). The results demonstrate that (R)- and (S)-DHNK and (2S,6S;2R,6R)-HNK are major circulating metabolites, that significant concentrations of these metabolites are present in the 24 h plasma samples and can be detected in the 48 h plasma of some patients (Figure 5). In addition to (2S,6S;2R,6R)-HNK, the plasma samples also contained measurable amounts of four of the other known positional and stereoisomeric HNKs [16, 28] with (2S,5R;2R,5S)-HNK as the most predominant. These findings confirm the extensive hydroxylation of norKet which was observed in the initial in vitro studies, in the urine of volunteer subjects after the administration of an oral dose of (R,S)-Ket [27] and in the plasma of CRPS patients on day 3 of a 5 day continuous infusion of (R,S)-Ket [28]. Interestingly, no significant concentrations of the hydroxylated metabolites of Ket, i.e. (2S,6S;2R,6R)-HK and (2S,6R;2R,6S)-HK, were detected in this study which is consistent with the data from the analysis of CRPS patients ([28], unpublished data). In addition, there were wide variations in the relative plasma concentrations of norKet, DHNK and (2S,6R;2R,6S)-HNK. For example, in the samples obtained 230 min post dose (R,S)-DHNK was the major metabolite in four out of nine patients, followed by (R,S)-norKET (three out of nine) and (2S,6S;2R,6R)-HNK (two out of nine). These results are consistent with previous data obtained from CRPS patients in which (2S,6S;2R,6R)-HNK and (2S,6R;2R,6S)-HNK were the predominant metabolites while the plasma concentrations of (R)- and (S)-DHNK were ∼five-fold greater than the corresponding norKet enantiomers.
In this study, the plasma concentrations of (R)- and (S)- DHNK and (2S,6S;2R,6R)-HNK increased from the end of the infusion to the 230 min sampling point, while the plasma concentrations of (R)- and (S)-norKet decreased. These results suggest that (R,S)-norKet is the source of (R,S)-DHNK and (2S,6S)-HNK and are consistent with the observation by Leung & Baillie [13] that the i.v. administration of (R,S)-norKet to rats resulted in increasing plasma and brain concentrations of (2S,6S;2R,6R)-HNK and decreasing (R,S)-norKet during the 10 min post dose sampling period. Bolze & Boulieu [23] also demonstrated that in an intensive care patient the plasma concentration of (R,S)-DHNK increased during a 2 h infusion of (R,S)-Ket and remained relatively constant during an 8 h sampling period. (R)-DHNK, (S)-DHNK and multiple HNK metabolites were also identified in the plasma and urine of Sheltand ponies after the i.v. administration of (R,S)-Ket or (S)-Ket [25, 26]. However the concentrations of these metabolites were not quantitatively determined nor was the identity of the HNK metabolite(s) established due to the lack of analytical standards.
Although the presence of down-stream Ket metabolites after administration of (R,S)-Ket and (S)-Ket has been established for over 10 years, all of the previous PK studies have been limited to the analysis of the plasma profiles of Ket and norKet. The present study reports the first simultaneous popPK model that includes (R)-DHNK, (S)-DHNK and (2S,6S;2R,6R)-HNK. Interestingly, the mean first order formation rate constant of (R)-DHNK is almost two-fold greater than that of (S)-DHNK, but with similar elimination rate constants (Table 1). This set of parameters results in greater systemic exposures to the R-enantiomer of DHNK (Figures 5 and 6C).
The clinical relevance of expanding the pharmacokinetic assessment of down-stream ketamine metabolites is suggested by the temporal disconnect in PK−PD studies seeking to establish a relationship between plasma concentrations of Ket or norKet, as the racemic mixtures or separate enantiomers, and therapeutic response. This was observed in PK−PD studies in CRPS patients receiving (R,S)-Ket [22] or (S)-Ket [10, 12] for subjective pain relief and in the antidepressant response determined in the initial study of the treatment-resistant bipolar depression patients re-analyzed in this study [8]. Although turnover of endogenous mediators likely plays a major role, one potential explanation for the apparent hysteresis in the PK−PD relationships is that one or more the hydroxylated metabolites or DHNK contribute to the efficacy of the drug. The pharmacological activities of the hydroxylated metabolites of Ket and norKet and DHNK are under investigation and data from these studies will be reported elsewhere.
In conclusion, the results of this study showed that after the administration of a single sub-anaesthetic dose of (R,S)-Ket to patients with treatment-resistant bipolar depression, significant plasma concentrations of DHNK and (2S,6S;2R,6R)-HNK were detected up to 48 h after administration. The data indicate that while norKet is the initial metabolite, it is not necessarily the major metabolite. The results also suggest that future PK, PK−PD and clinical studies of Ket should be expanded to include the analysis of the major Ket metabolites as well as patient covariates that influence inter-individual variability in exposures.
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Aging/NIH (IWW) and National Institute of Mental Health/NIH (CAZ) and NIH Grant GM57980 (DEM).
Competing Interests
Dr. Zarate is listed as co-inventor on a patent application for the use of ketamine in major depression. Dr. Zarate has assigned his rights in the patent but will share a percentage of any royalties that may be received by the government. CAZ, IWW, RM have submitted a patent for the use of ketamine metabolites in the treatment of bipolar disorder and major depression. The other authors have no competing interests to declare.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Comparison observed and individual model-fitted drug concentrations for a) ketamine, b) norketamine, c) dehydronorketamine and d) (2S,6S)-/(2R,6R)-HNK. Red symbols are data points for (S)-enantiomers, blue symbols are data points for (R)-enantiomers and the solid black line is the line of identity.
Figure S2 Individual standardized weighted residuals (iWRES) as a function of time for a) ketamine, b) norketamine, c) dehydronorketamine and d) (2S,6S)-/(2R,6R)-HNK. Red symbols are data points for (S)-enantiomers, blue symbols are data points for (R)-enantiomers and the solid black line is the line of identity. Time is shown on a log scale.
REFERENCES
- 1.Kohrs R, Durieux ME. Ketamine: teaching an old drug new tricks. Anesth Analg. 1998;87:1186–93. doi: 10.1097/00000539-199811000-00039. [DOI] [PubMed] [Google Scholar]
- 2.Domino EF. Taming the ketamine tiger. Anesthesiology. 2010;113:678–86. doi: 10.1097/ALN.0b013e3181ed09a2. [DOI] [PubMed] [Google Scholar]
- 3.Schwarztman RJ, Alexander GM, Grothusen JR. The use of ketamine in complex regional pain syndrome: possible mechanisms. Expert Rev Neurother. 2011;11:719–34. doi: 10.1586/ern.11.31. [DOI] [PubMed] [Google Scholar]
- 4.Sabia M, Hirsh RA, Torjman MC, Wainer IW, Cooper N, Domsky R, Goldberg ME. Advances in translational neuropathic pain research: example of enantioselective pharmacokinetic-pharmacodynamic modeling of ketamine-induced pain relief in complex regional pain syndrome. Curr Pain Headache Rep. 2011;15:207–14. doi: 10.1007/s11916-011-0185-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Loftus RW, Yeager MP, Clark JA, Brown JR, Abdu WA, Sengupta DK, Beach ML. Intraoperative ketamine reduces perioperative opiate consumption in opiate-dependent patients with chronic back pain undergoing back surgery. Anesthesiology. 2010;113:639–46. doi: 10.1097/ALN.0b013e3181e90914. [DOI] [PubMed] [Google Scholar]
- 6.Lester L, Braude DA, Niles C, Crandall CS. Low-dose ketamine for analgesia in the ED: a retrospective case series. Am J Emerg Med. 2010;28:820–7. doi: 10.1016/j.ajem.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Zarate CA, Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK. A randomized trial of an N-methyl-D-asparate antagonist in treatment resistant major depression. Arch Gen Psychiatry. 2006;63:856–64. doi: 10.1001/archpsyc.63.8.856. [DOI] [PubMed] [Google Scholar]
- 8.Diazgranados N, Ibraham L, Brutsche NE, Newberg A, Kronstein P, Khalife S, Kammerer WA, Quezado Z, Luckenbaugh DA, Salvadore G, Machado-Vieira R, Manji KM, Zarate CA. A randomized add-on trial of an N-methyl-D-asparate antagonist in treatment-resistant bipolar depression. Arch Gen Psychiatry. 2010;67:793–801. doi: 10.1001/archgenpsychiatry.2010.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Diazgranados N, Ibraham LA, Brutsche NE, Ameli RA, Henter ID, Luckenbaigh DA, Machado-Vieira R, Zarate CA. Rapid resolution of suicidal ideation after a single infusion of an N-methyl-D-asparate antagonist in patients with treatment-resistant major depressive disorder. J Clin Psychiatry. 2010;71:1605–11. doi: 10.4088/JCP.09m05327blu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigtermans MJ, van Hilten JJ, Bauer MC, Arbous MS, Marinus J, Sarton EY, Dahan A. Ketamine produces effective and long-term pain relief in patients with Complex Regional Pain Syndrome Type 1. Pain. 2009a;2009:304–11. doi: 10.1016/j.pain.2009.06.023. [DOI] [PubMed] [Google Scholar]
- 11.Sigtermans M, Dahan A, Mooren R, Bauer M, Kest B, Sarton E, Olofson ES. )-Ketamine effect on experimental pain and cardiac output. Anesthesiology. 2009;111:892–903. doi: 10.1097/ALN.0b013e3181b437b1. [DOI] [PubMed] [Google Scholar]
- 12.Dahan A, Olofsen E, Sigtermans M, Noppers I, Niesters M, Aarts L. Population pharmacokinetic-pharamcodynamic modeling of ketamine-induced pain relief of chronic pain. Eur. J. Pain. 2010;15:258–67. doi: 10.1016/j.ejpain.2010.06.016. [DOI] [PubMed] [Google Scholar]
- 13.Leung LY, Baillie TA. Comparative pharmacology in the rat of ketamine and its two principal metabolites, norketamine and (Z)-6-hydroxynorketamine. J Med Chem. 1986;29:2396–9. doi: 10.1021/jm00161a043. [DOI] [PubMed] [Google Scholar]
- 14.Herd DW, Anderson BJ, Holford NHG. Modeling the norketamine metabolite in children and the implications for analgesia. Pediatr Anesth. 2007;17:831–40. doi: 10.1111/j.1460-9592.2007.02257.x. [DOI] [PubMed] [Google Scholar]
- 15.Holtman JR, Jr, Crooks PA, Johnson-Hardy JK, Hojomat M, Kleven M, Wala EP. Effects of norketamine enantiomers in rodent models of persistent pain. Pharmacol Biochem Behavior. 2008;90:676–85. doi: 10.1016/j.pbb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Trevor AJ, Woolf TF, Baillie TA, Adams JD, Castagnoli N. In: Kamenka JM, Domino EF, Geneste P, editors. NPP Books; 1983. pp. 279–89. Stereoselective Metabolism of Ketamine Enantiomers, Phencyclidine and Related Arylcyclohexylamines: Present and Future Applications, eds . Ann Arbor, MI: [Google Scholar]
- 17.Portmann S, Kwan HT, Theurillat R, Schmitz A, Mevissen M, Thormann W. Enantioselective capillary electrophoresis for the identification and characterization of human cytochrome P450 enzymes which metabolize ketamine and norketamine in vitro. J Chromatog A. 2010;1217:7942–8. doi: 10.1016/j.chroma.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 18.Wieber J, Gugler R, Hengstmann JH, Dengler HJ. Pharmacokinetics of ketamine in man. Anaesthesist. 1975;24:260–3. [PubMed] [Google Scholar]
- 19.Geisslinger G, Hering W, Thomann P, Knoll R, Kamp HD. Pharmacokinetics and pharmacodynamics of ketamine enantiomers in surgical patients using a stereoselective analytical method. Br J Anaesth. 1993;70:666–71. doi: 10.1093/bja/70.6.666. [DOI] [PubMed] [Google Scholar]
- 20.Ihmsen H, Geisslinger G, Schuttler J. Stereoselective pharmacokinetics of ketamine: R( Clin Pharmacol Ther. 2001;70:431–8. doi: 10.1067/mcp.2001.119722. )-ketamine inhibits the elimination of S(+)-ketamine. [DOI] [PubMed] [Google Scholar]
- 21.Yanagihara Y, Ohtani M, Kariya S, Uchino K, Hiraishi T, Ashizawa N, Aoyama T, Yamamura Y, Yamada Y, Iga T. Plasma concentration profiles of ketamine and norketamine after administration of various ketamine preparations to healthy Japanese volunteers. Bopharm Drug Disp. 2003;24:37–43. doi: 10.1002/bdd.336. [DOI] [PubMed] [Google Scholar]
- 22.Goldberg ME, Torjman MC, Schwartzman RJ, Mager DE, Wainer IW. Enantioselective pharmacokinetics of (R)- and (S)-ketamine after a 5-day infusion in patients with complex regional pain syndrome. Chirality. 2011;23:138–43. doi: 10.1002/chir.20890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolze S, Boulieu R. HPLC determination of ketamine, norketamine and dehydronorketamine in plasma with a high-purity reversed-phase sorbent. Clin Chem. 1998;44:560–4. [PubMed] [Google Scholar]
- 24.Williams ML, Mager DE, Parenteau H, Gudi G, Tracy T, Mulheran M, Wainer IW. Effects of protein calorie malnutrition on the pharmacokinetics of ketamine in rats. Drug Metab Dispos. 2004;32:786–93. doi: 10.1124/dmd.32.8.786. [DOI] [PubMed] [Google Scholar]
- 25.Theurillat R, Knobloch M, Schmitz A, Lassahn P-G, Mevissen M, Thormann W. Enantioselective analysis of ketamine and its metabolites in equine plasma and urine by CE with multiple isomer sulfated β-CD. Electrophoresis. 2007;28:2748–57. doi: 10.1002/elps.200600820. [DOI] [PubMed] [Google Scholar]
- 26.Larenza MP, Peterbauer C, Landoni MF, Levionnois OL, Schatzmann U, Spadavecchia C, Thormann W. Stereoselective pharmacokinetics of ketamine and norketamine after constant rate infusion of a subanesthetic dose of racemic ketamine or S-ketamine in Shetland ponies. Am J Vet Res. 2009;70:831–9. doi: 10.2460/ajvr.70.7.831. [DOI] [PubMed] [Google Scholar]
- 27.Turfus SC, Parkin MC, Cowan DA, Halket JM, Smith NW, Braithwaite RA, Elliot SP, Steventon GB, Kicman AT. Use of human microsomes and deuterated substrates; an alternative approach for the identification of novel metabolites of ketamine by mass spectrometry. Drug Metab Dispos. 2009;37:1769–78. doi: 10.1124/dmd.108.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Moaddel R, Venkata SLV, Tanga MJ, Bupp JE, Green CE, LaIyer L, Furimsky A, Goldberg ME, Torjman MC, Wainer IW. A parallel chiral-achiral liquid chromatographic method for the determination of the stereoisomers of ketamine and ketamine metabolites in the plasma and urine of patients with complex regional pain syndrome. Talanta. 2010;82:1892–904. doi: 10.1016/j.talanta.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.White M, de Graaff P, Renshof B, van Kan E, Dzoljic M. Pharmacokinetics of S(+) ketamine derived from target controlled infusion. Br J Anaesth. 2006;96:330–4. doi: 10.1093/bja/aei316. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison observed and individual model-fitted drug concentrations for a) ketamine, b) norketamine, c) dehydronorketamine and d) (2S,6S)-/(2R,6R)-HNK. Red symbols are data points for (S)-enantiomers, blue symbols are data points for (R)-enantiomers and the solid black line is the line of identity.
Figure S2 Individual standardized weighted residuals (iWRES) as a function of time for a) ketamine, b) norketamine, c) dehydronorketamine and d) (2S,6S)-/(2R,6R)-HNK. Red symbols are data points for (S)-enantiomers, blue symbols are data points for (R)-enantiomers and the solid black line is the line of identity. Time is shown on a log scale.


