Abstract
TRP channels were first identified as membrane proteins mediating phototransduction in fruit flies. Astrocytes were initially referred to as the silent elements of the nervous system. At the time these discoveries were made, few would have suspected TRP channels and astrocytes could contribute significantly to our understanding of brain signalling. Recent findings, however, put TRP channels and astrocytes in the spotlight, describe their ability to modulate the activity of specific sets of synapses, and raise some interesting questions. What makes astrocytes capable of exerting cell-specific effects on interneuronal signals? How do different synapses respond to changes in astrocytic function and in the local micro-structure of the neuropil? Can astrocytes be considered good candidate targets for therapeutic intervention to treat neurological diseases? Here I discuss the recent developments on TRP channels and astrocytes that have made us aware of the many structural and functional features of synapses that still need to be discovered and that could lead a new avant-garde in decoding the cellular and molecular basis of brain (dys)function.
 |
Annalisa Scimemi works in the Synaptic Physiology Section at the National Institute of Neurological Disorders and Stroke, National Institutes of Health, in Bethesda (MD). She studies the basic mechanisms of synaptic transmission in the brain and the role of astrocytes in regulating independent processing of information at neighboring synaptic contacts, in health and disease. She obtained her PhD in Biophysics from the International School for Advanced Studies (SISSA/ISAS) in Trieste, Italy and did her post-doctoral studies in the laboratory of Dimitri Kullmann at University College London and Jeffrey Diamond at the National Institutes of Health.
TRP channels in a nutshell
The major organic compounds present in spices like chili pepper, mint, mustard oil, cinnamon and garlic (i.e. capsaicin, menthol, allyl isothiocyanate, cinnamaldehyde, and allicin, respectively) were first identified 70–150 years ago, but only recently the molecular mechanisms that allow them to act on particular molecular targets, the transient receptor potential (TRP) channels, have started to be elucidated. By sequence homology, in mammals, 28 different TRP channels have been identified and grouped in six subfamilies (TRPA, TRPC, TRPM, TRPML, TRPP, TRPV) (Wu et al. 2010). Each TRP channel assembles as a tetramer of six transmembrane domain subunits, and shows non-selective cation permeability and weak voltage sensitivity (Clapham, 2003). TRP channels are particularly abundant in epithelial cells and nerve endings in the skin and mouth, but are also present in a variety of peripheral tissues and brain regions like the hypothalamus, substantia nigra, locus coeruleus, amygdala, cortex, hippocampus and cerebellum (Mori et al. 1998). In the brain, TRP channels are activated by a wide range of stimuli including changes in temperature, pressure and inflammatory agents, and mediate diverse functions including thermoregulation, inflammatory hyperalgesia (Bautista et al. 2006) and mechanosensation (Kwan et al. 2006).
TRPA channels
Among all TRP channels, TRPA channels have a peculiar structural signature, characterized by the presence of at least 14 modular protein–protein interaction motifs (the ankyrin repeats) in their cytoplasmic N-terminal domain (Story et al. 2003) (Fig. 1A and B), which are suggested to act as gating springs for mechanotransduction (Sotomayor et al. 2005) and to be involved in trafficking the channels from intracellular organelles to the cell surface (Nilius et al. 2011). TRPA1 channels, the only members of the mammalian TRPA subfamily known so far, are activated directly by allyl isothiocyanate and cinnamaldehyde, and indirectly via G protein-coupled signalling cascades triggered by growth factors and proinflammatory peptides such as bradykinin. The jury is still out and with no prospect of immediate agreement on whether TRPA1 channels also play a major role in cold sensation (Story et al. 2003; Caspani & Heppenstall, 2009) (Fig. 1C), but while this is being evaluated, new important features of TRPA1 channels have emerged.
Figure 1. Architecture and functional properties of TRPA1 channels.
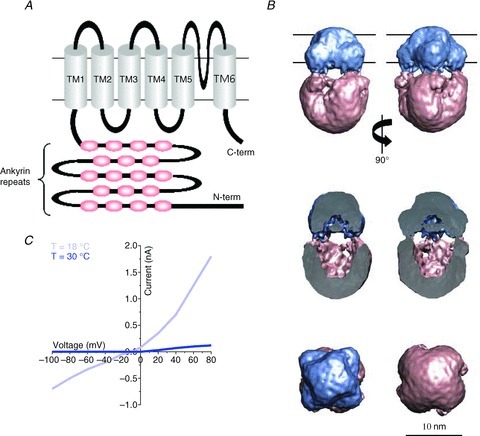
A, TRPA1 channels are composed of four identical subunits organized as the one schematized here. Each subunit contains six transmembrane domains (TM1–6), 14 intracellular ankyrin repeats, and a long intracellular N-terminal domain. B, side, cut-away, top and bottom views of a TRPA1 channel according to electron microscopy reconstructions. Modified with permission from Cvetkov et al. (2011); © 2011 The American Society for Biochemistry and Molecular Biology. C, the current–voltage relation for TRPA1 channels shows a weak outward rectification at 30°C. The inward and outward currents are both increased by lowering the temperature to 18°C. Modified with from Story et al. (2003) with permission from Elsevier (© 2003).
Shigetomi et al. (2011) recently reported that, in the hippocampus, spontaneous openings of TRPA1 channels in astrocytes shape fast inhibitory synaptic transmission in GABAergic interneurons (INs), but not in pyramidal cells (PCs). By monitoring the fluorescent signal of a genetically encoded Ca2+ indicator expressed in astrocytes (Lck-GCaMP3), the authors showed that spontaneous openings of TRPA1 channels mediate spatially confined (∼ 5 μm) and slow-decaying (∼4 s) increments in cytosolic Ca2+ (up to 0.5 μm) (Shigetomi et al. 2010a,b). Blocking TRPA1 channels prevented these events and led to a reduction in the astrocytic intracellular [Ca2+] from ∼100 nm (Kuchibhotla et al. 2009) to possibly ∼35 nm (a level of cytosolic Ca2+ similar to that measured with 13 mm intracellular BAPTA). This reduction in astrocytic baseline intracellular [Ca2+] levels was associated with clathrin-dependent internalization of the GABA transporter GAT3 in astrocytes and with increased amplitude of tonic inhibitory currents and reduced amplitude of GABAergic miniature inhibitory postsynaptic currents (mIPSCs) in INs.
This story provided valuable insights into possible novel roles of TRPA1 channels in a cell type where the expression and function of TRP channels (Pizzo et al. 2001; Song et al. 2005; Shibasaki et al. 2007), and in particular of TRPA channels (Golovina, 2005), had not been clearly resolved. Additionally, it drew attention to a number of potential mechanisms that, unexpectedly, could allow astrocytes to finely tune the activity of specific sets of synapses, rather than non-specifically modulating the activity of large groups of cells, as generally thought. Is this proposed synapse specificity due to the fact that astrocytic processes at IN–IN/IN–PC synapses have distinct structural and functional features or do IN–IN/IN–PC synapses have different sets of molecules that allow them to respond differently to similar changes in astrocytic function?
To answer this question, it is useful to review the currently available information on: (1) heterogeneous subcellular distribution, trafficking and cell regulation of GABA transporters in astrocytes; (2) differences in astrocytic coverage and membrane composition at IN–IN/IN–PC synapses; and (3) differences in (extra)synaptic GABAA receptor (GABAA-R) composition at IN–IN/IN–PC synapses.
GABA transporters: differences in expression and regulation
GAT3 and GAT1 are the two major types of GABA transporters in the brain: GAT3 is more abundant than GAT1 in astrocytes, whereas GAT1 prevails in neuronal inhibitory terminals (but is also present in astrocytes). In the developing brain, the subcellular distribution of GAT3 and GAT1 in astrocytes differs (Vitellaro-Zuccarello et al. 2003). During the first two postnatal weeks, when GABA is depolarizing (Ben-Ari et al. 1989; Rivera et al. 1999) and when the large extracellular space fraction of the neuropil favours extrasynaptic GABA diffusion, GAT3 is expressed evenly in distal, proximal and perisomatic astrocytic regions, while GAT1 is targeted to distal astrocytic processes close to synaptic contacts (Vitellaro-Zuccarello et al. 2003; Beenhakker & Huguenard, 2010). By the end of the third postnatal week, when GABA has become hyperpolarizing and when the structure of the neuropil resembles more closely that of the adult brain, GAT3 acquires its mature pattern of expression, and both GAT1 and GAT3 become confined to distal astrocytic processes and intermediate filaments adjacent to active synapses (Vitellaro-Zuccarello et al. 2003). This suggests that GAT3 undergoes more profound regulatory processes than GAT1. Biochemical studies have shown that surface expression of GAT1 in neurons can be regulated by extracellular [GABA] (Gadea & Lopez-Colome, 2001), tyrosine phosphorylation (Law et al. 2000) and G protein-coupled (Corey et al. 1994; Beckman et al. 1999), syntaxin 1A-mediated (Beckman et al. 1998) phosphorylation by protein kinase C, but it is not known whether similar modifications also target GAT3. More importantly, there is really no indication that one transporter may have different binding/transport efficiency than the other one at particular sets of synapses. Likewise, even though broad regional differences in the expression level of GAT1/3 have been observed (Engel et al. 1998), any direct evidence supporting different expression of these transporters at different sets of synapses is currently missing.
Astrocytic coverage of inhibitory synapses
Previous quantification of astrocytic coverage at excitatory synapses relied on serial section electron microscopy (EM) reconstructions (Ventura & Harris, 1999), but a systematic analysis of astrocytic coverage at inhibitory synapses is not currently available. Getting this information with traditional serial EM reconstructions is feasible, but the technique encounters some issues when one wants to go more into detail and resolve the anatomy on synapses onto specific target cells. It is not an intrinsic limitation of the technique, but is rather due to the fact that the technique is generally used to reconstruct tiny volumes of the neuropil. Identifying synapses onto particular target cells might need the reconstruction of larger volumes and ideally of entire cells. The recent advances in EM-based reconstruction techniques now allow reconstructing larger volumes of tissue (Denk & Horstmann, 2004; Micheva & Smith, 2007; Knott et al. 2008). This, combined with the use of cell- (Livet et al. 2007) and molecule-specific labelling strategies, may now provide an unprecedented opportunity to understand whether there is any unsuspected heterogeneity in the geometry of different sets of synaptic contacts and in the structure and membrane protein composition of astrocytic processes around them.
Extrasynaptic GABAA receptors in different cell types
The effects of varying astrocytic GABA transporter expression, function or localization are ultimately determined by the biophysical properties of the GABAA-Rs that are being activated. GABAA-Rs containing the δ subunit (δGABAA-Rs) are preferentially expressed peri- and extrasynaptically and are abundant in hippocampal (Wei et al. 2003) and cerebellar (Nusser et al. 1995) glutamatergic granule cells, not in glutamatergic and GABAergic cells of the hippocampus proper (Wei et al. 2003). Accordingly, tonic inhibitory signals, thought to be mediated primarily by extrasynaptic GABAA-Rs, largely rely on activation of δGABAA-Rs in hippocampal (Glykys et al. 2008) and cerebellar granule cells (Hamann et al. 2002), and on activation of γ2 and α5 subunit containing GABAA-Rs in hippocampal stratum radiatum INs (Semyanov et al. 2003) and CA1-PCs (Caraiscos et al. 2004; Scimemi et al. 2005), respectively. A distinguishing feature of γ subunit-containing GABAA-Rs (γGABAA-Rs) and δGABAA-Rs is their steady-state affinity for GABA (higher for δGABAA than γGABAA-Rs; Saxena & Macdonald, 1996). δGABAA-Rs are sensitive to small changes in extracellular [GABA], but can have a high level of occupancy/desensitization in physiological [GABA] (Scimemi et al. 2005; Santhakumar et al. 2006; Bright et al. 2011; Brickley & Mody, 2012). In contrast, γGABAA-Rs are better detectors of large changes in extracellular [GABA]. An apparently cell-specific effect of astrocytes, like the one reported by (Shigetomi et al. 2011), may therefore be consistent with a relatively higher abundance of extrasynaptic γGABAA-Rs in INs. The reason for focusing on extrasynaptic GABAA-Rs is that the subunit composition of synaptic GABAA-Rs in IN/PCs is fairly similar (i.e. γGABAA-Rs associated with α1, α2 or α3 subunits; Farrant & Nusser, 2005). Because synaptic GABAA-Rs are much closer to their presynaptic counterpart than to neighbouring astrocytic processes, they are also likely to be relatively insensitive to subtle functional/structural modifications in nearby astrocytic processes.
Can astrocytes be valuable therapeutical targets?
The ability to selectively dampen the inhibitory tone of INs (or maybe of specific sets of INs) with fairly gross manipulations of astrocytes could provide a remarkable tool to alter the responsiveness of these cells to excitatory inputs and change the balance of inhibition and excitation in the brain (Clarke & Attwell, 2011). However, the types of INs in the brain and the functions they serve are so diverse (Somogyi & Klausberger, 2005) that it is hard to get a good intuition of how changing astrocytic function can ultimately regulate the behaviour of the whole brain, in health and disease. A role of astrocytes in regulating the onset and amplification of epileptic seizures has been previously proposed, possibly due to synchronization of the activity of large neuronal networks via release of glutamate and other modulators of excitatory neurotransmission by astrocytic syncytia (Fellin & Haydon, 2005; Tian et al. 2005). Conversely, in pathological conditions associated with glutamate excitotoxicity and neurological damage, astrocytes have been proposed to be good candidate therapeutical targets due to their ability to rapidly remove glutamate from the extracellular space (Bergles & Jahr, 1997; Danbolt, 2001). In an extensive screen of 1040 FDA-approved drugs and nutritionals, Rothstein et al. 2005 showed that a number of β-lactam antibiotics that enhance the expression of the glutamate transporter GLT1 in astrocytes also delay the onset of neuronal loss in animal models of amyotrophic lateral sclerosis (Rothstein et al. 2005) and improve the survival of neurons and rats after stroke (Lipski et al. 2007; Thone-Reineke et al. 2008). However, for most neurodegenerative diseases, the potential therapeutic relevance of astrocytes has been related only to their ability to act as scavengers for reactive oxygen species which accumulate in the brain of patients affected by Parkison's and Alzheimer's disease. Despite the fact that multiple lines of evidence suggest the existence of correlations between changes in the physiology of astrocytes and the onset or progression of various disease states, the development of therapeutical approaches specifically aimed at regulating astrocytic function has not been extensively pursued yet. One of the main reasons is that many membrane proteins or signalling cascades present in astrocytes are also functional in neurons, so targeting selectively one or the other cell type, despite its great potential for future clinical applications, has so far remained a challenging task. Of course this trend may change dramatically if specific astrocytic molecules regulating synaptic function are being identified.
An integrated view of astrocytes and neurons
Astrocytes and neurons have often been studied as separate entities, and for a long time the only form of activity that astrocytes were thought to be able of generating were occasional (0.001–0.002 Hz) and slow-decaying (5–160 s) Ca2+ waves in cell bodies and thick primary processes (Hirase et al. 2004). Recent high-resolution imaging experiments suggest that smaller astrocytic processes generate much more frequent and fast Ca2+ signals (Di Castro et al. 2011). These fine processes are presumably closer to synaptic contacts, and here the Ca2+ signals have been suggested to regulate the probability of transmitter release at excitatory synaptic terminals (Di Castro et al. 2011). Other studies also indicate that the functional interactions between astrocytes and neurons may be subject to substantial remodelling (Theodosis et al. 2008), for example during development (Tashiro & Kawai, 2007), exposure to enriched environments (Jones et al. 1996), hormonal fluctuations (Panatier et al. 2006) and, over shorter periods of time, after induction of synaptic plasticity (Wenzel et al. 1991; see also Agulhon et al. 2010; Henneberger et al. 2010). These structural changes are likely to have important functional consequences on synaptic function, possibly regulating the ability of synapses to process information independently of each other, and of changing their strength in response to modulation by various neurotransmitter receptor agonists (Min & Nevian, 2012) and co-agonists that astrocytes release (Panatier et al. 2006).
The currently emerging view, therefore, is that astrocytes are far from being as silent and static as initially thought (Kuffler et al. 1966; Ransom & Goldring, 1973), but may have dynamic, subtle and diverse interactions with neurons. This requires to move from a descriptive to a more mechanistic understanding of astrocytic signalling.
Conclusions
A rapidly emerging body of work suggests that astrocytes might be able to exert synapse-specific effects and have a much finer control of brain function than previously thought. The time may have come to leave behind the long-standing contentions about astrocytes doing nothing or everything in the brain: the challenge now is to define the molecular mechanisms that underlie any dynamic interaction between neurons and astrocytes and how these can be programmed to modulate the activity of specific sets of synapses in particular disease states.
Acknowledgments
This work was supported by the NIH/NINDS Intramural Research Program (NS002986).
References
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–1254. doi: 10.1126/science.1184821. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Beckman ML, Bernstein EM, Quick MW. Protein kinase C regulates the interaction between a GABA transporter and syntaxin 1A. J Neurosci. 1998;18:6103–6112. doi: 10.1523/JNEUROSCI.18-16-06103.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman ML, Bernstein EM, Quick MW. Multiple G protein-coupled receptors initiate protein kinase C redistribution of GABA transporters in hippocampal neurons. J Neurosci. 1999;19:RC9. doi: 10.1523/JNEUROSCI.19-11-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenhakker MP, Huguenard JR. Astrocytes as gatekeepers of GABAB receptor function. J Neurosci. 2010;30:15262–15276. doi: 10.1523/JNEUROSCI.3243-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergles DE, Jahr CE. Synaptic activation of glutamate transporters in hippocampal astrocytes. Neuron. 1997;19:1297–1308. doi: 10.1016/s0896-6273(00)80420-1. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Mody I. Extrasynaptic GABAA receptors: their function in the CNS and implications for disease. Neuron. 2012;73:23–34. doi: 10.1016/j.neuron.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bright DP, Renzi M, Bartram J, McGee TP, MacKenzie G, Hosie AM, Farrant M, Brickley SG. Profound desensitization by ambient GABA limits activation of delta-containing GABAA receptors during spillover. J Neurosci. 2011;31:753–763. doi: 10.1523/JNEUROSCI.2996-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford KA, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by α5 subunit-containing γ-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspani O, Heppenstall PA. TRPA1 and cold transduction: an unresolved issue. J Gen Physiol. 2009;133:245–249. doi: 10.1085/jgp.200810136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham DE. TRP channels as cellular sensors. Nature. 2003;426:517–524. doi: 10.1038/nature02196. [DOI] [PubMed] [Google Scholar]
- Clarke LE, Attwell D. An astrocyte TRP switch for inhibition. Nat Neurosci. 2011;15:3–4. doi: 10.1038/nn.3010. [DOI] [PubMed] [Google Scholar]
- Corey JL, Davidson N, Lester HA, Brecha N, Quick MW. Protein kinase C modulates the activity of a cloned gamma-aminobutyric acid transporter expressed in Xenopus oocytes via regulated subcellular redistribution of the transporter. J Biol Chem. 1994;269:14759–14767. [PubMed] [Google Scholar]
- Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- Cvetkov TL, Huynh KW, Cohen MR, Moiseenkova-Bell VY. Molecular architecture and subunit organization of TRPA1 ion channel revealed by electron microscopy. J Biol Chem. 2011;286:38168–38176. doi: 10.1074/jbc.M111.288993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. 2004;2:e329. doi: 10.1371/journal.pbio.0020329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Castro MA, Chuquet J, Liaudet N, Bhaukaurally K, Santello M, Bouvier D, Tiret P, Volterra A. Local Ca2+ detection and modulation of synaptic release by astrocytes. Nat Neurosci. 2011;14:1276–1284. doi: 10.1038/nn.2929. [DOI] [PubMed] [Google Scholar]
- Engel D, Schmitz D, Gloveli T, Frahm C, Heinemann U, Draguhn A. Laminar difference in GABA uptake and GAT-1 expression in rat CA1. J Physiol. 1998;512:643–649. doi: 10.1111/j.1469-7793.1998.643bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Fellin T, Haydon PG. Do astrocytes contribute to excitation underlying seizures. Trends Mol Med. 2005;11:530–533. doi: 10.1016/j.molmed.2005.10.007. [DOI] [PubMed] [Google Scholar]
- Gadea A, Lopez-Colome AM. Glial transporters for glutamate, glycine, and GABA: II. GABA transporters. J Neurosci Res. 2001;63:461–468. doi: 10.1002/jnr.1040. [DOI] [PubMed] [Google Scholar]
- Glykys J, Mann EO, Mody I. Which GABAA receptor subunits are necessary for tonic inhibition in the hippocampus. J Neurosci. 2008;28:1421–1426. doi: 10.1523/JNEUROSCI.4751-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golovina VA. Visualization of localized store-operated calcium entry in mouse astrocytes. Close proximity to the endoplasmic reticulum. J Physiol. 2005;564:737–749. doi: 10.1113/jphysiol.2005.085035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Henneberger C, Papouin T, Oliet SH, Rusakov DA. Long-term potentiation depends on release of D-serine from astrocytes. Nature. 2010;463:232–236. doi: 10.1038/nature08673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirase H, Qian L, Bartho P, Buzsaki G. Calcium dynamics of cortical astrocytic networks in vivo. PLoS Biol. 2004;2:E96. doi: 10.1371/journal.pbio.0020096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones TA, Hawrylak N, Greenough WT. Rapid laminar-dependent changes in GFAP immunoreactive astrocytes in the visual cortex of rats reared in a complex environment. Psychoneuroendocrinology. 1996;21:189–201. doi: 10.1016/0306-4530(95)00041-0. [DOI] [PubMed] [Google Scholar]
- Knott G, Marchman H, Wall D, Lich B. Serial section scanning electron microscopy of adult brain tissue using focused ion beam milling. J Neurosci. 2008;28:2959–2964. doi: 10.1523/JNEUROSCI.3189-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchibhotla KV, Lattarulo CR, Hyman BT, Bacskai BJ. Synchronous hyperactivity and intercellular calcium waves in astrocytes in Alzheimer mice. Science. 2009;323:1211–1215. doi: 10.1126/science.1169096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler SW, Nicholls JG, Orkand RK. Physiological properties of glial cells in the central nervous system of amphibia. J Neurophysiol. 1966;29:768–787. doi: 10.1152/jn.1966.29.4.768. [DOI] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Law RM, Stafford A, Quick MW. Functional regulation of γ-aminobutyric acid transporters by direct tyrosine phosphorylation. J Biol Chem. 2000;275:23986–23991. doi: 10.1074/jbc.M910283199. [DOI] [PubMed] [Google Scholar]
- Lipski J, Wan CK, Bai JZ, Pi R, Li D, Donnelly D. Neuroprotective potential of ceftriaxone in in vitro models of stroke. Neuroscience. 2007;146:617–629. doi: 10.1016/j.neuroscience.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Livet J, Weissman TA, Kang H, Draft RW, Lu J, Bennis RA, Sanes JR, Lichtman JW. Transgenic strategies for combinatorial expression of fluorescent proteins in the nervous system. Nature. 2007;450:56–62. doi: 10.1038/nature06293. [DOI] [PubMed] [Google Scholar]
- Micheva KD, Smith SJ. Array tomography: a new tool for imaging the molecular architecture and ultrastructure of neural circuits. Neuron. 2007;55:25–36. doi: 10.1016/j.neuron.2007.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min R, Nevian T. Astrocyte signaling controls spike timing-dependent depression at neocortical synapses. Nat Neurosci. 2012;15:746–753. doi: 10.1038/nn.3075. [DOI] [PubMed] [Google Scholar]
- Montell C, Rubin GM. Molecular characterization of the Drosophila trp locus: a putative integral membrane protein required for phototransduction. Neuron. 1989;2:1313–1323. doi: 10.1016/0896-6273(89)90069-x. [DOI] [PubMed] [Google Scholar]
- Mori Y, Takada N, Okada T, Wakamori M, Imoto K, Wanifuchi H, Oka H, Oba A, Ikenaka K, Kurosaki T. Differential distribution of TRP Ca2+ channel isoforms in mouse brain. Neuroreport. 1998;9:507–515. [PubMed] [Google Scholar]
- Nilius B, Prenen J, Owsianik G. Irritating channels: the case of TRPA1. J Physiol. 2011;589:1543–1549. doi: 10.1113/jphysiol.2010.200717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusser Z, Roberts JD, Baude A, Richards JG, Somogyi P. Relative densities of synaptic and extrasynaptic GABAA receptors on cerebellar granule cells as determined by a quantitative immunogold method. J Neurosci. 1995;15:2948–2960. doi: 10.1523/JNEUROSCI.15-04-02948.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panatier A, Theodosis DT, Mothet JP, Touquet B, Pollegioni L, Poulain DA, Oliet SH. Glia-derived D-serine controls NMDA receptor activity and synaptic memory. Cell. 2006;125:775–784. doi: 10.1016/j.cell.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Pizzo P, Burgo A, Pozzan T, Fasolato C. Role of capacitative calcium entry on glutamate-induced calcium influx in type-I rat cortical astrocytes. J Neurochem. 2001;79:98–109. doi: 10.1046/j.1471-4159.2001.00539.x. [DOI] [PubMed] [Google Scholar]
- Ransom BR, Goldring S. Ionic determinants of membrane potential of cells presumed to be glia in cerebral cortex of cat. J Neurophysiol. 1973;36:855–868. doi: 10.1152/jn.1973.36.5.855. [DOI] [PubMed] [Google Scholar]
- Rivera C, Voipio J, Payne JA, Ruusuvuori E, Lahtinen H, Lamsa K, Pirvola U, Saarma M, Kaila K. The K+/Cl− co-transporter KCC2 renders GABA hyperpolarizing during neuronal maturation. Nature. 1999;397:251–255. doi: 10.1038/16697. [DOI] [PubMed] [Google Scholar]
- Rothstein JD, Patel S, Regan MR, Haenggeli C, Huang YH, Bergles DE, Jin L, Dykes Hoberg M, Vidensky S, Chung DS, Toan SV, Bruijn LI, Su ZZ, Gupta P, Fisher PB. β-Lactam antibiotics offer neuroprotection by increasing glutamate transporter expression. Nature. 2005;433:73–77. doi: 10.1038/nature03180. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Hanchar HJ, Wallner M, Olsen RW, Otis TS. Contributions of the GABAA receptor α6 subunit to phasic and tonic inhibition revealed by a naturally occurring polymorphism in the α6 gene. J Neurosci. 2006;26:3357–3364. doi: 10.1523/JNEUROSCI.4799-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Properties of putative cerebellar gamma-aminobutyric acid A receptor isoforms. Mol Pharmacol. 1996;49:567–579. [PubMed] [Google Scholar]
- Scimemi A, Semyanov A, Sperk G, Kullmann DM, Walker MC. Multiple and plastic receptors mediate tonic GABAA receptor currents in the hippocampus. J Neurosci. 2005;25:10016–10024. doi: 10.1523/JNEUROSCI.2520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semyanov A, Walker MC, Kullmann DM. GABA uptake regulates cortical excitability via cell type-specific tonic inhibition. Nat Neurosci. 2003;6:484–490. doi: 10.1038/nn1043. [DOI] [PubMed] [Google Scholar]
- Shibasaki K, Suzuki M, Mizuno A, Tominaga M. Effects of body temperature on neural activity in the hippocampus: regulation of resting membrane potentials by transient receptor potential vanilloid 4. J Neurosci. 2007;27:1566–1575. doi: 10.1523/JNEUROSCI.4284-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Kracun S, Khakh BS. Monitoring astrocyte calcium microdomains with improved membrane targeted GCaMP reporters. Neuron Glia Biol. 2010a:1–9. doi: 10.1017/S1740925X10000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Kracun S, Sofroniew MV, Khakh BS. A genetically targeted optical sensor to monitor calcium signals in astrocyte processes. Nat Neurosci. 2010b;13:759–766. doi: 10.1038/nn.2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shigetomi E, Tong X, Kwan KY, Corey DP, Khakh BS. TRPA1 channels regulate astrocyte resting calcium and inhibitory synapse efficacy through GAT-3. Nat Neurosci. 2011;15:70–80. doi: 10.1038/nn.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi P, Klausberger T. Defined types of cortical interneurone structure space and spike timing in the hippocampus. J Physiol. 2005;562:9–26. doi: 10.1113/jphysiol.2004.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song X, Zhao Y, Narcisse L, Duffy H, Kress Y, Lee S, Brosnan CF. Canonical transient receptor potential channel 4 (TRPC4) co-localizes with the scaffolding protein ZO-1 in human fetal astrocytes in culture. Glia. 2005;49:418–429. doi: 10.1002/glia.20128. [DOI] [PubMed] [Google Scholar]
- Sotomayor M, Corey DP, Schulten K. In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure. 2005;13:669–682. doi: 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Tashiro Y, Kawai Y. Glial coverage of the small cell somata in the rat nucleus of tractus solitarius during postnatal development. Glia. 2007;55:1619–1629. doi: 10.1002/glia.20577. [DOI] [PubMed] [Google Scholar]
- Theodosis DT, Poulain DA, Oliet SH. Activity-dependent structural and functional plasticity of astrocyte-neuron interactions. Physiol Rev. 2008;88:983–1008. doi: 10.1152/physrev.00036.2007. [DOI] [PubMed] [Google Scholar]
- Thone-Reineke C, Neumann C, Namsolleck P, Schmerbach K, Krikov M, Schefe JH, Lucht K, Hortnagl H, Godes M, Muller S, Rumschussel K, Funke-Kaiser H, Villringer A, Steckelings UM, Unger T. The beta-lactam antibiotic, ceftriaxone, dramatically improves survival, increases glutamate uptake and induces neurotrophins in stroke. J Hypertens. 2008;26:2426–2435. doi: 10.1097/HJH.0b013e328313e403. [DOI] [PubMed] [Google Scholar]
- Tian GF, Azmi H, Takano T, Xu Q, Peng W, Lin J, Oberheim N, Lou N, Wang X, Zielke HR, Kang J, Nedergaard M. An astrocytic basis of epilepsy. Nat Med. 2005;11:973–981. doi: 10.1038/nm1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura R, Harris KM. Three-dimensional relationships between hippocampal synapses and astrocytes. J Neurosci. 1999;19:6897–6906. doi: 10.1523/JNEUROSCI.19-16-06897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitellaro-Zuccarello L, Calvaresi N, De Biasi S. Expression of GABA transporters, GAT-1 and GAT-3, in the cerebral cortex and thalamus of the rat during postnatal development. Cell Tissue Res. 2003;313:245–257. doi: 10.1007/s00441-003-0746-9. [DOI] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel J, Lammert G, Meyer U, Krug M. The influence of long-term potentiation on the spatial relationship between astrocyte processes and potentiated synapses in the dentate gyrus neuropil of rat brain. Brain Res. 1991;560:122–131. doi: 10.1016/0006-8993(91)91222-m. [DOI] [PubMed] [Google Scholar]
- Wu LJ, Sweet TB, Clapham DE. International Union of Basic and Clinical Pharmacology. LXXVI. Current progress in the mammalian TRP ion channel family. Pharmacol Rev. 2010;62:381–404. doi: 10.1124/pr.110.002725. [DOI] [PMC free article] [PubMed] [Google Scholar]


