Abstract
Cell replacement therapy for Parkinson's disease has predominantly focused on ectopic transplantation of fetal dopamine (DA) neurons into the striatum as a means to restore neurotransmission, rather than homotopic grafts into the site of cell loss, which would require extensive axonal growth. However, ectopic grafts fail to restore important aspects of DA circuitry necessary for controlled basal ganglia output, and this may underlie the suboptimal and variable functional outcomes in patients. We recently showed that DA neurons in homotopic allografts of embryonic ventral mesencephalon (VM) can send long axonal projections along the nigrostriatal pathway in order to innervate forebrain targets, although the extent of striatal reinnervation remains substantially less than can be achieved with ectopic placement directly into the striatal target. Here, we examined the possible benefits of using younger VM donor tissue and over-expression of glial cell-derived neurotrophic factor (GDNF) in the striatal target to improve the degree of striatal innervation from homotopic grafts. Younger donor tissue, collected on embryonic day (E)10, generated 4-fold larger grafts with greater striatal targeting, compared to grafts generated from more conventional E12 donor VM. Over-expression of GDNF in the host brain also significantly increased DA axonal growth and striatal innervation. Furthermore, a notable increase in the number and proportion of A9 DA neurons, essential for functional recovery, was observed in younger donor grafts treated with GDNF. Behavioural testing confirmed functional integration of younger donor tissue and demonstrated that improved motor function could be attributed to both local midbrain and striatal innervation. Together, these findings suggest there is significant scope for further development of intra-nigral grafting as a restorative approach for Parkinson's disease.
Key points
Ectopic cell transplantation for Parkinson's disease improves dopamine neurotransmission but fails to restore the neural circuit important for controlled motor function, and may thereby underlie suboptimal and variable outcomes in patients. However, homotopic grafts, into the site of cell loss, survive poorly and show limited integration.
Here we explore intrinsic and extrinsic factors to improve homotopic grafting.
The use of fetal donor tissue, younger than conventionally used, generates significantly larger grafts with increased innervation and circuit reconstruction. These effects can be enhanced by exposure of the graft to glial derived neurotrophic factor.
Furthermore, improved homotypic grafts are capable of enhancing functional recovery in Parkinsonian mice.
These findings have important implications for the development of cell- and stem cell-based therapies to promote neural circuit reconstruction in the treatment of Parkinson's disease.
Introduction
The hallmark pathology in Parkinson's disease (PD) is the progressive degeneration of midbrain dopaminergic (DA) neurons, resulting in loss of striatal DA input and consequential motor disturbances. Experiments in animal models of PD, as well as a number of clinical trials in patients, have provided proof-of-principle for DA neuron replacement as a promising experimental therapy with significant scope for further development. Newly transplanted DA neurons isolated from the developing ventral midbrain (VM) are capable of surviving, releasing DA and improving motor function. However, extensive variability in the degree of functional recovery has been observed across patients (Winkler et al. 2005; Brundin et al. 2010). A number of factors are likely to contribute to this variability, including the choice of donor tissue, graft integration, patient selection and immune suppression (Winkler et al. 2005; Brundin et al. 2010), thereby highlighting the need for further development and standardization of this technology.
To date, the majority of studies have focused on the ectopic placement of new DA neurons into the target tissue (the striatum) as a means to restore DA neurotransmission, rather than homotopic placement into the site of cell loss (the substantia nigra pars compacta, SNpc), that would require extensive axonal growth. However, it has been widely speculated that limitations in the accurate restoration of DA circuitry inherent to the ectopic placement may underlie limitations in the degree of motor restoration that can be achieved. For example, DA neurons placed in the striatum would lack their normal pattern of afferent input, including reciprocal afferent striato-nigral projections that regulate DA axon arbour size, trajectory and DA delivery. Furthermore, ectopic grafts fail to establish dendritic DA control of the substantia nigra pars reticulata (SNpr), important in fine-tuning basal ganglia output and ultimately motor function (Yurek, 1997).
Initial efforts to restore DA circuitry and neurotransmission by homotopic grafting showed poor survival, integration and restoration of functional output compared to intrastriatal grafts, with little evidence of axonal growth along the nigrostriatal pathway (Bjorklund et al. 1983; Dunnett et al. 1983; Sauer et al. 1992; Nikkhah et al. 1994a, b; Bentlage et al. 1999). It was concluded from these studies that the adult brain is incapable of supporting the long-distance axonal growth required for re-construction of the nigrostriatal pathway following intranigral grafting (Bjorklund et al. 1983; Nikkhah et al. 1994a). Recently, however, studies using donor tissue from transgenic reporter mice have shown that this is not the case.
Using donor tissue from mice in which green fluorescent protein (GFP) is expressed in the DA neurons, two recent studies have shown that the transplanted neurons are capable of long-distance growth along the nigrostriatal pathway and innervation of appropriate forebrain targets, in adult mice (Gaillard et al. 2009; Thompson et al. 2009). Results from these studies suggested that, in addition to the use of improved tools to visualize graft connectivity, residual host fibres from partial rather than complete lesioning of the host nigrostriatal pathway may provide a growth-permissive environment capable of supporting newly grafted axons. Within these homotopic grafts, striatal reinnervation could be enhanced by over-expression of glial cell-derived neurotrophic factor (GDNF) (Thompson et al. 2009), a trophin known to promote the survival and axonal growth of DA neurons (Bjorklund et al. 1997). Although these findings demonstrate that restoration of the pathway is possible and can improve motor function, striatal reinnervation remained well below that of the intact brain, highlighting the need for efforts to improve the level of connectivity from these grafts.
Several studies have demonstrated the benefit of younger VM donor tissue for ectopic grafting. Donor tissue isolated prior to rather than at the peak phase of neurogenesis has been shown to produce larger grafts as a result of increased survival and proliferation of DA neuroblasts at the time of implantation (Brundin et al. 1985; Freeman et al. 1995; Gates et al. 2006; Torres et al. 2007; Bye et al. 2012). Furthermore, we recently showed that donor age could impact on the composition of DA subpopulations within the graft (Bye et al. 2012). Within the developing VM, and thereby donor tissue, progenitors for at least two main classes of DA neurons reside: the A9 cells of the SNpc and the A10 neurons of the ventral tegmental area (VTA). In the intact brain, the A9 DA neurons project via the nigrostriatal pathway as the main source of DA input to the dorsolateral striatum as a key regulator of motor function, whilst A10 DA neurons project predominately to cortical and limbic structures to regulate reward-related behaviour (Dahlstrom & Fuxe, 1964). Interestingly, these DA subtypes maintain their normal developmental target preference when grafted ectopically into the adult striatum (Mendez et al. 2005; Thompson et al. 2005) and specifically the A9 neurons appear to play a decisive role in improving motor function (Mendez et al. 2005; Kuan et al. 2007; O’Keeffe et al. 2008; Grealish et al. 2010). It is now recognized that during development, these A9 DA neurons precede the birth of A10 neurons (Joksimovic et al. 2009; Blaess et al. 2011; Hayes et al. 2011; Bye et al. 2012). Hence, the use of younger VM donor tissue can appropriately enrich for this A9 population (Bye et al. 2012). It now remains to be determined whether younger donor tissue may also benefit homotopic grafts and whether these younger cells remain responsive to GDNF.
In this study we have analysed specific donor- and host-related variables and their impact on the degree of nigrostriatal growth and striatal connectivity that can be achieved following homotopic grafting of embryonic VM. Specifically, we have assessed the effect of VM donor tissue age (embryonic day (E)10 versus‘conventional’ E12) and trophic tone of the host striatal target (over-expression of GDNF) on graft survival, integration, composition and functional recovery in Parkinsonian mice.
Methods
Ethical approval
All procedures were conducted in accordance with the Australian National Health and Medical Research Council's published Code of Practice for the Use of Animals in Research, and experiments were approved by the Florey Neuroscience Institute animal ethics committee.
All mice were housed on a 12 h light/dark cycle with ad libitum access to food and water. Adult female Swiss mice were used as graft recipients whilst tyrosine hydroxylase–GFP (TH-GFP) mice (Sawamoto et al. 2001) were used to generate embryos as a source of donor tissue for transplantation. In total, 109 adult mice were used in the present study and were divided into the following groups: intact controls (n= 15), lesion control (n= 20), GDNF controls (n= 10), animals receiving E10 donor tissue (n= 22), animals receiving E10 donor tissue + GDNF over-expression (n= 10), animals receiving E12 donor tissue (n= 21) and animals receiving E12 donor tissue + GDNF (n= 11).
Ventral mesencephalon cell suspensions
Within the developing mouse VM the first DA neurons appear at approximately E10 with DA neurogenesis peaking at E12.5 and ceasing around E14.5 (Bayer et al. 1995). Hence in the present study embryos were isolated from time-mated TH-GFP mice at E10 (early DA neurogenesis) and E12 (late neurogenesis and the ‘conventionally’ used donor age for most studies). Animals were time mated overnight and visualization of a vaginal plug on the following morning was taken as E0. At E10 or E12, pregnant female mice were deeply anaesthetized and killed using cervical dislocation before harvesting the embryos. The VM was microdissected from each TH-GFP embryo and prepared for transplantation as previously described (Dunnett & Bjorklund, 1997; Parish & Thompson, 2012). The final cell preparations, containing 1 × 105 viable cells μl−1, were prepared in magnesium- and calcium-free Hank's buffered salt solution containing 0.1% DNase. Cells were stored on ice for the duration of the transplantation procedure.
6-Hydroxydopamine lesions and transplantation
All surgeries were performed under general anaesthetic using 2% isoflurane inhalation (Baxter, Deerfield, IL, USA). Ninety-four mice received partial lesions of the VM DA neurons by unilateral injection of 6-hydroxydopamine (6-OHDA) into the SNpc. A single 3 μg stereotaxic injection (1.5 μl) of the toxin was made as previously described (Parish et al. 2001). Three weeks after lesioning host animals received intranigral grafts of a single cell VM suspension derived from either E10 or E12 TH-GFP donor embryos. Micro-transplantation, using a glass capillary attached to a 5 μl Hamilton syringe, was used to deliver a total of 1 μl of cell suspension into the SNpc at the following coordinates: 3.2 mm posterior and 1.4 mm lateral to bregma and 4.5 mm ventral from the surface of the dura (Franklin & Paxinos, 1997).
Viral delivery of GDNF
In order to over-express GDNF, a proportion of animals also received an intrastriatal injection of an adeno-associated viral vector carrying GDNF under the control of the chicken β-actin (CBA) promoter (rAAV2/5-CBA-GDNF; infectious titre of 1.0 × 1012), at the time of grafting. The virus (GDNF-AAV) was a kind donation from Professor Deniz Kirik (Lund University, Sweden) (Eslamboli et al. 2005). The virus (0.5 μl) was delivered via stereotaxic surgery at the following two coordinates (relative to bregma): AP, +1.0 mm; ML, −2.0 mm; DV, −3.2 mm and AP, +1.0 mm; ML, −2.0 mm; DV, −2.2 mm (Franklin & Paxinos, 1997). Confirmation of viral delivery, and expression in the target tissue (the striatum), was validated by immunostaining for GDNF.
Retrograde labelling of transplanted cells
For retrograde labelling of transplanted cells, a small subset of grafted mice received intrastriatal injections of 2% fluorogold (Fluorochrome, Denver, CO, USA) 11 weeks after intranigral grafting. Two injection sites were used to deliver small (2 × 0.25 μl) volumes of flurogold, to ensure maximal striatal labelling, without labelling adjacent nuclei. Injections were made at the following coordinates: AP, +0.7; ML, −1.9; DV, −2.6 and AP, +0.3; ML, −2.1; DV, −2.4 (flat skull position). The survival time after the fluorogold injections was 1 week.
Corridor testing
To assess the extent of 6-OHDA-induced SNpc lesions, as well as the degree of functional recovery after transplantation, sensorimotor function was measured using the corridor test. Testing was performed 3 weeks after 6-OHDA lesioning (pre-transplantation) and again at 12 weeks after grafting (post-transplantation), based on previously described methods (Smith et al. 2012). In brief, the apparatus consisted of a 60 cm-long, 5 cm-wide corridor with 10 pots containing sugar pellets placed at 5 cm intervals along the corridor. Mice underwent food restrictions with 1 g of food allocated per animal per day throughout the habituation and testing period. Mice were habituated in the corridors for 10 min day−1 over 3 days. Following habituation, animals were scored on three consecutive days for a period of 5 min. The numbers of ipsilateral and contralateral retrievals from the pots containing sugar pellets were counted. A ‘retrieval’ was defined as being >2 s that a mouse spent exploring a pot. Data were expressed as the percentage of contralateral retrievals relative to the total number of retrievals (contralateral and ipsilateral). Only those animals with less than 30% contralateral retrievals on average over the 3 days of testing were classified as lesioned and were included in subsequent behavioural testing at 12 weeks post-transplantation.
Tissue processing and immunohistochemistry
Animals received an overdose of sodium pentobarbitone (100 mg kg−1; Virbac, Peakhurst, Australia) and were transcardially perfused with warmed phosphate buffered saline (PBS), followed by chilled paraformaldehyde (4% w/v in 0.1 m phosphate buffer). Brains were post-fixed for 2 h in 4% paraformaldehyde and cryo-protected overnight in sucrose (30% w/v in 0.1 m PBS) before being sectioned on a freezing microtome (Leica, Wetzlar, Germany). Horizontal sections were collected in twelve series at a thickness of 40 μm.
Immunohistochemical procedures were performed as previously described (Thompson et al. 2005). Primary antibodies and dilution factors were as follows: chicken anti-GFP (1:1000; Abcam, Cambridge, MA, USA), rabbit anti-GFP (1:20,000; Abcam), mouse anti-calbindin (1:1500: Swant), rabbit anti-G-protein-gated inwardly rectifying K+ channel subunit 2 (GIRK2, 1:500; Chemicon, Temecula, CA, USA), rabbit anti-tyrosine hydroxylase (TH, 1:500; PelFreez) and goat anti-GDNF (1:2000; R&D Systems, Minneapolis, MN, USA). Secondary antibodies for (i) direct detection were used at a dilution of 1:200 – DyLight 488-, 549- or 649-conjugated donkey anti-mouse, anti-chicken, anti-rabbit or anti-rat (Jackson ImmunoResearch, West Grove, PA, USA); and (ii) indirect with streptavidin-biotin amplification – biotin-conjugated donkey anti-rabbit (1:500; Jackson ImmunoResearch) followed by peroxidase-conjugated streptavidin (Vectastain ABC kit, Vector Laboratories, Burlingame, CA, USA) or 649-conjugated streptavidin (1:200; Jackson ImmunoResearch). All fluorescent images were captured using a Zeiss Pascal confocal microscope system whilst bright- and dark-field images were obtained using a Leica DM6000 upright microscope.
Cell counting and fibre innervation
The extent of the midbrain lesion was determined by examination of TH labelling in lesioned, un-grafted mice, with comparisons made between the intact contralateral versus the lesioned ipsilateral hemispheres. The number of grafted DA neurons within each animal was quantified based upon GFP labelling. The numbers of GFP+ GIRK2+, GFP+ calbindin+ and GFP+GIRK2+ calbindin+ cells were estimated from confocal acquired images. For each set of stains, counts were performed on every sixth section (hence 240 μm between each section). The data are represented as total cell counts as well as the contribution of each of these cell types to the graft (as a percentage of the total number of GFP+ cells).
The number of graft-derived GFP+ DA fibres projecting along the nigrostriatal pathway was counted at a point midway between the edge of the graft (within the SN) and the globus palladis (GP)/striatal border, at an angle perpendicular to the fibre bundle. Counts were made from every sixth section through the entire dorsoventral axis of the pathway. The density of GFP+ fibres in the striatum of grafted animals was determined in the dorsolateral striatum, at a single point approximately 1.0 mm posterior and 3.0 mm lateral from bregma, and 2.8 mm below the surface of the dura (Franklin & Paxinos, 1997). Leica LAS Image Analysis Software was used to estimate fibre density from images captured at 40× on a Leica 6000 microscope. The area covered by GFP+ fibres was expressed as a percentage of the total area.
Statistical analysis
All data are expressed as mean ± standard error of the mean (SEM). All statistical analyses were conducted using Graph Pad Prism 5. One-way ANOVAs with Tukey's post hoc or Student's t tests were used where appropriate.
Results
Younger donor tissue produces larger grafts and enhanced innervation along the medial forebrain bundle
The use of TH-GFP reporter mice for VM donor tissue enabled a clear distinction between graft-derived DA neurons and the associated fibre outgrowth (GFP+; Figs 1 and 2) versus residual host (GFP-) DA patterns. At the time of death, TH immunohistochemistry confirmed robust ablation of the midbrain DA neurons in lesioned, un-grafted mice, with the vast majority of SNpc DA neurons lost, and relative sparing of DA neurons within the adjacent VTA and retrorubal field (data not shown). Twelve weeks post transplantation, 91% of transplanted animals showed viable grafts that were appropriately localized within the ventral midbrain.
Figure 1. Younger donor tissue results in larger grafts with increased axonal growth and striatal targeting.
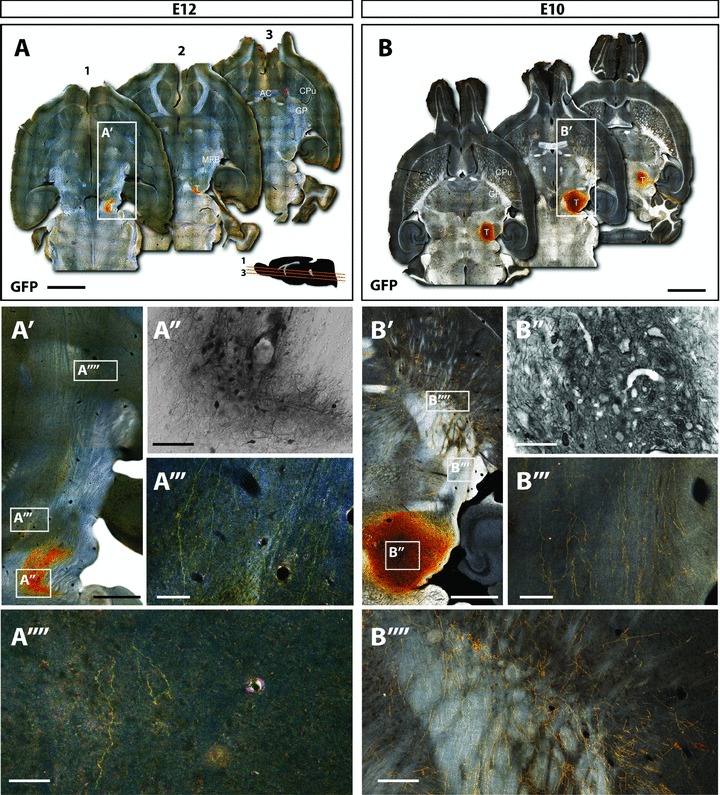
Immunohistochemical examination of mice 12 weeks after receiving intranigral grafts of embryonic day (E)12 or E10 VM donor tissue from TH-GFP reporter mice. A and B, dark-field photomontages providing a horizontal overview of the GFP+ grafts of E12 (A) and E10 (B) donor grafts. Insert in A shows a parasagittal schematic of the angle of cutting and approximate dorsoventral level of each section (1–3), with 480 μm between each section shown. A′ and B′, magnified overviews of the E12 and E10 donor grafts localized within the VM, their trajectory through the medial forebrain bundle and targeting the striatum. B′, bright field image illustrating that younger donor tissue showed larger grafts with notably more GFP+ cells and increased cellular density than E12 donor grafts. E10 donor tissue showed increased number of fibres coursing through the MFB (B″′) and entering the striatum (B″″) compared to E12 donor grafts (A″′ and A″″). AC, anterior commisure; CPu, caudate putamen; GP, globus pallidus; MFB, medial forebrain bundle; T, intranigral transplant. Scale bar: A,B= 2 mm; A′, A″, A″″, B′,B″,B″″= 500μm; A″′,B″′= 200μm.
Figure 2. GDNF over-expression promotes the integration of both older and younger age donor grafts.
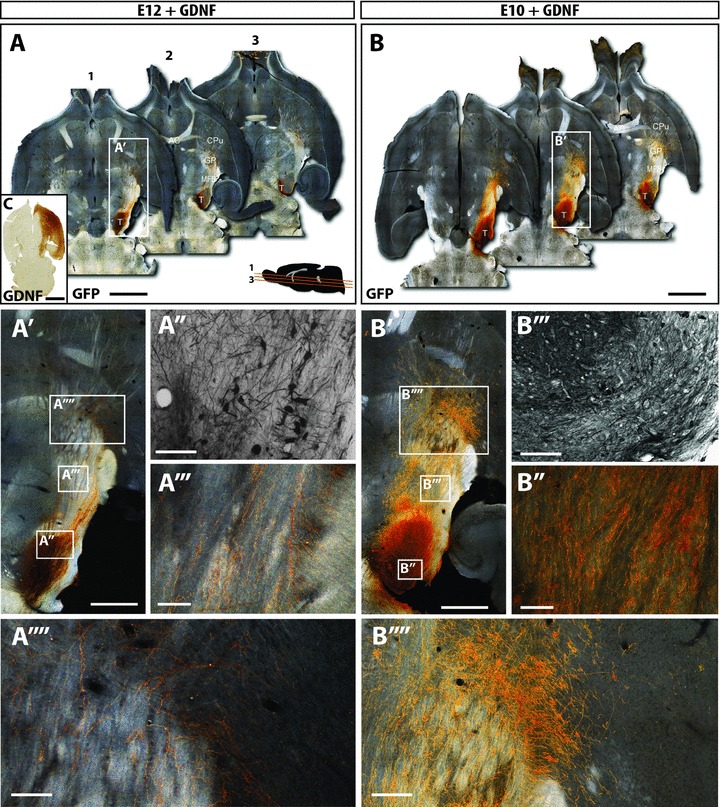
A and B, dark-field photomontages providing a horizontal overview of the GFP+ grafts of E12 (A) and E10 (B) donor grafts in the presence of GDNF over-expression, at 12 weeks post transplantation. C, GDNF was over-expressed by injection of AAV-GDNF into the host striatum at the time of grafting. Immunohistochemistry revealed robust GDNF expression throughout the target striatum and overlying cortex. Furthermore, GDNF was expressed within the efferent targets of striatal and cortical projection neurons, including the globus pallidus and substantia nigra. A′ and B′, magnified overviews of the E12+GDNF and E10+GDNF grafts localized within the VM, their trajectory through the medial forebrain bundle and targeting the striatum. Bright field images illustrating larger grafts within notably more GFP+ cells and increased cellular density in animals grafted with E10 donor tissue (+GDNF, A′), compared to E12 (+GDNF, B′) donor grafts. However, grafts were not notably larger than E10 or E12 grafts in the absence of GDNF (Fig. 1). A″′ and B″′, exposure of grafts to GDNF resulted in a marked increase in GFP+ fibres coursing through the MFB in both young and older donor grafts (compared to the absence of GDNF, Fig. 1). A″″ and B″″, additionally GDNF increased striatal innervation. The effects of GDNF on axonal growth and striatal targeting were more evident in younger than older donor grafts. AC, anterior commisure; CPu, caudate putamen; GP, globus pallidus; MFB, medial forebrain bundle; T, intranigral transplant. Scale bar: A,B= 2μm; A′, A″, A″″, B′,B′,B″″= 500μm; A″′,B″′= 200μm; C= 1μm.
Quantification of GFP+ labelled cells revealed that younger (E10) donor tissue produced significantly larger grafts (401%) compared to the older ‘conventional’ E12 donor tissue (2855 ± 396 and 711 ± 135, respectively, Figs 1 and 3A). In addition, younger donor grafts displayed increased GFP+ cell density within the graft (Fig. 1A′ and B′).
Figure 3. Quantification of the effects of donor age and GDNF over-expression on graft size and integration following intranigral transplantation.

A, quantification of GFP+ cells revealed significantly larger grafts from E10 donor tissue compared to E12. GDNF had little effect on the survival of either E10 or E12 donor tissue. P < 0.0001, F= 13.02. B, assessment of the number of graft-derived GFP+ axons at a point midway along the MFB revealed a significant increase in fibre density from younger versus older donor tissue. GDNF significantly increased the number of fibres within the MFB of both young and old donor grafts, with E10+GDNF showing the most robust innervation. P < 0.0001, F= 16.53. C, donor age had little effect on the proportion of cells within intranigral grafts capable of projecting through the MFB (GFP+ fibres within the MFB/GFP+ cells). However, exposure of the grafts to GDNF significantly increased the ability of E10 and E12 grafts to form long distance projections. P < 0.0001, F= 11.17. Open bars: E12 donor graft (n= 21), light grey bars: E10 donor graft (n= 22), dark grey bars: E12 donor graft+GDNF (n= 11), filled bars: E10 donor graft+GDNF (n= 10). Data represent mean + SEM. *P < 0.05, **P < 0.01, ***P < 0.001.
Within grafted animals, the GFP reporter enabled clear visualization of new DA fibres coursing from the site of implantation, along the nigrostriatal pathway and median forebrain bundle, exiting the GP and ramifying within the striatum (Fig. 1A′ and B′). GFP+ fibres were quantified at a point along the medial forebrain bundle (MFB) midway between the graft site and GP–striatal border. Whilst fibre innervation was poor in older donor grafts (10 ± 3 GFP+ fibres within the MFB), younger donor tissue showed a significant 23-fold increase in the number of GFP+ axons (237 ± 63; Fig. 3B). The increase in innervation was over and above that due to the increased cell number observed in younger E10 donor grafts, with an average of 8.9 ± 2.8% of GFP+ grafted cells capable of sending projections through the MFB, compared to only 1.1 ± 0.4% of older E12 donor cells (Fig. 3C). These findings demonstrate that younger donor tissue generates homotopic grafts that contain more DA neurons, and that these neurons have a significantly greater capacity for growth along the nigrostriatal pathway.
GDNF enhances the extent of innervation in young and older donor grafts
At the time of intranigral grafting, a subset of animals received intrastriatal injections of an adenovirus encoding GDNF. Immunohistochemistry for GDNF confirmed expression of the protein throughout the striatum and overlying cortex in 95% of animals. In each of these mice, GDNF expression was also observed along the MFB and in the VM, presumably due to efferent targeting from striatal projection neurons (Fig. 2C).
GDNF over-expression resulted in a modest (35%) increase in the number of DA neurons within the grafts of animals receiving E12 donor tissue (E12: 711 ± 135, E12+GDNF: 958 ± 161, Figs 1A″′, 2A″′ and 3A). More noteworthy was the 42-fold increase in the number of fibres coursing through the MFB in the presence of GDNF (E12: 10 ± 3, E12+GDNF: 416 ± 63, Figs 1A′, 2A′ and 3B), and demonstrating that approximately 66% of grafted cells were capable of long distance axonal growth along the MFB, compared to 1.1% of donor cells in the absence of GDNF (Fig. 3C).
GDNF had little effect (15% increase) on the survival of DA neurons from younger donor grafts (E10: 2855 ± 396, E10+GDNF: 3288 ± 496, Fig. 3A). However these younger donor cells were also responsive to GDNF, as demonstrated by the more extensive fibre growth along the nigrostriatal pathway compared to E10 grafts alone (1183 ± 287 and 237 ± 73, respectively, Figs 1B′, 2B′ and 3B). The fibre density within the MFB was not only significantly enhanced compared to E10 grafts (5-fold) but also showed a significant increase in innervation compared to E12+GDNF grafts (2.8-fold), suggesting that younger tissue provided an improved source of donor material for homotopic transplantations (Fig. 3B). Interestingly, despite the increased innervation observed from E10+GDNF grafts, only 38% of the transplanted DA neurons were capable of forming long distance projections (Fig. 3C).
Donor age and GDNF influence the phenotype of transplanted DA neurons
Within the intact midbrain and VM grafts, distinct subpopulations of DA neurons can readily be identified based upon their size, shape, forebrain targets and protein expression. Here we identified A9 DA neurons by co-expression of GFP and GIRK2, and A10 DA neurons by GFP and calbindin. Unlike intrastriatal grafts, we observed that the majority of GFP+ cells (>65%) within the intranigral grafts failed to adopt an A9 GIRK2+ or A10 calbindin+ phenotype. However, where present, calbindin+ cells were predominantly confined to the graft core whilst GIRK2+ cells were localized to the graft periphery (Fig. 4A–H), as previously described (Mendez et al. 2005; Thompson et al. 2005, 2009), demonstrating the ability for these grafts to maintain structural organization.
Figure 4. Younger donor grafts, exposed to GDNF, enriches for GIRK2+ A9 DA neurons.
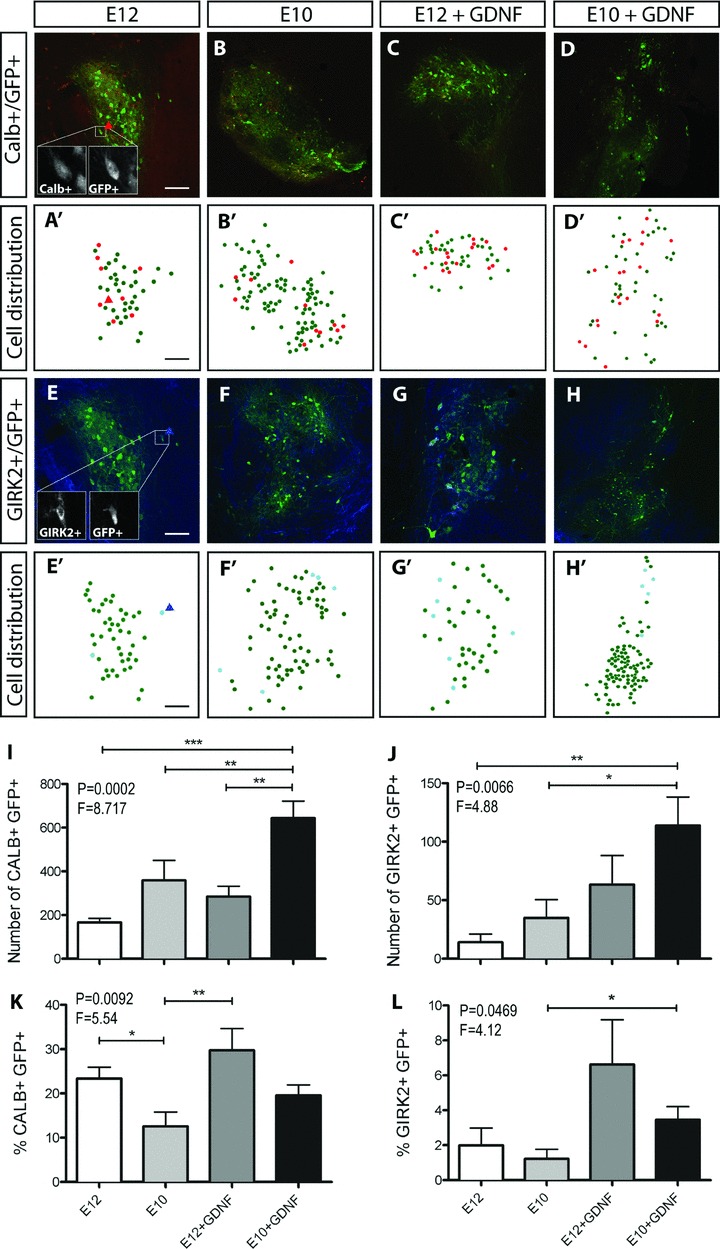
Horizontal sections through the VM illustrating GFP+ and calbindin+ cell bodies (A–D) as well as GFP+ and GIRK2+ (E–H) cells within intranigral grafts of differing donor age ± GDNF exposure. Dot plot representations of the distribution of GFP+ and GFP+calbindin+ cells (A′–D′) as well as GFP+ and GFP+GIRK2+ cells (E′–H′) within corresponding grafts depicted in A–H. I and J, exposure of E10 donor grafts to GDNF resulted in a significant increase in the total number of calbindin (I) and GIRK2+ (J) cells within the graft compared to E10, E12 or E12+GDNF grafts. K and L, proportion of calbindin+GFP+ (K) and GIRK2+GFP+ cells (L) (as a percentage of total GFP+ neurons) within the grafts revealed a depletion of calbindin cells in E10 versus E12 grafts. E10 grafts exposed to GDNF enriched the proportion of GIRK2+ A9 neurons. No significant difference was seen in the proportion of triple labelled (GIRK+/ calbindin+/GFP+) cells across the graft groups (data not shown). Data represent mean ± SEM. One-way ANOVA with Tukey's post hoc test, *P < 0.05, **P < 0.01, ***P < 0.001. E12 graft, n= 21; E10 graft, n= 22; E12+GDNF, n= 11; E10+GDNF, n= 10. Scale bar = 200μm.
Quantification of calbindin+ GFP+ and GIRK2+GFP+ cells revealed a significant increase in the number of DA neurons with a calbindin+ or GIRK2+ phenotype in younger E10 donor grafts exposed to GDNF, an effect not seen in E12+GDNF grafts or when comparing between donor ages alone (Fig. 4I and J). However, closer examination revealed that these increases in GIRK2+ and calbindin+ cells were a consequence of larger grafts in the E10+GDNF grafted animals. In fact, younger donor tissue significantly reduced the proportion of A10-like calbindin+ cells (Fig. 4K), as previously observed following intrastriatal grafting (Bye et al. 2012), yet had no effect on the proportion of GIRK2+ cells (Fig. 4L). Only exposure of younger donor tissue to GDNF was capable of enhancing the total number and proportion of A9-like GIRK2+ GFP+ DA neurons within the graft (Fig. 4H, J and L). These findings illustrate that in addition to larger grafts, E10 donor tissue exposed to GDNF can enhance the total number and proportion of A9 DA neurons.
Fluorogold (FG) was injected into the striatum of a subset of mice receiving homotopic nigral grafts to determine whether striatal targeting influenced phenotype (A9 or A10) acquisition (Supplemental Fig. S1A). Of the small number of retrograded filled grafted neurons (i.e. FG+ GFP+), 75% adopted an A9 GIRK2+ or A10 calbindin+ phenotype. Of those phenotypically marked FG+ GFP+ cells, three-quarters expressed calbindin (Supp. Fig. S1B), whilst one-quarter were GIRK2+, reflecting the greater propensity for calbindin cells within the grafts (Fig. 4I and J).
Graft donor age and exposure to GDNF influence motor function through altered local midbrain and striatal innervation
In light of the increased number of GFP+ and GIRK2+GFP+ neurons in younger grafts, as well as the enhanced number of fibres projecting through the MFB, we next examined the effect of these grafts on functional recovery. Corridor testing was performed to assess sensorimotor neglect in unilateral lesioned mice, with increases in contralateral retrievals at 12 weeks post transplantation reflecting improved motor function. Lesioning of the midbrain DA system was confirmed prior to transplantation with animals showing a reduction in contralateral retrievals compared to intact controls (white bars, Fig. 5A). No spontaneous improvement was observed in lesioned mice after 12 weeks, nor did GDNF over-expression, in the absence of a cell graft, have any effect on motor function (Fig. 5A).
Figure 5. Graft donor age and exposure to GDNF influence motor function through altered local midbrain and striatal innervation.
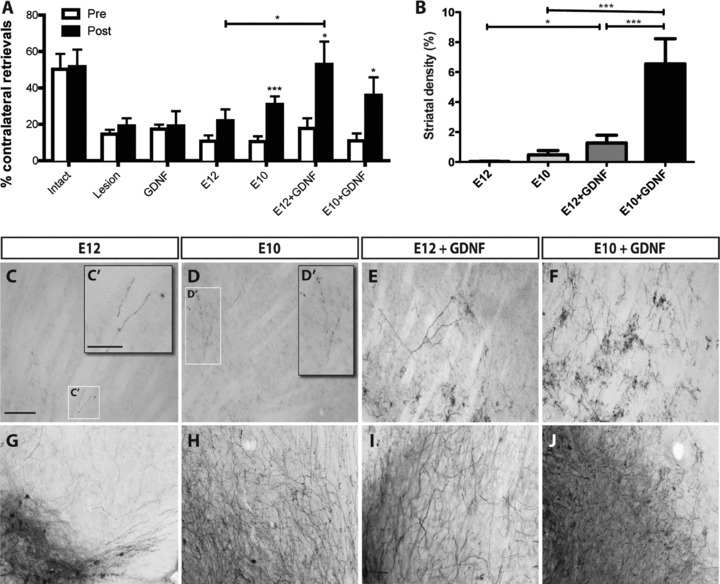
A, sensorimotor neglect was assessed using the corridor task in animals after lesioning and again at 12 weeks post transplantation. Intact animals showed no bias in motor function. Lesioning was confirmed in all relevant groups, as depicted by the low number of contralateral retrievals relative to intact controls. No spontaneous recovery was observed in lesioned controls or animals overexpressing GDNF in the absence of a graft at 12 weeks. E10 but not E12 donor tissue resulted in a significant improvement in motor function after 12 weeks. While GDNF provided no additional functional benefit to young E10 donor tissue grafts, contralateral retrievals were significantly increased in older E12-derived donor grafts. Open bars: pre-transplantation, filled bars: 12 weeks post transplantation. Intact, n= 15; lesion, n= 20; GDNF, n= 10; E12 graft, n= 21; E10 graft, n= 22; E12+GDNF, n= 11; E10+GDNF, n= 10. B, GDNF over-expression had a significant impact on the density of striatal innervation of E10 and E12 donor grafts. C–J, bright-field micrographs illustrating the density of GFP+ fibres in the dorsolateral striatum (C–F) and the ventral midbrain (G–J) of grafted animals. Data represent mean ± SEM. One-way ANOVA with Tukey's post hoc test, *P < 0.05, **P < 0.01, ***P < 0.001. Scale bar: C-J= 250μm, C′,D′= 100μm.
Younger donor age grafts resulted in a significant improvement (3-fold) in motor behaviour 12 weeks post transplantation, and was similarly observed following exposure to GDNF (3.3-fold). In contrast, older donor tissue grafts trended toward improved function at 12 weeks but did not reach significance. However, these older donor grafts responded to GDNF, with a significant increase (2-fold) in post- versus pre-transplantation contralateral retrievals, and notably increased from E12 grafts alone (2.4-fold, E12 vs. E12+GDNF post transplantation; Fig. 5A).
In the intact brain, DA innervation of the striatum, predominantly from SNpc A9 GIRK2+ neurons, is responsible for regulating motor function. However, additional DA release from dendritic innervation of the adjacent SNpr is also capable of modulating basal ganglia output and motor function. We therefore examined the extent of re-innervation within the SNr and dorsal caudate putamen (CPu) of grafted animals in order to gain further understanding of the motor improvements observed. GFP labelling enabled clear visualization of the extensive network of neuritic processes into neighbouring VM nuclei (e.g. SNr) as well as axonal arborization and synaptic varicosities within the striatum.
Poor innervation along the MFB of E12 donor age grafts (Fig. 1A) corresponded to poor striatal innervation, with less than 0.03% of the dorsolateral striatum innervated by GFP+ fibres (Fig. 5B and C). Additionally, these grafts showed low levels of GFP+ innervation within the VM (compared to E10 donor grafts or grafts exposed to GDNF) and may explain the lack of functional improvement in these animals (Fig. 5G–J). While E10 donor grafts showed improved striatal innervation (0.5%, Fig. 5B and D), more notable was the increased innervation within the SNpr (Fig. 5H).
Exposure of E12 donor age grafts to GDNF enhanced both striatal (42-fold) and midbrain innervation (Fig. 5E and I). Surprisingly, exposure of E10 grafts to GDNF, despite a notable increase in GFP+ fibres in the VM and a significant (14-fold) increase in striatal innervation (Fig. 5B, F and J), had no synergistic effect on function (Fig. 5A).
Discussion
Incomplete striatal innervation and restoration of motor function, combined with variable outcomes across patients, highlight the need for improved and standardized methods of cell transplantation as a restorative treatment for PD. Repair of normal basal ganglia circuitry and regulated DA neurotransmission is the ultimate goal for transplantation. Recent studies have highlighted the capacity for homotopically grafted cells to appropriately integrate into the host circuitry (Gaillard et al. 2009; Thompson et al. 2009). However, the degree of reinnervation, and consequential functional output, has remained poor. Here we sought to extend on this work and show that homotopic grafting can be improved by optimizing both the donor tissue and the host environment.
The majority of basic and clinical studies to date have focused on isolating donor tissue at an age correlating to the peak of VM neurogenesis. However, several studies have now demonstrated that younger donor tissue results in larger intrastriatal grafts in rodents (Sinclair et al. 1999; Torres et al. 2007; Hahn et al. 2009; Bye et al. 2012). While it has been suggested that these larger grafts were the result of increased cell survival (Torres et al. 2007), more recently we have demonstrated that the proliferation of DA neuroblasts at the time of implantation also contributes to larger grafts (Bye et al. 2012). Here we demonstrate for the first time that younger, E10, donor tissue also results in larger homotopic grafts, with 4-fold more DA neurons than conventional E12 donor tissue grafts. Additionally we previously demonstrated that intrastriatal transplantation of younger E10-derived VM tissue produced increased innervation within the striatum (Bye et al. 2012).
To date studies have favoured the ectopic placement of grafts because intranigral allografts (e.g. rat to rat) showed poor fibre growth along the mature nigrostriatal tract. Although we have shown that new axons are capable of regrowth and striatal targeting, we now demonstrate that younger tissue enhances the number of DA axons projecting through the MFB and innervating forebrain targets. It is plausible that younger donor tissue is less responsive to inhibitory cues present in white matter. As such, chondroitin sulphate proteoglycans, known to prevent axonal growth and to be upregulated in the adult and injured brain, signal through receptors not yet expressed at E10, but are present later in embryonic development (Koprivica et al. 2005; Shen et al. 2009). Alternatively, enhanced axonal growth seen in younger donor grafts may be attributed to trophic cues present within the VM tissue at this age. Studies by us and others have demonstrated the early responsiveness of DA neurites to such cues, with responsiveness down-regulated in older DA neurons (Van den Heuvel & Pasterkamp, 2008; Blakely et al. 2011).
A number of studies have demonstrated the ability of GDNF to promote DA neuron survival and neuritogenesis both in vitro and in vivo (Rosenblad et al. 1996; Bjorklund et al. 1997; Redmond et al. 2009; Rangasamy et al. 2010). While GDNF can promote the survival and connectivity of residual host DA neurons in Parkinsonian models as well as intrastriatally transplanted cells, more recently we showed that it could also impact on homotopic intranigral transplants (Thompson et al. 2009). In light of larger grafts and increased innervation observed using younger E10 donor tissue, we wished to determine whether these cells were also responsive to trophic cues that could enhance survival and neuritogenesis. Whereas GDNF exhibited moderate effects on the survival of E12 grafts (35% increase in GFP+ cells) it induced a 52-fold increase in the number of axons coursing through the MFB. However, in E10 donor grafts, GDNF had little effect on DA survival. Previous studies have demonstrated that the receptors for GDNF, c-ret and GFRa1, are not expressed in the developing brain until E11.5, which may explain the lack of responsiveness. Of relevance, Sonic Hedgehog (Shh) has been shown to be important for promoting survival of DA neurons during this early period of VM development, and of ectopic grafts using young VM donor tissue (Roussa et al. 2004; Torres et al. 2005). Despite this, in the present study, these young donor grafts were capable of responding to GDNF. A significant increase in DA axons within the MFB and dorsolateral striatum were observed, suggesting that these cells, whilst initially non-responsive to GDNF, may mature and acquire receptor expression in situ. It is important to note that whilst younger tissue grafts were responsive to GDNF, only a 4-fold increase was seen in striatal targeting (E10 vs. E10+GDNF, Fig. 3C) compared to a 66-fold increase for older donor tissue (E12 vs. E12+GDNF, Fig. 3C). These findings suggest that longer graft survival times, to enable maturation and GDNF responsiveness of younger DA neuroblasts within the transplants, and/or exposure of these grafts to additional cues such as Shh, to promote survival (Roussa et al. 2004) and connectivity of young neurons (Hammond et al. 2009), may be required to gain the full benefits of younger donor tissue.
The developing midbrain, and consequently VM donor tissue, consists of a heterogeneous population of cells, including progenitors for A9 and A10 subclasses of DA neurons. The A9 subpopulation, readily identifiable by GIRK2 expression, are known to be critical for their role in motor function in the intact brain and following transplantation in Parkinsonian models (Mendez et al. 2005; Bjorklund & Dunnett, 2007; Kuan et al. 2007; O’Keeffe et al. 2008; Grealish et al. 2010). Although we have previously demonstrated enrichment for A9 neurons following intrastriatal transplantation of younger (E10) versus older (E12) donor tissue, their relative contributions to intranigral VM grafts remained to be elucidated. Interestingly, we observed that the majority of GFP+ DA neurons, from both E10 and E12 donor grafts, failed to adopt an A9 or A10 phenotype. Regardless, younger donor grafts exposed to GDNF showed the greatest number of both GIRK2+ and calbindin+ DA neurons (Fig. 4I and J). Similar to previous intrastriatal grafting (Bye et al. 2012), younger donor tissue resulted in a significant reduction in the proportion of calbindin+ cells within the graft (Fig. 4K), but had no positive effect on the proportion of GIRK2+ cells within the graft (Fig. 4L). Exposure of these younger grafts to GDNF, however, significantly increased the number and proportion of GIRK2+ cells, suggesting that there is a pliable population, and that additional cues may be required to promote A9 phenotype acquisition.
Striatal targeting may also be required for grafted cells to adopt an A9 or A10 fate. In this regard, E12 donor grafts exposed to GDNF showed the greatest proportion of grafted cells projecting to forebrain targets (68%, Fig. 3C), and commensurately these grafts showed the greatest percentage of both calbindin+ and GIRK2+ DA neurons (>35%, Fig. 4K and L). In support of this, striatal injection of FG into the striatum of a subset of mice, whilst only retrogradely labelling a small number of GFP+ grafted neurons, demonstrated that 75% of FG+GFP+ backfilled grafted cells expressed either calbindin and/or GIRK2. Of the FG+GFP+ cells adopting a cellular phenotype, the majority expressed calbindin, reflecting the greater proportion of calbindin cells within the grafts. In light of the small number of FG+GFP+ cells labelled within the grafts, further studies are required to fully elucidate the importance of synaptic connectivity in phenotype acquisition of transplanted DA neurons, as well as the impact of additional, yet to be identified, cues/regulators of A9 versus A10 specification.
In light of the ability of E10 donor tissue (±GDNF) to generate larger grafts with greater midbrain and striatal innervation, we wished to assess how this may impact on functional recovery. Here we utilized the corridor task, given the sensitivity of this test in mice to distinguish between intact, lesion and subsequent graft-induced motor improvements. Furthermore, the sensorimotor co-ordination required for this task provides a more suitable test to examine improvements associated with local midbrain DA release. We found that E10, but not E12, donor tissue significantly improved behavioural deficits that manifested after SNpc lesioning. Although it is unlikely that this functional improvement can be attributed to the A9 cells within the graft (due to their low proportion under all graft conditions), it remains to be elucidated whether increased striatal innervation or local midbrain DA delivery are accountable. Behavioural tests that focus on examining synaptic DA transmission within the striatum (such as drug-induced rotational testing), in conjunction with sensorimotor testing, may better reveal this and should be followed up in animal models (e.g. rats) that are notably more responsive to such tests.
Surprisingly, despite the increase density of GFP+ fibres along the MFB and within the striatum, GDNF provided no further functional benefit to E10 donor grafts, suggesting that additional cues may be required to promote the maturation of these terminals and ensure functional synapses are formed. Further studies focused on the ultrastructure and electrochemistry of synaptic terminals may shed more light on the function of these younger donor cells. In contrast, E12 donor tissue was responsive to GDNF, showing a commensurate increase in functional recovery and striatal innervation, highlighting the possible importance of cell maturity.
While the present study focused on promoting homotopic transplantation using VM fetal tissue, these findings have broader implications for other donor tissue sources. Extensive efforts have been made to generate DA neurons from pluripotent stem cell sources and to demonstrate their capacity to survive upon ectopic transplantation. However, little attention has been paid to the ability of these cells to functionally integrate and importantly to restore normal circuitry. We have recently demonstrated that forebrain neurons derived from differentiated human embryonic stem cells expressing GFP are capable of appropriately integrating into the circuitry of the intact brain, forming long distance projections (Denham et al. 2012). It now remains to be seen how embryonic stem cell-derived DA neurons integrate within the brain, particularly in the context of homotopic grafting, and the stage of maturity of these cells that is most optimal for grafting.
In conclusion, our results demonstrate the capacity to improve homotopic grafting. Through careful selection of donor tissue and providing a more trophic host environment, graft size, composition and innervation can be enhanced to a level capable of inducing functional recovery. The further development of techniques based upon homotopic grafting and restoration of the normal circuitry, including the exploitation of additional trophic and axonal guidance cues as well as the use of stem cell-derived DA neurons, may significantly impact on the future of cell therapy for PD.
Acknowledgments
We wish to thank Dr Davor Stanic for fruitful discussions and critical reading of the manuscript. We thank Dr Chathurini Fernando, Ms Doris Tomas and Ms Mong Tien for their technical assistance. This research was supported by funding from the National Health and Medical Research Council, Australia (grant nos. 628542 and APP1022637). L.H.T was supported by NHMRC Career Development Awards. C.L.P was supported by an NHMRC Career Development Award, and subsequently a Senior Medical Research Fellowship provided by the Viertel Foundation, Australia.
Glossary
- CPu
caudate putamen
- DA
dopamine
- E
embryonic day
- GDNF
glial cell-derived neurotrophic factor
- FG
fluorogold
- GFP
green fluorescent protein
- GIRK2
G-protein-gated inwardly rectifying K+ channel subunit 2
- GP
globus pallidus
- MFB
medial forebrain bundle
- PD
Parkinson's disease
- SEM
standard error of the mean
- SNpc
substantia nigra pars compacta
- SNpr
substantia nigra pars reticulata
- TH
tyrosine hydroxylase
- TH-GFP
tyrosine hydroxylase green fluorescent protein
- VM
ventral mesencephalon/midbrain
- VTA
ventral tegmental area
Author contributions
All experiments were performed at the Florey Neuroscience Institute, The University of Melbourne, Parkville, Australia. J.K: collection and assembly of data, data analysis and interpretation, final approval of manuscript. L.H.T: concept and design, collection and assembly of data, data analysis and interpretation, final approval of manuscript. C.L.P.: concept and design, collection and assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript, financial support.
Supplementary material
Supplementry Material
References
- Bayer SA, Wills KV, Triarhou LC, Ghetti B. Time of neuron origin and gradients of neurogenesis in midbrain dopaminergic neurons in the mouse. Exp Brain Res. 1995;105:191–199. doi: 10.1007/BF00240955. [DOI] [PubMed] [Google Scholar]
- Bentlage C, Nikkhah G, Cunningham MG, Bjorklund A. Reformation of the nigrostriatal pathway by fetal dopaminergic micrografts into the substantia nigra is critically dependent on the age of the host. Exp Neurol. 1999;159:177–190. doi: 10.1006/exnr.1999.7110. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Rosenblad C, Winkler C, Kirik D. Studies on neuroprotective and regenerative effects of GDNF in a partial lesion model of Parkinson's disease. Neurobiol Dis. 1997;4:186–200. doi: 10.1006/nbdi.1997.0151. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Stenevi U, Schmidt RH, Dunnett SB, Gage FH. Intracerebral grafting of neuronal cell suspensions. II. Survival and growth of nigral cell suspensions implanted in different brain sites. Acta Physiol Scand Suppl. 1983;522:9–18. [PubMed] [Google Scholar]
- Blaess S, Bodea GO, Kabanova A, Chanet S, Mugniery E, Derouiche A, Stephen D, Joyner AL. Temporal–spatial changes in Sonic Hedgehog expression and signalling reveal different potentials of ventral mesencephalic progenitors to populate distinct ventral midbrain nuclei. Neural Dev. 2011;6:29. doi: 10.1186/1749-8104-6-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely BD, Bye CR, Fernando CV, Horne MK, Macheda ML, Stacker SA, Arenas E, Parish CL. Wnt5a regulates midbrain dopaminergic axon growth and guidance. PLoS One. 2011;6:e18373. doi: 10.1371/journal.pone.0018373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundin P, Barker RA, Parmar M. Neural grafting in Parkinson's disease: problems and possibilities. Prog Brain Res. 2010;184:265–294. doi: 10.1016/S0079-6123(10)84014-2. [DOI] [PubMed] [Google Scholar]
- Brundin P, Isacson O, Bjorklund A. Monitoring of cell viability in suspensions of embryonic CNS tissue and its use as a criterion for intracerebral graft survival. Brain Res. 1985;331:251–259. doi: 10.1016/0006-8993(85)91550-1. [DOI] [PubMed] [Google Scholar]
- Bye CR, Thompson LH, Parish CL. Birth dating of midbrain dopamine neurons identifies A9 enriched tissue for transplantation into Parkinsonian mice. Exp Neurol. 2012;236:58–68. doi: 10.1016/j.expneurol.2012.04.002. [DOI] [PubMed] [Google Scholar]
- Dahlström A, Fuxe K. Evidence for existence of monoamine-containing neurons in central nervous system. I. Demonstration of monoamines in cell bodies of brain stem neurons. Acta Physiol Scand. 1964;62:5–55. [PubMed] [Google Scholar]
- Denham M, Parish CL, Leaw B, Wright J, Reid CA, Petrou S, Dottori M, Thompson LH. Neurons derived from human embryonic stem cells extend long-distance axonal projections through growth along host white matter tracts after cerebral transplantation. Front Cell Neurosci. 2012;6:11. doi: 10.3389/fncel.2012.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A. Basic neural transplantation techniques. I. Dissociated cell suspension grafts of embryonic ventral mesencephalon in the adult rat brain. Brain Res Brain Res Protoc. 1997;1:91–99. doi: 10.1016/s1385-299x(96)00015-3. [DOI] [PubMed] [Google Scholar]
- Dunnett SB, Bjorklund A, Schmidt RH, Stenevi U, Iversen SD. Intracerebral grafting of neuronal cell suspensions. IV. Behavioural recovery in rats with unilateral 6-OHDA lesions following implantation of nigral cell suspensions in different forebrain sites. Acta Physiol Scand Suppl. 1983;522:29–37. [PubMed] [Google Scholar]
- Eslamboli A, Georgievska B, Ridley RM, Baker HF, Muzyczka N, Burger C, Mandel RJ, Annett L, Kirik D. Continuous low-level glial cell line-derived neurotrophic factor delivery using recombinant adeno-associated viral vectors provides neuroprotection and induces behavioral recovery in a primate model of Parkinson's disease. J Neurosci. 2005;25:769–777. doi: 10.1523/JNEUROSCI.4421-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin KBJ, Paxinos G. The Mouse Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- Freeman TB, Sanberg PR, Nauert GM, Boss BD, Spector D, Olanow CW, Kordower JH. The influence of donor age on the survival of solid and suspension intraparenchymal human embryonic nigral grafts. Cell Transplant. 1995;4:141–154. doi: 10.1177/096368979500400118. [DOI] [PubMed] [Google Scholar]
- Gaillard A, Decressac M, Frappe I, Fernagut PO, Prestoz L, Besnard S, Jaber M. Anatomical and functional reconstruction of the nigrostriatal pathway by intranigral transplants. Neurobiol Dis. 2009;35:477–488. doi: 10.1016/j.nbd.2009.07.003. [DOI] [PubMed] [Google Scholar]
- Gates MA, Torres EM, White A, Fricker-Gates RA, Dunnett SB. Re-examining the ontogeny of substantia nigra dopamine neurons. Eur J Neurosci. 2006;23:1384–1390. doi: 10.1111/j.1460-9568.2006.04637.x. [DOI] [PubMed] [Google Scholar]
- Grealish S, Jonsson ME, Li M, Kirik D, Bjorklund A, Thompson LH. The A9 dopamine neuron component in grafts of ventral mesencephalon is an important determinant for recovery of motor function in a rat model of Parkinson's disease. Brain. 2010;133:482–495. doi: 10.1093/brain/awp328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn M, Timmer M, Nikkhah G. Survival and early functional integration of dopaminergic progenitor cells following transplantation in a rat model of Parkinson's disease. J Neurosci Res. 2009;87:2006–2019. doi: 10.1002/jnr.22031. [DOI] [PubMed] [Google Scholar]
- Hammond R, Blaess S, Abeliovich A. Sonic hedgehog is a chemoattractant for midbrain dopaminergic axons. PLoS One. 2009;4:e7007. doi: 10.1371/journal.pone.0007007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes L, Zhang Z, Albert P, Zervas M, Ahn S. Timing of Sonic hedgehog and Gli1 expression segregates midbrain dopamine neurons. J Comp Neurol. 2011;519:3001–3018. doi: 10.1002/cne.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joksimovic M, Anderegg A, Roy A, Campochiaro L, Yun B, Kittappa R, McKay R, Awatramani R. Spatiotemporally separable Shh domains in the midbrain define distinct dopaminergic progenitor pools. Proc Natl Acad Sci U S A. 2009;106:19185–19190. doi: 10.1073/pnas.0904285106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koprivica V, Cho KS, Park JB, Yiu G, Atwal J, Gore B, Kim JA, Lin E, Tessier-Lavigne M, Chen DF, He Z. EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science. 2005;310:106–110. doi: 10.1126/science.1115462. [DOI] [PubMed] [Google Scholar]
- Kuan WL, Lin R, Tyers P, Barker RA. The importance of A9 dopaminergic neurons in mediating the functional benefits of fetal ventral mesencephalon transplants and levodopa-induced dyskinesias. Neurobiol Dis. 2007;25:594–608. doi: 10.1016/j.nbd.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Mendez I, Sanchez-Pernaute R, Cooper O, Vinuela A, Ferrari D, Bjorklund L, Dagher A, Isacson O. Cell type analysis of functional fetal dopamine cell suspension transplants in the striatum and substantia nigra of patients with Parkinson's disease. Brain. 2005;128:1498–1510. doi: 10.1093/brain/awh510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah G, Bentlage C, Cunningham MG, Bjorklund A. Intranigral fetal dopamine grafts induce behavioral compensation in the rat Parkinson model. J Neurosci. 1994a;14:3449–3461. doi: 10.1523/JNEUROSCI.14-06-03449.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikkhah G, Cunningham MG, Jodicke A, Knappe U, Bjorklund A. Improved graft survival and striatal reinnervation by microtransplantation of fetal nigral cell suspensions in the rat Parkinson model. Brain Res. 1994b;633:133–143. doi: 10.1016/0006-8993(94)91532-6. [DOI] [PubMed] [Google Scholar]
- O’Keeffe FE, Scott SA, Tyers P, O’Keeffe GW, Dalley JW, Zufferey R, Caldwell MA. Induction of A9 dopaminergic neurons from neural stem cells improves motor function in an animal model of Parkinson's disease. Brain. 2008;131:630–641. doi: 10.1093/brain/awm340. [DOI] [PubMed] [Google Scholar]
- Parish CL, Finkelstein DI, Drago J, Borrelli E, Horne MK. The role of dopamine receptors in regulating the size of axonal arbors. J Neurosci. 2001;21:5147–5157. doi: 10.1523/JNEUROSCI.21-14-05147.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish CL, Thompson LH. Neural progenitor cell transplantation in Parkinson's disease models. In: Reynolds LDaB., editor. Neural Progenitor Cells: Methods and Protocols. Totowa, NJ: Humana Press; 2012. [Google Scholar]
- Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson's disease. Prog Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- Redmond DE, Jr, Elsworth JD, Roth RH, Leranth C, Collier TJ, Blanchard B, Bjugstad KB, Samulski RJ, Aebischer P, Sladek JR., Jr Embryonic substantia nigra grafts in the mesencephalon send neurites to the host striatum in non-human primate after overexpression of GDNF. J Comp Neurol. 2009;515:31–40. doi: 10.1002/cne.22028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblad C, Martinez-Serrano A, Bjorklund A. Glial cell line-derived neurotrophic factor increases survival, growth and function of intrastriatal fetal nigral dopaminergic grafts. Neuroscience. 1996;75:979–985. doi: 10.1016/0306-4522(96)00343-0. [DOI] [PubMed] [Google Scholar]
- Roussa E, Farkas LM, Krieglstein K. TGF-β promotes survival on mesencephalic dopaminergic neurons in cooperation with Shh and FGF-8. Neurobiol Dis. 2004;16:300–310. doi: 10.1016/j.nbd.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Sauer H, Frodl EM, Kupsch A, ten Bruggencate G, Oertel WH. Cryopreservation, survival and function of intrastriatal fetal mesencephalic grafts in a rat model of Parkinson's disease. Exp Brain Res. 1992;90:54–62. doi: 10.1007/BF00229256. [DOI] [PubMed] [Google Scholar]
- Sawamoto K, Nakao N, Kobayashi K, Matsushita N, Takahashi H, Kakishita K, Yamamoto A, Yoshizaki T, Terashima T, Murakami F, Itakura T, Okano H. Visualization, direct isolation, and transplantation of midbrain dopaminergic neurons. Proc Natl Acad Sci U S A. 2001;98:6423–6428. doi: 10.1073/pnas.111152398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX, Liu K, He Z, Silver J, Flanagan JG. PTPσ is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 2009;326:592–596. doi: 10.1126/science.1178310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair SR, Fawcett JW, Dunnett SB. Dopamine cells in nigral grafts differentiate prior to implantation. Eur J Neurosci. 1999;11:4341–4348. doi: 10.1046/j.1460-9568.1999.00867.x. [DOI] [PubMed] [Google Scholar]
- Smith GA, Heuer A, Dunnett SB, Lane EL. Unilateral nigrostriatal 6-hydroxydopamine lesions in mice II: predicting l-DOPA-induced dyskinesia. Behav Brain Res. 2012;226:281–292. doi: 10.1016/j.bbr.2011.09.025. [DOI] [PubMed] [Google Scholar]
- Thompson L, Barraud P, Andersson E, Kirik D, Bjorklund A. Identification of dopaminergic neurons of nigral and ventral tegmental area subtypes in grafts of fetal ventral mesencephalon based on cell morphology, protein expression, and efferent projections. J Neurosci. 2005;25:6467–6477. doi: 10.1523/JNEUROSCI.1676-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson LH, Grealish S, Kirik D, Bjorklund A. Reconstruction of the nigrostriatal dopamine pathway in the adult mouse brain. Eur J Neurosci. 2009;30:625–638. doi: 10.1111/j.1460-9568.2009.06878.x. [DOI] [PubMed] [Google Scholar]
- Torres EM, Monville C, Gates MA, Bagga V, Dunnett SB. Improved survival of young donor age dopamine grafts in a rat model of Parkinson's disease. Neuroscience. 2007;146:1606–1617. doi: 10.1016/j.neuroscience.2007.03.037. [DOI] [PubMed] [Google Scholar]
- Torres EM, Monville C, Lowenstein PR, Castro MG, Dunnett SB. Delivery of sonic hedgehog or glial derived neurotrophic factor to dopamine-rich grafts in a rat model of Parkinson's disease using adenoviral vectors: Increased yield of dopamine cells is dependent on embryonic donor age. Brain Res Bull. 2005;68:31–41. doi: 10.1016/j.brainresbull.2005.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Heuvel DM, Pasterkamp RJ. Getting connected in the dopamine system. Prog Neurobiol. 2008;85:75–93. doi: 10.1016/j.pneurobio.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Winkler C, Kirik D, Bjorklund A. Cell transplantation in Parkinson's disease: how can we make it work. Trends Neurosci. 2005;28:86–92. doi: 10.1016/j.tins.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Yurek DM. Intranigral transplants of fetal ventral mesencephalic tissue attenuate D1-agonist-induced rotational behavior. Exp Neurol. 1997;143:1–9. doi: 10.1006/exnr.1996.6359. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


