Abstract
The paraventricular nucleus of the hypothalamus (PVN) plays a major role in central cardiovascular and volume control, and has been implicated in controlling sympathetic nerve activity (SNA) during volume expansion and in heart failure (HF). The objectives were to determine the role of the PVN on cardiac and renal SNA (CSNA and RSNA) in conscious normal sheep and sheep with pacing-induced heart failure. In normovolaemic sheep in the normal state and in HF, bilateral microinjection of the GABA agonist muscimol (2 mm, 500 nl), had no effects on resting mean arterial pressure (MAP), heart rate (HR), CSNA or RSNA. In addition, neither chemical inhibition of the PVN using the inhibitory amino acid glycine (0.5 m, 500 nl), nor electrolytic lesion of the PVN reduced the elevated level of CSNA in HF. Dysinhibition of the PVN with bilateral microinjection of bicuculline (1 mm, 500 nl) in normal sheep increased MAP, HR and CSNA, but decreased RSNA, whereas in HF bicuculline had no effects on MAP, HR or CSNA, but inhibited RSNA. During volume expansion in normal sheep, muscimol reversed the inhibition of RSNA, but not of CSNA. In summary, removal of endogenous GABAergic inhibition to the PVN indicated that CSNA is normally under inhibitory control. Although this inhibition was absent in HF, the responses to pharmacological inhibition, or lesion of the PVN, indicates that it does not drive the increased CSNA in HF. These findings indicate the PVN has a greater influence on RSNA than CSNA in the resting state in normal and HF sheep, and during volume expansion in normal sheep.
Key points
Heart failure is associated with large increases in sympathetic nerve activity to organs like the heart and kidney and this increase is detrimental to patients.
We explored the role played by the paraventricular nucleus of the hypothalamus (PVN), a central brain region, in mediating the increase in sympathetic drive during heart failure.
We show that neurons in the PVN selectively mediate changes in sympathetic drive to the kidney, but not to the heart when blood volume is increased.
In addition, neurons in the PVN do not contribute to the resting levels of sympathetic drive to the heart during normal conditions or in heart failure.
Our data demonstrates striking differences in the central mechanisms that control sympathetic drive to the heart and kidney during heart failure.
Introduction
The paraventricular nucleus of the hypothalamus (PVN) plays a major role in central cardiovascular and volume control. It has reciprocal neuronal connections to other cardiovascular and autonomic centres in the brain, and has been implicated in the increased sympathetic nerve activity (SNA) in cardiovascular disease states including heart failure (HF). In HF, the increases in SNA to the heart and kidneys are particularly damaging, and are independent predictors of mortality (Hasking et al. 1986; Kaye et al. 1995; Petersson et al. 2005). There is extensive evidence that the increased SNA in HF is mediated by central mechanisms, but these are not well defined, particularly those causing the high level of sympathetic drive to the heart.
An anatomical substrate for the actions of the PVN on SNA is indicated by studies showing transynaptic neuronal transport of pseudo-rabies virus from the kidney and stellate ganglion into neurons in the PVN (Jansen et al. 1995; Cano et al. 2004). There is also extensive evidence indicating a functional role for the PVN in mediating the responses of the sympathetic nervous system to changes in volume status (Deering & Coote, 2000; Pyner et al. 2002; Coote, 2005). Volume expansion and right atrial stretch increase the expression of the early gene marker c-fos in parvocellular neurons of the PVN (Akama et al. 1998), and inhibition of PVN neurons with muscimol attenuated the volume expansion-induced inhibition of renal SNA (RSNA) (Ng et al. 2004). Selective stretch of venous–atrial junctions in anaesthetised dogs and rats has been shown to inhibit RSNA (Karim et al. 1972; Pyner et al. 2002), with similar stimuli increasing cardiac SNA (CSNA) or sympathetically mediated heart rate (Karim et al. 1972; Kaufman et al. 1981). In contrast, our studies in conscious sheep indicate that volume expansion caused similar percentage decreases in CSNA and RSNA (Ramchandra et al. 2008, 2009b). It is important to note that in contrast to atrial stretch, volume expansion activates stretch receptors present outside the atria, and thus represents a more physiological stimulus. At present it is unknown whether the PVN mediates the inhibition of CSNA in response to volume expansion in the conscious state.
Considering the role of the PVN in integrating the sympathetic responses to volume change, there has been major interest in determining whether it mediates the increased SNA in HF, which is associated with volume expansion and large increases in sympathetic drive to the heart and the kidney (Gibson & Evans, 1937; Meneely & Kaltreider, 1943; Hasking et al. 1986; Kaye et al. 1994; Eisenhofer et al. 1996; Petersson et al. 2005). Activation of the PVN in HF is indicated by the increased expression of c-fos as well as Fos related antigens in neurons in the PVN (Vahid-Ansari & Leenen, 1998; Patel et al. 2000). In addition, studies in a rat model of HF indicate that the PVN contributes to the increased levels of RSNA (Patel, 2000; Li et al. 2003; Zheng et al. 2009), and that PVN neurons have a higher firing rate (Zhang et al. 2002b). A major factor leading to the increased sympathetic outflow to the kidneys from the PVN in HF is a reduced inhibitory GABAergic input (Zhang et al. 2002a; Li & Patel, 2003), but whether such a mechanism also accounts for the increase in CSNA in HF is unknown.
In the present study we first examined whether excitatory output from the PVN determines the resting level of CSNA and RSNA in the normal state and the increased CSNA in HF, by inhibition of neurons in the PVN with muscimol or glycine, or by ablation of the PVN. Secondly, in normal volume expanded sheep it was examined if the PVN mediates the inhibition of CSNA, as well as RSNA. Finally, we examined whether the control of CSNA and RSNA by tonic inhibitory GABAergic inputs to the PVN is altered in HF.
Methods
Adult merino ewes (35–45 kg body wt) were housed in individual metabolism cages in association with other sheep. Experiments were started when sheep were accustomed to laboratory conditions and human contact. Sheep were fed a diet of oaten chaff (800 g day−1), and water ad libitum. All experiments were approved by the Animal Experimentation Ethics Committee of the Howard Florey Institute under guidelines laid down by the National Health and Medical Research Council of Australia.
Surgical procedures
Prior to the studies, sheep underwent three aseptic surgical procedures, separated by a recovery period of at least 2 weeks. For all normal animals, anaesthesia was induced with intravenous sodium thiopental (15 mg kg−1), and following intubation anaesthesia was maintained with 1.5–2.0% isoflurane/O2. For HF animals, anaesthesia was induced and maintained with isoflurane. The first operation involved construction of a carotid arterial loop and in HF animals insertion of a cardiac pacemaker lead into the right ventricle (Excellence SS+, Viatron, Minneapolis, MN, USA). The construction of the carotid artery loop consisted of exteriorizing the carotid artery in a skin fold to facilitate arterial cannulation to measure arterial pressure. The right jugular vein was exposed, and the pacing lead was inserted and advanced to the apex of the right ventricle under fluoroscopic guidance. After 2 weeks, animals were placed in a stereotaxic frame and two stainless steel guide tubes were inserted so the tips were 5 mm above the lateral cerebral ventricles. Contrast media (0.6 ml, Omnipaque, GE Healthcare, Munich, Germany) was injected into the lateral ventricles, and two microinjection guide tubes were placed bilaterally 5 mm above the PVN using the ventricular anatomy on overhead and lateral radiographs to guide the placement (Frithiof et al. 2009). In one group of five sheep in HF, PVN lesion electrodes were inserted bilaterally, 1.0 mm either side of the sagittal midline with the tips positioned in the PVN. All cannulae were fixed in place with dental acrylic and sealed with obturators. After at least 2 weeks in normal animals, and following decrease of ejection fraction to <40% in the HF group (8–12 weeks), surgery was conducted to implant electrodes in the cardiothoracic and left renal sympathetic nerves as described previously (Watson et al. 2007; Ramchandra et al. 2009a). The left cardiothoracic nerves innervate the left and right ventricular muscle, the left atrium and septum, and the sinoatrial and atrioventricular nodes (Waites, 1957; McKibben & Getty, 1969). Briefly, an incision was made above the fourth rib, the periosteum was opened, and the rib was removed. The thoracic cardiac nerves were identified, and the facia over the nerves was removed. Up to five electrodes were inserted obliquely through the nerve sheath, ensuring that the tip was positioned in the centre of the nerve. Electrodes were fixed in place with cyanoacrylate glue, the implantation site was covered with a layer of Kwik-Sil (WPI, Glen Waverly, Vic, Australia), and the wires were exteriorized next to the sutured wound. A stainless-steel ring implanted subcutaneously was used as a ground. The renal artery was exposed via a paracostal retroperitoneal approach. The renal nerves were identified and up to five electrodes were implanted, as described for the cardiac sympathetic nerves. Antibiotic (900 mg procaine penicillin, Troy Laboratories, Glendenning NSW, Australia) was administered prophylactically at the start of surgery and for 2 days post-surgery. Post-surgical analgesia was maintained with intramuscular injection of flunixin meglumine (1 mg kg−1; Mavlab, Slacks Creek Qld, Australia) at the start of surgery, and after 24 h recovery.
A day prior to electrode placement surgery, using aseptic techniques, cannulae were inserted 20 cm into a jugular vein for measurement of central venous pressure (CVP) and for intravenous infusions. One day prior to experiments a cannula was inserted 3 cm proximally into the carotid artery exteriorized in a loop for measurement of arterial pressure. Experiments were started at least 4 days after implantation of electrodes.
Pacing-induced heart failure
Sheep with pacemaking leads underwent left ventricular echocardiograph measurements (Hewlett Packard Sonos 1000) prior to pacing, and were then paced at 200–220 beats min−1. Echocardiographs were repeated every 7 days with the pacing switched off to monitor progression of HF, and when ejection fraction was 30–40%, electrode placement surgery was performed. All experiments were conducted with the pacing switched off.
Nerve recording
Sympathetic nerve activity was recorded differentially between pairs of electrodes, and the pair with the best signal to noise ratio was selected. The signal was amplified (×100,000) and filtered (bandpass 400–1000 Hz), displayed on an oscilloscope and passed through an audio amplifier and loud speaker. Sympathetic nerve activity and blood pressure were recorded on computer using a CED micro 1401 interface and Spike2 software (Cambridge Electronic Design, Cambridge, UK). Sympathetic nerve activity was full-wave rectified and integrated using a time constant of 20 ms. For each heartbeat, the program determined diastolic, systolic, and mean arterial blood pressures (MAP), heart period, and the area of the rectified and integrated SNA signals between diastolic pressures. The smallest burst was identified in the entire recording from a spreadsheet of data, and its correct position in the cardiac cycle and absence of artifacts were confirmed visually. The rectified and integrated area between the corresponding diastolic pressures of this burst was noted, and this area was taken as the minimum area for the definition of a burst. When the rectified and integrated area between any heart beat was greater than the minimum area, this was determined to constitute a burst. The burst incidence was calculated as the number of bursts per 100 heart beats. For the determination of burst amplitude, the integrated area under the curve for each burst was taken as an index of burst amplitude for the associated heartbeat.
PVN microinjections of muscimol, bicuculline and glycine
These experiments were carried out on separate days in normal and HF animals (n= 5/group). After a 20 min baseline recording, either the GABA agonist muscimol (2 mm, 500 nl), the GABA antagonist bicuculline (1 mm, 500 nl), or aCSF (500 nl) was injected bilaterally into the PVN and recording was continued for a further 25 min.
In separate groups of normal (n= 5) and HF (n= 6) sheep, following a 10 min baseline recording, baroreflex responses were generated by measuring CSNA and HR responses to increasing doses of phenylephrine hydrochloride and sodium nitroprusside (Watson et al. 2007). Following control measurements, glycine (0.5 m, 500 nl) or artificial cerebrospinal fluid (500 nl) was injected bilaterally into the PVN. At 5 min after the microinjections, a second baseline recording was made and the baroreflex responses were retested. Baroreflex curves were drawn using a four-parameter sigmoidal logistic equation. Three of the normal animals were the same as those in a previous study in which we demonstrated that this dose of glycine significantly reduced the responses to i.c.v. infusion of hypertonic saline in conscious sheep (Frithiof et al. 2009), validating the effectiveness of the dose used.
Volume expansion and PVN microinjections
In conscious normal sheep (n= 6), resting CSNA, RSNA, arterial pressure and central venous pressure were recorded for 10 min. The animals were then subjected to a volume expansion protocol on two occasions, separated by a recovery period of at least 48 h. For volume expansion, Gelofusine (Braun Australia Pty Ltd, Bella Vista, NSW, Australia) was infused until the total volume infused reached 500 ml. Gelofusine was infused at 350–500 ml/30 min with the rate titrated to raise CVP by 4–6 mmHg without significantly increasing mean arterial pressure (MAP). When 300 ml of gelofusine had been infused, 500 nl of either aCSF (500 nl) or the GABA agonist muscimol (2 mm, 500 nl) was microinjected bilaterally into the PVN. The volume expansion protocol was continued until 500 ml had been infused.
PVN lesion experiments
These experiments were conducted in a separate group of sheep in HF (n= 5). Baseline levels of CSNA, HR and baroreflex responses (as described above) were measured at 4 days after surgery and for at least 2 days prior to PVN lesion. The lesion was created with radiofrequency current applied across the indwelling electrodes by means of a Grass LM-4 lesion maker, which raised the temperature at the electrode tips to 58–63°C, as measured by a thermistor, for 2 min. The sheep were conscious, remained quiet and did not show any unusual behaviour during this procedure. Following the lesion, baseline arterial pressure, CSNA, HR and baroreflex responses were measured for at least 3 days.
Verification of PVN cannulae placement and PVN lesion
All sheep were killed with an overdose of sodium pentobarbitone (100 mg kg−1). In the microinjection experiments, blue dye (100 nl) was microinjected into the PVN to verify cannulae position. In all animals, brains were instantly perfused via the carotid arteries with isotonic saline followed by 4% paraformaldehyde, and were later transferred to 20% sucrose. Two days later, brains sections (60 μm) were cut in a coronal plane. Alternate sections were stained with cresyl violet to determine the location of the injections.
Statistics
Results are expressed as means ± SEM. Two-way ANOVA was used to test for differences between aCSF and muscimol microinjections into the PVN. Two-way ANOVA was also used to test for differences before and after PVN bicuculline in the normal and HF animals. Student's unpaired t test was used to compare baseline values between normal and HF groups. A significant result was considered to be P < 0.05.
Results
Baseline haemodynamics in normal and HF sheep
Left ventricular ejection fraction and fractional shortening, measured in conscious sheep by echocardiography, gradually decreased over the 8–12 weeks of rapid ventricular pacing at 200–220 beats min−1. In HF animals, the day before implantation of recording electrodes, ejection fraction (36 ± 2%) and fractional shortening (18 ± 1%) were significantly reduced compared with the pre-pacing values (78 ± 2% and 35 ± 1%, respectively, both P < 0.001). MAP was lower (75 ± 2 vs. 82 ± 3 mmHg), while heart rate was higher (92 ± 8 vs. 76 ± 3 bpm) in the HF group. In normal animals, the resting burst incidence (bursts of pulse-related activity/100 heart beats) of CSNA (27 ± 6%) was significantly lower than the burst incidence of RSNA (77 ± 5%, P < 0.001). In HF there was a significant increase in burst incidence of CSNA to 78 ± 9%, but there was no change in the resting level of RSNA (78 ± 5%).
Effects of inhibition of the PVN with bilateral microinjections of muscimol or glycine in normal and HF sheep
Bilateral microinjection of muscimol (2 mm, 500 nl each side) in normovolemic sheep in the normal state and in HF had no effects on baseline levels of MAP, HR, CSNA or RSNA (Fig. 1). Microinjection of muscimol into areas adjacent to the PVN had no effects (data not shown).
Figure 1. Effects of bilateral microinjection of muscimol (2 mm, 500 nl) into the PVN on mean arterial blood pressure, heart rate, cardiac SNA and renal SNA in conscious normal (n= 5, continuous line) and HF sheep (n= 5, dashed lines).
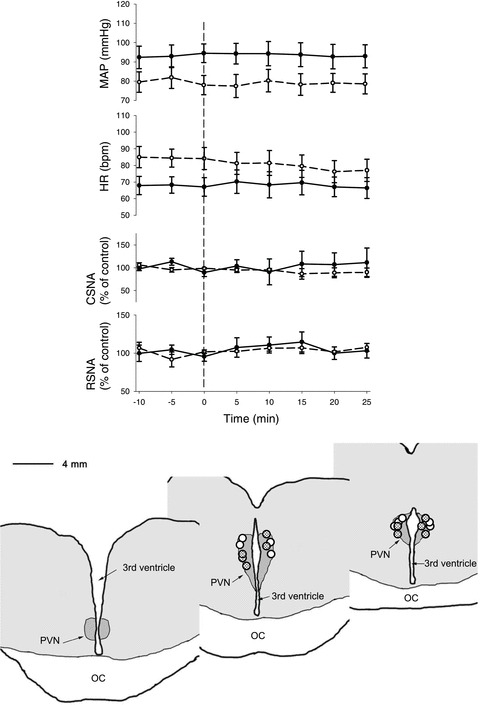
The distribution of all injection sites is shown in the schematic drawing adapted from coronal sections from 1 animal. The dots show the centre of injection sites as visualized by the distribution of blue dye. Dots indicate the paraventricular nucleus of the hypothalamus (PVN) muscimol injection sites in the normal (open circles) and heart failure animals. IC, optic chiasm.
In separate groups of normal sheep and sheep in HF, bilateral microinjections of glycine into the PVN (0.5 m, 500 nl each side) caused no change in the baseline levels of CSNA (Table 1). Nevertheless, glycine microinjection did significantly decrease the range of the baroreflex control of heart rate in normal animals (P < 0.05), though not in HF animals (Fig. 2). There was no change in the baroreflex control of CSNA in either group of animals. Microinjections of glycine outside the PVN in the normal animals had no effect on the range of the baroreflex control of HR (n= 3, data not shown).
Table 1.
Resting levels of cardiovascular variables from 10 min of resting data in conscious normal (n= 6) and HF (n= 5) sheep, before and after bilateral PVN microinjection of glycine
| Normal | Heart failure | |||
|---|---|---|---|---|
| Control | After glycine | Control | After glycine | |
| Mean arterial pressure (mmHg) | 78.1 ± 2.1 | 78.0 ± 3.7 | 72.8 ± 1.7 | 74.3 ± 1.7 |
| Heart rate (beats min−1) | 84.2 ± 3.1 | 82.8 ± 3.3 | 104.3 ± 9.5 * | 109.7 ± 12.5 |
| CSNA burst incidence (bursts (100 heart beats)−1) | 46.7 ± 5.5 | 43.3 ± 5.1 | 86.5 ± 4.1 * | 80.0 ± 2.5 |
| CSNA burst frequency (bursts min−1) | 38.7 ± 3.9 | 36.3 ± 4.2 | 91.2 ± 9.9 * | 91.6 ± 17.0 |
Data are means ± SEM; *P < 0.05 vs. control values in normal group.
Figure 2. Arterial baroreflex curves for cardiac SNA and heart rate (HR) during the control period (continuous line, filled circles) and after microinjection of glycine (1 mm) (dashed line, open circles) in normal and HF sheep.
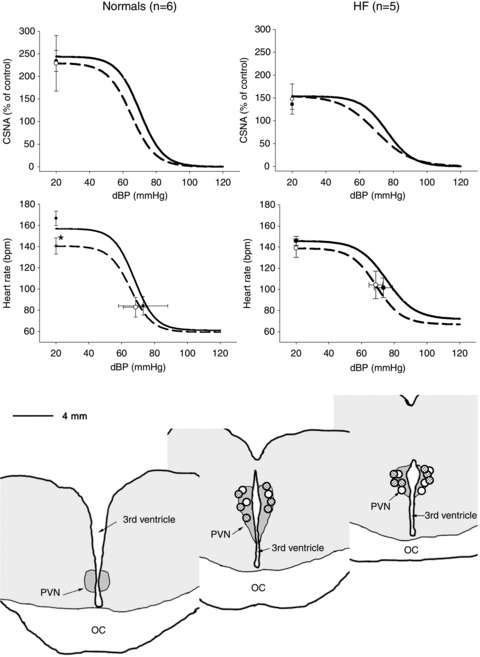
CSNA is presented as percentage of baseline activity measured. Resting points were taken from 5 min of recording during control periods. Values are means ± SEM (n= 6 in the normal and n= 5 in the HF group). The bottom panel indicates the distribution of microinjection sites in normal and HF sheep, dots show centre of injection sites as visualized by the distribution of blue dye in the normal (open circles) and heart failure animals. OC, optic chiasm.
Effect of bilateral radiofrequency lesion of the PVN in HF sheep
Large radio-frequency lesions were made bilaterally in the anterior medial part of the hypothalamus. Lesions were approximately symmetrical for 3–4 mm on either side of the lateral walls of the third ventricle and extended caudally from just caudal to the anterior commissure to the most caudal part of the hypothalamic PVN. The ablated regions that were common in all sheep are shown in Fig. 3. Lesions spared the ventral part of the lamina terminalis and the optic recess of the third ventricle and thus the most ventral part of the PVN was left intact, as was the OVLT, ventral median preoptic nucleus, suprachiasmatic nucleus and the supraoptic nucleus. The remainder of the hypothalamic PVN was almost entirely ablated, as was the medial anterior hypothalamic and periventricular regions. The dorsomedial, ventromedial, arcuate and lateral hypothalamic nuclei remained intact. Large parts of the anterior medial thalamic region were ablated in all sheep, reaching up to the level of the paraventricular nucleus of the thalamus.
Figure 3. Changes in levels of mean arterial pressure, heart rate, cardiac sympathetic nerve activity and baroreflex control of heart rate and CSNA pre- and post-lesion of the PVN in 5 HF sheep.
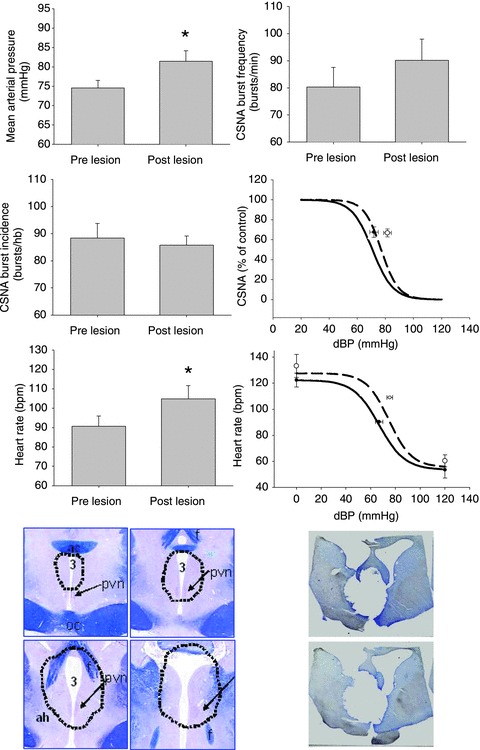
Data are means ± SEM; *P < 0.05 pre- vs. post-lesion. The straight lines represent the pre-lesion and the dotted lines represent the post-lesion baroreflex curves. The common area of lesion in all five sheep with HF is denoted below in the first 4 panels on the left. The panels on the right represent sections from one animal showing the lesioned area.
PVN lesions led to a significant increase in mean arterial pressure (from 75 ± 2 to 82 ± 3 mmHg, P < 0.05) and heart rate (from 90 ± 5 to 105 ± 7 bpm, P < 0.05), measured 2 days post-lesion (Fig. 3). There was no difference in baseline levels of CSNA, when measured either as burst incidence (from 88 ± 5 to 86 ± 3 bursts/100 heart beats) or as burst rate (from 80 ± 7 to 90 ± 8 bursts min−1). There was no change in any parameter of the baroreflex control of heart rate or CSNA (Fig. 3).
Effect of inhibition of the PVN with muscimol on the responses to volume expansion in normal sheep
This study was completed only in normal animals because, as we have shown previously, the reflex inhibition of CSNA and RSNA in response to volume expansion is abolished in HF (Ramchandra et al. 2009b). In normal sheep, volume expansion over 25 min caused a significant increase in CVP, without any change in MAP or HR, and a significant reduction in CSNA and RSNA (Fig. 4). Bilateral microinjection of aCSF into the PVN, after 300 ml of gelofusine had been infused, had no effect on the inhibition of CSNA or RSNA as the volume expansion continued. Inhibition of neurons in the PVN with bilateral microinjection of muscimol (2 mm, 500 nl each side) led to a reversal of the inhibition of RSNA, but in contrast there was no decrease in the inhibition of CSNA that continued until the completion of the volune expansion (Fig. 4).
Figure 4. Changes in mean arterial pressure, central venous pressure, cardiac SNA and renal SNA during volume expansion with infusion of gelofusine over 30 min in conscious sheep (n= 6).
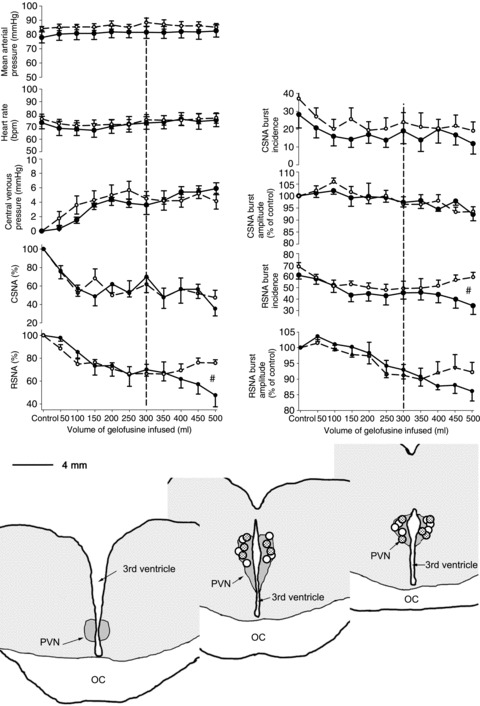
At an infused volume of 300 ml, sheep were treated with bilateral PVN microinjections of muscimol (2mM, 500 nL) (dashed line) or artificial CSF (continuous line). The dotted line signifies when the microinjection into the PVN was conducted. #Significant difference, P > 0.05 between treatments. The circles show the centre of injection sites as visualized by the distribution of bue dye. Circles indicate the paraventricular nucleus of the hypothalamus (PVN) muscimol injection sites in the normal (open circles) and heart failure animals. IC, optic chiasm.
The reduction in RSNA during infusion of gelofusine was mediated by decreases in both burst frequency (from 61 ± 3 to 34 ± 7%, P < 0.05) and burst amplitude (to 86 ± 3% of control, P < 0.05). Microinjection of muscimol to inhibit the PVN led to a reversal of the decreases in burst frequency and burst amplitude of RSNA (Fig. 4). In contrast, the reduction in CSNA was mediated primarily by a decrease in burst frequency (from 28 ± 8% to 12 ± 6%, P < 0.05); there was no change in CSNA burst amplitude.
Effects of dysinhibition of the PVN with bilateral microinjections of bicuculline in normal sheep
Microinjection of bicucilline (1 mm, 500 nl each side) was used to ascertain the effect of removing resting GABAergic inhibitory tone onto PVN neurons. In normal sheep, bicuculline caused large increases in MAP, HR and CSNA, accompanied by a significant inhibition of RSNA (Figs 5 and 6). In contrast, in HF sheep, there was no significant change in MAP, HR or CSNA after microinjection of bicuculline, but there was a similar inhibition of RSNA to that in normal animals (Figs 5 and 6). Microinjection of bicuculline outside the PVN had no effect on any parameter in normal or HF sheep (n= 3, data not shown).
Figure 5.
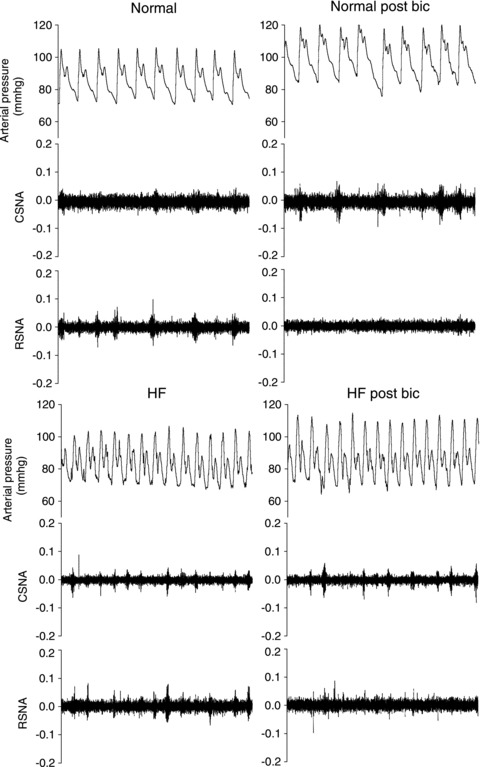
Original data showing changes in arterial pressure, cardiac SNA and renal SNA in normal (upper panels) and HF animals (lower panels) before (left panels) and 25 min post-bilateral microinjection of bicuculline (1 mM) into the PVN (right panels).
Figure 6. Effects of microinjection of bicuculline into the PVN on mean arterial blood pressure, heart rate, cardiac SNA and renal SNA in conscious normal (n= 5, continuous line) and HF sheep (n= 5, dashed lines).
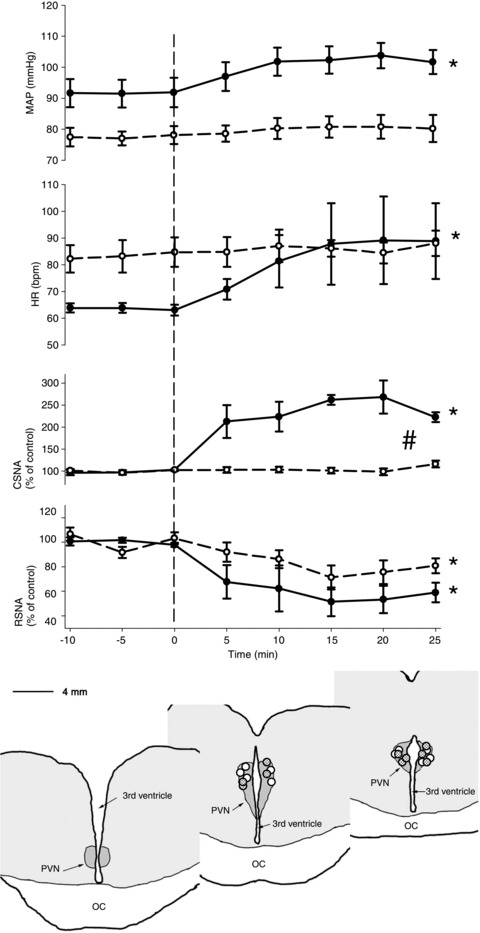
*Significant effect of time within group, P < 0.05; #significant difference between treatments, P < 0.05. The distribution of all injection sites is shown in the schematic drawing adapted from coronoal sections from 1 animal. The circles show the centre of injection sites as visualized by the distribution of bue dye. Circles indicate the paraventricular nucleus of the hypothalamus (PVN) muscimol injection sites in the normal (open circles) and heart failure animals. IC, optic chiasm.
Analysis of the sites of microinjection in normal and HF animals at post-mortem indicated that the sites of injection were localized in the PVN, as identified by the cresyl violet stains (Figs 1, 2, 4 and 6). There was no difference between the groups in terms of localization of injections sites.
Discussion
The present study examined the role of the PVN in the control of CSNA and RSNA in conscious sheep in the normal state and in HF. The novel findings of this study are as follows. (1) In normal sheep and sheep in HF, inhibition of the PVN with the GABA agonist muscimol had no effects on MAP, HR, CSNA or RSNA, suggesting that the PVN does not contribute to the resting level of CSNA or RSNA in the normovolaemic, healthy state or in HF. (2) In sheep in HF, inhibition of the PVN with the inhibitory amino acid glycine, or electrolytic lesion of the PVN, did not reduce CSNA, further indicating that the PVN does not drive the increased sympathetic activity to the heart in HF. (3) In volume expanded normal sheep, PVN microinjection of muscimol reversed the volume induced inhibition of RSNA, but not CSNA. (4) An excitatory pathway from the PVN to the heart was demonstrated in normal sheep by the large increase in CSNA, but not RSNA, in response to inhibition of endogenous GABAergic inputs into the PVN. In contrast, in HF bicuculline did not stimulate CSNA, indicating a loss of this GABAergic inhibitory tone.
Role of the PVN in the control of CSNA and RSNA in the normal state
Our findings that microinjections of muscimol or glycine into the PVN had no effects in conscious, normal sheep suggest that in the unanaesthetised healthy state tonic drive from the PVN does not maintain resting CSNA or RSNA. The role of the PVN in the control of CSNA has not been explored, but previous studies have found variable effects on RSNA. As in our study, muscimol injected in the PVN in conscious rabbits had no effect on RSNA, although in another study it increased RSNA (Badoer et al. 2002; Ng et al. 2004). In contrast, in anaesthetised rats acute inhibition of the PVN with muscimol decreased MAP, HR, RSNA (Zhang et al. 2002a; Akine et al. 2003; Wang et al. 2009) and lumbar SNA (Li & Pan, 2007). But this is not a universal finding as other studies in anaesthetized rats have observed no change in MAP or RSNA during microinjection of muscimol into the PVN (Stocker et al. 2004, 2005). These different RSNA responses are likely to be due to the effects of anaesthesia in previous studies, as anaesthesia has been reported to significantly modify the responses to stimulation of the PVN (Kannan et al. 1989).
The importance of a tonic inhibitory GABAergic input into the PVN in the normal state is indicated by significant increases in MAP, HR and RSNA after microinjection of bicuculline into the PVN in anaesthetized rats (Zhang et al. 2002a; Wang et al. 2009). In conscious normal sheep microinjection of bicuculline into the PVN increased MAP, HR and CSNA, but RSNA was inhibited, demonstrating a differential effect on these sympathetic outflows. This finding indicates the presence of pathways from the PVN that can stimulate CSNA, and shows that in the normal state CSNA is under tonic inhibitory control from the PVN. The bicuculline-induced inhibition of RSNA in conscious sheep is probably due to the greater baroreflex sensitivity in the conscious than the anaesthetised state. Based on our baroreflex data the observed increase in MAP with bicuculline would inhibit RSNA by approximately 50%, the change we observed. This suggests that there is no contribution by the PVN to baseline levels of RSNA, although we cannot categorically rule out a tonic inhibitory role of the PVN in mediating baseline levels of RSNA.
Role of the PVN in controlling CSNA and RSNA in HF
Although there are multiple studies indicating an important role for the PVN in determining the increased RSNA in HF (Patel, 2000; Coote, 2005; Yu et al. 2007; Kang et al. 2008), there are no studies that have investigated whether the PVN sets the high level of CSNA in HF. Such a role is not supported by our finding that microinjection of muscimol into the PVN did not reduce the elevated level of CSNA in HF. This finding is reinforced by the lack of inhibition of CSNA by microinjection of glycine into the PVN that we observed in HF. Since the bilateral PVN microinjections of muscimol and glycine may not have reached all the spinally projecting neurons, we also examined the effects of bilateral electrolytic lesions of the PVN. These large lesions also did not reduce the elevated baseline level of CSNA in HF, supporting the microinjection data. The technique of ablating the PVN in the conscious sheep, while recording CSNA immediately before and after the lesion, removes the possibility that there could have been reorganisation of central autonomic circuits to compensate for the lesion. Taken together these studies in conscious animals strongly suggest that while dysinhibition of neurons in the PVN can increase CSNA in normal animals, the high level of CSNA during HF is not dependent on excitatory tone from PVN neurons. Withdrawal of the GABAergic mediated inhibition of CSNA by PVN neurons may be important during other situations where cardio-sympathoexcitation occurs, for example during hypertonic saline resuscitation of haemorrhage (Frithiof et al. 2010).
Interestingly, in HF dysinhibition of PVN neurons with bicuculline did not increase MAP, HR or CSNA, suggesting that the tonic GABAergic inhibition of CSNA seen in normal sheep is abolished in HF. Although this absence of GABAergic input in HF could contribute to the excessive level of CSNA, our findings that inhibition of the PVN with muscimol or glycine, or lesion of the PVN, did not reduce CSNA in HF suggest this is not the case. In HF, bicuculline still inhibited RSNA, but importantly this occurred in the absence of an increase in MAP, suggesting that it was a direct consequence of dysinhibition of PVN neurons. Whether this reflects dysinhibition of GABAergic interneurons or an inhibitory projection from the PVN to the intermediolateral cell column of the spinal cord requires further investigation. Our data suggest that in conscious sheep, there is no tonic inhibition of RSNA arising from the PVN in normal animals, but this develops during HF.
Role of the PVN in mediating changes in SNA to volume expansion
Our finding in conscious sheep that the PVN is critical for the inhibition of RSNA by volume expansion is in accord with studies in other species (Patel, 2000; Badoer, 2001; Coote, 2005). For example, microinjection of muscimol into the PVN in conscious rabbits caused a similar attenuation of the inhibition of RSNA during volume expansion (Ng et al. 2004). Furthermore, lesions of the PVN in anaesthetized rats attenuated the inhibition of RSNA and renal vasodilatation following volume expansion (Lovick et al. 1993; Haselton et al. 1994). Together these data in three species indicate that in the normal state volume expansion stimulates a reflex that increases the level of GABAergic inhibitory input into the PVN and inhibits RSNA.
There is extensive evidence indicating that a reduction in GABAergic inhibitory input to the PVN leads to the increased RSNA in HF (Patel, 2000; Zhang et al. 2002a; Wang et al. 2009), and also the desensitization of the reflex inhibition of RSNA by increased volume that we and others have observed in HF (Dibner-Dunlap & Thames, 1992; Zheng et al. 2006; Ramchandra et al. 2009b). There is little information on the role of the PVN in the control of sympathetic outflow to the heart and our findings indicate that it has very different effects to those on RSNA. Our data indicate that GABAergic inhibitory inputs to neurons in the PVN contribute little if at all to the inhibition of CSNA during volume expansion. These findings in conscious animals highlight the differential central control of CSNA and RSNA that has been observed in other situations (May et al. 2010), and indicate that the inhibition of CSNA during volume expansion is mediated by a brain region(s) other than the PVN. Possible sites include the locus coeruleus and the medullary raphe, which show increased expression of c-fos following volume expansion, and have polysynaptic projections to the stellate ganglion (Jansen et al. 1995; Cunningham et al. 2002; Godino et al. 2005).
In earlier studies we demonstrated that the inhibition of CSNA and RSNA during volume expansion is determined by differential modulation of the amplitude and frequency of sympathetic bursts in the two nerves during volume expansion (Ramchandra et al. 2009b). Our present data confirm the previous findings that the inhibition of RSNA is due to decreases in both burst amplitude and burst frequency, while the inhibition of CSNA is due to a selective decrease in burst frequency. The attenuation of the volume induced RSNA inhibition by muscimol was due to effects on both burst amplitude and burst frequency of renal sympathetic bursts. These data suggest that the PVN modulates both the amplitude and the frequency of bursts in the renal sympathetic nerve.
In summary, these studies indicate that in conscious normal animals the resting levels of CSNA and RSNA were not altered by inhibition of the PVN with the GABA agonist muscimol. During volume expansion, however, the responses to muscimol demonstrate that the PVN selectively mediates the reflex inhibition of RSNA, but not CSNA. Interestingly, dysinhibition of the PVN with bicuculline stimulated CSNA, but not RSNA, indicating that the resting level of CSNA is normally under GABAergic inhibitory tone. In contrast, bicuculline did not increase CSNA in HF indicating removal of this GABAergic inhibitory tone. But in HF we found no evidence that the PVN stimulated CSNA as inhibition of the PVN with muscimol or glycine, as well as lesion of the PVN, did not reduce CSNA. These findings indicate that excitatory drive from the PVN does not contribute to the cardiac sympathoexcitation in HF. These studies demonstrate striking differences in the central mechanisms controlling CSNA and RSNA in HF, and during volume expansion. This is not surprising considering other evidence showing differential control of these two sympathetic outflows (Okada & Ninomiya, 1983; Matsukawa et al. 1993; May & McAllen, 1997; Watson et al. 2004; Frithiof et al. 2010; Ramchandra et al. 2012). Furthermore, the important finding in patients with HF that sympathetic activity to the heart increases before that to the kidney (Rundqvist et al. 1997) is further evidence that different mechanisms cause the increases in SNA to the heart and kidney in HF.
Acknowledgments
The authors acknowledge the expert technical assistance of Alan McDonald and Tony Dornom. This work was supported by National Health and Medical Research Council of Australia Grant 628573 and the Victorian Government's Operational Infrastructure Support Program. R. Ramchandra was the recipient of National Heart Foundation Postdoctoral Fellowship 09M 4930, and C. N. May was supported by National Health and Medical Research Council Research Fellowships 350328 and 566819.
Glossary
- CSNA
cardiac sympathetic nerve activity
- HF
heart failure
- PVN
paraventricular nucleus of the hypothalamus
- RSNA
renal sympathetic nerve activity
- SNA
sympathetic nerve activity
Author contributions
Conception and design of the experiments: R.R. and C.N.M. Collection, analysis and interpretation of data: R.R., S.G.H., R.F., M.J.M. and C.N.M. Drafting the article or revising it critically for important intellectual content: R.R., S.G.H., R.F., M.J.M. and C.N.M. No conflicts of interest are declared by the authors.
References
- Akama H, McGrath BP, Badoer E. Volume expansion fails to normally activate neural pathways in the brain of conscious rabbits with heart failure. J Auton Nerv Sys. 1998;73:54–62. doi: 10.1016/s0165-1838(98)00124-6. [DOI] [PubMed] [Google Scholar]
- Akine A, Montanaro M, Allen AM. Hypothalamic paraventricular nucleus inhibition decreases renal sympathetic nerve activity in hypertensive and normotensive rats. Auton Neurosci. 2003;108:17–21. doi: 10.1016/j.autneu.2003.08.009. [DOI] [PubMed] [Google Scholar]
- Badoer E. Hypothalamic paraventricular nucleus and cardiovascular regulation. Clin Exp Pharmacol Physiol. 2001;28:95–99. doi: 10.1046/j.1440-1681.2001.03413.x. [DOI] [PubMed] [Google Scholar]
- Badoer E, Ng CW, De Matteo R. Tonic sympathoinhibition arising from the hypothalamic PVN in the conscious rabbit. Brain Res. 2002;947:17–24. doi: 10.1016/s0006-8993(02)02901-3. [DOI] [PubMed] [Google Scholar]
- Cano G, Card JP, Sved AF. Dual viral transneuronal tracing of central autonomic circuits involved in the innervation of the two kidneys in rat. J Comp Neurol. 2004;471:462–481. doi: 10.1002/cne.20040. [DOI] [PubMed] [Google Scholar]
- Coote JH. A role for the paraventricular nucleus of the hypothalamus in the autonomic control of heart and kidney. Exp Physiol. 2005;90:169–173. doi: 10.1113/expphysiol.2004.029041. [DOI] [PubMed] [Google Scholar]
- Cunningham JT, Bruno SB, Higgs KA, Sullivan MJ. Intrapericardial procaine affects volume expansion-induced fos immunoreactivity in unanesthetized rats. Exp Neurol. 2002;174:181–192. doi: 10.1006/exnr.2002.7863. [DOI] [PubMed] [Google Scholar]
- Deering J, Coote JH. Paraventricular neurones elicit a volume expansion-like change of activity in sympathetic nerves to the heart and kidney in the rabbit. Exp Physiol. 2000;85:177–186. [PubMed] [Google Scholar]
- Dibner-Dunlap ME, Thames MD. Control of sympathetic nerve activity by vagal mechanoreflexes is blunted in heart failure. Circulation. 1992;86:1929–1934. doi: 10.1161/01.cir.86.6.1929. [DOI] [PubMed] [Google Scholar]
- Eisenhofer G, Friberg P, Rundqvist B, Quyyumi AA, Lambert G, Kaye DM, Kopin IJ, Goldstein DS, Esler MD. Cardiac sympathetic nerve function in congestive heart failure. Circulation. 1996;93:1667–1676. doi: 10.1161/01.cir.93.9.1667. [DOI] [PubMed] [Google Scholar]
- Frithiof R, Ramchandra R, Hood S, May C, Rundgren M. Hypothalamic paraventricular nucleus mediates sodium-induced changes in cardiovascular and renal function in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2009;297:R185–193. doi: 10.1152/ajpregu.00058.2008. [DOI] [PubMed] [Google Scholar]
- Frithiof R, Ramchandra R, Hood SG, May CN. Hypertonic sodium resuscitation after hemorrhage improves hemodynamic function by stimulating cardiac, but not renal, sympathetic nerve activity. Am J Physiol Heart Circ Physiol. 2010;300:H685–692. doi: 10.1152/ajpheart.00930.2010. [DOI] [PubMed] [Google Scholar]
- Gibson JG, Evans WA. Clinical studies of the blood volume. III. Changes in blood volume, venous pressure and blood velocity rate in chronic congestive heart failure. J Clin Invest. 1937;16:851–858. doi: 10.1172/JCI100911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godino A, Giusti-Paiva A, Antunes-Rodrigues J, Vivas L. Neurochemical brain groups activated after an isotonic blood volume expansion in rats. Neuroscience. 2005;133:493–505. doi: 10.1016/j.neuroscience.2005.02.035. [DOI] [PubMed] [Google Scholar]
- Haselton JR, Goering J, Patel KP. Parvocellular neurons of the paraventricular nucleus are involved in the reduction in renal nerve discharge during isotonic volume expansion. J Auton Nerv Sys. 1994;50:1–11. doi: 10.1016/0165-1838(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Hasking GJ, Esler MD, Jennings GL, Burton D, Johns JA, Korner PI. Norepinephrine spillover to plasma in patients with congestive heart failure: evidence of increased overall and cardiorenal sympathetic nervous activity. Circulation. 1986;73:615–621. doi: 10.1161/01.cir.73.4.615. [DOI] [PubMed] [Google Scholar]
- Jansen AS, Wessendorf MW, Loewy AD. Transneuronal labelling of CNS neuropeptide and monoamine neurons after pseudorabies virus injections into the stellate ganglion. Brain Res. 1995;683:1–24. doi: 10.1016/0006-8993(95)00276-v. [DOI] [PubMed] [Google Scholar]
- Kang YM, Ma Y, Elks C, Zheng JP, Yang ZM, Francis J. Cross-talk between cytokines and renin-angiotensin in hypothalamic paraventricular nucleus in heart failure: role of nuclear factor-κB. Cardiovas Res. 2008;79:671–678. doi: 10.1093/cvr/cvn119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kannan H, Hayashida Y, Yamashita H. Increase in sympathetic outflow by paraventricular nucleus stimulation in awake rats. Am J Physiol Regul Integr Comp Physiol. 1989;256:R1325–1330. doi: 10.1152/ajpregu.1989.256.6.R1325. [DOI] [PubMed] [Google Scholar]
- Karim F, Kidd C, Malpus CM, Penna PE. The effects of stimulation of the left atrial receptors on sympathetic efferent nerve activity. J Physiol. 1972;227:243–260. doi: 10.1113/jphysiol.1972.sp010030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman S, Mackay B, Kappagoda CT. Effect of stretching the superior vena cava on heart rate in rats. Am J Physiol Heart Circ Physiol. 1981;241:H248–254. doi: 10.1152/ajpheart.1981.241.2.H248. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Lambert GW, Lefkovits J, Morris M, Jennings G, Esler MD. Neurochemical evidence of cardiac sympathetic activation and increased central nervous system norepinephrine turnover in severe congestive heart failure. J Am Coll Cardiol. 1994;23:570–578. doi: 10.1016/0735-1097(94)90738-2. [DOI] [PubMed] [Google Scholar]
- Kaye DM, Lefkovits J, Jennings GL, Bergin P, Broughton A, Esler MD. Adverse consequences of high sympathetic nervous activity in the failing human heart. J Am Coll Cardiol. 1995;26:1257–1263. doi: 10.1016/0735-1097(95)00332-0. [DOI] [PubMed] [Google Scholar]
- Li DP, Pan HL. Role of gamma-aminobutyric acid (GABA)A and GABAB receptors in paraventricular nucleus in control of sympathetic vasomotor tone in hypertension. J Pharmacol Exp Therap. 2007;320:615–626. doi: 10.1124/jpet.106.109538. [DOI] [PubMed] [Google Scholar]
- Li YF, Cornish KG, Patel KP. Alteration of NMDA NR1 receptors within the paraventricular nucleus of hypothalamus in rats with heart failure. Circ Res. 2003;93:990–997. doi: 10.1161/01.RES.0000102865.60437.55. [DOI] [PubMed] [Google Scholar]
- Li YF, Patel KP. Paraventricular nucleus of the hypothalamus and elevated sympathetic activity in heart failure: the altered inhibitory mechanisms. Acta Physiol Scand. 2003;177:17–26. doi: 10.1046/j.1365-201X.2003.01043.x. [DOI] [PubMed] [Google Scholar]
- Lovick TA, Malpas S, Mahony MT. Renal vasodilatation in response to acute volume load is attenuated following lesions of parvocellular neurones in the paraventricular nucleus in rats. J Auton Nerv Sys. 1993;43:247–255. doi: 10.1016/0165-1838(93)90331-n. [DOI] [PubMed] [Google Scholar]
- Matsukawa K, Ninomiya I, Nishiura N. Effects of anaesthesia on cardiac and renal sympathetic nerve activities and plasma catecholamines. Am J Physiol Regul Integr Comp Physiol. 1993;265:R792–797. doi: 10.1152/ajpregu.1993.265.4.R792. [DOI] [PubMed] [Google Scholar]
- May CN, Frithiof R, Hood SG, McAllen RM, McKinley MJ, Ramchandra R. Specific control of sympathetic nerve activity to the mammalian heart and kidney. Exp Physiol. 2010;95:34–40. doi: 10.1113/expphysiol.2008.046342. [DOI] [PubMed] [Google Scholar]
- May CN, McAllen RM. Baroreceptor-independent renal nerve inhibition by intracerebroventricular angiotensin II in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 1997;273:R560–567. doi: 10.1152/ajpregu.1997.273.2.R560. [DOI] [PubMed] [Google Scholar]
- McKibben JS, Getty R. A comparative study of the cardiac innervation in domestic animals: sheep. Acta Anat. 1969;74:228–242. doi: 10.1159/000143379. [DOI] [PubMed] [Google Scholar]
- Meneely GR, Kaltreider NL. A Study of the volume of the blood in congestive heart failure. relation to other measurements in fifteen patients. J Clin Invest. 1943;22:521–530. doi: 10.1172/JCI101422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng CW, De Matteo R, Badoer E. Effect of muscimol and L-NAME in the PVN on the RSNA response to volume expansion in conscious rabbits. Am J Physiol Renal Physiol. 2004;287:F739–746. doi: 10.1152/ajprenal.00431.2003. [DOI] [PubMed] [Google Scholar]
- Okada Y, Ninomiya I. Different cardiac and renal inhibitory and excitatory areas in rabbit hypothalamus. Am J Physiol Heart Circ Physiol. 1983;244:H832–838. doi: 10.1152/ajpheart.1983.244.6.H832. [DOI] [PubMed] [Google Scholar]
- Patel KP. Role of paraventricular nucleus in mediating sympathetic outflow in heart failure. Heart Fail Rev. 2000;5:73–86. doi: 10.1023/A:1009850224802. [DOI] [PubMed] [Google Scholar]
- Patel KP, Zhang K, Kenney MJ, Weiss M, Mayhan WG. Neuronal expression of Fos protein in the hypothalamus of rats with heart failure. Brain Res. 2000;865:27–34. doi: 10.1016/s0006-8993(00)02186-7. [DOI] [PubMed] [Google Scholar]
- Petersson M, Friberg P, Eisenhofer G, Lambert G, Rundqvist B. Long-term outcome in relation to renal sympathetic activity in patients with chronic heart failure. Eur Heart J. 2005;26:906–913. doi: 10.1093/eurheartj/ehi184. [DOI] [PubMed] [Google Scholar]
- Pyner S, Deering J, Coote JH. Right atrial stretch induces renal nerve inhibition and c-fos expression in parvocellular neurones of the paraventricular nucleus in rats. Exp Physiol. 2002;87:25–32. doi: 10.1113/eph8702279. [DOI] [PubMed] [Google Scholar]
- Ramchandra R, Hood SG, Denton DA, Woods RL, McKinley MJ, McAllen RM, May CN. Basis for the preferential activation of cardiac sympathetic nerve activity in heart failure. Proc Natl Acad Sci U S A. 2009a;106:924–928. doi: 10.1073/pnas.0811929106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandra R, Hood SG, Frithiof R, May CN. Discharge properties of cardiac and renal sympathetic nerves and their impaired responses to changes in blood volume in heart failure. Am J Physiol Regul Integr Comp Physiol. 2009b;297:R665–674. doi: 10.1152/ajpregu.00191.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandra R, Hood SG, Watson AM, Allen AM, May CN. Central angiotensin type 1 receptor blockade decreases cardiac but not renal sympathetic nerve activity in heart failure. Hypertension. 2012;59:634–641. doi: 10.1161/HYPERTENSIONAHA.111.181131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandra R, Hood SG, Watson AM, May CN. Responses of cardiac sympathetic nerve activity to changes in circulating volume differ in normal and heart failure sheep. Am J Physiol Regul Integr Comp Physiol. 2008;295:R719–726. doi: 10.1152/ajpregu.00824.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundqvist B, Elam M, Bergmann-Sverrisdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95:169–175. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- Stocker SD, Hunwick KJ, Toney GM. Hypothalamic paraventricular nucleus differentially supports lumbar and renal sympathetic outflow in water-deprived rats. J Physiol. 2005;563:249–263. doi: 10.1113/jphysiol.2004.076661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stocker SD, Keith KJ, Toney GM. Acute inhibition of the hypothalamic paraventricular nucleus decreases renal sympathetic nerve activity and arterial blood pressure in water-deprived rats. Am J Physiol Regul Integr Comp Physiol. 2004;286:R719–725. doi: 10.1152/ajpregu.00494.2003. [DOI] [PubMed] [Google Scholar]
- Vahid-Ansari F, Leenen FH. Pattern of neuronal activation in rats with CHF after myocardial infarction. Am J Physiol Heart Circ Physiol. 1998;275:H2140–2146. doi: 10.1152/ajpheart.1998.275.6.H2140. [DOI] [PubMed] [Google Scholar]
- Waites GM. The course of the efferent cardiac nerves of the sheep. J Physiol. 1957;139:417–433. doi: 10.1113/jphysiol.1957.sp005902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang RJ, Zeng QH, Wang WZ, Wang W. GABA(A) and GABA(B) receptor-mediated inhibition of sympathetic outflow in the paraventricular nucleus is blunted in chronic heart failure. Clin Exp Pharmacol Physiol. 2009;36:516–522. doi: 10.1111/j.1440-1681.2008.05101.x. [DOI] [PubMed] [Google Scholar]
- Watson AM, Hood SG, Ramchandra R, McAllen RM, May CN. Increased cardiac sympathetic nerve activity in heart failure is not due to desensitization of the arterial baroreflex. Am J Physiol Heart Circ Physiol. 2007;293:H798–804. doi: 10.1152/ajpheart.00147.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson AM, Mogulkoc R, McAllen RM, May CN. Stimulation of cardiac sympathetic nerve activity by central angiotensinergic mechanisms in conscious sheep. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1051–1056. doi: 10.1152/ajpregu.00708.2003. [DOI] [PubMed] [Google Scholar]
- Yu Y, Kang YM, Zhang ZH, Wei SG, Chu Y, Weiss RM, Felder RB. Increased cyclooxygenase-2 expression in hypothalamic paraventricular nucleus in rats with heart failure: role of nuclear factor kappaB. Hypertension. 2007;49:511–518. doi: 10.1161/01.HYP.0000257356.20527.c5. [DOI] [PubMed] [Google Scholar]
- Zhang K, Li YF, Patel KP. Reduced endogenous GABA-mediated inhibition in the PVN on renal nerve discharge in rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2002a;282:R1006–1015. doi: 10.1152/ajpregu.00241.2001. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Francis J, Weiss RM, Felder RB. The renin-angiotensin-aldosterone system excites hypothalamic paraventricular nucleus neurons in heart failure. Am J Physiol Heart Circ Physiol. 2002b;283:H423–433. doi: 10.1152/ajpheart.00685.2001. [DOI] [PubMed] [Google Scholar]
- Zheng H, Li YF, Wang W, Patel KP. Enhanced angiotensin-mediated excitation of renal sympathetic nerve activity within the paraventricular nucleus of anaesthetized rats with heart failure. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1364–1374. doi: 10.1152/ajpregu.00149.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Li YF, Zucker IH, Patel KP. Exercise training improves renal excretory responses to acute volume expansion in rats with heart failure. Am J Physiol Renal Physiol. 2006;291:F1148–1156. doi: 10.1152/ajprenal.00501.2005. [DOI] [PubMed] [Google Scholar]


