Abstract
Taurine is one of the most abundant amino acids in the retina, throughout the CNS, and in heart and muscle cells. In keeping with its broad tissue distribution, taurine serves as a modulator of numerous basic processes, such as enzyme activity, cell development, myocardial function and cytoprotection. Despite this multitude of functional roles, the precise mechanism underlying taurine's actions has not yet been identified. In this study we report findings that indicate a novel role for taurine in the regulation of voltage-gated delayed rectifier potassium (KV) channels in retinal neurons by means of a metabotropic receptor pathway. The metabotropic taurine response was insensitive to the Cl− channel blockers, picrotoxin and strychnine, but it was inhibited by a specific serotonin 5-HT2A receptor antagonist, MDL11939. Moreover, we found that taurine enhanced KV channels via intracellular protein kinase C-mediated pathways. When 5-HT2A receptors were expressed in human embryonic kidney cells, taurine and AL34662, a non-specific 5-HT2 receptor activator, produced a similar regulation of KIR channels. In sum, this study provides new evidence that taurine activates a serotonin system, apparently via 5-HT2A receptors and related intracellular pathways.
Key points
Although taurine is one of the most abundant amino acids in human tissues, and serves a number of important functions ranging from cell development to cytoprotection, its precise mode of action is often obscure.
Here we present evidence that, in the vertebrate retina, taurine regulates voltage-gated potassium (KV) channels that are sensitive to the inhibitors of KV1, KV2 and KV4 subunits.
Taurine's effect was shown to be a metabotropic response, involving a G-protein linked, PKC-dependent intracellular pathway.
Noteworthy was the finding that responses to taurine were blocked by a specific antagonist of 5-HT2A receptors. Taurine activation of 5-HT2A receptors was further confirmed in HEK cells that expressed recombinant 5-HT2A receptors.
Taurine has been shown to be beneficial in the management of a number of brain disorders. Its interaction with serotonergic pathways suggests that taurine may also play a role in various cognitive functions of the CNS.
Introduction
Taurine (2-aminoethanesulfonic acid), is often referred to as a ‘non-essential’ amino acid because it is the only amino acid not involved in protein synthesis. This is clearly a misnomer considering its functional significance in cell development and survival. Indeed, taurine depletion leads to severe cardiomyopathy, renal dysfunction, pancreatic β-cell malfunction and to the loss of retinal photoreceptors (Heller-Stilb et al. 2002; Yamori et al. 2010; Zulli et al. 2011). Because a taurine-specific receptor has not yet been identified, it has been suggested that taurine may work as an agonist of chloride-permeable GABA and glycine receptors (Hussy et al. 1997; Jia et al. 2008). However, in many cases the effects of taurine are not mimicked by either GABA or glycine (Medina & De Robertis, 1984; Young & Cepko, 2004), implying that another class of taurine-sensitive receptor may exist. Interestingly, it has been reported that taurine acts on metabotropic GABAB receptors in the cerebral cortex (Kontro & Oja, 1990) and at similar sites in the cerebellum (Smith & Li, 1991), but the intracellular pathway(s) involved in the metabotropic response is largely unknown.
Taurine concentration in retinal tissues is extremely high, particularly in glutamatergic neurons (Cohen et al. 1973; Marc et al. 1995; Fletcher & Kalloniatis, 1996). Although taurine uptake has been observed in many retinal cell types at early stages of development (Kennedy & Voaden, 1976; Orr et al. 1976; Young & Cepko, 2004), its cellular distribution in the adult retina is far from uniform. Cohen and co-workers found that taurine exceeds by >10-fold the concentration of each of the other amino acids in the mouse retina, and a study of goldfish retina confirmed that the concentration of taurine is almost 20 times higher than that of glutamate, and as much as 25 times higher than GABA (Marc et al. 1995). Not surprisingly, animals that do not produce taurine metabolically experience severe degenerative changes in their photoreceptors and retinal pigment epithelium when deprived of dietary taurine (Pasantes-Morales et al. 1986; Heller-Stilb et al. 2002).
It is apparent that retinal cells provide an ideal venue in which to study the activity of taurine and, in the present study, we examined the action of taurine on the voltage-gated potassium channels (KV channels) that are critical for the generation of action potentials (APs) in the retina and CNS. Most significant was the effect of taurine on 5-HT2A receptors that regulate delayed rectifier KV channels via a metabotropic intracellular pathway involving protein kinases C and A (PKC and PKA).
Methods
All procedures were performed in accordance with the guidelines of National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Electrophysiological recording
Whole-cell patch-clamp recordings were performed on acutely isolated neurons from Larval tiger salamander (Ambystoma tigrinum) retina (Shen & Slaughter, 1999; Bulley & Shen, 2010), using an EPC-10 amplifier and HEKA Patchmaster software (HEKA Instruments Inc., Lambrecht/Pfalz, Germany). Low-resistance (5–10 MΩ) electrodes were pulled from borosilicate glass, and filled with a high K+ solution containing (in mm): potassium-gluconate, 100; MgCl2, 1; EGTA, 5; Hepes, 5; and an ATP regenerating cocktail consisting of (in mm): ATP, 20; phosphocreatine, 40; creatine phosphokinase, 2; pH 7.4. The Ringer solution contained the following (in mm): NaCl, 111; KCl, 2.5; CaCl2, 1.8; MgCl2, 1.0; Hepes, 5; dextrose, 10; pH 7.7. Cells were recorded 5–10 min after membrane rupture in order to allow the cells to stabilize after dialysis of the electrode solution. The isolated cells were constantly superfused with Ringer solution or drug solutions via a DAD-VM automated superfusion system (ALA Scientific Instruments, Farmingdale, NY, USA). All drugs were purchased from Tocris Bioscience (Minneapolis, MN, USA) and Sigma-Aldrich Co (St Louis, MO, USA), except AL34662 (a 5-HT2 receptor agonist), which was purchased from the Caymen Chemicals Company (Ann Arbor, MI, USA).
Cell selection
During the isolation process, cells often lost their typical morphological features, although photoreceptors exhibited their unique structure, and dissociated ganglion cells could be identified by the long axonal processes that extend from their cell somas. When amacrine and bipolar cells were not distinguishable, it was necessary to rely on physiological criteria, i.e. their distinctive transient Na+ currents in whole-cell recording. Unlike bipolar cells, which show extremely large inward rectifier currents, amacrine cells have relatively small transient Na+ currents as well as small inward rectifier currents. Because there are many types of amacrine cells in salamander retina, these criteria are less than ideal, but did not significantly influence the results. Nor can we exclude the possibility that some isolated cells we recorded might be bipolar cells, as some possess Na± channels (Ichinose et al. 2005).
Construction and expression of a 5-HT2A-GFP fusion protein
The complete coding region of the mouse 5-HT2A receptor (cDNA clone 40047362; GenBank accession number NM-172812.2) was obtained from Thermo Scientific Open Biosystems MGC (Pittsburgh, USA). Polymerase chain reaction (PCR) primers 5′-CCC AAG CTT CGC CAC C ATG GAA ATT CTC TGT G-3′ (forward) and 5′-CGC GGA TC C CA CAC ACA GCT AAC CTT TTC AT-3′ (reverse) were used to amplify cDNA, and both HindIII and BamHI restriction sites were introduced to the ends of the PCR products for in-frame insertion. The 1.4 kb PCR product was digested with HindIII and BamHI, and inserted into pEGFP-N1 (CLONTECH, Mountain View, USA). The orientation of the insert was confirmed by digestion with restriction enzyme, as well as sequenced to ensure that the 5-HT2A-GFP fusion plasmid was in frame.
Human embryonic kidney (HEK)293 cells were cultured in growth medium composed of Dulbecco's modified Eagle's medium (DMEM) and 10% fetal bovine serum in a 37°C, 5% CO2 incubator. Cells were grown to 65–70% confluency on coverslips in 12-well plates. Sixteen hours after plating, the cells were transfected with 1.5 mg 5-HT2A-GFP and 4.5 ml Lipofectamine LTX (Invitrogen, Grand Island, USA) in 1 ml medium according to the manufacturer's instructions. Transfected cells, identified by the expression of GFP, were incubated for 16–24 h before use.
Immunocytochemistry
To detect cells that express 5-HT2A receptors, retinal sections and transfected HEK293 cells were washed twice with PBS and fixed with 4% paraformaldehyde for 20 min at room temperature. The cells were permeabilized in 1% Triton-X100 in PBS for 10 min, and blocked in 5% goat serum–PBS for 1 h. HEK293 cells or retinal sections adherent to cover slips were immersed in either polyclonal anti-5-HT2A (Abcam, Cambridge, MA, USA) or polyclonal anti-glial fibrillary acidic protein (GFAP; Sigma-Aldrich Co.) at 1:500 and 1:80 dilution in 5% goat serum, respectively, and incubated overnight at 4°C. After washing three times in PBS, the tissues were incubated with goat-anti-rabbit Cy-3-conjugated secondary antibody at a concentration of 1:600 for 1 h in darkness. Immunostained cells were visualized with a Zeiss LSM 700 confocal microscope system (Munich, Germany).
Western blot assay
This assay is fairly well standardized (cf. Kurien et al. 2011). In brief, retinal tissues were lysed in a 2× Laemmli buffer, and the total protein content was obtained from homogenates. Protein concentrations were calibrated using a BCA™ Protein Assay Kit (Pierce, Rockford, IL, USA). Equal amounts of samples were loaded onto 3–8% Tris-acetate gels, and retinal proteins were separated by electrophoresis (100 V for 1.5 h). The proteins were transferred to a Hybond-ECL Nitrocellulose membrane (GE Healthcare Bio-Sciences Corp., Piscataway, NJ, USA), and then immersed in a block solution with 5% dry milk in PBS for 1.5 h at room temperature. 5-HT2A was detected with the specific antibody used at a concentration of 1:5000 in 5% milk solution and incubated overnight at 4°C. After washing in a Tris-buffered saline with 0.1% Tween (TBS-T) buffer and incubated for 45 min with a secondary antibody (horseradish peroxidase-conjugated goat anti-mouse IgG; 1:5000), positively stained bands were detected by a chemiluminescent blot assay with the ECL Plus Western blot reagent.
Results
Evidence that taurine regulates KV channels via a metabotropic mechanism
Figure 1A and B shows examples of the voltage–current relationship of an isolated amacrine cell in whole-cell recording. The cell was held at −60 mV and activated by brief (25 ms) voltage steps from −100 mV to +65 mV at 15 mV increments. The typical properties of amacrine cells are evident in response to the brief voltage steps, i.e. small, transient inward Na+ currents and large outward K+ currents at depolarizing voltages. When 100 μm Cd2+ combined with 1 μm tetrodotoxin (TTX), a Na+ channel blocker, was applied, the transient inward Na+ currents were almost completely suppressed, and the outward K+ currents for voltage steps greater than −10 mV were reduced by the Cd2+ (Fig. 1Ab). This is due to the fact that Cd2+ blocks Ca2+ influx through the voltage-gated Ca2+ channels, thereby also inhibiting Ca2+-mediated KCa channels (Sah & Davies, 2000).
Figure 1. Taurine regulates K+ currents and APs in isolated amacrine and ganglion cells.
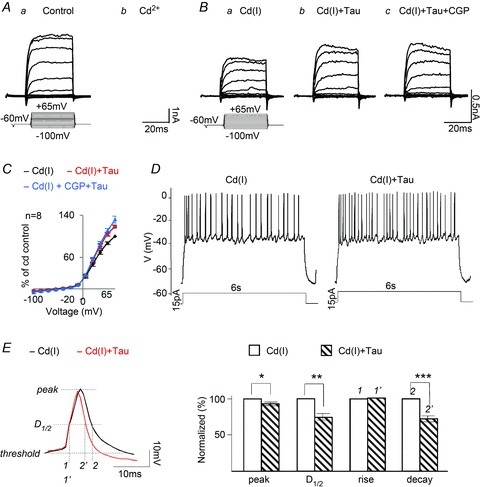
Aa and Ab, whole-cell recording of voltage-dependent currents from an amacrine cell in Ringer solution,100 μm Cd2+ + 1 μm TTX reduced both the K+ and Na+ currents. Ba, the addition of 100 μm picrotoxin and 10 μm strychnine, referred to as a Cd2+ inhibitory cocktail Cd(I), had little effect on the outward K+ currents. Bb, taurine markedly enhanced the K+ current amplitudes. Bc, the effect of taurine was insensitive to 10 μm CGP55845, a GABAB antagonist. C, the I–V curves measured and plotted from 8 cells in each of the test solutions. D, taurine increases the firing rate of APs generated by current injection (15 pA) in a ganglion cell superfused with the Cd(I) solution. Similar results were obtained from 7 of the 15 ganglion cells tested. The other 8 cells, in which injecting depolarizing currents generated spikes, rapidly accommodated and stopped firing within 1 s; these cells were not tested further. E, an expanded spike recording to indicate the points at which measurements were made of the peak, half-duration (D1/2), rise and decay times of APs in Cd(I) and with taurine (left). Bar graphs (right panel) show that taurine reduced the peak by 7.4 ± 2% (*P > 0.02, n = 189), D1/2 by 26.2 ± 7.2% (**P > 0.01, n = 189) and decay time by 27.1 ± 8.3% (***P > 0.01, n = 189).
To study the metabotropic taurine effect on KV channels, an inhibitory ‘cocktail’ containing (in μm): picrotoxin, 100; strychnine, 10; Cd2+, 100; TTX, 1; was added to the Ringer solution. These agents eliminated the effect of taurine on Cl−-permeable GABA and glycine receptors, but did not affect outward K+ channels. Figure 1Ba shows the steady state K+ currents in the presence of the Cd2+-containing inhibitory ‘cocktail’, referred to throughout the paper as Cd(I). When taurine was applied with Cd(I), the outward currents were increased (Fig. 1Bb). Because taurine-sensitive Cl−-permeable receptors were blocked by the inhibitory cocktail, we attribute the enhancement by taurine to a metabotropic effect, involving an intracellular pathway that regulates K+ channels.
Because taurine had been shown previously to act on metabotropic GABAB receptors (Smith & Li, 1991), we added CGP55845, a potent GABAB receptor antagonist, to the taurine + Cd(I) solution to test whether the taurine effect was mediated by this class of GABA receptor. Despite blocking GABAB receptors, the KV currents were still increased by taurine (Fig. 1Bc), indicating that the action of taurine was not mediated by metabotropic GABAB receptors. Voltage-dependent curves were plotted from eight cells in each of the test solutions used in Fig. 1B. The outward rectifying I–V curves derived from these 8 cells (Fig. 1C) confirm the fact that CGP55845 had no significant effect on the response of taurine.
The metabotropic effect of taurine was further investigated on the train of ganglion cell APs generated by injecting depolarizing currents in current-clamp mode. Figure 1D shows typical recordings from a ganglion cell with a resting potential of about −70 mV. Injecting 15 pA currents for 6 s evoked a train of APs in the cell exposed to the control Cd(I) inhibitory cocktail, and the firing rate of APs was increased with the addition of taurine. This suggests that taurine may increase the repolarization rate of APs by increasing K+ efflux through KV channels. The peak, half-duration (D1/2), rise and decay time of APs were compared in Cd(I) with and without taurine from seven cells, using the Minianalysis program (Synaptosoftware, Fort Lee, USA). A representative recording of an AP is shown on an expanded time scale to indicate the points at which kinetic measurements were made (Fig. 1E, left), and the histograms illustrate that taurine reduced the peak, D1/2 and decay time of APs, but had no effect on the rise time (Fig. 1E, right).
Pharmacological evidence that taurine responses were derived from various subtypes of delayed rectifier KV channels
To determine the specific subtypes of KV channels that are sensitive to taurine regulation, selective channel blockers for delayed rectifier KV channel subtypes were utilized. Margatoxin (MgTX) is a selective inhibitor of KV1.1 and KV1.3 channel complexes on retinal neurons (Koeberle & Schlichter, 2010), whereas stromatoxin-1 (ScTx-1) specifically inhibits channels formed by combinations of KV2 and KV4 subgroup-mediated channels (Guan et al. 2011). These antagonists allowed us to pharmacologically distinguish the subtypes of KV channels present in amacrine and ganglion cells that are regulated by taurine. Figure 2Aa shows that the sustained KV current evoked by a 25 ms voltage pulse in the Cd(I) solution (black trace) was largely suppressed by MgTX (50 nm), but it had little effect on the initial peak (grey trace); the addition of taurine had no effect on the currents (Fig. 2Ab). These findings were confirmed in recordings from five additional amacrine cells. Subtracting the control response from the current in MgTX revealed that the MgTX-sensitive currents resembled the slowly activated delayed rectifier KV current (Fig. 2Ac), thus indicating that KV1.1 and/or KV1.3 channels are enhanced by taurine.
Figure 2. The effect of taurine on KV current was inhibited by margatoxin (MgTX) and stromatoxin-1 (ScTx-1).
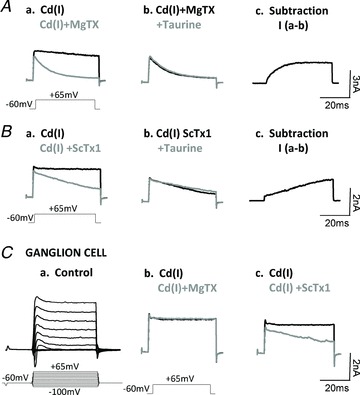
Aa, the KV current evoked by a 25 ms voltage pulse of −60 mV to +65 mV in the Cd(I) solution (black trace); MgTX (50 nm) suppressed the majority of the sustained KV currents with little effect on the initial peak (Grey trace); the addition of taurine had no effect on the currents (Ab). Taurine-sensitive KV1.1 and/or KV1.3 channel currents are revealed by subtracting the control response from the current in MgTX (Ac). Ba, ScTx-1 (50 nm) also suppressed delayed rectifier KV channels in amacrine cells, although to a lesser extent than MgTX. Bb, the addition of taurine following the ScTx-1 had no detectable effect on the KV currents. Bc, subtracting the control from the ScTx-1 current revealed that ScTx-1-sensitive KV2 and KV4 channels had a much slower activation rate compared with MgTX-sensitive currents. In approximately 50% of ganglion cells the KV currents were only sensitive to ScTx-1, but not MgTX, as shown in (Ca) K+ currents from a ganglion cell in Ringer solution; and the K+ currents sensitive to ScTx-1, but not MgTX (Cb and Cc).
Figure 2Ba and Cc shows that ScTx-1 (50 nm) also suppressed delayed rectifier KV channels in amacrine and ganglion cells, although to a lesser extent than MgTX. Here too the addition of taurine following the blockage of KV2 and KV4 channel subtypes had no detectable effect on the KV currents (Fig. 2Bb), evidence that taurine also regulates KV channels formed by KV2 subtypes. Subtracting the control from the ScTx-1 current revealed that ScTx-1-sensitive KV currents had a much slower activation rate compared with MgTX-sensitive currents (Fig. 2Bc). Clearly, the various subtypes of delayed rectifier KV channels exhibit distinct channel kinetics, and it appears that these various subgroups probably coexist in most amacrine cells. In contrast, KV channels in approximately 50% of the 25 ganglion cells tested were not blocked by MgTX (Fig. 2Cb), but were blocked by ScTx-1 (Fig. 2Cc), an indication that there is a subgroup of ganglion cells that express primarily KV2 subunits.
Intracellular pathways involved in the metabotropic taurine response
An intriguing issue that is difficult to address is the identification of the intracellular pathway mediating the metabotropic taurine response. PKC and PKA pathways are ubiquitous pathways that exist in retinal neurons (Gillette & Dacheux, 1996). Accordingly, we tested the effect of taurine on KV currents when selective kinase inhibitors interrupted these intracellular pathways. The peptide PKI 14–22 amide (10 μm), the specifically PKA inhibitor, was introduced intracellularly through the whole-cell recording electrodes. Following a series of control current–voltage recordings in the PKI 14–22 solution (Fig. 3Aa), the K+ currents were recorded following the addition of the Cd(I) cocktail (Fig. 3Ab); further addition of taurine greatly increased the KV currents (Fig. 3Ac). The I–V curves (n = 11) shown in Fig. 3B are a clear indication that blocking PKA does not inhibit the taurine enhancement of KV channel currents.
Figure 3. Potential intracellular pathways mediating taurine's metabotropic regulation of KV channels.
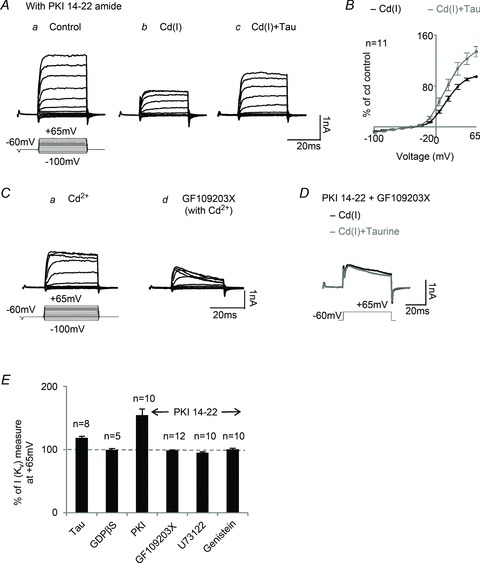
Aa and Ab, after intracellular dialysis of 10 μm PKI 14–22 amide (the PKA inhibitor), K+ currents were recorded in control Ringer and the Cd(I) solution. Ac, taurine still enhanced KV currents after blocking PKA. B, the I–V curves show that in the presence of PKI 14–22, taurine enhanced KV currents from 11 cells. Ca and Cb, 10 μm GF109203X, a selective PKC inhibitor, blocked sustained KV currents in amacrine cells. D, after blocking both PKA and PKC with PKI 14–22 and GF109203X, taurine no longer had an effect on KV currents. E, the effects of various intracellular protein inhibitors on the metabotropic taurine regulation of KV currents. The horizontal dashed line represents the control KV current recorded in the Cd(I) solution in response to a +65 mV voltage step. On average, taurine increased the KV currents by about 19% (n = 8); with PKI 14–22 to block PKA, taurine enhanced KV currents by approximately 48% (n = 11). While PKA was blocked, inhibition of PKC (with GF109203X) or PLC (with U73122) or tyrosine kinase (with genistein) eliminated the effect of taurine on KV currents. The effect of taurine was also blocked by intracellular application of GDPβS, an inhibitor of G-proteins (n = 6).
GF109203X, a membrane-permeable PKC inhibitor, was used to specifically block PKC and its related downstream pathways in amacrine and ganglion cells. After KV currents were recorded in a Cd2+-containing Ringer solution (Fig. 3Ca), superfusion of GF109203X (10 μm) greatly suppressed the KV currents (Fig. 3Cb); the effect of PKC inhibition was consistent in all the recordings from amacrine cells and most of the ganglion cells (n = 23 total). This indicates that PKC is critical for KV channel conductance in these neurons. Although suppression of PKA did not suppress the response to taurine, blocking both PKC and PKA pathways (applying PKI 14–22 with GF109203X) resulted in complete blockage of the current enhancement by taurine (Fig. 3D). The bar graphs of Fig. 3E illustrate the effects of various intracellular transduction pathway inhibitors on taurine enhancement of KV currents. Only taurine and taurine with the PKA inhibitor PKI 14–22 enhanced the KV currents; taurine increased the KV currents by 19% (n = 8), whereas in the presence of the PKA inhibitor, taurine enhanced the KV current by 48% (n = 11). Thus, blocking the PKA pathway significantly upregulated the taurine effect.
Because phospholipase C (PLC) is an upstream enzyme in the PKC cascade, it may be involved in the transduction pathway governing taurine regulation of KV channels. U73122, a specific PLC inhibitor, was applied with PKI 14–22 via internal dialysis to block the PLC/PKA pathway. The enhancement of KV currents by taurine was completely blocked by 10 μm of the PLC inhibitor, n = 10 (Fig. 3E). This suggests that PLC is probably an important stage in the taurine transduction pathway.
There is evidence that activation of delayed rectifier KV channels is regulated by tyrosine kinase phosphorylation (Strauss et al. 2002). Therefore, we applied genistein, a tyrosine kinase inhibitor, while PKA was inhibited with PKI 14–22. As shown in Fig. 3E, this effectively blocked taurine enhancement of KV currents, indicating that tyrosine kinase phosphorylation may also be involved in the taurine-activated transduction pathway.
GTP-binding protein (G-protein) often serves as an intracellular second messenger of neuronal metabotropic receptors. Indeed, this seems to be the case in regard to the taurine-induced activation of KV channels. To block G-protein activity and its downstream pathways, GDPβS (1 mm) was applied through the whole-cell recording electrodes. After intracellular application of GDPβS, taurine failed to enhance the KV currents (n = 5; Fig. 3E), demonstrating that a G-protein-linked metabotropic pathway is involved in the taurine response.
The involvement of 5-HT2A serotonin receptors
To investigate a potential site for taurine binding that subsequently triggers the intracellular metabotropic cascades that regulate KV currents, several metabotropic receptor antagonists were tested. Among these, MDL11939, a potent and selective antagonist for serotonin receptor 5-HT2A (Fox et al. 2010), was found to significantly block the effect of taurine (Fig. 4A and B).
Figure 4. Taurine activates 5-HT2A receptors in retinal neurons.
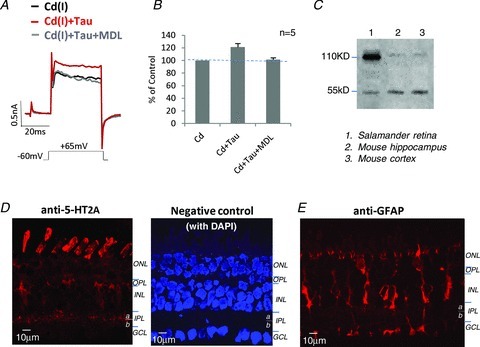
A, taurine enhanced KV currents in the Cd(I) solution, but the effect was blocked by 1 nm MDL11939, a selective 5-HT2A receptor antagonist. B, the bar graphs show the summary results of the effect of MDL11939 on blocked taurine-enhanced KV current amplitudes measured at +65 mV. C, Western blots show that the anti-5-HT2A detected protein bands at the predicted MW of 53 kDa in samples from mouse brain and salamander retina. It is likely that protein bands near MW of 110 kDa are dimers. D, confocal image of anti-5-HT2A labeling in a salamander retinal section, depicting the strong labeling in photoreceptors and within the inner plexiform layer (IPL); punctate labeling is also seen in the somas of the inner nuclear layer (INL) and ganglion cell layer (GCL); and the negative control (omitting 5-HT2A antibody) shows no labeling in retinal sections (the cell nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI)). E, Muller cells in a retinal section were labeled by the anti-glial fibrillary acidic protein (GFAP). ONL. outer nuclear layer; OPL, outer plexiform layer.
To localize 5-HT2A receptors in salamander retina, an antibody specific for 5-HT2A receptors was used; the antibody specificity was tested by Western blots in salamander retina and in the regions of mouse brain. The results demonstrated that the antibody recognized proteins with the same molecular weight (53 kDa) in these tissues (Fig. 4C); the secondary bands at 110 kDa are probably dimers. This antibody was also used to identify cells expressing 5-HT2A receptors by immunocytochemistry. Dense labeling was present in the proximal half (sublamina a) of the inner plexiform layer (IPL) where bipolar cell terminals, amacrine and ganglion cell processes are located. The antibody also lightly labeled some somas at the inner nuclear layer and ganglion cell layer, as well as photoreceptor outer-segments and possibly Muller cells (Fig. 4D, left). No positive labeling was observed in the negative control experiments performed using the same protocol, except the 5-HT2A (primary antibody) was omitted. 4′,6-Diamidino-2-phenylindole (DAPI) was used as a fluorescence marker to stain cell nuclei and to depict the retinal structure (Fig. 4D, right). Figure 4E shows Muller cells in a retinal section labeled by the GFAP antibody. Comparing labeling patterns with GFAP and 5-HT2A antibodies suggests that 5-HT2A labeling in the IPL and ganglion cell layer is probably not associated with Muller cells.
The effect of taurine on HEK cells expressing 5-HT2A
To further demonstrate that 5-HT2A receptors mediate the effects of taurine, we transfected HEK cells with GFP-tagged 5-HT2A receptors. Successful transfection was confirmed by 5-HT2A antibody-labeling of the cells. As illustrated in Fig. 5A, confocal images showed the presence of GFP-tagged 5-HT2A proteins (green) in both the cytosol and on the cell membrane. On the other hand, the 5-HT2A antibody labeled the protein solely on the plasma membrane (red). Superposition of the two images confirms that 5-HT2A receptors were expressed on the cell membranes of the HEK cells.
Figure 5. The effect of taurine on HEK cells expressing 5-HT2A receptors.
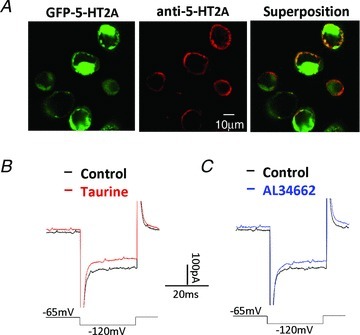
A, confocal images of HEK cells expressing GFP-tagged 5-HT2A receptors (green), and labeled with anti-5-HT2A receptors (red). Superposition of the images shows that 5-HT2A receptors are expressed in the plasma membrane of the transfected HEK cells. B, whole-cell recording with 80 μm taurine in the Cd(I) solution partially suppressed the endogenous inward rectifier (KIR) currents at −120 mV in a HEK cell transfected with 5-HT2A receptors. C, AL34662 mimicked the effect of taurine on the HEK cell.
The action of taurine on the 5-HT2A receptors in HEK cells was again tested in the Cd(I) solution. The inward rectifier KIR channels, endogenously expressed in HEK cells (Fig. 5B, black trace), were suppressed by 80 μm taurine (Fig. 5B, red trace). Similarly, Fig. 5C shows that the taurine response could be mimicked by the non-selective 5-HT2 receptor agonist AL34662. Approximately 20% of the currents were suppressed by AL34662 (n = 7) and taurine (n = 11). In HEK cells in which the 5-HT2A receptor was not expressed, neither AL34662 nor taurine affected the inward rectifier KIR currents (n = 16, data not shown).
Discussion
This study shows for the first time that taurine activates 5-HT2A receptors, which, via a metabotropic intracellular pathway, increases the currents through delayed rectifier KV channels in retinal neurons. In the vertebrate retina, these channels are essential for the generation of APs in amacrine and ganglion cells. Although in some systems it appears that taurine acts on metabotropic GABAB receptors (Kontro & Oja, 1990; Smith & Li, 1991), the GABAB receptor antagonist CGP55845 had no effect on the response to taurine. In contrast, MDL11939, a potent and selective antagonist of the 5-HT2A serotonin receptor, was found to significantly block the effect of taurine.
Overall, the pharmacological results indicate that the metabotropic taurine response is via an intracellular G-protein-mediated pathway involving PLC, PKC and tyrosine kinase. These features correspond to the regulatory pathway by which serotonin regulates KV channels in pulmonary artery smooth muscle through 5-HT2A receptors (Varghese et al. 2006). Although a PKC-sensitive cascade represents the major intracellular pathway for taurine enhancement of delayed rectifier KV channels, we found that inhibition of PKA increases the taurine effect on the channel currents. Thus, a PKA-mediated pathway may represent a negative control mechanism for taurine regulation in amacrine and ganglion cells.
Mechanistic studies of channel regulation will contribute to our understanding of how the myriad KV channels respond to physiological inputs in neural signaling. The KV family, with 12 known subfamilies, is widely expressed in the CNS, including retina. Among these, the delayed rectifier K+ channels, assembled by KV1 and KV2, are the major K+ channels that directly influence the firing rates of APs. The responses to MgTX and ScTx-1 revealed that KV1, KV2 and KV4 subtypes of delayed rectifier KV channels are the principal sustained K+ channels in amacrine and ganglion cells.
There is evidence that PKC phosphorylation of KV1 and KV2 channels is a feature of delayed rectifier KV channels in various cell types (Cai & Douglass, 1993; Murakoshi et al. 1997; Boland & Jackson, 1999; Strauss et al. 2002), and that tyrosine kinase directly interacts with these channels (Huang et al. 1993; Holmes et al. 1996). Our findings show that these internal proteins are critical for the metabotropic regulation of KV channels by taurine in retinal neurons. It is noteworthy that most amacrine cells have at least two subtypes of KV channel that can be blocked by MgTX and ScTx-1. However, there are other ganglion cells that are sensitive only to ScTx-1, suggesting that different neuronal subtypes express a unique set of KV channels, which probably subserve distinct physiological functions and allow the neurons to control their unique pattern of excitability (Leung, 2010).
As mentioned earlier, taurine has been shown to be a major cytoprotectant, and essential for the normal function of heart, kidney, pancreas and other organs. In addition, taurine supplementation has been found useful for various brain disorders, for example epilepsy and addiction (Wu et al. 2005; Junyent et al. 2009; Olive, 2010). Clearly, it would be of significant interest to know the mechanisms of the action of taurine in the CNS. The present findings suggest that its effects may be mediated by 5-HT2A receptors, one of the various serotonin receptor subtypes that have been extensively studied as targets for the development of antipsychotic drugs.
Acknowledgments
The authors thank Dr Changlong Nan for guidance in the use of the HEK-cell expression system. This study was supported by research grants from the National Science Foundation (IOS-1021646, W.S.), and the National Eye Institute, NIH (EY14161, W.S.).
Glossary
- AP
action potential
- GFAP
glial fibrillary acidic protein
- HEK
human embryonic kidney
- IPL
inner plexiform layer
- KV channel
voltage-gated potassium channel
- MgTX
margatoxin
- PCR
polymerase chain reaction
- PKA
protein kinase A
- PKC
protein kinase C
- PLC
phospholipase C
- ScTx-1
stromatoxin-1
- TTX
tetrodotoxin
Author contributions
S.B., H.R. and W.S. were involved in the conception and design of the experiments, and in the collection and analysis of data; S.B. conducted the major experiments depicted in Figs 1–4. W.S. conducted the experiments in Figs 1D, 5B and C. Y.L. performed the experiments of Figs 4C and D, 5A. W.S. and H.R. wrote the manuscript. The manuscript was read and approved by all the authors. The experiments were conducted in the Department of Biomedical Science, Charles E Schmidt College of Medicine at Florida Atlantic University.
Author's present address
S. Bulley: Department of Physiology, University of Tennessee Health Science Center, Memphis, TN 38163, USA. Email: sbulley@uthsc.edu
Supplementary material
Supplemental figures
References
- Boland LM, Jackson KA. Protein kinase C inhibits Kv1.1 potassium channel function. Am J Physiol Cell Physiol. 1999;277:C100–C110. doi: 10.1152/ajpcell.1999.277.1.C100. [DOI] [PubMed] [Google Scholar]
- Bulley S, Shen W. Reciprocal regulation between taurine and glutamate response via Ca2+-dependent pathways in retinal third-order neurons. J Biomed Sci. 2010;17(Suppl 1):S5. doi: 10.1186/1423-0127-17-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai YC, Douglass J. In vivo and in vitro phosphorylation of the T lymphocyte type n (Kv1.3) potassium channel. J Biol Chem. 1993;268:23720–23727. [PubMed] [Google Scholar]
- Cohen AI, McDaniel M, Orr H. Absolute levels of some free amino acids in normal and biologically fractionated retinas. Invest Ophthalmol. 1973;12:686–693. [PubMed] [Google Scholar]
- Fletcher EL, Kalloniatis M. Neurochemical architecture of the normal and degenerating rat retina. J Comp Neurol. 1996;376:343–360. doi: 10.1002/(SICI)1096-9861(19961216)376:3<343::AID-CNE1>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Fox MA, Stein AR, French HT, Murphy DL. Functional interactions between 5-HT2A and presynaptic 5-HT1A receptor-based responses in mice genetically deficient in the serotonin 5-HT transporter (SERT) B J Pharmacol. 2010;159:879–887. doi: 10.1111/j.1476-5381.2009.00578.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillette MA, Dacheux RF. Protein kinase modulation of GABAA currents in rabbit retinal rod bipolar cells. J Neurophysiol. 1996;76:3070–3086. doi: 10.1152/jn.1996.76.5.3070. [DOI] [PubMed] [Google Scholar]
- Heller-Stilb B, van Roeyen C, Rascher K, Hartwig HG, Huth A, Seeliger MW, Warskulat U, Haussinger D. Disruption of the taurine transporter gene (taut) leads to retinal generation in mice. FASEB J. 2002;16:231–233. doi: 10.1096/fj.01-0691fje. [DOI] [PubMed] [Google Scholar]
- Holmes TC, Fadool DA, Levitan IB. Tyrosine phosphorylation of the Kv1.3 potassium channel. J Neurosci. 1996;16:1581–1590. doi: 10.1523/JNEUROSCI.16-05-01581.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XY, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G protein coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- Hussy N, Deleuze C, Pantaloni A, Michel G, Desarmenien M, Moos F. Agonist action of taurine on glycine receptors in rat supraoptic magnocellular neurones: possible role in osmoregulation. J Physiol. 1997;502:609–621. doi: 10.1111/j.1469-7793.1997.609bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose T, Shields CR, Lukasiewics PD. Sodium channels in transient retinal bipolar cells enhance visual responses in ganglion cells. J Neurosci. 2005;25:1856–1865. doi: 10.1523/JNEUROSCI.5208-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Yue M, Chandra D, Keramidas A, Goldstein PA, Homanics GE, Harrison NL. Taurine is a potent activator of extrasynaptic GABAA receptors in the thalamus. J Neurosci. 2008;28:106–115. doi: 10.1523/JNEUROSCI.3996-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junyent F, Utrera J, Romero R, Pallàs M, Camins A, Duque D, Auladell C. Prevention of epilepsy by taurine treatments in mice experimental model. J Neurosci Res. 2009;87:1500–1508. doi: 10.1002/jnr.21950. [DOI] [PubMed] [Google Scholar]
- Kennedy AJ, Voaden MJ. Studies on the uptake and release of radioactive taurine by the frog retina. J Neurochem. 1976;27:131–137. doi: 10.1111/j.1471-4159.1976.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Kontro P, Oja SS. Interactions of taurine with GABAB binding sites in mouse brain. Neuropharmacology. 1990;29:243–247. doi: 10.1016/0028-3908(90)90008-f. [DOI] [PubMed] [Google Scholar]
- Kurien BT, Dorri Y, Dillon S, Dsouza A, Scofield RH. An overview of Western blotting for determining antibody specificities for immunohistochemistry. Methods Mol Biol. 2011;717:55–67. doi: 10.1007/978-1-61779-024-9_3. [DOI] [PubMed] [Google Scholar]
- Leung YM. Voltage-gated K+ channel modulators as neuroprotective agents. Life Sci. 2010;86:775–780. doi: 10.1016/j.lfs.2010.04.004. [DOI] [PubMed] [Google Scholar]
- Marc RE, Murry RF, Basinger SF. Pattern recognition of amino acid signatures in retinal neurons. J Neurosci. 1995;15:5106–5129. doi: 10.1523/JNEUROSCI.15-07-05106.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina JH, De Robertis E. Taurine modulation of the benzodiazepine-gamma-aminobutyric acid receptor complex in brain membranes. J Neurochem. 1984;42:1212–1217. doi: 10.1111/j.1471-4159.1984.tb02774.x. [DOI] [PubMed] [Google Scholar]
- Murakoshi H, Shi G, Scannevin RH, Trimmer JS. Phosphorylation of the Kv2.1 K+ channel alters voltage-dependent activation. Mol Pharmacol. 1997;52:821–828. doi: 10.1124/mol.52.5.821. [DOI] [PubMed] [Google Scholar]
- Olive ME. Pharmacotherapies for alcoholism: the old and the new. CNS Neurol Disord Drug Targets. 2010;9:2–4. doi: 10.2174/187152710790966722. [DOI] [PubMed] [Google Scholar]
- Orr HT, Cohen AI, Lowry OH. The distribution of taurine in the vertebrate retina. J Neurochem. 1976;26:609–611. doi: 10.1111/j.1471-4159.1976.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Pasantes-Morales H, Domínguez L, Campomanes MA, Pacheco P. Retinal degeneration induced by taurine deficiency in light-deprived cats. Exp Eye Res. 1986;43:55–60. doi: 10.1016/s0014-4835(86)80045-8. [DOI] [PubMed] [Google Scholar]
- Sah P, Davies P. Calcium-activated potassium currents in mammalian neurons. Clin Exp Pharmacol Physiol. 2000;27:657–663. doi: 10.1046/j.1440-1681.2000.03317.x. [DOI] [PubMed] [Google Scholar]
- Shen W, Slaughter MM. Metabotropic GABA receptors facilitate L-type and inhibit N-type calcium channels in single salamander retinal neurons. J Physiol. 1999;516:711–718. doi: 10.1111/j.1469-7793.1999.0711u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Li J. GABAB receptor stimulation by baclofen and taurine enhances excitatory amino acid induced phosphatidylinositol turnover in neoanatal rat cerebellum. Neurosci Lett. 1991;132:59–64. doi: 10.1016/0304-3940(91)90433-t. [DOI] [PubMed] [Google Scholar]
- Strauss O, Rosenthal R, Dey D, Beninde J, Wollmann G, Thieme H, Wiederbolt M. Effects of protein kinase C on delayed rectifier K+ channel regulation by tyrosine kinase in rat retinal pigment epithelial cells. Invest Ophthal Vis Sci. 2002;43:1645–1654. [PubMed] [Google Scholar]
- Varghese A, Hong Z, Wier ED. Serotonin-induced inhibition of Kv current. Circ Res. 2006;98:860–862. doi: 10.1161/01.RES.0000219683.65556.74. [DOI] [PubMed] [Google Scholar]
- Wu H, Jin Y, Wei J, Jin H, Sha D, Wu JY. Mode of action of taurine as a neuroprotector. Brain Res. 2005;1038:123–131. doi: 10.1016/j.brainres.2005.01.058. [DOI] [PubMed] [Google Scholar]
- Xu H, Zhou KQ, Huang YN, Chen L, Xu TL. Taurine activates strychnine-sensitive glycine receptors in neurons of the rat inferior colliculus. Brain Res. 2004;1021:232–240. doi: 10.1016/j.brainres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Young TL, Cepko CL. A role for ligand-gated ion channels in rod photoreceptor development. Neuron. 2004;41:867–879. doi: 10.1016/s0896-6273(04)00141-2. [DOI] [PubMed] [Google Scholar]
- Zulli A. Taurine in cardiovascular disease. Curr Opin Clin Nutr Metab Care. 2011;14:57–60. doi: 10.1097/MCO.0b013e328340d863. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


