Abstract
While substantial alterations in myelination and axonal growth have been described during maturation, their interactions with the configuration and activity of axonal membrane ion channels to achieve impulse conduction have not been fully elucidated. The present study utilized axonal excitability techniques to compare the changes in nerve function across healthy infants, children, adolescents and adults. Multiple excitability indices (stimulus–response curve, strength–duration time constant, threshold electrotonus, current–threshold relationship and recovery cycle) combined with conventional neurophysiological measures were investigated in 57 subjects (22 males, 35 females; age range 0.46–24 years), stimulating the median motor nerve at the wrist. Maturational changes in conduction velocity were paralleled by significant alterations in multiple excitability parameters, similarly reaching steady values in adolescence. Maturation was accompanied by reductions in threshold (P < 0.005) and rheobase (P= 0.001); depolarizing and hyperpolarizing electrotonus progressively reduced (P < 0.001), or ‘fanned-in’; resting current–threshold slope increased (P < 0.0001); accommodation to depolarizing currents prolonged (P < 0.0001); while greater threshold changes in refractoriness (P= 0.001) and subexcitability (P < 0.01) emerged. Taken together, the present findings suggest that passive membrane conductances and the activity of K+ conductances decrease with formation of the axo-glial junction and myelination. In turn, these functional alterations serve to enhance the efficiency and speed of impulse conduction concurrent with the acquisition of motor skills during childhood, and provide unique insight into the evolution of postnatal human peripheral nerve function. Significantly, these findings bring the dynamics of axonal development to the clinical domain and serve to further illuminate pathophysiological mechanisms that occur during development.
Key points
The evolution of human peripheral nerve function after birth to facilitate more complex neural tasks has not been fully elucidated.
The present study has established the changes that occur in nerve function in developing humans using specialized non-invasive excitability techniques in infants, children, adolescents and young adults for the first time.
The activity of axonal K+ conductances reduces with formation of the axo-glial junction. This occurs simultaneously with alterations in passive membrane properties and conductance (axonal diameter and myelination).
These functional alterations serve to enhance the efficiency and speed of impulse conduction, whilst maintaining membrane stability, concurrent with the acquisition of motor skills in childhood.
Significantly, these findings bring the dynamics of axonal development to the clinical domain and serve to further illuminate pathophysiological mechanisms that occur during development.
Introduction
The intricate structure and molecular organization of the human myelinated axon is important in determining the efficient conduction of nerve impulses. Development of the peripheral nerve commences between 4 and 6 weeks gestation in humans, with nerve fibres growing out from neuroblasts and neural crest cells in the spinal cord and dorsal root ganglia (Sadler, 1990). Schwann cells migrate from the neural crest to sites adjacent to nerve fibres, and myelination begins at about 15 weeks of gestation.
Histopathological studies have described the postnatal development of peripheral nerves, with a doubling of axonal diameter between 5 months and 5 years (Schroder et al. 1978; Jacobs & Love, 1985). While myelin thickness is related to axonal diameter, it increases proportionately more than axonal caliber, by a factor of 2.5 times initial postnatal values until between 5 and 14 years old (Gutrecht & Dyck, 1970; Schroder et al. 1978; Jacobs & Love, 1985). After myelination has commenced, some peripheral nerves elongate by more than a factor of four, with internodal lengths increasing until the second decade (Jacobs & Love, 1985).
As the speed of impulse conduction is related to the diameter of the largest myelinated fibres (Rushton, 1951; Waxman, 1980), these morphological changes coincide with marked increases in conduction velocities. The motor conduction velocities in a full-term infant are approximately half those of adult values, increasing to approach adult values at slightly over the age of 4 years (Gamstorp, 1963; Baer & Johnson, 1965; Wagner & Buchthal, 1972; Lang et al. 1985). A further gradual increase until 16 years has been variably observed (Baer & Johnson, 1965).
Molecular interactions between myelinating glial cells, axonal membrane and cytoskeletal proteins have recently been shown to have prominent roles in the molecular specialization of the axon (Susuki & Rasband, 2008; Thaxton et al. 2011). Distinct nodal and juxtaparanodal regions are produced, physically separated by axo-glial junctions at the paranode. Importantly, the clustering of voltage-gated Na+ channels at nodes of Ranvier enables efficient and rapid propagation of impulses (Hille, 1972; Scholz et al. 1993; Waxman & Ritchie, 1993). In mature myelinated axons, fast K+ channels are concentrated under the myelin at the juxtaparanode and may limit re-excitation of the node following conduction of an action potential or participate in the generation of the internodal resting potential (Baker et al. 1987; Waxman & Ritchie, 1993).
The configuration and activity of a variety of ion channels, exchangers and pumps activated during impulse conduction in maturing human peripheral axons and their interactions with myelination and axonal growth have not yet been elucidated. While nerve conduction studies provide very limited information about the physiology and function of such ion channels, axonal excitability techniques have been established in adults that provide insights into axonal ion channel function in health and disease (Krishnan et al. 2009). As such, the present study utilized in vivo axonal excitability studies in healthy infants, children, adolescents and young adults for the first time to provide insight into the maturation of axonal biophysical properties that develop with growth.
Methods
Conventional nerve conduction and specialized axonal excitability studies were undertaken in 57 subjects (22 males, 35 females; age range 0.46–24 years). All subjects gave written informed consent, assent or parental consent to the procedures, in accordance with the Declaration of Helsinki, which were approved by the South Eastern Sydney and Illawarra Area Health Service Human Research Ethics Committee.
No subject had a history of illness known to be associated with neurological dysfunction, or medication use known to potentially affect axonal excitability; for example, anticonvulsants, local anaesthetics or anti-arrhythmics (Krishnan et al. 2009). Neurophysiological studies were undertaken at the conclusion of planned medical procedures in 22 children (age range 0.46–4.9 years) admitted to the Sydney Children's Hospital day unit for sedation. The majority of admissions (78%) were for follow up of previous urinary tract infection. Serum electrolytes and creatinine and renal function were normal on laboratory measurement. Children were sedated with oral chloral hydrate ± intramuscular morphine and droperidol according to standard protocol. These medications are not known to affect peripheral nerve function or excitability. A further 35 healthy subjects (age range 4.5–24 years) were recruited from the community, and studies undertaken whilst conscious with distraction and play therapy. Mean temperature was 32.9 ± 0.2°C and did not demonstrate significant changes across different cohorts.
Axonal excitability studies were performed using previously described threshold tracking protocols applied to the median nerve in adults (Kiernan et al. 2000). The compound motor action potential (CMAP) was recorded using surface electrodes (4620M; Unomedical Ltd, Birkerød, Denmark) positioned over the abductor pollicis brevis muscle, with the active electrode at the motor point and the reference electrode distal over the tendon insertion. An electrosurgical neutral earth plate (2406M; Unomedical Ltd) was placed in the palm, with Redux electrolyte creme (Parker Laboratories, Fairfield, USA). Bipolar electrodes were utilized to locate the optimal stimulation site of the median nerve at the wrist for each patient. The cathode was located over the median nerve at the wrist and the anode 5–10 cm proximally, depending on arm size. Median nerve motor conduction velocities were calculated following proximal stimulation at the elbow (Medelec Synergy System, Oxford Instruments, Oxfordshire, UK; Kimura, 1983). Skin was prepared with Nuprep abrasive skin prepping gel (Weaver and Company, Aurora, USA).
Data acquisition and stimulation delivery were controlled by QTRACS software (Institute of Neurology, UK). Recordings of CMAP were amplified and filtered (3 Hz–3 kHz) using a Medelec Sapphire 4ME amplifier (Medelec AA6 MK III, Surrey, UK), with electronic noise removed (Hum Bug 50/60 Hz Noise Eliminator, Quest Scientific Instruments, North Vancouver, Canada) and digitized by computer via a data acquisition device (DAQ PCI-6221; Shielded connector block BNC-2110; Cable SHC-68-68-EPM; National Instruments, Austin, USA). Electrical stimulation was converted to current using an isolated linear bipolar constant current simulator (maximal output 50 mA; DS5, Digitimer, Welwyn Garden City, UK). Temperature was measured with a surface probe at the wrist (Digitech, Jaycar, Rydalmere, Australia). The amplitude of the CMAP was measured from baseline to negative peak and the threshold tracking target set to 40% of the maximum, utilizing the area of steepest slope of the stimulus–response curve. Threshold tracking follows the changes in the intensity of a test stimulus required to produce the target potential. Excitability testing incorporated a number of assessments designed to reflect different nodal and internodal properties, from which various excitability measures could be extracted for analysis. These included the following.
Stimulus–response relationship
To commence the protocol, a stimulus–response curve was generated by increasing the stimulus intensity in a stepwise fashion from zero until maximal CMAP amplitude was achieved. The following parameters were measured: (i) the stimulus intensity (mA), defined as the current required to elicit a target response set to 50% of maximal CMAP for a stimulus of 1 ms duration; (ii) stimulus/response slope.
Strength–duration relationship
The strength–duration relationship was recorded by tracking the threshold to a stimulus as it is reduced from 1 ms to 0.2 ms duration. The measurements generate the charge–duration plot, in which the points are almost linear, of which the slope indicates the rheobase and the intercept on the x-axis indicates the strength–duration time constant (TSD). TSD is an apparent membrane time constant representing the relationship between stimulus intensity and width, as described by Weiss’ formula (Weiss, 1901; Bostock, 1983; Mogyoros et al. 1996). Rheobase is defined as the threshold current required to produce a target response for a stimulus of infinitely long duration (Bostock et al. 1998).
Threshold electrotonus (TE) and current–threshold (I–V) relationship
In this part, threshold tracking was used to record the changes in excitability in response to prolonged 100 ms subthreshold polarizing currents, set to ±40% of the control (1 ms) threshold current (Bostock et al. 1998; Kiernan et al. 2000). The changes in threshold at different time intervals before, during and after the conditioning stimuli were measured. TE is used to provide information about internodal properties and conductances in addition to an estimate of resting membrane potential. The recorded TE was plotted in conventional format as threshold reduction, so that the response to depolarizing current was plotted upwards (Bostock & Baker, 1988; Bostock et al. 1998). Depolarizing TE threshold change was assessed at multiple intervals, in particular, as the average between 10 and 20 ms (TEd (10–20)), and 90 and 100 ms (TEd (90–100)). The parameter accommodation half-time was measured as the time from the start of the 40% depolarizing current until the threshold reduction returned to half way between the peak and plateau levels. The threshold change to the 40% hyperpolarizing current was also assessed at multiple intervals, as the average between 10 and 20 ms (TEh (10–20)), and 90 and 100 ms (TEh (90–100)).
The current–threshold relationship is comparable to TE and reflects the rectifying properties of the axon. The I–V relationship was assessed by measuring the change in threshold following the injection of polarizing currents of 200 ms duration, the strength of which was altered in 10% steps from +50% (depolarizing) to −100% (hyperpolarizing) of the control 1 ms threshold. Similarly, the I–V relationship was plotted in conventional format as threshold reduction, so that the response to depolarizing current was plotted upwards. From the I–V graph, the following parameters were recorded: (i) resting I–V slope, calculated from polarizing currents between +10% to −10%; (ii) hyperpolarizing I–V slope, calculated from polarizing current between 0 and 100%.
Recovery cycle
This protocol records the excitability changes occurring at various interstimulus intervals, decreasing from 200 ms to 2.5 ms, after a supramaximal conditioning stimulus (Kiernan et al. 1996, 2000). Three stimulus combinations were recorded: (i) the control 1 ms threshold; (ii) supramaximal conditioning stimulus alone; (iii) conditioning and tracking test stimuli in combination. The response in (ii) was subtracted on-line from the response in (iii) so that only the response to the second stimulus was measured. The recovery cycle normally includes three phases: refractoriness; superexcitable period; and the late subexcitable phase. The following parameters were measured: (i) refractoriness, which reflects the inactivation of nodal voltage-gated sodium (Na+) channels following an impulse, was measured as the percentage threshold increase at the 2.5 ms interstimulus interval; (ii) superexcitability (%), defined as the largest mean reduction in threshold of three adjacent points, peaking at a conditioning-test interval of 5–15 ms; (iii) subexcitability (%), defined as the largest increase in threshold after an interstimulus interval of 15 ms.
Data analysis
Neurophysiological results were summarized across four groups: late infancy and early childhood, 0.5–3 years; late childhood, 3–8 years; adolescence, 8–15 years; and young adulthood, 15–25 years. These groups and age ranges were selected as they were similar to previous conventional neurophysiological studies on the evolution of nerve conduction velocity (Gamstorp, 1963; Lang et al. 1985). All results were expressed as mean ± standard error of the mean (SEM). Regression analyses and curve estimation were performed using SPSS 20 for Windows XP (SPSS Inc., Chicago, IL, USA) to determine whether age-related changes were best fitted by a linear or logarithmic function. Where more than one function was significant, the one with the higher R2 value has been reported. The one-way ANOVA test was used to compare mean differences between groups. Correlations between excitability measures and motor conduction velocity were analysed by Spearman's rank correlation coefficient. A probability (P) value of <0.05 was considered statistically significant.
Mathematical computer model of excitability
To model the excitability changes in motor axons with maturation and the effects of altered axonal conductances and passive membrane properties, mathematical simulations were undertaken using a model of the human axon (Bostock et al. 1991, 1995; Kiernan et al. 2005). Transient Na+ channels were modelled using the voltage-clamp data (Schwarz et al. 1995), and persistent Na+ currents were added (Bostock & Rothwell, 1997). The equations for a single node and internode, representing a spatially uniform axon, were assessed by integration over successive small time steps (Euler's method; Press et al. 1992; Kiernan et al. 2005; Lin et al. 2008; Boland et al. 2009; Farrar et al. 2011). At times corresponding to those in human nerve excitability recordings, the excitability of the model nerve was tested repeatedly to determine threshold with an accuracy of 0.5%. The discrepancy between the thresholds determined for the model and those determined from a sample of real nerves was scored as the weighted sum of the error terms: [(xm−xn)/sn]2, where xm is the threshold of the model, xn the mean and sn the standard deviation of the thresholds for the real nerves. The weights were the same for all threshold measurements of the same type (e.g. recovery cycle), and chosen to give an equal total weight to the different types of threshold measurement: current–threshold relationship, TE and the recovery cycle. The standard model was obtained by minimizing the discrepancy between the model and the young adulthood data with an iterative least squares procedure, so that alteration of any of the above parameters would make the discrepancy worse.
Results
Conventional neurophysiological assessment
Motor conduction velocity increased from 40.6 ± 2.3 m s−1 during late infancy and early childhood to reach mean maximal values of 55.6 ± 0.9 m s−1 at 8–15 years, and remained constant thereafter (Table 1; Fig. 1; logarithmic function, R2= 0.74, P < 0.0001). The most rapid increase in motor conduction velocity was observed during late infancy and early childhood, while smaller increases occurred during late childhood. Adult values (greater than 50 m s−1) were reached between 3 and 5 years. Similarly, CMAP amplitude increased from 4.3 ± 0.3 mV during late infancy and early childhood to 8.4 ± 0.5 mV in adolescence, then became relatively constant with age (Fig. 2; logarithmic function, R2= 0.43, P < 0.0001). The mean temperature was 32.9 ± 0.2°C and did not significantly differ between groups (P= 0.2).
Table 1.
Demographic and conventional neurophysiological measures
| Late infancy and early childhood | Late childhood | Adolescence | Young adulthood | Change with age | |
|---|---|---|---|---|---|
| Age range (years) | 0.5–3 | 3–8 | 8–15 | 15–25 | |
| Number | 13 | 12 | 19 | 13 | |
| Mean age ± SEM | 1.6 ± 0.2 | 4.4 ± 0.3 | 10.3 ± 0.4 | 19.4 ± 1.1 | |
| Sex ratio M:F | 4:9 | 6:6 | 11:8 | 5:8 | |
| CMAP amplitude (mV) | 4.3 ± 0.3 | 6.4 ± 0.7 | 8.4 ± 0.5 | 8.6 ± 0.6 | Logarithmic R2= 0.43, P < 0.0001 |
| Median nerve MCV (m s−1) | 40.6 ± 2.3 | 50.9 ± 1.9 | 55.6 ± 0.9 | 57.2 ± 0.4 | Logarithmic R2= 0.74, P < 0.0001 |
| Temperature (°C) | 32.7 ± 0.4 | 32.5 ± 0.3 | 33.2 ± 0.3 | 33.2 ± 0.3 | NS |
Values are represented as mean ± SEM. CMAP, compound motor action potential; MCV, motor conduction velocity.
Figure 1.
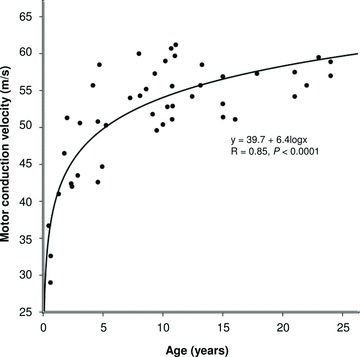
Median nerve motor conduction velocity changes with age
Figure 2.
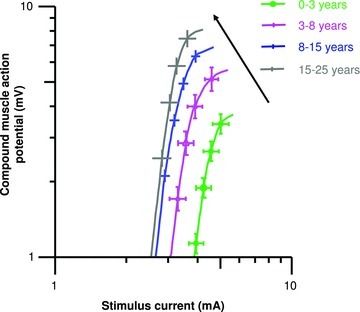
Mean stimulus–response relations for healthy subjects grouped by chronological age
Measures of axonal excitability
Multiple measures of excitability showed significant changes with age, paralleling the maturation of motor conduction velocity, with the greatest changes over younger ages (Table 2).
Table 2.
Measures of axonal excitability for each age group
| Late infancy and early childhood (0.5–3 years) | Late childhood (3–8 years) | Adolescence (8–15 years) | Young adulthood (15–25 years) | Change with age | |
|---|---|---|---|---|---|
| Mean threshold 50% CMAP (mA) | 4.1 ± 1.1 | 3.6 ± 1.1 | 3.2 ± 1.0 | 3.1 ± 1.1 | Logarithmic R2= 0.14, P < 0.005 |
| TSD (ms) | 0.33 ± 0.02 | 0.42 ± 0.03 | 0.39 ± 0.01 | 0.39 ± 0.03 | Logarithmic R2= 0.05, P= 0.09 |
| Rheobase (mA) | 3.1 ± 1.1 | 2.4 ± 1.1 | 2.2 ± 1.1 | 2.1 ± 1.1 | Logarithmic R2= 0.2, P= 0.001 |
| TEd40 (10–20 ms) (%) | 74.0 ± 1.3 | 72.1 ± 1.4 | 66.0 ± 1.0 | 65.0 ± 1.6 | Logarithmic R2= 0.32, P < 0.0001 |
| Accommodation half-time (ms) | 31.8 ± 0.7 | 35.3 ± 1.0 | 42.8 ± 0.7 | 43.0 ± 1.3 | Logarithmic R2= 0.61, P < 0.0001 |
| TEd40 (90–100 ms) (%) | 50.2 ± 1.3 | 47.7 ± 1.0 | 42.4 ± 0.7 | 43.9 ± 1.1 | Logarithmic R2= 0.29, P < 0.0001 |
| TEh40 (10–20 ms) (%) | −99.8 ± 3.1 | −86.6 ± 2.9 | −73.4 ± 1.6 | −68.4 ± 2.2 | Logarithmic R2= 0.62, P < 0.0001 |
| TEh40 (90–100 ms) (%) | −181.0 ± 10.7 | −145.8 ± 8.1 | −108.8 ± 3.8 | −108.0 ± 5.0 | Logarithmic R2= 0.50, P < 0.0001 |
| Resting I–V slope | 0.50 ± 0.03 | 0.53 ± 0.02 | 0.66 ± 0.02 | 0.64 ± 0.03 | Logarithmic R2= 0.33, P < 0.0001 |
| Hyperpolarizing I–V slope | 0.43 ± 0.02 | 0.42 ± 0.04 | 0.38 ± 0.01 | 0.31 ± 0.01 | Logarithmic R2= 0.24, P < 0.001 |
| RRP (ms) | 2.4 ± 1.0 | 2.6 ± 1.1 | 3.0 ± 1.0 | 3.0 ± 1.0 | Logarithmic R2= 0.27, P < 0.0001 |
| Refractoriness at 2.5 ms (%) | −10.2 ± 2.0* | 2.7 ± 5.6 | 24.3 ± 4.6 | 21.6 ± 3.6 | Logarithmic R2= 0.27, P= 0.001 |
| Peak superexcitability (%) | −25.7 ± 0.9 | −24.0 ± 1.4 | −24.8 ± 1.0 | −25.8± 1.0 | NS |
| Subexcitability (%) | 8.8 ± 1.1 | 11.0 ± 1.1 | 12.8 ± 0.7 | 13.0 ± 1.0 | Logarithmic R2= 0.13, P < 0.01 |
Values are mean ± SEM. CMAP, compound motor action potential; TSD, strength–duration time constant; RRP, relative refractory period; TEd(10–20), depolarizing change at 10–20 ms in threshold electrotonus; TEd(90–100), depolarizing change at 90–100 ms; TEh(10–20), hyperpolarizing change at 10–20 ms; TEh(90–100), hyperpolarizing change at 90–100 ms. *Refractoriness was not present at the 2.5 ms interstimulus interval as a mean threshold reduction was observed.
Changes in stimulus–response and strength–duration relationships with maturation
The stimulus threshold reduced significantly with age, becoming relatively constant during adolescence, indicating that more current was required to excite axons at younger ages (Table 2; Fig. 2). In addition, the stimulus–response slope did not significantly change with age, confirming a consistent reduction in threshold with age for axons of both low and high thresholds (late infancy and early childhood, 5.3 ± 1.1; late childhood, 4.4 ± 1.1; adolescence, 4.6 ± 1.1; young adulthood, 5.5 ± 1.1, P= 0.9). The reduction in current threshold was associated with a reduction in rheobase with age (Table 2). Again, the greatest reduction in rheobase was during late infancy and early childhood, becoming steady by adolescence. While TSD, an indirect measure of nodal persistent Na+ conductances (Bostock & Rothwell, 1997), did not significantly change with age (P= 0.09), the previously established negative correlation with rheobase was maintained (R=−0.4, P < 0.005; Mogyoros et al. 1998).
Changes in TE and current–threshold relationship with maturation
TE waveforms showed significant changes with age, and again the most prominent occurred over younger ages (Table 2; Fig. 3). Early and late depolarizing electrotonus responses progressively reduced until adolescence, and accommodation to depolarizing currents was markedly faster at younger ages (accommodation half-time). In the same way, hyperpolarizing electrotonus recordings at younger ages demonstrated greater threshold changes. Together the more prominent changes in TE waveforms at younger ages have previously been described as ‘fanning-out’, related to their resemblance to a Japanese fan (Kaji, 1997). In addition, the ratio of TEd(10–20) to TEh(10–20) increased with maturation (logarithmic function, R= 0.79, P < 0.0001; Fig. 3F), suggesting simultaneous alterations in several distinct biophysical properties of the axon (see Discussion).
Figure 3. Comparison of TE measures with age.
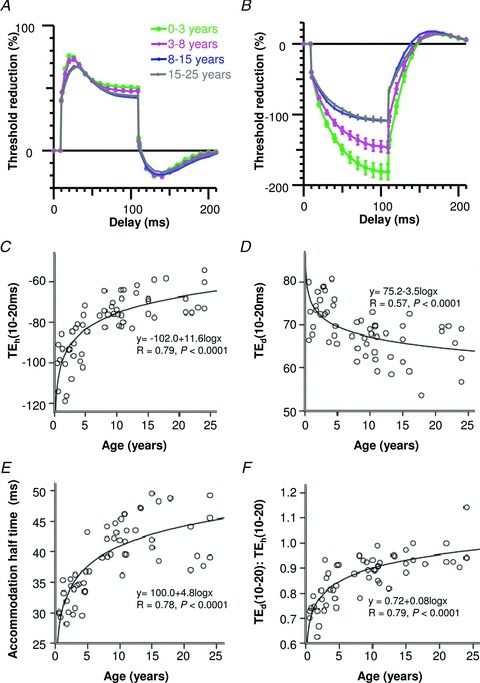
A, depolarizing TE curves for healthy subjects grouped by age. B, hyperpolarizing TE curves for healthy subjects grouped by age. C, hyperpolarizing changes at 10–20 ms (TEh(10–20 ms)) reduced with age, P < 0.0001. D, depolarizing changes at 10–20 ms (TEd(10–20 ms)) reduced with age, P < 0.0001. E, accommodation half-time increased with age, P < 0.0001. F, the ratio of TEd(10–20) to TEh(10–20) increased with age, P < 0.0001.
Similar to the ‘fanning-in’ of TE waveforms with age, the I–V relationship showed reductions in threshold changes for both depolarization and hyperpolarization with age (Table 2; Fig. 4). Additionally, the slope of the I–V curve at low current strengths, termed resting I–V slope, increased with age.
Figure 4.
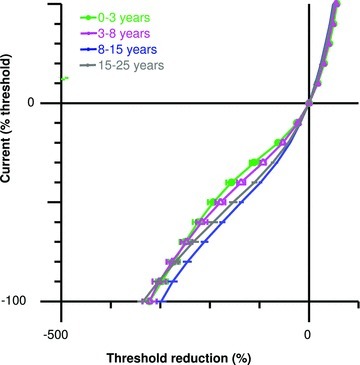
Comparison of current–threshold relationship for healthy subjects grouped by chronological age
Changes in recovery cycle with maturation
The recovery cycle showed significant changes in membrane excitability in response to a supramaximal conditioning stimulus with age (Table 2; Fig. 5). Notably refractoriness, the initial phase of the recovery cycle in which threshold increases related to the inactivation of transient Na+ channels, was not observed at the 2.5 ms interstimulus in one-third of the youngest subjects during late infancy and early childhood with threshold reductions occurring. Refractoriness and subexcitability similarly showed significant increases with age, while peak superexcitability did not significantly change. The greatest changes occurred during late infancy and early childhood, and excitability measures stabilized during adolescence.
Figure 5. Comparison of recovery cycle of excitability measures with age.
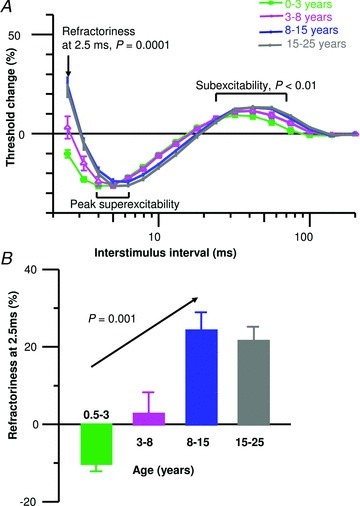
A, recovery cycle of excitability curves. B, mean group data illustrating that refractoriness at 2.5 ms significantly increased with age; refractoriness was not present for the youngest group as a mean threshold reduction was observed.
Correlations with conduction velocity
Combining measures of axonal excitability and motor conduction velocity, it was evident that specific excitability parameters were significantly associated with increases in conduction velocity (Fig. 6); including reductions in TEh(10–20) (R= 0.67, P < 0.0001) and TEh(90–100) (R= 0.63, P < 0.0001), and increases in accommodation half-time (R= 0.56, P < 0.0001).
Figure 6. The relationships between axonal excitability parameters and motor conduction velocity.
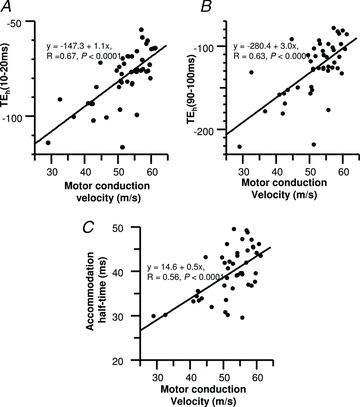
A, threshold changes to subthreshold 40% hyperpolarizing currents at 10–20 ms (TEh(10–20 ms)). B, threshold changes to subthreshold 40% hyperpolarizing currents at 90–100 ms (TEh(90–100 ms)). C, accommodation half-time.
Mathematical modelling of altered excitability properties with maturation
To assist in interpreting the complex changes observed in clinical nerve excitability with maturation, a mathematical model of the human motor axon was first adjusted to provide a close match to the recordings from the young adulthood group. The model was then used to explore whether changes in any membrane parameter could reproduce the changes seen in each of the younger age groups. Taken in isolation, no single parameter change could account for the younger subject recordings satisfactorily. The best match was an increase in internodal fast K+ channels (GKfi) with immaturity; from 100 to 172 units during late infancy and early childhood, which reduced the discrepancy by 25.3%. The best fit and most plausible model was determined by changing two parameters, including GKfi and Barrett and Barrett conductance (GBB), and was consistent for the two younger age groups. Simulating the axonal excitability recordings with this model is depicted in Fig. 7. In late infancy and early childhood GKfi increased from 100 to 309 units and GBB increased from 33.9 to 48.2 units, which reduced the discrepancy by 83.1%. Importantly the magnitude of change for these parameters reduced with maturation. In late childhood, GKfi increased from 100 to 218 units and GBB increased from 33.9 to 40.4 units, which reduced the discrepancy by 79.6%. In contrast, modelling these parameter changes in adolescence produced a poor match with subject recordings, GKfi increased from 100 to 194 units and GBB increased from 33.9 to 36.3 units, which reduced the discrepancy by 48.3%. As such, mathematical simulations support the hypothesis that maturation produces changes in passive cable conductance and axonal ion channel function to reach steady values during adolescence, with a reduction in GKfi as the most important channel alteration with maturation.
Figure 7. Simulation of the excitability changes in clinical nerve excitability with maturation using the mathematical model.
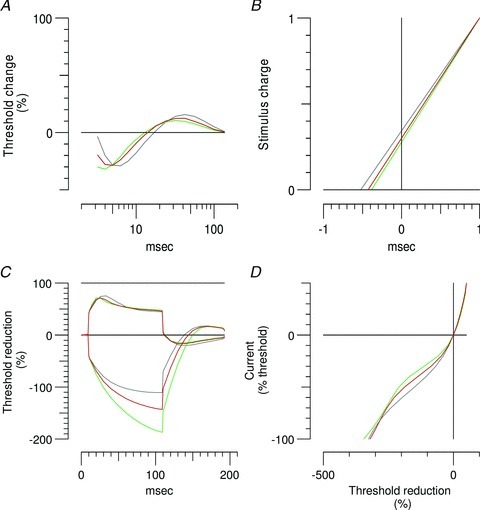
Grey lines represent the model generated by the young adulthood group. Green lines were generated by the model by increasing GBB from 33.9 to 48.2 units and GKfi from 100 to 309 units, which reduced the discrepancy in late infancy and early childhood by 83.1%. Red lines were generated by the model by increasing GBB from 33.9 to 40.4 units and GKfi from 100 to 218 units, which reduced the discrepancy in late childhood by 79.6%. A, recovery cycles. B, charge–duration plot based on stimuli of 0.2 ms and 1 ms duration, with the negative intercept on the x-axis equating to TSD, and the slope equal to the rheobase. C, TE for 100 ms polarizing currents ±40% of the resting threshold. D, current–threshold relationship.
Discussion
The present study has identified striking changes in motor axonal membrane properties associated with maturation of the peripheral nerve in humans, utilizing in vivo nerve excitability testing for the first time. Specifically, the present findings suggest that the activity of K+ conductances decreases with formation of the axo-glial junction, and myelination reduces the GBB. Importantly, concurrent assessment of peripheral nerve motor conduction velocities established that alterations that were paralleled by significant changes across multiple excitability parameters, similarly reached steady values during adolescence. Taken in total, these functional alterations serve to enhance the efficiency and speed of impulse conduction concurrent with the acquisition of motor skills during childhood.
A distinctive pattern of changes occurred in peripheral axonal excitability with maturation, typified by reductions in threshold, rheobase and TE, with waveforms ‘fanning-in’, while resting current–threshold slope increased, all indicative of a relative reduction in current to excite the axon and evolution to a more efficient state. Overall, this pattern of excitability changes with development may reflect interactions between passive structural factors, such as axonal diameter and myelination, and active variables, including ion channel function and membrane potential. Significantly, changes in motor conduction velocities were in general agreement with previous studies, indicating the present cohort was representative of the normal population (Gamstorp, 1963; Baer & Johnson, 1965; Wagner & Buchthal, 1972; Lang et al. 1985).
Maturation in passive axonal properties
Mathematical models examining the effects of passive membrane properties on TE have previously confirmed that the degree of early ‘fanning-out’ increases with reduced axon diameter or internodal resistance through and under the myelin sheath, as occurs with thin myelin or immaturity of the axo-glial junction (GBB, confirmed by modelling in the present study; Yang et al. 2000; Nodera et al. 2004). Accordingly, excitability changes with maturation may reflect alterations in passive membrane properties related to smaller axonal diameter, shortened internodes and thin myelin at younger ages, and are similar to previous axonal excitability studies in immature animal nerves (Yang et al. 2000; Boerio et al. 2009). However, not all elements predicted by alteration of passive membrane properties in in vivo animal models have been observed in the present study, suggesting additional alterations in axonal biophysical properties with growth. Further, while animal models have attempted to outline and provide an understanding of these changes, the present studies using intact human ‘preparations’ provide definitive information and indicate that animal models may be inaccurate.
Alterations in membrane potential and conductances – K+ conductances
The present results also suggest modulation of ion channel function with maturation, with faster accommodation at younger ages indicating increased activation of fast K+ conductances. Altered organization of fast K+ conductances at the axonal membrane and immaturity of the axo-glial junction during development may enable their activation in response to nodal depolarization. Increased activation of fast K+ conductances alone may be expected to ‘clamp’ the ‘fanning-out’ of depolarizing TE, yet in the present study this may have been masked by the added effect of passive axonal properties. In support of this, the ratio TEd(10–20) to TEh(10–20), which is related to nodal K+ channel activity, increased with maturation. Insights into the developmental changes of fast K+ channels and the effects of myelination have been provided by studies that have demonstrated that the sensitivity of 4-aminopyridine, a blocker of fast K+ channels, attenuated during development (Ritchie, 1982; Eng et al. 1988; Yang et al. 2000). These findings suggest that fast K+ channels gradually shift from the node to the juxtaparanode during maturation, where they are covered by myelin and segregated from the effects of 4-amiopyridine. Recent studies have demonstrated that the correct clustering of juxtaparanodal K+ channels depends on several distinct interactions between myelinating glial cells and axons (Rasband, 2011). Specifically, juxtaparanodal K+ channels are part of a larger protein complex comprising the glial and axonal cell adhesion molecules contactin2 (TAG-1) and contactin-associated protein-2 (caspr2) and the cytoskeletal protein 4.1B (Bhat et al. 2001; Boyle et al. 2001; Poliak et al. 2003; Horresh et al. 2010). In addition, the development of paranodal axo-glial junctions containing the axonal proteins contactin and caspr with glial neurofascin-155 function as a physical barrier to lateral diffusion (Einheber et al. 1997; Menegoz et al. 1997; Rios et al. 2000; Tait et al. 2000). These molecular studies provide an understanding of the simultaneous maturation of passive membrane properties and K+ conductances shown in the present study, which are inextricably linked. Taken together these factors influence increases in motor conduction velocity as reflected by correlations with the appropriate excitability parameters.
In addition, a nodal representation of fast K+ channels in immature nerves may influence action potential repolarization, as occurs in the Hodgkin–Huxley giant squid axon model (Hodgkin & Huxley, 1952); their activation may reduce action potential duration and the refractory period, thereby advancing the ensuing period of increased membrane excitability, also known as superexcitability. A striking finding in the present study was the leftwards shift of the recovery cycle of excitability, such that at younger ages superexcitability was more prominent at short interstimulus intervals and its peak earlier, yet the peak magnitude was not significantly altered. Molecular and electrophysiological studies in the developing rat have confirmed a dynamic distribution of fast K+ channels, functioning to ensure stable and secure impulse propagation during development. Specifically, during early development nodal fast K+ channels were directly involved in speeding action potential repolarization, to allow trains of impulses at high frequency (Vabnick et al. 1999). At intermediate stages of myelination, fast K+ channels were shown to have a role in the prevention of repetitive discharges.
Taken together it may be expected that changes in passive and active (voltage-dependent) axonal membrane properties will produce complex changes in the recovery cycle of excitability, as indicated by the modelling. Previous animal and computer models have established that smaller diameter axons along with lower internodal resistances generated greater depolarizing afterpotentials, as measured by superexcitability (David et al. 1995; McIntyre et al. 2002). In contrast, the activation of ‘nodal’ fast K+ channels limits the amplitude and duration of the depolarizing afterpotential and superexcitability (Stephanova & Mileva, 2000).
Alterations in membrane potential and Na+ conductances
The shape of the recovery cycle is also dependent on the kinetics of Na+ conductances (McIntyre et al. 2002; Kiernan et al. 2005), and previous studies have suggested that nodal Na+ channel density may be a factor involved in maturation (Ritchie, 1982; Boerio et al. 2009). The changes in the recovery cycle are difficult to interpret in the present study and not solely in keeping with alterations in passive membrane properties, membrane potential or ion conductances alone. TSD, an indirect measure of persistent Na+ conductances, did not significantly change with maturation, and suggested that the time frame of nodal Na+ channel maturation is early. The early maturity of persistent Na+ conductances at nodes of Ranvier critically enables saltatory conduction of nerve impulses along myelinated fibres (Hille, 1972; Scholz et al. 1993; Waxman & Ritchie, 1993). Recent molecular studies have revealed that nodal organization occurs independently of paranodes, with several molecules enriched at the node regulating voltage-gated Na+ channel localization, stabilization and function, including neurofascin 186, Ankyrin-G and βIV-spectrin (Rasband & Trimmer, 2001; Girault & Peles, 2002; Komada & Soriano, 2002; Thaxton et al. 2011). Accordingly, it may be expected that the configuration and activity of nodal Na+ conductances may occur independently of myelination and development of the axo-glial junction (Utzschneider et al. 1993), as suggested in the present study.
In addition to assessing voltage-independent properties of nerve fibres, TE also provides information about voltage-dependent internodal conductances and an estimate of axonal membrane potential (Baker & Bostock, 1989; Kiernan & Bostock, 2000). The raised thresholds, fanning out of TE, together with significantly reduced refractoriness and subexcitability at younger ages in the present study are consistent with the alterations in excitability established in axonal membrane hyperpolarization (Kiernan & Bostock, 2000; Kiernan et al. 2002). However, this seems unlikely given that other excitability parameters were not appropriately altered. Furthermore, mathematical modelling clarified the complex interpretation of the excitability data and did not predict shifts in membrane potential.
Excitability changes in developing nerves in the present study share some similarities to those in regenerating axons, explained by changes in fibre morphology and functional organization of K+ channels, yielding increased conductances (Kocsis et al. 1982; Kocsis & Waxman, 1983; Moldovan & Krarup, 2004b, 2007; Sawai et al. 2008). Importantly, during development nerve growth is achieved by increasing the internodal length with preservation of the number of nodes (Vizoso & Young, 1948). In contrast, following regeneration/remyelination the number of nodes per unit length, where voltage-gated Na+ channels are located in highest concentration, increases by up to a factor of four, and there is a corresponding increase in the Na+ channel content (Rosenbluth, 1976; Ritchie, 1982; Nakata et al. 2008). In response to the greater Na+ load there is increased Na+/K+ pump activity, which in turn distinctly produces membrane hyperpolarization in regenerated nerves (Moldovan & Krarup, 2004a,b, 2007; Sawai et al. 2008).
Clinical implications
The changes of multiple excitability measures with age in the present study illustrate the time course of postnatal axonal growth, myelination and maturation of the paranodal axo-glial junction, together with its effect on the organization and activity of fast K+ conductances. Together these changes induced increases in conduction velocity and provide unique insight into the evolution of postnatal human peripheral nerve function, serving to ensure stable and secure impulse propagation, whilst enhancing efficiency. Altogether these processes were simultaneous, concurrent with the acquisition of motor skills in childhood. Changes were greatest during late infancy and early childhood, and while adult values were reached between 3 and 5 years, small alterations continued during late childhood to reach constant values in adolescence.
Unlike conventional diagnostic nerve conduction studies, usually including several nerves, axonal excitability testing focuses on a single nerve and may be completed in less than 7 minutes. Similar to undertaking routine diagnostic procedures in young children, patients may not be able to cooperate with axonal excitability testing, being unable to sit still or tolerate mild discomfort during the procedure. Consequently careful preparation utilizing an arm board and securing electrodes with surface taping to reduce movement, together with distraction play therapy or sedation may assist with successful completion of studies. Understanding normal maturation through infancy and childhood may provide a platform for developing a further understanding of the pathophysiology of various childhood-onset neuromuscular conditions throughout the disease course (Farrar et al. 2010). As such, the present study has established the feasibility and potential importance of novel nerve excitability testing in children.
Acknowledgments
M.A.F. received grant support from the National Health and Medical Research Council of Australia: Medical Postgraduate Scholarship, ID568915. The authors wish to thank James Howells for his assistance with mathematical modelling.
Glossary
- CMAP
compound motor action potential
- GBB
Barrett and Barrett conductance
- GKfi
internodal fast K+ channels
- TSD
strength–duration time constant
- TE
threshold electrotonus
- TEd(10–20)
depolarizing change at 10–20 ms in threshold electrotonus
- TEd(90–100)
depolarizing change at 90–100 ms
- TEh(10–20)
hyperpolarizing change at 10–20 ms
- TEh(90–100)
hyperpolarizing change at 90–100 ms
Author contributions
The studies were undertaken at Sydney Children's hospital, Randwick, Australia. M.A.F.: conception and design of the study, data collection, data analysis, data interpretation and drafting of the manuscript; S.B.P.: data collection, data analysis, data interpretation and revision of the manuscript; C.S.-Y.L.: data collection, data analysis, data interpretation and revision of the manuscript; and M.C.K.: conception and design of the study, data analysis, data interpretation and revision of the manuscript. All authors approved the final version of the manuscript.
References
- Baer RD, Johnson EW. Motor nerve conduction velocities in normal children. Arch Phys Med Rehabil. 1965;46:698–704. [PubMed] [Google Scholar]
- Baker M, Bostock H. Depolarization changes the mechanism of accommodation in rat and human motor axons. J Physiol. 1989;411:545–561. doi: 10.1113/jphysiol.1989.sp017589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker M, Bostock H, Grafe P, Martius P. Function and distribution of three types of rectifying channel in rat spinal root myelinated axons. J Physiol. 1987;383:45–67. doi: 10.1113/jphysiol.1987.sp016395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat MA, Rios JC, Lu Y, Garcia-Fresco GP, Ching W, St Martin M, Li J, Einheber S, Chesler M, Rosenbluth J, Salzer JL, Bellen HJ. Axon-glia interactions and the domain organization of myelinated axons requires neurexin IV/Caspr/Paranodin. Neuron. 2001;30:369–383. doi: 10.1016/s0896-6273(01)00294-x. [DOI] [PubMed] [Google Scholar]
- Boerio D, Greensmith L, Bostock H. Excitability properties of motor axons in the maturing mouse. J Peripher Nerv Syst. 2009;14:45–53. doi: 10.1111/j.1529-8027.2009.00205.x. [DOI] [PubMed] [Google Scholar]
- Boland RA, Bostock H, Kiernan MC. Plasticity of lower limb motor axons after cervical cord injury. Clin Neurophysiol. 2009;120:204–209. doi: 10.1016/j.clinph.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Bostock H. The strength-duration relationship for excitation of myelinated nerve: computed dependence on membrane parameters. J Physiol. 1983;341:59–74. doi: 10.1113/jphysiol.1983.sp014792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Baker M. Evidence for two types of potassium channel in human motor axons in vivo. Brain Res. 1988;462:354–358. doi: 10.1016/0006-8993(88)90564-1. [DOI] [PubMed] [Google Scholar]
- Bostock H, Baker M, Reid G. Changes in excitability of human motor axons underlying post-ischaemic fasciculations: evidence for two stable states. J Physiol. 1991;441:537–557. doi: 10.1113/jphysiol.1991.sp018766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Cikurel K, Burke D. Threshold tracking techniques in the study of human peripheral nerve. Muscle Nerve. 1998;21:137–158. doi: 10.1002/(sici)1097-4598(199802)21:2<137::aid-mus1>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- Bostock H, Rothwell JC. Latent addition in motor and sensory fibres of human peripheral nerve. J Physiol. 1997;498:277–294. doi: 10.1113/jphysiol.1997.sp021857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostock H, Sharief MK, Reid G, Murray NM. Axonal ion channel dysfunction in amyotrophic lateral sclerosis. Brain. 1995;118:217–225. doi: 10.1093/brain/118.1.217. [DOI] [PubMed] [Google Scholar]
- Boyle ME, Berglund EO, Murai KK, Weber L, Peles E, Ranscht B. Contactin orchestrates assembly of the septate-like junctions at the paranode in myelinated peripheral nerve. Neuron. 2001;30:385–397. doi: 10.1016/s0896-6273(01)00296-3. [DOI] [PubMed] [Google Scholar]
- David G, Modney B, Scappaticci KA, Barrett JN, Barrett EF. Electrical and morphological factors influencing the depolarizing after-potential in rat and lizard myelinated axons. J Physiol. 1995;489(Pt 1):141–157. doi: 10.1113/jphysiol.1995.sp021037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Zanazzi G, Ching W, Scherer S, Milner TA, Peles E, Salzer JL. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eng DL, Gordon TR, Kocsis JD, Waxman SG. Development of 4-AP and TEA sensitivities in mammalian myelinated nerve fibers. J Neurophysiol. 1988;60:2168–2179. doi: 10.1152/jn.1988.60.6.2168. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Lin CS, Krishnan AV, Park SB, Andrews PI, Kiernan MC. Acute, reversible axonal energy failure during stroke-like episodes in MELAS. Pediatrics. 2010;126:e734–e739. doi: 10.1542/peds.2009-2930. [DOI] [PubMed] [Google Scholar]
- Farrar MA, Vucic S, Lin CS, Park SB, Johnston HM, du Sart D, Bostock H, Kiernan MC. Dysfunction of axonal membrane conductances in adolescents and young adults with spinal muscular atrophy. Brain. 2011;134:3185–3197. doi: 10.1093/brain/awr229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamstorp I. Normal conduction velocity of ulnar, median and peroneal nerves in infancy, childhood and adolescence. Acta Paediatr Suppl. 1963;146:168–176. doi: 10.1111/j.1651-2227.1963.tb05519.x. [DOI] [PubMed] [Google Scholar]
- Girault JA, Peles E. Development of nodes of Ranvier. Curr Opin Neurobiol. 2002;12:476–485. doi: 10.1016/s0959-4388(02)00370-7. [DOI] [PubMed] [Google Scholar]
- Gutrecht JA, Dyck PJ. Quantitative teased-fibre and histologic studies of human sural nerve during postnatal development. J Comp Neurol. 1970;138:117–129. doi: 10.1002/cne.901380109. [DOI] [PubMed] [Google Scholar]
- Hille B. Ionic permeability changes in active axon membranes. Arch Intern Med. 1972;129:293–298. [PubMed] [Google Scholar]
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horresh I, Bar V, Kissil JL, Peles E. Organization of myelinated axons by Caspr and Caspr2 requires the cytoskeletal adapter protein 4.1B. J Neurosci. 2010;30:2480–2489. doi: 10.1523/JNEUROSCI.5225-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs JM, Love S. Qualitative and quantitative morphology of human sural nerve at different ages. Brain. 1985;108:897–924. doi: 10.1093/brain/108.4.897. [DOI] [PubMed] [Google Scholar]
- Kaji R. Physiological and technical basis of peripheral nerve and motoneurons testing. In: Kimura J, Kaji R, editors. Physiology of ALS and Related Diseases. Amsterdam: Elsevier; 1997. pp. 15–41. [Google Scholar]
- Kiernan MC, Bostock H. Effects of membrane polarization and ischaemia on the excitability properties of human motor axons. Brain. 2000;123:2542–2551. doi: 10.1093/brain/123.12.2542. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Burke D, Andersen KV, Bostock H. Multiple measures of axonal excitability: a new approach in clinical testing. Muscle Nerve. 2000;23:399–409. doi: 10.1002/(sici)1097-4598(200003)23:3<399::aid-mus12>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Guglielmi JM, Kaji R, Murray NM, Bostock H. Evidence for axonal membrane hyperpolarization in multifocal motor neuropathy with conduction block. Brain. 2002;125:664–675. doi: 10.1093/brain/awf041. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Isbister GK, Lin CS, Burke D, Bostock H. Acute tetrodotoxin-induced neurotoxicity after ingestion of puffer fish. Ann Neurol. 2005;57:339–348. doi: 10.1002/ana.20395. [DOI] [PubMed] [Google Scholar]
- Kiernan MC, Mogyoros I, Burke D. Differences in the recovery of excitability in sensory and motor axons of human median nerve. Brain. 1996;119:1099–1105. doi: 10.1093/brain/119.4.1099. [DOI] [PubMed] [Google Scholar]
- Kimura J. Electrodiagnosis in Diseases of Nerve and Muscle: Principles and Practice. Philadelphia: Davis FA; 1983. [Google Scholar]
- Kocsis JD, Waxman SG. Long-term regenerated nerve fibres retain sensitivity to potassium channel blocking agents. Nature. 1983;304:640–642. doi: 10.1038/304640a0. [DOI] [PubMed] [Google Scholar]
- Kocsis JD, Waxman SG, Hildebrand C, Ruiz JA. Regenerating mammalian nerve fibres: changes in action potential waveform and firing characteristics following blockage of potassium conductance. Proc R Soc Lond B Biol Sci. 1982;217:77–87. doi: 10.1098/rspb.1982.0095. [DOI] [PubMed] [Google Scholar]
- Komada M, Soriano P. βIV-spectrin regulates sodium channel clustering through ankyrin-G at axon initial segments and nodes of Ranvier. J Cell Biol. 2002;156:337–348. doi: 10.1083/jcb.200110003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan AV, Lin CS, Park SB, Kiernan MC. Axonal ion channels from bench to bedside: a translational neuroscience perspective. Prog Neurobiol. 2009;89:288–313. doi: 10.1016/j.pneurobio.2009.08.002. [DOI] [PubMed] [Google Scholar]
- Lang HA, Puusa A, Hynninen P, Kuusela V, Jäntti V, Sillanpää M. Evolution of nerve conduction velocity in later childhood and adolescence. Muscle Nerve. 1985;8:38–43. doi: 10.1002/mus.880080108. [DOI] [PubMed] [Google Scholar]
- Lin CS, Krishnan AV, Lee MJ, Zagami AS, You HL, Yang CC, Bostock H, Kiernan MC. Nerve function and dysfunction in acute intermittent porphyria. Brain. 2008;131:2510–2519. doi: 10.1093/brain/awn152. [DOI] [PubMed] [Google Scholar]
- McIntyre CC, Richardson AG, Grill WM. Modelling the excitability of mammalian nerve fibers: influence of afterpotentials on the recovery cycle. J Neurophysiol. 2002;87:995–1006. doi: 10.1152/jn.00353.2001. [DOI] [PubMed] [Google Scholar]
- Menegoz M, Gaspar P, Le Bert M, Galvez T, Burgaya F, Palfrey C, Ezan P, Arnos F, Girault JA. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D. Strength-duration properties of human peripheral nerve. Brain. 1996;119:439–447. doi: 10.1093/brain/119.2.439. [DOI] [PubMed] [Google Scholar]
- Mogyoros I, Kiernan MC, Burke D, Bostock H. Strength-duration properties of sensory and motor axons in amyotrophic lateral sclerosis. Brain. 1998;121:851–859. doi: 10.1093/brain/121.5.851. [DOI] [PubMed] [Google Scholar]
- Moldovan M, Krarup C. Mechanisms of hyperpolarization in regenerated mature motor axons in cat. J Physiol. 2004a;560:807–819. doi: 10.1113/jphysiol.2004.069443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan M, Krarup C. Persistent abnormalities of membrane excitability in regenerated mature motor axons in cat. J Physiol. 2004b;560:795–806. doi: 10.1113/jphysiol.2004.069476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldovan M, Krarup C. Internodal function in normal and regenerated mammalian axons. Acta Physiol. 2007;189:191–200. doi: 10.1111/j.1748-1716.2006.01654.x. [DOI] [PubMed] [Google Scholar]
- Nakata M, Baba H, Kanai K, Hoshi T, Sawai S, Hattori T, Kuwabara S. Changes in Na+ channel expression and nodal persistent Na+ currents associated with peripheral nerve regeneration in mice. Muscle Nerve. 2008;37:721–730. doi: 10.1002/mus.21031. [DOI] [PubMed] [Google Scholar]
- Nodera H, Bostock H, Kuwabara S, Sakamoto T, Asanuma K, Jia-Ying S, Ogawara K, Hattori N, Hirayama M, Sobue G, Kaji R. Nerve excitability properties in Charcot-Marie-Tooth disease type 1A. Brain. 2004;127:203–211. doi: 10.1093/brain/awh020. [DOI] [PubMed] [Google Scholar]
- Poliak S, Salomon D, Elhanany H, Sabanay H, Kiernan B, Pevny L, Stewart CL, Xu X, Chiu SY, Shrager P, Furley AJ, Peles E. Juxtaparanodal clustering of Shaker-like K+ channels in myelinated axons depends on Caspr2 and TAG-1. J Cell Biol. 2003;162:1149–1160. doi: 10.1083/jcb.200305018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Press W, Teukolsky S, Vetterling W, Flannery B. Numerical Recipes in C – The Art of Computing. Cambridge: Cambridge University Press; 1992. [Google Scholar]
- Rasband MN. Composition, assembly, and maintenance of excitable membrane domains in myelinated axons. Semin Cell Dev Biol. 2011;22:178–184. doi: 10.1016/j.semcdb.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN, Trimmer JS. Developmental clustering of ion channels at and near the node of Ranvier. Dev Biol. 2001;236:5–16. doi: 10.1006/dbio.2001.0326. [DOI] [PubMed] [Google Scholar]
- Rios JC, Melendez-Vasquez CV, Einheber S, Lustig M, Grumet M, Hemperly J, Peles E, Salzer JL. Contactin-associated protein (Caspr) and contactin form a complex that is targeted to the paranodal junctions during myelination. J Neurosci. 2000;20:8354–8364. doi: 10.1523/JNEUROSCI.20-22-08354.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie JM. Sodium and potassium channels in regenerating and developing mammalian myelinated nerves. Proc R Soc Lond B Biol Sci. 1982;215:273–287. doi: 10.1098/rspb.1982.0042. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol. 1976;5:731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]
- Rushton WA. A theory of the effects of fibre size in medullated nerve. J Physiol. 1951;115:101–122. doi: 10.1113/jphysiol.1951.sp004655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadler TW. Langman's Medical Embryology. Baltimore: Williams and Wilkins; 1990. [Google Scholar]
- Sawai S, Kanai K, Nakata M, Hiraga A, Misawa S, Isose S, Hattori T, Kuwabara S. Changes in excitability properties associated with axonal regeneration in human neuropathy and mouse Wallerian degeneration. Clin Neurophysiol. 2008;119:1097–1105. doi: 10.1016/j.clinph.2008.01.022. [DOI] [PubMed] [Google Scholar]
- Scholz A, Reid G, Vogel W, Bostock H. Ion channels in human axons. J Neurophysiol. 1993;70:1274–1279. doi: 10.1152/jn.1993.70.3.1274. [DOI] [PubMed] [Google Scholar]
- Schroder JM, Bohl J, Brodda K. Changes of the ratio between myelin thickness and axon diameter in the human developing sural nerve. Acta Neuropathol. 1978;43:169–178. doi: 10.1007/BF00685012. [DOI] [PubMed] [Google Scholar]
- Schwarz JR, Reid G, Bostock H. Action potentials and membrane currents in the human node of Ranvier. Pflugers Arch. 1995;430:283–292. doi: 10.1007/BF00374660. [DOI] [PubMed] [Google Scholar]
- Stephanova DI, Mileva K. Different effects of blocked potassium channels on action potentials, accommodation, adaptation and anode break excitation in human motor and sensory myelinated nerve fibres: computer simulations. Biol Cybern. 2000;83:161–167. doi: 10.1007/s004220000151. [DOI] [PubMed] [Google Scholar]
- Susuki K, Rasband MN. Molecular mechanisms of node of Ranvier formation. Curr Opin Cell Biol. 2008;20:616–623. doi: 10.1016/j.ceb.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait S, Gunn-Moore F, Collinson JM, Huang J, Lubetzki C, Pedraza L, Sherman DL, Colman DR, Brophy PJ. An oligodendrocyte cell adhesion molecule at the site of assembly of the paranodal axo-glial junction. J Cell Biol. 2000;150:657–666. doi: 10.1083/jcb.150.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaxton C, Pillai AM, Pribisko AL, Dupree JL, Bhat MA. Nodes of Ranvier act as barriers to restrict invasion of flanking paranodal domains in myelinated axons. Neuron. 2011;69:244–257. doi: 10.1016/j.neuron.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Utzschneider DA, Thio C, Sontheimer H, Ritchie JM, Waxman SG, Kocsis JD. Action potential conduction and sodium channel content in the optic nerve of the myelin-deficient rat. Proc Biol Sci. 1993;254:245–250. doi: 10.1098/rspb.1993.0153. [DOI] [PubMed] [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J Neurosci. 1999;19:747–758. doi: 10.1523/JNEUROSCI.19-02-00747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vizoso AD, Young JZ. Internode length and fibre diameter in developing and regenerating nerves. J Anat. 1948;82:110–134. [PMC free article] [PubMed] [Google Scholar]
- Wagner AL, Buchthal F. Motor and sensory conduction in infancy and childhood: reappraisal. Dev Med Child Neurol. 1972;14:189–216. doi: 10.1111/j.1469-8749.1972.tb02576.x. [DOI] [PubMed] [Google Scholar]
- Waxman SG. Determinants of conduction velocity in myelinated nerve fibers. Muscle Nerve. 1980;3:141–150. doi: 10.1002/mus.880030207. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Molecular dissection of the myelinated axon. Ann Neurol. 1993;33:121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]
- Weiss G. Sur la possibilité de rendre comparables entre eux les appareils servant l’excitation électrique. Arch Ital Biol. 1901;35:413–446. [Google Scholar]
- Yang Q, Kaji R, Hirota N, Kojima Y, Takagi T, Kohara N, Kimura J, Shibasaki H, Bostock H. Effect of maturation on nerve excitability in an experimental model of threshold electrotonus. Muscle Nerve. 2000;23:498–506. doi: 10.1002/(sici)1097-4598(200004)23:4<498::aid-mus7>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]


