Abstract
In Drosophila, the mushroom body (MB) is a critical brain structure for olfactory associative learning. During aversive conditioning, the MBs are thought to associate odour signals, conveyed by projection neurons (PNs) from the antennal lobe (AL), with shock signals conveyed through ascending fibres of the ventral nerve cord (AFV). Although synaptic transmission between AL and MB might play a crucial role for olfactory associative learning, its physiological properties have not been examined directly. Using a cultured Drosophila brain expressing a Ca2+ indicator in the MBs, we investigated synaptic transmission and plasticity at the AL–MB synapse. Following stimulation with a glass micro-electrode, AL-induced Ca2+ responses in the MBs were mediated through Drosophila nicotinic acetylcholine receptors (dnAChRs), while AFV-induced Ca2+ responses were mediated through Drosophila NMDA receptors (dNRs). AL–MB synaptic transmission was enhanced more than 2 h after the simultaneous ‘associative-stimulation’ of AL and AFV, and such long-term enhancement (LTE) was specifically formed at the AL–MB synapses but not at the AFV–MB synapses. AL–MB LTE was not induced by intense stimulation of the AL alone, and the LTE decays within 60 min after subsequent repetitive AL stimulation. These phenotypes of associativity, input specificity and persistence of AL–MB LTE are highly reminiscent of olfactory memory. Furthermore, similar to olfactory aversive memory, AL–MB LTE formation required activation of the Drosophila D1 dopamine receptor, DopR, along with dnAChR and dNR during associative stimulations. These physiological and genetic analogies indicate that AL–MB LTE might be a relevant cellular model for olfactory memory.
Key points
During olfactory aversive conditioning in Drosophila, odour and shock information are delivered to the mushroom bodies (MBs) through projection neurons in the antennal lobes (ALs) and ascending fibres of the ventral nerve cord (AFV), respectively.
Using an isolated cultured brain expressing a Ca2+ indicator in the MBs, we demonstrated that the simultaneous stimulation of the ALs and AFV establishes long-term enhancement (LTE) in AL-induced Ca2+ responses.
The physiological properties of LTE, including associativity, input specificity and persistence, are highly reminiscent of those of olfactory memory.
Similar to olfactory aversive memory, LTE requires the activation of nicotinic acetylcholine receptors that mediate the AL-evoked Ca2+ response, NMDA receptors that mediate the AFV-induced Ca2+ response, and D1 dopamine receptors during the simultaneous stimulation of the ALs and AFV.
Considering the physiological and genetic analogies, we propose that LTE at the AL–MB synapse can be a relevant cellular model for olfactory memory.
Introduction
After olfactory aversive conditioning, Drosophila selectively increases avoidance toward the conditioned odour (conditioned stimulus, CS), which was previously presented with foot-shock (unconditioned stimulus, US). Because chemical ablation of the mushroom bodies (MBs) completely prevents olfactory learning (de Belle & Heisenberg, 1994) and blocking of the synaptic output from MBs disrupts retrieval (Dubnau et al. 2001; McGuire et al. 2001), the MBs are considered as a critical neuronal structure for integrating odour and foot-shock information. Consistent with this model, recent in vivo imaging studies have demonstrated the formation of memory traces in the MBs after conditioning; Ca2+ responses in the MBs to an odour, which were previously paired with foot-shock, are increased for more than 1 h (Wang et al. 2008; Tan et al. 2010). However, the synaptic mechanisms involved in neural plasticity in the MBs remain unknown.
In Drosophila, odour information is delivered to the MBs through projection neurons (PNs) in the antennal lobe (AL) (Marin et al. 2002; Wong et al. 2002), whereas foot-shock information from the body is delivered to the MBs via the ascending fibres of the ventral nerve cord (AFV) (Fig. 1A). Although in vivo imaging has suggested that the physiological properties of the AL–MB synapses are potentially important for neuronal plasticity in the MB, current in vivo imaging methods are unsuited to analysing AL–MB synaptic transmission by stimulating AL directly. Furthermore, considerable brain movement during in vivo recording reduces the signal-to-noise ratio, thereby hindering the kinetic analysis of MB responses.
Figure 1. Preparation of isolated cultured Drosophila brains for imaging.
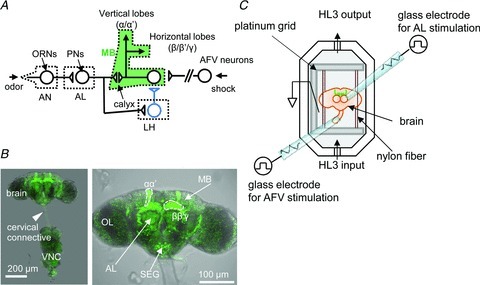
A, odour signalling pathway in Drosophila. An odour signal perceived through the dendrites of the ORNs in the AN is transmitted to PNs in the AL and subsequently to MB or LH neurons that project inhibitory GABAergic terminals onto MB neurons. The foot-shock signal from the body is delivered through the AFV. B, a c309;UAS-G-CaMP fly brain with the VNC. The VNC was cut at the cervical connection (arrowhead) for stimulating AFV. G-CaMP expression was observed throughout the α, α′, β, β′ and γ lobes of the MBs. OL, optic lobe; SEG, sub-oesophageal ganglion. C, schematic diagram of the recording setup. Glass micro-electrodes were placed on the AL, and the cut ends of the AFV were suctioned for stimulation.
To overcome these difficulties and analyse AL–MB synaptic transmission and plasticity, we employed in vitro imaging (Wang et al. 2008; Tomchik & Davis, 2009) using an isolated cultured Drosophila brain to directly stimulate the AL and AFV without brain movement during recording of the Ca2+ responses in the MBs. Using this in vitro imaging system in conjunction with high-speed scanning confocal microscopy, we observed that cholinergic AL–MB synaptic transmission was enhanced for more than 2 h after the simultaneous stimulation of the AL and AFV. Strikingly, the physiological properties and genetic requirements of this long-term enhancement (LTE) at the AL–MB synapse are highly reminiscent of the characteristics of olfactory memory. We propose that AL–MB LTE might be a reasonable cellular model for learning and memory similar to long-term potentiation (LTP) at the mammalian hippocampal synapses.
Methods
Fly stocks
All fly stocks were maintained at 25 ± 2°C and 60 ± 10% humidity under a 12/12 h light–dark cycle. All transgenic flies and mutants were outcrossed to our wild-type control line w(CS10) (Dura et al. 1993; Tamura et al. 2003). We used female flies for imaging analyses, and both male and female flies for behavioural tests.
Imaging analysis
Brains with attached ventral nerve cords (VNCs) (Fig. 1B) were dissected in 0 mm Ca2+ HL3 medium (in mm, NaCl, 70; sucrose, 115; KCl, 5; MgCl2, 20; NaHCO3, 10; trehalose, 5; Hepes, 5; pH 7.3) (Stewart et al. 1994). The isolated brains were immobilized by placing their optic lobes between two nylon fibre bundles attached to the platinum grid and were placed in a bath chamber (Fig. 1C). We cut the VNC at the cervical connective. The ALs and AFV were electrically stimulated using glass micro-electrodes with a stimulator (SEN-7103; Nihon Kohden, Tokyo, Japan) and an isolator (SS-104J for AL, SS202J for AFV, Nihon Kohden). During the experiments, fresh HL3 medium (in mm, NaCl, 70; sucrose, 115; KCl, 5; MgCl2, 20; CaCl2, 1.8; NaHCO3, 10; trehalose, 5; Hepes, 5; pH 7.3) was infused into the chamber using a peristaltic pump (2 ml min−1, MiniPuls3, Gilson, Inc., Middleton, WI, USA).
Images were captured using a high-speed scanning confocal microscope system (A1R, Nikon Corp., Tokyo, Japan) with a 20× water-immersion lens (numerical aperture 0.5; Nikon Corp.). In this imaging system, 512 × 512 pixel images can be captured at 30 Hz frequency. Before the experiments, the offset value was set to obtain a background intensity of approximately 0. The F value was calculated for each pixel in the region of interest using NIS-elements software (NIS-Elements Ar; Nikon Corp.).
To record AL- and AFV-induced Ca2+ responses, we stimulated the AL or AFV with three trains of 30 pulses (100 Hz, 1.0 ms pulse duration, intensity 1–2 × threshold current) with an inter-train interval of 10 s. We obtained initial fluorescence values (F0) by averaging the F values obtained in the five sequential frames before stimulation onset. We averaged the three fluorescent traces obtained after stimulation and calculated (F–F0)/F0 to obtain ΔF/F0. To evaluate the relative Ca2+ responses induced through the associative stimulation of the AL and AFV or intensive AL stimulation, the ΔF/F0 obtained after associative or intensive stimulation was divided by the ΔF/F0 obtained prior to associative or intensive stimulation.
Electrical stimulation protocols
For ‘associative stimulation,’ the AL and AFV were simultaneously stimulated with 12 trains at an interval of 5 s, and the average of the first three responses was considered as the relative Ca2+ response during the associative stimulation. For unpaired stimulation, AL stimuli were induced at 3 min after the end of the AFV stimuli. In intensive AL-alone stimulation, the pulse duration for AL stimulation was extended from 1.0 to 1.5 ms to obtain a Ca2+ response during intensive stimulation comparable with that elicited during associative stimulation.
Behavioural tests
The procedure for measuring olfactory memory has been previously described (Tully & Quinn, 1985). Briefly, two aversive odours (OCT or MCH) were sequentially delivered to approximately 100 flies for 1 min at an interval of 45 s between each odour exposure. When the flies were exposed to the first treatment, CS odour (either OCT or MCH), they were also subjected to 1.5 s pulses of 60 V DC electric shocks every 5 s. To test olfactory memory, the flies were placed at the choice point of a T-maze where both odours were delivered and were allowed to choose between the odours. After 1.5 min, memory was calculated as a performance index, such that a 50:50 distribution (no memory) yielded a performance index of zero and a 0:100 distribution away from the CS yielded a performance index of 100.
For extinction, the flies were sequentially exposed to the CS odours for 1 min at 5 min intervals in the training chamber after conditioning to expose the conditioned flies to the CS odours 11 times before examining 1 h memory.
LexA/LexAop system in DopR rescue flies
To construct the pMBp-LexA construct, which contains the LexA gene under the control of an MB promoter/enhancer, a DNA fragment containing 247 bp of the 5′ flanking sequence from the Mef2 gene (which is expressed in the MBs) and the hsp70Bb minimal promoter was subcloned into pCasper-W-LexA::GAD (Diegelmann et al. 2008) using the Gateway system (Life Technologies Corp., Carlbad, CA, USA) according to the manufacturer's instructions. To obtain LexAop-G-CaMP2 for expression of G-CaMP2 from a LexA driver, G-CaMP2 cDNA amplified from pN1-G-CaMP2 was subcloned into pCasper-lexAop-W (Diegelmann et al. 2008). The two constructs were injected into embryos from our standard w(CS10) strain to obtain w;MBp-LexA and w;LexAop-G-CaMP2 transgenic flies.
In the DopRf02676 mutant, DopR expression is inhibited by insertion of a piggyBac construct in the first intron of the DopR gene (Kim et al. 2007). Because the piggyBac construct carries a UAS sequence (Thibault et al. 2004), DopR expression can be induced from an in-frame ATG in the second exon using a GAL4 driver (Kim et al. 2007). Using this system, we generated w;MBp-LexA,LexAop-G-CaMP2/+;DopRf02676/DopRf02676 and w;MBp-LexA/c309,LexAop-G-CaMP2;DopRf02676/DopRf02676 flies.
Statistics
All data are expressed as mean ± SEM. Student's t test for paired data was used to evaluate the statistical significance between two data sets. For multiple comparisons, one-way ANOVA was used, followed by Bonferroni post hoc analyses. The time constant for decay kinetics (see Fig. 3I) was calculated by fitting a single exponential curve, using Prism (GraphPad Software, Inc., La Jolla, CA, USA).
Figure 3. LTE of AL-induced Ca2+ responses in the MB.
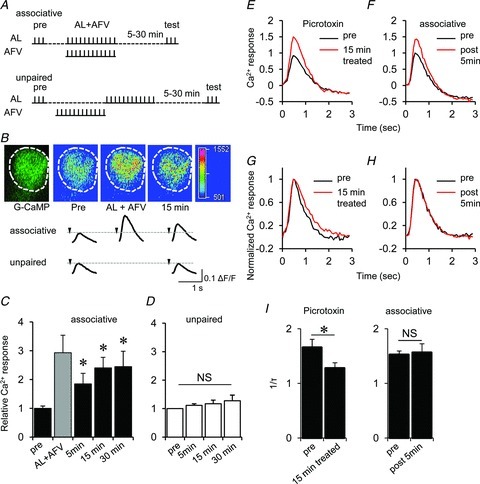
A, stimulation protocol. In associative stimulation, AL and AFV were stimulated simultaneously with 12 stimulus trains (AL + AFV). In unpaired stimulation, AL stimulation was induced after the end of AFV stimulation. B, typical changes in G-CaMP fluorescence at the distal end of the MB vertical lobes (α/α′ lobes), and the traces of AL-induced Ca2+ responses in the MBs before and after associated or unpaired stimulation. The arrowhead indicates stimulation onset. C and D, summary of AL-induced Ca2+ responses in the MBs measured before (pre) and after associative (C) and unpaired (D) stimulation. The shaded column in C indicates Ca2+ responses during associative stimulation (AL + AFV) (*P < 0.05 compared with ‘pre’ responses). E, averaged traces of AL-induced Ca2+ responses before and after treatment with 50 μm picrotoxin for 15 min. F, averaged traces of AL-induced Ca2+ responses before and 5 min after associative AL + AFV stimulation. G and H, peak Ca2+ responses were normalized to 1.0 to evaluate the effects of picrotoxin (G) and associative stimulations (H) on decay kinetics. I, picrotoxin treatment reduced the decay kinetics (1/τ, where τ represents the time constant) of the Ca2+ response, while associative stimulations did not (*P < 0.05 by t test). n= 6–8 for all data.
Results
Imaging analyses of AL–MB synaptic transmission
We isolated Drosophila brains expressing the Ca2+ indicator G-CaMP in the MBs using a GAL4/UAS binary system (Brand & Perrimon, 1993) and immobilized the isolated brains in the recording bath chamber (Fig. 1B and C). To measure synaptic transmission between PNs and MB neurons, we stimulated the AL using a glass micro-electrode (Fig. 1C).
The MBs contain approximately 5000 intrinsic neurons called Kenyon cells. Kenyon cells receive the presynaptic terminals of the PNs at the calyx, dendritic regions of the MBs, and project terminals to vertical and horizontal lobes. Kenyon cells are classified as α/β, α′/β′ and γ neurons using functional and anatomical criteria (Ito et al. 1997; Crittenden et al. 1998). While AL-induced Ca2+ responses were consistently observed in the distal ends of the vertical lobes (α/α′), these responses were weak and infrequent in the horizontal lobes (β/β′/γ) (Fig. 2A and B) and in the calyx (Fig. 2C and D).
Figure 2. Ca2+ responses in the MBs.
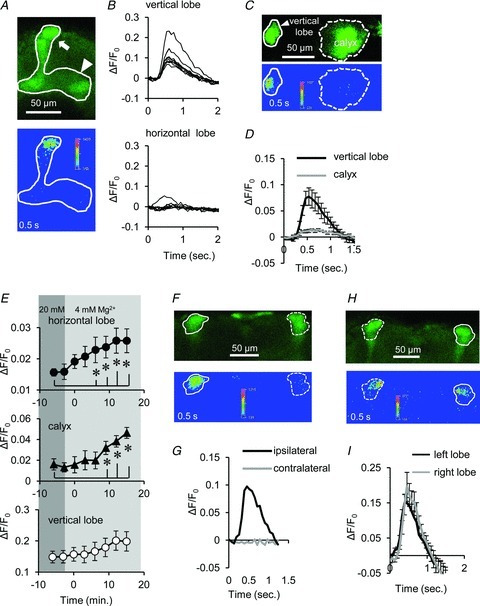
A, Ca2+ responses induced by AL stimulation in the vertical (arrow) and horizontal (arrowhead) lobes of the MB. While robust Ca2+ responses were observed in the distal end of the vertical lobe, the significant responses were not observed in the horizontal lobe (33 of 38 brains). B, nine traces of AL-induced Ca2+ responses in the vertical (upper) and horizontal (lower) lobes of the MB. C, the calyx exhibited weak AL-induced Ca2+ responses. D, summary of Ca2+ responses in the vertical lobes (black line) and the calyx (grey line) of the MB (P < 0.05 between the vertical lobe and the calyx; n= 6). E, AL-induced Ca2+ responses were increased in the horizontal lobes (upper) and in the calyx (middle) with decreasing extracellular Mg2+ concentration from 20 to 4 mm (*P < 0.05, n= 5). F and G, AL stimulation produced Ca2+ responses in the ipsilateral but not in the contralateral MB lobe. H and I, AFV stimulation produced Ca2+ responses in both sides of MB lobes (n= 5). All data in this study are shown as mean ± SEM.
In contrast, previous imaging studies have demonstrated that odour and electrical stimulation to antennae (AN) induces significant Ca2+ responses not only in vertical lobes but also in horizontal lobes and the calyx (Martin et al. 2007; Wang et al. 2008). Notably, while our external recording solution contains 20 mm Mg2+, these previous studies employed lower concentrations of Mg2+ (≤4 mm). Therefore, we suspect that the robust Ca2+ responses in the horizontal lobe observed in other studies are due to the low concentration of Mg2+.
To test this possibility, we examined AL-induced responses in 4 mm Mg2+ recording solution. As suspected, we observed a significant increase in responses in the horizontal lobes and in the calyx in recording solution containing 4 mm Mg2+ (Fig. 2E). This result suggests that Ca2+ responses in the horizontal lobe and in the calyx are more sensitive to external Mg2+ than Ca2+ responses in the vertical lobes.
Consistent with anatomical studies indicating that PNs of each AL send their axons to the ipsilateral MB (Marin et al. 2002; Wong et al. 2002), stimulation of an AL induced Ca2+ responses in the ipsilateral, but not the contralateral, MB (Fig. 2F and G). In contrast to AL stimuli, AFV stimulation induced Ca2+ responses in the MBs of both brain hemispheres (Fig. 2H and I). We employed 20 mm Mg2+ in the recording solution in this study, as this concentration lies within the physiologically relevant range (see Discussion), and we primarily focused on Ca2+ responses in the vertical lobes in subsequent experiments.
LTE of AL–MB synaptic transmission
In our in vitro imaging system, we observed the LTE of AL-induced Ca2+ responses in the MB after the simultaneous stimulation of AL and AFV for 1 min (Fig. 3A–C). In contrast to associative AL and AFV stimulation, unpaired AL and AFV stimulation did not enhance the Ca2+ responses (Fig. 3A, B and D). In this study, we primarily used the c309 Gal4 driver to measure the Ca2+ response in the MBs because we observed the most significant LTE with this MB driver. For example, although the OK107 MB driver generated higher UAS transgene expression than c309 (Aso et al. 2010), the LTE observed with OK107 was lower than that observed with c309; the relative Ca2+ responses at 15 min after the associative stimuli were 1.48 with OK107 (see Fig. 4H) and 2.40 with c309 (Fig. 3C).
Figure 4. LTE occurred in both α and α′ lobes but not in the calyx.
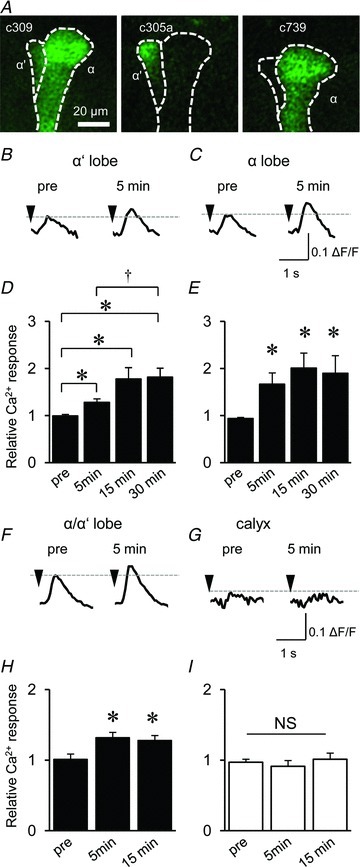
A, G-CaMP was expressed in each α′ and α lobe by the c305a and c739 GAL4 drivers, respectively. B and C, typical traces of AL-induced Ca2+ responses in the α′ (B) and α lobes (C) before (pre) and 5 min after associative AL and AFV stimulation. D and E, AL-induced Ca2+ responses were enhanced after associative stimulation in both α′ (D), and α (E) lobes (*P < 0.05 compared with ‘pre’ responses, and †P < 0.05 comparison between 5 and 30 min). F and G, typical traces of AL-induced Ca2+ responses in the vertical lobe (F) and the calyx (G) before (pre) and after associative AL and AFV stimulation. Both traces were obtained from identical OK107-GAL4/UAS-G-CaMP brains. H and I, LTE was observed after associative stimulation in the vertical lobe (H) but not in the calyx (I) (*P < 0.05 compared with ‘pre’ responses). n= 6–7 for all data.
These results suggest that the associative AL and AFV stimulation produces the LTE of AL–MB synaptic transmission. However, PNs in the AL also convey olfactory signalling to lateral horn (LH) neurons (Marin et al. 2002; Wong et al. 2002), which in turn project their GABAergic terminals onto MB neurons (Perez-Orive et al. 2002; Yasuyama et al. 2002; Fig. 1A). Therefore, it is possible that the enhanced Ca2+ responses in the MBs reflect the inhibition of LH neurons rather than increases in excitatory transmission between the AL and the MB.
To test this possibility, we recorded AL-induced Ca2+ responses in the brain following treatment with picrotoxin, a GABA receptor blocker (Su & O’Dowd, 2003). Picrotoxin treatment increased the amplitude of the AL-induced Ca2+ response (Fig. 3E) but significantly decreased the decay kinetics of the Ca2+ response (Fig. 3G and I). However, after associative AL and AFV stimulation, the AL-induced Ca2+ response amplitudes increased (Fig. 3F) without any changes in the decay kinetics (Fig. 3H and I). These results support the idea that LTE in the MBs results from an increase in excitatory synaptic transmission between PNs and MB neurons.
The vertical lobes in Drosophila consist of α and α′ lobes (Fig. 4A; Ito et al. 1997). Although previous studies demonstrate that the associative stimulation of the AN and VNC enhances AN-induced Ca2+ responses in α′ but not α lobes (Wang et al. 2008), we observed that the associative AL and AFV stimulation enhances AL-induced Ca2+ responses in both lobes. To confirm the enhancement in both the α and the α′ lobes, we employed the α and α′ lobe-specific GAL4 drivers c739 and c305a, respectively (Yang et al. 1995; Krashes et al. 2007).
As shown in Fig. 4A and B, LTE was significantly induced in both lobes after associative AL and AFV stimulations. Notably, the PNs in the AL received olfactory information from the olfactory receptor neurons (ORNs) in the maxillary palps (MPs) in addition to the AN (Rajashekar & Shamprasad, 2004), and AN stimuli evoked much higher Ca2+ responses in the α′ lobe than in the α lobe (Wang et al. 2008). Because we stimulated the entire AL, AL-induced Ca2+ responses and LTE in the α lobe might reflect the activation of PNs that receive input from ORNs in the MPs. Consistent with the results of a previous study (Wang et al. 2008), we did not observe substantial LTE at the calyx (Fig. 4F and G), although pre-synaptic PNs form synapses with post-synaptic MB neurons at the calyx (Fig. 1A).
Associativity, input specificity and persistence of LTE
LTP in the mammalian hippocampus and post-tetanic potentiation in the Drosophila neuromuscular junction (Zhong & Wu, 1991) can be induced through the intensive stimulation of fibres of a single input pathway. Therefore, we examined whether the association of AL and AFV stimuli is essential for LTE formation or whether intense AL stimulation alone is sufficient (Fig. 5A). During associative stimulation, robust increases in Ca2+ responses were observed in MB neurons (Fig. 5B and C). To increase Ca2+ responses in MB neurons during intense AL stimulation alone, we extended the pulse duration from 1.0 to 1.5 ms. Although this intense AL stimulation produced increases in Ca2+ responses comparable with associative stimulation (Fig. 5B–D), no subsequent enhancement of AL-induced Ca2+ responses was observed (Fig. 5B and D). Thus, LTE at the AL–MB synapse probably requires the association of AL and AFV stimuli.
Figure 5. Associative stimulation of AL and AFV induces LTE in the AL–MB synaptic transmission.
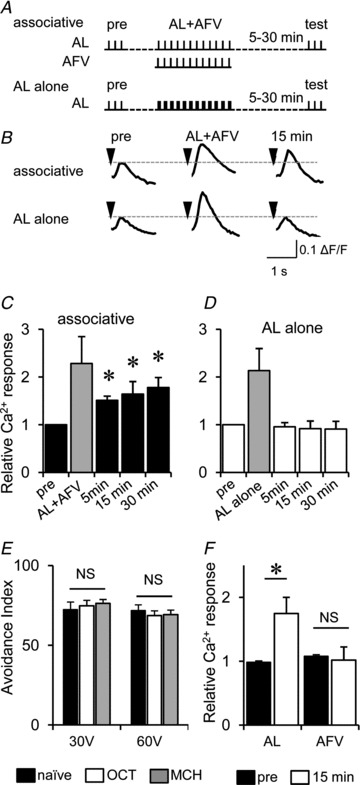
A, stimulation protocols for associative stimulations (associative) and intense AL stimulations (AL alone). B, typical traces of AL-induced Ca2+ responses before, during and after associative or AL-alone stimulation. C and D, summary of AL-induced Ca2+ responses in the MB before and after associative (C) or AL-alone (D) stimulation. The shaded columns indicate Ca2+ responses during associative and intense AL-alone stimulation (*P < 0.05 compared with ‘pre’ responses). E, avoidance of flies to 30 and 60 V electric shocks. Flies that had been previously trained to associate 3-octanol (OCT) or 4-methylcyclohexanol (MCH) with foot-shocks did not exhibit altered shock avoidance. Avoidance tests were performed at 15 min after conditioning. F, AL- and AFV-induced Ca2+ responses before (pre) and 15 min after the associative stimulation (*P < 0.05). NS, not significant; n= 6–8 for all data.
The association of an odour with foot-shock selectively increases avoidance to the odour but not to the shock (Fig. 5E). This observation prompted us to test the input specificity of LTE in the MBs. As shown in Fig. 5F, the simultaneous stimulation of AL and AFV enhanced AL-induced responses but not AFV-induced responses. This indicates that although stimulation of the AFV is required for LTE induction, LTE is selectively induced at AL–MB synapses but not at AFV–MB synapses.
To further assess the physiological properties of LTE at the AL–MB synapse, we examined the persistence of this LTE. When we stimulated AL at 120 min after the induction of LTE, we still observed a significant enhancement in the Ca2+ response (Fig. 6A, filled circles). However, when we stimulated AL repeatedly every 5 min after the associative stimulations, LTE decayed rapidly and disappeared within 1 h (Fig. 6B, filled circles). This decay was not the result of synaptic transmission fatigue between the AL and MB or the desensitization of postsynaptic responses, as repeated AL stimulation every 5 min did not decrease AL-induced Ca2+ responses (Fig. 6B, open circles).
Figure 6. LTE is extinguished through repetitive post-associative AL stimulation.
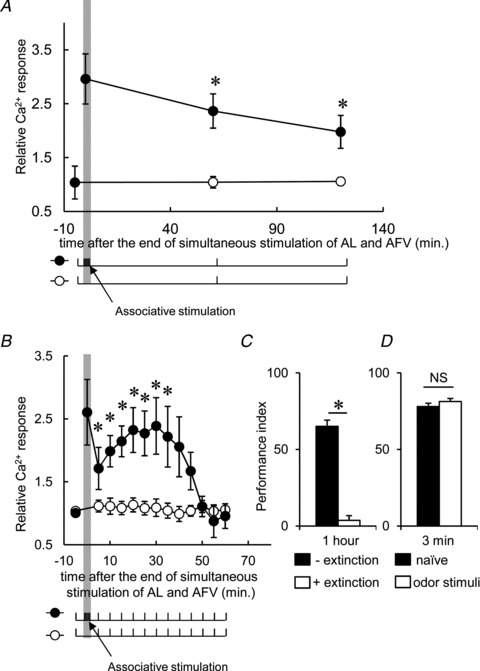
A, AL-induced Ca2+ responses in the MB measured at 60 and 120 min after associative stimulation (filled circles) were still significantly higher than the control responses (open circles), indicating that LTE persists for at least 120 min. B, the enhanced Ca2+ responses declined within 60 min through repetitive AL stimuli, induced every 5 min, after associative stimulation (filled circles). Ca2+ responses were not diminished by repetitive AL stimuli in the control MBs (open circles). C, extinction of olfactory memory in trained flies through repetitive presentation of the conditioned odour. D, the extinction protocol administered prior to olfactory conditioning did not affect olfactory learning (*P < 0.05 compared with pre-associative responses). n= 6–8 for all data.
Notably, the persistence of LTE is highly reminiscent of the persistence of olfactory memory. Similar to LTE, the olfactory memory formed by olfactory conditioning is extinguished through subsequent repeated exposure to the CS odours (Quinn et al. 1974; Qin & Dubnau, 2010). Indeed, the 1 h memory after olfactory conditioning was extinguished through repetitive CS presentations applied every 5 min (Fig. 6C). This effect was not due to desensitization, as repeated exposure to the CS odours every 5 min for 1 h prior to olfactory conditioning did not affect the 3 min memory, which represents learning ability and is lowered by desensitization to the odour used in subsequent olfactory conditioning (Fig. 6D).
nAChR and NMDA receptors mediate Ca2+ responses and LTE in the MBs
In previous studies, it was demonstrated that the cholinergic synaptic currents in MB neurons are mediated through Drosophila nicotinic acetylcholine receptors (dnAChRs), which are widely expressed in Drosophila brain including MBs (Jonas et al. 1994; Su & O’Dowd, 2003). Consistent with this result, we observed that AL-induced Ca2+ responses were suppressed in the presence of mecamylamine, an nAChR antagonist (Kazama & Wilson, 2008; Fig. 7A–C). However, the AFV-induced Ca2+ responses were not suppressed by mecamylamine (Fig. 7A and B).
Figure 7. dnAChR and dNR activities during associative stimulation are required for LTE.
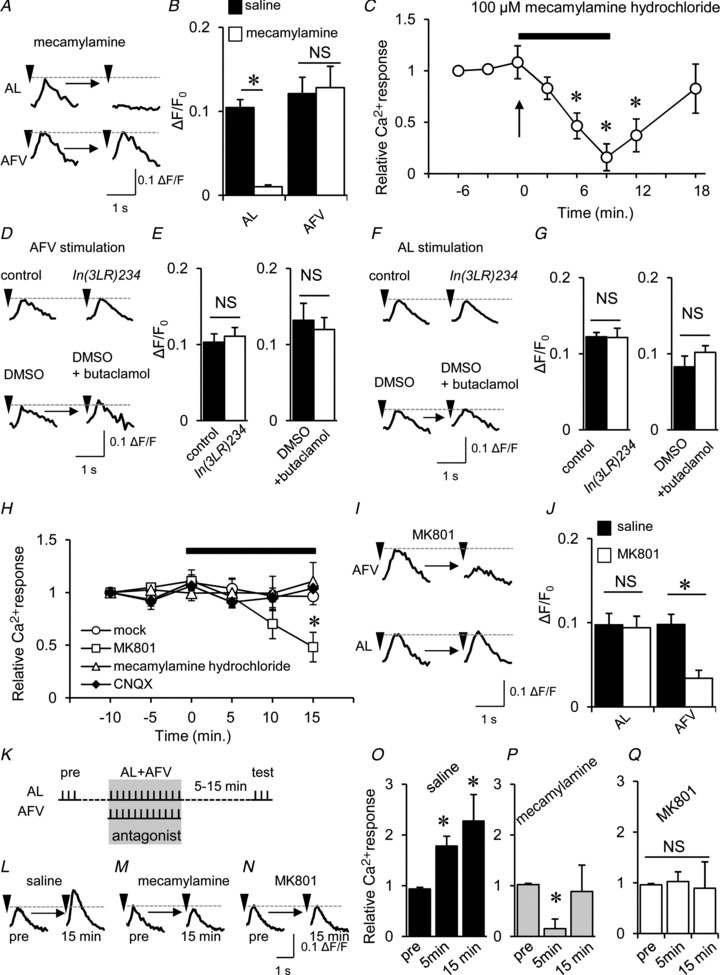
A, typical traces of AL- and AFV-induced Ca2+ responses before and after the application of 100 μm mecamylamine. B, summary of the effects of mecamylamine on AL- and AFV-induced Ca2+ responses (*P < 0.05). C, after bath application of 100 μm mecamylamine, AL-induced Ca2+ responses were decreased and abolished within 9 min. Subsequently, the responses were restored at 10 min after washout (18 min). D and E, neither DopR mutations nor the DopR antagonist (50 μm butaclamol) altered AFV-induced Ca2+ responses. Typical traces of AFV-induced Ca2+ responses (D) and the summary of the effects of DopR inhibitions (E). F and G, neither DopR mutations nor the DopR antagonist altered AL-induced Ca2+ responses. H, AFV-induced Ca2+ responses were suppressed using 100 μm MK801, while mecamylamine and the AMPA receptor antagonist CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) had no effect (*P < 0.05 compared with the response at 0 min). I and J, MK801 suppressed AFV-induced Ca2+ responses but not AL-induced Ca2+ responses. Typical AL- and AFV-induced Ca2+ traces (I), and averaged peak responses in saline (filled columns) and after (open columns) application of 100 μm MK801 (J) (*P < 0.05). K–Q, both dnAChR and dNR activities during associative stimulation are required for LTE formation. K, experimental design. While LTE was induced in control conditions (L, O), the application of mecamylamine (M, P) and MK801 (N, Q) during associative stimulation prevented LTE induction (*P < 0.05 compared with pre-associative responses (pre). n= 6–8 for all data.
In olfactory aversive conditioning, foot-shock information, which is conveyed through the AFV, can be substituted by artificial stimulation of dopaminergic (DAergic) neurons (Claridge-Chang et al. 2009; Aso et al. 2010). DA neurons innervate the MBs (Mao & Davis, 2009), and the dopamine D1 receptor, DopR (Sugamori et al. 1995), is required in the MBs for olfactory aversive memory formation (Kim et al. 2007). Therefore, we were curious to test whether AFV-induced Ca2+ responses are mediated by DopR, although it is classified as G-protein coupled receptor (GPCR) (Sugamori et al. 1995). However, AFV-induced Ca2+ responses were not suppressed by DopRIn(3LR)234 mutations (Kim et al. 2007) or exposure to butaclamol, a DopR antagonist (Sugamori et al. 1995; Fig. 7D and E). AL-induced Ca2+ responses were also unaffected by DopRIn(3LR)234 mutations and butaclamol (Fig. 7F and G). These results suggest that neither AL- nor AFV-induced Ca2+ responses in the MBs are mediated through DopR.
Drosophila NMDA receptors (dNRs) are expressed in the MBs and are involved in olfactory memory formation (Xia et al. 2005; Wu et al. 2007; Sinakevitch et al. 2010; Miyashita et al. 2012); therefore, we hypothesized that dNRs might mediate the AFV-induced Ca2+ response. Consistent with this hypothesis, AFV-induced Ca2+ responses were suppressed in response to exposure to the NR antagonist MK801 (Fig. 7H–J). These results indicate that while AL-induced Ca2+ responses are mediated through dnAChRs, AFV-induced Ca2+ responses are mediated through dNRs.
To test whether dnAChRs and dNRs are required for LTE formation, each receptor antagonist was applied during associative stimulations (Fig. 7K). As shown in Fig. 7L–Q, each antagonist suppressed LTE formation. Because AL-induced Ca2+ responses are mediated through dnAChRs and the effects of mecamylamine last approximately 10 min after washout (Fig. 7C), mecamylamine treatment reduced AL-induced Ca2+ responses at 5 min after associative stimulation (Fig. 7P).
DopR in the MBs is required for PAE formation
As reported previously (Kim et al. 2003, 2007), DopR is preferentially expressed in MB and required for olfactory aversive memory formation (Fig. 8A), although it mediates neither AL nor AFV-induced Ca2+ responses. To address whether DopRs are necessary for inducing LTE, we examined LTE in DopR mutants. Consistent with the behavioural data, LTE was not produced in DopR mutants (Fig. 8B and C). Furthermore, LTE formation in control brains was suppressed in response to butaclamol treatment (Fig. 8D and E) during associative stimulations (see Fig. 7K), indicating that DopR is physiologically required for LTE formation. Interestingly, Ca2+ responses during the associative stimulation were enhanced above control levels as a result of the DopR mutation (Fig. 8F) and in response to butaclamol treatment (Fig. 8G), although AL- and AFV-induced Ca2+ responses were not different from those in the control (Fig. 7D–G).
Figure 8. DopR activity during associative stimulation is required for LTE.
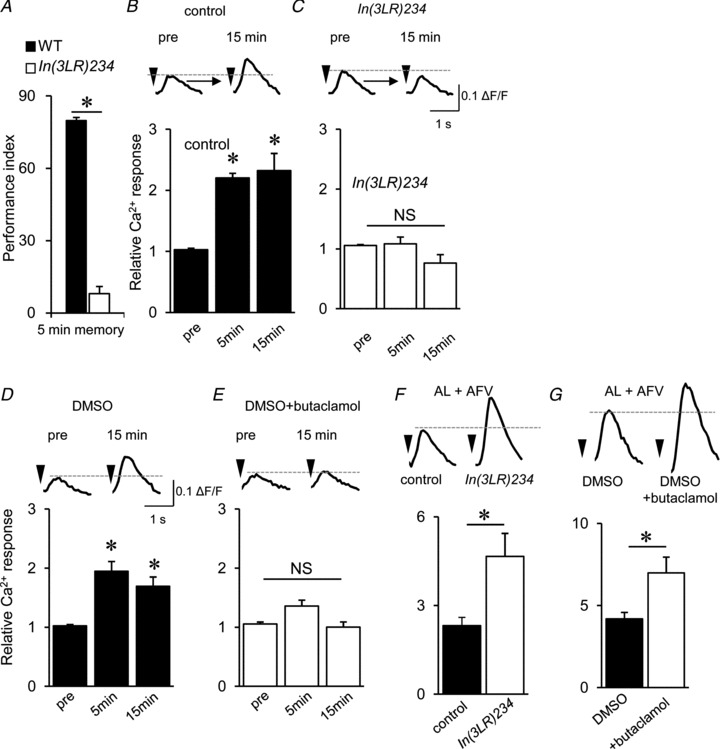
A, 5 min memory was impaired in the DopR mutant, DopRIn(3LR)234. B and C, DopR mutants are defective for AL–MB LTE formation. D and E, application of 50 μm butaclamol during associative stimulation blocked LTE induction. F and G, DopR mutations and butaclamol treatment further increased Ca2+ responses during associative stimulation compared with the wild-type control and DMSO treatment (*P < 0.05). n= 5–8 for all data.
Considering that DopR mutations do not affect AFV-induced Ca2+ responses in the MBs, DopRs expressed in areas outside of MB neurons might be required for LTE in contrast to the DopR requirement for olfactory aversive memory formation. To test this possibility, we used rescue experiments with DopRf02676 mutants (Kim et al. 2007). DopRf02676 is a null allele as a result of interference of transcription from its endogenous promoter by the insertion of piggyBac in the first intron. This piggyBac insertion acts as a carrier of rescue construct in the presence of GAL4, as the inserted piggyBac contains UAS, which functions as an exogenous enhancer/promoter when bound by GAL4 to initiate transcription of the downstream gene (Thibault et al. 2004). We used both the GAL4/UAS (Brand & Perrimon, 1993) and the LexA/LexAop binary systems (Lai & Lee, 2006; Fig. 9A and B) to generate transgenic flies expressing both the DopR transgene and G-CaMP2 (Tallini et al. 2006) independently in the MBs of DopRf02676. Similar to hypomorphic DopRIn(3LR)234 mutants, LTE did not occur in the DopRf02676-null mutants (Fig. 9C and D). However, in a rescue line expressing DopRs in the MBs, LTE was restored to the control level (Fig. 9E). Notably, while LTE was restored in this transgenic line, the hyper-enhancement of Ca2+ responses during associative stimulation was not reduced to normal levels (Fig. 9F). These results suggest that while DopR in the MBs is essential for LTE formation, the larger Ca2+ response during associative stimulation results from defects in DopR expressed in areas outside of the MBs.
Figure 9. DopRs expressed in the MBs are required for LTE formation.
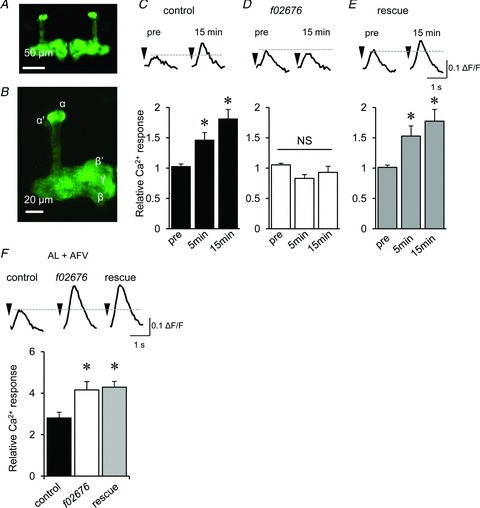
A, G-CaMP2 fluorescence observed in the MBs of an MBp-LexA;LexAop-G-CaMP2 fly. B, G-CaMP2 fluorescence is observed in all α, α′, β, β′ and γ lobes of an MBp-Lex;LexAop-G-CaMP2 brain. C–E, AL–MB LTE (C) disrupted in DopR mutants (D) was restored through expression of the DopR+ transgene in the MBs (E). AL-induced Ca2+ responses before (pre) and after associative stimulation were recorded in the MBs from control MBp-Lex,LexAop-G-CaMP2/+ (C), MBp-Lex,LexAop-GCaMP2/+;DopRf02676/DopRf02676 (D) and MBp-Lex,LexAop-GCaMP2/c309;DopRf02676/DopRf02676 flies (E) (*P < 0.05 compared with pre-associative stimulation). F, the larger increase in Ca2+ responses during associative stimulation in DopRf02676 (open column) were not decreased in the rescue line (shaded column) (*P < 0.05 compared with control brains). n= 6–7 for all data.
Discussion
Using in vitro imaging, we directly measured AL–MB synaptic transmission and successively observed the induction, persistence and decline of LTE at the AL–MB synapse. Previous studies have shown that odour-induced Ca2+ responses in the MBs were increased more than 1 h after single-cycle olfactory aversive conditioning (Wang et al. 2008; Tan et al. 2010). However, the synaptic basis of such neural plasticity in the MBs is not understood. Odour information received through ORNs in the AN and MPs is transmitted to PNs in the AL, which in turn convey signals to MB neurons. Although synaptic transmission from ORNs to PNs is also increased after olfactory conditioning, this increase disappears within 10 min (Yu et al. 2004). Thus, it has been suggested that memory trace might be formed through the enhancement of AL–MB synaptic transmission. Consistent with this hypothesis, LTE at the AL–MB synapse lasts more than 2 h.
AL–MB LTE exhibits characteristic physiological properties, including associativity, input specificity and persistence. Importantly, these properties are also observed in olfactory aversive memory. While AL–MB LTE formation requires the correlated activity of AL and AFV inputs, olfactory aversive memory formation requires the correlated presentation of CS odour and US foot-shock. In olfactory aversive conditioning, odour and foot-shock signals are associated in the MBs. In this study, we demonstrated that signals delivered from the AL and AFV are associated in the MBs and produce LTE at AL–MB synapses. Trained flies increase avoidance to CS odour but not to US foot-shock. Similarly, LTE is specifically formed at AL–MB but not AFV–MB synapses. This input specificity of LTE might explain why avoidance to foot-shock is not increased following olfactory aversive conditioning. Furthermore, both AL–MB LTE and olfactory memory were reduced after subsequent repetitive AL stimulations and CS odour presentations, respectively. This suggests that attenuated AL–MB synaptic transmission might be involved in the memory extinction process. In addition to these physiological similarities, both AL–MB LTE and olfactory aversive memory require the activity of dNRs and DopRs during association. These results demonstrate that LTE at the AL–MB synapse can be an appropriate cellular model for olfactory memory.
In this study, we employed 20 mm Mg2+ in the external recording solution because various groups have shown that the Mg2+ concentration in the Drosophila haemolymph ranges between 20 and 33 mm (Croghan & Lockwood, 1960; Begg & Cruickshank, 1963; Stewart et al. 1994). In mammals, the Mg2+ concentration is higher in the cerebrospinal fluid than in the plasma (McKee et al. 2005). Furthermore, while ≤10 mm Mg2+ concentration is not sufficient for the Mg2+ blockade of Drosophila NMDA receptors, 20 mm Mg2+ is sufficient to produce this effect (Miyashita et al. 2012). We thus propose that the 20 mm Mg2+ concentration used in our study lies within the physiologically relevant range. We employed a much higher Mg2+ concentration (20 mm) than those used in previous in vivo and in vitro imaging studies (<4 mm). Therefore, we only observed unconvincing or weak AL-induced Ca2+ responses in the horizontal lobes and the calyx. In contrast, previous studies employing lower Mg2+ concentrations reveal significant Ca2+ responses in these MB domains upon odour presentation or stimulation of the AN (Yu et al. 2006; Wang et al. 2008). These differential sensitivities in Ca2+ responsiveness to external Mg2+ implicate the heterogeneous expression or property of Ca2+ channels in these MB domains.
As the D1 receptor is a GPCR but not ionotropic receptor (Sugamori et al. 1995), it is reasonable that AFV-induced Ca2+ responses were not suppressed by DopR mutations or butaclamol treatment. Instead of DopRs, we observed that AFV-induced Ca2+ responses were mediated through ionotropic dNRs. Our data demonstrated that dNR was not dispensable for LTE formation. Consistently, dNR is reported to be essential for olfactory memory formation (Xia et al. 2005; Miyashita et al. 2012), further suggesting that the coincident activation of the dnAChRs, dNRs and DopRs during associative stimulations is required for LTE formation. As US foot-shock can be substituted by the artificial activation of DA neurons (Claridge-Chang et al. 2009; Aso et al. 2010), AFV stimulation might directly or indirectly activate DA neurons and thereby activate DopR in the MBs.
Interestingly, Ca2+ responses during associative AL and AFV stimulations were increased above control levels following DopR mutations and butaclamol treatment. Previous in vivo and in vitro studies suggest that cholinergic excitatory postsynaptic currents and Ca2+ influx in MB neurons are suppressed in response to dopamine through the D1 receptor (Yuan & Lee, 2007; Tsydzik & Wright, 2009). Hence, DopR inhibition might disinhibit cholinergic transmission, causing a hyper-enhancement of the Ca2+ response during associative AL and AFV stimulations. However, this is unlikely to be the case because the cholinergic AL-induced Ca2+ response in the MBs was not increased by DopR mutations or butaclamol treatment. Significantly, while expressing DopR in MB neurons restored LTE, it did not reduce the hyper-enhancement of Ca2+ responses during associative stimulations, suggesting that the hyper-enhancement of Ca2+ responses is not mediated through DopRs in MB neurons and that DopRs which suppress hyper-enhancement are not involved in LTE formation.
The physiological properties of AL–MB LTE are similar to those of mammalian hippocampal LTP (Daw et al. 1993; Gruart & Delgado-Garcia, 2007). For example, both require the coincident activation of pre- and post-synaptic cells and NR activity during the induction period. However, several aspects of LTE are different from LTP. For LTE induction, the simultaneous stimulation of two different input pathways is required, while the intense tetanic stimulation of a single input pathway is sufficient to induce LTP. In addition, repeated AL stimulation after LTE induction extinguishes LTE, while this extinction property has not been reported for LTP.
Due to deleterious effects of stimulations by glass micro-electrodes, we did not measure Ca2+ responses over 2 h. However, in our culture conditions, we could record AL- and AFV-induced Ca2+ responses in the MBs, even in brains cultured for over 1 week (data not shown). Previous studies demonstrate the formation of memory trace in the MBs after training which forms long-term memory (Yu et al. 2006; Akalal et al. 2010). Thus, we expect to examine LTP further, such as late-phase LTP, successively throughout induction and retention using an in vitro imaging system in conjunction with photo-activation of neurons expressing Channelrhodopsin-2. Because of its small size, the Drosophila brain has been a difficult target for traditional electrophysiological analyses. However, the development of optogenetic and Ca2+ imaging techniques has made the small size and high transparency of Drosophila brains advantageous, allowing the simultaneous stimulation and recording in various brain regions. Using appropriate imaging probes to measure the activity of second messengers, kinases and transcription factors, in vitro imaging with isolated brains has the potential for elucidating the dynamics of these molecules in neural networks.
Acknowledgments
We thank J. Dubnau (Cold Spring Harbor Laboratory) for DopR mutants, K. Scott (University of California, Berkeley) for UAS-G-CaMP transgenic flies, S. Diegelmann (University of Cambridge, UK) for pCasper-W-LexA::GAD and pCasper-lexAop-W vectors and J. Nakai (Saitama University, Japan) for G-CaMP2. We also thank Ms Fukuda and Ms Ofusa for stock maintenance and rearing flies. This research was supported by a Grant-in-Aid for Scientific Research in Innovative Areas ‘Systems Molecular Ethology’ to M.S., and MEXT/JSPS KAKENHI Grant (21700376 and 23700405) to K.U.
Glossary
- AFV
ascending fibres of the ventral nerve cord
- AL
antennal lobe
- AN
antennae
- CS
conditioned stimulus
- DA
dopamine
- dnAChR
Drosophila nicotinic acetylcholine receptor
- dNR
Drosophila NMDA receptor
- DopR
Drosophila D1 dopamine receptor
- GPCR
G-protein coupled receptor
- LH
lateral horn
- LTE
long-term enhancement
- LTP
long-term potentiation
- MB
mushroom body
- MP
maxillary palp
- ORN
olfactory receptor neuron
- PN
projection neuron
- US
unconditioned stimulus
- VNC
ventral nerve cord
Author contributions
K.U. designed and performed most of the experiments. S.N. contributed to the imaging study, and Y.H. contributed to the genetics and behaviour studies. M.S. supervised and wrote the manuscript with K.U. and J.H.
References
- Akalal DB, Yu D, Davis RL. A late-phase, long-term memory trace forms in the γ neurons of Drosophila mushroom bodies after olfactory classical conditioning. J Neurosci. 2010;30:16699–16708. doi: 10.1523/JNEUROSCI.1882-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aso Y, Siwanowicz I, Bracker L, Ito K, Kitamoto T, Tanimoto H. Specific dopaminergic neurons for the formation of labile aversive memory. Curr Biol. 2010;20:1445–1451. doi: 10.1016/j.cub.2010.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg M, Cruickshank WJ. A partial analysis of Drosophila larval haemolymp. Proc Roy Soc Edinburgh. 1963;68:215–236. [Google Scholar]
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Claridge-Chang A, Roorda RD, Vrontou E, Sjulson L, Li H, Hirsh J, Miesenbock G. Writing memories with light-addressable reinforcement circuitry. Cell. 2009;139:405–415. doi: 10.1016/j.cell.2009.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crittenden JR, Skoulakis EM, Han KA, Kalderon D, Davis RL. Tripartite mushroom body architecture revealed by antigenic markers. Learn Mem. 1998;5:38–51. [PMC free article] [PubMed] [Google Scholar]
- Croghan PC, Lockwood APM. The composition of the haemolyph of the larva of Drosophila melanogaster. J Exp Biol. 1960;37:339–343. [Google Scholar]
- Daw NW, Stein PS, Fox K. The role of NMDA receptors in information processing. Annu Rev Neurosci. 1993;16:207–222. doi: 10.1146/annurev.ne.16.030193.001231. [DOI] [PubMed] [Google Scholar]
- de Belle JS, Heisenberg M. Associative odour learning in Drosophila abolished by chemical ablation of mushroom bodies. Science. 1994;263:692–695. doi: 10.1126/science.8303280. [DOI] [PubMed] [Google Scholar]
- Diegelmann S, Bate M, Landgraf M. Gateway cloning vectors for the LexA-based binary expression system in Drosophila. Fly (Austin) 2008;2:236–239. doi: 10.4161/fly.6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau J, Grady L, Kitamoto T, Tully T. Disruption of neurotransmission in Drosophila mushroom body blocks retrieval but not acquisition of memory. Nature. 2001;411:476–480. doi: 10.1038/35078077. [DOI] [PubMed] [Google Scholar]
- Dura JM, Preat T, Tully T. Identification of linotte, a new gene affecting learning and memory in Drosophila melanogaster. J Neurogenet. 1993;9:1–14. doi: 10.3109/01677069309167272. [DOI] [PubMed] [Google Scholar]
- Gruart A, Delgado-Garcia JM. Activity-dependent changes of the hippocampal CA3-CA1 synapse during the acquisition of associative learning in conscious mice. Genes Brain Behav. 2007;6(Suppl 1):24–31. doi: 10.1111/j.1601-183X.2007.00319.x. [DOI] [PubMed] [Google Scholar]
- Ito K, Awano W, Suzuki K, Hiromi Y, Yamamoto D. The Drosophila mushroom body is a quadruple structure of clonal units each of which contains a virtually identical set of neurones and glial cells. Development. 1997;124:761–771. doi: 10.1242/dev.124.4.761. [DOI] [PubMed] [Google Scholar]
- Jonas PE, Phannavong B, Schuster R, Schroder C, Gundelfinger ED. Expression of the ligand-binding nicotinic acetylcholine receptor subunit D alpha 2 in the Drosophila central nervous system. J Neurobiol. 1994;25:1494–1508. doi: 10.1002/neu.480251203. [DOI] [PubMed] [Google Scholar]
- Kazama H, Wilson RI. Homeostatic matching and nonlinear amplification at identified central synapses. Neuron. 2008;58:401–413. doi: 10.1016/j.neuron.2008.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Han KA. D1 dopamine receptor dDA1 is required in the mushroom body neurons for aversive and appetitive learning in Drosophila. J Neurosci. 2007;27:7640–7647. doi: 10.1523/JNEUROSCI.1167-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Lee HG, Seong CS, Han KA. Expression of a D1 dopamine receptor dDA1/DmDOP1 in the central nervous system of Drosophila melanogaster. Gene Expr Patterns. 2003;3:237–245. doi: 10.1016/s1567-133x(02)00098-4. [DOI] [PubMed] [Google Scholar]
- Krashes MJ, Keene AC, Leung B, Armstrong JD, Waddell S. Sequential use of mushroom body neuron subsets during Drosophila odour memory processing. Neuron. 2007;53:103–115. doi: 10.1016/j.neuron.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai SL, Lee T. Genetic mosaic with dual binary transcriptional systems in Drosophila. Nat Neurosci. 2006;9:703–709. doi: 10.1038/nn1681. [DOI] [PubMed] [Google Scholar]
- Mao Z, Davis RL. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: anatomical and physiological heterogeneity. Front Neural Circuits. 2009;3:5. doi: 10.3389/neuro.04.005.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin EC, Jefferis GS, Komiyama T, Zhu H, Luo L. Representation of the glomerular olfactory map in the Drosophila brain. Cell. 2002;109:243–255. doi: 10.1016/s0092-8674(02)00700-6. [DOI] [PubMed] [Google Scholar]
- Martin JR, Rogers KL, Chagneau C, Brulet P. In vivo bioluminescence imaging of Ca signalling in the brain of Drosophila. PLoS One. 2007;2:e275. doi: 10.1371/journal.pone.0000275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire SE, Le PT, Davis RL. The role of Drosophila mushroom body signalling in olfactory memory. Science. 2001;293:1330–1333. doi: 10.1126/science.1062622. [DOI] [PubMed] [Google Scholar]
- McKee JA, Brewer RP, Macy GE, Phillips-Bute B, Campbell KA, Borel CO, Reynolds JD, Warner DS. Analysis of the brain bioavailability of peripherally administered magnesium sulfate: a study in humans with acute brain injury undergoing prolonged induced hypermagnesemia. Crit Care Med. 2005;33:661–666. doi: 10.1097/01.ccm.0000156293.35868.b2. [DOI] [PubMed] [Google Scholar]
- Miyashita T, Oda Y, Horiuchi J, Yin Jerry CP, Morimoto T, Saitoe M. Mg2+ block of Drosophila NMDA receptors is required for long-term memory formation and CREB-dependent gene expression. Neuron. 2012;74:887–898. doi: 10.1016/j.neuron.2012.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Orive J, Mazor O, Turner GC, Cassenaer S, Wilson RI, Laurent G. Oscillations and sparsening of odour representations in the mushroom body. Science. 2002;297:359–365. doi: 10.1126/science.1070502. [DOI] [PubMed] [Google Scholar]
- Qin H, Dubnau J. Genetic disruptions of Drosophila Pavlovian learning leave extinction learning intact. Genes Brain Behav. 2010;9:203–212. doi: 10.1111/j.1601-183X.2009.00548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn WG, Harris WA, Benzer S. Conditioned behaviour in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1974;71:708–712. doi: 10.1073/pnas.71.3.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajashekar KP, Shamprasad VR. Maxillary palp glomeruli and ipsilateral projections in the antennal lobe of Drosophila melanogaster. J Biosci. 2004;29:423–429. doi: 10.1007/BF02712114. [DOI] [PubMed] [Google Scholar]
- Sinakevitch I, Grau Y, Strausfeld NJ, Birman S. Dynamics of glutamatergic signalling in the mushroom body of young adult Drosophila. Neural Dev. 2010;5:10. doi: 10.1186/1749-8104-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart BA, Atwood HL, Renger JJ, Wang J, Wu CF. Improved stability of Drosophila larval neuromuscular preparations in haemolymph-like physiological solutions. J Comp Physiol A. 1994;175:179–191. doi: 10.1007/BF00215114. [DOI] [PubMed] [Google Scholar]
- Su H, O’Dowd DK. Fast synaptic currents in Drosophila mushroom body Kenyon cells are mediated by α-bungarotoxin-sensitive nicotinic acetylcholine receptors and picrotoxin-sensitive GABA receptors. J Neurosci. 2003;23:9246–9253. doi: 10.1523/JNEUROSCI.23-27-09246.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI. Imaging cellular signals in the heart in vivo: cardiac expression of the high-signal Ca2+ indicator GCaMP2. Proc Natl Acad Sci U S A. 2006;103:4753–4758. doi: 10.1073/pnas.0509378103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura T, Chiang AS, Ito N, Liu HP, Horiuchi J, Tully T, Saitoe M. Aging specifically impairs amnesiac-dependent memory in Drosophila. Neuron. 2003;40:1003–1011. doi: 10.1016/s0896-6273(03)00732-3. [DOI] [PubMed] [Google Scholar]
- Tan Y, Yu D, Pletting J, Davis RL. Gilgamesh is required for rutabaga-independent olfactory learning in Drosophila. Neuron. 2010;67:810–820. doi: 10.1016/j.neuron.2010.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thibault ST, Singer MA, Miyazaki WY, Milash B, Dompe NA, Singh CM, Buchholz R, Demsky M, Fawcett R, Francis-Lang HL, Ryner L, Cheung LM, Chong A, Erickson C, Fisher WW, Greer K, Hartouni SR, Howie E, Jakkula L, Joo D, Killpack K, Laufer A, Mazzotta J, Smith RD, Stevens LM, Stuber C, Tan LR, Ventura R, Woo A, Zakrajsek I, Zhao L, Chen F, Swimmer C, Kopczynski C, Duyk G, Winberg ML, Margolis J. A complementary transposon tool kit for Drosophila melanogaster using P and piggyBac. Nat Genet. 2004;36:283–287. doi: 10.1038/ng1314. [DOI] [PubMed] [Google Scholar]
- Tomchik SM, Davis RL. Dynamics of learning-related cAMP signalling and stimulus integration in the Drosophila olfactory pathway. Neuron. 2009;64:510–521. doi: 10.1016/j.neuron.2009.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsydzik V, Wright NJ. Dopamine modulation of the in vivo acetylcholine response in the Drosophila mushroom body. Dev Neurobiol. 2009;69:705–714. doi: 10.1002/dneu.20716. [DOI] [PubMed] [Google Scholar]
- Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- Wang Y, Mamiya A, Chiang AS, Zhong Y. Imaging of an early memory trace in the Drosophila mushroom body. J Neurosci. 2008;28:4368–4376. doi: 10.1523/JNEUROSCI.2958-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong AM, Wang JW, Axel R. Spatial representation of the glomerular map in the Drosophila protocerebrum. Cell. 2002;109:229–241. doi: 10.1016/s0092-8674(02)00707-9. [DOI] [PubMed] [Google Scholar]
- Wu CL, Xia S, Fu TF, Wang H, Chen YH, Leong D, Chiang AS, Tully T. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci. 2007;10:1578–1586. doi: 10.1038/nn2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S, Miyashita T, Fu TF, Lin WY, Wu CL, Pyzocha L, Lin IR, Saitoe M, Tully T, Chiang AS. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang MY, Armstrong JD, Vilinsky I, Strausfeld NJ, Kaiser K. Subdivision of the Drosophila mushroom bodies by enhancer-trap expression patterns. Neuron. 1995;15:45–54. doi: 10.1016/0896-6273(95)90063-2. [DOI] [PubMed] [Google Scholar]
- Yasuyama K, Meinertzhagen IA, Schurmann FW. Synaptic organization of the mushroom body calyx in Drosophila melanogaster. J Comp Neurol. 2002;445:211–226. doi: 10.1002/cne.10155. [DOI] [PubMed] [Google Scholar]
- Yu D, Akalal DB, Davis RL. Drosophilaα/β mushroom body neurons form a branch-specific, long-term cellular memory trace after spaced olfactory conditioning. Neuron. 2006;52:845–855. doi: 10.1016/j.neuron.2006.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu D, Ponomarev A, Davis RL. Altered representation of the spatial code for odors after olfactory classical conditioning; memory trace formation by synaptic recruitment. Neuron. 2004;42:437–449. doi: 10.1016/s0896-6273(04)00217-x. [DOI] [PubMed] [Google Scholar]
- Yuan N, Lee D. Suppression of excitatory cholinergic synaptic transmission by Drosophila dopamine D1-like receptors. Eur J Neurosci. 2007;26:2417–2427. doi: 10.1111/j.1460-9568.2007.05870.x. [DOI] [PubMed] [Google Scholar]
- Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]


