Abstract
Nectins are Ca2+-independent immunoglobulin (Ig)-like cell-cell adhesion molecules. The trans-interactions of nectins recruit cadherins to the nectin-based cell-cell adhesion, resulting in formation of cell-cell adherens junctions (AJs) in epithelial cells and fibroblasts. The trans-interaction of E-cadherin induces activation of Rac small G protein, whereas the trans-interactions of nectins induce activation of not only Rac but also Cdc42 small G protein. We showed by the fluorescent resonance energy transfer (FRET) imaging that the trans-interaction of E-cadherin induced dynamic activation and inactivation of Rac, which led to dynamic formation and retraction of lamellipodia. Moreover, we found here that the nectins, which did not trans-interact with other nectins (non–trans-interacting nectins), inhibited the E-cadherin–induced activation of Rac and reduced the velocity of the formation of the E-cadherin-based cell-cell AJs. The inhibitory effect of non–trans-interacting nectins was suppressed by the activation of Cdc42 induced by the trans-interactions of nectins. These results indicate a novel role of nectins in regulation of the E-cadherin–induced activation of Rac and formation of cell-cell AJs.
INTRODUCTION
Cadherins are Ca2+-dependent cell-cell adhesion molecules that form AJs (Takeichi, 1991; Gumbiner, 1996; Yagi and Takeichi, 2000). E-Cadherin, like other classical cadherins, is a single-pass transmembrane protein whose extracellular domain mediates homophilic recognition and adhesive binding (Takeichi, 1991). The cytoplasmic tail of E-cadherin is linked to the actin cytoskeleton through many peripheral membrane proteins, including α-catenin, β-catenin, vinculin, and α-actinin, which strengthen the cell-cell adhesion activity of E-cadherin (Nagafuchi, 2001).
Nectins are Ca2+-independent Ig-like cell-cell adhesion molecules that comprise a family of four members, nectin-1, -2, -3, and -4 (Takai and Nakanishi, 2003; Takai et al., 2003). Nectins form homo-cis-dimers and then homo-trans-dimers (trans-interactions), causing cell-cell adhesion. Nectin-3 furthermore forms hetero-trans-dimers with either nectin-1 or -2. Nectin-4 also forms hetero-trans-dimers with nectin-1. All the nectin family members, except nectin-1β, -1γ, -3γ, and -4, have a conserved motif of four amino acid (aa) residues (Glu/Ala-X-Tyr-Val) at their carboxyl termini, and this binds the PDZ domain of afadin, an actin-filament (F-actin)-binding protein. Although nectin-4 lacks this conserved motif, it binds afadin. Evidence has accumulated that the trans-interactions of nectins recruit cadherins to the nectin-based cell-cell adhesion, resulting in formation of AJs in epithelial cells and fibroblasts and in formation of synapses in neurons (Takai and Nakanishi, 2003). Furthermore, the trans-interactions of nectins recruit first junctional adhesion molecules (JAMs) and then claudins and occludin to the apical side of AJs in cooperation with the trans-interactions of cadherins, resulting in formation of tight junctions (Fukuhara et al., 2002a, 2002b). Claudins are key Ca2+-independent cell-cell adhesion molecules at tight junctions (Tsukita and Furuse, 1999). The function of occludin has not yet been established, but JAMs are Ca2+-independent Ig-like cell-cell adhesion molecules, which recruit the cell polarity protein complex, consisting of Par-3, atypical PKC, and Par-6, by directly binding Par-3 (Ohno, 2001). We have recently demonstrated that Par-3 binds to the C-terminal four aa of nectin-1 and -3, but not nectin-2, through its PDZ domain (Takekuni et al., 2003).
Recent studies have demonstrated that the trans-interaction of E-cadherin induces activation of Rac small G protein, but not that of Cdc42 small G protein, resulting in formation of lamellipodia, in several cell lines including epithelial cells and fibroblasts (Nakagawa et al., 2001; Noren et al., 2001; Betson et al., 2002; Kovacs et al., 2002). Although two studies (Betson et al., 2002; Ehrlich et al., 2002) have shown that activation of Rac upon the E-cadherin–mediated formation of cell-cell adhesion is independent of phosphatidylinositol-3 (PI3) kinase, two other studies (Nakagawa et al., 2001; Kovacs et al., 2002) have shown that PI3 kinase is required for the full activation of Rac by the trans-interaction of E-cadherin.
On the other hand, the trans-interactions of nectins induce activation of not only Rac but also Cdc42 in a PI3 kinase-independent manner, resulting in formation of lamellipodia and filopodia, respectively, and activation of c-Jun N-terminal kinase, but not p38 mitogen-activated kinase or extracellular signal-regulated kinase (Kawakatsu et al., 2002; Honda et al., 2003b). It has previously been shown that formation and maintenance of AJs are regulated by Cdc42 and Rac in epithelial cells (Braga et al., 1997; Kuroda et al., 1997; Takaishi et al., 1997; Jou and Nelson, 1998; Kodama et al., 1999; Ehrlich et al., 2002). Although the modes of action of Cdc42 and Rac for the formation and maintenance of AJs still remain to be elucidated, the activation of Cdc42 and Rac upon the trans-interactions of cadherins and/or those of nectins may be involved in regulation of the formation and/or maintenance of AJs.
We have studied here the role of nectins in the E-cadherin–induced activation of Rac and formation of cell-cell AJs and found that the nectins, which do not trans-interact with other nectins (non–trans-interacting nectins), inhibit both the E-cadherin–induced activation of Rac and formation of cell-cell AJs. Taken together with our previous observations, the present results indicate that nectins positively and negatively regulate the E-cadherin functions through regulation of the Rac activity. The present results have provided a novel molecular mechanism of how nectins play an important role in the formation of AJs in cooperation with E-cadherin in epithelial cells.
MATERIALS AND METHODS
Expression Plasmids
Expression vectors for green fluorescent protein (GFP)-tagged NWASP-Cdc42 and Rac interactive binding domain (CRIB) (pEGFP-NWASP-CRIB), FLAG-tagged nectin-1-ΔC (pCAGIPuro-FLAG-nectin-1-ΔC), FLAG-tagged nectin-2α (pCAGIPuro-FLAG-nectin-2α), FLAG-tagged nectin-3α (pCAGIPuro-FLAG-nectin-3α), Myc-tagged V12Cdc42 (pEFBOS-myc-V12Cdc42), and FLAG-tagged N17Rac1 (pEFBOS-FLAG-N17Rac1) were prepared as described (Kodama et al., 1999; Takahashi et al., 1999; Satoh-Horikawa et al., 2000; Kawakatsu et al., 2002). The expression vector for a point-mutated form of nectin-2α (pm-nectin-2α), which contained the following sequence: substitution of TTT at codon 136 with CTT (Phe to Leu) (pCAGIPuro-pm-nectin-2α), was prepared as described (Miyahara et al., 2000). The expression vectors for GFP-tagged V12Rac1 (pEGFP-V12Rac1) and GFP-tagged N17Rac1 (pEGFP-N17Rac1) were kind gifts from Dr. S. Narumiya (Kyoto University, Kyoto, Japan). pRaichu-Rac1, pRaichu-Rac1-Y40C, pRaichu-Cdc42, and pRaichu-Cdc42-Y40C were constructed as described (Itoh et al., 2002). The retrovirus expression vector for GFP (pMXII-GFPN) was prepared as described (Ono et al., 2000). pMXII-E-cadherin-GFPN for E-cadherin fused to GFP was prepared by inserting the cDNA fragment encoding mouse E-cadherin into the 5′ end of the cDNA fragment encoding GFP in pMXII-GFPN. The cDNA of E-cadherin was a kind gift from Dr. Sh. Tsukita (Kyoto University, Kyoto, Japan). All the constructs used here were confirmed by sequencing.
Antibodies and Proteins
A mouse anti-afadin mAb was prepared as described (Sakisaka et al., 1999). A rat anti–E-cadherin mAb (ECCD-2) was kindly supplied from Dr. M. Takeichi (RIKEN Center for Developmental Biology, Kobe, Japan). A mouse anti–ZO-1 mAb was kindly supplied from Dr. Sh. Tsukita (Kyoto University, Kyoto, Japan). Hybridoma cells expressing the mouse anti-myc mAb (9E10) were obtained from American Type Culture Collection (Manassas, VA) as described (Kodama et al., 1999). A rabbit anti-GFP polyclonal antibody (pAb; BD Biosciences Clontech, Palo Alto, CA) and secondary antibodies (Chemicon, Temecula, CA) were purchased from commercial sources. The extracellular fragments of E-cadherin and nectin-1, -2, or -3 fused to the Fc portion of human IgG (Cef and Nef-1, -2, or -3, respectively) were prepared as described (Honda et al., 2003c).
Cell Culture and DNA Transfection
L and EL cells were supplied from Dr. Sh. Tsukita (Kyoto University, Kyoto, Japan). EL cells were cloned by introduction of the exogenous E-cadherin cDNA to cadherin-deficient L cells (Nagafuchi et al., 1987). EL cells stably expressing exogenous full-length nectin-1α and C-terminal 4 aa–deleted nectin-1α (nectin-1-EL and nectin-1-ΔC-EL cells, respectively) were prepared as described (Tachibana et al., 2000). Nectin-2- and -3-EL cells were prepared by transfecting pCAGIPuro-FLAG-nectin-2α and pCAGIPuro-FLAG-nectin-3α, respectively, and by selecting for transfectants with 5 μg/ml puromycin. MDCK cells were kindly supplied from Dr. W. Birchmeier (Max-Delbruck-Center for Molecular Medicine, Berlin, Germany). MDCK cells stably expressing exogenous full-length nectin-1α (nectin-1-MDCK cells) were prepared as described (Takahashi et al., 1999). MDCK cells stably expressing both exogenous full-length nectin-1α and pm-nectin-2α (pm-nectin-2-nectin-1-MDCK cells) were prepared by transfecting pCAGIPuro-pm-nectin-2α in nectin-1-MDCK cells and selecting for a transfectant with 5 μg/ml puromycin. The transfection was done with the plasmids as described (Takahashi et al., 1999). Ecotropic retrovirus competent MDCK cells were prepared as described (Nishimura et al., 2002). MDCK cells stably expressing E-cadherin-GFPN (E-cadherin-GFPN-MDCK cells) were prepared by infecting ecotropic retrovirus competent MDCK cells with pMXII-E-cadherin-GFPN as described (Nishimura et al., 2002).
Cell Adhesion Assay
The cell adhesion assay was performed as described (Kawakatsu et al., 2002) with some modifications. Briefly, coverslips were coated with 50 μg/ml Cef, 50 μg/ml Nef-3, a mixture of 50 μg/ml Cef and 50 μg/ml Nef-3, a mixture of 50 μg/ml Cef, 50 μg/ml Nef-1, 50 μg/ml Nef-2, and 50 μg/ml Nef-3, or 50 μg/ml human IgG as a control and blocked with 1% BSA in HBSS plus 5 mM CaCl2. For EL cells and EL cells stably expressing exogenous nectins, these EL cells were washed with PBS, incubated with 0.01% trypsin and 1 mM CaCl2 at 37°C for 40 min, and dispersed by pipetting. For wild-type MDCK cells, E-cadherin-GFPN-MDCK cells, and E-cadherin-GFPN-MDCK cells transiently expressing exogenous nectins, these MDCK cells were washed with PBS, incubated with 0.25% trypsin and 1 mM EDTA at 37°C for 15 min, and dispersed by pipetting. The dispersed MDCK cells were placed on culture dishes and cultured for 6 h. The signal for E-cadherin at cell-cell contact sites in these MDCK cells were concentrated at 4 h after the plating (our unpublished results). After the 6-h culture, the MDCK cells were washed with PBS, incubated with 0.01% trypsin and 1 mM CaCl2 at 37°C for 40 min, and dispersed vigorously by pipetting. The dispersed EL and MDCK cells were then suspended in DMEM containing 10% FCS. The cells (1.0 × 104) were placed on the coverslips coated with Cef, Nef-3, a mixture of Cef and Nef-3, a mixture of Cef, Nef-1, Nef-2, and Nef-3, or human IgG in a 24-well dish and cultured for indicated periods of time. The cells were then fixed, followed by immunostaining. Immunofluorescence microscopy of cultured cells was done as described (Mandai et al., 1997; Takahashi et al., 1999).
Adenovirus Infection
An adenovirus vector encoding a constitutively active form of p110 (Ax-CAMyr-p110) was kindly supplied by Dr. M. Kasuga (Kobe University, Kobe, Japan; Kitamura et al., 1999). EL and nectin-1-EL cells were infected with AxCAMyr-p110 at a multiplicity of infection of 0.1 PFU/cell. After a 36-h culture, the cells were subjected to the cell adhesion assay. More than 95% of the infected EL cells formed lamellipodia to a small extent on the IgG-coated coverslips (our unpublished results).
FRET Imaging
The FRET imaging was performed as described (Honda et al., 2003b) with some modifications. EL or nectin-1-EL cells were transfected with pRaichu-Rac1, pRaichu-Rac1-Y40C, pRaichu-Cdc42, or pRaichu-Cdc42-Y40C. The FRET probes for wild-type Rac1 and Cdc42 consisted of a CRIB domain of PAK, Rac1 or Cdc42, a pair of GFP mutants, and a CAAX box of Ki-Ras (Itoh et al., 2002). In Y40C mutants, Cys was substituted for Tyr40 in the effector domain of Rac and Cdc42, respectively, which is essential for the binding to PAK-CRIB (Itoh et al., 2002). Twenty-four hours after the transfection, the cells were replated on the dishes coated with 50 μg/ml Cef, a mixture of 50 μg/ml Cef and 50 μg/ml Nef-3, or 50 μg/ml IgG. The cells were then imaged with an Olympus IX71 inverted microscope equipped with a cooled charge-coupled device camera, CoolSNAP HQ (Roper Scientific, Trenton, NJ), controlled by MetaMorph software (Universal Imaging, West Chester, PA; Miyawaki et al., 1997; Mochizuki et al., 2001). For dual-emission ratio imaging, we used a 440AF21 excitation filter, a 455DRLP dichroic mirror, and two emission filters, 480AF30 for CFP and 535AF25 for YFP (Omega Optical Inc., Brattleboro, VT). The cells were illuminated with a 75-W xenon lamp through a 6% neutral density filter (Omega Optical Inc.) and a 60× oil immersion objective lens. Exposure times for 3 × 3 binning were 200 ms to obtain images of CFP and YFP, and 50 ms to obtain images of a differential interference contrast (DIC). After background subtraction, the ratio image of YFP/CFP was created with the MetaMorph software and used to represent FRET efficiency.
Ca2+ Switch Assay
Cell-cell adhesion of nectin-1-MDCK and pm-nectin-2-nectin-1-MDCK cells was assayed as described (Honda et al., 2003c) with some modifications. Both nectin-1-MDCK and pm-nectin-2-nectin-1-MDCK cells were cultured in Ca2+-free DMEM with 10% FCS dialyzed against PBS. After an 18-h culture, these cells were cultured in DMEM containing 5 mM EGTA (at a final concentration of 2 μM Ca2+) for 2 h and then washed with DMEM (at a final concentration of 2 mM Ca2+). The cells were further cultured in DMEM (at a final concentration of 2 mM Ca2+) for 2, 4, and 8 h. The cells were then fixed, followed by immunostaining.
RESULTS
Formation of Lamellipodia by Cef in EL Cells
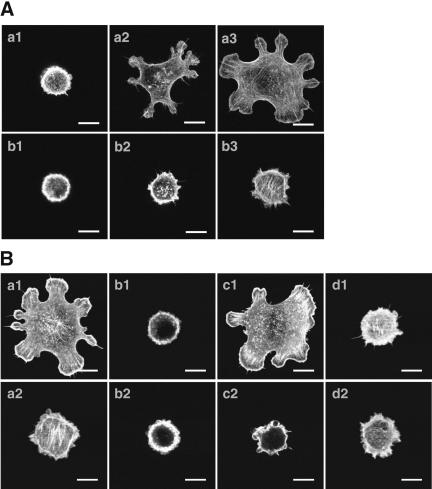
We first confirmed by analyzing morphological changes whether the trans-interaction of E-cadherin activates Rac in EL cells (L cells expressing exogenous E-cadherin). For this purpose, we used a recombinant protein (Cef, an extracellular fragment of E-cadherin fused to the Fc portion of IgG), which trans-interacts with cellular E-cadherin in nectin-1-EL cells (L cells expressing exogenous E-cadherin and nectin-1; Honda et al., 2003c). EL cells cultured on the Cef-coated coverslips formed lamellipodia to a medium extent at 60 min and to a large extent at 90 min (Figure 1, A, a1–a3, and Ba1), whereas EL cells cultured on the IgG-coated coverslips formed lamellipodia to a small extent even at 90 min (Figure 1, A, b1–b3, and Ba2). These results indicate that the trans-interaction of Cef with cellular E-cadherin results in the formation of lamellipodia. Overexpression of N17Rac1, a dominant negative mutant of Rac, in EL cells reduced the Cef-induced formation of lamellipodia (Figure 1B, b1 and b2). In contrast, overexpression of NWASP-CRIB, a specific inhibitor of GTP-Cdc42 (Takenawa and Miki, 2001), did not affect the Cef-induced formation of lamellipodia (Figure 1B, c1 and c2). Wortmannin, a PI3 kinase inhibitor (Kovacs et al., 2002), reduced the Cef-induced formation of lamellipodia (Figure 1B, d1 and d2). LY294002, another PI3 kinase inhibitor (Kovacs et al., 2002), also reduced the Cef-induced formation of lamellipodia (our unpublished results). These results indicate that the trans-interaction of E-cadherin induces the formation of lamellipodia in Rac- and PI3 kinase–dependent manners in EL cells.
Figure 1.
Formation of lamellipodia by Cef in EL cells. (A) Time course of formation of lamellipodia. EL cells were cultured on the Cef- or IgG-coated coverslips for indicated periods of time. The cells were fixed and stained with rhodamine-phalloidin. (a1) Cef at 30 min (n = 100, N:S:M = 90:10:0); (a2) Cef at 60 min (n = 100, N:S:M = 55:0:45); (a3) Cef at 90 min (n = 100, N:S:M = 9:21: 70); (b1) IgG at 30 min (n = 100, N:S:M = 93:7:0); (b2) IgG at 60 min (n = 100, N:S:M = 92:8:0); and (b3) IgG at 90 min (n = 100, N:S:M = 75:25:0). Bars, 10 μm. (B) Effect of N17Rac1 and wortmannin. EL or EL cells transiently expressing GFP, GFP-N17Rac1, or GFP-NWASP-CRIB were cultured on the Cef- or IgG-coated coverslips for 90 min in the presence or absence of 60 nM wortmannin. The cells were fixed and stained with rhodamine-phalloidin. (a1) GFP and Cef (n = 100, N:S:M = 25:0:75); (a2) GFP and IgG (n = 100, N:S:M = 77:23:0); (b1) GFP-N17Rac1 and Cef (n = 100, N:S:M = 99:1:0); (b2) GFP-N17Rac1 and IgG (n = 100, N:S: M = 99:1:0); (c1) GFP-NWASP-CRIB and Cef (n = 100, N:S:M = 41:0:59); (c2) GFP-NWASP-CRIB and IgG (n = 100, N:S:M = 98:2:0); (d1) Cef in the presence of wortmannin (n = 100, N:S:M = 99:1:0); and (d2) IgG in the presence of wortmannin (n = 100, N:S:M = 97:3:0). N, no lamellipodia; S, formation of lamellipodia to a small extent; and M, formation of lamellipodia to a medium or large extent. Bars, 10 μm. The results shown are representative of three independent experiments.
Activation of Rac by Cef in EL Cells
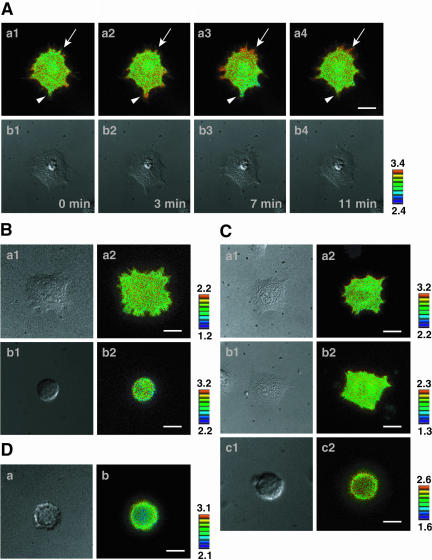
We examined whether the trans-interaction of E-cadherin indeed induces activation of Rac. We could not measure the level of GTP-Rac or GTP-Cdc42 in EL cells by the pull-down assay with the glutathione-S-transferase-PAK-CRIB fusion protein, because the expression levels of the endogenous Rac and Cdc42 proteins in EL cells were too low for this assay (our unpublished results; Kawakatsu et al., 2002). Therefore, we used the FRET probes, Raichu-Rac1 and -Cdc42, to examine the activation of Rac1 and Cdc42 induced by the trans-interaction of E-cadherin (Itoh et al., 2002; Honda et al., 2003b). Cef induced activation of Rac1 (Figure 2A, a1–a4 and b1–b4), but not that of Cdc42 (Figure 2C, a1 and a2), as shown by the FRET imaging. Furthermore, we found that the trans-interaction of E-cadherin induced dynamic formation of lamellipodia and activation of Rac1. The activation of Rac led to formation of lamellipodia (Figure 2A, a1–a4, arrows), whereas the inactivation of Rac1 led to retraction of lamellipodia (Figure 2A, a1–a4, arrowheads), as shown by the FRET imaging (see also Video 1). IgG did not induce activation of Rac1 or Cdc42 (Figure 2, Bb1, Bb2, Cc1, and Cc2). The cells expressing Raichu-Rac1-Y40C or Raichu-Cdc42-Y40C on the Cef-coated dishes did not show any activation of these small G proteins, confirming the intramolecular binding of CRIB to Rac1 generating the gradient of FRET efficiency (Figure 2, Ba1, Ba2, Cb1, and Cb2). To confirm that the trans-interaction of E-cadherin induces the activation of Rac through activation of PI3 kinase, EL cells expressing Raichu-Rac1 were cultured on the Cef-coated dishes in the presence of wortmannin. Wortmannin markedly reduced the Cef-induced activation of Rac1 (Figure 2D, a and b). These results are consistent with the earlier observations (Nakagawa et al., 2001; Kovacs et al., 2002) and indicate that the trans-interaction of E-cadherin induces activation of Rac, but not that of Cdc42, through PI3 kinase, resulting in formation of lamellipodia.
Figure 2.
Activation of Rac, but not Cdc42, by Cef in EL cells. EL cells expressing either Raichu-Rac1, Raichu-Rac1-Y40C, Raichu-Cdc42, or Raichu-Cdc42-Y40C were cultured on the Cef- or IgG-coated dishes. After incubation for 60 min in the presence or absence of wortmannin, the cells were imaged for YFP, CFP, and DIC. A ratio image of YFP/CFP was created to represent FRET efficiency. In the intensity-modulated display (IMD) mode images, eight colors from red to blue were used to represent the YFP/CFP ratio, with the intensity of each color indicating the mean intensity of YFP and CFP. High YFP/CFP ratio shown in red color indicates high FRET efficiency of the probe, reflecting high GTP/GDP ratio of Cdc42 or Rac1. The upper and lower limits of ratio range are shown. (A) and (B) Rac1. (Aa1-Aa4, Ab1-Ab4, Bb1, and Bb2) Raichu-Rac1; (Ba1 and Ba2) Raichu-Rac1-Y40C; (Aa1-Aa4, Ab1-Ab4, Ba1, and Ba2) Cef; (Bb1 and Bb2) IgG; (Aa1-Aa4, Ba2, and Bb2) IMD mode images; and (Ab1-Ab4, Ba1, and Bb1) DIC images. (C) Cdc42. (a and c) Raichu-Cdc42; (b) Raichu-Cdc42-Y40C; (a and b) Cef; (c) IgG; (1) DIC images; and (2) IMD mode images. (D) Rac1 in the presence of wortmannin. (a) a DIC image and (b) an IMD image. Bars, 10 μm. We selected here representative figures in which the activation of these small G proteins were easily observed in lamellipodia. The results shown are representative of three independent experiments.
Inhibition of the Cef-induced Formation of Lamellipodia by Non–trans-interacting Nectin-1 in EL Cells
We have previously shown by use of Nef-1, -2, and -3 (extracellular fragments of nectin-1, -2, and -3 fused to the Fc portion of IgG, respectively) that the trans-interactions of Nefs with cellular nectins induce activation of Cdc42 and Rac in a PI3 kinase–independent manner, resulting in formation of filopodia and lamellipodia, respectively, in nectin-1-L, nectin-2-L, or nectin-3-L cells (L cells stably expressing exogenous nectin-1α, -2 α, or -3 α, respectively; Kawakatsu et al., 2002; Honda et al., 2003b). We next examined the effect of nectins on the Cef-induced activation of Rac. Cef induced formation of lamellipodia in EL cells in a time-dependent manner (Figure 3A, a1 and a2; also see Figure 1A). In contrast, when nectin-1-EL, nectin-2-EL, or nectin-3-EL cells (EL cells expressing exogenous nectin-1α, -2α, or -3α, respectively) were cultured on the Cef-coated coverslips, the Cef-induced formation of lamellipodia was markedly reduced in these nectin-expressing EL cells, which did not adhere to each other, even after the 90-min culture (Figure 3A, b1, b2, c1, c2, d1, and d2). The Cef-induced activation of Rac was also markedly reduced in nectin-1-EL cells as shown by the FRET imaging (Figure 3B, a and b; also see Figure 2A, a1–a4 and b1–b4). The same results were obtained in nectin-2-EL and nectin-3-EL cells as that in nectin-1-EL cells (our unpublished results). These results indicate that the nectins, which do not trans-interact with other nectins (non–trans-interacting nectins), inhibit the Cef-induced activation of Rac, resulting in inhibition of the formation of lamellipodia.
Figure 3.
Inhibitory effect of non–trans-interacting nectins on the Cef-induced formation of lamellipodia and activation of Rac in EL cells. (A) Inhibition of the formation of lamellipodia. EL, nectin-1-EL, nectin-2-EL, and nectin-3-EL cells were cultured on the Cef- or IgG-coated coverslips for 90 min. The cells were fixed and stained with rhodamine-phalloidin. (a1) EL cells and Cef (n = 100, N:S:M = 21:0:79); (a2) EL cells and IgG (n = 100, N:S:M = 74:26:0); (b1), nectin-1-EL cells and Cef (n = 100, N:S:M = 68:32:0); (b2) nectin-1-EL cells and IgG (n = 100, N:S:M = 68:32:0); (c1) nectin-2-EL cells and Cef (n = 100, N:S:M = 70:30:0); (c2) nectin-2-EL cells and IgG (n = 100, N:S:M = 84:16:0); (d1) nectin-3-EL cells and Cef (n = 100, N:S:M = 78:22:0); and (d2) nectin-3-EL cells and IgG (n = 100, N:S:M = 87:13:0). N, no lamellipodia; S, formation of lamellipodia to a small extent; M, formation of lamellipodia to a medium or large extent. (B) Inhibition of the activation of Rac. Nectin-1-EL cells expressing Raichu-Rac1 were cultured on the Cef-coated dishes. After incubation for 60 min, cell images were obtained as in Figure 2. (a) a DIC image; and (b) an IMD mode image. Bars, 10 μm. The results shown are representative of three independent experiments.
Suppression of the Inhibitory Effect of Non–trans-interacting Nectin-1 by Overexpression of a Constitutively Active Mutant of Rac or PI3 Kinase in EL Cells
We examined which step in the E-cadherin-Rac signaling pathway is inhibited by non–trans-interacting nectins. The inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia was suppressed by overexpression of a constitutively active mutant of Rac1 (V12Rac1; Figure 4A, a1 and b1), indicating that the step of the inhibitory effect of non–trans-interacting nectin-1 is upstream of Rac. Overexpression of V12Rac1 also induced formation of lamellipodia in nectin-1-EL cells on the control IgG-coated coverslips (Figure 4A, a2 and b2), consistent with our previous result that overexpression of V12Rac1 induces formation of lamellipodia in L cells (Ono et al., 2000). We next examined using an adenovirus expressing a constitutively active mutant of PI3 kinase (Myr-p110) whether the step of the inhibitory effect of non–trans-interacting nectin-1 is upstream or downstream of PI3 kinase. Overexpression of Myr-p110 induced formation of lamellipodia to a small extent in EL cells on the IgG-coated coverslips (Figure 4B, a3 and a4). Overexpression of Myr-p110 also induced formation of lamellipodia to a small extent in nectin-1-EL cells on the IgG-coated coverslips (Figure 4B, b3 and b4). Furthermore, the inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia was mostly suppressed by overexpression of Myr-p110 (Figure 4B, b1 and b2). Overexpression of Myr-p110 did not affect the Cef-induced formation of lamellipodia in EL cells (Figure 4B, a1 and a2). These results indicate that the step of the inhibitory action of non–trans-interacting nectin-1 is upstream of PI3 kinase and Rac.
Figure 4.
Suppression of the inhibitory effect of non–trans-interacting nectin-1 by a constitutively active mutant of Rac1 or PI3 kinase in EL cells. EL and nectin-1-EL cells or EL and Nectin-1-EL cells transiently expressing either GFP, GFP-V12Rac1, or Myr-p110 were cultured on the Cef- or IgG-coated coverslips for 90 min. The cultured cells were fixed and stained with rhodamine-phalloidin. (A) Effect of V12Rac1 in nectin-1-EL cells. (a1) GFP and Cef (n = 100, N:S:M = 72:28:0); (a2) GFP and IgG (n = 100, N:S:M = 67:33:0); (b1) GFP-V12Rac1 and Cef (n = 100, N:S:M = 8:0:92); and (b2) GFP-V12Rac1 and IgG (n = 100, N:S:M = 33:0:67). Bars, 10 μm. (B) Effect of Myr-p110. (a1) EL cells, Cef, and Myr-p110 (n = 100, N:S:M = 4:0:96); (a2) EL cells and Cef (n = 100, N:S:M = 45:0:55); (b1) nectin-1-EL cells, Cef, and Myr-p110 (n = 100, N:S:M = 46:0:54); (b2) nectin-1-EL cells and Cef (n = 100, N:S:M = 83:17:0); (a3) EL cells, IgG, and Myr-p110 (n = 100, N:S:M = 4:96:0); (b3) nectin-1-EL cells, IgG, and Myr-p110 (n = 100, N:S:M = 70:30:0); (a4) EL cells and IgG (n = 100, N:S:M = 82:18:0); and (b4) nectin-1-EL cells and IgG (n = 100, N:S:M = 81:19:0). N, no lamellipodia; S, formation of lamellipodia to a small extent; and M, formation of lamellipodia to a medium or large extent. Bars, 10 μm. The results shown are representative of three independent experiments.
Requirement of the C-Terminal Four aa of Nectin-1 for Its Inhibitory Effect on the Cef-induced Formation of Lamellipodia in EL Cells
We next examined whether the C-terminal four aa of nectin-1, which are responsible for the binding of afadin or Par-3 (Takahashi et al., 1999; Takekuni et al., 2003), are necessary for the inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia. EL cells on the Cef-coated coverslips formed lamellipodia (Figure 5A, 1 and 2). When EL cells expressing nectin-1, of which C-terminal four aa were deleted (nectin-1-ΔC-EL cells), were cultured on the Cef-coated coverslips, nectin-1-ΔC-EL cells formed lamellipodia (Figure 5B, 1 and 2), whereas nectin-1-EL cells on the Cef-coated coverslips did not form lamellipodia (Figure 5C, 1 and 2). These results indicate that the C-terminal four aa are necessary for the inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia and suggest that the binding of afadin to nectins is necessary for the inhibitory effect of non–trans-interacting nectins. The binding of Par-3 to nectins is not likely to be involved in this inhibitory effect, because nectin-2 shows its inhibitory effect (Figure 3A, c1 and c2), but does not bind Par-3 (Takekuni et al., 2003).
Figure 5.
Requirement of the C-terminal four aa of nectin-1 for its inhibitory effect in EL cells. EL, nectin-1-ΔC-EL, and nectin-1-EL cells were cultured on the Cef- or IgG-coated coverslips for 90 min. The cells were then fixed and stained with rhodamine-phalloidin. (A1) EL cells and Cef (n = 100, N:S:M = 27:0:73); (A2) EL cells and IgG (n = 100, N:S:M = 74:26:0); (B1) nectin-1-ΔC-EL cells and Cef (n = 100, N:S:M = 32:0:68); (B2) nectin-1-ΔC-EL cells and IgG (n = 100, N:S:M = 65:35:0); (C1) nectin-1-EL cells and Cef (n = 100, N:S:M = 67:33:0); and (C2) nectin-1-EL cells and IgG (n = 100, N:S:M = 77:23:0). N, no lamellipodia; S, formation of lamellipodia to a small extent; and M, formation of lamellipodia to a medium or large extent. Bars, 10 μm. The results shown are representative of three independent experiments.
Suppression of the Inhibitory Effect of Non–trans-interacting Nectin-1 by Its trans-interaction with Nef-3 in EL Cells
We then examined whether the trans-interaction of nectin-1 with Nef-3 affects the inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced phenotypes. Nectin-1-EL cells cultured on the Cef-coated coverslips did not form lamellipodia (Figure 6Aa; also see Figure 3Ab1). In contrast, nectin-1-EL cells cultured on the Nef-3-coated coverslips formed filopodia and lamellipodia markedly as described (Kawakatsu et al., 2002; Figure 6Ab), and this result indicates that non–trans-interacting E-cadherin does not affect the nectin-induced formation of filopodia and lamellipodia. When nectin-1-EL cells were cultured on the coverslips coated with the same amounts of Cef and Nef-3, not only lamellipodia but also filopodia were formed in a time-dependent manner (Figure 6Ac and our unpublished results). The extents of the formation of lamellipodia were more marked than those of nectin-1 EL cells cultured on the coverslips coated with Nef-3 alone (Figure 6A, b and c). Rac1 as well as Cdc42 was activated in nectin-1-EL cells on the dishes coated with both Cef and Nef-3 as shown by the FRET imaging (Figure 6B, a1, a2, b1, and b2). These results indicate that the trans-interaction of nectin-1 with Nef-3 suppresses the inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia through the activation of Rac.
Figure 6.
Suppression of the inhibitory effect of non–trans-interacting nectin-1 by Nef-3 or a constitutively active mutant of Cdc42 in EL cells. (A) Effect of Nef-3 on the formation of lamellipodia. Nectin-1-EL cells were cultured on the coverslips coated with Cef, Nef-3, or a mixture of Cef and Nef-3 for 90 min. The cells were then fixed and stained with rhodamine-phalloidin. (a) Cef (n = 100, N:S:M = 63:37:0); (b) Nef-3 (n = 100, N:S:M = 27:0:73); and (c) a mixture of Cef and Nef-3 (n = 100, N:S:M = 26:0:74). (B) Effect of Nef-3 on the activation of Rac and Cdc42. Nectin-1-EL cells expressing either Raichu-Rac1 or -Cdc42 were cultured on the dishes coated with a mixture of Cef and Nef-3. After incubation for 60 min, cell images were obtained as in Figure 2. (a) Raichu-Rac1; (b) Raichu-Cdc42; (1) DIC images; and (2) IMD mode images. We selected here representative figures in which the activation of these small G proteins were easily observed in filopodia and lamellipodia. (C) Effect of V12Cdc42 on the formation of lamellipodia. Nectin-1-EL cells transiently expressing myc-tagged V12Cdc42 were cultured on the Cef- or IgG-coated coverslips for 90 min. The cells were then fixed and stained for F-actin and myc-tagged V12Cdc42 using rhodamine-phalloidin and the antimyc mAb. (a) IgG (n = 100, N:S:M = 99:1:0); and (b) Cef (n = 100, N:S:M = 4:0:96). The figures represent the rhodamine-phalloidin staining. N, no lamellipodia; S, formation of lamellipodia to a small extent; and M, formation of lamellipodia to a medium or large extent. Bars, 10 μm. The results shown are representative of three independent experiments.
Suppression of the Inhibitory Effect of Non–trans-interacting Nectin-1 by Overexpression of a Constitutively Active Mutant of Cdc42 in EL Cells
We have previously shown that the trans-interactions of nectins induce activation of Cdc42 and Rac (Kawakatsu et al., 2002). It is possible that the trans-interaction of nectin-1 with Nef-3 suppresses the inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia through activation of Cdc42. Therefore, we examined whether overexpression of a constitutively active mutant of Cdc42 (V12Cdc42) suppresses the inhibitory effect of non–trans-interacting nectin-1. Overexpression of V12Cdc42 alone did not induce the formation of lamellipodia in nectin-1-EL cells on the control IgG-coated coverslips, whereas it induced the formation of filopodia (Figure 6Ca), consistent with our previous result (Ono et al., 2000). In contrast, overexpression of V12Cdc42 induced the formation of not only filopodia but also lamellipodia in nectin-1-EL cells on the Cef-coated coverslips (Figure 6Cb). These results suggest that activation of Cdc42 by the trans-interaction of nectin-1 with Nef-3 is sufficient for suppression of the inhibitory effect of non–trans-interacting nectin-1 on the Cef action.
Inhibition of the Cef-induced Formation of Lamellipodia through Rac by Non–trans-interacting Nectins in MDCK Cells
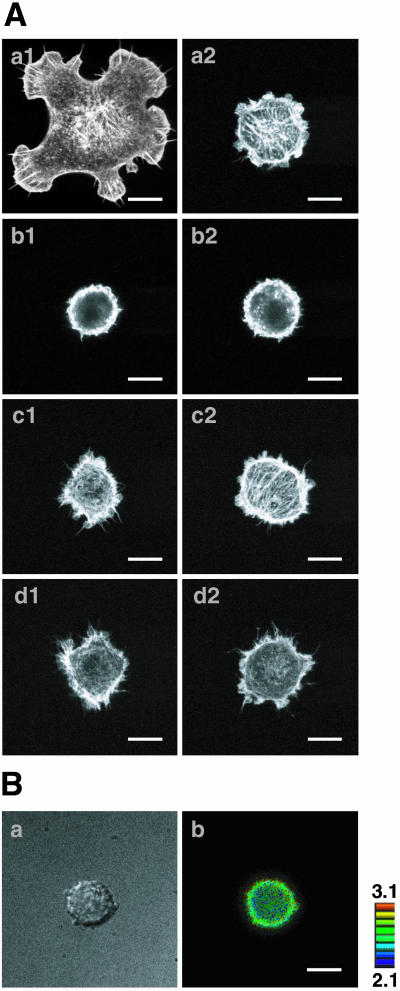
We next examined using MDCK cells whether non–trans-interacting nectins inhibit the Cef-induced formation of lamellipodia in epithelial cells. When wild-type MDCK cells were cultured on the coverslips coated with Cef or with a mixture of Cef and Nef-1, Nef-2, and Nef-3, the wild-type MDCK cells did not form lamellipodia (our unpublished results). The expression level of E-cadherin in wild-type MDCK cells was far less than that in EL cells (Figure 7A, a1 and a2), suggesting that the expression level of endogenous E-cadherin in wild-type MDCK cells is not high enough to observe the Cef-induced formation of lamellipodia in this assay system. Therefore, we established E-cadherin-GFPN-MDCK cells (MDCK cells stably overexpressing E-cadherin-GFPN). We confirmed that the expression level of the E-cadherin-GFPN protein in E-cadherin-GFPN-MDCK cells was similar to that of E-cadherin in EL cells (Figure 7A, a1, a3, b1, and b3). When E-cadherin-GFPN-MDCK cells were cultured on the Cef- or IgG-coated coverslips, Cef induced formation of lamellipodia to a medium extent at 60 min and to a large extent at 90 min (Figure 7B, a1–a3), whereas IgG-induced formation of lamellipodia to a small extent even at 90 min (Figure 7B, b1–b3). Overexpression of N17Rac1 in E-cadherin-GFPN-MDCK cells reduced the Cef-induced formation of lamellipodia (Figure 7C, a and d). When E-cadherin-GFPN-MDCK cells transiently overexpressing either exogenous nectin-1, -2, or -3 or a point-mutated nectin-2α, which is incapable of trans-interacting (pm-nectin-2), were cultured on the Cef-coated coverslips, the Cef-induced formation of lamellipodia was markedly reduced in these nectin-expressing E-cadherin-GFPN-MDCK cells (Figure 7C, a and b, and our unpublished results). In contrast, when nectin-1–overexpressing E-cadherin-GFPN-MDCK cells were cultured on the coverslips coated with the same amounts of Cef and Nef-3, not only lamellipodia but also filopodia were formed (Figure 7Cc). These results indicate that non–trans-interacting nectins inhibit the Cef-induced formation of lamellipodia through Rac in MDCK cells in the same way as in L fibroblasts.
Figure 7.
Inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia through Rac in MDCK cells. (A) The expression level of E-cadherin-GFPN. EL, wild-type MDCK, and E-cadherin-GFPN-MDCK cells were homogenized and 20 μg of each homogenate was subjected to SDS-PAGE, followed by Western blotting using the anti-E-cadherin mAb and anti-GFP pAb. (a) the anti-E-cadherin mAb; (b) the anti-GFP pAb; (1) EL cells; (2) wild-type MDCK cells; and (3) E-cadherin-GFPN-MDCK cells. The arrow and arrowhead indicate E-cadherin and E-cadherin-GFPN, respectively. (B) Effect of Cef on the formation of lamellipodia. E-cadherin-GFPN-MDCK cells were cultured on the Cef- or IgG-coated coverslips for indicated periods of time. The cells were fixed and stained with rhodamine-phalloidin. (a1) Cef at 30 min (n = 100, N:S:M = 92:8:0); (a2) Cef at 60 min (n = 100, N:S:M = 80:0:20); (a3) Cef at 90 min (n = 100, N:S:M = 35:0:65); (b1) IgG at 30 min (n = 100, N:S:M = 93:7:0); (b2) IgG at 60 min (n = 100, N:S:M = 88:12:0); and (b3) IgG at 90 min (n = 100, N:S:M = 85:15:0). (C) Effect of nectin-1, Nef-3, and N17Rac1 on the formation of lamellipodia. E-Cadherin-GFPN-MDCK cells or FLAG-nectin-1- or FLAG-N17Rac1-overexpressing E-cadherin-GFPN-MDCK cells were cultured on the coverslips coated with Cef or a mixture of Cef and Nef-3 for 90 min. The cells were then fixed and stained for F-actin and FLAG-nectin-1 or -N17Rac1 using rhodamine-phalloidin and the anti-FLAG pAb. The figures represent the rhodamine-phalloidin staining. (a) E-cadherin-GFPN-MDCK cells on the Cef-coated coverslips (n = 100, N:S:M = 44:0:56); (b) nectin-1-overexpressing E-cadherin-GFPN-MDCK cells on the Cef-coated coverslips (n = 100, N:S:M = 79:21: 0); (c) nectin-1-overexpressing E-cadherin-GFPN-MDCK cells on the coverslips coated with a mixture of Cef and Nef-3 (n = 100, N:S:M = 20:0:80); and (d) N17Rac1-overexpressing E-cadherin-GFPN-MDCK cells on the Cef-coated coverslips (n = 100, N:S:M = 95:5:0). N, no lamellipodia; S, formation of lamellipodia to a small extent; and M, formation of lamellipodia to a medium or large extent. Bars, 10 μm. The results shown are representative of three independent experiments.
Inhibition of the Formation of AJs by Non–trans-interacting Nectin-2 in MDCK Cells
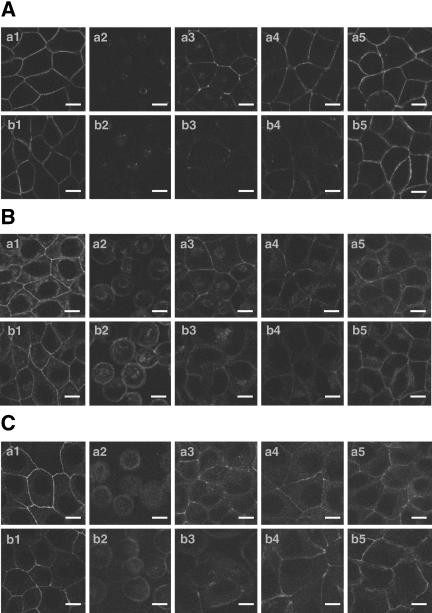
It has been shown that activation of Rac is required for the formation of the E-cadherin–based AJs in keratinocytes and MDCK cells (Braga et al., 1997; Takaishi et al., 1997; Jou and Nelson, 1998) and that activation of Rac increases the velocity of initiation of the E-cadherin–based cell-cell adhesion and leads to the maturation of the E-cadherin–based cell-cell AJs in MDCK cells (Ehrlich et al., 2002). Therefore, if non–trans-interacting nectins indeed inhibit the E-cadherin–induced activation of Rac, overexpression of non–trans-interacting nectins should reduce maturation of the E-cadherin–based cell-cell AJs. To examine this possibility, we prepared nectin-1-MDCK cells stably expressing pm-nectin-2 (a point-mutated nectin-2α that is incapable of trans-interacting; pm-nectin-2-nectin-1-MDCK cells; Miyahara et al., 2000). When nectin-1-MDCK and pm-nectin-2-nectin-1-MDCK cells were cultured at 2 mM Ca2+, the immunofluorescence signal for E-cadherin was concentrated at the cell-cell junctions (Figure 8A, a1 and b1). When the two types of cells were cultured at low Ca2+ for 20 h, the signal for E-cadherin was not observed at any site along the plasma membrane in both nectin-1-MDCK and pm-nectin-2-nectin-1 MDCK cells (Figure 8A, a2 and b2). When these two types of cells were recultured at 2 mM Ca2+, the signal for E-cadherin was reconcentrated at the cell-cell junctions in time-dependent manners, but the time course of accumulation of E-cadherin in pm-nectin-2-nectin-1-MDCK cells was much slower than that in nectin-1-MDCK cells (Figure 8A, a3–a5 and b3–b5). Moreover, the time courses of accumulation of afadin, ZO-1, and β-catenin in pm-nectin-2-nectin-1-MDCK cells were much slower than those in nectin-1-MDCK cells (Figure 8, B and C, and our unpublished results). The reason why cell-cell junctions were kept and formed in pm-nectin-2-nectin-1-MDCK cells might be due to endogenous nectin-1 and -2 and exogenous nectin-1. Taken together with the observation that non–trans-interacting nectins inhibited the Cef-induced formation of lamellipodia through Rac in MDCK cells, these results suggest that non–trans-interacting nectin-2 (pm-nectin-2) overexpressed in MDCK cells inhibits the activation of Rac induced by the trans-interaction of E-cadherin, resulting in a reduction of the velocity of the formation of the E-cadherin-based cell-cell AJs. It may be noted that there was an increase in the nuclear staining for afadin when pm-nectin-2-nectin-1-MDCK and nectin-1-MDCK cells were treated at a low Ca2+ concentration (Figure 8B). We previously observed the similar increase in the nuclear staining for afadin in wild-type MDCK and nectin-1-MDCK cells using the same anti-afadin mAb (Sakisaka et al., 1999; Fukuhara et al., 2002a). In these articles we concluded that the nuclear staining for afadin is non-specific. Therefore, the increase in the nuclear staining for afadin is not a specific phenotype for pm-nectin-2-nectin-1-MDCK cells.
Figure 8.
Inhibitory effect of non–trans-interacting nectin-2 on the formation of AJs in MDCK cells. Both nectin-1-MDCK and pm-nectin-2-nectin-1-MDCK cells were cultured at 2 mM Ca2+. After culture for 20 h at low Ca2+, the cells were washed with DMEM and recultured at 2 mM Ca2+ for indicated periods of time. The cells were fixed, followed by immunostaining for E-cadherin, afadin, and ZO-1 with the anti-E-cadherin, anti-afadin, and anti-ZO-1 mAbs. (A) E-cadherin; (B) afadin; (C) ZO-1; (a) nectin-1-MDCK cells; (b) pm-nectin-2-nectin-1-MDCK cells. (1) at 2 mM Ca2+; (2) at low Ca2+; (3) 2 h after the reculture at 2 mM Ca2+; (4) 4 h after the reculture at 2 mM Ca2+; and (5) 8 h after the reculture at 2 mM Ca2+. Bars, 10 μm. The results shown are representative of three independent experiments.
DISCUSSION
We have first confirmed by the FRET imaging that the previous reports that the trans-interaction of E-cadherin induces activation of Rac (Nakagawa et al., 2001; Noren et al., 2001). One report shows, however, that the E-cadherin–mediated cell-cell adhesion induces activation of Cdc42 in MCF-7 and EL cells (Kim et al., 2000), but this result has not been reproduced using MDCK cells by another group (Nakagawa et al., 2001). We have shown that overexpression of NWASP-CRIB does not inhibit the Cef-induced formation of lamellipodia and that the trans-interaction of E-cadherin with Cef induces activation of Rac, but not that of Cdc42, in EL cells as shown by the FRET imaging. Kim's group (Kim et al., 2000) used cultured cells that might express endogenous nectins, because nectins are ubiquitously expressed (Takai and Nakanishi, 2003; Takai et al., 2003). Therefore, we cannot rule out the possibility that Kim's group (Kim et al., 2000) evaluated the activation of Cdc42 by the trans-interactions of endogenous nectins as that by the trans-interaction of E-cadherin. Taken together, it is likely that the trans-interaction of E-cadherin induces activation of Rac, but not that of Cdc42.
Moreover, we have demonstrated by the FRET imaging that the trans-interaction of E-cadherin induces dynamic formation of lamellipodia and activation of Rac. The activated Rac localizes to newest protruding lamellipodia, whereas the inactivated Rac localizes to the retracting lamellipodia. It has been shown that Rac and lamellipodia are transiently concentrated at newest cell-cell contact sites, but decrease at older, stabilized cell-cell contact sites during the formation of cell-cell AJs in MDCK cells (Ehrlich et al., 2002). Taken together, our observations indicate that Rac is activated by the trans-interaction of E-cadherin at newest cell-cell contact sites and forms lamellipodia to rapidly expand the cell-cell adhesion, whereas the activated Rac is dynamically downregulated, resulting in a reduction of the formation of lamellipodia and stabilization of matured cell-cell contact sites. However, it remains unknown how the trans-interaction of E-cadherin induces dynamic formation of lamellipodia and activation of Rac.
We have then confirmed here the previous observation that PI3 kinase is required for the full activation of Rac induced by the trans-interaction of E-cadherin using the PI3 kinase inhibitors, LY294002 and/or wortmannin (Nakagawa et al., 2001; Kovacs et al., 2002). In contrast to these results, two groups have recently demonstrated that PI3 kinase is not involved in the activation of Rac upon the formation of cell-cell contact in keratinocytes and MDCK cells (Betson et al., 2002; Ehrlich et al., 2002). The exact reason for this inconsistency is not known, but may be due to the involvement of endogenous nectin-induced activation of Rac. We have recently found that the nectin-induced activation of Cdc42 and Rac is independent of PI3 kinase (Honda et al., 2003b). Kovacs's group (Kovacs et al., 2002) evaluated the E-cadherin–induced activation of Rac in CHO cells stably expressing E-cadherin using a recombinant Cef, whereas the latter two groups evaluated the activation of Rac upon the formation of cell-cell contact of keratinocytes or MDCK cells without using Cef (Betson et al., 2002; Ehrlich et al., 2002). Therefore, they might evaluate the endogenous nectin-induced activation of Rac independent of PI3 kinase as the E-cadherin–induced activation of Rac.
We have shown here that the nectin-1, which does not trans-interact with other nectins (non–trans-interacting nectin-1), inhibits the E-cadherin–induced activation of Rac in EL cells. The other nectin family members, nectin-2 and -3, also show the same inhibitory effect. The step of the inhibitory action of nectin-1 is upstream of PI3 kinase and Rac. The inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia and/or activation of Rac is suppressed by the trans-interaction of nectin-1 with Nef-3 or overexpression of a constitutively active mutant of Cdc42. We have previously shown that the trans-interactions of nectins induce activation of not only Rac but also Cdc42 in nectin-1-MDCK and nectin-1-EL cells (Kawakatsu et al., 2002; Honda et al., 2003b). Taken together, these results indicate that the inhibitory effect of non–trans-interacting nectins is suppressed by the activation of Cdc42 induced by the trans-interaction of nectin-1 with Nef-3.
We have furthermore demonstrated here that non–trans-interacting nectin-1 inhibits the E-cadherin–induced formation of lamellipodia through Rac in MDCK cells as well as in EL cells. The other nectin family members, nectin-2 and -3, also show the same inhibitory effect in MDCK cells. The inhibitory effect of non–trans-interacting nectin-1 on the Cef-induced formation of lamellipodia is suppressed by the trans-interaction of nectin-1 with Nef-3 in MDCK cells. Moreover, non–trans-interacting nectin-2 reduces the velocity of formation of the E-cadherin–based cell-cell AJs in MDCK cells. Because it has been shown that activation of Rac increases the velocity of formation of the E-cadherin–based cell-cell adhesion and leads to the maturation of the E-cadherin-based cell-cell AJs in MDCK cells (Ehrlich et al., 2002), it is likely that nectins regulate the E-cadherin–induced activation of Rac and thereby the velocity of the formation of the E-cadherin–based cell-cell AJs in both fibroblasts and epithelial cells.
The inhibitory effect of non–trans-interacting nectin-1 requires its C-terminal four aa. Afadin directly binds to the C-terminal four aa of nectin-1, -2, and -3 through its PDZ domain (Takahashi et al., 1999), whereas Par-3 binds to the C-terminal four aa of nectin-1 and -3, but not to those of nectin-2, through its PDZ domain (Takekuni et al., 2003). Because non–trans-interacting nectin-2 inhibits the E-cadherin–induced activation of Rac, it is likely that afadin, but not Par-3, plays a key role in the inhibitory effect of non–trans-interacting nectins. We have previously shown that nectins and E-cadherin are associated through afadin and α-catenin (Yokoyama et al., 2001). Afadin and α-catenin directly interact with each other, but this association is not strong. Additional factors or the posttranslational modification(s) of these proteins, such as protein phosphorylation, may be involved in this association. Consistently, we have identified two connector units for the nectin and E-cadherin systems. One is a ponsin-vinculin unit (Mandai et al., 1999). Ponsin is an afadin- and vinculin-binding protein and vinculin is an F-actin- and α-catenin–binding protein. The other is an ADIP-α-actinin unit (Asada et al., 2003). It is possible that afadin bound to non–trans-interacting nectins is associated with α-catenin through these additional factors or the posttranslational modification(s) and inhibits the activation of PI3 kinase induced by the trans-interaction of E-cadherin. Once non–trans-interacting nectins trans-interact, Cdc42 is activated and may affect the interaction of afadin with α-catenin, resulting in the E-cadherin–induced activation of PI3 kinase, an upstream molecule of Rac.
We have recently shown that the trans-interaction of nectin-1 increases the velocity of the formation of the E-cadherin–based AJs in EL and MDCK cells (Honda et al., 2003a). In contrast, we have shown here that non–trans-interacting nectin-2 reduces the velocity of the formation of the E-cadherin–based AJs in MDCK cells. We have previously proposed a model for the role and mode of action of nectins in the formation of the E-cadherin–based AJs (Takai and Nakanishi, 2003; Takai et al., 2003). Nectins and E-cadherin are diffusely distributed on the free surface of the plasma membrane of migrating cells. When the cells touch each other, nectins and E-cadherin separately form trans-dimers that form respective microclusters at cell-cell contact sites. The nectin-based microclusters may be more rapidly formed than the E-cadherin–based microclusters. The nectin-based microclusters then recruit E-cadherin, which then forms trans-dimers at this site, and this results in the formation of a mixture of the nectin- and E-cadherin–based microclusters.
On the other hand, the E-cadherin–based microclusters that form slowly and independently of the nectin-based microclusters may rapidly recruit the nectin-afadin complex to form microclusters. These microclusters fuse with each other to develop into maturated AJs. In this way, the nectin-afadin unit may regulate the formation of the E-cadherin–based cell-cell AJs. Based on the present results, it is likely that non–trans-interacting nectins around the E-cadherin–based microclusters, that form slowly and independently of the nectin-based microclusters, inhibit the activation of Rac induced by the trans-interaction of E-cadherin and the Rac-mediated maturation of AJs. Thus, AJs may be mainly maturated around the initially formed nectin-based microcluster. In this way, both trans-interacting and non–trans-interacting nectins play roles in regulation of the formation of cell-cell AJs.
Supplementary Material
Acknowledgments
We thank Drs. M. Takeichi, S. Narumiya, Sh. Tsukita, M. Kasuga, and W. Birchmeier for their generous gifts of reagents. This work was supported by grants-in-aid for Scientific Research and for Cancer Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan (2001–2003).
Abbreviations used: aa, amino acid(s); AJs, adherens junctions; Cef, the extracellular fragment of E-cadherin fused to the IgG Fc; CRIB, Cdc42 and Rac interactive binding domain; DIC, differential interference contrast; F-actin, actin filaments; FRET, fluorescent resonance energy transfer; GFP, green fluorescent protein; IMD, intensity modulated display; mAb, monoclonal antibody; MDCK, Madin-Darby canine kidney; Nef, the extracellular fragment of nectin fused to the IgG Fc; pAb, polyclonal Ab; PI3 kinase, phosphatidylinositol-3 kinase.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–05–0321. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-05-0321.
Online version of this article contains video material. Online version is available at www.molbiolcell.org.
References
- Asada, M., Irie, K., Morimoto, K., Yamada, A., Ikeda, W., Takeuchi, M., and Takai, Y. (2003). ADIP: a novel afadin- and α-actinin-binding protein localized at cell-cell adherens junctions. J. Biol. Chem. 278, 4103-4111. [DOI] [PubMed] [Google Scholar]
- Betson, M., Lozano, E., Zhang, J., and Braga, V.M.M. (2002). Rac activation upon cell-cell contact formation is dependent on signaling from the epidermal growth factor receptor. J. Biol. Chem. 277, 36962-36969. [DOI] [PubMed] [Google Scholar]
- Braga, V.M., Machesky, L.M., Hall, A., and Hotchin, N.A. (1997). The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J. Cell Biol. 137, 1421-1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich, J.S., Hansen, M.D.H., and Nelson, W.J. (2002). Spatio-temporal regulation of Rac1 localization and lamellipodia dynamics during epithelial cell-cell adhesion. Dev. Cell 3, 259-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara, A. et al. (2002a). Involvement of nectin in the localization of junctional adhesion molecule at tight junctions. Oncogene 21, 7649-7655. [DOI] [PubMed] [Google Scholar]
- Fukuhara, A., Irie, K., Yamada, A., Katata, T., Honda, T., Shimizu, K., Nakanishi, H., and Takai, Y. (2002b). Roles of nectin in organization of tight junctions in epithelial cells. Genes Cells 7, 1059-1072. [DOI] [PubMed] [Google Scholar]
- Gumbiner, B.M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345-357. [DOI] [PubMed] [Google Scholar]
- Honda, T., Shimizu, K., Fukuhara, A., Irie, K., and Takai, Y. (2003a). Regulation by nectin of the velocity of the formation of adherens junctions and tight junctions. Biochem. Biophys. Res. Commun. 306, 104-109. [DOI] [PubMed] [Google Scholar]
- Honda, T., Shimizu, K., Kawakatsu, T., Fukuhara, A., Irie, K., Nakamura, T., Matsuda, M., and Takai, Y. (2003b). Cdc42 and Rac small G proteins activated by trans-interactions of nectins are involved in activation of c-Jun N-terminal kinase, but not in association of nectins and cadherin to form adherens junctions, in fibroblasts. Genes Cells 8, 481-491. [DOI] [PubMed] [Google Scholar]
- Honda, T. et al. (2003c). Antagonistic and agonistic effects of an extracellular fragment of nectin on formation of E-cadherin-based cell-cell adhesion. Genes Cells 8, 51-63. [DOI] [PubMed] [Google Scholar]
- Itoh, R.E., Kurokawa, K., Ohba, Y., Yoshizaki, H., Mochizuki, N., and Matsuda, M. (2002). Activation of rac and cdc42 video imaged by fluorescent resonance energy transfer-based single-molecule probes in the membrane of living cells. Mol. Cell. Biol. 22, 6582-6591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou, T.S., and Nelson, W.J. (1998). Effects of regulated expression of mutant RhoA and Rac1 small GTPases on the development of epithelial (MDCK) cell polarity. J. Cell Biol. 142, 85-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakatsu, T., Shimizu, K., Honda, T., Fukuhara, T., Hoshino, T., and Takai, Y. (2002). Trans-interactions of nectins induce formation of filopodia and lamellipodia through the respective activation of Cdc42 and Rac small G proteins. J. Biol. Chem. 277, 50749-50755. [DOI] [PubMed] [Google Scholar]
- Kim, S.H., Li, Z., and Sacks, D.B. (2000). E-cadherin-mediated cell-cell attachment activates Cdc42. J. Biol. Chem. 275, 36999-37005. [DOI] [PubMed] [Google Scholar]
- Kitamura, T. et al. (1999). Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serin-threonine kinase Akt. Mol. Cell. Biol. 19, 6286-6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodama, A., Takaishi, K., Nakano, K., Nishioka, H., and Takai, Y. (1999). Involvement of Cdc42 small G protein in cell-cell adhesion, migration and morphology of MDCK cells. Oncogene 18, 3996-4006. [DOI] [PubMed] [Google Scholar]
- Kovacs, E.M., Ali, R.G., McCormack, A.J., and Yap, A.S. (2002). E-Cadherin homophilic ligation directly signals through rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277, 6708-6718. [DOI] [PubMed] [Google Scholar]
- Kuroda, S., Fukata, M., Fujii, K., Nakamura, T., Izawa, I., and Kaibuchi, K. (1997). Regulation of cell-cell adhesion of MDCK cells by Cdc42 and Rac1 small GTPases. Biochem. Biophys. Res. Commun. 240, 430-435. [DOI] [PubMed] [Google Scholar]
- Mandai, K. et al. (1997). Afadin: a novel actin filament-binding protein with one PDZ domain localized at cadherin-based cell-to-cell adherens junction. J. Cell Biol. 139, 517-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai, K., Nakanishi, H., Satoh, A., Takahashi, K., Satoh, K., Nishioka, H., Mizoguchi, A., and Takai, Y. (1999). Ponsin/SH3P 12, an l-afadin- and vinculin-binding protein localized at cell-cell and cell-matrix adherens junctions. J. Cell Biol. 144, 1001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyahara, M., Nakanishi, H., Takahashi, K., Satoh-Horikawa, K., Tachibana, K., and Takai, Y. (2000). Interaction of nectin with afadin is necessary for its clustering at cell-cell contact sites but not for its cis dimerization or trans interaction. J. Biol. Chem. 275, 613-618. [DOI] [PubMed] [Google Scholar]
- Miyawaki, A., Llopis, J., Heim, R., McCaffery, J.M., Adams, J.A., Ikura, M., and Tsien, R.Y. (1997). Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 388, 882-887. [DOI] [PubMed] [Google Scholar]
- Mochizuki, N., Yamashita, S., Kurokawa, K., Ohba, Y., Nagai, T., Miyawaki, A., and Matsuda, M. (2001). Spatio-temporal images of growth-factor-induced activation of Ras and Rap1. Nature 411, 1065-1068. [DOI] [PubMed] [Google Scholar]
- Nagafuchi, A. (2001). Molecular architecture of adherens junctions. Curr. Opin. Cell Biol. 13, 600-603. [DOI] [PubMed] [Google Scholar]
- Nagafuchi, A., Shirayoshi, Y., Okazaki, K., Yasuda, K., and Takeichi, M. (1987). Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 329, 341-343. [DOI] [PubMed] [Google Scholar]
- Nakagawa, M., Fukata, M., Yamaga, M., Itoh, N., and Kaibuchi, K. (2001). Recruitment and activation of Rac1 by the formation of E-cadherin-mediated cell-cell adhesion sites. J. Cell Sci. 114, 1829-1838. [DOI] [PubMed] [Google Scholar]
- Nishimura, M., Kakizaki, M., Ono, Y., Morimoto, K., Takeuchi, M., Inoue, Y., Imai, T., and Takai, Y. (2002). JEAP, a novel component of tight junctions in exocrine cells. J. Biol. Chem. 277, 5583-5587. [DOI] [PubMed] [Google Scholar]
- Noren, N.K., Niessen, C.M., Gumbiner, B.M., and Burridge, K. (2001). Cadherin engagement regulates Rho family GTPases. J. Biol. Chem. 276, 33305-33308. [DOI] [PubMed] [Google Scholar]
- Ohno, S. (2001). Intercellular junctions and cellular polarity: the PAR-aPKC complex, a conserved core cassette playing fundamental roles in cell polarity. Curr. Opin. Cell Biol. 13, 641-648. [DOI] [PubMed] [Google Scholar]
- Ono, Y., Nakanishi, H., Nishimura, M., Kakizaki, M., Takahashi, K., Miyahara, M., Satoh-Horikawa, K., Mandai, K., and Takai, Y. (2000). Two actions of frabin: direct activation of Cdc42 and indirect activation of Rac. Oncogene 19, 3050-3058. [DOI] [PubMed] [Google Scholar]
- Sakisaka, T., Nakanishi, H., Takahashi, K., Mandai, K., Miyahara, M., Satoh, A., Takaishi, K., and Takai, Y. (1999). Different behavior of l-afadin and neurabin-II during the formation and destruction of cell-cell adherens junction. Oncogene 18, 1609-1617. [DOI] [PubMed] [Google Scholar]
- Satoh-Horikawa, K., Nakanishi, H., Takahashi, K., Miyahara, M., Nishimura, M., Tachibana, K., Mizoguchi, A., and Takai, Y. (2000). Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 275, 10291-10299. [DOI] [PubMed] [Google Scholar]
- Tachibana, K., Nakanishi, H., Mandai, K., Ozaki, K., Ikeda, W., Yamamoto, Y., Nagafuchi, A., Tsukita, S., and Takai, Y. (2000). Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150, 1161-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K. et al. (1999). Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145, 539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai, Y., Irie, K., Shimizu, K., Sakisaka, T., and Ikeda, W. (2003). Nectins and nectin-like molecules: roles in cell adhesion, migration, and polarization. Cancer Sci. 94, 655-667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai, Y., and Nakanishi, H. (2003). Nectin and afadin: novel organizers of intercellular junctions. J. Cell Sci. 116, 17-27. [DOI] [PubMed] [Google Scholar]
- Takaishi, K., Sasaki, T., Kotani, H., Nishioka, H., and Takai, Y. (1997). Regulation of cell-cell adhesion by rac and rho small G proteins in MDCK cells. J. Cell Biol. 139, 1047-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi, M. (1991). Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451-1455. [DOI] [PubMed] [Google Scholar]
- Takekuni, K., Ikeda, W., Fujito, T., Morimoto, K., Takeuchi, M., Monden, M., and Takai, Y. (2003). Direct binding of cell polarity protein PAR-3 to cell-cell adhesion molecule nectin at neuroepithelial cells of developing mouse. J. Biol. Chem. 278, 5497-5500. [DOI] [PubMed] [Google Scholar]
- Takenawa, T., and Miki, H. (2001). WASP and WAVE family proteins: key molecules for rapid rearrangement of cortical actin filaments and cell movement. J. Cell Sci. 114, 1801-1809. [DOI] [PubMed] [Google Scholar]
- Tsukita, S., and Furuse, M. (1999). Occludin and claudins in tight-junction strands: leading or supporting players? Trends. Cell Biol. 9, 268-273. [DOI] [PubMed] [Google Scholar]
- Yagi, T., and Takeichi, M. (2000). Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes Dev. 14, 1169-1180. [PubMed] [Google Scholar]
- Yokoyama, S., Tachibana, K., Nakanishi, H., Yamamoto, Y., Irie, K., Mandai, K., Nagafuchi, A., Monden, M., and Takai, Y. (2001). alpha-Catenin-independent recruitment of ZO-1 to nectin-based cell-cell adhesion sites through afadin. Mol. Biol. Cell 12, 1595-1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.