Abstract
The photic resetting response of the human circadian pacemaker depends on the timing of exposure, and the direction and magnitude of the resulting shift is described by a phase response curve (PRC). Previous PRCs in humans have utilized high-intensity polychromatic white light. Given that the circadian photoreception system is maximally sensitive to short-wavelength visible light, the aim of the current study was to construct a PRC to blue (480 nm) light and compare it to a 10,000 lux white light PRC constructed previously using a similar protocol. Eighteen young healthy participants (18–30 years) were studied for 9–10 days in a time-free environment. The protocol included three baseline days followed by a constant routine (CR) to assess initial circadian phase. Following this CR, participants were exposed to a 6.5 h 480 nm light exposure (11.8 μW cm−2, 11.2 lux) following mydriasis via a modified Ganzfeld dome. A second CR was conducted following the light exposure to re-assess circadian phase. Phase shifts were calculated from the difference in dim light melatonin onset (DLMO) between CRs. Exposure to 6.5 h of 480 nm light resets the circadian pacemaker according to a conventional type 1 PRC with fitted maximum delays and advances of −2.6 h and 1.3 h, respectively. The 480 nm PRC induced ∼75% of the response of the 10,000 lux white light PRC. These results may contribute to a re-evaluation of dosing guidelines for clinical light therapy and the use of light as a fatigue countermeasure.
Key points
The human ∼24 h circadian pacemaker ensures appropriate timing of physiological, behavioural and metabolic events and is synchronized to the 24 h day primarily by the 24 h light–dark cycle.
The direction and magnitude of photic resetting depend on the timing of light exposure, and are described by a phase response curve (PRC).
The human circadian photoreception system is functionally and anatomically distinct from the visual system and employs a novel photoreceptor, melanopsin, which is maximally sensitive to short-wavelength (blue) visible light.
We constructed a PRC to 6.5 h of blue (480 nm) light and compared it with a prior 6.7 h white light PRC; the blue light PRC achieved ∼75% of the resetting response of the white light PRC.
This study suggests that short-wavelength visible light exposures may be more efficient than traditional high-intensity white light exposures for treatment of circadian rhythm sleep disorders.
Introduction
In mammals, multiple physiological, endocrine, behavioural and molecular rhythms cycle with a period of approximately 24 h, and are governed by an endogenous clock located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Stephan & Zucker, 1972; Klein et al. 1991). In order to ensure that rhythms are appropriately synchronized with the solar day, the SCN is entrained daily by the environmental light–dark cycle. Retinal light exposure is the preeminent synchronizer of circadian rhythms in mammals, including humans (Czeisler et al. 1989; Czeisler, 1993). Photic input is detected exclusively by the eyes and transmitted via the retinohypothalamic tract (RHT) to the SCN. Light elicits multiple circadian, neuroendocrine and neurobehavioural responses, sometimes termed ‘non-visual responses’, including melatonin suppression and circadian phase resetting. These responses depend on light timing, intensity, wavelength, pattern, duration and light exposure history (Czeisler & Gooley, 2007).
The effect of light timing on the phase resetting response is described by a phase response curve (PRC) which expresses the magnitude and direction of the phase shift as a function of the time that the stimulus is given (Hastings & Sweeney, 1958; DeCoursey, 1961; Winfree, 1980). PRCs can be classified as ‘type 1’ (weak) or ‘type 0’ (strong) based on the average slope of the plot of the initial and final circadian phase following a resetting stimulus. Published light PRCs in humans to date have employed high-intensity polychromatic white light sources (intensity range: 3000–10,000 lux). While direct comparison of these PRCs is difficult due to differences in methodology, they are generally consistent and show that light exposures occurring in the early biological night induce a phase delay shift of the circadian pacemaker, whereas light exposures occurring in the late biological night/early morning induce a phase advance shift. Most of these PRCs report phase delays of less than 12 h, which classifies them as type 1 (weak) (Honma & Honma, 1988; Minors et al. 1991; Van Cauter et al. 1994; Khalsa et al. 2003). One study (Czeisler et al. 1989; Jewett et al. 1994) showed type 0 resetting and accompanying amplitude suppression following three consecutive days with light pulses.
Light wavelength also determines the magnitude of photic phase resetting. In addition to rod and cone photoreceptors, the mammalian retina contains intrinsically photosensitive retinal ganglion cells (ipRGCs), which contain the opsin-based photopigment melanopsin and are most sensitive to short-wavelength light (λmax = ∼480 nm) (Provencio et al. 2000; Berson et al. 2002; Hattar et al. 2002; Berson, 2003; Melyan et al. 2005). This non-rod, non-cone photoreceptor system is anatomically and functionally distinct from those used for vision and in the absence of the classical photoreceptors, melanopsin-containing ipRGCs can account for all ‘non-visual’ photoresponses in mammals, e.g. circadian entrainment (Panda et al. 2002; Ruby et al. 2002; Hattar et al. 2003; Güler et al. 2008), pupil constriction (Lucas et al. 2001; Hattar et al. 2003) and melatonin suppression (Lucas et al. 1999), even in totally visually blind subjects (Czeisler et al. 1995; Klerman et al. 2002b; Zaidi et al. 2007), although at a reduced sensitivity in dim light (Gooley et al. 2010). Analytic action spectra for melatonin suppression are consistent with a novel non-rod, non-cone photoreceptor system with peak sensitivity in the visible short-wavelength range of 446–477 nm (Brainard et al. 2001; Thapan et al. 2001). When controlled for photon density, exposure to short-wavelength 460 nm light at night induces greater phase delay shifts, melatonin suppression and alerting responses as compared to mid-wavelength 555 nm light, the peak of photopic (colour) vision (Lockley et al. 2003, 2006; Cajochen et al. 2005). The phase-resetting effects of short-wavelength light across different times of day have not been studied, however, and therefore the aim of this study was to construct a PRC to a 6.5 h monochromatic light pulse (480 nm).
Methods
Ethical approval
The study was approved by the Human Research Committee at Partners HealthCare System, in compliance with the Declaration of Helsinki. All participants gave written informed consent prior to enrolling in the study and were paid for their participation.
Participants
Twenty-one participants (10 females, mean age ± SD: 23.10 ± 3.43 years) were randomized and admitted for the study to the Intensive Physiological Monitoring Unit (IPM) of the Center for Clinical Investigation (CCI) at the Brigham and Women's Hospital (BWH; Boston, MA, USA) and were studied for 9–10 days in an environment free of time cues between May and December 2009.
Participants were screened for medical and psychological health via examination, questionnaires, interview, and comprehensive urine and blood tests. Routine ophthalmology examinations were performed before and after the study. The absence of colour-blindness was confirmed before the study using the Ishihara test (Ishihara, 1996). For at least 3 weeks prior to admission, participants kept a self-selected, regular sleep–wake schedule of 8 h of sleep and 16 h of wake that was verified by calls to a time- and date-stamped voicemail in addition to actigraphy for at least 1 week prior to admission (Actiwatch-L, Philips-Respironics, The Netherlands). Participants were required to abstain from caffeine, nicotine, alcohol and other foreign substances for the duration of the study from the beginning of screening until completion, and compliance was evaluated by toxicology tests during screening and upon admission. In addition, female participants were confirmed to be non-pregnant during screening and upon admission. Female participants not using oral contraceptives were tested in the luteal phase of their menstrual cycle (based on menstrual history), and female participants taking oral contraceptives were tested during times when they took a contraceptive with a stable hormone concentration.
Experimental protocol
During the 9–10 day protocol, participants remained in an individual time-free suite. The protocol started with three baseline days consisting of 8 h of scheduled sleep in darkness (<0.02 lux, <0.00006 W m−2; black bars, Fig. 1) and 16 h of scheduled wake in ambient light (white bars, Fig. 1). Ambient light was provided by 4100K fluorescent lamps (Philips Lighting, The Netherlands) with digital ballasts (Lutron Electronics Co., Inc., PA, USA) transmitted through a UV-stable filter (Lexan 9030 with prismatic lens, GE Plastics, MA, USA) and light levels were approximately 90 lux at the cornea [0.23 W m−2 (∼89 lux) at 137 cm from the floor in the vertical plane and had a maximum of 0.48 W m−2 (∼150 lux) when measured in the horizontal plane at a height of 187 cm anywhere in the room]. Halfway through day 3, the light intensity was dimmed to ∼0.5 lux (0.001 W m−2) [at 137 cm from the floor in the vertical plane with a maximum <3 lux (0.01 W m−2) at 187 cm from the floor in the horizontal plane anywhere in the room] for the remainder of the study (grey bars, Fig. 1). Participants were in darkness during scheduled sleep (black bars, Fig. 1). At wake time on day 4, participants began an initial ∼30–52 h constant routine (CR) in <3 lux during which time participants remained awake in a semi-recumbent position in bed, with consumption of equal isocaloric snacks at hourly intervals while being constantly monitored by a technician to maintain wakefulness (Duffy & Dijk, 2002).
Figure 1. Study protocol.
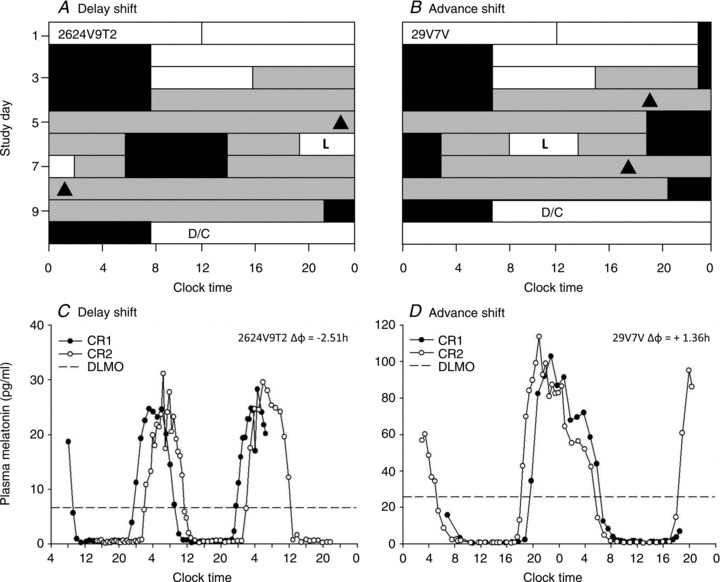
Example of a raster plot for a phase delay protocol (A) and a phase advance protocol (B). The y-axis depicts consecutive study days and the x-axis clock time. White and grey bars indicate wake episodes in ambient light of <150 lux or <3 lux, respectively; black bars indicate scheduled sleep in 0 lux. ‘L’ indicates the 6.5 h light exposure and the black triangles show the timing of dim light melatonin onset (DLMO) before and after the light exposure. C and D show the corresponding plasma melatonin profiles during constant routines (CR) for the subjects plotted in A and B, respectively. The dashed line indicates the 25% value of the fitted peak-to-trough-amplitude DLMO.
Following an 8 h sleep episode, participants were exposed to monochromatic 480 nm light (11.8 μW cm−2; 2.8 × 1013 photons cm−2 s−1; ≤15 nm half-peak bandwidth) for 6.5 h via a modified Ganzfeld dome (Brainard et al. 2001) centred in the 16 h wake episode (Lockley et al. 2003; Gooley et al. 2010). The monochromatic light source was generated by using a 1200 W xenon arc lamp and a grating monochromator. Power readings were taken regularly throughout the light exposure to ensure constant irradiance, using an IL-1400 radiometer with an SEL-033/F/W detector (International Light, Inc., Peabody, MA, USA) by fixing the light meter to the front of the dome at approximately eye level. The average corneal irradiance achieved during the light exposure was 11.75 ± 0.16 μW cm−2 (mean ± SD).
From 95 min prior to and throughout the light exposure, participants remained seated (Deacon & Arendt, 1994), and 15 min prior to the start of the light exposure, one drop of 0.5% cyclopentolate HCI (Cyclogyl, Alcon Laboratories, Fort Worth, TX, USA) was administered in each eye to dilate the pupil (Gaddy et al. 1993). Participants wore black-out goggles after the pupil dilator was administered until the start of the light exposure. Throughout the monochromatic light exposure, participants were asked to keep their gaze fixed for 90 min, while resting their head on a chin rest, before changing to a free gaze for 10 min, repeated throughout the exposure. Participants were monitored continuously by a technician to ensure compliance. Light timing was randomized to 1 of 18 circadian phases separated by 80 min (=20 deg intervals) according to habitual wake-time. Inter-individual differences in phase angle of entrainment contributed to a slightly varied interval of final light exposure phases. Following another 8 h sleep episode, participants started a second CR (∼32–55 h), followed by a 10 h recovery sleep episode before discharge. Figure 1 shows an example of a phase delay (A), and a phase advance (B) protocol, and the corresponding individual melatonin profiles (C and D, respectively).
Melatonin
Starting on day 2, an indwelling, intravenous catheter was inserted in each participant's forearm vein and plasma samples were collected every 20–60 min. Samples were analysed by radioimmunoassay (Buehlmann Laboratories AG, Schoenebuch, Switzerland). The limit of detection was 0.01 pg ml−1; the inter-assay coefficient of variation (CV) was 10.79% at a level of 1.80 pg ml−1 and 8.01% at a level of 14.20 pg ml−1. The intra-assay coefficient of variation (CV) was 5.8% at a level of 1.98 pg ml−1 and 10.64% at a level of 13.96 pg ml−1.
Phase shifts (mean ± SD) were calculated as the difference between initial phase and final phase of the melatonin rhythm measured during the first and second CR, respectively (Fig. 1). Melatonin phase was defined as the dim-light melatonin onset (DLMO) calculated from 25% of the fitted three-harmonic peak-to-trough amplitude of the melatonin rhythm during the first constant routine (Fig. 1) (Klerman et al. 2002a).
Figure 2. Phase response curve to 6.5 h of 480 nm light.
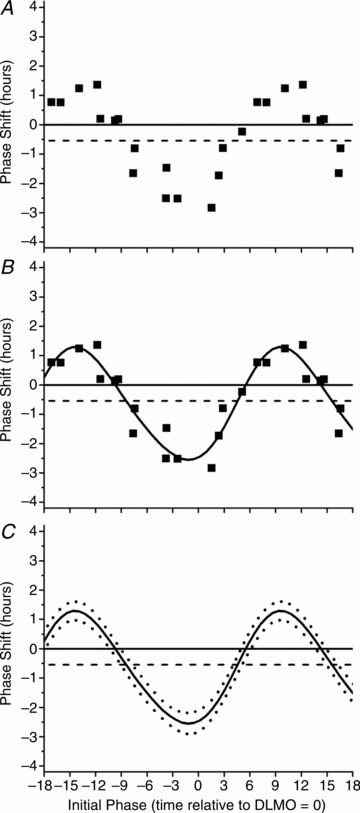
Raw phase shifts in plasma melatonin DLMO are plotted as a function of circadian phase (A). Circadian phase was computed as the time at which light onset occurred relative to the DLMO estimate from CR1. Per convention phase delays are negative on the y-axis and phase advances are positive. Circadian phase 0 on the abscissa refers to light onset coinciding with DLMO on CR1 with a negative value indicating that the light onset was timed prior to CR1 DLMO and a positive value indicating that light onset occurred after CR1 DLMO. Phase shifts were fitted with a two-harmonic function (continuous line, B). According to the 95% confidence intervals generated from the fit two-harmonic function (dotted lines, C), phase shifts were significantly different from 0 at all phases (continuous horizontal line, A–C) with no evidence of a dead zone. The estimated drift due to intrinsic circadian period is illustrated by the dashed line (−0.54 h over 3 cycles; Czeisler et al. 1999).
For PRC construction, the circadian phase of light timing was determined as the time at which the onset of the light exposure was administered relative to the DLMO estimate from CR1. Per convention, phase delays are plotted as negative values and phase advances as positive values on the ordinate (Fig. 2).
Melatonin suppression
Melatonin suppression was calculated during the 6.5 h monochromatic light exposure by calculating the area under the curve (AUC) during the light exposure, expressed as a percentage of the AUC during the corresponding clock time 24 h earlier (Lockley et al. 2003). Participants were categorized as having received their light exposure either during their biological ‘night’ or biological ‘day’, with biological night defined as any portion of the 6.5 h light exposure occurring between DLMO and DLMOff 24 h earlier.
Core body temperature
Core body temperature (CBT) was recorded every minute via rectal thermistor (Yellow Springs Instruments, Inc., Yellow Springs, OH, USA). CBT data were visually inspected and non-physiological values due to sensor movement and removals were excluded. The phase of CBT minimum (CBTmin) was determined for CR1 and CR2 separately using a two-harmonic-regression statistical model (Brown & Czeisler, 1992). Phase shifts were calculated for the clock time difference in CBTmin between CR1 and CR2.
Phase response curve
The PRC was fitted with a two-harmonic function of the form
| (1) |
where x is initial phase and y is phase shift. Parameters μ, A, φA, B and φB, represent the mean phase shift and the amplitudes and phases of the first and second harmonics, respectively. These parameters were estimated using a Levenberg–Marquardt algorithm and adjusted R2 and 95% confidence intervals were computed from the resulting fit function using the non-linear curve fitting implementation in OriginPro 8.5 (Northampton, MA, USA). Statistical comparisons of the monochromatic 480 nm 6.5 h PRC with a 6.7 h bright white light PRC constructed previously under similar conditions (Khalsa et al. 2003) were based on the 95% confidence intervals of the two-harmonic fits of each PRC. Non-overlapping confidence intervals indicated significant differences between the PRCs.
Results
Participants
Two participants were excluded during the first constant routine for failure to comply with the protocol, and a third subject inadvertently received polychromatic light exposure due to technical failure of the monochromatic light source and was also excluded from this analysis. Of the remaining 18 participants (9 female, mean age ± SD: 23.72 ± 3.3 years), two participants did not have sufficient data during one of the CRs for accurate phase assessment using melatonin (29P9V; 26T2V9T2) or temperature (29K6V, 26T2V9T2), and one participant had insufficient data for calculation of melatonin suppression (26T2V9T2).
Phase resetting
Exposure to a 6.5 h monochromatic light pulse resulted in a conventional type 1 PRC (Fig. 2). Applying a two-harmonic fit, the PRC showed a peak-to-trough amplitude of 3.8 h with a maximum fitted advance of 1.3 h and a maximum fitted delay of −2.6 h (adjusted R2 = 0.88) (Fig. 2B). The 95% confidence intervals show that phase shifts were significantly different from zero across all circadian phases with no evidence of a dead zone (Fig. 2C). There was a significant correlation between phase shifts in DLMO and CBTmin (N = 15; Pearson R = 0.69; P = 0.004) (SPSS, version 15; IBM, Armonk, NY, USA).
In Fig. 3, the 480 nm PRC is plotted together with a previously published white light PRC, which used a ∼10,000 lux exposure in a similar experimental protocol (4100K, Philips Lighting, The Netherlands) (Khalsa et al. 2003). The white light PRC shows a peak-to-trough magnitude of 5.5 h with maximum advances and delays of 2.0 h and −3.4 h, respectively (adjusted R2 = 0.89) (Fig. 3B). Figure 3C plots the 95% confidence intervals for both the monochromatic 480 nm and polychromatic 10,000 lux white light PRCs. The two PRCs are not significantly different except for a small window around the maximal delay region (i.e. light onset ∼2 h before DLMO) and maximal advance region (i.e. light onset occurring between 3 and 6 h after DLMO).
Figure 3. Comparison of phase response curve to 6.5 h of 480 nm light and 6.7 h of 10,000 lux white light.
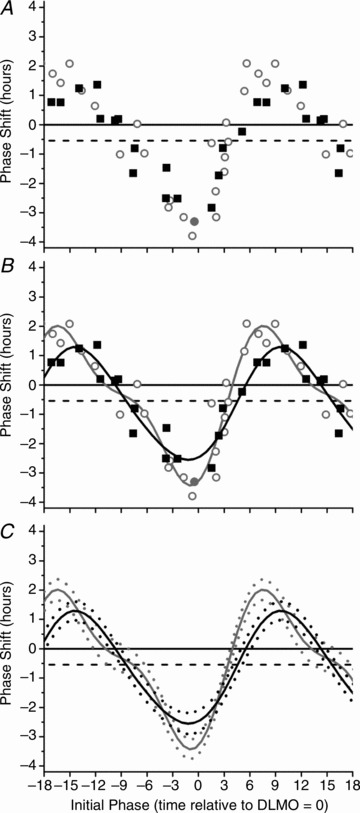
The phase response curve to 6.5 h of 480 nm light and 6.7 h of 10,000 lux white light for phase shifts calculated over three circadian cycles using the DLMO estimate from CR1. The raw data (A) and two-harmonic fits (B) for both the monochromatic 480 nm light (black squares, black line) and 6.7 h polychromatic white light (open grey circles, grey line) PRCs are shown. The 95% confidence intervals generated from the fit two-harmonic functions (monochromatic blue, dashed black line; polychromatic white, dashed grey line, C) show that the two PRCs are not significantly different except for a small window around the maximal delay and advance region. Phase shifts were computed using either plasma (open symbols) or salivary (filled symbols) melatonin for the 6.7 h polychromatic white PRC. The plotting convention is described in the legend for Fig. 2.
Inherent in any phase-shifting response is a contribution of the ‘drift’ due to the intrinsic period of the endogenous circadian pacemaker, which equals a shift of −0.54 h over three cycles (dashed horizontal line, Figs 2 and 3) based on published averages (24.18 h; Czeisler et al. 1999). In order to reduce the variance due to the intrinsic circadian period, the two PRCs are also plotted based on the phase shift calculated over two circadian cycles (Lockley et al. 2003). The 95% confidence intervals of the two PRCs overlap at all circadian phases and thus the PRCs are indistinguishable (Fig. 4).
Figure 4. Comparison of phase response curve to 6.5 h of 480 nm light and 6.7 h of 10,000 lux white light.
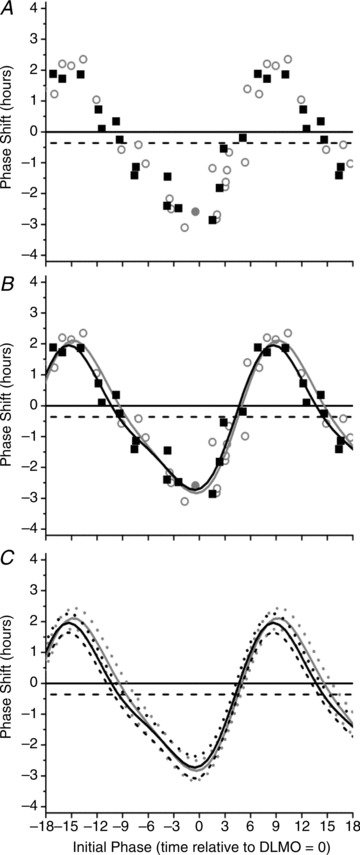
The phase response curve to 6.5 h of 480 nm light and 6.7 h of 10,000 lux white light for phase shifts calculated over two circadian cycles. The raw data (A) and two-harmonic fits (B) for both the monochromatic blue light (black squares) and 6.7 h polychromatic white light (open grey circles) PRCs are shown. Both PRCs were fitted with a two-harmonic function (monochromatic blue, continuous black line; polychromatic white, continuous grey line, B). Due to insufficient data to estimate DLMO, one subject was removed from the 6.7 h PRC for this analysis. The 95% confidence intervals generated from the fitted two-harmonic functions (monochromatic blue, dashed black line; polychromatic white, dashed grey line, C) show that the two PRCs are not significantly different. The plotting convention is described in the legend for Fig. 2.
Melatonin suppression
Ten participants received light exposure during their biological day and seven during their biological night. Figure 5A illustrates the individual and average percentage of melatonin suppression during the 6.5 h of blue light occurring during the biological night (N = 10) ordered by timing of light exposure onset. When the light exposure began early in the biological night, close to DLMO, the greatest amount of melatonin suppression was achieved with the suppression staying fairly consistent across the entire night (Fig. 5A, a and d). As the onset of the light exposure progressed towards the early morning hours, the amount of melatonin suppression is reduced as the light exposure occurs at only the tail end of the melatonin production window (Fig. 5Bb).
Figure 5. Melatonin suppression throughout the biological night in order of timing of light exposure.
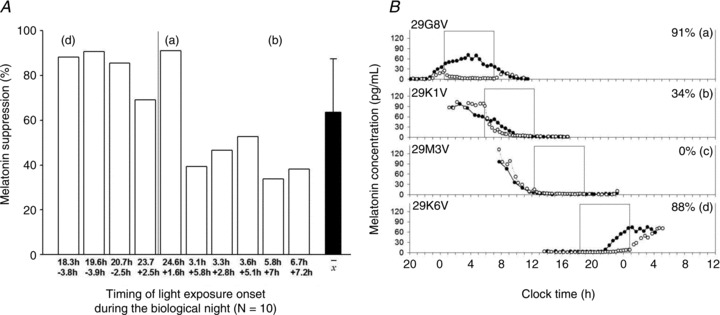
A shows individual and average percentage melatonin suppression during the 6.5 h of blue light ordered by the timing of the light exposure in relation to the timing of CR1 DLMO (numbers in the upper row represent the timing of LE, the lower numbers represent the timing of CR1 DLMO in decimal hours). Melatonin suppression is expressed as the area under the curve (AUC) during the light exposure expressed as the difference from the AUC during CR1 for the corresponding clock times for individuals (open bars; N = 10) and as a group (filled bar: mean ± SD). The vertical line indicates light exposures starting after midnight. B depicts four individual melatonin profiles approximately 90 deg (∼6 h) apart during CR (filled symbols) and light exposure (open symbols). The open box indicates the timing of the 6.5 h light exposure. In B, a, b and d correspond to the same individual indicated in A.
Discussion
This study demonstrates that exposure to a 6.5 h 480 nm monochromatic light pulse resets the circadian system according to a conventional type 1 PRC, with a maximal delay of −2.8 h and a maximal advance of 1.4 h in circadian melatonin rhythm (raw data). A two-harmonic function fitted to the raw data resulted in a PRC with a fitted peak-to-trough amplitude of 3.8 h. The PRC is in accordance with the results reported in previous PRC studies using 3–4 h white light pulses (Minors et al. 1991; Dawson et al. 1993; Van Cauter et al. 1994) although shifts are smaller than those reported in PRC studies using ∼6 h white light pulses (Honma & Honma, 1988; Jewett et al. 1994; Khalsa et al. 2003). Previous studies that employed a 6.5 h monochromatic light exposure of 460 nm with equal photons under similar experimental conditions also achieved comparable results, i.e. a phase delay shift of ∼3 h of the circadian melatonin rhythm following light exposure administered in the biological night (Lockley et al. 2003; Gooley et al. 2010).
In the original publication of the polychromatic 6.7 h PRC (Khalsa et al. 2003), circadian phase was computed as the midpoint of the light exposure relative to the DLMO estimate on CR1, and adjusted for the phase relationship between DLMO and CBTmin such that circadian phase 0 corresponded to the midpoint of the light exposure occurring at CBTmin. After replotting the polychromatic 6.7 h PRC using the circadian phase convention introduced in Fig. 2, the PRCs calculated over two circadian cycles are virtually indistinguishable and show the same magnitude of response (Fig. 4). Neither PRC showed evidence of a ‘dead zone’, a time during the day when light has no discernible effect on the circadian pacemaker, which has been observed primarily in nocturnal species (Pohl, 1982; Johnson, 1990; Jewett et al. 1997; Comas et al. 2006).
While the monochromatic 480 nm PRC and the 10,000 lux white light PRC are similar, it should be noted that the Khalsa et al. (2003) study differed in several ways: (a) no pupil dilator was used; (b) the current study had a fixed/free gaze interval of 90/10 min, whereas Khalsa et al. used a fixed/free gaze interval of 6/6 min; (c) the background light intensity for the last 8 h of baseline day 3 did not change in the study of Khalsa et al. but was reduced to <3 lux in the current study; (d) the light source in Khalsa et al. was ceiling-mounted whereas a modified Ganzfeld source was used in the current study; and (e) the CR was conducted under <15 lux background light in Khalsa et al. as compared with <3 lux in the current study. The use of a pupil dilator is presumed to increase retinal light exposure by a factor of ∼10, as it has been demonstrated that high-intensity light constricts an untreated pupil to an average diameter of 2 mm (surface area 4 π mm2) from an unconstricted diameter of 6–7 mm (surface area of 36 π–49 π mm2) (Spring & Stiles, 1949; deGroot & Gebhard, 1952). Thus, after adjusting for pupillary constriction, it appears that the 480 nm PRC (∼12 μW cm−2 × 10) required only ∼4% of the energy of the 10,000 lux white light PRC (3000 μW cm−2) yet induced ∼75% of the response. While these results suggest that the 480 nm light was more efficient at phase resetting the clock, the relative efficacy cannot be fully quantified from a single irradiance comparison with methodological differences in light geometry and pupil dilator use.
Most previous comparisons of the efficacy of different light stimuli to induce photic circadian resetting suffer from some of the same limitations as our current study. For example, Smith et al. (Smith & Eastman, 2009; Smith et al. 2009) compared the phase-shifting properties of blue-enriched white light (17,000 K, Philips Lighting, Eindhoven, The Netherlands) to standard fluorescent white light (4100K), using commercially available light boxes, and found no difference in the magnitude of induced phase shifts, despite the fact that the blue-enriched lamps were of lower illuminance (4000 versus 6000 lux) and irradiance (1640 versus 1741 μW cm−2) than the white light lamps. One interpretation of these results is that the blue-enriched 17,000 K lamps are more efficient in inducing circadian resetting. Another explanation might be that the responses were saturated in both cases, precluding a comparison of their relative efficacy. Indeed, Zeitzer and colleagues (2000) showed that a 6.5 h light pulse of ∼1000 lux was sufficient to induce maximum phase delays of the circadian system and that increasing the intensity to 9100 lux did not increase the response of the circadian system. Importantly, however, a recent study by Brainard and colleagues that compared fluence response curves for melatonin suppression following exposure to narrow-band blue LED light (λmax 469 nm) and polychromatic 4000 K white fluorescent light, demonstrated a greater efficiency for the blue LED light (West et al. 2011), consistent with our current findings. Future studies aiming to quantify the relative circadian efficacy of different light spectra require construction of comparable fluence response curves controlled for timing, duration, and other confounding factors (Gooley et al. 2010).
There is some evidence that a spectrally targeted light source will enhance efficiency and effectiveness of light treatments by reducing the light irradiance and potentially the duration of the treatment required. For example, studies employing a spectrally targeted blue light source in the treatment of seasonal affective disorder (SAD) can achieve clinical benefits comparable with higher-intensity white light typically employed (2500–10,000 lux), at lower irradiances and with relatively short exposures (45 min) (Glickman et al. 2006; Anderson et al. 2009; Strong et al. 2009; Meesters et al. 2011). The current study suggests that similar benefits may be obtained when circadian phase resetting is desired in the treatment of circadian rhythm sleep disorders by using spectrally targeted light sources to reduce exposure to unnecessarily high light intensities.
Acknowledgments
We thank Elizabeth Lydon, research staff and research participants at the Division of Sleep Medicine, Brigham and Women's Hospital (BWH); the technical, dietary, nursing and medical staff at the Center for Clinical Investigation at BWH in partnership with the Harvard Catalyst, Jonathan Williams MD, for medical supervision; Ralph Todesco (BWH), John Hanifin (Thomas Jefferson University), Ron Kovak and Jon Cooke (PTI, Inc.) for technical support for the generation of monochromatic light; Vincent Ricchiuti, PhD and the Core Laboratory staff (BWH) for melatonin assays. This work was supported by National Institute of Mental Health grant 5R01MH45130. M.R., G.C.B., C.A.C. and S.W.L. were supported, in part, by the National Space Biomedical Research Institute grants NBPF02001 (M.R., S.W.L.), HPF00001 (G.C.B., S.W.L.), HPF01605 and HPF01701 (G.C.B.), HFP01601 (C.A.C.) and HPF01301 (S.W.L.) through NASA NCC 9-58. M.A.St.H. was supported by an NHLBI training grant T32-HL07901 and S.-B.S.K. by NHLBI NRSA F33-HL009588.
Glossary
- CBT
core body temperature
- CBTmin
core body temperature minimum
- CR
constant routine
- DLMO
dim light melatonin onset
- DLMOff
dim light melatonin offset
- ipRGCs
intrinsically photosensitive retinal ganglion cells
- LE
light exposure
- PRC
phase response curve
- SCN
suprachiasmatic nucleus
- RHT
retinohypothalamic tract
Author contributions
Conception and design of the experiments: G.C.B., C.A.C. and S.W.L. Collection, analysis and interpretation of data: M.R., M.A.St.H. and S.W.L. Drafting the article or revising it critically for important intellectual content: M.R., M.A.St.H., G.C.B., S.-B.S.K., R.E.K., C.A.C. and S.W.L. All authors approved the final version.
References
- Anderson JL, Glod CA, Dai J, Cao Y, Lockley SW. Lux vs. wavelength in light treatment of Seasonal Affective Disorder. Acta Psychiat Scand. 2009;120:203–212. doi: 10.1111/j.1600-0447.2009.01345.x. [DOI] [PubMed] [Google Scholar]
- Berson DM. Strange vision: ganglion cells as circadian photoreceptors. Trends Neurosci. 2003;26:314–320. doi: 10.1016/S0166-2236(03)00130-9. [DOI] [PubMed] [Google Scholar]
- Berson DM, Dunn FA, Takao M. Phototransduction by retinal ganglion cells that set the circadian clock. Science. 2002;295:1070–1073. doi: 10.1126/science.1067262. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Hanifin JP, Greeson JM, Byrne B, Glickman G, Gerner E, Rollag MD. Action spectrum for melatonin regulation in humans: Evidence for a novel circadian photoreceptor. J Neurosci. 2001;21:6405–6412. doi: 10.1523/JNEUROSCI.21-16-06405.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown EN, Czeisler CA. The statistical analysis of circadian phase and amplitude in constant-routine core-temperature data. J Biol Rhythm. 1992;7:177–202. doi: 10.1177/074873049200700301. [DOI] [PubMed] [Google Scholar]
- Cajochen C, Munch M, Kobialka S, Krauchi K, Steiner R, Oelhafen P, Orgul S, Wirz-Justice A. High sensitivity of human melatonin, alertness, thermoregulation, and heart rate to short wavelength light. J Clin Endocrinol Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythm. 2006;21:362–372. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- Czeisler CA. Light. In: Carskadon MA, editor. Encyclopedia of Sleep and Dreaming. New York: Macmillan Publishing Co; 1993. pp. 328–331. [Google Scholar]
- Czeisler CA, Duffy JF, Shanahan TL, Brown EN, Mitchell JF, Rimmer DW, Ronda JM, Silva EJ, Allan JS, Emens JS, Dijk DJ, Kronauer RE. Stability, precision, and near-24-hour period of the human circadian pacemaker. Science. 1999;284:2177–2181. doi: 10.1126/science.284.5423.2177. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Gooley JJ. Sleep and circadian rhythms in humans. Cold Spring Harb Symp Quant Biol. 2007;72:579–597. doi: 10.1101/sqb.2007.72.064. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Kronauer RE, Allan JS, Duffy JF, Jewett ME, Brown EN, Ronda JM. Bright light induction of strong (type 0) resetting of the human circadian pacemaker. Science. 1989;244:1328–1333. doi: 10.1126/science.2734611. [DOI] [PubMed] [Google Scholar]
- Czeisler CA, Shanahan TL, Klerman EB, Martens H, Brotman DJ, Emens JS, Klein T, Rizzo JF., III Suppression of melatonin secretion in some blind patients by exposure to bright light. New Engl J Med. 1995;332:6–11. doi: 10.1056/NEJM199501053320102. [DOI] [PubMed] [Google Scholar]
- Dawson D, Lack L, Morris M. Phase resetting of the human circadian pacemaker with use of a single pulse of bright light. Chronobiol Int. 1993;10:94–102. doi: 10.3109/07420529309059697. [DOI] [PubMed] [Google Scholar]
- De Groot SG, Gebhard JW. Pupil size as determined by adapting luminance. J Opt Soc Am. 1952;42:492–495. doi: 10.1364/josa.42.000492. [DOI] [PubMed] [Google Scholar]
- Deacon S, Arendt J. Posture influences melatonin concentrations in plasma and saliva in humans. Neurosci Lett. 1994;167:191–194. doi: 10.1016/0304-3940(94)91059-6. [DOI] [PubMed] [Google Scholar]
- DeCoursey PJ. Effect of light on the circadian activity rhythm of the flying squirrel, Glaucomys volans. J Comp Physiol. 1961;44:331–354. [Google Scholar]
- Duffy JF, Dijk DJ. Getting through to circadian oscillators: why use constant routines. J Biol Rhythm. 2002;17:4–13. doi: 10.1177/074873002129002294. [DOI] [PubMed] [Google Scholar]
- Gaddy JR, Rollag MD, Brainard GC. Pupil size regulation of threshold of light-induced melatonin suppression. J Clin Endocrinol Metab. 1993;77:1398–1401. doi: 10.1210/jcem.77.5.8077340. [DOI] [PubMed] [Google Scholar]
- Glickman G, Byrne B, Pineda C, Hauck WW, Brainard GC. Light therapy for seasonal affective disorder with blue narrow-band light-emitting diodes (LEDs) Biol Psychiat. 2006;59:502–507. doi: 10.1016/j.biopsych.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Gooley JJ, Rajaratnam SMW, Brainard GC, Kronauer RE, Czeisler CA, Lockley SW. Spectral responses of the human circadian system depend on the irradiance and duration of exposure to light. Sci Transl Med. 2010;2:31ra33. doi: 10.1126/scitranslmed.3000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Güler AD, Ecker JL, Lall GS, Haq S, Altimus CM, Liao HW, Barnard AR, Cahill H, Badea TC, Zhao H, Hankins MW, Berson DM, Lucas RJ, Yau KW, Hattar S. Melanopsin cells are the principal conduits for rod-cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hastings JW, Sweeney BM. A persistent diurnal rhythm of luminescence in Gonyaulax polyedra. Biol Bull. 1958;115:440–458. [Google Scholar]
- Hattar S, Liao H-W, Takao M, Berson DM, Yau K-W. Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science. 2002;295:1065–1070. doi: 10.1126/science.1069609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattar S, Lucas RJ, Mrosovsky N, Thompson S, Douglas RH, Hankins MW, Lem J, Biel M, Hofmann F, Foster RG, Yau K-W. Melanopsin and rod-cone photoreceptive systems account for all major accessory visual functions in mice. Nature. 2003;424:76–81. doi: 10.1038/nature01761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honma K, Honma S. A human phase response curve for bright light pulses. Jpn J Psychiatr Neurol. 1988;42:167–168. [Google Scholar]
- Ishihara S. The Series of Plates Designed as a Test for Colour-Deficiency. Tokyo, Japan: Kanehara & Co., Ltd; 1996. [Google Scholar]
- Jewett ME, Kronauer RE, Czeisler CA. Phase-amplitude resetting of the human circadian pacemaker via bright light: a further analysis. J Biol Rhythm. 1994;9:295–314. doi: 10.1177/074873049400900310. [DOI] [PubMed] [Google Scholar]
- Jewett ME, Rimmer DW, Duffy JF, Klerman EB, Kronauer RE, Czeisler CA. Human circadian pacemaker is sensitive to light throughout subjective day without evidence of transients. Am J Physiol Regul Integr Comp Physiol. 1997;273:R1800–R1809. doi: 10.1152/ajpregu.1997.273.5.r1800. [DOI] [PubMed] [Google Scholar]
- Johnson CH. An Atlas of Phase Response Curves for Circadian and Circatidal Rhythms. Nashville, TN: Department of Biology, Vanderbilt University; 1990. [Google Scholar]
- Khalsa SBS, Jewett ME, Cajochen C, Czeisler CA. A phase response curve to single bright light pulses in human subjects. J Physiol. 2003;549:945–952. doi: 10.1113/jphysiol.2003.040477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein DC, Moore RY, Reppert SM. Suprachiasmatic Nucleus: The Mind's Clock. New York: Oxford University Press; 1991. [Google Scholar]
- Klerman EB, Gershengorn HB, Duffy JF, Kronauer RE. Comparisons of the variability of three markers of the human circadian pacemaker. J Biol Rhythm. 2002a;17:181–193. doi: 10.1177/074873002129002474. [DOI] [PubMed] [Google Scholar]
- Klerman EB, Shanahan TL, Brotman DJ, Rimmer DW, Emens JS, Rizzo JF, III, Czeisler CA. Photic resetting of the human circadian pacemaker in the absence of conscious vision. J Biol Rhythm. 2002b;17:548–555. doi: 10.1177/0748730402238237. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Brainard GC, Czeisler CA. High sensitivity of the human circadian melatonin rhythm to resetting by short wavelength light. J Clin Endocrinol Metab. 2003;88:4502–4505. doi: 10.1210/jc.2003-030570. [DOI] [PubMed] [Google Scholar]
- Lockley SW, Evans EE, Scheer FAJL, Brainard GC, Czeisler CA, Aeschbach D. Short-wavelength sensitivity for the direct effects of light on alertness, vigilance, and the waking electroencephalogram in humans. Sleep. 2006;29:161–168. [PubMed] [Google Scholar]
- Lucas RJ, Douglas RH, Foster RG. Characterization of an ocular photopigment capable of driving pupillary constriction in mice. Nat Neurosci. 2001;4:621–626. doi: 10.1038/88443. [DOI] [PubMed] [Google Scholar]
- Lucas RJ, Freedman MS, Muñoz M, Garcia-Fernandez JM, Foster RG. Regulation of the mammalian pineal by non-rod, non-cone, ocular photoreceptors. Science. 1999;284:505–507. doi: 10.1126/science.284.5413.505. [DOI] [PubMed] [Google Scholar]
- Meesters Y, Dekker V, Schlangen LJ, Bos EH, Ruiter MJ. Low-intensity blue-enriched white light (750 lux) and standard bright light (10,000 lux) are equally effective in treating SAD. A randomized controlled study. BMC Psychiatry. 2011;11:17. doi: 10.1186/1471-244X-11-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melyan Z, Tarttelin EE, Bellingham J, Lucas RJ, Hankins MW. Addition of human melanopsin renders mammalian cells photoresponsive. Nature. 2005;433:741–745. doi: 10.1038/nature03344. [DOI] [PubMed] [Google Scholar]
- Minors DS, Waterhouse JM, Wirz-Justice A. A human phase-response curve to light. Neurosci Lett. 1991;133:36–40. doi: 10.1016/0304-3940(91)90051-t. [DOI] [PubMed] [Google Scholar]
- Panda S, Sato TK, Castrucci AM, Rollag MD, Degrip WJ, Hogenesch JB, Provencio I, Kay SA. Melanopsin (Opn4) requirement for normal light-induced circadian phase shifting. Science. 2002;298:2213–2215. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- Pohl H. Characteristics and variability in entrainment of circadian rhythms to light in diurnal rodents. In: Aschoff J, Daan S, Groos GA, editors. Vertebrate Circadian Systems: Structure and Physiology. Berlin: Springer-Verlag; 1982. pp. 339–346. [Google Scholar]
- Provencio I, Rodriguez IR, Jiang G, Hayes WP, Moreira EF, Rollag MD. A novel human opsin in the inner retina. J Neurosci. 2000;20:600–605. doi: 10.1523/JNEUROSCI.20-02-00600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby NF, Brennan TJ, Xie X, Cao V, Franken P, Heller HC, O’Hara BF. Role of melanopsin in circadian responses to light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- Smith MR, Eastman CI. Phase delaying the human circadian clock with blue-enriched polychromatic light. Chronobiol Int. 2009;26:709–725. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MR, Revell VL, Eastman CI. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–294. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring KH, Stiles WS. Variation of pupil size with change in the angle at which the light stimulus strikes the retina. Br J Ophthalmol. 1948;32:340–346. doi: 10.1136/bjo.32.6.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behaviour and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong RE, Marchant BK, Reimherr FW, Williams E, Soni P, Mestas R. Narrow-band blue-light treatment of seasonal affective disorder in adults and the influence of additional nonseasonal symptoms. Depress Anxiety. 2009;26:273–278. doi: 10.1002/da.20538. [DOI] [PubMed] [Google Scholar]
- Thapan K, Arendt J, Skene DJ. An action spectrum for melatonin suppression: evidence for a novel non-rod, non-cone photoreceptor system in humans. J Physiol. 2001;535:261–267. doi: 10.1111/j.1469-7793.2001.t01-1-00261.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Cauter E, Sturis J, Byrne MM, Blackman JD, Leproult R, Ofek G, L’Hermite-Balériaux M, Refetoff S, Turek FW, Van Reeth O. Demonstration of rapid light-induced advances and delays of the human circadian clock using hormonal phase markers. Am J Physiol Endocrinol Metab. 1994;266:E953–E963. doi: 10.1152/ajpendo.1994.266.6.E953. [DOI] [PubMed] [Google Scholar]
- West KE, Jablonski MR, Warfield B, Cecil KS, James M, Ayers MA, Maida J, Bowen C, Sliney DH, Rollag MD, Hanifin JP, Brainard GC. Blue light from light-emitting diodes elicits a dose-dependent suppression of melatonin in humans. J Appl Physiol. 2011;110:619–626. doi: 10.1152/japplphysiol.01413.2009. [DOI] [PubMed] [Google Scholar]
- Winfree AT. The Geometry of Biological Time. New York: Springer-Verlag; 1980. [Google Scholar]
- Zaidi FH, Hull JT, Peirson SN, Wulff K, Aeschbach D, Gooley JJ, Brainard GC, Gregory-Evans K, Rizzo JF, III, Czeisler CA, Foster RG, Moseley MJ, Lockley SW. Short-wavelength light sensitivity of circadian, pupillary, and visual awareness in humans lacking an outer retina. Curr Biol. 2007;17:2122–2128. doi: 10.1016/j.cub.2007.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitzer JM, Dijk DJ, Kronauer RE, Brown EN, Czeisler CA. Sensitivity of the human circadian pacemaker to nocturnal light: melatonin phase resetting and suppression. J Physiol. 2000;526:695–702. doi: 10.1111/j.1469-7793.2000.00695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


