Abstract
Lexical-semantic knowledge is a core language component that undergoes prolonged development throughout childhood and is therefore highly amenable to developmental studies. Most previous lexical-semantic functional MRI (fMRI) studies have been limited to single-word or word-pair tasks, outside a sentence context. Our objective was to investigate the development of lexical-semantic language networks in typically developing children using a more ecological sentence-embedded semantic task that permitted performance monitoring while minimizing head movement by avoiding overt speech. Sixteen adults and 23 children completed two fMRI runs of an auditory lexical-semantic decision task with a button-press response, using reverse speech as control condition. Children and adults showed similar activation in bilateral temporal and left inferior frontal regions. Greater activation in adults than in children was seen in left inferior parietal, premotor, and inferior frontal regions, and in bilateral supplementary motor area (SMA). Specifically for semantically incongruous sentences, adults also showed greater activation than children in left inferior frontal cortex, possibly related to enhanced top-down control. Age-dependent activation increases in motor-related regions were shown to be unrelated to overt motor responses, but could be associated with covert speech accompanying semantic decision. Unlike previous studies, age-dependent differences were not detected in posterior sensory cortices (such as extrastriate cortex), nor in middle temporal gyrus.
1. Introduction
Semantic comprehension refers to the meaningful interpretation of language elements (morphemes, words, sentences). Lexical semantics more specifically relates to single word meanings. From lesion literature, left posterior superior temporal gyrus (corresponding to part of classical Wernicke’s area) has been implicated in the processing of lexical semantics. It has been considered that the temporal lobes may “store” semantic representations (Bookheimer, 2002; Fiez, 1997). However, the more recent neuroimaging literature has tended to suggest a distributed network for lexical-semantic processing, also including inferior parietal regions, the cerebellum, and some regions related to category specific (potentially sensorimotor-based) components of lexical representations (Hwang, Palmer, Basho, Zadra, & Müller, 2009; Martin, 2007). Prominent among those additional regions is left inferior frontal cortex (Broca’s area), which may provide top-down control and on-line manipulation of elements of semantic representations.
Functional magnetic resonance imaging (fMRI) studies of lexical semantic processing in adults have implicated activation in the inferior frontal gyrus (IFG) (Binder, 1997; Binder et al., 1997) and more specifically pars orbitalis of the left inferior frontal lobe (Brodmann Area [BA] 47) in semantic processing and retrieval of semantic information (Bookheimer, 2002). Ruff and colleagues (Ruff, Blumstein, Myers, & Hutchison, 2008) demonstrated that prefrontal cortex is recruited in both lexical decision and semantic judgment tasks and additionally found increased activation in the superior temporal gyrus (STG) associated with both the storage and retrieval of lexical-semantic information.
While there is a large literature describing the distributed organization of the lexical-semantic system in adults, developmental changes of lexical-semantic organization in childhood are less understood. Brain development is characterized by a complex sequence of constructive and regressive events that aid the formation of highly specialized neural networks (Kandel, Jessell, & Sanes, 2000; Quartz & Sejnowski, 1997; Rakic, Ang, & Breunig, 2004). Cognitive development is a protracted process during which changes in ability are seen in diverse domains such as language, memory, and executive functioning throughout childhood, adolescence, and – for some domains – even young adulthood. Several characteristics of language acquisition are pertinent to the study of brain development as language acquisition has a relatively late onset and a protracted development, and behavioral studies have shown that language development benefits heavily from developmental plasticity.
Lexical-semantic skills undergo prolonged development throughout childhood and adolescence, thus making these tasks highly amenable to developmental studies (Holland et al., 2001; Vannest, Karunanayaka, Schmithorst, Szaflarski, & Holland, 2009). FMRI provides a non-invasive method for developmental studies in children. A better understanding of the mechanisms of cognitive change may broaden our understanding of cognitive development, elucidate the causes of atypical development, and inform the diagnosis, treatment, and prevention of developmental disorders.
Few studies have examined lexical semantic processing in children using complete sentences. Brauer and Friederici (2007) had young children (ages 5 and 6 years) passively listen to correct, syntactically incorrect, and semantically incongruous sentences. Cortical activation in children was less left-lateralized than in adults, although no significant between-group differences were reported. In both children and adults they observed bilateral activation in STG for all three conditions, and in left lateral IFG and frontal operculum for the semantically incongruous condition.
Virtually all other lexical semantic fMRI tasks have been limited to single-word or word-pair tasks, outside a sentence context. Frequently used paradigms include lexical association, generation, or naming. Word-pair tasks, for example, typically require participants to make decisions about word pairs (e.g., do the words rhyme?) or complete a word-pair through generation of an antonym, rhyme, or verb in response to a noun (Brown et al., 2005). These single word and word-pair tasks have been associated with mostly similar activation patterns in young adults and children in left inferior frontal, superior and middle temporal, and anterior cingulate gyri (Blumenfeld, Booth, & Burman, 2006; Bookheimer, 2002; Chou et al., 2006; Gaillard et al., 2000; Gaillard et al., 2003; Holland et al., 2001; Kotz, Cappa, von Cramon, & Friederici, 2002; Schlaggar et al., 2002).
Despite overall strong similarities, differences between children and adults have been found in several respects. Some studies have reported generally greater left lateralization in adults than in children (Holland et al., 2001; Holland et al., 2007; Szaflarski, Holland, Schmithorst, & Byars, 2006). Other studies have yielded more region-specific findings of greater activation for adults in left dorsal frontal cortex (Schlaggar et al., 2002), left inferior and middle frontal gyri (Gaillard et al., 2003), left middle temporal gyrus (MTG) (Chou et al., 2006) and left parietal cortex (Brown et al., 2005). Conversely, children have shown greater activation than adults in left extrastriate regions (Schlaggar et al., 2002). In a more recent study by this latter group that combined large sample size with thorough isolation of performance and age-related effects, greater activation in children (inverse correlation between activity and age) was detected in medial frontal and anterior cingulate cortex, right inferior frontal gyrus, medial parietal and posterior cingulate cortex, and bilateral occipitoparietal cortex (Brown et al., 2005).
Intriguingly, although the study by Brown and colleagues (2005) also reported age-dependent increases in lateral frontal cortex of the left hemisphere, the effect did not occur in inferior frontal gyrus, as to be expected from several previous studies (Brauer & Friederici, 2007; Gaillard et al., 2003; Schlaggar et al., 2002; Szaflarski, Holland et al., 2006), but rather in BA 6, which is considered premotor cortex. The question remains to what extent this finding may relate to overt speech responses. Many previous lexical-semantic studies have resorted to covert responses (Gaillard et al., 2000; Gaillard et al., 2003; Szaflarski, Holland et al., 2006) given that overt speech can cause artifacts related to increased head motion and changes in magnetic susceptibility, which is particularly problematic in a pediatric population where minimizing movement is already challenging. However, there is a serious trade-off since covert word generation prevents response monitoring, which is clearly needed in children who may not always be as task-compliant as the investigator hopes.
Single-word and word-pair paradigms are generally non-ecological, creating highly artificial task demands that differ dramatically from actual language use in linguistic environments encountered by children. In the attempt to overcome some of the methodological issues of studies described above, the present study used a sentence-embedded semantic task that was easy enough for children and that required participants to respond with a manual button press, thus avoiding speech-related artifacts. Based on findings from previous studies, as presented above, we posed three questions: (1) Can age-dependent activation increases in left lateral frontal cortex be replicated and will these localize to classical Broca’s area (left inferior frontal gyrus) or to premotor cortex (BA 6)? In case of the latter finding, can we detect indication of this age-related difference to be related to the effector of task response; i.e., is the peak of the effect superior to the one seen by Schlaggar et al. (2002) and Brown et al. (2005), corresponding to somatotopic organization of motor cortex? (2) Can greater activation in non-perisylvian sensory cortices (in particular extrastriate cortex) in children compared to adults be replicated for our sentence-embedded manual response paradigm (Brown et al., 2005)? (3) Can increased activation in left MTG in adults, attributed to richer semantic representations (Blumenfeld et al., 2006), be replicated? All three of the above questions are based on findings from some studies that have not been consistently replicated. We therefore considered them open questions rather than directional hypotheses.
2. Methods
2.1. Participants
Twenty-three typically developing children (12 girls) ranging in age from 7.0 to 10.0 years (M = 8.79 years, SD = 1.08 years) and 16 adult volunteers (7 females) ranging in age from 21 to 25 years (M = 22.9 years, SD = 1.31) were recruited for this study from San Diego county via community and online bulletin boards using Internal Review Board (IRB) approved fliers. All participants were native monolingual English speakers, and those who were bilingual or had significant exposure to languages other than English before the age of five years were excluded from the study. All adult participants were confirmed to be right-handed by the Edinburgh Handedness Inventory (Oldfield, 1971), which was slightly modified to be age-appropriate for children; all of the children were also right-handed. All participants were screened for the presence of bodily ferromagnetic materials. None of them had a history of learning disabilities, psychiatric disorders, neurological disease or psychostimulant medication use.
2.2. Stimuli
In the lexical-semantic decision task, which was adapted from the paradigm used by Gaillard and colleagues (2007), a descriptive statement was followed by a noun (e.g., “Something that tells time is a clock” or “Something you sit on is spaghetti”) and participants were asked to respond via button press and push one button if the sentence was congruous and a different button if the sentence was incongruous (with button assignments counterbalanced across participants). Participants responded using their non-dominant left hand (as discussed below in 4.1). All stimuli were presented binaurally for 2.75 seconds through noise-reduction headphones (Resonance Technology; www.mrivideo.com) specially designed for use with fMRI, followed by 1.25 seconds to provide time to respond.
Words used in the semantic task ranged in log of frequency from 4.3 to 11.5, as determined using Hyperspace Analogue to Language (HAL) word frequency norms (Lund & Burgess, 1996). Control trials consisted of 2.75 seconds of reverse speech to control for auditory and motor processing. Participants were instructed to push the button for “incorrect” when they heard the reverse speech.
All participants completed two 6-minute runs of the lexical-semantic decision task. Each run consisted of 40 lexical-semantic stimuli (20 semantically congruous, 20 incongruous) and 20 control stimuli. Temporal jittering with 60 two-second null baseline trials (presenting only a visual crosshair) was optimally randomized through both runs using Optseq (surfer.nmr.mgh.harvard.edu/optseq/).
2.3. Procedure
After explanation of the study, adult participants gave written informed consent and child participants gave oral and written assent and their parents gave written informed consent. Participants were assured of confidentiality according to guidelines established and approved by the IRB and Human Subjects Committees of both the University of California, San Diego and San Diego State University. Participants received monetary compensation for their time and effort.
At the first session, participants completed a battery of neurocognitive measures to ensure that they were at age-appropriate developmental levels and free of learning disorders. At the end of the first session, participants practiced in a mock scanner to acclimate them to the scanning environment and teach them the lexical-semantic decision task. Different stimuli (matched in their stimulus characteristics) were used in the practice and fMRI sessions. At the fMRI session, participants were screened for metal and practiced the lexical-semantic decision task a second time before going into the scanning room.
2.4. Data Acquisition
Participants were scanned at the Center for Functional Magnetic Resonance Imaging at the University of California, San Diego using a GE 3 Tesla HD Signa Excite magnet with an 8-channel gradient head coil. All participants completed two 6-minute runs of a lexical-semantic decision task during which blood-oxygen level-dependent (BOLD) fMRI data were acquired for 180 image volumes with 39 interleaved axial slices (3 mm slice thickness, and 4mm2 in-plane voxel size) using a single-shot, gradient-recalled, echo-planar (EPI) pulse sequence (TR 2000 ms; TE 30 ms; flip angle 90; matrix 64 × 64). High-resolution anatomical images were acquired using a standard FSPGR T1-weighted sequence. Participants heads were stabilized with foam padding to reduce motion. The experiment was presented on a MacBook 2 GHz Intel Core 2 Duo laptop with Mac OS X operating system, using PsyScope software (Cohen, MacWhinney, Flatt, & Provost, 1993). Behavioral responses were recorded using an MRI compatible response box. Participants viewed a movie while the FSPGR was acquired and saw a crosshair during the fMRI tasks, both of which were displayed on a back-projection screen at their feet using a mirror attached to the head coil.
2.5.1. fMRI Data Analysis
Child participants had been selected from a larger pool (n=27), based on their compliance with head motion restraints. Each participant (including adults) reported here completed two functional EPI runs with less than 2.0 mm of motion per run. The first five volumes of each run were not collected to remove signal equilibration effects. Each run was then corrected for intra-run motion and field inhomogenieties using FSL (Smith et al., 2004; Woolrich et al., 2009). Additional preprocessing and data analysis were completed with Analysis of Functional Neuroimages (AFNI) software (Cox, 1996). Each volume was slice-time corrected for inter-run motion by registering each volume to the middle (90th) volume of the first run. The two runs were then concatenated to create a single time-series with 360 volumes and smoothed with a 6-mm3 full-width at half-maximum Gaussian kernel. The hemodynamic response function for each stimulus type was estimated using a general linear model that included separate regressors to estimate the BOLD response at the onset of each stimulus and at each of the next 6 time-points (0 – 12 s poststimulus onset). Impulse response functions (IRFs) were estimated across time points 2 through 4 (4–8 s). A multiple regression analysis was performed on the estimated IRFs and the stimulus time series. Performance-related effects were examined by the use of separate regressors for incorrect trials in the experimental condition, adapted specifically to each participant’s responses. The six motion parameters corresponding to translation and rotation were also used as orthogonal regressors. Activation maps were normalized into standard space (Talairach & Tournoux, 1988) using AFNI auto-Talairach procedures and interpolated to 3 mm3 isotropic voxels.
Within-group differences for experimental and control trials were assessed using one-sample t-tests. Additional two-sample independent t-tests were used to compare adult and child groups for the experimental and control trials. Finally, a follow-up analysis was performed for semantically congruous and incongruous trials, using a one-sample t-test for within group differences and a two-sample independent t-test for a between-group comparison. A minimum cluster size of 624 mm3, a voxel connectivity distance of 5.20 mm, and a single voxel threshold of t(22) ≥ 3.786; p < .001 (child within-group) and a minimum cluster size of 576 mm3, a voxel connectivity distance of 5.20 mm, and a single voxel threshold of t(15) ≥ 4.033; p < .001 (adult within-group) and t(38) ≥ 3.539; p < .005 (group comparison) was used to correct for multiple comparisons. All cluster corrections yielded a corrected threshold of p < .05, as determined by Monte Carlo simulation (AFNI program AlphaSim; (Forman et al., 1995)).
2.5.2. Behavioral data analysis
Behavioral data were analyzed using SPSS 14.0. Two-sample independent t-tests were used to compare the adult and child groups for their hit rate and overall accuracy (hits and correct rejections) for the experimental condition.
3. Results
3.1. Behavioral results
All participants were able to complete both runs of the lexical-semantic decision task. Mean overall accuracy was 99% (SD = 2.0%) in the adults and 93% (SD = 6.9%) in the children. A two-sample independent t-test showed that adults performed significantly better than the children (t(37) = 3.536; p = .001). Mean hit rate was 99% (SD = 2.6%) in the adults and 93% (SD = 6.1%) in the children, with adults again performing significantly better than children (t(37) = 3.827; p < .001).
3.2. fMRI results
For all contrasts reported below, results are illustrated in Figure 1 and Supplementary Figures 2–5, with full cluster listings in Tables 1–4 and Supplementary Tables 5 and 6.
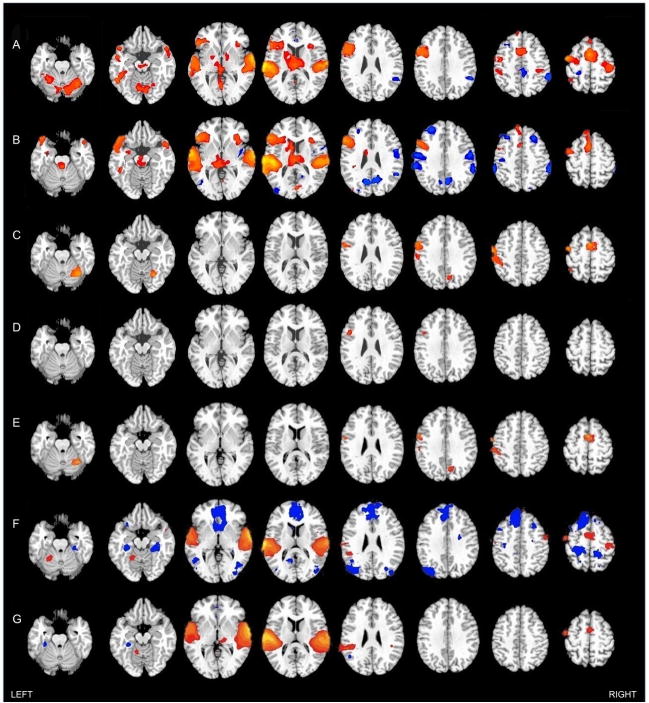
Figure 1.
Significant clusters of activation effects for the contrast lexical-semantic decision vs. reverse speech within the (A) adult group, (B) child group. (C) Between-group effects showing greater activation in the adult compared to the child group. (D) Significant clusters of between-group effects (adults > children) for the contrast of semantically incongruous vs. congruous trials. No inverse effects (children > adults) were detected for either between-group analysis (C & D). (E) Results for secondary analyses for a child subsample of the 16 children with least head movement, in comparison to full adult sample (all effects: adults > children). Significant main effects of control condition (reverse speech) compared to null baseline in adults (F), and children (G). All clusters shown are significant (p<.05; corr.). Red represents positive T-scores, blue represents negative T-scores.
Table 1.
Significant clusters (p<05; corr.) detected in the adult group for the contrast lexical-semantic decision vs. reverse speech. For clusters with volume greater than 1000 μl, subregions are listed as the percentage of total cluster volume. Subregions are contiguous areas of cluster activation that extend beyond the peak activation (Eickhoff et al., 2007).
| Peak location (Brodmann area) | |||||
|---|---|---|---|---|---|
| Regions included in cluster (% volume of cluster) | Talairach coordinates | Volume (μl) | T-score | ||
| x | y | z | |||
| Activations | |||||
| L superior temporal gyrus (BA 22) | −56 | −14 | 6 | 64098 | 15.96 |
| L inferior frontal gyrus [p. triangularis] (14.5%) | |||||
| L superior temporal gyrus (13.9%) | |||||
| L middle temporal gyrus (10.6%) | |||||
| L precentral gyrus (8.2%) | |||||
| L thalamus (6.1%) | |||||
| R superior temporal gyrus (BA 22) | 58 | −8 | 2 | 16767 | 13.02 |
| R superior temporal gyrus (61.8%) | |||||
| R temporal pole (9.8%) | |||||
| R middle temporal gyrus (9.4%) | |||||
| R Heschls gyrus (7.2%) | |||||
| R rolandic operculum (6.0%) | |||||
| L medial frontal gyrus (BA 6) | −8 | −2 | 60 | 14067 | 11.91 |
| L SMA (53.5%) | |||||
| R SMA (27.8%) | |||||
| R middle cingulate cortex (5.8%) | |||||
| R cerebellum | 8 | −70 | −16 | 14040 | 9.05 |
| R cerebellum (41.4%) | |||||
| cerebellar vermis 6 (10.6%) | |||||
| L cerebellum (10.1%) | |||||
| cerebellar vermis 4/5 (5.3%) | |||||
| R precentral gyrus (BA 4) | 34 | −22 | 50 | 6048 | 8.24 |
| R precentral gyrus (58.8%) | |||||
| R postcentral gyrus (31.3%) | |||||
| L cerebellum | −26 | −46 | −18 | 5103 | 8.30 |
| L inferior temporal gyrus (42.6%) | |||||
| L cerebellum (32.5%) | |||||
| L fusiform gyrus (16%) | |||||
| R insula (BA 13) | 28 | 20 | 6 | 2214 | 7.74 |
| L superior medial gyrus (BA 8) | −2 | 28 | 50 | 1485 | 5.69 |
| L superior medial gyrus (77.8%) | |||||
| L superior frontal gyrus (8.2%) | |||||
| L inferior parietal lobule (BA 40) | −46 | −32 | 44 | 1188 | 6.25 |
| L inferior parietal lobule (68.9%) | |||||
| L postcentral gyrus (21%) | |||||
| Deactivations | |||||
| R inferior parietal lobule (BA 40) | 56 | −44 | 42 | 3294 | −7.76 |
| R supramarginal gyrus (46.4%) | |||||
| R inferior parietal lobule (32.8%) | |||||
| R angular gyrus (14.9%) | |||||
| R precuneus (BA 7) | 8 | −34 | 42 | 945 | −5.36 |
| R middle cingulate cortex (81.7%) | |||||
| L middle cingulate cortex (10.2%) | |||||
| L anterior cingulate cortex (BA 24) | −4 | 34 | 6 | 945 | −4.88 |
| L anterior cingulate cortex (84.4%) | |||||
| R anterior cingulate cortex (10.1%) | |||||
| L postcentral gyrus (BA 3) | −22 | −34 | 54 | 675 | −6.22 |
| L middle frontal gyrus (BA 8) | −22 | 16 | 48 | 594 | −5.83 |
Table 4.
Clusters with significantly greater activation (p<05; corr.) in the adult than in the child group for the contrast semantically incongruous trials vs. semantically congruous trials.
| Peak location (Brodmann area) | |||||
|---|---|---|---|---|---|
| Regions included in cluster (% volume of cluster) | Talairach coordinates | Volume (μl) | T-score | ||
| x | y | z | |||
| L inferior frontal gyrus (BA 44/45) | −44 | 14 | 26 | 702 | 5.02 |
| L inferior frontal gyrus [p. triangularis] (75.4%) | |||||
| L inferior frontal gyrus [p. opercularis] (24.6%) | |||||
3.2.1. Lexical-semantic vs. control condition
Adults showed a large activation cluster that extended from the left inferior frontal gyrus to the left middle and superior temporal gyrus (Table 1; Figure 1A). Significant activation was also detected in the right hemisphere including a large cluster peaking in the right superior temporal gyrus and extending to the right temporal pole. The adult group also showed activation in bilateral medial frontal cortex (mostly in SMA), right insula, bilateral pre- and postcentral gyri, as well as in left inferior temporal and inferior parietal regions. Activation outside cerebral cortex was observed in left thalamus and in the cerebellum (predominantly the right hemisphere). Deactivations (greater activity for the reverse speech control condition) were detected in right inferior parietal lobe, precuneus and posterior cingulate gyrus (mostly in the right hemisphere), as well as in anterior cingulate, middle frontal, and postcentral gyri of the left hemisphere.
Similar to the adults, children showed a large activation cluster that extended from the left middle and superior temporal gyri to the left inferior frontal gyrus (Table 2; Figure 1B). A cluster in the right hemisphere peaked in superior temporal gyrus, extending to the temporal pole, middle temporal gyrus, insula, and inferior frontal gyrus. Children further showed activation in left superior and medial frontal regions (including SMA), left inferior temporal gyrus and cuneus, as well as lingual and fusiform gyri bilaterally. Outside cerebral cortex, activation was seen in bilateral thalamus and cerebellum. Deactivations were observed in bilateral inferior parietal lobules, extending into pericentral cortex and supramarginal gyri, bilateral middle frontal gyri, right precuneus and cuneus, as well as left middle occipital gyrus.
Table 2.
Significant clusters (p<05; corr.) detected in the child group for the contrast lexical-semantic decision vs. reverse speech.
| Peak location (Brodmann area) | |||||
|---|---|---|---|---|---|
| Regions included in cluster (% volume of cluster) | Talairach coordinates | Volume (μl) | T-score | ||
| x | y | z | |||
| Activations | |||||
| L superior temporal gyrus (BA 22) | −50 | −32 | 6 | 57672 | 12.25 |
| L inferior frontal gyrus [p. triangularis] (17.3%) | |||||
| L superior temporal gyrus (16.6%) | |||||
| L middle temporal gyrus (15.1%) | |||||
| L inferior frontal gyrus [p. orbitalis] (9%) | |||||
| L temporal pole (5.7%) | |||||
| R superior temporal gyrus (BA 41/42) | 56 | −16 | 8 | 24867 | 11.33 |
| R superior temporal gyrus (48.1%) | |||||
| R temporal pole (8.9%) | |||||
| R inferior frontal gyrus [p. orbitalis] (7.5%) | |||||
| R insula (6.6%) | |||||
| R middle temporal gyrus (5.9%) | |||||
| R Hechls gyrus (5.1%) | |||||
| L thalamus | −8 | −14 | 18 | 19872 | 8.28 |
| L thalamus (21.1%) | |||||
| L caudate nucleus (9.4%) | |||||
| R thalamus (8.7%) | |||||
| L superior frontal gyrus (BA 6) | −4 | 4 | 50 | 6696 | 8.63 |
| L medial frontal gyrus (42.7%) | |||||
| L SMA (41.1%) | |||||
| L superior frontal gyrus (12.9%) | |||||
| Vermis | −2 | −58 | −4 | 2214 | 4.59 |
| R lingual gyrus (7.7%) | |||||
| L lingual gyrus (5.1%) | |||||
| R insula (BA 13) | 32 | 20 | 12 | 2160 | 6.56 |
| R insula (64.2%) | |||||
| R inferior frontal gyrus (13.6%) | |||||
| L cuneus | −2 | −94 | 8 | 2079 | 4.97 |
| L cuneus (64.5%) | |||||
| L calcarine gyrus (26.1%) | |||||
| L parahippocampal gyrus (BA 34) | −26 | −2 | −16 | 1512 | 5.07 |
| L inferior temporal gyrus (48.2%) | |||||
| L amygdala (16.4%) | |||||
| L fusiform gyrus (8.1%) | |||||
| R fusiform gyrus (BA 36) | 32 | −4 | −28 | 675 | 5.15 |
| R fusiform gyrus (86%) | |||||
| R inferior temporal gyrus (7.1%) | |||||
| Deactivations | |||||
| R inferior partietal lobule (BA 40) | 58 | −38 | 48 | 11853 | −7.55 |
| R supramarginal gyrus (33.5%) | |||||
| R postcentral gyrus (17.4%) | |||||
| R inferior parietal lobule (8.7%) | |||||
| R precentral gyrus (7.6%) | |||||
| L precentral gyrus (BA 4) | −56 | −10 | 32 | 9747 | −8.56 |
| L postcentral gyrus (26.9%) | |||||
| L supramarginal gyrus (17.3%) | |||||
| L inferior parietal lobule (16.4%) | |||||
| L precentral gyrus (6%) | |||||
| L middle occipital gyrus (BA 19) | −38 | −76 | 8 | 3105 | −6.16 |
| R middle frontal gyrus (BA 8) | 26 | 26 | 42 | 2079 | −6.12 |
| R middle frontal gyrus (77.6%) | |||||
| R superior frontal gyrus (22.3%) | |||||
| L middle frontal gyrus (BA 8) | −32 | 26 | 38 | 1890 | −5.59 |
| R precuneus (BA 7) | 16 | −76 | 38 | 675 | −5.57 |
| R precuneus (54.6%) | |||||
| R superior occipital gyrus (19.7%) | |||||
| R cuneus (12.8%) | |||||
In a direct between-group comparison, adults showed greater activation than children in left pre- and postcentral gyri, extending into inferior parietal lobule and supramarginal gyrus and inferior frontal gyrus (pars opercularis), as well as in bilateral SMA, right cuneus/precuneus, and right cerebellum (Table 3; Figure 1C).
Table 3.
Clusters with significantly greater activation (p<05; corr.) in the adult than in the child group for the contrast lexical-semantic decision vs. reverse speech.
| Peak location (Brodmann area) | |||||
|---|---|---|---|---|---|
| Regions included in cluster (% volume of cluster) | Talairach coordinates | Volume (μl) | T-score | ||
| x | y | z | |||
| L inferior parietal lobule (BA 40) | −50 | −32 | 42 | 9531 | 6.06 |
| L precentral gyrus (28.2%) | |||||
| L inferior parietal lobule (23.4%) | |||||
| L postcentral gyrus (17.6%) | |||||
| L supramarginal gyrus (8.7%) | |||||
| L inferior frontal gyrus [p. opercularis] (2.7%) | |||||
| R cerebellum | 28 | −56 | −18 | 3159 | 5.88 |
| R cerebellum (84.8%) | |||||
| R fusiform gyrus (14.2%) | |||||
| L medial frontal gyrus (BA 6) | −4 | −2 | 56 | 2727 | 6.07 |
| L SMA (67.7%) | |||||
| R SMA (32.2%) | |||||
| R cuneus/precuneus (BA 7) | 8 | −68 | 32 | 594 | 4.54 |
We also inspected the group-averaged hemodynamic response time courses for latency differences between adults and children. Peak activated voxels within the left IFG and MTG were selected for each group and independent sample t-tests indicated that the time courses were not significantly different between groups for either region (Supplementary Figure 2).
3.2.2. Semantically congruous vs. incongruous sentences
Adults showed greater activation in the left inferior frontal gyrus (pars triangularis) for incongruous trials (Supplementary Table 5; Supplementary Figure 3A). Inverse effects (greater activation for congruous than for incongruous sentences) were found in the left superior frontal gyrus, right SMA, postcentral and supramarginal gyrus, as well as bilateral insula, rolandic operculum, precuneus, and cingulate cortex.
Children showed bilateral activation for congruous trials in pre- and postcentral gyri, rolandic operculum, SMA, supramarginal gyrus, cingulate cortex, and putamen (Supplementary Table 6; Supplementary Figure 3B). Left lateralized activation was seen in superior frontal gyrus, inferior parietal cortex, and superior occipital gyrus. Right lateralized activation for congruous trials was found in the insula and superior temporal gyrus. No significant effects for incongruous trials were detected in the child group. Finally, in a between-group comparison, adults displayed significantly greater activation than children in left inferior frontal gyrus (pars triangularis and pars opercularis) for semantically incongruous trials (Table 4; Figure 1D).
3.2.3. Performance- and motion-adjusted between-group comparison
In view of differences in performance and head motion between groups, we carried out additional comparisons for a subset of the children. Note that children generally performed at slightly lower levels and moved slightly more than adults. It was therefore not possible to fully match subsamples without sacrificing too much statistical power. As a compromise, we excluded only 7 children with lowest performance and strongest head motion, respectively. The sixteen highest performing children, with a mean overall accuracy of 94.8% (SD = .06) and a mean hit rate of 94.7% (SD = .06%), were compared to the adults. While the adults still performed better than the children (M = 99%, SD = 2.0; t(30) = 9.135; p = .005), the groups were better matched than in the comparison of full samples. The sixteen children with the smallest amounts of head movement, M = .58mm (SD = .34) were also compared to the adults (M = .27mm, SD = .11; t(30) = 15.66; p < .001). Comparisons using performance- and motion-adjusted child subsamples yielded regional effects of group differences (performance-adjusted Supplementary Figure 4A, motion-adjusted Figure 1E) very similar to the comparison of full samples (Figure 1C), highlighting that effects for the full samples were probably not driven by differences in performance or head movement.
4. Discussion
Our findings suggest that towards the end of the first decade of life, children employ overall similar cortical networks as adults for semantic processing. Both children and adults showed extensive activation in bilateral superior temporal, and left inferior frontal and middle temporal gyri. However, in direct group comparisons greater activation was observed in adults (compared to children) in several regions, including left inferior parietal lobule, supramarginal gyrus, and IFG. Adults also showed more activation than children in sensorimotor cortices in the left pre- and postcentral gyri and bilateral supplementary motor areas, as well as in the right cerebellum. The results of the between-group comparison were replicated when adults were compared to the 16 highest performing children and to the 16 children with the least head motion, suggesting that our findings were not driven by differences in performance or movement.
4.1. Inferior frontal and premotor cortex
Our sentence-embedded semantic paradigm replicated the findings of single-word and word-pair tasks showing age-dependent activation increases in left lateral frontal cortex with localized clusters in both premotor cortex (BA 6) and classical Broca’s area. Specifically, adults had greater activation in the pars opercularis of the left inferior frontal gyrus (BA 44). This is consistent with a model of age-dependent increase in top-down control mechanisms during language processing (Brown et al., 2005; Schlaggar et al., 2002). The model of progressive neural scaffolding (Petersen, van Mier, Fiez, & Raichle, 1998) posits that in the process of learning, when performance is still immature, large sets of lower-level (e.g., sensorimotor) brain regions are recruited for novel tasks. As tasks become learned, increasing top-down support is provided through higher-level control mechanisms. This scaffolding model is not specific to language, but relates to domain-general mechanisms of learning.
We also performed comparisons between incongruous and congruous trials in the experimental condition. In children, only effects of greater activation for the congruous condition were observed, which were predominantly located in inferior parietal, perirolandic, and cingulate cortices bilaterally. Effects of greater activation for congruous trials were observed in similar regions in adults who, however, also showed one inverse effect (greater for incongruous trials) in left IFG. Our findings are consistent with those by Brauer and Friederici (2007) who reported significantly stronger activation for sentences with semantic violations compared to correct sentences in the posterior portion of the left frontal operculum in adults. Conversely, 5–7 year old children showed slightly (though not significantly) reduced activity in the left frontal operculum for the same contrast. Unfortunately, no direct group comparison was presented in this study. Brauer and Friederici also reported a larger total volume of cortical activation (outside IFG) for correct sentences than for semantic violations – a difference that was more pronounced in children than in adults. This is consistent with our findings of greater activation for congruous sentences, especially in the child group. Overall, the predominantly greater activation for semantically congruous compared to incongruous sentences we observed in both groups suggests that effects for our main comparison (lexico-semantic vs. control) were largely driven by congruous trials, except for one site in BA 44 of the left hemisphere, which showed greater activity in adults for semantic incongruity.
Event-related potentials (ERPs) have also been used to examine lexical-semantic processes that are reflected in a centro-parietally distributed negativity around 400 ms (i.e., N400). Hahne and colleagues (Hahne, Eckstein, & Friederici, 2004) found that semantic anomalies in a single-word study elicited an N400 component in both children (ages 6–13 years) and adults, but the latency of the N400 decreased with age. Aside from this latency difference, increased N400 amplitude has been reported in children (compared to adults) for semantic incongruity in word pairs, with an adult-like pattern developing around the age of 12 years (Wang, Dong, Ren, & Yang, 2009). These differences in N400 amplitude and latency may reflect reduced processing demands and faster cognitive processing of semantic incongruities in adults. Our finding of greater activation in left IFG for semantically incongruous sentences in adults may suggest age-dependent increase in top-down control. The N400 findings from ERP studies and our fMRI findings in LIFG likely reflect different, though complementary, aspects of age-dependent change in response to semantic violations. Differential findings may be attributed to differences in spatial and temporal resolution between ERP and fMRI.
For analyses of the main comparison (all lexico-semantic trials vs. control), frontal effects of greater activation in adults than in children extended beyond Broca’s area into several motor-related regions. One such cluster was seen in bilateral SMA. Activation in SMA has been frequently seen for language tasks in both children and adults (Gaillard et al., 2003) and is often assumed to be due to response-related motion, such as overt speech or button press (Chee, O’Craven, Bergida, Rosen, & Savoy, 1999). In some language studies with covert responses, activation was observed in SMA and attributed to “inner speech” (Wise et al., 1991). Chee and colleagues noted anterior SMA activation in a semantic task relative to a non-semantic task that was matched for motor response, suggesting a linguistic component to the activation. Consistent results were also observed by Binder and colleagues (Binder et al., 1997) who found SMA activation for a semantic decision task. It has been hypothesized that activation in SMA may be due to the rehearsal component in verbal working memory (Chee et al., 1999; Fiez et al., 1996). Verbal working memory is inherent in most language tasks as participants need to hold a word “online” while making a decision or generating a response. Our lexical-semantic decision task required the participant to keep the definition sentence online (e.g., “Something you sit on is a…”) while processing the final word of the sentence (e.g., “spaghetti”). Of note in the context of motor versus linguistic role of SMA in our task is that children showed activation in this region only in the left hemisphere, whereas activity in adults was bilateral (Figures 1A–B).
Additionally, we found an extensive cluster of greater activation in adults than in children in left precentral gyrus extending into left postcentral and supramarginal gyri and inferior parietal lobule. Two studies by Schlaggar and colleagues (Brown et al., 2005; Schlaggar et al., 2002) previously reported concordant effects (activity increasing with age) in similar regions. One of our research questions was whether effects in motor cortices may have been related to overt speech responses required in the above studies. Since we also found effects of greater activation in adults than in children in motor and premotor cortex (for a paradigm without speech response), it appears unlikely that such effects are selectively tied to overt speech. Note that our tasks also required a motor response, albeit a manual button press. Peak coordinates in BA 6 detected in our study were, however, not distinctly superior to those seen in the overt speech study by Brown and colleagues (2005), as would be expected based on somatotopic organization (Hauk, Johnsrude, & Pulvermüller, 2004) if activation sites were linked to the response effector (hand vs. mouth and vocal tract).
It should be further considered that in the present study participants used their left hands for button presses. While responses by the non-dominant hand may be slightly more effortful, this allowed us to better segregate activation effects of motor-response (expected in the right hemisphere) from those related to lexical processing (predominantly expected in the left hemisphere). It is therefore unlikely that effects in motor and premotor cortex of the left hemisphere detected in our study were directly tied to button presses. One may argue that inhibition of a preferential response with the dominant hand could have still contributed to effects in motor cortex. However, it is hard to construe how this would have resulted in the group differences observed by us. While children might have found responding with the non-dominant hand (possibly while inhibiting a right-hand response) more difficult than adults, such a scenario would have resulted in greater activation in motor and premotor cortex (reflecting more effortful processing), which is the inverse of our actual finding.
Based on existing findings, greater activity in (pre)motor cortices in adults is therefore probably not related to motor response, although it remains possible that it reflects developmental changes in covert speech processes that may have accompanied performance on our semantic decision task. Our present results appear to suggest that such covert speech components are more pronounced in adults than in children. While this interpretation may seem counterintuitive, it is supported by concordant age-dependent effects in left premotor cortex (BA 6) detected in studies by Szaflarski and colleagues (2006; 2006), who used covert word generation. As mentioned above, Schlaggar, Petersen, and colleagues (Brown et al., 2005; Schlaggar et al., 2002) also found consistent age-dependent effects in (pre)motor cortex for word generation tasks using overt speech. In our view, a more general interpretation of the pattern of findings suggests that in lexical stimulus-response tasks children may direct more attentional resources to the perceptual part of the task – reflected by greater activity in extrastriate cortices as observed by Brown et al. (Brown et al., 2005; see 4.2) – whereas adults tend to be more response-oriented, reflected in greater activity in motor, premotor, and supplementary motor cortices. However, this interpretation remains tentative as no language fMRI study designed to isolate effects of motor planning, covert speech, or other factors that may contribute to age-dependent changes in premotor cortex is currently available, to our knowledge.
4.2. Sensory cortex
Some previous single-word and word-pair studies have shown greater activation in non-perisylvian sensory cortices (in particular extrastriate cortex) in children compared to adults (Brown et al., 2005; Schlaggar et al., 2002), arguably related to progressive neural scaffolding (as discussed above) and bottom-up language development emerging from sensorimotor abilities. This finding was not replicated in our sentence-embedded paradigm. In fact, we found no cortical areas of greater activation in children than in adults.
Our between-group comparison may have been affected by more extensive negative effects in children than in adults, including deactivations in sensory cortices (Figure 1B). For instance, the between-group comparison yielded greater activation in the right cuneus/precuneus and the left supramarginal gyrus in the adults. This finding does not reflect significant activation in the precuneus or the supramarginal gyrus for the adult group, but was instead driven by negative effects in these regions in children.
To explore the reasons of greater deactivation in children for the contrast semantic decision vs. reverse speech, we examined effects of reverse speech (vs. null baseline). As seen in Figure 1F–G, positive activation patterns for the reverse speech control condition were similar between children and adults. Direct group comparisons yielded no clusters of significant difference for this contrast (therefore not shown in Figure 1). Nonetheless, inspection of within-group effects showed widespread regions of deactivation for the control condition (compared to baseline) for adults (Figure 1F) that were not seen in the child group (with the sole exception of the left medial temporal lobe; Figure 1G). Most of the deactivated regions seen in the adults (such as medial prefrontal cortex, lateral parietal cortex, medial temporal lobe) coincided with the default network, i.e., a brain system that is active during task-free periods and is considered to relate to self-reflective processing (Fair et al., 2008; Fransson, 2005; Greicius, Supekar, Menon, & Dougherty, 2009). A recent functional connectivity study by Fair et al. (2008) showed reduced connectivity between nodes of the default network in children ages 7–9 years, suggesting that the default network does not mature until adolescence, which could account for the absence of deactivations in this network in our child group.
However, it remains unclear whether findings related to the default network could explain the widespread deactivations we observed in children for the higher-order contrast (semantic decision vs. reverse speech). Except for the precuneus, these deactivations were observed in regions outside the default network, i.e., in dorsolateral prefrontal and pericentral cortices and in the inferior parietal lobe. As mentioned, there was also no obvious explanation related to our hierarchical subtractive task design. In particular, regions with robust deactivations for the higher-level contrast (semantic vs. reverse) did not show significantly greater activation for the lower-level contrast (reverse vs. null) in the child group. It thus remains open whether deactivations observed in the child group may have been indirectly affected by age-dependent differences in the default network, or by subthreshold differences in the response to the control condition. Note that use of reverse speech as a control condition is not uncommon in language studies (e.g., Burton, Noll, & Small, 2001; Perani et al., 1996; Röder, Stock, Neville, Bien, & Rösler, 2002), including those in children (Dehaene-Lambertz, Dehaene, & Hertz-Pannier, 2002; Gaillard et al., 2003; Redcay, Haist, & Courchesne, 2008).
A further aspect that may have affected direct group comparisons relates to variability in the child group. Though children performed at high levels on the lexical-semantic decision task, they exhibited significantly greater variability in performance than the adults, possibly reflecting different cognitive strategies applied to the task. The children also ranged in age from 7 to 10 years and maturational changes during this age window are expected to add to within-group variability. Groupwise effects in some regions, such as SMA, may reflect greater variability (as opposed to consistent absence of activity).
4.3. Middle temporal gyrus
Some previous studies found age-dependent increases of activation in left MTG, which was attributed to increasing richness and complexity of semantic representations (Blumenfeld et al., 2006; Chou et al., 2006). Our results were not consistent with this hypothesis. Although both children and adults showed extensive left MTG activation in within-group analyses, there was no significant group difference in this region. The null finding could be due to the age of the child participants or the lexical stimuli used. As both children and adults performed near ceiling on the semantic task, it is possible that semantic representations for the specific lexical items used in our study were equally rich in children and adults. In the study by Blumenfeld et al. (2006), the child group, aged 9 to 12 years, had a slightly wider range of variability in performance accuracy (72.9% to 100%) than in the present study (82.5% to 100%) and a slightly lower mean accuracy for the auditory condition (M = 89.5%) than in the present study (M = 93%). However, these performance differences were subtle and since our child cohort was actually younger than the one studied by Blumenfeld et al., it remains possible that the non-replication of age-dependent MTG effects was instead related to our task paradigm, in which a prompt word was preceded by a complete sentence.
4.4. Additional findings: Inferior parietal lobule and cerebellum
Studies in adults, indicate a role of the left inferior parietal lobule (IPL) in both phonological (Hickok & Poeppel, 2000) and semantic processing (Kuperberg, Sitnikova, & Lakshmanan, 2008). Our adult group showed significantly greater activation than the child group in left IPL for lexical-semantic decision (Figure 1C, Table 3). This is consistent with findings of age-dependent activation increases for overt word generation in left IPL by Brown et al. (Brown et al., 2005). Chou and colleagues (Chou et al., 2006) further observed that activity in left IPL was stronger for semantically associated (compared to unrelated) words in children ages 9–15 years. Correspondingly, we found activation in left IPL for the contrast of congruent vs. incongruent sentences in our child group, whereas adults showed activation for this contrast in right IPL (Supplementary Tables 5–6). This may suggest developmental changes in hemispheric asymmetry of the IPL response to semantic congruency and anomaly.
The adult group also showed significantly stronger activation for semantic decision in the right cerebellar hemisphere, compared to the child group (Figure 1C). The laterality of this finding is consistent with concordant effects (adults > children) in inferior frontal, pericentral, and inferior parietal regions of the left hemisphere, given the known crossed connectivity between cerebral cortex and cerebellum (Schmahmann, 1996). Robust cerebellar activation in the adult group is consistent with previous imaging studies reporting cerebellar participation in a wide range of non-motor tasks, including learning, working memory, and language (Desmond & Fiez, 1998). The significantly reduced cerebellar activity in our child group may reflect ongoing maturation of cerebro-cerebellar networks. This is supported by results from a recent study on verbal working memory showing reduced load-dependent effects in the cerebellum in children (ages 7–10 years), compared to adolescents (ages 11–15years; O’Hare, Lu, Houston, Bookheimer, & Sowell, 2008).
4.5 Conclusion
Using a sentence-embedded semantic decision paradigm, we replicated only some of the findings from previous single-word or word-pair studies. We found greater activation in adults than in children, not only in left inferior frontal cortex (attributed to age-dependent increase in top-down control), but also in premotor and motor cortex of the left hemisphere. The latter finding was most likely related to the language task itself (possibly covert speech), rather than motor response. Our study did not replicate previous findings of increased semantic activations in MTG in adults, nor did it replicate increased activation in posterior sensory cortices in children. Reduced deactivations of the default network and reduced activations in the cerebellum were also noted in children, compared to adults, which may be attributed to incomplete maturation of connectivity within default and cerebro-cerebellar networks.
Supplementary Material
Significant clusters (p<05; corr.) detected in the adult group for the contrast semantically incongruous vs. semantically congruous trials.
Significant clusters (p<05; corr.) detected in the child group for the contrast semantically incongruous vs. semantically congruous trials.
Hemodynamic response time courses for peak voxels in (A) left MTG and (B) left IFG. Independent sample t-tests for each timepoint indicated that the time courses were not significantly different between groups for either MTG or IFG. Talairach coordinates for peak voxels in MTG were [−53 −39 5] in the adult, and [−52 −32 4] in the child group. For IFG, they were [−44 7 28] in the adult, and [−47, 25, 14] in the child group.
Significant clusters of activation effects (p<.05; corr.) for the contrast semantically incongruous trials vs. semantically congruous trials within the adult group (A) and child group (B). Red represents greater activation for incongruous trials, blue represents greater activation for congruous trials.
Results for the secondary analyses for a child subsample of the 16 highest-performing children, in comparison to full adult sample. For all images, red represents significant (p<.05; corr.) clusters where adults had greater activation than children. No inverse effects were detected.
Significant clusters of activation effects for the main effect of lexical-semantic decision compared to null baseline within the adult group (A) and child group (B). (C) Between-group effects showing greater activation in the adult compared to the child group for the contrast of lexical-semantic decision vs. null baseline. All clusters shown are significant (p<.05; corr.). Red represents positive T-scores, blue represents negative T-scores. significant (p<.05; corr.) clusters where adults had greater activation than children. No inverse effects were detected.
Acknowledgments
This study was supported by the National Institutes of Health, grants R01-NS43999 and F31-NS059094 (author ENMP). Thanks to our participants and their families for their time and effort, and to William D. Gaillard, MD, and Madison Berl, PhD, for permission to adapt their lexical-semantic paradigm.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Binder JR. Neuroanatomy of language processing studied with functional MRI. Clin Neurosci. 1997;4(2):87–94. [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. J Neurosci. 1997;17(1):353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld HK, Booth JR, Burman DD. Differential prefrontal-temporal neural correlates of semantic processing in children. Brain Lang. 2006;99(3):226–235. doi: 10.1016/j.bandl.2005.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annu Rev Neurosci. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Brauer J, Friederici AD. Functional neural networks of semantic and syntactic processes in the developing brain. J Cogn Neurosci. 2007;19(10):1609–1623. doi: 10.1162/jocn.2007.19.10.1609. [DOI] [PubMed] [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL. Developmental changes in human cerebral functional organization for word generation. Cereb Cortex. 2005;15(3):275–290. doi: 10.1093/cercor/bhh129. [DOI] [PubMed] [Google Scholar]
- Burton MW, Noll DC, Small SL. The anatomy of auditory word processing: individual variability. Brain Lang. 2001;77(1):119–131. doi: 10.1006/brln.2000.2444. [DOI] [PubMed] [Google Scholar]
- Chee MW, O’Craven KM, Bergida R, Rosen BR, Savoy RL. Auditory and visual word processing studied with fMRI. Hum Brain Mapp. 1999;7(1):15–28. doi: 10.1002/(SICI)1097-0193(1999)7:1<15::AID-HBM2>3.0.CO;2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TL, Booth JR, Burman DD, Bitan T, Bigio JD, Lu D, et al. Developmental changes in the neural correlates of semantic processing. Neuroimage. 2006;29(4):1141–1149. doi: 10.1016/j.neuroimage.2005.09.064. [DOI] [PubMed] [Google Scholar]
- Cohen JD, MacWhinney B, Flatt M, Provost J. PsyScope: A new graphic interactive environment for designing psychology experiments. Behavioral Research Methods, Instruments, and Computers. 1993;25(2):257–271. [Google Scholar]
- Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Dehaene-Lambertz G, Dehaene S, Hertz-Pannier L. Functional neuroimaging of speech perception in infants. Science. 2002;298(5600):2013–2015. doi: 10.1126/science.1077066. [DOI] [PubMed] [Google Scholar]
- Desmond JE, Fiez JA. Neuroimaging studies of the cerebellum: language, learning and memory. Trends in Cognitive Sciences. 1998;2(9):355–362. doi: 10.1016/s1364-6613(98)01211-x. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, et al. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. Neuroimage. 2007;36(3):511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Fair DA, Cohen AL, Dosenbach NU, Church JA, Miezin FM, Barch DM, et al. The maturing architecture of the brain’s default network. Proc Natl Acad Sci U S A. 2008;105(10):4028–4032. doi: 10.1073/pnas.0800376105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiez JA. Phonology, semantics, and the role of the left inferior prefrontal cortex [editorial] Human Brain Mapping. 1997;5(2):79–83. [PubMed] [Google Scholar]
- Fiez JA, Raife EA, Balota DA, Schwarz JP, Raichle ME, Petersen SE. A positron emission tomography study of the short-term maintenance of verbal information. Journal of Neuroscience. 1996;16(2):808–822. doi: 10.1523/JNEUROSCI.16-02-00808.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC. Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): use of a cluster-size threshold. Magn Reson Med. 1995;33(5):636–647. doi: 10.1002/mrm.1910330508. [DOI] [PubMed] [Google Scholar]
- Fransson P. Spontaneous low-frequency BOLD signal fluctuations: an fMRI investigation of the resting-state default mode of brain function hypothesis. Hum Brain Mapp. 2005;26(1):15–29. doi: 10.1002/hbm.20113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, et al. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69(18):1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Hertz-Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH. Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology. 2000;54(1):180–185. doi: 10.1212/wnl.54.1.180. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, et al. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18(3):176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne A, Eckstein K, Friederici AD. Brain signatures of syntactic and semantic processes during children’s language development. J Cogn Neurosci. 2004;16(7):1302–1318. doi: 10.1162/0898929041920504. [DOI] [PubMed] [Google Scholar]
- Hauk O, Johnsrude I, Pulvermüller F. Somatotopic representation of action words in human motor and premotor cortex. Neuron. 2004;41(2):301–307. doi: 10.1016/s0896-6273(03)00838-9. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4(4):131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS., Jr Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14(4):837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Holland SK, Vannest J, Mecoli M, Jacola LM, Tillema JM, Karunanayaka PR, et al. Functional MRI of language lateralization during development in children. Int J Audiol. 2007;46(9):533–551. doi: 10.1080/14992020701448994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang K, Palmer ED, Basho S, Zadra JR, Müller RA. Category specific activations during word generation reflect experiential sensorimotor modalities. Neuroimage. 2009;48:717–725. doi: 10.1016/j.neuroimage.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel ER, Jessell TM, Sanes JR. Sensory experience and the fine tuning of synaptic connections. In: Kandel ER, Schwartz JH, Jessell TM, editors. Principles of Neural Science. New York: Elsevier; 2000. pp. 1115–1130. [Google Scholar]
- Kotz SA, Cappa SF, von Cramon DY, Friederici AD. Modulation of the lexical-semantic network by auditory semantic priming: an event-related functional MRI study. Neuroimage. 2002;17(4):1761–1772. doi: 10.1006/nimg.2002.1316. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Sitnikova T, Lakshmanan BM. Neuroanatomical distinctions within the semantic system during sentence comprehension: evidence from functional magnetic resonance imaging. Neuroimage. 2008;40(1):367–388. doi: 10.1016/j.neuroimage.2007.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund K, Burgess C. Producing high-dimensional semantic spaces from lexical co-occurrences. Behavioral Research Methods, Instruments, and Computers. 1996;28:203–208. [Google Scholar]
- Martin A. The representation of object concepts in the brain. Annu Rev Psychol. 2007;58:25–45. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- O’Hare ED, Lu LH, Houston SM, Bookheimer SY, Sowell ER. Neurodevelopmental changes in verbal working memory load-dependency: an fMRI investigation. Neuroimage. 2008;42(4):1678–1685. doi: 10.1016/j.neuroimage.2008.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Perani D, Dehaene S, Grassi F, Cohen L, Cappa SF, Dupoux E, et al. Brain processing of native and foreign languages. Neuroreport. 1996;7(15–17):2439–2444. doi: 10.1097/00001756-199611040-00007. [DOI] [PubMed] [Google Scholar]
- Petersen SE, van Mier H, Fiez JA, Raichle ME. The effects of practice on the functional anatomy of task performance. Proc Natl Acad Sci U S A. 1998;95(3):853–860. doi: 10.1073/pnas.95.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quartz SR, Sejnowski TJ. The neural basis of cognitive development: a constructivist manifesto. Behav Brain Sci. 1997;20(4):537–556. doi: 10.1017/s0140525x97001581. discussion 556–596. [DOI] [PubMed] [Google Scholar]
- Rakic P, Ang ESBC, Breunig J. Setting the stage for cognition: Genesis of the primate cerebral cortex. In: Gazzaniga MS, editor. The Cognitive Neurosciences. Cambridge (Mass.): MIT Press; 2004. pp. 33–49. [Google Scholar]
- Redcay E, Haist F, Courchesne E. Functional neuroimaging of speech perception during a pivotal period in language acquisition. Dev Sci. 2008;11(2):237–252. doi: 10.1111/j.1467-7687.2008.00674.x. [DOI] [PubMed] [Google Scholar]
- Röder B, Stock O, Neville H, Bien S, Rösler F. Brain Activation Modulated by the Comprehension of Normal and Pseudo-word Sentences of Different Processing Demands: A Functional Magnetic Resonance Imaging Study. Neuroimage. 2002;15(4):1003–1014. doi: 10.1006/nimg.2001.1026. [DOI] [PubMed] [Google Scholar]
- Ruff I, Blumstein SE, Myers EB, Hutchison E. Recruitment of anterior and posterior structures in lexical-semantic processing: an fMRI study comparing implicit and explicit tasks. Brain Lang. 2008;105(1):41–49. doi: 10.1016/j.bandl.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE. Functional neuroanatomical differences between adults and school-age children in the processing of single words. Science. 2002;296(5572):1476–1479. doi: 10.1126/science.1069464. [DOI] [PubMed] [Google Scholar]
- Schmahmann JD. From movement to thought: anatomic substrates of the cerebellar contribution to cognitive processing. Human Brain Mapping. 1996;4:174–198. doi: 10.1002/(SICI)1097-0193(1996)4:3<174::AID-HBM3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Holland SK, Schmithorst VJ, Byars AW. fMRI study of language lateralization in children and adults. Hum Brain Mapp. 2006;27(3):202–212. doi: 10.1002/hbm.20177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szaflarski JP, Schmithorst VJ, Altaye M, Byars AW, Ret J, Plante E, et al. A longitudinal functional magnetic resonance imaging study of language development in children 5 to 11 years old. Ann Neurol. 2006;59(5):796–807. doi: 10.1002/ana.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York: Thieme; 1988. [Google Scholar]
- Vannest J, Karunanayaka PR, Schmithorst VJ, Szaflarski JP, Holland SK. Language networks in children: evidence from functional MRI studies. AJR Am J Roentgenol. 2009;192(5):1190–1196. doi: 10.2214/AJR.08.2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Dong X, Ren Y, Yang Y. The development of semantic priming effect in childhood: an event-related potential study. Neuroreport. 2009;20(6):574–578. doi: 10.1097/WNR.0b013e328329f215. [DOI] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R. Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain. 1991;114 ( Pt 4):1803–1817. doi: 10.1093/brain/114.4.1803. [DOI] [PubMed] [Google Scholar]
- Woolrich MW, Jbabdi S, Patenaude B, Chappell M, Makni S, Behrens T, et al. Bayesian analysis of neuroimaging data in FSL. Neuroimage. 2009;45(1 Suppl):S173–186. doi: 10.1016/j.neuroimage.2008.10.055. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Significant clusters (p<05; corr.) detected in the adult group for the contrast semantically incongruous vs. semantically congruous trials.
Significant clusters (p<05; corr.) detected in the child group for the contrast semantically incongruous vs. semantically congruous trials.
Hemodynamic response time courses for peak voxels in (A) left MTG and (B) left IFG. Independent sample t-tests for each timepoint indicated that the time courses were not significantly different between groups for either MTG or IFG. Talairach coordinates for peak voxels in MTG were [−53 −39 5] in the adult, and [−52 −32 4] in the child group. For IFG, they were [−44 7 28] in the adult, and [−47, 25, 14] in the child group.
Significant clusters of activation effects (p<.05; corr.) for the contrast semantically incongruous trials vs. semantically congruous trials within the adult group (A) and child group (B). Red represents greater activation for incongruous trials, blue represents greater activation for congruous trials.
Results for the secondary analyses for a child subsample of the 16 highest-performing children, in comparison to full adult sample. For all images, red represents significant (p<.05; corr.) clusters where adults had greater activation than children. No inverse effects were detected.
Significant clusters of activation effects for the main effect of lexical-semantic decision compared to null baseline within the adult group (A) and child group (B). (C) Between-group effects showing greater activation in the adult compared to the child group for the contrast of lexical-semantic decision vs. null baseline. All clusters shown are significant (p<.05; corr.). Red represents positive T-scores, blue represents negative T-scores. significant (p<.05; corr.) clusters where adults had greater activation than children. No inverse effects were detected.