Abstract
Objective
Sublingual buprenorphine/naloxone (Bup/Nx) is approved for addiction treatment and may be useful for pain management, particularly in opioid-treated pain patients with nonadherence behaviors. The transition of opioid-treated pain patients to buprenorphine carries the risk of precipitated withdrawal and increased pain. This study convened pain and addiction specialists to develop and pilot a clinical protocol for safe transitioning to Bup/Nx.
Design
The protocol was revised three times based on outside expert review and pilot study observations. The pilot was conducted with a prospective cohort of 12 patients with moderate to severe chronic pain, who were receiving long-term opioid therapy with any full μ-agonist drug, and had exhibited one or more aberrant drug-related behaviors. Patients were followed up for 3 to 6 months with the expectation that they would experience few adverse events and report lower pain severity.
Results
The three patients on the highest baseline opioid dose (equivalent to 303–450 mg of oral morphine) and the three on the lowest doses (≤20 mg) had early adverse events (AEs) when switched to Bup/Nx and did not complete the trial. Of the remaining six, one withdrew due to AEs; one responded well, then withdrew; and four completed a three-month trial. A mixed effects model controlling for dropouts found that average and worst pain significantly decreased after the switch to Bup/Nx (both p < .01).
Conclusion
Based on this experience, the protocol recommends Bup/Nx for pain only when baseline opioid doses are within bounds that reduce AEs at transition and incorporates dose flexibility to further reduce risks. This protocol warrants further testing.
Keywords: Buprenorphine, chronic pain, treatment guidelines, opioid therapy, withdrawal
INTRODUCTION
Opioid therapy, the mainstay treatment for chronic pain due to serious medical illnesses,1 has been used increasingly for common types of chronic non-cancer pain.2,3 This treatment is controversial, however, because supporting evidence for efficacy and safety is limited and rising rates of prescription drug abuse and overdose have paralleled increased prescribing.4–7 Risk assessment prior to treatment, adherence monitoring for those who receive treatment, and strategies aimed at minimizing risk or managing adverse outcomes are now viewed as necessary elements for safe and effective therapy.8,9
Risk-related clinical strategies are particularly important when opioid therapy is considered for pain patients who have a history of drug abuse or have already demonstrated non-adherence behaviors during opioid therapy.9,10 Among the strategies proposed to minimize risk is the use of an opioid that may have a lower abuse potential and lower risk profile than conventional pure μ-agonists. One such drug is buprenorphine, a partial μ-agonist, kappa-antagonist, and ORL-1 partial agonist that has been approved in the U.S. as a sublingual treatment for opioid dependence and as a parenteral and transdermal analgesic.
Buprenorphine’s pharmokinetic/pharmodynamic profile suggests that it may be valuable as a pain medication.3,11–12 Buprenorphine has a high affinity for and slow dissociation from the μ-opioid receptor, which may help to explain its blocking activity from many opioid effects and its relatively mild withdrawal.3 There is a ceiling effect for respiratory depression, but clinical evidence suggests a lack of a ceiling effect for analgesia.13 At therapeutic levels, buprenorphpine and its main metabolite norbuprenorphine do not appear to significantly inhibit cytochromes, and, as a result, are typically associated with fewer adverse effects than full μ-opioids especially when these medications are prescribed at high doses.14 Unlike some other opioids, buprenorphine’s pharmacokinetics are not altered with age,15 and its pharmacodynamics appear to have little or no effect on sex hormones or the immune system.16 Pre-clinical observations encourage speculation that buprenorphine may favorably influence clinical outcomes related to opioid tolerance or hyperalgesia or may be relatively useful for neuropathic pain.17,18 Analgesic trials will be necessary to characterize the clinical potential suggested by these findings.
There have been several reports (from outside of the U.S.) of buprenorphine associated deaths.19 These deaths appear to represent a pharmacodynamic interaction between buprenorphine and a benzodiazepine (or other central nervous system depressant) especially when the two are injected together,19,20 although deaths from buprenorphine alone have also been reported.21 Like other opioids, buprenorphine abuse and diversion have increased in the United States,22 however, in contrast to other countries,23 data are lacking whether (at the national level), there has been a corresponding increase in deaths in the U.S. attributed to buprenorphine. Difficulty in detecting a trend may be partially attributed to the manner in which the U.S. Centers for Disease Control codes buprenorphine deaths (combining deaths involving fentanyl with those from buprenorphine) (N. Dasgupta, MPH, Personal Communication, August 14, 2012). Although the morbidity risk of buprenorphine is unclear and may be comparatively low (especially with in the treatment setting), a protocol for treating chronic pain patients with buprenorphine would be helpful to decrease morbidity and mortality.
For opioid dependence, the sublingual formulations are available at relatively high doses (starting at 2 mg). For pain, transdermal formulations are available in Europe24,25 and the U.S.26 The U.S. transdermal formulation (Butrans®) provides a relatively low dose (starting at 5 mcg/hr), which can be initiated in those who are opioid-naïve or are receiving relatively low doses of another opioid. Transdermal therapy with the U.S. formulation is not recommended when patients are taking more than the equivalent of oral morphine 30 mg/day because of concerns about precipitated withdrawal when buprenorphine is substituted for a full μ-agonist.27 Transition to the low-dose transdermal formulation from treatment with another opioid also raises concerns about pain control, because pain can intensify due to the withdrawal itself or due to the use of a buprenorphine dose too low to provide analgesia comparable to the baseline opioid.
Although sublingual buprenorphine (either as a single entity or combined with naloxone) does not have a FDA indication for pain in the U.S., it could be used off-label for medical purposes. Patients who are receiving an opioid and are being considered for a change to buprenorphine therapy, perhaps because of concerns about aberrant drug taking behavior, may be candidates for a trial of one of the sublingual formulations. The transition to the sublingual formulation can precipitate withdrawal and pain, however, and a protocol that minimizes this risk may have clinical utility. A large experience in the initiation of sublingual therapy for addiction management (known as “induction”) suggests that the change can be made safely, and clinical protocols have been developed to reduce the risk of precipitated withdrawal during treatment of addiction.28
A recent study by Daitch and colleagues (published after our study had been completed) reported significant reductions in pain severity among chronic pain patients who transferred from full-opioid agonists to sublingual buprenorphine/naloxone (Bup/Nx).29 That study did not address the potential for adverse effects during the transition from a prior opioid regimen to buprenorphine. It reported outcomes among patients who had been treatment adherent and had continued Bup/Nx for at least 60 days, and did not report the percentage of patients who discontinued Bup/Nx or experienced adverse effects earlier than this. Two other observational studies30,31 and one randomized clinical trial32 also suggested that sublingual buprenorphine can be used as an analgesic, but did not establish the efficacy of buprenorphine in terms of reduced risk of non-adherence behaviors during pain therapy. Collectively, these four studies did not provide sufficient guidance to fully inform recommendations regarding a safe transition from the baseline opioid to buprenorphine in heterogeneous pain populations.
The primary aim of this work was to develop a protocol for the use of sublingual buprenorphine in opioid-treated chronic pain populations at relatively high risk of non-adherence behavior. To accomplish this, an initial protocol created by an expert panel of pain and addiction specialists was repeatedly revised, based on (1) outside review and, (2) the results of a pilot observational study.
MATERIALS AND METHODS
Development of the Clinical Protocol
A panel comprising specialists in addiction medicine (authors AR, HJ, LAM, LFM, SM, and ES) and specialists in pain medicine (RAC, RKP, and SS) were tasked with creating a clinical protocol for the administration of sublingual buprenorphine in populations of opioid-treated chronic pain patients at risk of non-adherence behavior. The protocol focused on patient selection, safe transition to buprenorphine, and subsequent dose adjustments for long-term therapy. The panel reviewed existing guidelines for the use of this drug in the treatment of addiction,28 an article that described inducting patients on relatively high methadone doses33 and the few pertinent publications30–32 relevant to the treatment of pain. During the course of a year, the panel convened several times by teleconference to propose, discuss or review interim drafts of the protocol. Decisions about the elements of the protocol were made by consensus.
The panel chose to develop the protocol for the commercial formulation containing buprenorphine and naloxone (ratio: 4 mg buprenorphine/1 mg naloxone; Suboxone® [Bup/Nx]). The decision to select Bup/Nx was determined by its comparatively lower abuse liability.34 Naloxone, an opioid antagonist, has minimal bioavailability by the sublingual and oral routes, and is presumed to have no significant effect if the Bup/Nx is taken sublingually. If the tablet is dissolved and injected, however, the naloxone would have the potential to reverse opioid agonist effects in an opioid dependent person (i.e., to precipitate withdrawal). The panel initially considered using the single entity buprenorphine formulation to transition from the baseline opioid but felt that any advantages inherent in avoiding the naloxone during this time were offset by the confusion that could complicate a second transition from a single entity buprenorphine to Bup/Nx. Neither the film formulation of Bup/Nx (Suboxone® Sublingual Film) nor transdermal buprenorphine were commercially available in the U.S. at the time that this work was undertaken.
The clinical protocol developed by the expert panel was condensed into a one-page guideline for patient selection, transitioning to Bup/Nx, and monitoring. After consensus within the expert panel was achieved on the specific elements of this algorithm, it was reviewed by three other specialists in addiction medicine, each of whom had experience in buprenorphine induction for opioid addiction. The reviewers unanimously endorsed the approach as designed.
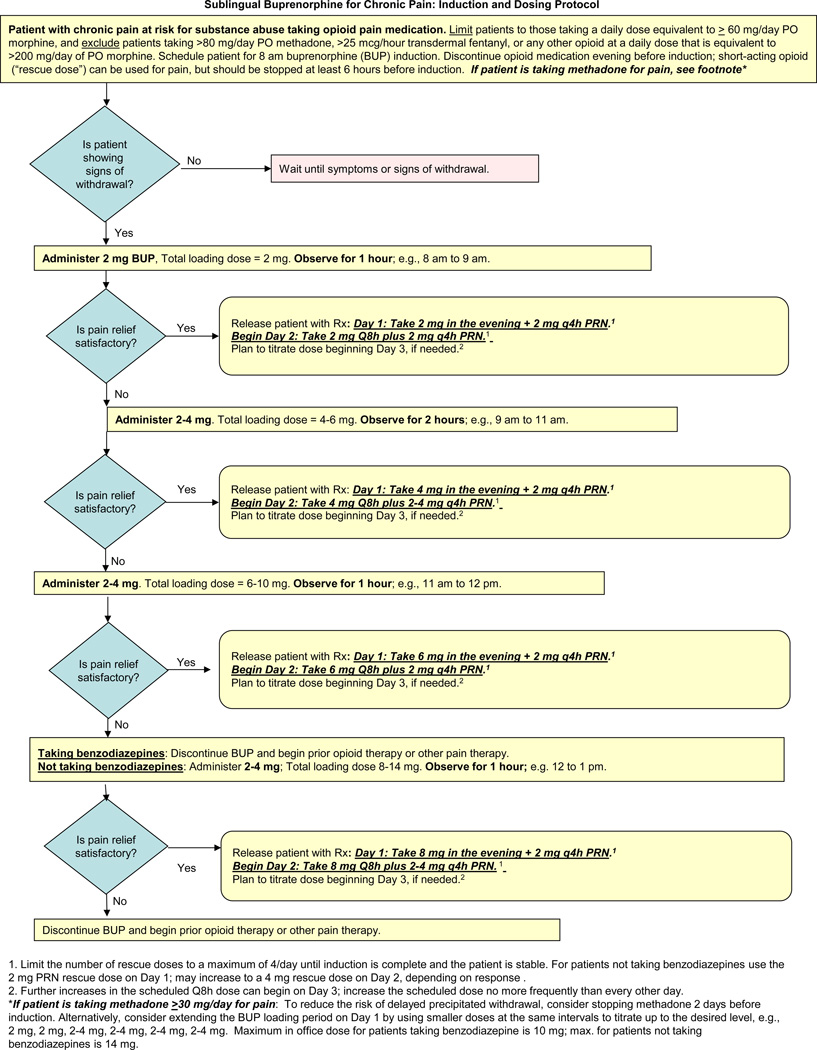
Revision of this initial guideline then occurred based on formal evaluations by regulators at the U.S. Food and Drug Administration (FDA) and the experience gained during a pilot study. There were three separate modifications. The version of the protocol incorporating all these modifications was created as the final product of this project (Figure 1); there has been no testing of this last version.
Figure 1.
The first modification was performed in response to a review by the FDA. This review occurred prior to the pilot study in response to an application for Investigational New Drug (IND) status. The agency placed a clinical “hold” on the study, expressing concerns about the safety of Bup/Nx dosing and several aspects of the methodology proposed for the pilot study, such as inclusion of patients who were taking benzodiazepines (raising concerns about drug-drug interaction) and lack of a ceiling for the maximum Bup/Nx dose (raising concerns about undesirable effects due to cumulative naloxone). Although none of the agency’s concerns had been raised earlier by panel members or the three additional reviewers, the guideline and methodology were revised in response to these concerns. The clinical hold was then lifted and the pilot study began.
The second modification to the protocol was performed after treatment of the first patient in the pilot study. The initial dosing algorithm indicated that the period of transition to Bup/Nx should begin with doses observed in the physician’s office. The first buprenorphine dose was 2 mg, and subsequent doses of 4 mg, 6 mg, and then 8 mg (representing a possible total loading dose of 20 mg) could be given at intervals if the patient experienced either a high level of pain or inadequate control of withdrawal symptoms. After the first patient was treated in this fashion, concerns were raised about the lack of flexibility needed to react quickly and calibrate treatment to patient need. The panel therefore modified the approach, recommending that the starting dose of 2 mg buprenorphine should be followed, if needed, by 2–4 mg for each of 2–3 subsequent doses at one or two hour intervals. The decision to exclude benzodiazepine-treated patients from the pilot also was revisited with the FDA at this time, given the frequency with which benzodiazepines and opioids were co-administered in practice, and the agency agreed that these patients could be included, with the proviso of a lower Bup/Nx maximial dose.
The third modification occurred after the 12th patient entered the pilot study. This patient required hospitalization after experiencing both severe withdrawal symptoms and severe pain on the first day. This event was reported to the FDA, which required a hold on patient recruitment. Because the planned end of the recruitment period had passed, the decision was made to close the study. The expert panel then evaluated the data from all 12 patients in parallel with the FDA review. Based on this evaluation (see below), the panel concluded that the initial guideline for patient selection did not adequately minimize risk for some types of patients. For those receiving a relatively high dose of a μ-agonist opioid or an opioid with a longer half-life, such as methadone and transdermal fentanyl, the initial doses required by the dosing algorithm did not appear to satisfactorily reduce the risk of precipitated withdrawal. For those receiving a very low dose of a μ-agonist opioid, the initial doses appeared too high, potentially causing side effects immediately after the transition.
Although these observations reflected experience in a limited number of patients, the expert panel believed that they were consistent with buprenorphine pharmacology and justified another modification. Accordingly, the third and final change in the protocol recommended against treatment with Bup/Nx when the baseline opioid dose was low (proposed as <60 oral morphine equivalent mg/d), very high (proposed as >200 oral morphine equivalent mg/d) or when patients had been receiving moderate or higher doses of either oral methadone (proposed as >80 mg/d) or transdermal fentanyl (proposed as >25 mcg/hour). The proposal to reduce risk by narrowing the eligibility for the sublingual Bup/Nx dosing algorithm was discussed with the FDA and the agency indicated that the new approach (Figure 1) was acceptable for further study.
Pilot Study
A small longitudinal observational study was designed to pilot test the clinical protocol in the population for which it was intended. As described previously, most of the patients who participated in the pilot study were treated using the penultimate draft of the clinical protocol. The study commenced after approval of the FDA’s IND application and approval from the Institutional Review Boards of Beth Israel Medical Center (in NYC) and the National Development and Research Institutes. Patients provided signed informed consent prior to participation.
Eligibility
A convenience sample was recruited from the ambulatory pain practice of Beth Israel Medical Center. Patients were eligible for the pilot if they had moderate to severe chronic pain, were receiving long-term opioid therapy with any full μ-agonist drug, and had exhibited one or more aberrant drug-related behaviors, as assessed using the Current Opioid Misuse Measure (COMM).35 At the discretion of the investigator, patients who had taken opioids in the past and had a history of substance abuse (including opioid abuse) also could be included in the study. Patients were excluded if they met criteria for a current substance use disorder defined by the Diagnostic and Statistical Manual-IV of the American Psychiatric Association, as determined by The MINI International Neuropsychiatric Interview.36 Patients with moderate-severe cardiopulmonary disease or liver function tests more than three times normal also were excluded.
Procedures
Patients who signed the study consent were asked to complete a baseline interview, participate in the protocol for Bup/Nx therapy, and complete periodic assessments over the next 3–6 months. Bup/Nx sublingual tablets were provided by the National Institute on Drug Abuse, Research Technology Branch (Rockville, MD) from a supply provided by Reckitt Benckiser Pharmaceuticals, Inc (Hull, England).
Patients were instructed to discontinue their opioid therapy the night before Bup/Nx treatment (although they were permitted to take short acting opioids until 2:00 am of the day of their Bup/Nx transition). To reduce the risk of delayed precipitated withdrawal, patients taking methadone ≥30 mg/day were instructed to stop this drug at least 2 days before. On arrival to the physician’s office, patients underwent an assessment that included measurement of pain and withdrawal phenomena (see below). The goal was to administer the first dose of Bup/Nx, containing 2 mg of buprenorphine, at the time that mild withdrawal signs were observed or reported.
As described previously, subsequent in-office doses were administered in response to repeated assessment of pain, withdrawal symptoms, and other adverse events (AEs). Patients could leave when the assessment suggested that withdrawal phenomena and pain were both tolerable and stable or improving. The patient was given instructions to take one more dose of Bup/Nx during the evening or night, and begin regular, three times per day dosing on the following day. The standing dose was suggested in the guideline, but could be changed as early as the following day if monitoring of the patient revealed that it should be either higher or lower. The patient also was told that extra tablets of Bup/Nx could be used for breakthrough pain; this dose ranged from 2 mg to 4 mg, with a maximum frequency of 4 times a day.
Baseline Assessment
Patients completed questionnaires after consenting to the study. Selected parts of the patient version of the national Buprenorphine Waiver Evaluation of office-based buprenorphine treatment for opioid dependence37 were used to collect data on socio-demographics. A checklist (constructed by the investigators) was used to assess self-reported chronic illnesses and pain medications that had been taken during the past six months. Psychiatric distress was measured with the SCL-6,38 a six item version of the Symptom Checklist 90 (SCL-90).39 The Individual Assessment Profile40 was adapted to collect information on addiction and mental health treatments and services, as well as lifetime and past 30 day use of alcohol, tobacco, illegal drugs and non-medical use of prescribed drugs. Worst pain intensity, average pain intensity and pain interference with function and related quality-of-life concerns were measured on 0–10 numeric scales using the Brief Pain Inventory (BPI).41
Day 1 and Subsequent Assessments
The BPI pain severity scale for pain right now was administered prior to the first Bup/Nx dose and approximately one hour after each in-office dose. At the same times, withdrawal severity was assessed with both the clinician-rated Clinical Opiate Withdrawal Scale (COWS) and the patient-rated Subjective Opiate Withdrawal Scale (SOWS). The COWS includes 11 signs or symptoms and the SOWS includes 16 symptoms.42,43 COWS scores can range from 0 to 48 and categorizes withdrawal as mild (5–12), moderate (13–24), moderately severe (25–36) and severe (>36). The SOWS scores range from 0 to 64.
Scheduled visit frequency was weekly for the first 2 weeks, biweekly for weeks 3–6, and then once every four weeks. Telephone calls between visits could be arranged by the clinician as needed. The initial plan to follow patients for up for 6 months was changed to 3 months due to concerns about time to complete the trial. At each visit, assessments included vital signs, pain intensity (using the scales from the BPI), sedation (a 0 to 10 single-item rating of sleepiness), opioid withdrawal (with the COWS and SOWS), and other AEs (a side-effects measure constructed by the investigators). Illicit drug use was monitored with a urine drug screen at each visit or less frequently.
Statistical analysis
Descriptive statistics were used to summarize the data. The primary aim of the analysis was to compare baseline average and worst pain with average and worst pain reports during Bup/Nx treatment. A mixed-effects model with random intercepts was used to evaluate the results from all patients, including those who did not complete the three-month pilot. The nlme package44 of the freely available, open-source R program45 was used to fit the mixed-effects model. All other statistical procedures were conducted with SPSS version 6.14 (Chicago, IL).
RESULTS
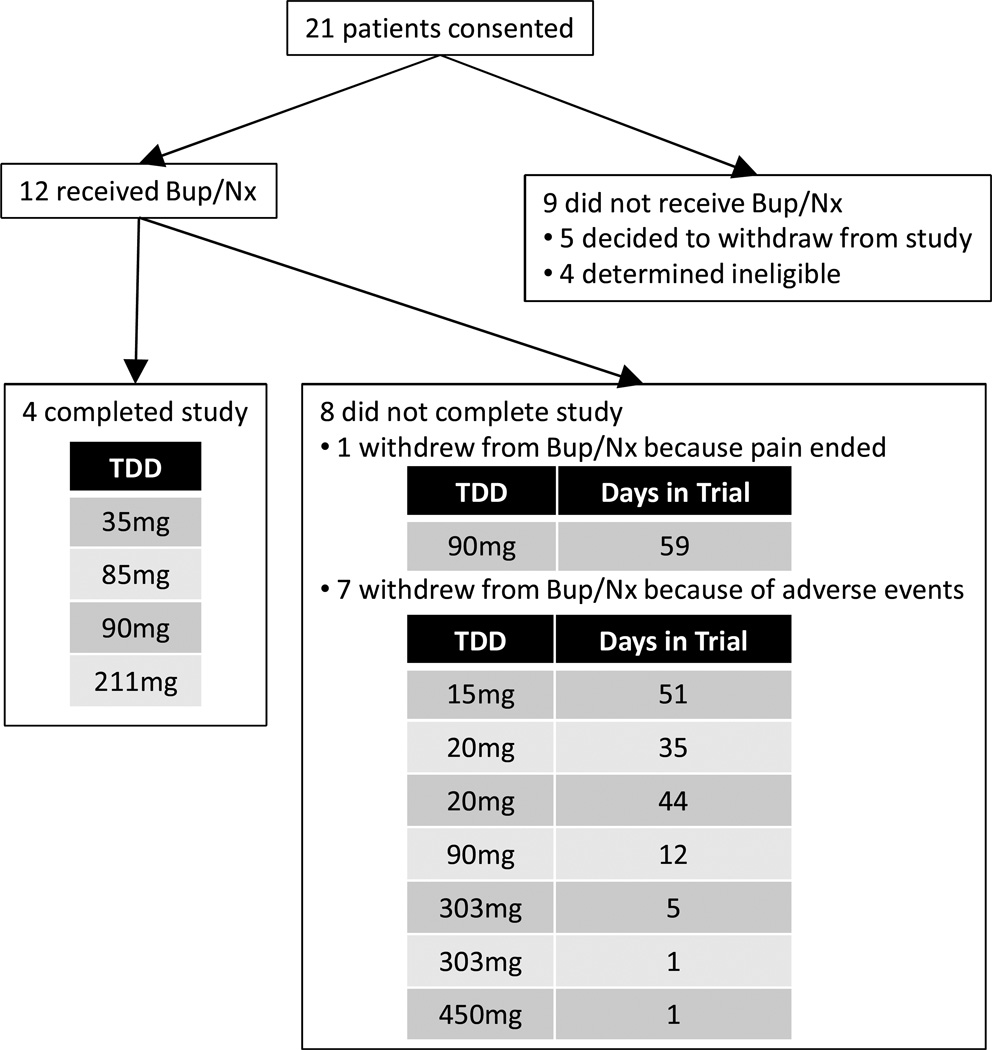
As noted, the results of the pilot study informed the final modification of the clinical protocol. A sample of 40 patients had been planned, but the strict inclusion criteria related to the combination of opioid therapy, recent aberrant behavior, and no diagnosable substance use disorder slowed recruitment. During the study period (July, 2008 to February, 2010), only 21 patients provided signed informed consent and only 12 patients were able to receive Bup/Nx. Of the nine consenting patients who did not receive study drug, five withdrew after signing consent and four were determined to be ineligible. During the study, eight patients discontinued prior to the three month follow-up and four completed the trial (See Figure 2).
Figure 2.
Consort diagram of patient flow through study.
Bup/Nx = Sublingual Buprenorphine/Naloxone (Suboxone®).
TDD = total daily dose of opioids (morphine equivalent) prior to taking Bup/Nx.
Characteristics at Baseline
The mean age of the 12 patients was 50 years (SD=9.62; range 40 to 66 years), 58% were male, and 67% were White, 25% Black and 8% Hispanic. Slightly more than two-fifths (42%) were married, 33% were on disability (Social Security Disability and Supplemental Security Income) and 42% were employed.
Most patients (83%) reported a chronic illness and 58% reported taking medication for a chronic illness. Fifty percent rated their general health as good or very good and 50% rated their health as fair or poor. Three patients (25%) reported that they were taking medication for a psychiatric disorder. On the SCL6 (where responses to psychiatric symptoms could range from 0 "not at all" to 4 "extremely"), the mean was relatively low (.955; SD = 1.33), with 10 patients scoring ≤1.33 and 2 scoring at 3.67 (between “quite a bit” and “extremely”).
Four patients (33%) reported a past history of a DSM-IV substance use disorder and three of these had an addiction treatment history. Three patients (25%) reported one or two days during the past 30 days of heavy drinking (≥ 4 drinks per day) or the use of marijuana or medications that had not been prescribed to them; 16% reported using tobacco. The number of items endorsed on the COMM at baseline ranged from one to 12, with a median of five. A non-parametric Wilcoxon-Mann-Whitney test showed that subjects who had a substance use disorder history, compared to those without such a history, endorsed significantly more COMM items (mean rank 9.88 vs. 4.81, p =.02).
Type of opioids and opioid dose at the time of Bup/Nx transition are in Table 1. Pain diagnoses were diverse and included degenerative disk disease, diabetic neuropathy, chemotherapy-induced neuropathy, post-polio syndrome, neuropathic post-surgical pain, intractable migraine, and complex regional pain syndrome. Half of the patients (N=6) reported a pain duration of more than five years. The duration of opioid therapy for chronic pain ranged from one to 25 years, with a mean of 8.5 years (SD=7.3). Opioids that had been prescribed to patients during the six months prior to study enrollment included codeine, methadone, morphine, oxycodone, hydrocodone, fentanyl and hydromorphone. Half (50%) of the sample had been prescribed two or more of these opioids during this period. When asked to rate the extent to which the opioids relieved pain, 58% said "a lot," 17% "moderately", and 25% "a little". Most patients also took non-opioid prescription or over-the-counter pain medications, and most used non-pharmacological therapies for pain.
Table 1.
Baseline opioids, induction and post-induction among pain patients transferred to sublingual buprenorphine
| ID | Baseline opioids | TDDa,b | Side effects; clinical notes | Days in trial |
Total office release dose |
Total Day 1 dose |
Highest post induction dose |
|---|---|---|---|---|---|---|---|
| 10 | Codeine 90mg | 15 | Side effects: drowsiness, muscle twitching. Notes: Excellent pain relief but not able to tolerate the side effects even after multiple dose adjustments and additional medications. Patient requested to be withdrawn from the study. | 51 | 2 | 6 | 6 |
| 8 | Oxycodone 13.5mg | 20 | Side effects: pain, difficulty breathing, sweating, confusion, disorientation, dry mouth, nausea, anxiety, nervousness, increased back spasms, auditory hallucinations. Note: Went to ED after experiencing symptoms. At ED Pt. stabilized & instructed to continue w/bup w/addition of Metformin HCI. Pain not well controlled; withdrawn from bup and switched to methadone. | 35 | 2 | 6 | 14 |
| 9 | Morphine 20mg | 20 | Side effects: irritability, hyperactive. Notes: Initially had exceptionally good response w/excellent pain control; low profile of side effects. Effectiveness decreased after several weeks. Terminated from study. | 44 | 2 | 2 | 8 |
| 6 | Morphine 20mg; oxycodone 10mg | 35 | Side effects: Occasional dry mouth, blurry vision, constipation, loss of appetite and trouble sleeping. Note: Pt. completed the study (3 months). Reported adequate pain control with no major side effects. Patient started exercise regimen to increase her quality of life. Overall, patient reported good health. | >90 | 4 | 10 | 8 |
| 3 | Oxycodone 30mg; fentanyl 12.5mcg/48hr, morphine | 85 | Side effects: None. Note: Although pt. initially took 2mg tid + up to 6mg bup as rescue medication, pt eventually responded well to medication and would take 2mg bid on pain free days and 2mg tid when pain was present. | >90 | 2 | 5c | 12 |
| 1 | Morphine 30mg | 90 | Side effects: chest pain, anxiety, severe perspiration, loss of appetite. Note: Stopped bup on his own; 4 days later went to ED. No abnormalities found. Terminated from study; returned to regular primary care physician for pain control. | 12 | 2 | 6 | 10 |
| 5 | Oxycodone 60mg | 90 | Side effects: None. Note: Pt. chose to withdraw from bup after becoming pain free. Remained pain free and did not resume opioids. | 59 | 2 | 6 | 8 |
| 4 | Oxycodone 60mg | 90 | Side effects: nausea, depression, weakness, drowsy, irritation. Note: Patient stopped bup on his own, experienced more severe symptoms and resumed bup 3 days later at a reduced level (6mg tid, no PRN). Patient sought counseling for depression. Despite difficult transition onto bup the patient was successfully stabilized, reported reduced pain, no subsequent adverse events and completed the study. | >90 | 6 | 18 | 24 |
| 7 | Methadone 70mg; morphine, 105mg | 211 | Side effects: None. Note: Pt. called physician to report severe distress. On her own had reduced amount and frequency of bup dose for fear of adverse drug-drug interactions (had been taking a benzodiazepine). Symptoms improved after resuming bup and taking clonazepam. Continued w/study and reported effective pain control most of the time. | >90 | 4 | 8 | 12 |
| 2 | Oxycodone 30mg; fentanyl 125mcg/48hr | 303 | Side effects: nausea, vomiting, sleeplessness, loss of appetite, fatigue, pain, restlessness. Note: Stopped bup & resumed fentanyl patch on his own. Terminated from study. | 5 | 8 | 16 | 24 |
| 12 | Fentanyl 225mcg/48hr | 450 | Side effects: Intense withdrawal symptoms & increased pain. Note: Pt. given Ativan and morphine sc, but symptoms persisted. Was transferred to ER & later admitted to hospital. Withdrawal symptoms controlled later that evening, but kept in hospital for pain control. | 1 | 6 | n/a | n/a |
| 11 | Methadone 120–200mg | ~303 | Side effects: anxiety, slurred speech, nausea, hallucinations, vomiting, restlessness, tingling, nervousness, hot/cold flashes, sweating, shaking uscle twitching, runny nose, teary eyes. Notes: Patient experienced pain and intense/severe withdrawal during induction period. Received morphine sc, Opana ER and Xanax to help control symptoms. Notes: Patient experience pain and intense/severe withdrawal effects during the induction period. Received morphine sc, Opana ER and Xanax to help control symptoms. Gradually, the withdrawal symptoms abated. Patient was discharged 6 hours after first induction dose and instructed to resume methadone regimen (max of 200 mg/day) he was on prior to the study. | 1 | 8 | n/a | n/a |
TDD: Total daily dose. Subjects are sorted by TDD.
Oral morphine conversion ratios for codeine, methadone and oxycodone were derived from Fine & Portenoy;47,48 and for TD fentanyl from Levy (Levy’s Rule).49 The insert in the fentanyl package has a different conversion than the one used here, and also has significant variability.50
Subject had taken half of a 2mg tablet in the evening of Day 1.
At the time of transition to Bup/Nx, the 12 patients were receiving codeine, oxycodone, morphine, methadone, or fentanyl. Daily opioid consumption was 15–35 oral morphine equivalent mg in four patients, 85–90 mg in four patients, and 200–450 mg in four patients. The mean worst pain intensity during the week prior to initiation of Bup/Nx was 8.4 (SD=1.9; range 3–10) and the mean average pain intensity was 6.5 (SD=2.5; range 2–10). The mean on the BPI pain interference scale was 6.7 (SD=2.3; range 1.7–8.6).
Transitioning to Bup/Nx
The mean in-office buprenorphine dose administered was 4 mg (range 2 mg to 8 mg). The highest dose (8 mg) in the office was lower than the highest dose permitted by the algorithm (14 mg). From Day 2 onward, the average total daily buprenorphine dose was 12 mg (range 8 mg to 24 mg). This was lower than the highest daily dose (scheduled plus supplemental doses for breakthrough pain) permitted by the protocol (40 mg).
Prior to the first dose of Bup/Nx, the COWS scores ranged from 1 to 30 (mean=11.25; SD=7.24). After the first dose, the scores ranged from 1 to 23 (mean=7.58; SD=5.99), and for all subsequent in-office doses, the range was 0–25 (M=8.21; SD=6.91). Similarly, prior to the first dose, the SOWS scores ranged from 4 to 56 (mean=21.50; SD=14.64); after the first dose, the range was 1 to 60 (mean=14.83; SD=16.63), and for all subsequent doses, the range was 0 to 39 (mean=13.38; SD=13.74). The COWS and SOWS scores were highly intercorrelated (r=.90, p < .001).
Before Bup/Nx was given, pain severity scores ranged from 2 to 10 (mean=7.33; SD=2.38). After the first dose, in-office pain severity scores ranged from 0 to 9 (mean=4.71; SD=2.91). The correlation between pain severity and COWS scores was r=.55 (p<.001) and the correlation with the SOWS scores was r=.59 (p=<.001).
Adverse Events and Discontinuations
Ten of the 12 patients transitioned to Bup/Nx experienced AEs, including seven patients who stopped treatment as a result (Table 1). Patients reported sedation, dizziness, confusion, thirst/dry mouth, slurred speech, depression, hot flushes, chills, nausea, headache, heart racing, vomiting, sweating, blurred vision, trouble swallowing, fatigue/weakness, nervousness, constipation, diarrhea, ringing in ears, loss of appetite, trouble sleeping, skin rash, and feeling groggy. As noted, one patient was hospitalized during the first day of treatment due to intense withdrawal symptoms and increased pain.
Three patients stopped Bup/Nx within seven days, five stopped between 12 and 60 days, and four completed three months of treatment (Table 1). In addition to the seven patients who stopped treatment because of AEs, one was withdrawn from Bup/Nx 59 days after treatment began because she had become pain free. She had been taking the equivalent of 90 mg oral morphine/day prior to the switch to Bup/Nx and did not experience side effects after transitioning to Bup/Nx.
Of the patients who withdrew from the study due to AEs, three were receiving relatively high opioid doses (>300 mg oral morphine equivalent per day) prior to Bup/Nx treatment (Table 1). Two of these patients stopped treatment on the first day due to intense withdrawal symptoms and one stopped five days later after experiencing vomiting, sleeplessness, pain, and restlessness. The three patients who had been receiving the lowest opioid doses prior to Bup/Nx (≤ 20 oral morphine equivalent mg/day) also experienced AEs after the switch; symptoms included pain, difficulty breathing, anxiety, drowsiness and muscle twitching. These patients withdrew from the study between 12 and 60 days after Bup/Nx was initiated. The seventh patient who withdrew from the study due to AEs was receiving the equivalent of 90 mg of oral morphine per day and stopped Bup/Nx after 12 days of treatment due to chest pain, anxiety, perspiration and loss of appetite; he was evaluated at an emergency department and no pathology was identified.
The four patients who completed the trial were receiving daily opioid doses equivalent to 35 mg to 211 mg oral morphine prior to the switch to Bup/Nx. One of these patients, who was also receiving a benzodiazepine, reduced the dose of Bup/Nx on her own after successfully transitioning to this therapy; she experienced withdrawal symptoms, which abated when she resumed her prescribed dose of Bup/Nx.
Pain and Other Outcomes
A mixed-effects model with random intercepts was used to compare baseline pain with pain reports after the transition to Bup/Nx. Due to subjects leaving the trial and non-adherence to the assessment schedule, data collection after baseline was variable. There were 18 assessments (for 10 subjects) during the first 2 weeks, 15 assessments (8 subjects) during weeks 3–6, and 16 assessments (7 subjects) for 7 or more weeks. Average pain significantly declined from baseline (mean=6.6; SD=2.7) to after baseline (mean=2.1; SD=2.5), t(48) = −6.44, P < .01). Worst pain also showed a significant decline from baseline (mean=8.2; SD=1.9) to after baseline (mean=4.8; SD=3.2), t(48) = −3.77, P < .01). As a sensitivity analysis, the missing pain scores for the two pain patients who were withdrawn from the trial on the first day were replaced with the highest possible average pain and worst pain scores (i.e., a value of 10). (This sensitivity analysis allowed us to impute the worst possible post-baseline pain severity scores for these two patients.) For all 12 patients, average pain significantly declined from baseline (mean=6.6; SD=2.7) to after baseline (mean=3.4; SD=3.7), t(58)= −5.38, p < .01), and worst pain significantly declined from baseline (mean=8.2; SD=1.9) to after baseline (mean=5.7; SD=3.5), t(58), = −3.04, p<.01).
The four patients who completed three months of treatment recorded a mean pain interference score of 6.00 (SD=2.61) at baseline and 2.86 (2.77) at the last follow-up. Two had decreased pain interference (from 7.57 to 1.14; and from 8.43 to 0) and two had increased interference (from 2.57 to 4.29; and from 5.43 to 6.00). These four patients also had little aberrant drug-related behavior; the follow-up COMM scores ranged from 0 to 3, compared to baseline scores that ranged from 3 to 6.
Eight patients underwent urine drug screening. Of 42 tests, 37 were negative for indications of drug abuse. Two were positive for THC, one for THC and ethyl glucuronide (EtG; an alcohol metabolite), and two for EtG alone. No subject had more than one positive drug test.
DISCUSSION
The primary aim of this project was to develop a clinical protocol for the use of sublingual Bup/Nx in opioid-treated chronic pain patients at high risk of aberrant drug-related behavior. This protocol was initially created by a panel of experts in substance abuse and pain, and underwent several modifications based on review by a federal agency and experience gained during a pilot test. The pilot suggests that a sublingual formulation of buprenorphine, which contains 2 mg of this drug (its lowest dose form available in the U.S.), can be efficacious in reducing severity of pain among chronic pain patients. Although consistent with other studies that have evaluated the analgesic effect of sublingual buprenorphine (Bup/Nx or buprenorphine only), the small number of subjects in our study (12) may represent a highly selected sample. The effect of Bup/Nx on other important behaviors, such as pain interference with function and drug-related aberrant behavior, could not be ascertained due to the small sample size and limited number of within subject observations.
The primary implication of this study is that there are challenges in transitioning patients from a full μ-agonist opioid. Ten of the 12 patients switched to Bup/Nx experienced AEs and seven had to stop treatment. The six patients receiving the highest and lowest opioid doses prior to the switch had the most significant AEs.
More study will be necessary to confirm the observation that baseline opioid regimens within a high-dose range and a low-dose range increase the risk of AEs when transitioning opioid-treated pain patients to Bup/Nx. The expert panel hypothesized that the three patients receiving the highest doses experienced significant withdrawal after receiving Bup/Nx, and the three patients receiving the lowest doses experienced side effects from a buprenorphine dose that was relatively too high. This hypothesis provided the rationale for the final modification of the clinical protocol (see Figure 1), which recommends that Bup/Nx be considered for pain management only when the baseline opioid regimen is within bounds that reduce the risk of AEs at transition.
Specifically, the protocol favors a trial of Bup/Nx only if total daily baseline opioid dose is equivalent to ≥ 60 mg and ≤ 200 mg of oral morphine; it also discourages treatment if patients are receiving methadone at a dose >80 mg/day or transdermal fentanyl at a dose >25 mcg/hour. The 60 mg “floor” was suggested because standard equianalgesic dose tables indicate that a single dose of buprenorphine 2 mg is approximately equianalgesic to 60 mg oral morphine. The “ceiling” and the decision to discourage treatment of patients taking more than the lowest dose of transdermal fentanyl were suggested by the observed AEs of patients in the pilot study. The 80 mg limit recommended for methadone was informed by a small study with buprenorphine induction of patients receiving methadone maintenance.33 Further studies will be needed to evaluate the risk of withdrawal when transition is attempted during prior treatment with various drugs and doses.
Research will also need to determine whether there are safe and effective methods to transition patients who fall outside of the presumed high and low dose range for opioid doses. For example, it is possible that patients on higher doses of opioids such as methadone or transdermal fentanyl could be successfully transitioned by tapering their methadone/fentanyl dose into the target morphine equivalent range (60–200mg per day) prior to transitioning to Bup/Nx. Alternatively, waiting a longer period of time after the last dose of opioid before starting Bup/Nx would be less likely to result in precipitated withdrawal. However, both of these approaches risk increased or more prolonged withdrawal discomfort or pain for the patient between stopping the original opioid and starting the Bup/Nx, so these approaches need to be studied to determine if there is a net reduction in distress with transition. For patients in the presumed low-dose range, a 1 mg divided tablet of Bup/Nx (1mg/.25mg) could be considered. One of our panel members (SS) reported some success initiating Bup/Nx at 1mg in patients discontinued from lower doses of other opioids. Since our pilot did not utilize these methods, they are not part of our current protocol.
Three previous studies reported the use of sublingual buprenorphine (Bup/Nx and/or buprenorphine only) to treat chronic pain. All included patients who were either abusing or dependent on opioids,30,32 or were being referred for detoxification following the failure of opioid therapy.31 By contrast, patients in our study were receiving prescribed opioid therapy for pain and did not have a current opioid dependence or abuse disorder. The objective in developing the protocol was not to manage addiction or ultimately discontinue opioid therapy, but rather to explore another option for long-term pain treatment using an opioid that may be particularly suited in the management of high-risk patients. A fourth study evaluated ongoing use of sublingual buprenorphine (Bup/Nx) for chronic pain patients who experienced continued or worsening pain despite being treated with full μ-agonist medications.29 Although the prevalence of aberrant behaviors in the latter study population is unclear, the experience reported by Daitch et al. appears to have included a population similar to ours, with patients’ pre-conversion morphine equivalent dose ranging from 10–840mg (Mean/SD 180 ± 160). However, the data reported are limited to patients who had continued buprenorphine for more than 60 days and there is no description of patients who stopped taking burprenorphine or experienced adverse events prior to this 60 day interval. All of these four studies report a reduction in pain severity and few or no adverse events following successful treatment with sublingual buprenorphine; none adequately describe the potential for adverse effects associated with the conversion to buprenorphine during pain treatment with a pure μ-agonist. A separate study of prescription opioid dependent patients (which excluded patients who required ongoing pain management with opioids) reported equally favorable drug use outcomes for patients with and without chronic pain.46
The difficulties encountered in transitioning pain patients from their prescribed opioids to Bup/Nx suggests that this population may be more challenging to treat with sublingual buprenorphine than populations who are being treated for abuse or dependence, or are scheduled for opioid discontinuation. Additional research is needed to clarify the predictors of both favorable and unfavorable outcomes when chronic pain patients with aberrant drug-related behavior are switched to buprenorphine with the intent of continuing long-term therapy for pain.
The process that created, modified and pilot tested the clinical protocol had significant limitations. The lack of a relevant experience or evidence base to inform the initial creation of the protocol, the need to modify the dosing algorithm after only one patient, and the decision to restrict eligibility based on the results of a small pilot trial all underscore the tentative nature of the guideline. Indeed, the decision to develop a protocol in the absence of formative research that clarifies the clinical pharmacology of Bup/Nx in opioid-treated pain patients and empirically explores risks and benefits in groups receiving different drugs and doses can be questioned. This path was justified, however, by a confluence of factors, including the favorable outcomes observed with increasing experience with sublingual buprenorphine in addiction management, the commercialization of transdermal buprenorphine (anticipated in the U.S. and available in Europe when the current project was planned) indicated specifically for chronic pain, and the need to rapidly develop new strategies to address the increasing problem of prescription drug abuse. From these perspectives, a consensus process involving both pain specialists and addiction medicine specialists held the promise that a safe approach could be proposed for the use of Bup/Nx in opioid-treated chronic pain patients, which if confirmed by further testing, has the promise to expand access to a potentially valuable therapy.
Acknowledgments
This study was supported by two grants from the National Institute on Drug Abuse (R21 DA022675 and K24 DA023186). We wish to thank Howard Heit, Walter Ling, Randy Seewald, who served as raters for our treatment protocol. Reckitt Benckiser provided medication for the study.
References
- 1.World Health Organization (WHO) Cancer pain relief, with a guide to opioid availability. 2nd ed. Geneva: 1996. [Accessed on February 26, 2012]. Available at http://whqlibdoc.who.int/publications/9241544821.pdf. [Google Scholar]
- 2.Boudreau D, Von Korff M, Rutter CM, et al. Trends in long-term opioid therapy for chronic non-cancer pain. Pharmacoepidemiol Drug Saf. 2009;18:1166–1175. doi: 10.1002/pds.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson RE, Fudala PJ, Payne R. Buprenorphine: considerations for pain management. J Pain Symptom Manage. 2005;29:297–326. doi: 10.1016/j.jpainsymman.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 4.Compton WM, Volkow ND. Major increases in opioid analgesic abuse in the United States: concerns and strategies. Drug Alcohol Depend. 2006;81:103–107. doi: 10.1016/j.drugalcdep.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 5.Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981–1985. doi: 10.1056/NEJMp1011512. [DOI] [PubMed] [Google Scholar]
- 6.Rosenblum A, Marsch LA, Joseph H, et al. Opioids and the treatment of chronic pain: controversies, current status, and future directions. Exp Clin Psychopharmacol. 2000;16:405–416. doi: 10.1037/a0013628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Webster LR. Ending Unnecessary Opioid-Related Deaths: A National Priority. Pain Medicine. 2011;12:S13–S15. doi: 10.1111/j.1526-4637.2011.01124.x. [DOI] [PubMed] [Google Scholar]
- 8.Passik SD, Kirsh KL, Konaghy KB, et al. Pain and aberrant drug-related behaviors in medically ill patients with and without histories of substance abuse. Clin J Pain. 2006;22:173–181. doi: 10.1097/01.ajp.0000161525.48245.aa. [DOI] [PubMed] [Google Scholar]
- 9.Portenoy RK. Acute and chronic pain. In: Ruiz P, Strain E, editors. Lowinson & Ruiz’s Substance Abuse: A Comprehensive Textbook. 5th edition. Philadelphia: Lippincott, Williams & Wilkins; 2011. pp. 695–720. [Google Scholar]
- 10.Savage SR, Kirsh KL, Passik SD. Challenges in using opioids to treat pain in persons with substance use disorders. Addict Sci Clin Pract. 2008;42:4–25. doi: 10.1151/ascp08424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson RE, Strain EC, Amass L. Buprenorphine: how to use it right. Drug Alcohol Depend. 2003;70:S59–S77. doi: 10.1016/s0376-8716(03)00060-7. [DOI] [PubMed] [Google Scholar]
- 12.Walsh SL, Preston KL, Bigelow GE, et al. Acute administration of buprenorphine in humans: Partial agonist and blockade effects. J Pharmacol Exp Ther. 1995;274:361–372. [PubMed] [Google Scholar]
- 13.Dahan A, Yassen A, Romberg R, et al. Buprenorphine induces ceiling in respiratory depression but not in analgesia. Br J Anaesth. 2006;96:627–632. doi: 10.1093/bja/ael051. [DOI] [PubMed] [Google Scholar]
- 14.Umehara K, Shimokawa Y, Miyamoto G. Inhibition of human drug metabolizing cytochrome P450 by buprenorphine. Biol Pharm Bull. 2002;25:682–685. doi: 10.1248/bpb.25.682. [DOI] [PubMed] [Google Scholar]
- 15.Kress HG. Clinical update on the pharmacology, efficacy and safety of transdermal buprenorphine. Eur J Pain. 2009;13:219–230. doi: 10.1016/j.ejpain.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 16.Pergolizzi, Aloisi, Dahan, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010;10:428–450. doi: 10.1111/j.1533-2500.2010.00378.x. [DOI] [PubMed] [Google Scholar]
- 17.Davis 2012: Twelve reasons for considering buprenorphine as a frontline analgesic in the management of pain. [Accessed on August 24, 2012];J Supportive Oncology. doi: 10.1016/j.suponc.2012.05.002. In press. ( http://www.oncologypractice.com/fileadmin/content_images/jso/PDF/AIP_Burprenorphine_Review.pdf). [DOI] [PubMed] [Google Scholar]
- 18.Lutfy K, Cowan A. Buprenorphine: a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2:395–402. doi: 10.2174/1570159043359477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seldén T, Ahlner J, Druid H, Kronstrand R. Toxicological and pathological findings in a series of buprenorphine related deaths. Possible risk factors for fatal outcome. Forensic Sci Int. 2012;220:284–290. doi: 10.1016/j.forsciint.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 20.Obadia Y, Perrin V, Feroni I, Vlahov D, Moatti JP. Injecting misuse of buprenorphine among French drug users. Addiction. 2001;96:267–272. doi: 10.1046/j.1360-0443.2001.96226710.x. [DOI] [PubMed] [Google Scholar]
- 21.Pelissier-Alicot AL, Sastre C, Baillif-Couniou V, et al. Buprenorphine-related deaths: unusual forensic situations. Int J Legal Med. 2010;124:647–651. doi: 10.1007/s00414-010-0449-1. [DOI] [PubMed] [Google Scholar]
- 22.Dasgupta N, Bailey EJ, Cicero T, et al. Post-marketing surveillance of methadone and buprenorphine in the United States. Pain Med. 2010;11:1078–1091. doi: 10.1111/j.1526-4637.2010.00877.x. [DOI] [PubMed] [Google Scholar]
- 23.Lai SH, Teo CE. Buprenorphine-associated deaths in Singapore. Ann Acad Med Singapore. 2006;35:508–511. [PubMed] [Google Scholar]
- 24.Gatti A, Dauri M, Leonardis F, et al. Transdermal buprenorphine in non-oncological moderate-to-severe chronic pain. Clin Drug Investig. 2010;30(Suppl 2):31–38. doi: 10.2165/1158409-S0-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 25.Griessinger N, Sittl R, Likar R. Transdermal buprenorphine in clinical practice--a post-marketing surveillance study in 13,179 patients. Curr Med Res Opin. 2005;21:1147–1156. doi: 10.1185/030079905X53315. [DOI] [PubMed] [Google Scholar]
- 26.Gordon A, Rashiq S, Moulin DE, et al. Buprenorphine transdermal system for opioid therapy in patients with chronic low back pain. Pain Res Manag. 2010;15:169–178. doi: 10.1155/2010/216725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Butrans Full Product Information. Stamford, CT: Purdue Pharma L.P.; [Accessed November 28, 2012]. http://www.purduepharma.com/pi/prescription/ButransPI.pdf. [Google Scholar]
- 28.SAMHSA. Substance Abuse and Mental Health Services Administration. Rockville, MD: 2004. [accessed on February, 26, 2012]. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction, Treatment Improvement Protocol (TIP) Series 40. DHHS Publication No. (SMA) 04-3939. Available at: http://www.ncbi.nlm.nih.gov/bookshelf/br.fcgi?book=hssamhsatip&part=A72564. [PubMed] [Google Scholar]
- 29.Daitch J, Frey ME, Silver D, et al. Conversion of chronic pain patients from full-opioid agonists to sublingual buprenorphine. Pain Physician. 2012;15:ES59–ES66. [PubMed] [Google Scholar]
- 30.Heit HA, Gourlay DL. Buprenorphine: new tricks with an old molecule for pain management. Clin J Pain. 2008;24:93–97. doi: 10.1097/AJP.0b013e31815ca2b4. [DOI] [PubMed] [Google Scholar]
- 31.Malinoff HL, Barkin RL, Wilson G. Sublingual buprenorphine is effective in the treatment of chronic pain syndrome. Am J Ther. 2005;12:379–384. doi: 10.1097/01.mjt.0000160935.62883.ff. [DOI] [PubMed] [Google Scholar]
- 32.Blondell RD, Ashrafioun L, Dambra CM, et al. A clinical trial comparing tapering doses of buprenorphine with steady doses for chronic pain and co-existent opioid addiction. J Addict Med. 2010;4:140–146. doi: 10.1097/ADM.0b013e3181ba895d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosado J, Walsh SL, Bigelow GE, et al. Sublingual buprenorphine/naloxone precipitated withdrawal in subjects maintained on 100 mg of daily methadone. Drug Alcohol Depend. 2007;90:261–269. doi: 10.1016/j.drugalcdep.2007.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Comer SD, Sullivan MA, Vosburg SK, et al. Abuse liability of intravenous buprenorphine/naloxone and buprenorphine alone in buprenorphine-maintained intravenous heroin abusers. Addiction. 2010;105:709–718. doi: 10.1111/j.1360-0443.2009.02843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butler SF, Budman SH, Fernandez KC, et al. Development and validation of the Current Opioid Misuse Measure. Pain. 2007;130:144–156. doi: 10.1016/j.pain.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): The development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. quiz 34–57. [PubMed] [Google Scholar]
- 37.Stanton A, McLeod CC, Kissin W, et al. Presented at CPDD. Orlando, FL: 2005. [Accessed on February 26, 2012]. Results from SAMHSA/CSAT's national evaluation of the buprenorphine waiver program. Available at http://buprenorphine.samhsa.gov/ASAM_06_Final_Results.pdf. [Google Scholar]
- 38.Rosen CS, Drescher KD, Moos RH, et al. Six- and ten-item indexes of psychological distress based on the Symptom Checklist-90. Assessment. 2000;7:103–111. doi: 10.1177/107319110000700201. [DOI] [PubMed] [Google Scholar]
- 39.Derogatis LR, Lipman RS, Covi L. The SCL-90: An outpatient psychiatric rating scale – Preliminary report. Psychopharmacol Bull. 1973;9:13–28. [PubMed] [Google Scholar]
- 40.Flynn PM, Hubbard RL, Luckey JW, et al. Individual Assessment Profile (IAP): Standardizing the assessment of substance abusers. J Subst Abuse Treat. 1995;12:213–221. doi: 10.1016/0740-5472(95)00023-X. [DOI] [PubMed] [Google Scholar]
- 41.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23:129–138. [PubMed] [Google Scholar]
- 42.Wesson DR, Ling W. The Clinical Opiate Withdrawal Scale (COWS) J Psychoactive Drugs. 2003;35:253–259. doi: 10.1080/02791072.2003.10400007. [DOI] [PubMed] [Google Scholar]
- 43.Handelsman L, Cochrane KJ, Aronson MJ, et al. Two new rating scales for opiate withdrawal. Am J. Drug Alcohol Abuse. 1987;13:293–308. doi: 10.3109/00952998709001515. [DOI] [PubMed] [Google Scholar]
- 44.Pinheiro J, Bates D, DebRoy S, et al. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1–102. 2011 [Google Scholar]
- 45.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. [Accessed on February 27, 2012]. ISBN 3-900051-07-0, Available at: http://www.R-project.org/ [Google Scholar]
- 46.Weiss RD, Potter JS, Fiellin DA, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68:1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fine PG, Portenoy RK. A Clinical Guide to Opioid Analgesia. New York, NY: Vendome Group, LLC; 2007. [Google Scholar]
- 48.Fine PG, Portenoy RK. Establishing “best practices” for opioid rotation: conclusions of an expert panel. J Pain Symptom Manage. 2009;38:418–425. doi: 10.1016/j.jpainsymman.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levy MH. Pharmacologic treatment of cancer pain. New Engl J Med. 1996;335:1124–1132. doi: 10.1056/NEJM199610103351507. [DOI] [PubMed] [Google Scholar]
- 50.Duragesic insert. Raritan, NJ: Ortho-McNeil-Janssen Pharmaceuticals, Inc.; 2009. [Google Scholar]