Abstract
Activation induced deaminase (AID) is globally targeted to immunoglobulin loci, preferentially focused to switch (S) regions and variable (V) regions, and prone to attack hotspot motifs. Nevertheless, AID deamination is not exclusive to Ig loci and the rules regulating AID targeting remain unclear. Transcription is critically required for class switch recombination and somatic hypermutation. Here, I consider the unique features associated with S region transcription leading to RNA polymerase II pausing, that in turn promote the introduction of activating chromatin remodeling, histone modifications and recruitment of AID to targeted S regions. These findings allow for a better understanding of the interplay between transcription, AID targeting and mistargeting to Ig and non-Ig loci.
Keywords: AID targeting, Germline transcription, Epigenetic modifications, RNA polymerase II pausing
1. Introduction
Immunoglobulin (Ig) antigen binding specificity is determined by assembly of a mature V region via V(D)J recombination during early B cell development. Antibody binding to antigen undergoes affinity maturation by means of somatic hypermutation (SHM), a mutational process, that is focused to rearranged V(D)J region genes in germinal center B cells. IgH effector function, determined by constant (CH) genes, is also diversified in mature B cells through class switch recombination (CSR), while maintaining the original antigen binding specificity arising from V(D)J recombination. The mouse immunoglobulin heavy chain (Igh) locus contains a series of eight constant (CH) genes (µ, δ, γ3, γ1, γ2b, γ2ba, ε, and α) that are located downstream of the V, D and JH segments (Fig. 1A). Each CH gene, with the exception of Cδ, is paired with a repetitive DNA switch (S) region and is a target for CSR. CSR involves the introduction and joining of double strand breaks (DSBs) in Sµ and a downstream S region with the concomitant deletion of the intervening genomic material and generation of Sµ–SX hybrid junctions. Activation induced deaminase (AID) deaminates dC residues in transcribed V(D)J exons or S regions and is critical for both SHM and CSR, respectively [1,2]. The means by which DNA lesions or DSBs initiated by AID deamination are repaired and culminate in SHM at V(D)J exons or CSR targeted to S regions have been extensively reviewed elsewhere [3–11].
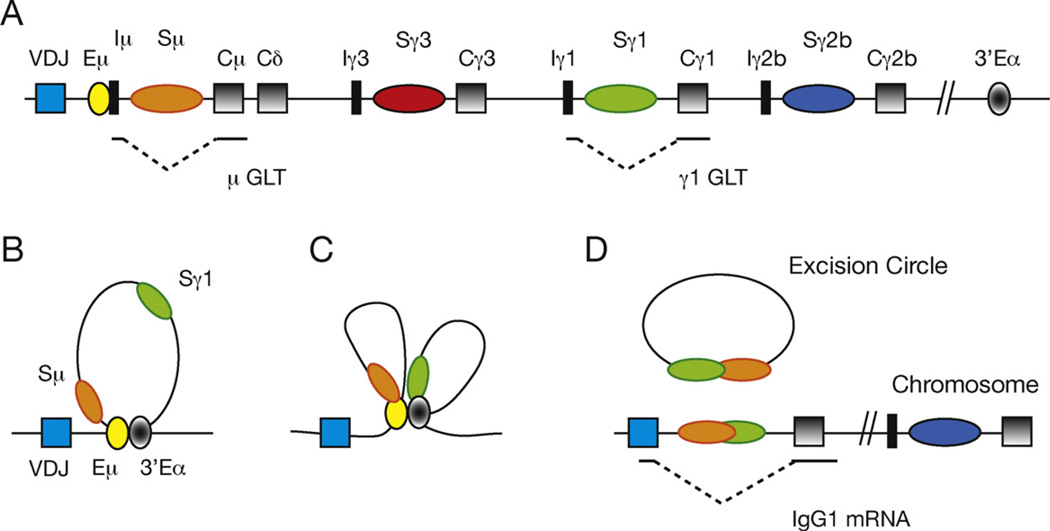
Fig. 1.
AID creates DSBs in a looped Igh locus. The diagram (not to scale) shows VDJ exons (blue box), the intronic µ enhancer, Eµ (yellow circle), Sµ, Sγ3, Sγ1 and Sγ2b regions (orange, red, green and blue ovals, respectively), I exons (black rectangles), CH genes (gray boxes) and the 3′Eα regulatory regions (gray filled circle). (A) Following B cell activation with LPS and IL4 both the µ and γ1 GLTs are expressed. (B) In resting B cells the Igh locus is in a looped configuration tethered by long range interactions between Eµ and 3′Eα. (C) Upon treatment with LPS and IL4 the γ1 GLT promoter interacts with 3′Eα and brings Sγ1 into proximity with Sµ (D) B cell activation also activates AID that in turn generates DSBs in Sµ and Sγ1. The DSBs are resolved by a reciprocal recombination event which generates an excision circle and a new hybrid Sµ/Sγ1 junction along the chromosome, thereby facilitating IgG1 expression.
The requirement for transcription is a hallmark of SHM and CSR [12,13]. VH region transcription is directed from a promoter 5′ of the rearranged VHDJH exon and terminates downstream of the Cµ exons. In VHDJH exons, AID induced mutations are most prevalent within 2 kb of the transcription start site (TSS) and are rectified by error prone repair [3,8,11,13]. These and other studies suggest that the transcription apparatus or transcription per se is a requirement for the process of SHM [14–16]. For CSR, a transcriptional promoter coupled with an I (intervening) exon is situated immediately upstream of a S region paired with each CH gene [17]. Transcription initiates at the 5′ end of the I exon, proceeds through the S region and terminates downstream of the corresponding CH gene (Fig. 1A). The transcribed mRNA products are termed sterile or germline transcripts (GLTs) since they lack an open reading frame and are not translated [6,17]. Combinations of specific cytokines and B cell activators stimulate transcription from particular GLT promoters and target the paired S region for CSR [17]. Gene targeting studies have highlighted the critical requirement for genetic element directing GLT expression as a prerequisite for CSR [18].
At the primary DNA sequence level, AID deamination occurs preferentially at the WRC motifs (W = A/T, R = A/G) (reviewed in [19]). WRC motifs are scattered throughout the genome but AID deamination is preferentially focused to Ig genes. Nevertheless, AID mistargeting to non-Ig genes is nonrandom and occurs at measurable frequencies in a set of highly transcribed genes [20,21]. In vivo, AID induces DSBs at hundreds of non-Ig sites many of which are syntenic with sites of translocations, deletions, and amplifications found in human B cell lymphomas and are not necessarily restricted to transcribed regions [22]. AID targeting becomes promiscuous upon ectopic overexpression [23–26] or as a consequence of environmental events that elevate AID expression [27]. Aberrant AID targeting may be responsible for SHM of non-Ig genes in B cell tumors [28] and Igh:myc translocations in AID expressing mature B cells [29,30]. Mutations in TP53 gene accumulate following infection of human gastric epithelial cells with Helicobacter pylori which causes induction of AID expression [31]. The preferential focus of AID to Ig loci and the nonrandom aspect of AID attack on non-Ig genes imply a determinant set of rules governing AID targeting. Here, I explore new integrating principles regarding the mechanism of transcription, its relationship to epigenetic chromatin modifications, chromatin accessibility, and its influence on three dimensional Igh chromatin organization, which together provide new insights regarding AID targeting in CSR and SHM and the potential for genomewide mistargeting.
2. Formation of the S/S synaptosome relies on transcriptional elements
S regions targeted for recombination are dispersed over 220 kb and this distance will impede formation of S/S synaptosome required for CSR. Chromosome conformation capture (3C) techniques permit the quantitative measurement of long range interactions along chromatin fibers [32,33]. Studies of the Igh locus using 3C methods have made two important observations pertinent to CSR. First, in the Igh locus the Eµ enhancer directly interacts with the downstream 3′Eα LCR to form a unique chromosomal loop in vivo [34–36] (Fig. 1B). Second, inducible long range intra-chromosomal interactions between the GLT promoter region and the downstream 3′Eα [34,36] are in accord with the requirement for 3′Eα element to enable inducible GLT expression [37,38]. The effect of simultaneous Eµ:3′Eα:GLT promoter interactions is that Sµ, proximal to Eµ is also in close contact with a downstream S region located adjacent to its GLT promoter (Fig. 1C and D). This three dimensional spatial organization of S regions targeted for CSR assures formation of the S/S synaptosome required for successful recombination and highlights the highly integrated requirements of CSR for transcription.
3. Transcription and the distribution of AID induced mutation
The distribution of mutations across V(D)J exons has a sharp 5′ boundary located about 120 bp downstream of the TSSs but a less defined 3′ boundary approximately 1 kb downstream of the promoter [39,40]. Promoter proximal transcription has been strongly linked to induction of AID dependent mutations because alteration of the promoter position leads to a corresponding displacement of transcription initiation and perturbation of mutation distribution [12,41]. Consequently, Peters and Storb hypothesized that mutations are introduced by a mutator, now known to be AID, that associates with the transcription initiation apparatus, tracks with the transcription elongation complex and dissociates stochastically [12]. This model was later applied to CSR, because similar to the mutation pattern found for V exons, S region mutations begin 150 bp downstream of the I exon transcription start site [42]. However, the 3′ boundary for CSR mutations is located downstream of S regions, a distance of up to 10 kb from the TSS, a distribution distinct from that found for SHM [42]. Recent studies support the Peters and Storb hypothesis and indicate that ectopically expressed AID binds with RNAP II in co-immunoprecipitation assays [43] and interacts with the transcription apparatus in vitro [44] implying that AID targeting is closely aligned with transcription.
4. Regional targeting of AID to S regions and exclusion from CH regions
AID targeting is strikingly asymmetric within the I-S-CH region. Whereas S regions are substrates for AID attack as evidenced by acquisition of mutations and DSBs, CH regions within the same transcription unit are protected [42,45,46]. Studies concerning transcription through chromatin provide a mechanistic perspective on how specific DNA targets become differentially accessible. Eukaryotic DNA is wrapped around histone octamers to form nucleosomes that are organized into higher order chromatin fibers which precludes access of trans acting factors to DNA [47–49]. Eukaryotic transcription begins at the promoter with the assembly of the preinitiation complex (PIC) composed of hypophosphorylated RNA polymerase II (RNAP II) and general transcription factors followed by nucleosome displacement, chromatin accessibility, and transcription initiation [49–52]. Early elongating RNAP II clears the promoter and transits to the promoter proximal pause site. The paused RNAP II complex becomes increasingly phosphorylated at Ser5 (p-ser5) on the C-terminal domain (CTD). RNAP II escapes from the pause site by entering productive elongation whereupon the CTD loses p-ser5 and becomes enriched for p-ser2. There is an important link between the phosphorylation status of RNAP II CTD, the recruitment of histone modifying enzymes and acquisition of chromatin hyperaccessibility in S regions [48,49,53,54].
Genome wide analyses indicate that in transcriptionally active genes, promoter proximal sites are enriched for tri-methyl histone H3 lysine 4 (H3K4me3), hyper-acetylated (Ac) H3K9, and with RNAP II p-ser5 whereas, H3K36me3 and elongating RNAP II p-ser2 are elevated in the downstream coding regions [55–58]. H3K4me3 is directly bound by a component of the NuA3 histone acetytransferase (HAT) complex that coordinates transcription activation with histone Ac (reviewed in [59]). DNA accessibility increases in response to histone Ac which changes the net charge of nucleosomes and alters chromatin fiber folding properties [60]. Histone Ac may also create binding surfaces for factor-histone contacts that then facilitate recruitment of transcription regulators [48]. In yeast, H3K36me3, is recognized by the histone deactylase complex (HDAC), Rpd3S, which acts to diminish histone Ac and thereby repress transcription initiation in downstream coding regions [61,62]. Thus, H3K4me3 and H3K36me3 modifications provide for recruitment of HATs and HDACs, respectively, which in turn modulate chromatin accessibility to assure spatially appropriate transcription initiation within the transcription unit. Collectively, these studies have significant implications for understanding the AID targeting to hyperaccessible S regions and the exclusion of the CH regions from AID attack.
Within the Igh locus, transcribed I-S regions are a focus for increased H3K4me3 and histone Ac while CH regions accumulate the repressive countermarks, H3K36me3 and H4K20mel [43,63–65]. In chromatin, I-S regions undergoing active transcription are nuclease hypersensitive whereas CH regions are relatively inaccessible [63,64]. Antisense RNA transcripts are detected in S regions but not in CH regions reflecting the reciprocal zones of accessible or repressed chromatin [66]. Modulation of H3Ac at S regions using tricostatin A, an inhibitor of HDACs, leads to greater chromatin accessibility and increased CSR indicating a direct link between this histone mark and switching frequency [63]. B cells with PTIP (PAX interaction with transcription activation domain protein-) deficiency display reduced H3K4me3 and histone Ac marks and impaired transcription initiation at several downstream S regions [67]. PTIP is a constituent of several histone methyl transferases (HMTases) including MLL3 (mixed lineage leukemia 3)–MLL4 complex that associate with RNAPII p-Ser5 and introduce H3K4me3 modifications [67,68]. PTIP also indirectly interacts with p300/CBP HAT suggesting that PTIP regulates both histone Ac and Me [69]. These findings support the conclusion that histone Ac status and chromatin hyperaccessibility are patterned across the I-S regions by means of H3K4me3 modifications linked to transcription. Long range interactions between GLT promoters and the 3′Eα enhancer are impaired in PTIP deficient B cells which reflects the failure of GLT transcription initiation [70].
5. S region sequence mediates RNAP II occupancy
S regions extend 1–10 kb downstream of the I exons [71], and when transcribed are decorated with H3K4me3, H3Ac, H4Ac, activating histone modifications, and reside in hyperaccessible chromatin throughout their lengths [63,64]. In contrast, genome wide ChlP-seq studies show that these histone marks are largely restricted to promoter proximal locations and rarely extend more that 1 kb downstream of the TSS [55,56]. The observation that RNAP II p-ser5 collaborates with an HMTase to introduce H3K4me3 at the 5′ end of active genes [55,56,72] suggested that the unusual distribution of H3K4me3 in S regions might be explained by stalled RNAP II p-ser5. ChIP studies demonstrated a significant enrichment of RNAP II p-ser5 throughout the Sµ, and Sγ3 regions implying that RNAP II has stalled at multiple sites in S DNA [63]. In Sµ deleted B cells, RNAP II p-ser5 occupancy and H3K4me3 marks remained promoter proximal, demonstrating that S region sequence mediates RNAP II enrichment [63,73]. DNA breaks inhibit transcription by RNAP I [74] but are not the cause of high occupancy RNAP II since retention in S regions occurs equivalently in AID proficient and deficient B cells [63].
Transcribed S regions are unique in that they contain long stretches of R loops that enhance CSR efficiency in vivo [75,76]. R-loops are composed of RNA:DNA hybrids hybridized to the template strand, impede transcription elongation [77,78] and could be responsible for RNAP II stalling detected in S regions. Enrichment of RNAP II in S regions could occur by another non-mutually exclusive mechanism. Broadly distributed H3K4me3 is detected across multiple Hox-coding and intergenic regions, and is correlated with the RNAP II transcription of noncoding RNAs [55,58]. Accordingly, anti-sense RNAs have been detected immediately 5′ of transcriptionally active S regions [66]. It is possible that antisense RNA expression initiate from multiple alternative promoters within the S regions, attract RNAP II which becomes stalled and hyperphosphorylated at p-ser5, then recruits PTIP to introduce H3K4me3. Thus, there are two plausible mechanisms to account for the unique distribution of RNAP II p-ser5 in S DNA. The presence of sense and antisense transcription could create head to head collisions between RNA pol II molecules that in turn might amplify RNA pol II stalling.
6. The R-loop dilemma
AID activity is specific for ssDNA but lacks activity on dsDNA [79–82]. In vivo, AID deaminates dC residues nearly equivalently on both strands of DNA raising the question of how access is gained to the transcribed strand (reviewed in [3,6,11,19]). SHM and CSR require transcription through dsDNA to generate ssDNA template which could be targeted by AID. During GLT expression R-loops are formed when the transcribed template strand hybridizes with the nascent S region transcript and the nontemplate strand is looped out as a long stretch of ssDNA [75,76,83]. Biochemical studies indicate that transcription generated R-loops within dsDNA allow DNA deamination primarily on the ssDNA nontemplate strand [82]. AID, phosphorylated on serine 38 by protein kinase A, can access transcribed dsDNA substrates that lack R-loops, as for V(D)J exons, but primarily on the nontemplate strand [84] (see also Vuong and Chaudhuri, this issue). Ectopic expression of AID in bacteria or yeast introduces mutations, mainly on the nontemplate strand [80,85]. RNAP II generated supercoiling occurs immediately upstream and downstream of the transcription complex [86]. Certain transcribed plasmids acquire AID induced mutations on both strands due to supercoils [87]. However, this does not explain how AID accesses the template strand when it is blocked by the nascent RNA transcript that will form the R-loop [19].
Evidence indicates that the RNA exosome, a cellular RNA-processing degradation complex, associates with AID, accumulates on S regions in an AID dependent manner and is required for CSR [88]. The RNA exosome complex contains 3′-5′ exoribonucleases that are implicated in the processing of structural RNA and in the degradation of improperly processed pre-mRNA [89]. The RNA exo-some is tethered to the RNAP II via the transcription elongation factors Spt5 and Spt6 [89]. Biochemical analyses demonstrate that the RNA exosome core complex facilitates AID- and transcription dependent deamination to both the template and nontemplate stands in vitro [88]. Phosphorylation of AID on Ser38 is a precondition for association with replication protein A (RPA) a complex which binds ssDNA as a trimolecular ring [90,91]. RPA associates with the S region prior to AID binding. It has been suggested that the ssDNA targets generated on the template strand by the RNA exosome might be stabilized by RPA which in turn mediates the accumulation of AID p-Ser38 by direct interaction [88] (see also Vuong and Chaudhuri, this issue).
7. AID targeted to paused RNAP II in S regions
Is RNAP II pausing simply a byproduct of transitioning through difficult R-loop territory? Or, does RNAP II pausing in S DNA confer some advantage to CSR? Transcription initiation begins with clearance of RNAP II from promoter bound factors and is then paused 25–50 nucleotides from the TSS by 5,6-dichloro-1-b-d-ribofuranosyl-benzimidazole (DRB) sensitivity inducing factor (DSIF) composed of Spt4 and Spt5, and negative elongation factor (NEF) [50]. Current models suggest that positive transcription elongation factor b (P-TEFb) kinase drives the transition from the paused to the actively elongating state by phosphorylating RNAP II and Spt5 leading to the release of NELF [50,53]. A shRNA screen recently identified Spt5 as a critical regulator of CSR [92]. Co-immunoprecipitation studies demonstrated that Spt5 interacts with AID and facilitates association with RNAP II [92]. ChlP-seq experiments show a strong correlation between positions of Spt5 and RNAP II stalling [92,93] and between sites of Spt5 and AID binding genomewide [92]. Technical concerns regarding the quality of the AID ChlP-seq and its interpretation, however, make this aspect of the study somewhat controversial. Collectively, the data suggest that AID accumulates at sites of RNAP II pausing by means of its association with Spt5. The high density of paused RNAP II and Spt5 in transcribed S regions appears to confer preferential AID targeting to these sites.
8. Links between Spt5, co-transcriptional factors and CSR
Earlier studies showed that pre-mRNA splicing may be integral to CSR since deletion of the GLT γ1 I exon splice donor abolished µ→γ1 switching [94,95], however the mechanism linking RNA processing to CSR was obscure. Transcription proceeds through a series of transitions moving from initiation, early elongation, promoter proximal pausing, active elongation and termination with pre-mRNA processing and splicing occurring as a series of functionally coupled co-transcriptional events [50,96,97]. Spt5 interacts with a number of co-transcriptional factors, and may thereby serve as a functional adaptor to the transcription machinery. Spt5 links RNAP II to splicing factors [98], capping enzyme [99,100] and the RNA exosome [89]. Accordingly, several RNA processing and splicing factors have been functionally linked to CSR and could be recruited to S DNA via Spt5. Polypyrimidine tract binding protein 2 (PTBP2), identified based on its biochemical association with AID, is a critical regulator of CSR [101]. PTBP2 binds S region RNA transcripts and DNA, and targets AID to S regions [101]. PTBP2 is highly homologous with the well characterized PTBP1, a regulator of splicing that binds RNA polypyrimidine tracts, blocks spliceosome assembly and facilitates exon exclusion during alternative splicing [102,103]. CTNNBL1, a spliceosome associated factor, interacts with AID in vivo and in vitro and may have a partially redundant role in nuclear trafficking or retention of AID [104,105]. Although targeted deletion of CTNNBL1 has little effect on CSR [106] this may be due to other proteins which facilitate AID nuclear transport [107]. Together, the data suggest that Spt5 is a critical adaptor which anchors co-transcriptional factors to RNAP II and recruits AID to the transcriptional complex. It is intriguing to speculate that the I exon associated splice donor acts as an anchor for the co-transcriptional machinery and is required for appropriate association of Spt5 with RNAP II. These findings suggest a multilayered relationship between Spt5 and paused RNAP II and/or binding of co-transcriptional factors to enable recruitment of AID to S regions. However, this model must be considered incomplete since other factors modulate AID binding to S DNA including, KRAB domain-associated protein 1 (KAP1) [108] and the 14-3-3 scaffolding protein [109]. It is currently unclear whether KAP1 and 14-3-3 are related to RNAP II pausing or other co-transcriptional activities.
9. Cis-regulatory elements as recruiters for AID
Transcriptional elements are candidates for providing specificity for AID attack. Transcription of Ig H and L chain genes is driven by well characterized promoters and enhancers [110], how-ever, a specific role for each in SHM has been difficult to define. AID attack of Ig loci has been shown to occur in close proximity to either the V [3,19] and S region promoters [42]. Earlier studies showed that heterologous promoters functioned well for both SHM and CSR suggesting that these transcription elements are fungible [3,95]. Evidence also indicates that mutation frequency correlates with transcription rates in manipulated transgenes [3,19]. However, substitution of the endogenous Ig promoter with the highly transcribed elongation factor 1-a promoter leads to a reduction in SHM in the chicken DT40 B cell line indicating that not all promoters are equally capable of supporting SHM [111]. An enhancer has been identified within the DT40 IgL locus that confers mutability on heterologous genes and is distinct from the known transcriptional enhancer elements [112]. Targeting of AID activity to Ig loci may occur by species specific mechanisms since the murine IgLk transcriptional enhancer located in the context of the DT40 IgL locus supports transcription but not SHM [113].
Recently, the E-box motif, CAGCTC, a binding site for the transcription factor E2A, was shown to facilitate SHM in a GFP mutable transgenic substrate driven by a CMV promoter and the mouse Lk intronic enhancer [114]. These findings suggest that E-box motifs and E2A binding collaborate with the Ig enhancer to specify AID attack, although the method by which this occurs remains unclear. Intriguingly, E-box motifs are found at high density in S regions targeted for CSR and engage with E2A factors in vitro [115]. More work is required to clarify the involvement of E2A in AID targeting.
10. Concluding remarks
Models seeking to understand AID targeting must incorporate the observation that AID both focuses preferentially to Ig loci but also targets other non-Ig genes. Chromatin remodeling, histone modifications and accessibility in S regions are linked to transcriptionally active but paused RNAP II. AID may be preferentially recruited to the S region by means of several variables which in combination provide a unique context. RNAP II pausing in S DNA may be severe due to R-loop formation, which is an intrinsic feature of transcribed S regions. The expanse over which RNAP II pausing occurs is long, 1–10 kb, and may provide a platform for substantial Spt5 recruitment. AID may also be recruited by PTBP2, 14-3-3 and KAP1, which bind to transcribed S regions. The compounding effect of these individual events may provide the specificity required for AID targeting to S regions. Thus, non-Ig genes with several of the features characteristic of S regions may attract AID at lower but still detectable levels. The mechanism by which AID attacks V genes during SHM is unclear because V genes lack R-loops. It will be of great interest to determine whether RNAP II pausing occurs in V genes as a necessary feature of SHM.
Acknowledgments
This work was supported in part by the National Institutes of Health (AI052400 to A.L.K.). I thank Drs. I. Achour, S. Kumar and R. Wuerffel for the critical reading of this manuscript. The author has no competing financial interests.
References
- 1.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 2.Revy P, Muto T, Levy Y, Geissmann F, Plebani A, Sanal O, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the Hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 3.Peled JU, Kuang FL, Iglesias-Ussel MD, Roa S, Kalis SL, Goodman MF, et al. The biochemistry of somatic hypermutation. Annual Review of Immunology. 2008;26:481–511. doi: 10.1146/annurev.immunol.26.021607.090236. [DOI] [PubMed] [Google Scholar]
- 4.Neuberger MS, Di Noia JM, Beale RC, Williams GT, Yang Z, Rada C. Somatic hypermutation at A.T. pairs: polymerase error versus dUTP incorporation. Nature Reviews Immunology. 2005;5:171–178. doi: 10.1038/nri1553. [DOI] [PubMed] [Google Scholar]
- 5.Saribasak H, Rajagopal D, Maul RW, Gearhart PJ. Hijacked DNA repair proteins and unchained DNA polymerases. Philosophical Transactions of the Royal Society of London, Series B: Biological Sciences. 2009;364:605–611. doi: 10.1098/rstb.2008.0188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chaudhuri J, Basu U, Zarrin A, Yan C, Franco S, Perlot T, et al. Evolution of the immunoglobulin heavy chain class switch recombination mechanism. Advances in Immunology. 2007;94:157–214. doi: 10.1016/S0065-2776(06)94006-1. [DOI] [PubMed] [Google Scholar]
- 7.Teng G, Papavasiliou FN. Immunoglobulin somatic hypermutation. Annual Review of Genetics. 2007;41:107–120. doi: 10.1146/annurev.genet.41.110306.130340. [DOI] [PubMed] [Google Scholar]
- 8.Stavnezer J, Guikema JE, Schrader CE. Mechanism and regulation of class switch recombination. Annual Review of Immunology. 2008;26:261–292. doi: 10.1146/annurev.immunol.26.021607.090248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kenter AL. Class switch recombination: an emerging mechanism. Current Topics in Microbiology and Immunology. 2005;290:171–199. doi: 10.1007/3-540-26363-2_8. [DOI] [PubMed] [Google Scholar]
- 10.Stavnezer J. Complex regulation and function of activation-induced cytidine deaminase. Trends in Immunology. 2011;32:194–201. doi: 10.1016/j.it.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Di Noia JM, Neuberger MS. Molecular mechanisms of antibody somatic hypermutation. Annual Review of Biochemistry. 2007;76:1–22. doi: 10.1146/annurev.biochem.76.061705.090740. [DOI] [PubMed] [Google Scholar]
- 12.Peters A, Storb U. Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity. 1996;4:57–65. doi: 10.1016/s1074-7613(00)80298-8. [DOI] [PubMed] [Google Scholar]
- 13.Storb U, Shen HM, Longerich S, Ratnam S, Tanaka A, Bozek G, et al. Targeting of AID to immunoglobulin genes. Advances in Experimental Medicine and Biology. 2007;596:83–91. doi: 10.1007/0-387-46530-8_8. [DOI] [PubMed] [Google Scholar]
- 14.Goyenechea B, Klix N, Yelamos J, Williams GT, Riddell A, Neuberger MS, et al. Cells strongly expressing Ig(kappa) transgenes show clonal recruitment of hypermutation: a role for both MAR and the enhancers. EMBO Journal. 1997;16:3987–3994. doi: 10.1093/emboj/16.13.3987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tumas-Brundage K, Manser T. The transcriptional promoter regulates hypermutation of the antibody heavy chain locus. Journal of Experimental Medicine. 1997;185:239–250. doi: 10.1084/jem.185.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fukita Y, Jacobs H, Rajewsky K. Somatic hypermutation in the heavy chain locus correlates with transcription. Immunity. 1998;9:105–114. doi: 10.1016/s1074-7613(00)80592-0. [DOI] [PubMed] [Google Scholar]
- 17.Stavnezer J. Molecular processes that regulate class switching. Current Topics in Microbiology and Immunology. 2000;245:127–168. doi: 10.1007/978-3-642-59641-4_6. [DOI] [PubMed] [Google Scholar]
- 18.Manis JP, Tian M, Alt FW. Mechanism and control of class-switch recombination. Trends in Immunology. 2002;23:31–39. doi: 10.1016/s1471-4906(01)02111-1. [DOI] [PubMed] [Google Scholar]
- 19.Maul RW, Gearhart PJ. AID and somatic hypermutation. Advances in Immunology. 2010;105:159–191. doi: 10.1016/S0065-2776(10)05006-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shen HM, Peters A, Baron B, Zhu X, Storb U. Mutation of BCL-6 gene in normal B cells by the process of somatic hypermutation of Ig genes. Science. 1998;280:1750–1752. doi: 10.1126/science.280.5370.1750. [DOI] [PubMed] [Google Scholar]
- 21.Liu M, Duke JL, Richter DJ, Vinuesa CG, Goodnow CC, Kleinstein SH, et al. Two levels of protection for the B cell genome during somatic hypermutation. Nature. 2008;451:841–845. doi: 10.1038/nature06547. [DOI] [PubMed] [Google Scholar]
- 22.Staszewski O, Baker RE, Ucher AJ, Martier R, Stavnezer J, Guikema JE. Activation-induced cytidine deaminase induces reproducible DNA breaks at many non-Ig Loci in activated B cells. Molecular Cell. 2011;41:232–242. doi: 10.1016/j.molcel.2011.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martin A, Bardwell PD, Woo CJ, Fan M, Shulman MJ, Scharff MD. Activation-induced cytidine deaminase turns on somatic hypermutation in hybridomas. Nature. 2002;415:802–806. doi: 10.1038/nature714. [DOI] [PubMed] [Google Scholar]
- 24.Martin A, Scharff MD. Somatic hypermutation of the AID transgene in B and non-B cells. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12304–12308. doi: 10.1073/pnas.192442899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Okazaki IM, Hiai H, Kakazu N, Yamada S, Muramatsu M, Kinoshita K, et al. Constitutive expression of AID leads to tumorigenesis. Journal of Experimental Medicine. 2003;197:1173–1181. doi: 10.1084/jem.20030275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshikawa K, Okazaki IM, Eto T, Kinoshita K, Muramatsu M, Nagaoka H, et al. AID enzyme-induced hypermutation in an actively transcribed gene in fibroblasts. Science. 2002;296:2033–2036. doi: 10.1126/science.1071556. [DOI] [PubMed] [Google Scholar]
- 27.Rosenberg BR, Papavasiliou FN. Beyond SHM and CSR: AID and related cytidine deaminases in the host response to viral infection. Advances in Immunology. 2007;94:215–244. doi: 10.1016/S0065-2776(06)94007-3. [DOI] [PubMed] [Google Scholar]
- 28.Pasqualucci L, Neumeister P, Goossens T, Nanjangud G, Chaganti RS, Kuppers R, et al. Hypermutation of multiple proto-oncogenes in B-cell diffuse large-cell lymphomas. Nature. 2001;412:341–346. doi: 10.1038/35085588. [DOI] [PubMed] [Google Scholar]
- 29.Kuppers R, Dalla-Favera R. Mechanisms of chromosomal translocations in B cell lymphomas. Oncogene. 2001;20:5580–5594. doi: 10.1038/sj.onc.1204640. [DOI] [PubMed] [Google Scholar]
- 30.Takizawa M, Tolarova H, Li Z, Dubois W, Lim S, Callen E, et al. AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. Journal of Experimental Medicine. 2008;205:1949–1957. doi: 10.1084/jem.20081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, et al. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nature Medicine. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 32.Dekker J, Rippe K, Dekker M, Kleckner N. Capturing chromosome conformation. Science. 2002;295:1306–1311. doi: 10.1126/science.1067799. [DOI] [PubMed] [Google Scholar]
- 33.Miele A, Dekker J. Mapping cis- and trans-chromatin interaction networks using chromosome conformation capture (3C) Methods in Molecular Biology. 2009;464:105–121. doi: 10.1007/978-1-60327-461-6_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wuerffel R, Wang L, Grigera F, Manis J, Selsing E, Perlot T, et al. SS synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity. 2007;27:711–722. doi: 10.1016/j.immuni.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ju Z, Volpi SA, Hassan R, Martinez N, Giannini SL, Gold T, et al. Evidence for physical interaction between the immunoglobulin heavy chain variable region and the 3′ regulatory region. Journal of Biological Chemistry. 2007;282:35169–35178. doi: 10.1074/jbc.M705719200. [DOI] [PubMed] [Google Scholar]
- 36.Sellars M, Reina-San-Martin B, Kastner P, Chan S. Ikaros controls isotype selection during immunoglobulin class switch recombination. Journal of Experimental Medicine. 2009;206:1073–1087. doi: 10.1084/jem.20082311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manis JP, van der Stoep N, Tian M, Ferrini R, Davidson L, Bottaro A, et al. Class switching in B cells lacking 3′ immunoglobulin heavy chain enhancers. Journal of Experimental Medicine. 1998;188:1421–1431. doi: 10.1084/jem.188.8.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pinaud E, Khamlichi AA, Le Morvan C, Drouet M, Nalesso V, Le Bert M, et al. Localization of the 3′ IgH locus elements that effect long-distance regulation of class switch recombination. Immunity. 2001;15:187–199. doi: 10.1016/s1074-7613(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 39.Lebecque SG, Gearhart PJ. Boundaries of somatic mutation in rearranged immunoglobulin genes: 5′ boundary is near the promoter, and 3′ boundary is approximately 1 kb from V(D)J gene. Journal of Experimental Medicine. 1990;172:1717–1727. doi: 10.1084/jem.172.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rada C, Yelamos J, Dean W, Milstein C. The 5′ hypermutation boundary of kappa chains is independent of local and neighbouring sequences and related to the distance from the initiation of transcription. European Journal of Immunology. 1997;27:3115–3120. doi: 10.1002/eji.1830271206. [DOI] [PubMed] [Google Scholar]
- 41.Bachl J, Carlson C, Gray-Schopfer V, Dessing M, Olsson C. Increased transcription levels induce higher mutation rates in a hypermutating cell line. Journal of Immunology. 2001;166:5051–5057. doi: 10.4049/jimmunol.166.8.5051. [DOI] [PubMed] [Google Scholar]
- 42.Xue K, Rada C, Neuberger MS. The in vivo pattern of AID targeting to immunoglobulin switch regions deduced from mutation spectra in msh2−/− ung−/− mice. Journal of Experimental Medicine. 2006;203:2085–2094. doi: 10.1084/jem.20061067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nambu Y, Sugai M, Gonda H, Lee CG, Katakai T, Agata Y, et al. Transcription-coupled events associating with immunoglobulin switch region chromatin. Science. 2003;302:2137–2140. doi: 10.1126/science.1092481. [DOI] [PubMed] [Google Scholar]
- 44.Besmer E, Market E, Papavasiliou FN. The transcription elongation complex directs activation-induced cytidine deaminase-mediated DNA deamination. Molecular and Cellular Biology. 2006;26:4378–4385. doi: 10.1128/MCB.02375-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wuerffel RA, Du J, Thompson RJ, Kenter AL. Ig Sgamma3 DNA-specifc double strand breaks are induced in mitogen-activated B cells and are implicated in switch recombination. Journal of Immunology. 1997;159:4139–4144. [PubMed] [Google Scholar]
- 46.Schrader CE, Linehan EK, Mochegova SN, Woodland RT, Stavnezer J. Inducible DNA. breaks in Ig S regions are dependent on AID and UNG. Journal of Experimental Medicine. 2005;202:561–568. doi: 10.1084/jem.20050872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Woodcock CL, Dimitrov S. Higher-order structure of chromatin and chromosomes. Current Opinion in Genetics and Development. 2001;11:130–135. doi: 10.1016/s0959-437x(00)00169-6. [DOI] [PubMed] [Google Scholar]
- 48.Li B, Carey M, Workman JL. The role of chromatin during transcription. Cell. 2007;128:707–719. doi: 10.1016/j.cell.2007.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Suganuma T, Workman JL. Signals and combinatorial functions of histone modifications. Annual Review of Biochemistry. 2011;80:473–499. doi: 10.1146/annurev-biochem-061809-175347. [DOI] [PubMed] [Google Scholar]
- 50.Fuda NJ, Ardehali MB, Lis JT. Defining mechanisms that regulate RNA polymerase II transcription in vivo. Nature. 2009;461:186–192. doi: 10.1038/nature08449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belotserkovskaya R, Saunders A, Lis JT, Reinberg D. Transcription through chromatin: understanding a complex FACT. Biochimica et Biophysica Acta. 2004;1677:87–99. doi: 10.1016/j.bbaexp.2003.09.017. [DOI] [PubMed] [Google Scholar]
- 52.Orphanides G, Reinberg D. RNA polymerase II elongation through chromatin. Nature. 2000;407:471–475. doi: 10.1038/35035000. [DOI] [PubMed] [Google Scholar]
- 53.Saunders A, Core LJ, Lis JT. Breaking barriers to transcription elongation. Nature Reviews Molecular Cell Biology. 2006;7:557–567. doi: 10.1038/nrm1981. [DOI] [PubMed] [Google Scholar]
- 54.Henikoff S, Shilatifard A. Histone modification: cause or cog? Trends in Genetics. 2011;27:389–396. doi: 10.1016/j.tig.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 55.Bernstein BE, Kamal M, Lindblad-Toh K, Bekiranov S, Bailey DK, Huebert DJ, et al. Genomic maps and comparative analysis of histone modifications in human and mouse. Cell. 2005;120:169–181. doi: 10.1016/j.cell.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Pokholok DK, Harbison CT, Levine S, Cole M, Hannett NM, Lee TI, et al. Genome-wide map of nucleosome acetylation and methylation in yeast. Cell. 2005;122:517–527. doi: 10.1016/j.cell.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 57.Barski A, Cuddapah S, Cui K, Roh TY, Schones DE, Wang Z, et al. Highresolution profiling of histone methylations in the human genome. Cell. 2007;129:823–837. doi: 10.1016/j.cell.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 58.Guenther MG, Levine SS, Boyer LA, Jaenisch R, Young RA. A chromatin landmark and transcription initiation at most promoters in human cells. Cell. 2007;130:77–88. doi: 10.1016/j.cell.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Berger SL. The complex language of chromatin regulation during transcription. Nature. 2007;447:407–412. doi: 10.1038/nature05915. [DOI] [PubMed] [Google Scholar]
- 60.Shahbazian MD, Grunstein M. Functions of site-specific histone acetylation and deacetylation. Annual Review of Biochemistry. 2007;76:75–100. doi: 10.1146/annurev.biochem.76.052705.162114. [DOI] [PubMed] [Google Scholar]
- 61.Carrozza MJ, Li B, Florens L, Suganuma T, Swanson SK, Lee KK, et al. Histone H3 methylation by Set2 directs deacetylation of coding regions by Rpd3S to suppress spurious intragenic transcription. Cell. 2005;123:581–592. doi: 10.1016/j.cell.2005.10.023. [DOI] [PubMed] [Google Scholar]
- 62.Lieb JD, Clarke ND. Control of transcription through intragenic patterns of nucleosome composition. Cell. 2005;123:1187–1190. doi: 10.1016/j.cell.2005.12.010. [DOI] [PubMed] [Google Scholar]
- 63.Wang L, Wuerffel R, Feldman S, Khamlichi AA, Kenter AL. S region sequence, RNA polymerase II, and histone modifications create chromatin accessibility during class switch recombination. Journal of Experimental Medicine. 2009;206:1817–1830. doi: 10.1084/jem.20081678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang L, Whang N, Wuerffel R, Kenter AL. AID-dependent histone acetylation is detected in immunoglobulin S regions. Journal of Experimental Medicine. 2006;203:215–226. doi: 10.1084/jem.20051774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Li Z, Luo Z, Scharff MD. Differential regulation of histone acetylation and generation of mutations in switch regions is associated with Ig class switching. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:15428–15433. doi: 10.1073/pnas.0406827101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perlot T, Li G, Alt FW. Antisense transcripts from immunoglobulin heavy-chain locus V(D)J and switch regions. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:3843–3848. doi: 10.1073/pnas.0712291105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Daniel JA, Santos MA, Wang Z, Zang C, Schwab KR, Jankovic M, et al. PTIP promotes chromatin changes critical for immunoglobulin class switch recombination. Science. 2010;329:917–923. doi: 10.1126/science.1187942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Munoz IM, Rouse J. Control of histone methylation and genome stability by PTIP. EMBO Reports. 2009;10:239–245. doi: 10.1038/embor.2009.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoffmeister A, Ropolo A, Vasseur S, Mallo GV, Bodeker H, Ritz-Laser B, et al. The HMG-I/Y-related protein p8 binds to p300 and Pax2 trans-activation domain-interacting protein to regulate the trans-activation activity of the Pax2A and Pax2B transcription factors on the glucagon gene promoter. Journal of Biological Chemistry. 2002;277:22314–22319. doi: 10.1074/jbc.M201657200. [DOI] [PubMed] [Google Scholar]
- 70.Schwab KR, Patel SR, Dressler GR. Role of PTIP in class switch recombination and long-range chromatin interactions at the immunoglobulin heavy chain locus. Molecular and Cellular Biology. 2011;31:1503–1511. doi: 10.1128/MCB.00990-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gritzmacher CA. Molecular aspects of heavy-chain class switching. Critical Reviews in Immunology. 1989;9:173–200. [PubMed] [Google Scholar]
- 72.Hampsey M, Reinberg D. Tails of intrigue: phosphorylation of RNA polymerase II mediates histone methylation. Cell. 2003;113:429–432. doi: 10.1016/s0092-8674(03)00360-x. [DOI] [PubMed] [Google Scholar]
- 73.Rajagopal D, Maul RW, Ghosh A, Chakraborty T, Khamlichi AA, Sen R, et al. Immunoglobulin switch mu sequence causes RNA polymerase II accumulation and reduces dA hypermutation. Journal of Experimental Medicine. 2009;206:1237–1244. doi: 10.1084/jem.20082514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kruhlak M, Crouch EE, Orlov M, Montano C, Gorski SA, Nussenzweig A, et al. The ATM repair pathway inhibits RNA polymerase I transcription in response to chromosome breaks. Nature. 2007;447:730–734. doi: 10.1038/nature05842. [DOI] [PubMed] [Google Scholar]
- 75.Huang FT, Yu K, Balter BB, Selsing E, Oruc Z, Khamlichi AA, et al. Sequence dependence of chromosomal R-loops at the immunoglobulin heavy-chain Smu class switch region. Molecular and Cellular Biology. 2007;27:5921–5932. doi: 10.1128/MCB.00702-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yu K, Chedin F, Hsieh CL, Wilson TE, Lieber MR. R-loops at immunoglobulin class switch regions in the chromosomes of stimulated B cells. Nature Immunology. 2003;4:442–451. doi: 10.1038/ni919. [DOI] [PubMed] [Google Scholar]
- 77.Huertas P, Aguilera A. Cotranscriptionally formed DNA:RNA hybrids mediate transcription elongation impairment and transcription-associated recombination. Molecular Cell. 2003;12:711–721. doi: 10.1016/j.molcel.2003.08.010. [DOI] [PubMed] [Google Scholar]
- 78.Tous C, Aguilera A. Impairment of transcription elongation by R-loops in vitro. Biochemical and Biophysical Research Communications. 2007;360:428–432. doi: 10.1016/j.bbrc.2007.06.098. [DOI] [PubMed] [Google Scholar]
- 79.Sohail A, Klapacz J, Samaranayake M, Ullah A, Bhagwat AS. Human activation-induced cytidine deaminase causes transcription-dependent, strand-biased C to U deaminations. Nucleic Acids Research. 2003;31:2990–2994. doi: 10.1093/nar/gkg464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ramiro AR, Stavropoulos P, Jankovic M, Nussenzweig MC. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nature Immunology. 2003;4:452–456. doi: 10.1038/ni920. [DOI] [PubMed] [Google Scholar]
- 81.Dickerson SK, Market E, Besmer E, Papavasiliou FN. AID mediates hypermutation by deaminating single stranded DNA. Journal of Experimental Medicine. 2003;197:1291–1296. doi: 10.1084/jem.20030481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chaudhuri J, Tian M, Khuong C, Chua K, Pinaud E, Alt FW. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature. 2003;422:726–730. doi: 10.1038/nature01574. [DOI] [PubMed] [Google Scholar]
- 83.Shinkura R, Tian M, Smith M, Chua K, Fujiwara Y, Alt FW. The influence of transcriptional orientation on endogenous switch region function. Nature Immunology. 2003;4:435–441. doi: 10.1038/ni918. [DOI] [PubMed] [Google Scholar]
- 84.Vuong BQ, Lee M, Kabir S, Irimia C, Macchiarulo S, McKnight GS, et al. Specific recruitment of protein kinase A to the immunoglobulin locus regulates class-switch recombination. Nature Immunology. 2009;10:420–426. doi: 10.1038/ni.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gomez-Gonzalez B, Aguilera A. Activation-induced cytidine deaminase action is strongly stimulated by mutations of the THO complex. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8409–8414. doi: 10.1073/pnas.0702836104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Collins I, Weber A, Levens D. Transcriptional consequences of topoisomerase inhibition. Molecular and Cellular Biology. 2001;21:8437–8451. doi: 10.1128/MCB.21.24.8437-8451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shen HM, Storb U. Activation-induced cytidine deaminase (AID) can target both DNA strands when the DNA is supercoiled. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:12997–3002. doi: 10.1073/pnas.0404974101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Basu U, Meng FL, Keim C, Grinstein V, Pefanis E, Eccleston J, et al. The RNA exosome targets the AID cytidine deaminase to both strands of transcribed duplex DNA substrates. Cell. 2011;144:353–363. doi: 10.1016/j.cell.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andrulis ED, Werner J, Nazarian A, Erdjument-Bromage H, Tempst P, Lis JT. The RNA processing exosome is linked to elongating RNA polymerase II in Drosophila. Nature. 2002;420:837–841. doi: 10.1038/nature01181. [DOI] [PubMed] [Google Scholar]
- 90.Cheng HL, Vuong BQ, Basu U, Franklin A, Schwer B, Astarita J, et al. Integrity of the AID serine-38 phosphorylation site is critical for class switch recombination and somatic hypermutation in mice. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2717–2722. doi: 10.1073/pnas.0812304106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chaudhuri J, Khuong C, Alt FW. Replication protein A interacts with AID to promote deamination of somatic hypermutation targets. Nature. 2004;430:992–998. doi: 10.1038/nature02821. [DOI] [PubMed] [Google Scholar]
- 92.Pavri R, Nussenzweig MC. AID targeting in antibody diversity. Advances in Immunology. 2011;110:1–26. doi: 10.1016/B978-0-12-387663-8.00005-3. [DOI] [PubMed] [Google Scholar]
- 93.Rahl PB, Lin CY, Seila AC, Flynn RA, McCuine S, Burge CB, et al. c-Myc regulates transcriptional pause release. Cell. 2010;141:432–445. doi: 10.1016/j.cell.2010.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hein K, Lorenz MG, Siebenkotten G, Petry K, Christine R, Radbruch A. Processing of switch transcripts is required for targeting of antibody class switch recombination. Journal of Experimental Medicine. 1998;188:2369–2374. doi: 10.1084/jem.188.12.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lorenz M, Jung S, Radbruch A. Switch transcripts in immunoglobulin class switching. Science. 1995;267:1825–1828. doi: 10.1126/science.7892607. [DOI] [PubMed] [Google Scholar]
- 96.Brookes E, Pombo A. Modifications of RNA polymerase II are pivotal in regulating gene expression states. EMBO Reports. 2009;10:1213–1219. doi: 10.1038/embor.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Oesterreich FC, Bieberstein N, Neugebauer KM. Pause locally, splice globally. Trends in Cell Biology. 2011;21:328–335. doi: 10.1016/j.tcb.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 98.Pei Y, Shuman S. Interactions between fission yeast mRNA capping enzymes and elongation factor Spt5. Journal of Biological Chemistry. 2002;277:19639–19648. doi: 10.1074/jbc.M200015200. [DOI] [PubMed] [Google Scholar]
- 99.Wen Y, Shatkin AJ. Transcription elongation factor hSPT5 stimulates mRNA capping. Genes and Development. 1999;13:1774–1779. doi: 10.1101/gad.13.14.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Glover-Cutter K, Kim S, Espinosa J, Bentley DL. RNA polymerase II pauses and associates with pre-mRNA processing factors at both ends of genes. Nature Structural & Molecular Biology. 2008;15:71–78. doi: 10.1038/nsmb1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nowak U, Matthews AJ, Zheng S, Chaudhuri J. The splicing regulator PTBP2 interacts with the cytidine deaminase AID and promotes binding of AID to switch-region DNA. Nature Immunology. 2011;12:160–166. doi: 10.1038/ni.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma S. Isolation of a sequence-specific RNA binding protein, polypyrimidine tract binding protein, using RNA affinity chromatography. Methods in Molecular Biology. 2008;488:1–8. doi: 10.1007/978-1-60327-475-3_1. [DOI] [PubMed] [Google Scholar]
- 103.Sawicka K, Bushell M, Spriggs KA, Willis AE. Polypyrimidine-tract-binding protein: a multifunctional RNA-binding protein. Biochemical Society Transactions. 2008;36:641–647. doi: 10.1042/BST0360641. [DOI] [PubMed] [Google Scholar]
- 104.Conticello SG, Ganesh K, Xue K, Lu M, Rada C, Neuberger MS. Interaction between antibody-diversification enzyme AID and spliceosome-associated factor CTNNBL1. Molecular Cell. 2008;31:474–484. doi: 10.1016/j.molcel.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 105.Ganesh K, Adam S, Taylor B, Simpson P, Rada C, Neuberger M. CTNNBL1 is a novel nuclear localization sequence-binding protein that recognizes RNA-splicing factors CDC5L and Prp31. Journal of Biological Chemistry. 2011;286:17091–17102. doi: 10.1074/jbc.M110.208769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han L, Masani S, Yu K. Cutting edge: CTNNBL1 is dispensable for Ig class switch recombination. Journal of Immunology. 2010;185:1379–1381. doi: 10.4049/jimmunol.1001643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Maeda K, Singh SK, Eda K, Kitabatake M, Pham P, Goodman MF, et al. GANP-mediated recruitment of activation-induced cytidine deaminase to cell nuclei and to immunoglobulin variable region DNA. Journal of Biological Chemistry. 2010;285:23945–23953. doi: 10.1074/jbc.M110.131441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jeevan-Raj BP, Robert I, Heyer V, Page A, Wang JH, Cammas F, et al. Epigenetic tethering of AID to the donor switch region during immunoglobulin class switch recombination. Journal of Experimental Medicine. 2011;208:1649–1660. doi: 10.1084/jem.20110118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xu Z, Fulop Z, Wu G, Pone EJ, Zhang J, Mai T, et al. 14-3-3 adaptor proteins recruit AID to 5′-AGCT-3′-rich switch regions for class switch recombination. Nature Structural & Molecular Biology. 2010;17:1124–1135. doi: 10.1038/nsmb.1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Perlot T, Alt FW. Cis-regulatory elements and epigenetic changes control genomic rearrangements of the IgH locus. Advances in Immunology. 2008;99:1–32. doi: 10.1016/S0065-2776(08)00601-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Yang SY, Fugmann SD, Gramlich HS, Schatz DG. Activation-induced cytidine deaminase-mediated sequence diversification is transiently targeted to newly integrated DNA substrates. Journal of Biological Chemistry. 2007;282:25308–25313. doi: 10.1074/jbc.M704231200. [DOI] [PubMed] [Google Scholar]
- 112.Blagodatski A, Batrak V, Schmidl S, Schoetz U, Caldwell RB, Arakawa H, et al. A cis-acting diversification activator both necessary and sufficient for AID-mediated hypermutation. PLoS Genetics. 2009;5:e1000332. doi: 10.1371/journal.pgen.1000332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kothapalli NR, Norton DD, Fugmann SD. Classical Mus musculus Igkappa enhancers support transcription but not high level somatic hypermutation from a V-lambda promoter in chicken DT40 cells. PLoS One. 2011;6:e18955. doi: 10.1371/journal.pone.0018955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Tanaka A, Shen HM, Ratnam S, Kodgire P, Storb U. Attracting AID to targets of somatic hypermutation. Journal of Experimental Medicine. 2010;207:405–415. doi: 10.1084/jem.20090821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ma L, Hu B, Kenter AL. Ig S gamma-specific DNA binding protein SNAP is related to the helix-loop-helix transcription factor E47. International Immunology. 1997;9:1021–1029. doi: 10.1093/intimm/9.7.1021. [DOI] [PubMed] [Google Scholar]