Abstract
Purpose.
To investigate neuroprotective and axogenic properties of pigment epithelium-derived factor (PEDF) in retinal ganglion cells (RGC) in vitro and in vivo.
Methods.
Adult rat retinal cultures were treated with combinations of PBS and PEDF with or without a cell permeable analogue of cAMP, and RGC survival and neurite lengths quantified. The optic nerves of anesthetised rats were also crushed intraorbitally to transect all RGC axons followed by intravitreal injections of either PBS, PEDF, or cAMP+PEDF every 7 days. RGC were back filled with FluoroGold to quantify RGC survival and longitudinal optic nerve sections were stained with GAP43 antibodies to detect regenerating RGC axons.
Results.
An optimal dose of 2.5 × 10−5 μg/μL, promoted 65% more RGC survival than controls in vitro, increasing by 4.4- and 5-fold the number of RGC with neurites and the mean neurite length, respectively. Addition of cAMP with or without PEDF did not potentiate RGC survival or the mean number of RGC with neurites, but enhanced RGC neurite length by 1.4-fold, compared with PEDF alone. After optic nerve crush (ONC), PEDF protected RGC from apoptosis and increased the numbers of regenerating RGC axons in the optic nerve by 4.6- and 3.4-fold, respectively when compared with controls. cAMP did not enhance PEDF-induced RGC neuroprotection, but potentiated its neuroregenerative effects by 2- to 3-fold, increasing the number of RGC axons regenerating at 500 and 1000 μm from the lesions site.
Conclusions.
This study is the first to demonstrate that PEDF enhances both RGC survival and axon regeneration in vitro and in vivo.
Keywords: PEDF, retinal ganglion cells, neuroprotection, axon regeneration, optic nerve
Pigment epithelium-derived factor promotes retinal ganglion cell survival and neurite outgrowth/axon regeneration in vitro and in vivo.
Introduction
Retinal ganglion cells (RGC) rapidly die after axotomy, but are protected by combinatorial treatments with neurotrophic factors (NTF), including brain-derived NTF (BDNF), neurotrophin-3/4 (NT-3/4), ciliary neurotrophic factor (CNTF), glial cell line–derived neurotrophic (GDNF), and basic fibroblast growth factor (FGF2).1–7 NTF combinations also activate intrinsic axon growth signalling pathways that initiate RGC axon regeneration.1–7 Pigment epithelium-derived factor (PEDF) is a 50 kDa NTF glycoprotein belonging to the serpin superfamily, that protects a wide range of central nervous system (CNS) neurons.8–15 Although first isolated from fetal human retinal pigment (choroid) epithelial cells, like many other NTF, PEDF is expressed in a wide variety of tissues including brain, spinal cord, skeletal muscle, heart, endothelial cells, and osteoblasts.16–20 PEDF has an array of unique properties including neuroprotective, anti-angiogenic, anti-inflammatory, anti-oxidative, and antitumorigenic activities.21–27
PEDF is a multifaceted NTF that is active in a variety of ocular disorders including diabetic retinopathy and ischemic degeneration.13,21,28,29 Although major sources of PEDF are choroid, ciliary body, and corneal epithelium, constitutive expression is also detected in RGC and photoreceptors.30–36 The upregulation of endogenous PEDF in activated Müller cells and astrocytes may become an RGC neuroprotective source of PEDF during the early stages of degenerative conditions.13,34,35,37 Although the mechanisms underlying PEDF-mediated neuroprotection remain unclear, PEDF does promote the survival of photoreceptors and RGC in many retinal pathologies.34,38–41
In this present study, we evaluated, in vitro and in vivo, the RGC neuroprotective and axogenic properties of PEDF and compared the results with the effects of CNTF, a neurotrophic factor with well documented RGC survival and neurite/axon growth promoting properties.42–46 We also studied the effects of combining PEDF with cAMP (which raises the intrinsic ability of RGC to grow their neurites and axons),47–50 predicting that PEDF-induced RGC axon regeneration would be enhanced by cAMP. We confirm that PEDF is RGC neuroprotective after axotomy and also promotes RGC neuritogenesis/axon regeneration both in vitro and in vivo. cAMP+PEDF did not enhance PEDF-induced RGC neuroprotection, but did enhance RGC axon regeneration when compared with PEDF treatment alone. Our results imply that PEDF, with or without cAMP, is a promising novel neuroprotective/axogenic therapy applicable to a wide range of ocular disorders.
Materials and Methods
Experimental Design: In Vitro Experiments
For all in vitro experiments, 12 adult female Sprague-Dawley rats were used and experimental conditions included retinal cells treated with: (1) Neurobasal-A (NBA) medium alone (Control), (2) 1 × 10−5 μg/μL PEDF (Peprotech, London, UK), (3) 2.5 × 10−5 μg/μL PEDF, (4) 5 × 10−5 μg/μL PEDF, (5) 1 × 10−4 μg/μL PEDF, (6) 2.5 × 10−5 μg/μL PEDF+Chlorophenylthio adenosine cyclic monophosphate (CPT-cAMP [referred to as cAMP from here on]; Sigma, Poole, UK), and (7) 2.5 × 10−5 μg/μL CNTF (our ‘gold standard' RGC neuroprotective/axogenic factor; Peprotech).2,49 All experiments were performed in triplicate and on three independent occasions.
Adult Retinal Cultures
Neuronal enriched, mixed adult rat retinal cultures were prepared from 12, 6- to 8-week-old adult female Sprague Dawley rats, as described by us previously.49,51,52 Briefly, retinal cells were dissociated from dissected retinae using a Papain dissociation kit following the manufacturer's instructions (Worthington Biochemical, Lakewood, NJ). Retinal cells (125 × 103/well) were plated in 8-well chamber slides precoated with poly-D-lysine and laminin and cultured in NBA supplemented with B27 supplement and gentimicin (all from Invitrogen, Paisley, UK). Cells were incubated for 4 days at 37°C and 5% CO2, and cultured for 4 days before fixation in 4% paraformaldehyde for immunocytochemistry as described by us previously.49,51–53
Immunocytochemistry of Retinal Cultures
Fixed cells were washed in three changes of PBS before permeabilisation and blocking in PBS containing 3% BSA and 0.1% Triton X-100. Cells were then washed three times in PBS, incubated with monoclonal anti-βIII tubulin (1:200 dilution; Sigma) to localise RGC and their neurites for 1 hour at room temperature, washed three times in PBS, incubated with Alexa 488 anti-Mouse Immunoglobulin G (IgG; 1:400 dilution; Invitrogen) for 1 hour at room temperature, washed three times in PBS and mounted using Vectamount containing DAPI (Vector Laboratories, Peterborough, UK). Cells were viewed under an epi-fluorescent microscope (Zeiss Axioplan 2; Zeiss, Hertfordshire, UK). Immunocytochemistry controls, with primary antibody omitted, did not stain, and were used to set the background threshold levels of nonspecific staining (not shown), prior to image capture.
RGC Neurite Outgrowth and Survival
The mean number of surviving βIII-tubulin+ RGC, RGC with neuritis, and the mean RGC neurite length were quantified as described by us previously.49,51,52 Briefly, each chamber slide was anonymised and partitioned into nine quadrants and images of RGC and their neurites captured randomly from each quadrant. Axiovision image analysis software (Axiovision, version 4.8; Zeiss) was used to measure neurite lengths and ImagePro (Version 6.3; Media Cybernetics, Bethesda, MD) was used to quantify the number of βIII-tubulin+ RGC with neurites and the total number of DAPI+ cells. Neurite outgrowth of at least 180 RGC was measured from nine different wells/treatment, except for control (NBA) cultures, in which a total of 108 RGC were measured (i.e., all RGC that grew neurites).
Optic Nerve Crush (ONC)
Animal procedures were licensed and approved by the United Kindom Home Office and the University of Birmingham ethical review committee. The optic nerves (ON) of anesthetised adult female 200 to 250 g Sprague-Dawley rats were exposed surgically through a supraorbital approach and crushed bilaterally 2 mm from the lamina cribrosa, using calibrated watchmaker's forceps as described by us previously.51–57
Experimental Design: In Vivo Experiments
For the in vivo experiments, a total of 102 adult female Sprague-Dawley rats were used. In the preliminary PEDF neuroprotection dose-finding experiments, groups comprised six rats/treatment (i.e., 12 eyes/treatment): (1) Intact, (2) ONC+vehicle (PBS; 0 μg/μL PEDF), (3) 0.2 μg/μL PEDF, (4) 0.5 μg/μL PEDF, (5) 1 μg/μL PEDF, (6) 1.5 μg/μL PEDF, and (7) 2 μg/μL of PEDF dissolved in 5 μL of sterile saline. In further experiments, six rats/treatment (i.e., 12 eyes/treatment) were used for the FluroGold (FG) backfilling of RGC experiments, while a separate six rats/treatment (i.e., 12 eyes and ON/treatment) were used for growth associated protein-43 (GAP43) immunostaining in the ON and glial fibrillary acidic protein (GFAP)/PEDF immunostaining in the retina. In these experiments, treatment groups comprised: (1) Intact, (2) ONC+PBS, (3) ONC+cAMP, (4) ONC+PEDF, and (5) ONC+cAMP+PEDF.
Intravitreal Injections
PEDF (Peprotech) was dissolved in sterile PBS and injected intravitreally in a final volume of 5 μl. In a preliminary experiment, the eyes of animals (n = 6 rats/treatment; 12 eyes/treatment) were bilaterally injected intravitreally with either PBS, or 0.2, 0.5, 1, 1.5, or 2 μg/μL PEDF dissolved in 5 μL of sterile saline immediately after ONC (0 days) and intravitreal injections were repeated at 7 and 14 days after ONC with the same dose of PEDF. PEDF was injected every 7 days based on our previous experiments showing that a single intravitreal injection of BDNF at 0 days sustained near 100% RGC survival for 7 days after ONC.53 Animals were allowed to survive for 21 days and killed by raising the levels of CO2. Retinae were whole mounted for immunohistochemistry as described in the relevant sections below. None of the animals developed cataracts, confirming that the lens had not been injured either during surgery or after repeated intravitreal injections. These experiments established that 5 μL of 1 μg/μL PEDF was the optimal dose for significant RGC neuroprotection.
The definitive experiments comprised rats treated with 5 μL intravitreal injections of PBS (vehicle control), 0.25 μg/μL cAMP,58 1 μg/μL PEDF, and 0.25 μg/μL cAMP+1 μg/μL PEDF groups (n = 6 rats/treatment [i.e., 12 eyes/ON/group]) immediately after ONC at day 0 and repeated at day 7 and 14 with the same doses of PEDF/PBS control.
Retinal Whole Mounts
At 19 days after ONC, 2 μL of 4% FG (Cambridge Bioscience, Cambridge, UK), was injected into the ON, between the lamina cribrosa and the site of ONC in PBS controls and experimental groups to avoid widespread diffusion of FG and entrapment within lesion sites, while intact controls were injected 2 mm from the lamina cribrosa since these animals did not possess a lesion.52,53 Animals were killed 2 days later by overdose of CO2, and retinae immersion-fixed for 2 hours in 4% formaldehyde (TAAB Laboratories, Aldermaston, UK), flattened onto Superfrost Plus microscope slides (VWR International, Lutterworth, UK) by making four equidistal radial cuts to obtain four equally sized quadrants, attached together around the optic disc. Retinal whole mounts were air dried and mounted in Vectamount (Vector Laboratories). Retinae were randomised and photographs were captured at ×200 magnification using a Zeiss epi-fluorescent microscope (Zeiss Axioplan 2) equipped with a digital camera (Axiocam HRc) in Axiovision 4 (all from Zeiss). The number of FG-labelled RGC were then counted blind using ImagePro Version 6.0 (Media Cybernetics) from captured images of 12 rectangular areas (0.36 × 0.24 mm), 3 from each quadrant, placed at radial distances from the centre of the optic disc of the inner (1/6 eccentricity), midperiphery (1/2 eccentricity), and outer retina (5/6 eccentricity), as described by us previously.53 The number of FG-labelled cells in the 12 images were divided by the area of the counting region and pooled together to calculate mean densities of FG-labelled RGC/mm2 for each retina.59
Tissue Preparation and Sectioning
Animals were killed with an overdose of CO2 and perfused intracardially with 4% formaldehyde, eyes and ON were immersion-fixed in 4% formaldehyde (TAAB Laboratories) for 2 hours, washed for 10 minutes in PBS, immersed in 10% and 20% sucrose (Sigma), each for 1 hour, and finally immersed in 30% sucrose overnight. Cryoprotected eyes and ON were embedded in optimal cutting temperature (OCT) compound (Raymond A Lamb Ltd., Eastbourne, UK) and stored at −80°C until required. Subsequently, 15-μm thick sections of eyes in the plane through the optic disc and longitudinal sections of ON were cut on a cryostat (Bright Instruments, Huntingdon, UK), adhered onto glass slides, and stored at −20°C until required.
Immunohistochemistry of ON and Retinae
Immunohistochemistry was performed as described by us previously.52,53 Briefly, sections were washed in PBS and nonspecific binding blocked in 3% bovine serum albumin (BSA) in PBS, containing 0.1% Triton X-100 for 20 minutes before incubation with primary antibodies diluted in PBS containing 3% BSA and 0.05% Tween 20, overnight at 4°C (16–18 hours). A polyclonal goat antihuman PEDF antibody (R&D Systems, Oxford, UK), monoclonal mouse anti-GFAP and monoclonal mouse anti–βIII-tubulin (both from Sigma) were all used at 1:500 dilution in the retina to stain for PEDF, glia, and RGC, respectively. Regenerating axons were stained with a monoclonal anti-GAP43 antibody (Invitrogen) at 1:500 dilution. Immunohistochemistry controls included sections treated as above except that primary antibodies were omitted, which showed an absence of positive staining (not shown). Sections were then washed three times in PBS, incubated with appropriate Alexa Fluor 488 or Texas Red-labelled secondary antibodies (1:400 dilution; Invitrogen) for 1 hour at room temperature, washed, and mounted using Vectashield mounting medium containing DAPI (Vector Laboratories), examined under an Axioplan-2 epi-fluorescent microscope, and photomicrographs captured using Axiovision Software (all from Zeiss). Negative control sections were used to set the background threshold levels of nonspecific staining, prior to image capture.
Quantification of Axon Regeneration
The number of regenerating GAP43+ axons was counted at ×400 magnification in ON sections after drawing a vertical line through the axons and counting the number of axons extending beyond this line, using previously published methods.60,61 Briefly an observer, blinded to the identity of each sample, counted the number of GAP43+ axons at 250, 500, 1000, 1500, 2000, 2500 μm distal to the lesion site in four longitudinal sections of each nerve (n = 6 rats/12 ON/treatment). The diameter of the nerve at each counting distance was also measured using Axiovision Software (Zeiss) and the number of axons per millimeter of nerve width calculated and averaged over the sections and the total number of axons (Σad) extending distances d, in an ON of radius r estimated by summing over all sections with a thickness (t) of 15 μm using the following formula:
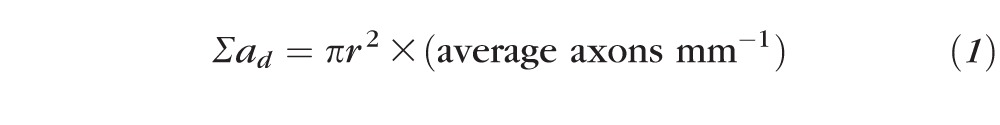 |
Statistical Analysis
Unless otherwise stated, 6 rats (i.e., 12 eyes/ON) were used for each in vivo experiment. For in vitro experiments, each condition was tested in triplicate and repeated on three independent occasions. For all experiments, sample means and SEM were calculated and tested for significance by one-way ANOVA, followed by Bonferroni's multiple comparison test to compare differences using GraphPad Prism software (GraphPad Software Inc., San Diego, CA).
Results
In Vitro Experiments
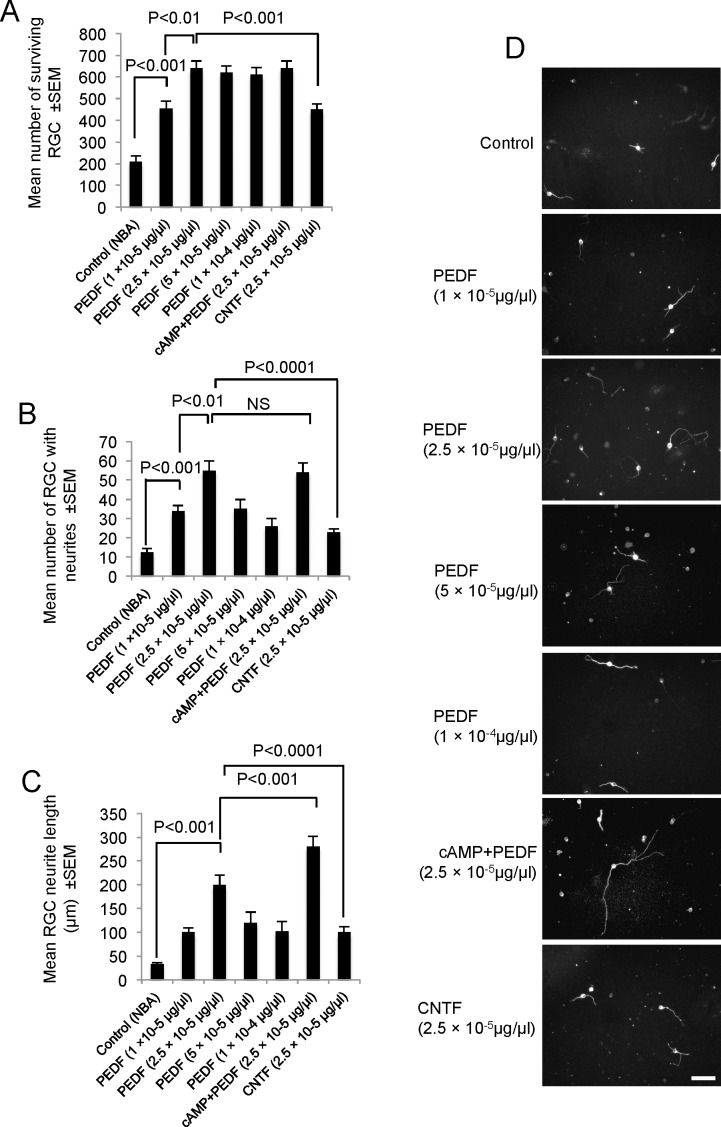
PEDF Promoted RGC Survival and Neurite Outgrowth In Vitro After 4 Days in Culture.
At doses of 1 × 10−5 μg/μL and 2.5 × 10−5 μg/μL, PEDF increased RGC survival from 210 ± 27 cells in controls to 455 ± 34 cells and 640 ± 33 cells, respectively (P < 0.0001; Fig. 1A). A dose of 2.5 × 10−5 μg/μL PEDF was optimal for RGC survival in vitro since increasing concentrations did not improve RGC survival (Fig. 1). Comparing the effects of 2.5 × 10−5 μg/μL PEDF with a similar dose of CNTF, the former promoted higher (640 ± 33 cells) RGC survival than the latter (450 ± 33 cells; P < 0.001), that is increased RGC survival with 2.5 × 10−5 μg/μL PEDF and CNTF was 65% and 30%, respectively, compared with controls. The dose of 2.5 × 10−5 μg/μL CNTF induced a similar level of RGC protection as that observed with the lowest concentration of PEDF used in our study (Fig. 1A). Combining cAMP with 2.5 × 10−5 μg/μL PEDF did not increase RGC survival beyond that observed with PEDF alone (Fig. 1A).
Figure 1.
RGC survival and neurite outgrowth after addition of PEDF with or without cAMP in vitro. (A) RGC survival with increasing concentrations of PEDF and CNTF. (B) The number of RGC with neurites, (C) the mean neurite length, and (D) representative images of βIII-tubulin+ RGC to demonstrate neurite outgrowth. NS, not significant; scale bar: 50 μm (D).
Compared with control cultures, treatment of retinal cell cultures with 2.5 × 10−5 μg/μL PEDF, increased the number of RGC with neurites from 12 ± 3 cells to 55 ± 5 cells and the mean neurite length from 34 ± 3 μm to 200 ± 20 μm (Figs. 1C, 1D; P < 0.001). The optimal concentration of 2.5 × 10−5 μg/μL PEDF was also more RGC neuritogenic than an equivalent concentration of CNTF, which showed that the mean number of RGC with neurites was 23 ± 3 cells (Fig. 1B) with a mean neurite length of 100 ± 12 μm (Figs. 1C, 1D; P < 0.001). Treatment with cAMP+PEDF had no effect on the number of RGC with neurites (Fig. 1B), but did increase mean neurite length to 280 ± 22 μm (Figs. 1C, 1D) compared with PEDF alone. In summary, 2.5 × 10−5 μg/μL of PEDF was a more potent promoter of RGC survival and neurite outgrowth in vitro compared with the equivalent concentrations of CNTF. Furthermore, cAMP+PEDF neither enhanced RGC survival nor the mean number of RGC with neuritis, but did increase mean RGC neurite length.
In Vivo Experiments
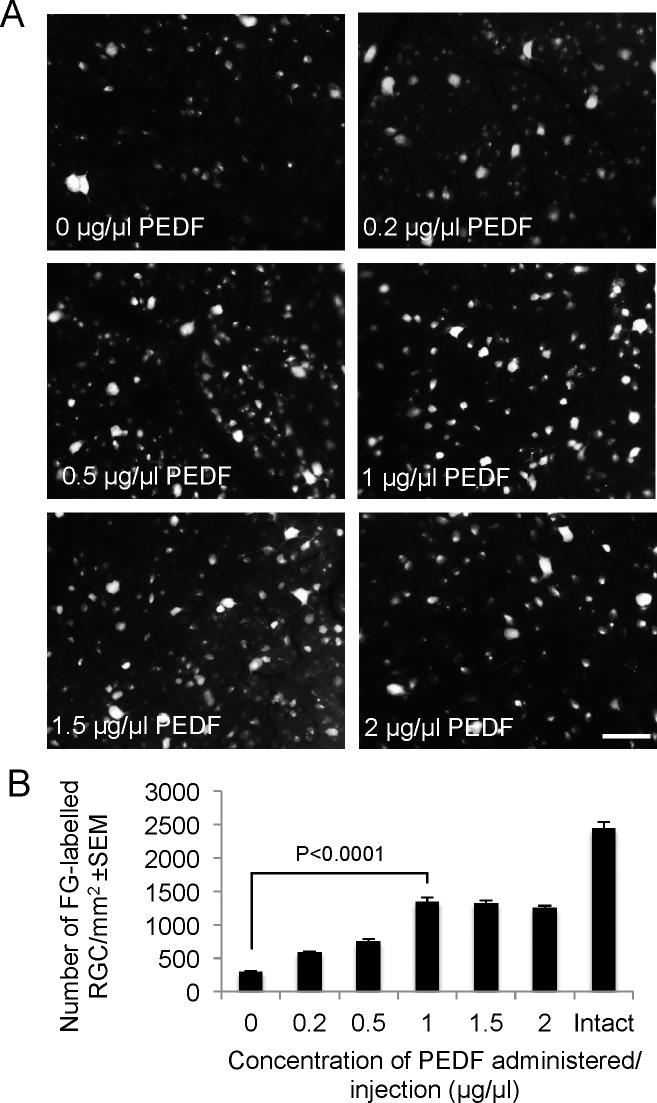
Determination of Optimal RGC Neuroprotective Dose of PEDF 21 Days After ONC.
After intravitreal injection of 5 μL PBS, 292 ± 12 FG+ RGC/mm2 survived 21 days after ONC. Doses of 0.2, 0.5, and 1 μg/μL PEDF caused a dose-dependent increase in the number of FG+ RGC, achieving maximal survival of 1345 ± 63 RGC/mm2 using 1 μg/μL PEDF (Figs. 2A, 2B; P < 0.0001). RGC survival was not improved by increasing the dose of PEDF to 1.5 and 2 μg/μL (Figs. 2A, 2B). These results show that the optimal dose of 1 μg/μL PEDF promoted 55% RGC survival compared with intact controls.
Figure 2.
PEDF-mediated RGC neuroprotection is dose-dependent in vivo. (A) Representative images show FG-labelled RGC after intravitreal injection of 0, 0.2, 0.5, 1, 1.5, and 2 μg/μL of PEDF at 21 days after ONC. (B) The number of FG+ RGC at the different doses of PEDF and in intact controls. Scale bar: 50 μm (A).
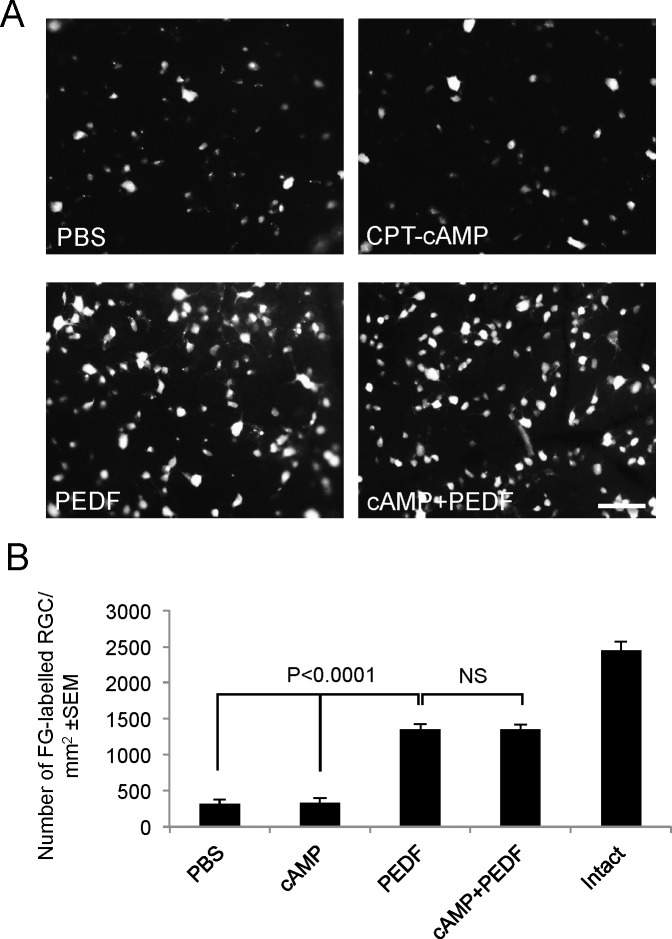
Intravitreal Injection of 1 μg/μL of PEDF Combined With 0.25 μg/μL cAMP Did Not Improve RGC Survival Over PEDF Alone 21 Days After ONC.
In PBS vehicle- and cAMP-treated (0.25 μg/μL) animals, 353 ± 38 (14% of intact) and 360 ± 55 (14% of intact) FG+ RGC/mm2, respectively, remained at 21 days after ONC (Figs. 3A, 3B). After 1 μg/μL PEDF and cAMP+PEDF treatment, 55% of intact FG+ RGC/mm2 (1350 ± 48 and 1348 ± 32 RGC/mm2, respectively) survived ONC at 21 days (Figs. 3A, 3B). Thus, the combination of cAMP+PEDF did not improve RGC survival beyond that afforded by PEDF alone (i.e., 55% RGC neuroprotection compared with intact controls).
Figure 3.
Combining the optimal dose of PEDF with cAMP enhances RGC protection after ONC. (A) Representative images of FG+ RGC in PBS-, cAMP-, PEDF-, and cAMP+PEDF-treated retinae. (B) The number of FG+ RGC/mm2 in the treatment control groups. NS, not significant; scale bar: 50 μm (A).
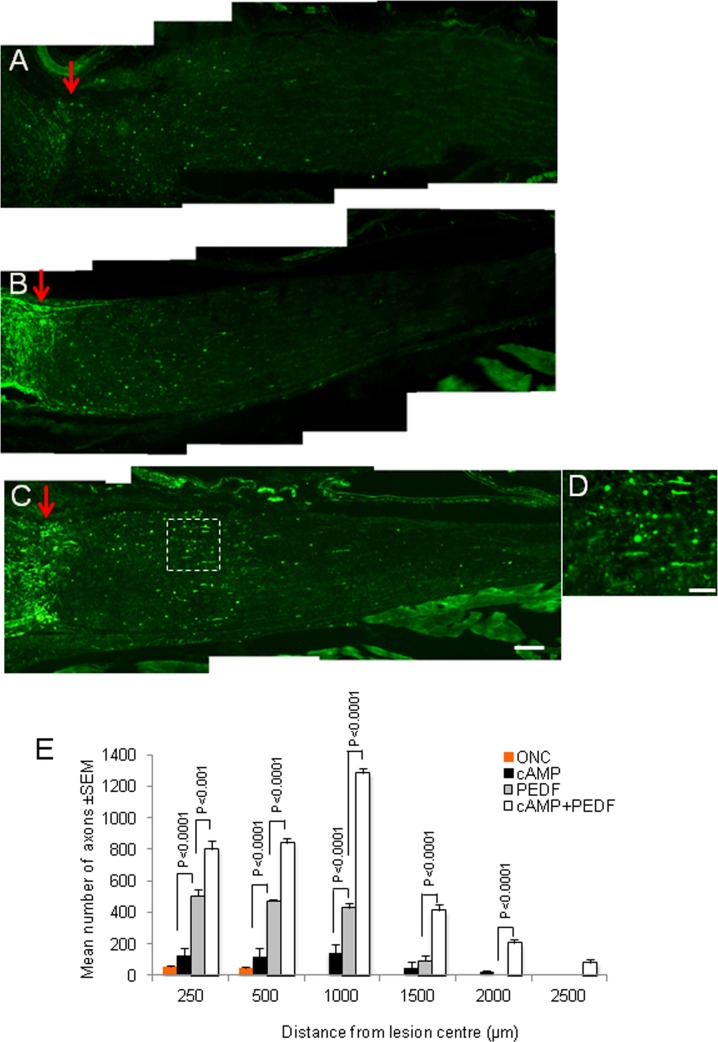
PEDF Promoted RGC Axon Regeneration.
After ONC, small numbers of RGC axons remained in the proximal ON stump and few regenerated across the lesion site into the distal ON after both intravitreal injection of PBS (not shown) and cAMP (maximum of 145 ± 54 axons 500 μm distal to the lesion site) (Figs. 4A, 4E). By contrast after injecting 1 μg/μL PEDF intravitreally, many GAP43+ axons had regenerated 500 μm into the distal ON (P < 0.0001) and some had extended 1500 μm from the lesion site (Figs. 4B, 4E). After cAMP+PEDF treatment (0.25 μg/μL cAMP+1 μg/μL PEDF), the numbers of GAP43+ axons in the proximal ON stump was similar to those observed in the PEDF-treated animals, although 840 ± 32, 1283 ± 33, and 415 ± 33 axons had regenerated into the distal ON over distances of 500, 1000, and 1500 μm, respectively (Figs. 4C, 4D [high power of boxed region in (C)], 4E), with a few extending to 1500 and 2500 μm (Figs. 4C, 4E). These results demonstrate that PEDF promoted RGC axon regeneration; a response that was potentiated by the addition of cAMP.
Figure 4.
PEDF promotes RGC axon regeneration at 21 days after ONC. Representative GAP43+ immunohistochemistry after (A) cAMP-, (B) PEDF-, and (C) cAMP+PEDF-treatment (red arrow demarcates lesion site; scale bar: 200 μm). (D) High power image of boxed region in (C). (E) The mean number of axons present at different distances from the lesion center.
Glial and RGC Expression of PEDF in the Retina.
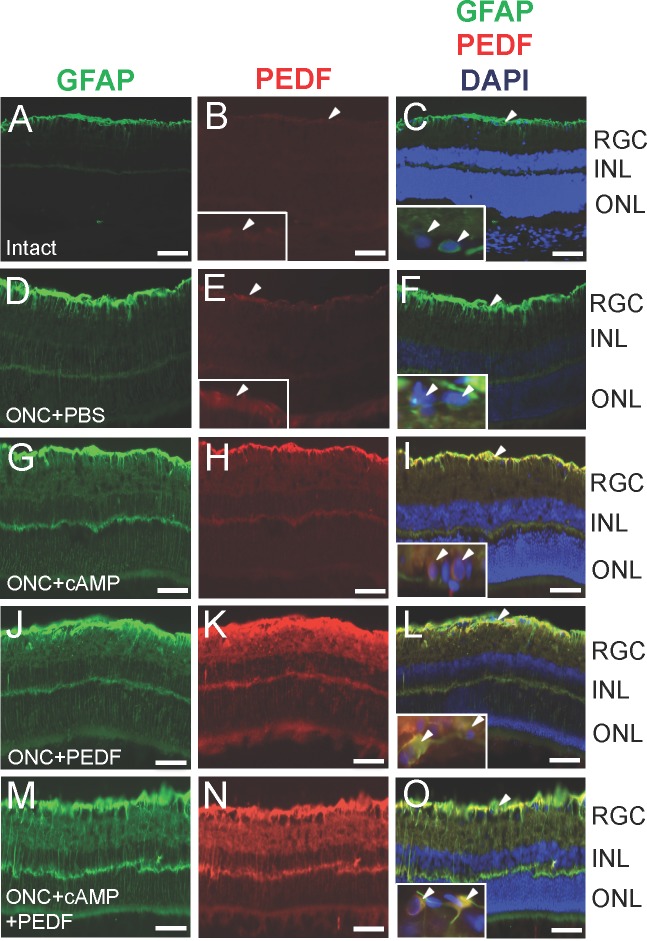
In the nerve fibre layer of intact retinae, astrocytes were faintly GFAP+ and there was little or no PEDF immunoreactivity (Figs. 5A–C, inset Fig. 5B). After ONC and intravitreal injection of PBS, GFAP+ astrocytes became activated in the nerve fibre layer and GFAP+ Müller cell processes traversed the inner plexiform layer. PEDF staining was increased in astrocytes, compared with intact controls (Figs. 5D–F, inset Fig. 5E). GFAP immunoreactivity was increased in the nerve fibre layer and in Müller cell processes after ONC+cAMP treatment and strong PEDF immunoreactivity that colocalized to GFAP+ astrocyte and Müller cells (Figs. 5G–I). In ONC+PEDF (Figs. 5J–L) and ONC+cAMP+PEDF (Figs. 5M–O) groups, PEDF immunostaining was substantially enhanced in the nerve fibre layer and the inner and outer plexiform layers and colocalized in GFAP+ astrocytes, Müller cell end feet, and radial processes that spanned the entire retina.
Figure 5.
PEDF localises to GFAP+ astrocytes and Müller cells. Double immunohistochemistry for GFAP (green) and PEDF (red) and merged images with DAPI counterstain to show GFAP immunolocalisation in cells of the retina in (A–C) intact controls and after (D–F) ONC, (G–I) ONC+cAMP, (J–L) ONC+PEDF, and (M–O) ONC+cAMP+PEDF. Insets show high power images of areas pointed to by arrowheads in the low power images, demonstrating PEDF localisation to RGC (arrowheads). Scale bar: 50 μm; inset scale bars: 20 μm.
In intact and ONC+PBS groups, no immunoreactivity for PEDF was observed in βIII-tubulin+ RGC (Figs. 5C, inset; 5F). However after intravitreal injection of CPT-cAMP, occasional βIII-tubulin+ RGC expressed PEDF (Fig. 5I, inset) and PEDF (Figs. 5L, inset; 5O, inset). These results show that PEDF is expressed in GFAP+ astrocytes/Müller cells and occasional RGC after PEDF injection with and without co-incident elevation of cAMP levels in the injured eye.
Discussion
In retinal cultures, we confirmed the original observations of Unterlauft et al.34 and Pang et al.36 that PEDF had RGC neuroprotective properties, but was surprisingly more neuroprotective than equivalent concentrations of CNTF. We also observed that PEDF in vivo was more neuroprotective than CNTF.45,62,63 PEDF can protect RGC from death after glutamate toxicity, ischemia, trophic factor withdrawal, and in mouse models of glaucoma and inherited retinal degeneration.35,38,64–67 In vitro and in vivo, optimal RGC neuroprotective dosages of PEDF were 2.5 × 10−5 μg/μL and 1 μg/μL, respectively. Others report that in vivo, 0.001 μg/μL is required to protect RGC from excitotoxicity and serum withdrawal,36 a concentration of PEDF that is also neuroprotective for photoreceptors, cerebellar granule cells, and hippocampal neurons.9,38,68 After ON transection in vivo, 1 μg/μL intravitreal PEDF and combined cAMP+PEDF treatment promoted 55% RGC survival, similar to the levels of neuroprotection seen after grafting peripheral nerves onto the cut end of the ON, combined with intraocular injections of cAMP+CNTF.69
Our results suggest that PEDF alone may trigger neuroprotective pathways activated by combined cAMP+CNTF, since combined cAMP+PEDF did not potentiate RGC protection and CNTF delivery on its own did not rescue more than 43% of RGC from death.44,62,63 Exogenous PEDF may promote RGC neuroprotection by down regulating the apoptotic genes caspase-2, calpain-1, and MAPK kinase-1 after binding to one or more high affinity PEDF receptors, and stimulating downstream phospholipase A2 enzymatic activity.12 Although PEDF signalling has not been fully elucidated, inhibition of both nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) and ERK1/2 pathways abolishes the protective effects of PEDF in cultured RGC.12,30,36,70 For example, activation of the NFκB pathway induces the expression of BDNF, NGF, Bcl-2, Bcl-x, and superoxide dismutase,71,72 but PEDF-activated ERK1/2 modulates kinases, phosphatases, transcription factors, and regulators of apoptosis.73,74
RGC neuritogenesis/axogenesis were stimulated by PEDF and, after combined cAMP+PEDF treatments, neurites/axons grew for greater distances than after PEDF alone. CNTF+cAMP also promotes longer RGC neurite outgrowth than CNTF alone.68 Thus, although CNTF and PEDF act singly as initiators and elongators of neurite outgrowth,75,76 their effects become synergistic when each factor is administered in combination with cAMP. Both extrinsic and intrinsic mechanisms regulate axon sprouting and elongation and are often signalled by the same molecules. Most NTF activate ras-raf-MAP kinase (MAPK) and PI3-kinase-Akt (PI3K) signalling pathways that contribute to axon outgrowth, including CNTF and PEDF.77 When apoptosis is inhibited in neurons by Bax knockout or over expression of Bcl-2, inhibition of either MAPK or PI3K partially reduces axon outgrowth while, inhibiting both together, completely suppresses elongation.78 Others have reported that stimulating either PI3K or Akt increased axon thickness and branching, although blocking raf, PI3K or Akt retards elongation.79 Thus, like other NTF, PEDF is likely to activate the above pathways and stimulate the initiation of axon sprouting and sustain elongation, although it is unclear if axogenesis is promoted by the direct effects of PEDF on neurons or indirectly through glia.
It is widely accepted that different signalling pathways subserve neuron survival and axogenesis since both responses can be separated. For example, NGF administered to either the cell body or axon in separate Campenot chamber compartments of cultured sympathetic neurons supports survival and axon growth, but only the latter when applied to somata.80 Similarly, application of NTF to RGC somata in vivo promotes both RGC survival and axon regeneration51,54,55,57 and both intravitreal and intra-ON inflammation are RGC neuroprotective, but only the former promotes axon regeneration,52 suggesting that a key RGC axon regeneration stimulatory factor is absent from the transected ON and exclusively secreted by retinal cells.
PEDF is constitutively expressed in pigment, ciliary, and corneal epithelial cells, and also in Müller glia, retinal astrocytes, and RGC.12,22,34,81,82 The increased PEDF immunoreactivity of retinal cells after intravitreal PEDF/cAMP+PEDF injections is probably explained by both cellular uptake of exogenous PEDF, and by increased autocrine-induced PEDF transcription in RGC, astrocytes and Müller cells potentiated by raised retinal levels of cAMP. Secreted retinal glia-derived PEDF may also supplement endogenous RGC titres to promote RGC neuroprotection and axon regeneration after ON transection. PEDF may, thus, function both directly and indirectly in the retina as a Müller cell-derived RGC axogenic factor after ON transection and also protect RGC by suppressing caspase activity, preserving glutamine synthetase levels, preventing the accumulation of reactive oxygen species and counteracting the detrimental effects of gliosis.71–74,83–85
Other commonly used RGC neuroprotective factors include BDNF, GDNF, NT-3, NT-4/5, TrkB gene transfer, as well as caspase inhibitors.45,53,59,75–80,83–86 Although we showed that BDNF administration in our ONC model supported nearly 100% survival of RGC at 7 days,53 long-term studies with BDNF peptide or gene transfer showed that BDNF supported approximately 60% of RGC survival at 2 weeks after ON transection.77,78 Most neuroprotective strategies including other NTF and caspase inhibitors commonly support 60% of RGC survival at 2 weeks.45,76,83,84 However, combinations of neurotrophic agents such as GDNF+BDNF and BDNF+TrkB support nearly 80% RGC from death at 2 weeks after ON transection suggesting that combinations of neurotrophic factors may support synergistic RGC protection.77 Despite this optimism, very few of these strategies promote RGC axon regeneration. Therefore, the reported effects of PEDF in this current study on RGC protection and axon regeneration demonstrate the potential of PEDF in future therapeutics in disease of the eye and injuries that affect RGC death and axon integrity.
In conclusion, we confirm previous reports that PEDF promoted RGC survival in vitro and in vivo but demonstrate for the first time that PEDF also promotes RGC neurite outgrowth/axon regeneration. In addition, combining PEDF with cAMP enhanced RGC axon regeneration but did not promote RGC survival. The neuroprotective properties of PEDF were also more potent than those of CNTF both in vitro and in vivo. Our findings suggest that PEDF is a potential RGC neuroprotective and axogenic therapeutic for optic neuropathies and diseases such as glaucoma in which RGC death occurs.
Acknowledgments
Supported by Wellcome Trust Grant 092539/Z/10/Z (ZA).
Disclosure: V. Vigneswara, None; M. Berry, None; A. Logan, None; Z. Ahmed, None
References
- 1. Yip HK, So KF. Axonal regeneration of retinal ganglion cells: effect of trophic factors. Prog Retin Eye Res. 2000; 19: 559–575 [DOI] [PubMed] [Google Scholar]
- 2. Logan A, Ahmed Z, Baird A, Gonzalez AM, Berry M. Neurotrophic factor synergy is required for neuronal survival and disinhibited axon regeneration after CNS injury. Brain. 2006; 129: 490–502 [DOI] [PubMed] [Google Scholar]
- 3. Watanabe M, Tokita Y, Kato M, Fukuda Y. Intravitreal injections of neurotrophic factors and forskolin enhance survival and axonal regeneration of axotomized beta ganglion cells in cat retina. Neuroscience. 2003; 116: 733–742 [DOI] [PubMed] [Google Scholar]
- 4. Cui Q, So KF, Yip HK. Major biological effects of neurotrophic factors on retinal ganglion cells in mammals. Biol Signals Recept. 1998; 7: 220–226 [DOI] [PubMed] [Google Scholar]
- 5. Song DL, Zhong Y. Research advancement of optic nerve injury therapy by transgenic expression neurotrophic factors [in Chinese]. Zhonghua Yan Ke Za Zhi. 2008; 44: 858–861 [PubMed] [Google Scholar]
- 6. Hellstrom M, Harvey AR. Retinal ganglion cell gene therapy and visual system repair. Curr Gene Ther. 2011; 11: 116–131 [DOI] [PubMed] [Google Scholar]
- 7. Benowitz LI, Yin Y. Combinatorial treatments for promoting axon regeneration in the CNS: strategies for overcoming inhibitory signals and activating neurons' intrinsic growth state. Dev Neurobiol. 2007; 67: 1148–1165 [DOI] [PubMed] [Google Scholar]
- 8. Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc Natl Acad Sci U S A. 1993; 90: 1526–1530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Taniwaki T, Becerra SP, Chader GJ, Schwartz JP. Pigment epithelium-derived factor is a survival factor for cerebellar granule cells in culture. J Neurochem. 1995; 64: 2509–2517 [DOI] [PubMed] [Google Scholar]
- 10. Bilak MM, Corse AM, Bilak SR, Lehar M, Tombran-Tink J, Kuncl RW. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. J Neuropathol Exp Neurol. 1999; 58: 719–728 [DOI] [PubMed] [Google Scholar]
- 11. Houenou LJ, D'Costa AP, Li L, et al. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. J Comp Neurol. 1999; 412: 506–514 [PubMed] [Google Scholar]
- 12. Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nat Rev Neurosci. 2003; 4: 628–636 [DOI] [PubMed] [Google Scholar]
- 13. Li H, Tran VV, Hu Y. Mark Saltzman W, Barnstable CJ, Tombran-Tink J. A PEDF N-terminal peptide protects the retina from ischemic injury when delivered in PLGA nanospheres. Exp Eye Res. 2006; 83: 824–833 [DOI] [PubMed] [Google Scholar]
- 14. Sanagi T, Yabe T, Yamada H. Gene transfer of PEDF attenuates ischemic brain damage in the rat middle cerebral artery occlusion model. J Neurochem. 2008; 106: 1841–1854 [DOI] [PubMed] [Google Scholar]
- 15. Sanagi T, Yabe T, Yamada H. Adenoviral gene delivery of pigment epithelium-derived factor protects striatal neurons from quinolinic acid-induced excitotoxicity. J Neuropathol Exp Neurol. 2010; 69: 224–233 [DOI] [PubMed] [Google Scholar]
- 16. Tombran-Tink J, Mazuruk K, Rodriguez IR, et al. Organization, evolutionary conservation, expression and unusual Alu density of the human gene for pigment epithelium-derived factor, a unique neurotrophic serpin. Mol Vis. 1996; 2: 11 [PubMed] [Google Scholar]
- 17. Yabe T, Sanagi T, Yamada H. The neuroprotective role of PEDF: implication for the therapy of neurological disorders. Curr Mol Med. 2010; 10: 259–266 [DOI] [PubMed] [Google Scholar]
- 18. Longeras R, Farjo K, Ihnat M, Ma JXA. PEDF-derived peptide inhibits retinal neovascularization and blocks mobilization of bone marrow-derived endothelial progenitor cells. Exp Diabetes Res. 2012; 2012: 518426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Rychli K, Kaun C, Hohensinner PJ, et al. The anti-angiogenic factor PEDF is present in the human heart and is regulated by anoxia in cardiac myocytes and fibroblasts. J Cell Mol Med. 2010; 14: 198–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Akiyama T, Dass CR, Shinoda Y, Kawano H, Tanaka S, Choong PF. PEDF regulates osteoclasts via osteoprotegerin and RANKL. Biochem Biophys Res Commun. 2010; 391: 789–794 [DOI] [PubMed] [Google Scholar]
- 21. Zhu XF, Zou HD. PEDF in diabetic retinopathy: a protective effect of oxidative stress. J Biomed Biotechnol. 2012; 2012: 580687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dawson DW, Volpert OV, Gillis P, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999; 285: 245–248 [DOI] [PubMed] [Google Scholar]
- 23. Duh EJ, Yang HS, Suzuma I, et al. Pigment epithelium-derived factor suppresses ischemia-induced retinal neovascularization and VEGF-induced migration and growth. Invest Ophthalmol Vis Sci. 2002; 43: 821–829 [PubMed] [Google Scholar]
- 24. Zhang SX, Wang JJ, Gao G, Shao C, Mott R, Ma JX. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006; 20: 323–325 [DOI] [PubMed] [Google Scholar]
- 25. Cao W, Tombran-Tink J, Chen W, Mrazek D, Elias R, McGinnis JF. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J Neurosci Res. 1999; 57: 789–800 [PubMed] [Google Scholar]
- 26. Ho TC, Chen SL, Shih SC, et al. Pigment epithelium-derived factor (PEDF) promotes tumor cell death by inducing macrophage membrane tumor necrosis factor-related apoptosis-inducing ligand (TRAIL). J Biol Chem. 2011; 286: 35943–35954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Feng CC, Wang PH, Ding Q, et al. Expression of pigment epithelium-derived factor and tumor necrosis factor-alpha is correlated in bladder tumor and is related to tumor angiogenesis. Urol Oncol. 2013; 31: 241–246 [DOI] [PubMed] [Google Scholar]
- 28. Ramirez-Castillejo C, Sanchez-Sanchez F, Andreu-Agullo C, et al. Pigment epithelium-derived factor is a niche signal for neural stem cell renewal. Nat Neurosci. 2006; 9: 331–339 [DOI] [PubMed] [Google Scholar]
- 29. Ma X, Pan L, Jin X, et al. Microphthalmia-associated transcription factor acts through PEDF to regulate RPE cell migration. Exp Cell Res. 2012; 318: 251–261 [DOI] [PubMed] [Google Scholar]
- 30. Barnstable CJ, Tombran-Tink J. Neuroprotective and antiangiogenic actions of PEDF in the eye: molecular targets and therapeutic potential. Prog Retin Eye Res. 2004; 23: 561–577 [DOI] [PubMed] [Google Scholar]
- 31. Tombran-Tink J, Barnstable CJ. Therapeutic prospects for PEDF: more than a promising angiogenesis inhibitor. Trends Mol Med. 2003; 9: 244–250 [DOI] [PubMed] [Google Scholar]
- 32. Ogata N, Wada M, Otsuji T, Jo N, Tombran-Tink J, Matsumura M. Expression of pigment epithelium-derived factor in normal adult rat eye and experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002; 43: 1168–1175 [PubMed] [Google Scholar]
- 33. Karakousis PC, John SK, Behling KC, et al. Localization of pigment epithelium derived factor (PEDF) in developing and adult human ocular tissues. Mol Vis. 2001; 7: 154–163 [PubMed] [Google Scholar]
- 34. Unterlauft JD, Eichler W, Kuhne K, et al. Pigment epithelium-derived factor released by Müller glial cells exerts neuroprotective effects on retinal ganglion cells. Neurochem Res. 2012; 37: 1524–1533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lange J, Yafai Y, Reichenbach A, Wiedemann P, Eichler W. Regulation of pigment epithelium-derived factor production and release by retinal glial (Müller) cells under hypoxia. Invest Ophthalmol Vis Sci. 2008; 49: 5161–5167 [DOI] [PubMed] [Google Scholar]
- 36. Pang IH, Zeng H, Fleenor DL, Clark AF. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci. 2007; 8: 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Yang XM, Yafai Y, Wiedemann P, et al. Hypoxia-induced upregulation of pigment epithelium-derived factor by retinal glial (Müller) cells. J Neurosci Res. 2012; 90: 257–266 [DOI] [PubMed] [Google Scholar]
- 38. Cao W, Tombran-Tink J, Elias R, Sezate S, Mrazek D, McGinnis JF. In vivo protection of photoreceptors from light damage by pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2001; 42: 1646–1652 [PubMed] [Google Scholar]
- 39. Takita H, Yoneya S, Gehlbach PL, Duh EJ, Wei LL, Mori K. Retinal neuroprotection against ischemic injury mediated by intraocular gene transfer of pigment epithelium-derived factor. Invest Ophthalmol Vis Sci. 2003; 44: 4497–4504 [DOI] [PubMed] [Google Scholar]
- 40. Miyazaki M, Ikeda Y, Yonemitsu Y, et al. Pigment epithelium-derived factor gene therapy targeting retinal ganglion cell injuries: neuroprotection against loss of function in two animal models. Hum Gene Ther. 2011; 22: 559–565 [DOI] [PubMed] [Google Scholar]
- 41. Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000; 20: 7149–7157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mey J, Thanos S. Intravitreal injections of neurotrophic factors support the survival of axotomized retinal ganglion cells in adult rats in vivo. Brain Res. 1993; 602: 304–317 [DOI] [PubMed] [Google Scholar]
- 43. Cui Q, Harvey AR. CNTF promotes the regrowth of retinal ganglion cell axons into murine peripheral nerve grafts. Neuroreport. 2000; 11: 3999–4002 [DOI] [PubMed] [Google Scholar]
- 44. Hu Y, Leaver SG, Plant GW, et al. Lentiviral-mediated transfer of CNTF to schwann cells within reconstructed peripheral nerve grafts enhances adult retinal ganglion cell survival and axonal regeneration. Mol Ther. 2005; 11: 906–915 [DOI] [PubMed] [Google Scholar]
- 45. Monnier PP, D'Onofrio PM, Magharious M, et al. Involvement of caspase-6 and caspase-8 in neuronal apoptosis and the regenerative failure of injured retinal ganglion cells. J Neurosci. 2011; 31: 10494–10505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Leaver SG, Cui Q, Plant GW, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006; 13: 1328–1341 [DOI] [PubMed] [Google Scholar]
- 47. Kurimoto T, Yin Y, Omura K, et al. Long-distance axon regeneration in the mature optic nerve: contributions of oncomodulin, cAMP, and pten gene deletion. J Neurosci. 2010; 30: 15654–15663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Lima S, Koriyama Y, Kurimoto T, et al. Full-length axon regeneration in the adult mouse optic nerve and partial recovery of simple visual behaviors. Proc Natl Acad Sci U S A. 2012; 109: 9149–9154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ahmed Z, Berry M, Logan A. ROCK inhibition promotes adult retinal ganglion cell neurite outgrowth only in the presence of growth promoting factors. Mol Cell Neurosci. 2009; 42: 128–133 [DOI] [PubMed] [Google Scholar]
- 50. Park KK, Hu Y, Muhling J, et al. Cytokine-induced SOCS expression is inhibited by cAMP analogue: impact on regeneration in injured retina. Mole Cell Neurosci. 2009; 41: 313–324 [DOI] [PubMed] [Google Scholar]
- 51. Ahmed Z, Suggate EL, Brown ER, et al. Schwann cell-derived factor-induced modulation of the NgR/p75NTR/EGFR axis disinhibits axon growth through CNS myelin in vivo and in vitro. Brain. 2006; 129: 1517–1533 [DOI] [PubMed] [Google Scholar]
- 52. Ahmed Z, Aslam M, Lorber B, Suggate EL, Berry M, Logan A. Optic nerve and vitreal inflammation are both RGC neuroprotective but only the latter is RGC axogenic. Neurobiol Dis. 2010; 37: 441–454 [DOI] [PubMed] [Google Scholar]
- 53. Ahmed Z, Kalinski H, Berry M, et al. Ocular neuroprotection by siRNA targeting caspase-2. Cell Death Dis. 2011; 2: e173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Berry M, Carlile J, Hunter A. Peripheral nerve explants grafted into the vitreous body of the eye promote the regeneration of retinal ganglion cell axons severed in the optic nerve. J Neurocytol. 1996; 25: 147–170 [DOI] [PubMed] [Google Scholar]
- 55. Berry M, Carlile J, Hunter A, Tsang W, Rosenstiel P, Sievers J. Optic nerve regeneration after intravitreal peripheral nerve implants: trajectories of axons regrowing through the optic chiasm into the optic tracts. J Neurocytol. 1999; 28: 721–741 [DOI] [PubMed] [Google Scholar]
- 56. Ahmed Z, Dent RG, Leadbeater WE, Smith C, Berry M, Logan A. Matrix metalloproteases: degradation of the inhibitory environment of the transected optic nerve and the scar by regenerating axons. Mol Cell Neurosci. 2005; 28: 64–78 [DOI] [PubMed] [Google Scholar]
- 57. Douglas MR, Morrison KC, Jacques SJ, et al. Off-target effects of epidermal growth factor receptor antagonists mediate retinal ganglion cell disinhibited axon growth. Brain. 2009; 132: 3102–3121 [DOI] [PubMed] [Google Scholar]
- 58. Crowder RJ, Freeman RS. The survival of sympathetic neurons promoted by potassium depolarization, but not by cyclic AMP, requires phosphatidylinositol 3-kinase and Akt. J Neurochem. 1999; 73: 466–475 [PubMed] [Google Scholar]
- 59. Peinado-Ramon P, Salvador M, Villegas-Perez MP, Vidal-Sanz M. Effects of axotomy and intraocular administration of NT-4, NT-3, and brain-derived neurotrophic factor on the survival of adult rat retinal ganglion cells. A quantitative in vivo study. Invest Ophthalmol Vis Sci. 1996; 37: 489–500 [PubMed] [Google Scholar]
- 60. Leon S, Yin Y, Nguyen J, Irwin N, Benowitz LI. Lens injury stimulates axon regeneration in the mature rat optic nerve. J Neurosci. 2000; 20: 4615–4626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lorber B, Howe ML, Benowitz LI, Irwin N. Mst3b, an Ste20-like kinase, regulates axon regeneration in mature CNS and PNS pathways. Nat Neurosci. 2009; 12: 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Weise J, Isenmann S, Klocker N, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000; 7: 212–223 [DOI] [PubMed] [Google Scholar]
- 63. Zhang CW, Lu Q, You SW, et al. CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci. 2005; 46: 1497–1503 [DOI] [PubMed] [Google Scholar]
- 64. Cayouette M, Smith SB, Becerra SP, Gravel C. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol Dis. 1999; 6: 523–532 [DOI] [PubMed] [Google Scholar]
- 65. Yafai Y, Lange J, Wiedemann P, Reichenbach A, Eichler W. Pigment epithelium-derived factor acts as an opponent of growth-stimulatory factors in retinal glial-endothelial cell interactions. Glia. 2007; 55: 642–651 [DOI] [PubMed] [Google Scholar]
- 66. Tsao YP, Ho TC, Chen SL, Cheng HC. Pigment epithelium-derived factor inhibits oxidative stress-induced cell death by activation of extracellular signal-regulated kinases in cultured retinal pigment epithelial cells. Life Sci. 2006; 79: 545–550 [DOI] [PubMed] [Google Scholar]
- 67. Park K, Jin J, Hu Y, Zhou K, Ma JX. Overexpression of pigment epithelium-derived factor inhibits retinal inflammation and neovascularization. Am J Pathol. 2011; 178: 688–698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. DeCoster MA, Schabelman E, Tombran-Tink J, Bazan NG. Neuroprotection by pigment epithelial-derived factor against glutamate toxicity in developing primary hippocampal neurons. J Neurosci Res. 1999; 56: 604–610 [DOI] [PubMed] [Google Scholar]
- 69. Cui Q, Yip HK, Zhao RC, So KF, Harvey AR. Intraocular elevation of cyclic AMP potentiates ciliary neurotrophic factor-induced regeneration of adult rat retinal ganglion cell axons. Mol Cell Neurosci. 2003; 22: 49–61 [DOI] [PubMed] [Google Scholar]
- 70. Tombran-Tink J. The neuroprotective and angiogenesis inhibitory serpin, PEDF: new insights into phylogeny, function, and signaling. Front Bioscie. 2005; 10: 2131–2149 [DOI] [PubMed] [Google Scholar]
- 71. Courtois G. The NF-kappaB signaling pathway in human genetic diseases. Cell Mole Life Sci. 2005; 62: 1682–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wu JT, Kral JG. The NF-kappaB/IkappaB signaling system: a molecular target in breast cancer therapy. J Surg Res. 2005; 123: 158–169 [DOI] [PubMed] [Google Scholar]
- 73. Yoon S, Seger R. The extracellular signal-regulated kinase: multiple substrates regulate diverse cellular functions. Growth Factors. 2006; 24: 21–44 [DOI] [PubMed] [Google Scholar]
- 74. Rubinfeld H, Seger R. The ERK cascade: a prototype of MAPK signaling. Mol Biol. 2005; 31: 151–174 [DOI] [PubMed] [Google Scholar]
- 75. Zhi Y, Lu Q, Zhang CW, Yip HK, So KF, Cui Q. Different optic nerve injury sites result in different responses of retinal ganglion cells to brain-derived neurotrophic factor but not neurotrophin-4/5. Brain Res. 2005; 1047: 224–232 [DOI] [PubMed] [Google Scholar]
- 76. Zhang CW, Lu Q, You SW, et al. CNTF and BDNF have similar effects on retinal ganglion cell survival but differential effects on nitric oxide synthase expression soon after optic nerve injury. Invest Ophthalmol Vis Sci. 2005; 46: 1497–1503 [DOI] [PubMed] [Google Scholar]
- 77. Koeberle PD, Ball AK. Neurturin enhances the survival of axotomized retinal ganglion cells in vivo: combined effects with glial cell line-derived neurotrophic factor and brain-derived neurotrophic factor. Neuroscience. 2002; 110: 555–567 [DOI] [PubMed] [Google Scholar]
- 78. Mo X, Yokoyama A, Oshitari T, et al. Rescue of axotomized retinal ganglion cells by BDNF gene electroporation in adult rats. Invest Ophthalmol Vis Sci. 2002; 43: 2401–2405 [PubMed] [Google Scholar]
- 79. Kurokawa T, Katai N, Shibuki H, et al. BDNF diminishes caspase-2 but not c-Jun immunoreactivity of neurons in retinal ganglion cell layer after transient ischemia. Invest Ophthalmol Vis Sci. 1999; 40: 3006–3011 [PubMed] [Google Scholar]
- 80. Cheng L, Sapieha P, Kittlerova P, Hauswirth WW, Di Polo A. TrkB gene transfer protects retinal ganglion cells from axotomy-induced death in vivo. J Neurosci. 2002; 22: 3977–3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Behling KC, Surace EM, Bennett J. Pigment epithelium-derived factor expression in the developing mouse eye. Mol Vis. 2002; 8: 449–454 [PubMed] [Google Scholar]
- 82. Eichler W, Yafai Y, Keller T, Wiedemann P, Reichenbach A. PEDF derived from glial Muller cells: a possible regulator of retinal angiogenesis. Exp Cell Res. 2004; 299: 68–78 [DOI] [PubMed] [Google Scholar]
- 83. Kermer P, Klocker N, Labes M, Bahr M. Inhibition of CPP32-like proteases rescues axotomized retinal ganglion cells from secondary cell death in vivo. J Neurosci. 1998; 18: 4656–4662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Vigneswara V, Berry M, Logan A, Ahmed Z. Pharmacological inhibition of caspase-2 protects axotomised retinal ganglion cells from apoptosis in adult rats. PloS One. 2012; 7: e53473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Parrilla-Reverter G, Agudo M, Sobrado-Calvo P, Salinas-Navarro M, Villegas-Perez MP, Vidal-Sanz M. Effects of different neurotrophic factors on the survival of retinal ganglion cells after a complete intraorbital nerve crush injury: a quantitative in vivo study. Exp Eye Res. 2009; 89: 32–41 [DOI] [PubMed] [Google Scholar]
- 86. Lucius R, Sievers J. YVAD protect post-natal retinal ganglion cells against axotomy-induced but not free radical-induced axonal degeneration in vitro. Brain Res Mol Brain Res. 1997; 48: 181–184 [DOI] [PubMed] [Google Scholar]