Abstract
Purpose.
We demonstrated that eyes of young animals of various species (chick, tree shrew, marmoset, and rhesus macaque) can shorten in the axial dimension in response to myopic defocus.
Methods.
Chicks wore positive or negative lenses over one eye for 3 days. Tree shrews were measured during recovery from induced myopia after 5 days of monocular deprivation for 1 to 9 days. Marmosets were measured during recovery from induced myopia after monocular deprivation, or wearing negative lenses over one or both eyes, or from wearing positive lenses over one or both eyes. Rhesus macaques were measured after recovery from induced myopia after monocular deprivation, or wearing negative lenses over one or both eyes. Axial length was measured with ultrasound biometry in all species.
Results.
Tree shrew eyes showed a strong trend to shorten axially to compensate for myopic defocus. Of 34 eyes that recovered from deprivation-induced myopia for various durations, 30 eyes (88%) shortened, whereas only 7 fellow eyes shortened. In chicks, eyes wearing positive lenses reduced their rate of ocular elongation by two-thirds, including 38.5% of eyes in which the axial length became shorter than before. Evidence of axial shortening in rhesus macaque (40%) and marmoset (6%) eyes also occurred when exposed to myopic defocus, although much less frequently than that in eyes of tree shrews. The axial shortening was caused mostly by the reduction in vitreous chamber depth.
Conclusions.
Eyes of chick, tree shrew, marmoset, and rhesus macaque can shorten axially when presented with myopic defocus, whether the myopic defocus is created by wearing positive lenses, or is the result of axial elongation of the eye produced by prior negative lens wear or deprivation. This eye shortening facilitates compensation for the imposed myopia. Implications for human myopia control are significant.
Keywords: emmetropization, myopia, hyperopia, ocular length, chick, tree shrew, marmoset, rhesus macaque
We have shown that, in 4 different animal models of refractive development, eyes shorten axially to compensate for myopic defocus. This has potentially important implications for control of myopia in the growing eye of children.
Introduction
Many animal studies have shown that eyes can compensate for imposed defocus by changing choroidal thickness and the rate of ocular elongation, above or below that found in normal untreated growing eyes. For instance, when wearing a positive lens that puts the focal plane in front of the photoreceptors, the eye decreases its rate of ocular elongation and increases choroidal thickness, thereby pushing the retina forward to meet the focal plane; the opposite happens in the case of wearing a negative lens. Among the various animal species used, chick eyes have been shown to be able to compensate for the widest range of defocus.1
It usually is assumed that, when eyes compensate for myopic defocus imposed by positive lenses, their rate of ocular elongation is reduced, so the eye either elongates at a slower rate than normal or, at the most, stops its growth. Even though it seems more natural that an eye in a growing animal should elongate rather than actually shorten (reduced length from the front of the cornea to the back of the sclera), there seems no obvious reason why an eye experiencing myopic defocus cannot axially shorten or shrink through a mechanism, such as extracellular matrix remodeling of the sclera, thereby further facilitating compensation. Given that tissues are remodeled continuously under homeostatic control, we ask why should axial shortening or shrinkage be more implausible than elongation or enlargement.
Previous studies have shown that organ size can fluctuate drastically under physiologic conditions. In Burmese pythons, which typically feed once every a couple of months, the heart, lungs, liver, intestinal mucosa, and kidneys all alternate between a large and a small size. After a large meal, the increase in mass of these organs ranges between 50% and 150% (as percentage of fasted mass).2 In many seasonally breeding birds, the gonads can shrink by 87% when the day length decreases from 13 to 12 hours (e.g., spotted antbirds3). If other organs can fluctuate in size, perhaps eyes as well can shrink when needed. In this study, we demonstrate that eyes of chick, tree shrew, marmoset, and rhesus macaque also can shorten axially in response to myopic defocus when wearing positive lenses, or recovering from wearing negative lenses or form deprivation, by summarizing earlier data from four independent laboratories of Josh Wallman, Neville A. McBrien, David Troilo, and Earl L. Smith III.
Some of the results from chicks have been presented previously either in a preliminary form (Zhu X and Wallman J. IOVS 2009;50:ARVO E-Abstract 3929) or in separate studies for different purposes.4–6 For the tree shrew, data relating to scleral metabolism and induced myopia in the same animals have been reported in a separate study for different purposes.7 For the marmoset data8–10 and rhesus macaque data11–15 some findings have been presented in separate reports for different purposes related to recovery from myopia.
Materials and Methods
Animals
White Leghorn chicks were obtained from either Cornell University (Cornell K-strain; Ithaca, NY) or Truslow Farms (Hyline-W98-strain; Chestertown, MD). Chicks were housed in a heated, sound-attenuated chamber (76 × 61 cm), with a 14:10 hour light–dark cycle in the Wallman laboratory. Maternally reared tree shrews (Tupaia belangeri) from the breeding colony of the McBrien laboratory were used. Animals were transferred from the breeding colony 15 days after natural eye opening, on the day experimental procedures commenced. Eye opening occurred at 20 ± 3 days (mean ± SD) after birth. Animals were housed individually in large stainless steel cages and kept on a 15:9 hour light–dark cycle. Maternally reared marmosets (Callithrix jacchus) from the breeding colony in the Troilo laboratory were used. Animals were kept in group enclosures on a 10:14 light–dark cycle. Rhesus macaques (Macaca mulatta) were obtained at 1 to 3 weeks of age and housed in the primate nursery in the Smith laboratory. They were maintained on a 12:12 hour light–dark cycle. Care and use of all animals adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and the local animal ethics committees at the respective investigators' institutions.
Experimental Procedure
The Table includes the treatment details and sample sizes for each treatment for each species.
Table.
Details and Sample Sizes for Each Treatment for All Species
|
Species |
Treatment |
Ocularity |
Sample Size |
| Chicks | −7 D lens, continuous | Monocular | 24 |
| +6 or +7 D lens, continuous | Monocular | 36 | |
| +7 D lens with a weak diffuser, continuous | Monocular | 13 | |
| +6 D lens, 5 s every 5 min | Monocular | 10 | |
| +6 D lens, 20 s every 20 min | Monocular | 9 | |
| +7 D lens, 2 min every 10 min | Monocular | 7 | |
| +6 D lens, 2 min every h | Monocular | 14 | |
| +10 D lens, 5 min every 4 h | Monocular | 6 | |
| +6 D lens, 30 min every 2 h | Monocular | 6 | |
| +6 or +10 D lens, 30 min every 4 h | Monocular | 76 | |
| +6 D lens, 30 min every 12 h | Monocular | 6 | |
| +6 and −6 D lenses, each worn alternately for 15 min every 4 h | Monocular | 12 | |
| Tree shrews | 1 d of recovery after 5 d of form deprivation | Monocular | 5 |
| 3 d of recovery after 5 d of form deprivation | Monocular | 8 | |
| 5 d of recovery after 5 d of form deprivation | Monocular | 10 | |
| 7 d of recovery after 5 d of form deprivation | Monocular | 5 | |
| 9 d of recovery after 5 d of form deprivation | Monocular | 6 | |
| Marmosets | Recovery from −5 D contact lens wear | Monocular | 15 |
| Recovery from −3 or −5 D spectacle lens wear | Binocular | 24 | |
| Recovery from form deprivation | Monocular | 17 | |
| +3 or +5 D spectacle lens wear | Binocular | 18 | |
| +5 D contact lens wear | Monocular | 20 | |
| Untreated, normal marmosets | N.A. | 20 | |
| Rhesus macaques | Recovery from form deprivation | Monocular | 9 |
| Recovery from −3 D lens wear on the right eye (plano lens on the left eye) | Monocular | 9 | |
| Recovery from −3 D lens wear on both eyes | Binocular | 10 | |
| Recovery from −6 D lens wear on both eyes | Binocular | 3 | |
| Recovery from wearing negative lenses sequentially | Binocular | 4 | |
| Untreated, normal rhesus macaques | N.A. | 40 |
Details and sample sizes for each treatment for all species (except for chicks whose untreated fellow eyes were measured repeatedly within an hour and form-deprived tree shrews without recovery; these values are given in the text).
For chicks, PMMA plastic lenses (12 mm diameter with a back optic radius of 7 mm) or glass lenses (not conspicuously curved) of −7, +6, +7, or +10 diopters (D) were used. Each lens was glued between a rigid plastic ring and a Velcro ring, and attached to a mating Velcro ring glued to the feathers around the chicks' eyes. Lenses were cleaned at least twice a day. The majority of chicks wore a lens over one eye for 3 days. Some chicks wore negative lenses (−7 D) continuously, and the rest of them wore positive lenses (+6, +7, or +10 D) either continuously (with or without a weak diffuser) or for various durations (specifically, 20 seconds per 20 minutes, 5 seconds per 5 minutes, 2 minutes per 10 minutes or hour, 5 minutes per 4 hours, and 30 minutes per 2, 4, or 12 hours) with darkness between episodes. These chicks were measured by ultrasound biometry before and after 3 days of treatment. Another set of the chicks wore various lenses on one eye and had the fellow control eyes measured by ultrasound biometry repeatedly within 1 hour. Data from only the untreated fellow eyes were used to calculate the SD, an index of measurement error in chicks. The starting age of all chicks was one week old in all the experiments.
For tree shrews, one of the paired eyes was deprived of vision by a translucent occluder fitted to a head-mounted goggle 15 days after natural eye opening. The translucent occluder remained in place for 5 days, while the fellow eye was left untreated. After 5 days deprivation was discontinued, all tree shrews (n = 39) had ultrasound biometry (A-scan ultrasound) performed immediately on removal of the head-mounted goggle holding the occluder. A total of 34 animals were allowed to recover from the induced myopia for periods of 1, 3, 5, 7, or 9 days, with n ≥ 5 in each recovery group.
For marmosets, the conditions examined that might result in reduced eye growth included rearing some with either positive contact lenses (+5 D) over one eye or spectacle lenses over both eyes (+3 D or +5 D) starting at the age of 4 months (mean age = 128 days) for various durations to impose myopic defocus. Details of the lenses used are reported in the original studies.8–10 The rest of the animals used in this analysis had myopia induced by wearing either a translucent occluder or negative contact lens (−5 D) over one eye, or spectacle lenses over both eyes (−3 D or −5 D) starting at the age of 10 weeks, and these devices were removed when the marmosets were roughly 4 months old (mean age = 112 days), and the eyes were allowed to recover. Another group of untreated marmosets also were measured by ultrasonography periodically.
Rhesus macaques wore negative lenses (specifically, −3 D over one eye [OD −3 D], −3 D over both eyes [OU −3 D], −6 D over both eyes [OU −6 D], negative sequential lenses over both eyes [OU NS], or occluders over one eye [FD]) starting at the age of 3 or 4 weeks (mean age = 25 days) for roughly 4 months (mean duration = 135 days), after which the eyes were allowed to recover. Another group of untreated rhesus macaques also were measured by ultrasonography periodically.
Axial Biometry Measurements
Internal ocular dimensions were measured with A-scan ultrasonography from the anterior surface of the cornea for all four species, but to different tissues at the back of the eye with different measuring intervals, for different animals.
For chicks, A-scan ultrasonography was conducted with a 30 MHz transducer (Model 176599; Panametrics, Waltham, MA) and sampled at 100 MHz with a Sonix 8100 A/D board (Sonix, Inc., Springfield, VA) on a computer.16 The internal ocular dimensions (from the anterior surface of the cornea to the outer surface of the sclera) were measured with chicks anesthetized with 1.5% of isoflurane.17 Ocular length was defined as the sum of anterior chamber depth, lens thickness, vitreous chamber depth, and the thicknesses of the retina, choroid, and sclera. For chicks that wore various lenses for 3 days, the eyes were measured at the beginning and end of each experiment, and the rest of the chicks were measured repeatedly within an hour.
For tree shrews, the length of the eye (from the anterior surface of cornea to the inner surface of the sclera) was measured before and after recovery using A-Scan ultrasonography. In tree shrews, ultrasound measures were made using a 10 MHz, 6.35 mm diameter ultrasound transducer focused at 22 mm and driven by a Panametrics 5052 pulser/receiver that was coupled to a 15 mm Perspex (Lucite International, Southhampton, United Kingdom) standoff perfused continuously with 0.9% saline (flow rate 0.8 mL/min). The standoff was positioned by hand so the saline column contacted the anesthetized cornea (0.5% proxymetacaine HC1) without any applanation. Waveform echoes passed from the pulser/receiver into a LeCroy 9400 digital storage oscilloscope (sample rate 100 megasamples/s; LeCroy, Geneva, Switzerland). To enhance the signal-to-noise ratio, each stored waveform was the average of 20 single incoming waveforms. Six stored waveforms from independent positioning of the transducer were collected for each eye and transferred to PC for subsequent measurement. Conversion of time to distance used previously reported values for the tree shrew eye.18 At the end of the recovery period equatorial dimensions (superior-inferior and nasal-temporal) of the enucleated tree shrew eyes were measured with a digital caliper.
For marmosets, the length of the eye (from the anterior surface of the cornea to the inner surface of the sclera) was measured repeatedly using A-scan ultrasonography during periods of positive lens wear, or recovery from deprivation or from negative lens-induced myopia. A 33 MHz piezoelectric immersion transducer (model PZ25-0.25-SU-R1.00; Panametrics) driven by an ultrasound pulser/receiver (model 5072 PR-15U; Panametrics) was used. The transducer was coupled to the eye with a 16-mm water-filled plexiglass stand-off that positioned the focal zone of the sound wave inside the vitreous chamber of marmoset eyes for all ages. The ultrasound signal was digitized for analysis using a 100 MHz analogue-to-digital conversion board (model STR-8100; Sonix, Inc.; or model NI-PCI-5922; National Instruments, Austin, TX).
Similarly, for rhesus macaques, the length of the eye also was measured repeatedly by A-scan ultrasonography during recovery from either form-deprivation or wearing negative lenses. However, the length of the eye (or “axial length”) was from the anterior surface of the cornea to the anterior surface of the retina. Thus, axial length can be affected by changes in choroidal thickness. The great majority (67%) of data (n = 4, 3, and 26 in the groups of FD, OD −3 D, and untreated, respectively, and all of the binocularly-treated animals) were obtained with an instrument (Mentor Image 2000, 7 MHz transducer; Mentor, Norwell, MA) that provided information on individual ocular components, in particular vitreous chamber depth. This instrument (Mentor Image 2000, 7 MHz transducer; Mentor) provided the average of 10 separate measures. The instrument (Mentor Image 2000, 7 MHz transducer; Mentor) used a weighted average velocity of sound in the ocular media of 1550 m/s to calculate intraocular distances. The rest of the data (n = 5, 6, and 14 in the groups of FD, OD −3 D, and untreated, respectively) were obtained with the OTI A-scan (OTI scan 1000, 12 MHz transducer).
Statistics
Data are presented as mean ± SD. Two different statistical methods were used to compare the number of eyes that shortened axially versus the number of eyes that did not in the treated eyes and control eyes, respectively.
For chicks and tree shrews, the number of treated eyes that shortened while wearing positive lenses (chicks) or recovering from deprivation (tree shrews) versus those that did not was compared to their fellow control eyes with χ2 tests.
For marmosets and rhesus macaques, the hypothesis that more eyes that wore positive lenses, or recovered from deprivation or wearing negative lenses shortened compared to eyes from normal, untreated animals was tested using a bootstrapping method19 (Matlab, version R2010b; Mathworks, Natick, MA). Analysis consisted of the following steps: Firstly, the change in axial length in treated eyes between either the initiation of positive lens wear, or recovery from deprivation or negative lenses and the next time the animals were measured (mean = 31 and 14 days for marmosets and rhesus macaques, respectively) was calculated. For animals that wore devices over both eyes, change in axial length from both treated eyes was used for analysis. Data from monocularly- versus binocularly-treated animals were analyzed separately.
Secondly, data from eyes of untreated animals were used for comparison. Since the eyes of the treated and untreated animals were measured on different days, the hypothetical axial length of the eyes of the untreated animals on the same day that the treated animals were measured was interpolated based on the axial length data from that particular normal animal (Igor Pro, version 5.02; Wavemetrics, Lake Oswego, OR), provided that this normal animal was measured frequently enough. Therefore, for x treated animals, x sets of axial length can be calculated for each normal, untreated animal. Furthermore, if there were x treated animals and y untreated animals, a total of xy sets of axial length can be calculated. Specifically, for marmosets, there were a total of 94 treated animals and 20 untreated animals (note that data from some treated and untreated animals were not included for calculation due to either lack of ultrasound data or infrequent measurements), thus leaving a total of 1334 sets of axial length that were calculated (see Fig. 6). For rhesus macaques, there were 35 treated animals and 40 normal animals. Hence, a total of 1400 (35 × 40 = 1400) sets of axial length was calculated. Since these data are not independent from each other, bootstrapping methods, instead of χ2 tests, were used to compare the numbers of eyes that shortened versus those that did not for treated and untreated animals.
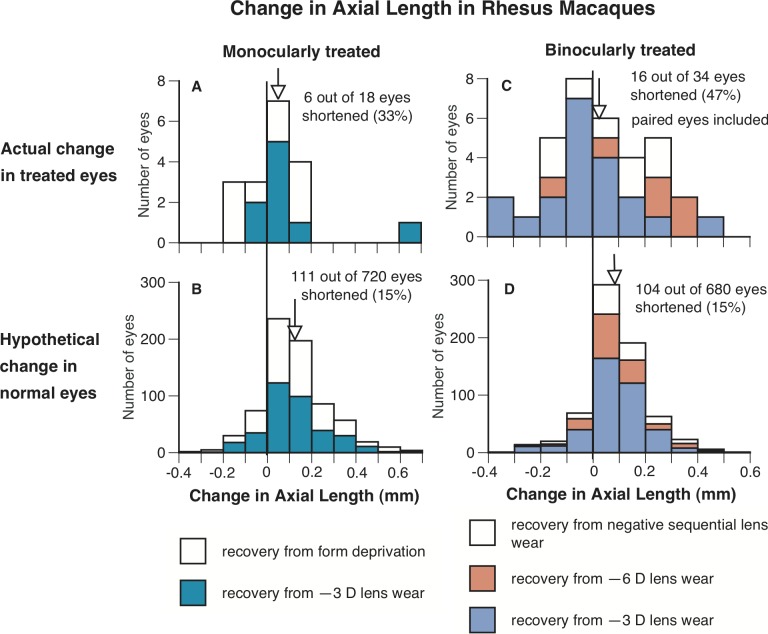
Figure 6.
The frequency distributions of change in axial length (from the anterior surface of the cornea to the anterior surface of the retina) in the treated eyes of rhesus macaques that recovered from form deprivation, or wearing negative lenses (A, C) and in untreated eyes in normal animals grouped to match the same duration of visual exposure as the treated eyes through interpolation (B, D). Left and right represent the change in axial length in the eyes of monocularly- and binocularly-treated eyes in rhesus macaques (and the corresponding interpolated untreated eyes), respectively. It is clear that a higher percentage of the treated eyes shortened (axial change below zero) than the calculated data from age-matched normal eyes. The vertical lines indicate where zero is x-axes, and the arrows indicate the mean change in axial length for each group of eyes.
Thirdly, the number of treated eyes that shortened and those that did not was compared to the number of eyes from untreated animals that shortened during the same duration (calculated with interpolation) and those that did not, using the bootstrapping procedure. Specifically, having observed that, for a given treatment, a certain number, n, out of m treated eyes shortened, we analyzed whether such an event could have occurred by chance. To estimate the probability of observing n out of m treated eyes shortening axially, we used the bootstrapping procedure to build a distribution representing the probability of observing an arbitrary number of shortening eyes in a group of m. To build that distribution, we pooled measurements from untreated eyes, and drew random samples of m measurements 50,000 times. For each randomly selected sample, we counted the number of measurements less than 0 (eyes that shortened). The resulting distribution allowed us to calculate the probability of observing at least n shortening eyes in a sample of size m by counting the number of times out of 50,000 that we had drawn random samples of size m that also contained at least n measurements less than 0. If we observed fewer than 2500 such occurrences (less than 5% of the bootstrapped samples), we concluded that the observed number of eyes that shortened axially could not be attributed to chance.
Furthermore, the 95% confidence intervals (CIs) for A-scan ultrasonography measures of axial length for the different species was used to assess whether the observed axial shortening in response to myopic defocus could be accounted for by measurement error. For chicks, the 95% CIs were calculated from control eyes (n = 145) that were measured twice within an interval of one hour, during which time their fellow eyes wore various lenses. For rhesus macaques the repeatability of measures from 20 eyes was measured to establish 95% CIs. For tree shrews18 and marmosets8 the 95% CIs were used from previously reported data on normal animals from the two laboratories.
Results
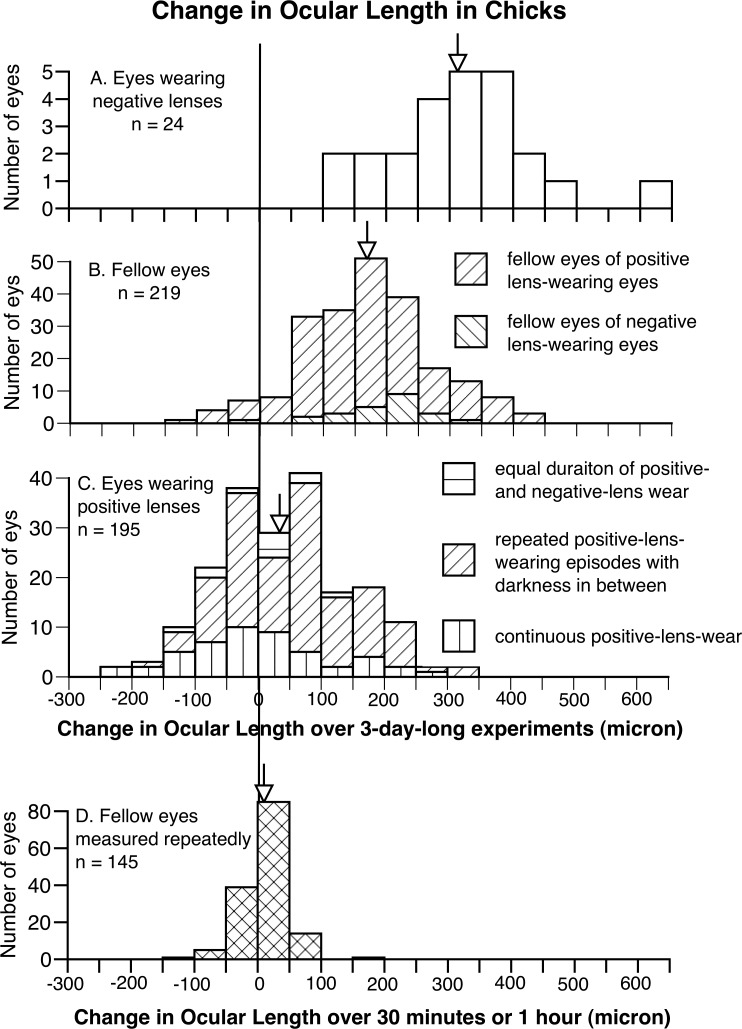
For chicks, as expected, negative and positive spectacle lenses increased and decreased the rate of ocular elongation, respectively (Fig. 1). Eyes wearing negative lenses for 3 days (n = 24) elongated twice as much as fellow eyes of positive and negative lens–wearing eyes (n = 219, mean change in ocular length, 314 vs. 171 μm, P < 0.001, unpaired 1-tailed Student's t-test, Figs. 1A, 1B), whereas eyes wearing positive lenses for 3 days (n = 195) elongated less than a quarter as much as these fellow eyes (mean 40 vs. 171 μm, P < 0.001, Figs. 1B, 1C). In chicks wearing positive lenses, 75 out of 195 (38.5%) positive lens–wearing eyes became shorter than at the start of the experiment (mean shortening ± SD −63 ± 49 μm, Fig. 1C), whereas only 10 fellow eyes shortened (−58 ± 34 μm, Fig. 1B). The frequency of eye shortening in the positive lens–wearing eyes and their fellow eyes was significantly different (P < 0.001, χ2 test).
Figure 1.
The frequency distributions of change in ocular length (front of cornea to back of sclera) in negative lens–wearing eyes (A), all the fellow eyes (B), positive lens–wearing eyes (C), all measured 3 days apart, and untreated eyes measured repeatedly within an hour from another group (D), in chicks. Arrows indicate the average of each group. It is clear that, while negative lenses increased the rate of ocular elongation, positive lenses decreased it, with 38.5% of the positive lens–wearing eyes shortening during the course of the experiment (on the left side of zero). Furthermore, data from eyes measured repeatedly (D) show the error of biometry measurements in ocular length. When very little eye growth was expected within an hour, most of the points are close to zero.
The 95% CI for ocular length measures was estimated from repeated measures on 145 fellow eyes, each measure separated by 1 hour. This provided a SD of 26 μm, resulting in 95% CIs of ±51 μm. As a matter of fact, the SD of these measurements (SD = 26 μm) overestimated the measurement error because it was based on a heterogeneous sample of experimental animals measured at different times of day. Using this SD and supposing the changes in the length of individual positive lens–wearing eyes approximated a normal distribution (Fig. 1D), zero change in ocular length in eyes wearing positive lenses would be 1.54 SDs below the mean (40 μm). Therefore, if measurement error were the only cause, we would expect 6.2% of these 195 eyes (12 eyes) to have shortened, rather than 38.5% (75 eyes) that were encountered (P < 0.0001, Fisher's exact test).
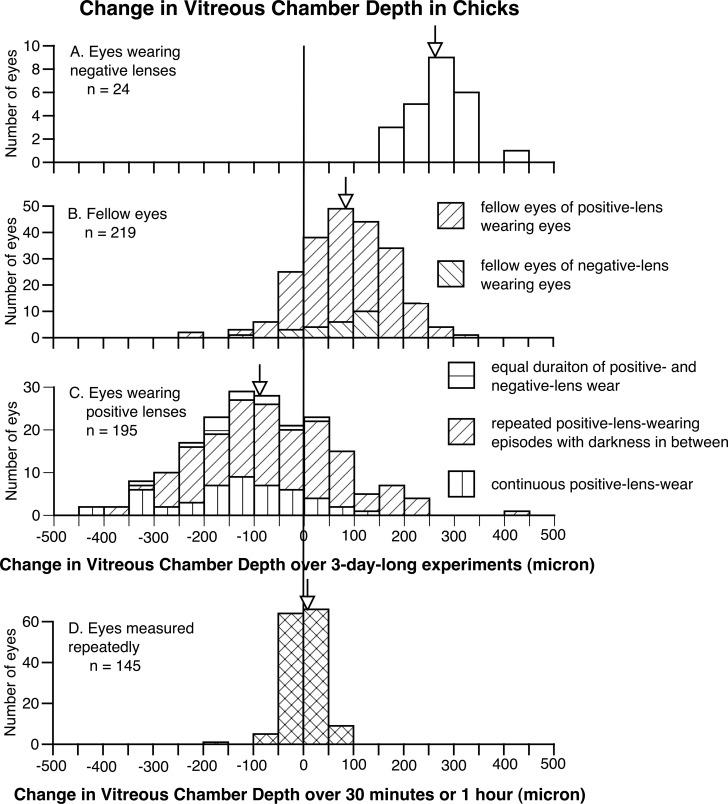
Not surprisingly, the shortening in ocular length also resulted in a decrease in the depth of the vitreous chamber (Fig. 2). The vitreous chamber depth in positive lens–wearing eyes decreased by −84 μm over 3-day–long experiments (Fig. 2C), whereas the vitreous chamber depth in the fellow eyes of positive and negative lens–wearing eyes elongated by the same amount (Fig. 2B, P < 0.001, unpaired 1-tailed Student's t-test). Furthermore, significantly reduced axial enlargement in anterior chamber depth and lens thickness also was found in positive lens–wearing eyes, although to a smaller degree (anterior chamber depth, positive lens–wearing eyes versus all fellow eyes 1 vs. 15 μm, P < 0.01; lens thickness 89 vs. 102 μm, P < 0.01). The choroids in positive lens–wearing eyes thickened significantly more than those in the fellow eyes (32 vs. −26 μm, P < 0.001). This change, however, only caused a reduction in vitreous chamber depth without changing ocular length. Indeed, the shortening of the vitreous chamber in positive lens–wearing eyes cannot be explained fully by choroidal thickening in these same eyes (vitreous chamber shortening versus choroidal thickening 84 vs. 32 μm), but is a consequence of the reduced ocular length. No significant change was found in retinal or scleral thickness during the course of experiments.
Figure 2.
The frequency distributions of change in vitreous chamber depth in negative lens–wearing eyes (A), all the fellow eyes (B), positive lens–wearing eyes (C), all measured 3 days apart, and untreated eyes measured repeatedly within an hour from another group (D), in chicks. Arrows indicate the average of each group. Similar to Figure 1, it is clear that, while negative lenses increased the vitreous chamber depth, positive lenses decreased it (wearing positive lenses caused the vitreous chamber to shorten in approximately two thirds of the eyes). Again, data from eyes measured repeatedly (D) show the accuracy and validity of biometric measurements of vitreous chamber depth.
To rule out the possibility of abnormal growth in the chicks whose positive lens–wearing eyes shortened, the ocular growth of the fellow eyes in these chicks was compared to the rest of the fellow eyes, since a systemic pathologic condition could have reduced eye growth not only in the lens-wearing eye, but also in the fellow eye. Among 195 positive lens–wearing chicks, while 75 treated eyes shortened (38.5%), only 10 fellow eyes shortened (5.1%). This percentage was not significantly different from the percentage of eyes that shortened in all the fellow eyes (11 of 219 fellow eyes or 5.0%, P = 0.26, Fisher's exact test). Although the fellow eyes of those that had shortened lengthened slightly less on average than untreated fellow eyes in general (mean 131 vs. 171 μm), this may be related to the known yoking between eyes.20–22 This difference cannot explain the shortening of the lens-wearing eyes. In addition, wearing positive lenses caused the eyes to shorten axially over a wide range of paradigms, suggesting that axial shortening of positive lens–wearing eyes in chicks was not the result of pathology, but was the product of an active compensatory mechanism for superimposed myopic defocus.
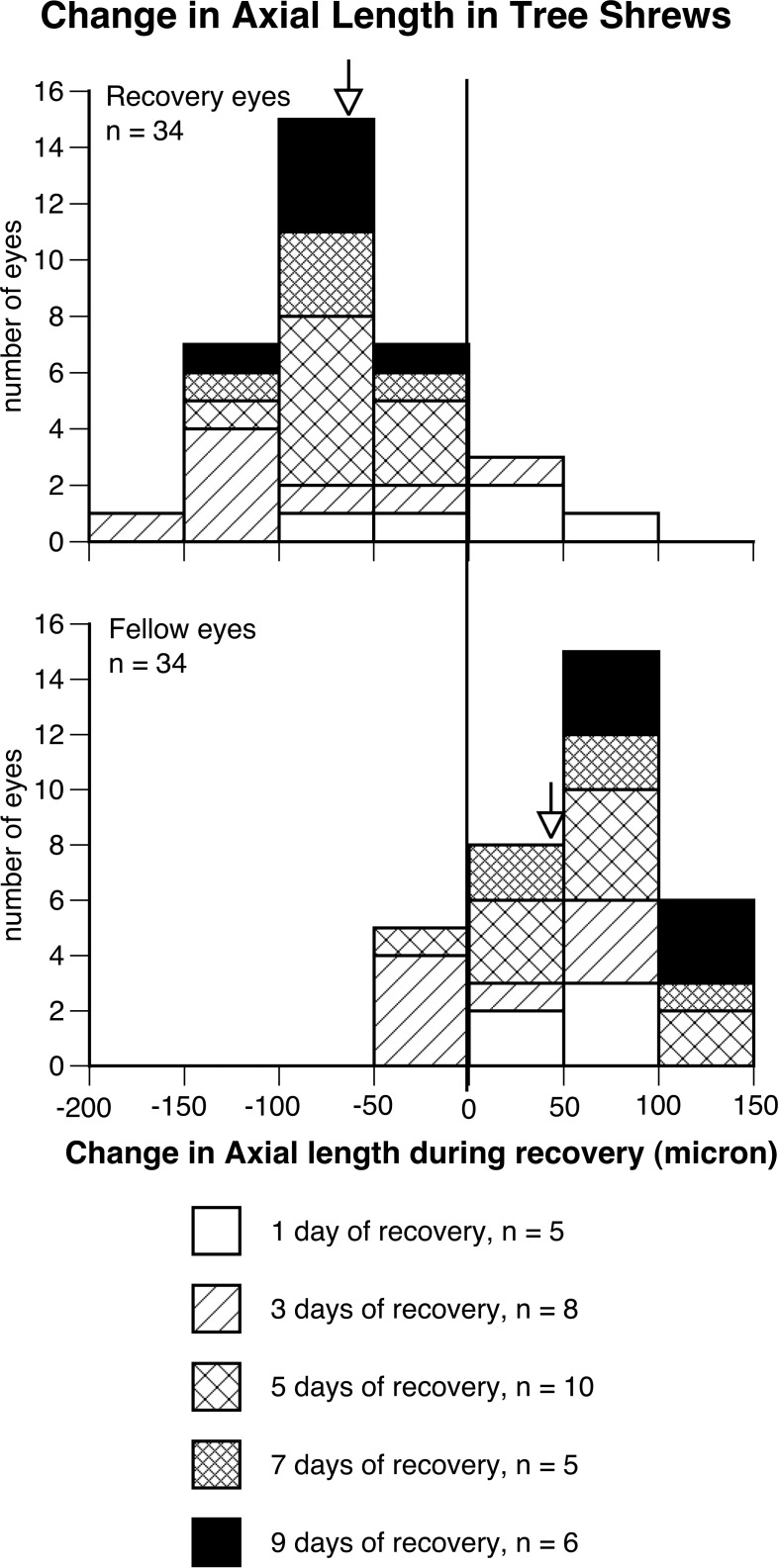
For tree shrews, similarly, eyes recovering from deprivation-induced myopia shortened axially to compensate for myopic defocus (Fig. 3): After 1, 3, 5, 7, or 9 days of recovery following 5 days of monocular deprivation, 30 of 34 (88%) treated eyes shortened (mean change in axial length from cornea to the inner surface of the sclera during recovery −60 ± 53 μm, mean ± SD), whereas only 5 fellow eyes shortened axially (mean change 49 ± 48 μm, P < 0.0001, χ2 test, Fig. 3). The shortening in axial length also resulted in a decrease in the depth of vitreous chamber (data not shown): The vitreous chamber depth in recovering eyes decreased by 89 μm, whereas the vitreous chamber depth in the fellow eyes elongated by 16 μm (P < 0.0001, paired 1-tailed Student's t-test). Furthermore, small but significantly reduced growth in anterior chamber depth also was found (recovery eyes versus fellow eyes −3 vs. 16 μm, P < 0.05, paired 2-tailed Student's t-test). The 95% CIs for axial length measures in tree shrew have been reported previously to be ±40 μm,18 which is markedly less than the mean axial shortening of 60 μm observed in the tree shrew eyes recovering from experimentally-induced myopia in our study.
Figure 3.
The frequency distributions of change in axial length (from the anterior surface of cornea to the inner surface of sclera) in the treated eyes that recovered from deprivation-induced myopia for various durations (top) and in their untreated, fellow eyes within the same duration (below) in tree shrews. A total of 30 treated eyes shortened during recovery, whereas 5 fellow eyes shortened within the same duration (P < 0.0001, χ2 test).
Another striking finding in tree shrews is that the percentage of eyes that shortened axially was correlated positively with the recovery duration (Figs. 3, 4). After 1 day of recovery (n = 5), 2 treated and 2 fellow eyes (from different animals) shortened, respectively. After 3 (n = 8) and 5 (n = 10) days of recovery, 7 and 10 treated eyes shortened, respectively, versus 4 and 1 fellow eye that shortened (P < 0.0001 for the 5-day recovery group, χ2 test). After 7 and 9 days of recovery (n = 5 and 6, respectively), all of the treated eyes shortened and all of the fellow eyes grew (P < 0.0001). Furthermore, it is clear from Figure 4 that more than 68% of treated eyes (mean ± 1 SD) in groups that had more than 3 days of recovery (5–9 days of recovery) shortened, reinforcing the finding that the majority of eyes of tree shrews shorten axially to compensate for myopic defocus. Figure 4 also shows that the greatest relative difference between recovering and fellow control eyes occurred in the 9-day recovery group (≈ 150 μm), although the largest mean shortening in treated eyes (mean = 92 μm) occurred in the 3-day recovery group.
Figure 4.
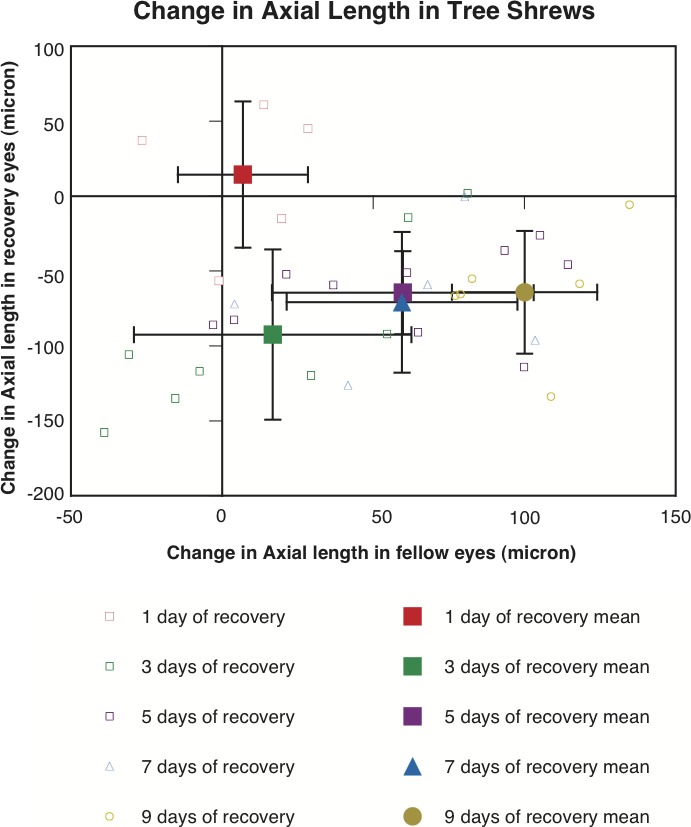
Change in axial length (from the anterior surface of cornea to the inner surface of sclera) in the recovery eyes (y-axis) plotted against that in the fellow eyes (x-axis) in tree shrews. Small and open symbols represent individual eyes, and large and solid symbols represent averages for each group (mean ±1 SD). It is clear that most of the recovery eyes shortened (below zero on the y-axis), whereas their fellow eyes grew (above zero on the x-axis). The figure also shows that the largest mean difference between treated and fellow control eyes occurred in the 9 day recovery group (≈150 μm), while the largest mean degree of axial shortening in treated eyes (92 μm) occurred in the 3 days recovery group.
As eyes did shorten axially in absolute terms during compensation for myopic defocus, it is possible that, during recovery, eye growth was reduced in the axial direction, but could have increased in the equatorial direction. However, measurements, using digital caliper, of equatorial dimensions (superior/inferior and medial/lateral) from these enucleated tree shrews' eyes demonstrated that the equatorial enlargement observed after 5 days of induced myopia (mean difference of superior/inferior + medial/lateral between treated versus control was 90 ± 24 μm) incrementally reduced the longer the recovery period (group mean differences between treated and fellow control eyes for 1-, 3-, 5-, 7-, and 9-day recovery groups were 91 ± 18, 77 ± 15, 20 ± 13, 16 ± 14, and 17 ± 21 μm, respectively). Thus, equatorial enlargement from induced myopia reduced gradually during recovery from myopia, but did not show absolute shortening in the equatorial dimension, suggesting that the change in eye growth is predominantly in the axial direction.
For marmosets, eyes that either wore positive lenses to impose myopia, or recovered from induced myopia from deprivation or from wearing negative lenses also could shorten axially to compensate for myopic defocus, although this was seen with much less frequency compared to eyes of tree shrews, chicks, or rhesus macaque (Fig. 5). For monocularly treated marmosets that either wore +5 D contact lenses, or recovered from myopia induced by wearing −5 D contact lenses or occluders (n = 15 to 18 in each group), 8% of the treated eyes shortened axially (4 treated eyes of 48 eyes consisting of 2 eyes recovering from −5 D contact lenses and 2 eyes recovering from form-deprivation; mean change in axial length ± SD 165 ± 185 μm, Fig. 5A), whereas only 3% of the calculated, age-matched (interpolated) normal eyes shortened (22 of 643 calculated eyes, Fig. 5B; P < 0.05 for pooled data, bootstrapping; when data from each group were analyzed separately, only recovery from wearing occluders reached statistical significance, P < 0.05). For binocularly-treated marmosets that either wore +3 D or +5 D spectacle lenses, or recovered from myopia induced by wearing −3 D or −5 D spectacles lenses (n = 18 and 24, respectively), 4% of treated eyes shortened axially (3 treated eyes of 76 eyes, all 3 eyes from animals recovering from wearing −3 D or −5 D lenses; mean change in axial length 173 ± 180 μm, Fig. 5C), whereas only 1% of calculated, age-matched (interpolated) normal eyes shortened (9 of 691 eyes, Fig. 5D, P > 0.05 for pooled data; when data from each group were analyzed separately, P < 0.001 for the binocular negative lens–wearing group). The 95% CIs for axial length measures in marmoset have been reported previously to be ±33 μm.8
Figure 5.
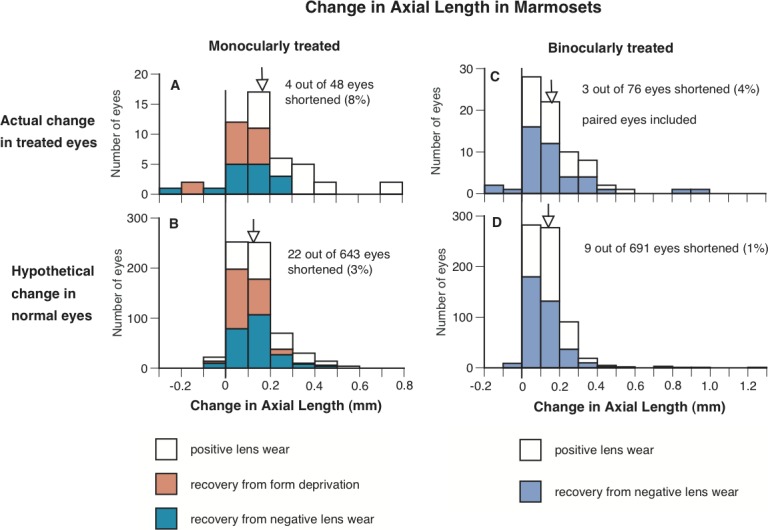
The frequency distributions of change in axial length (from the anterior surface of cornea to the posterior surface of sclera) in the treated eyes of marmosets that either wore positive lenses, or recovered from form deprivation or wearing negative lenses (A, C), and in untreated eyes in normal animals grouped to match the same duration of visual exposure as the treated eyes through interpolation (B, D). Left and right represent the change in axial length in the eyes of monocularly- and binocularly-treated marmosets (and the corresponding interpolated untreated eyes), respectively. It is clear that a higher percentage of the treated eyes shortened (axial change below zero) than the calculated data from age-matched normal eyes. The vertical lines indicate where zero is on the x-axes, and the arrows indicate the mean change in axial length for each group of eyes.
Axial length data from eyes of rhesus macaques (from the anterior corneal surface to the anterior surface of retina) were analyzed in a similar fashion as the marmoset eyes, and it showed that rhesus macaque eyes also can shorten axially to compensate for myopic defocus (Fig. 6). For monocularly treated macaques that recovered from deprivation-induced myopia (FD, n = 9) or wearing −3 D lenses (OD −3D, n = 9), a total of 33% of eyes shortened axially (6 of 18 eyes, mean change in axial length ± SD 57 ± 179 μm, Fig. 6A), whereas only 15% (111 of 720 eyes) of calculated, age-matched (interpolated) normal eyes shortened (Fig. 6B, P > 0.05 for pooled data, bootstrapping; when data from each group was analyzed separately, the FD group showed a significant difference, P < 0.05).
The frequency of eye shortening in binocularly-treated macaques was stronger than that observed in monocularly-treated macaques, although it still was just under half the treated eyes (47%). These rhesus macaques recovered from wearing either −3 D (OU −3 D, n = 10) or −6 D lenses (OU −6 D, n = 3), or negative sequential lenses (OU NS, n = 4) over both eyes. A total of 47% of the binocularly-treated recovering eyes shortened axially (16 of 34 eyes; 12, 1, and 3 eyes from the groups of OU −3 D, OU −6 D, and OU NS, respectively; mean ± SD 22 ± 181 μm, change in axial length, Fig. 6C), whereas only 15% (104 of 680) of calculated, age-matched (interpolated) normal eyes shortened (Fig. 6D, P < 0.001 for pooled data, bootstrapping; when data from each group were analyzed separately, the groups of OU −3 D and OU −6 D also showed a significant difference of P < 0.001 and P < 0.05, respectively). The 95% CIs for axial length measures for rhesus macaque were calculated from repeated measures on 20 rhesus macaque eyes and found to be ±47 μm.
Since the axial length in rhesus macaques was measured to the anterior surface of the retina, there is the possibility that the axial shortening was caused by choroidal thickening. If that were the case, one would expect that the amount of choroidal thickening would equal the amount of eye shortening. However, previous findings suggest that the axial shortening discovered in these rhesus macaque eyes was not caused exclusively by choroidal thickening. Choroidal thickening in rhesus macaques has been suggested to be on average 50 μm, with the largest amount of thickening noted to be 102 μm.23 Among the rhesus macaque eyes that shortened axially, in more than half of the eyes the shortening was more than the maximal choroidal thickening reported previously.23 Of the 22 macaque eyes that shortened axially, 11 shortened by more than 100 μm, ranging from 120 to 310 μm (mean shortening in these 22 eyes −122 ± 85 μm), amounts substantially larger than could be accounted for by choroidal thickening.
Therefore, although it is likely that choroidal thickening in rhesus macaque eyes contributed to the observed axial shortening measured in these eyes, roughly in half of the eyes that shortened, the magnitude of shortening could not be accounted for by choroidal thickening alone, indicating that rhesus macaque eyes also can shorten axially to compensate for myopic defocus.
Discussion
Our data provided evidence that avian and mammalian eyes can shorten axially to compensate for myopic defocus. That eye shortening was found in a variety of species, regardless of the structural differences in the sclera and eye sizes, suggests that the same mechanism modulating eye growth is conserved evolutionarily.
In tree shrews, where the majority of treated eyes (88%) exposed to myopic defocus shortened axially, the finding that the enlarged equatorial diameters observed after 5 days of induced myopia reduced incrementally during recovery from myopia, such that there was no statistical difference between treated and fellow eyes across all recovery groups, supports the likelihood that the treated eyes compensating for myopic defocus actually shrank (reduction in surface area). For chicks, marmosets, and rhesus macaques we can only state that eyes of these species can shorten axially in response to myopic defocus as no equatorial measures were available. However, it would seem likely a similar process occurs across all species.
It might be considered that it would be more difficult for chick eyes to shorten because the chick sclera has a more rigid cartilaginous layer composed of chondrocytes, whereas the outer layer of mammalian eyes has only fibrous sclera composed of fibroblasts and myofibroblasts,24 which theoretically should make it easier to remodel. However, an earlier study by Kusakari et al. found evidence of scleral remodeling in the posterior sclera during induced myopia in chicks.25 Specifically, after deprivation-induced myopia, the boundary between the cartilaginous and fibrous sclera became indistinct, and some spindle-shaped transitional mesenchymal cells that showed morphologic features of fibroblasts and chondrocytes were discovered between the two layers, suggesting possible transformation of the two cell types during altered eye growth. These findings support the possibility of remodeling during compensation that could lead to actual eye shortening in chicks.
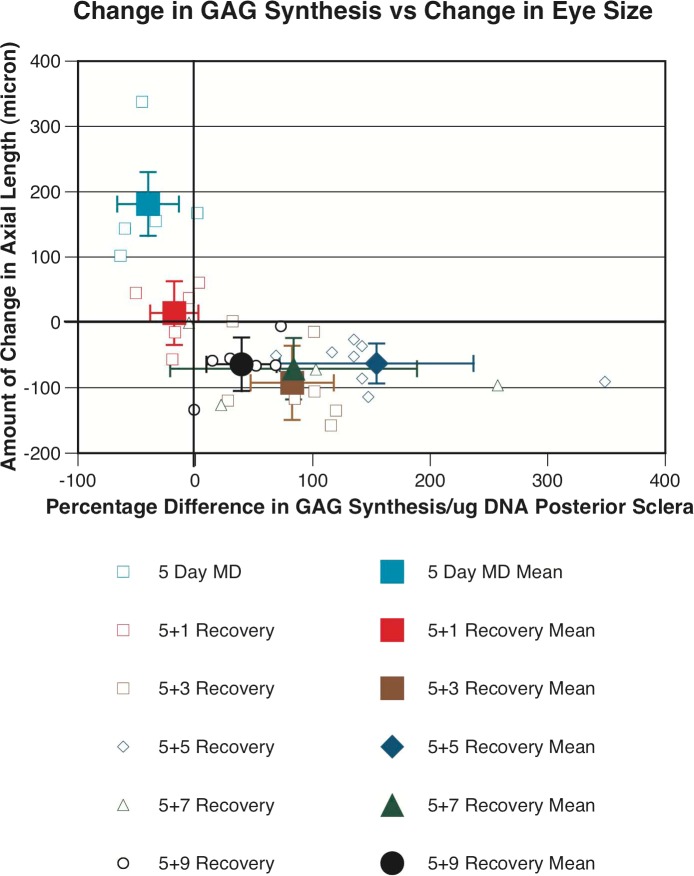
In tree shrews we have direct evidence that the eyes that shortened axially also underwent active sclera remodeling, which was in the opposite direction to that found for eyes that were enlarging due to developing myopia. On the morning of the day when final biometric measures were collected on each tree shrew, animals were given an intraperitoneal injection of radiolabeled sulfate (35S) to label glycosaminoglycans (GAGs) in the sclera of tree shrews. Six hours after injection of radiolabeled sulfate (when sulfate incorporation in the sclera is at its peak7), collection of in vivo biometric measures was completed, then animals were given a terminal dose of anesthesia, and scleral tissue was collected and processed for measurement of GAG synthesis using procedures described previously.7 Of the 30 tree shrew eyes that actually underwent axial shortening during recovery from induced myopia, 27 eyes (90%) underwent an increase in GAG synthesis in the posterior sclera (central 5 mm). Of the 4 eyes recovering from myopia that did not show shortening, 3 eyes had been recovering for only 24 hours and only 1 of these eyes had an increase in GAG synthesis (Fig. 7). On the contrary, for the tree shrews that had been deprived of pattern vision monocularly for 5 days, but not allowed any recovery from myopia, all treated eyes underwent enlargement over the 5 days of MD with an average elongation of the axial length of 181 ± 90 μm, with 4 of the 5 myopic eyes undergoing a significant reduction in GAG synthesis (−40.3 ± 26%, n = 5, P < 0.01). Interestingly, it also was found that the same 27 recovering eyes that showed an increase in GAG synthesis in the posterior sclera also showed an increase in GAG synthesis in the equatorial sclera, although to a smaller degree, giving further evidence that tree shrew eyes recovering from myopic defocus underwent eye shrinkage and not just axial shortening. This relationship between changes in eye size and GAG synthesis, such that eyes that shortened underwent increased GAG synthesis in the sclera and eyes that elongated underwent reduced GAG synthesis in the sclera, provides strong evidence for active regulation of scleral metabolism to facilitate eye size changes in both directions.
Figure 7.
Change in axial length in the treated eyes of tree shrew (y-axis) plotted against the percentage difference in glycosaminoglycan synthesis in the posterior sclera between treated and control eyes (x-axis) in tree shrews. Small and open symbols represent individual eyes, and large and solid symbols represent averages for each group (mean ± SD). It is clear that most of the recovery eyes that shortened axially (27 of 30—below zero on the y-axis) had an increase in glycosaminoglycan synthesis in the posterior sclera compared to their fellow eyes, whereas tree shrew eyes that elongated due to deprivation-induced myopia (above zero on the y-axis) underwent a decrease in glycosaminoglycan synthesis in the posterior sclera, when compared to their fellow eyes.
The frequency of eyes that shortened axially to compensate for myopic defocus shows marked differences across the different animal species evaluated. Using the not unreasonable criterion for axial shortening as any reduction in ocular length (as in fellow eyes and normal eyes the norm is for axial elongation) then 88% of treated eyes in tree shrews recovering from myopia shortened axially, 38.5% of chick eyes treated with positive lenses for 3 days shortened, 47% of binocularly-treated and 33% of monocularly-treated rhesus macaque eyes shortened axially, and 8% of monocularly-treated and 4% of binocularly-treated marmoset eyes shortened axially. While the percentage values for axial shortening in response to myopic defocus of treated eyes of tree shrews and chicks are very different from the response of their fellow eye data, with only 5 fellow eyes (6.8%) of tree shrews shortened axially and 10 fellow eyes (5.1%) of chicks shortened axially, it is pertinent also to review the frequency of axial shortening in relation to 95% CIs for A-scan ultrasonography of axial length on the four species. Using the 95% CI values reported for each species in the results section, it is found that 71% of treated tree shrew eyes, 19.5% of treated chick eyes, 35% of binocularly- and 33% of monocularly-treated rhesus macaque eyes, and 6.2% of monocularly- and 4% of binocularly-treated marmoset eyes shortened axially more than the 95% CI value for axial length measures. Thus, under the specific experimental paradigms used with each species, the vast majority of eyes shortened axially in response to myopic defocus in tree shrew, while in chicks and rhesus macaques 20% to 35% of eyes shortened axially in response to myopic defocus and around 5% of marmoset eyes shortened axially.
We consider the above differences in the frequency of axial shortening between species were likely due to the experimental paradigms used, in particular the relatively older age of the primates when recovery or positive lens wear began and in the case of chicks the very short period of positive lens wear of only 3 days. For chicks and tree shrews, either positive lens wear or recovery from myopia was initiated during the most susceptible period for influencing postnatal refractive development and eye size,26,27 and at considerably younger ages (especially in relative developmental terms) than marmosets or rhesus macaques, whereas for marmosets and rhesus macaques recovery from monocular deprivation or negative lens wear only started after 4 or 5 months, respectively, at which time the rate of postnatal eye size changes has past their most susceptible period for influencing refractive development.8 Although positive lens wear in chicks was initiated at the most susceptible period, the duration of lens wear of only 3 days is likely to have limited the degree and frequency of eyes that shortened axially compared to tree shrew eyes that had recovery periods up to 9 days. Compared to the monocularly-treated macaques, the binocularly-treated rhesus macaques showed a stronger trend of axial eye shortening. This may have been caused by the yoking effect, an interaction between paired eyes that drives both eyes to change in the same direction.
Eye growth is controlled by local retinal mechanisms, as demonstrated by the fact that after the eye and brain are disconnected, either by optic nerve section28 or blocking the action potentials of retinal cells by tetrodotoxin,29,30 chick and tree shrew eyes still develop deprivation-induced myopia. Chick eyes also have been shown to maintain the ability to compensate for positive or negative spectacle lenses after optic nerve section.21,31 These results all suggest that the retina can modulate eye growth in response to altered visual stimuli without input from the brain. In addition, this local mechanism can alter eye growth selectively within a limited region or quadrant of the eye when diffusers32 or lenses33 degrade the retinal image in that part of the eye, while leaving the rest of the retinal image relatively intact. It seems highly likely that active shortening of eyes, as reported in our study, also is controlled by local ocular mechanisms.
In summary, we have presented an analysis of data from various established animal models of refractive error development showing the capacity of eyes in young, rapidly growing animals to shorten axially to facilitate compensation for myopic defocus. It would be interesting to determine if this phenomenon also exists in children or adolescents, since older adult human eyes have been shown to shorten axially, possibly in response to the increased refractive power of the cornea and the lens.34 These results suggest the possibility that combining distance correction with some myopic defocus in the correct amount and duration might cause the developing human eye to shorten axially or shrink. If this were the case, then strategies for preventing or reducing the axial elongation of the eye that results in high myopia in human, and the associated ocular pathologies, might be treated feasibly.
Acknowledgments
Supported by National Institute of Health Grants EY-02727 and RR-03060 (JW); National Health & Medical Research Council of Australia Grant 454602 (NMcB); National Institutes of Health Grants EY-03611 and EY-07551, Vision Cooperative Research Centre, Sydney, Australia (ELS III); and National Institute of Health Grant EY-011228 (DT).
Disclosure: X. Zhu, None; N.A. McBrien, None; E.L. Smith III, None; D. Troilo, None; J. Wallman, None
References
- 1. Irving EL, Sivak JG, Callender MG. Refractive plasticity of the developing chick eye. Ophthalmic Physiol Opt. 1992; 12: 448–456 [PubMed] [Google Scholar]
- 2. Secor SM, Diamond J. A vertebrate model of extreme physiological regulation. Nature. 1998; 395: 659–662 [DOI] [PubMed] [Google Scholar]
- 3. Hau M, Wikelski M, Wingfield JC. A neotropical forest bird can measure the slight changes in tropical photoperiod. Proc R Soc Lond B. 1998; 265: 89–95 [Google Scholar]
- 4. Winawer J, Wallman J. Temporal constraints on lens compensation in chicks. Vision Res. 2002; 42: 2651–2668 [DOI] [PubMed] [Google Scholar]
- 5. Zhu X, Winawer J, Wallman J. The potency of myopic defocus in lens-compensation. Invest Ophthalmol Vis Sci. 2003; 44: 2818–2827 [DOI] [PubMed] [Google Scholar]
- 6. Zhu X, Park TW, Winawer J, Wallman J. In a matter of minutes, the eye can know which way to grow. Invest Ophthalmol Vis Sci. 2005; 46: 2238–2241 [DOI] [PubMed] [Google Scholar]
- 7. McBrien NA, Lawlor P, Gentle A. Scleral remodeling during the development of and recovery from axial myopia in the tree shrew. Invest Ophthalmol Vis Sci. 2000; 41: 3713–3719 [PubMed] [Google Scholar]
- 8. Troilo D, Nickla DL. The response to visual form deprivation differs with age in marmosets. Invest Ophthalmol Vis Sci. 2005; 46: 1873–1881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Troilo D, Quinn N, Baker K. Accommodation and induced myopia in marmosets. Vision Res. 2007; 47: 1228–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Troilo D, Totonelly K, Harb E. Imposed anisometropia, accommodation, and regulation of refractive state. Optom Vis Sci. 2009; 86: E31–E39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith EL III, Hung LF. The role of optical defocus in regulating refractive development in infant monkeys. Vision Res. 1999; 39: 1415–1435 [DOI] [PubMed] [Google Scholar]
- 12. Smith EL III, Hung LF. Form-deprivation myopia in monkeys is a graded phenomenon. Vision Res. 2000; 40: 371–381 [DOI] [PubMed] [Google Scholar]
- 13. Qiao-Grider Y, Hung LF, Kee CS, Ramamirtham R, Smith EL III. Recovery from form-deprivation myopia in rhesus monkeys. Invest Ophthalmol Vis Sci. 2004; 45: 3361–3372 [DOI] [PubMed] [Google Scholar]
- 14. Huang J, Hung LF, Ramamirtham R, et al. Effects of form deprivation on peripheral refractions and ocular shape in infant rhesus monkeys (Macaca mulatta). Invest Ophthalmol Vis Sci. 2009; 50: 4033–4044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith EL III, Hung LF, Huang J, Blasdel TL, Humbird TL, Bockhorst KH. Effects of optical defocus on refractive development in monkeys: evidence for local, regionally selective mechanisms. Invest Ophthalmol Vis Sci. 2010; 51: 3864–3873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nickla DL, Wildsoet C, Wallman J. Visual influences on diurnal rhythms in ocular length and choroidal thickness in chick eyes. Exp Eye Res. 1998; 66: 163–181 [DOI] [PubMed] [Google Scholar]
- 17. Nickla DL. Diurnal Rhythms and Eye Growth in Chicks. New York, NY: City University of New York; 1996. [Google Scholar]
- 18. Norton TT, McBrien NA. Normal development of refractive state and ocular component dimensions in the tree shrew (Tupaia belangeri). Vision Res. 1992; 32: 833–842 [DOI] [PubMed] [Google Scholar]
- 19. Efron B, Tibshirani RJ. An Introduction to the Bootstrap. New York, NY: Chapman and Hall/CRC; 1994. [Google Scholar]
- 20. Hung LF, Crawford ML, Smith EL. Spectacle lenses alter eye growth and the refractive status of young monkeys. Nature Med. 1995; 1: 761–765 [DOI] [PubMed] [Google Scholar]
- 21. Wildsoet C, Wallman J. Choroidal and scleral mechanisms of compensation for spectacle lenses in chicks. Vision Res. 1995; 35: 1175–1194 [DOI] [PubMed] [Google Scholar]
- 22. Schmid KL, Wildsoet CF. Effects on the compensatory responses to positive and negative lenses of intermittent lens wear and ciliary nerve section in chicks. Vision Res. 1996; 36: 1023–1036 [DOI] [PubMed] [Google Scholar]
- 23. Hung LF, Wallman J, Smith EL III. Vision-dependent changes in the choroidal thickness of macaque monkeys. Invest Ophthalmol Vis Sci. 2000; 41: 1259–1269 [PubMed] [Google Scholar]
- 24. McBrien NA, Jobling AI, Gentle A. Biomechanics of the sclera in myopia: extracellular and cellular factors. Optom Vis Sci. 2009; 86: E23–E30 [DOI] [PubMed] [Google Scholar]
- 25. Kusakari T, Sato T, Tokoro T. Regional scleral changes in form-deprivation myopia in chicks. Exp Eye Res. 1997; 64: 465–476 [DOI] [PubMed] [Google Scholar]
- 26. Wallman J, Adams JI. Developmental aspects of experimental myopia in chicks: susceptibility, recovery and relation to emmetropization. Vision Res. 1987; 27: 1139–1163 [DOI] [PubMed] [Google Scholar]
- 27. McBrien NA, Norton TT. The development of experimental myopia and ocular component dimensions in monocularly lid-sutured tree shrews (Tupaia belangeri). Vision Res. 1992; 32: 843–852 [DOI] [PubMed] [Google Scholar]
- 28. Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res. 1987; 6: 993–999 [DOI] [PubMed] [Google Scholar]
- 29. Norton TT, Essinger JA, McBrien NA. Lid-suture myopia in tree shrews with retinal ganglion cell blockade. Vis Neurosci. 1994; 11: 143–153 [DOI] [PubMed] [Google Scholar]
- 30. McBrien NA, Moghaddam HO, Cottriall CL, Leech EM, Cornell LM. The effects of blockade of retinal cell action potentials on ocular growth, emmetropization and form deprivation myopia in young chicks. Vision Res. 1995; 35: 1141–1152 [DOI] [PubMed] [Google Scholar]
- 31. Wildsoet C. Neural pathways subserving negative lens-induced emmetropization in chicks—insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res. 2003; 27: 371–385 [DOI] [PubMed] [Google Scholar]
- 32. Wallman J, Gottlieb MD, Rajaram V, Fugate-Wentzek LA. Local retinal regions control local eye growth and myopia. Science. 1987; 237: 73–77 [DOI] [PubMed] [Google Scholar]
- 33. Diether S, Schaeffel F. Local changes in eye growth induced by imposed local refractive error despite active accommodation. Vision Res. 1997; 37: 659–668 [DOI] [PubMed] [Google Scholar]
- 34. Grosvenor T. Reduction in axial length with age: an emmetropizing mechanism for the adult eye? Am J Optom Physiol Opt. 1987; 64: 657–663 [PubMed] [Google Scholar]