Abstract
Apoptotic cells are cleared by phagocytosis during development, homeostasis, and pathology. However, it is still unclear how necrotic cells are removed. We compared the phagocytic uptake by macrophages of variants of L929sA murine fibrosarcoma cells induced to die by tumor necrosis factor-induced necrosis or by Fas-mediated apoptosis. We show that apoptotic and necrotic cells are recognized and phagocytosed by macrophages, whereas living cells are not. In both cases, phagocytosis occurred through a phosphatidylserine-dependent mechanism, suggesting that externalization of phosphatidylserine is a general trigger for clearance by macrophages. However, uptake of apoptotic cells was more efficient both quantitatively and kinetically than phagocytosis of necrotic cells. Electron microscopy showed clear morphological differences in the mechanisms used by macrophages to engulf necrotic and apoptotic cells. Apoptotic cells were taken up as condensed membrane-bound particles of various sizes rather than as whole cells, whereas necrotic cells were internalized only as small cellular particles after loss of membrane integrity. Uptake of neither apoptotic nor necrotic L929 cells by macrophages modulated the expression of proinflammatory cytokines by the phagocytes.
INTRODUCTION
During embryonic development, tissue homeostasis, immune regulation, and other physiological processes, superfluous and harmful cells are eliminated through an ordered cellular process called apoptosis that involves proteolytic activation or inactivation of proteins by caspases (Earnshaw et al., 1999; Lamkanfi et al., 2002a,b). During apoptosis, the plasma membrane blebs, chromatin is condensed, the nucleus is fragmented, DNA is degraded, and apoptotic bodies are formed (Earnshaw et al., 1999). Nevertheless, the plasma membrane of the dying cell typically remains intact until the late stages of apoptosis, preventing leakage of cell contents (Denecker et al., 2001a). Rapid recognition, uptake, and degradation of apoptotic cells by phagocytes limits potential tissue injury by avoiding the spilling of cellular contents and prevents the occurrence of (auto)immune responses to intracellular autoantigens associated with dying cells (Ren and Savill, 1998). Apoptotic cells undergo specific surface changes that signal professional or nonprofessional phagocytes to bind and engulf them. Among these markers is most notably the surface exposure of phosphatidylserine (PS) due to loss of plasma membrane asymmetry (Henson et al., 2001; Scott et al., 2001). PS exposure is crucial for the uptake of apoptotic cells (Fadok et al., 2001b) and precedes DNA degradation, zeiosis, and cell lysis (Verhoven et al., 1999; Denecker et al., 2000), indicating that it is an early event. When apoptotic cells are phagocytosed in an early stage of the cell death process, all of the subsequent apoptotic events occur inside the phagocytes, including the DNA-fragmentation by the macrophage lysosomal DNaseII instead of the caspase-activated DNase CAD (McIlroy et al., 2000).
Necrotic cell death has classically been described in severe and acute physicochemical injury. However, necrosis can also occur when a cell death stimulus is given under conditions where the apoptotic pathway is blocked by absence of caspase-8 and overexpression of Bcl-2, or by the presence of viral or synthetic caspase-inhibitors (Vercammen et al., 1998; Matsumura et al., 2000; Kalai et al., 2002). Typically during necrosis, plasma membrane integrity is lost, the cell and organelles swell, and eventually the cytosolic content is released in the surrounding tissue (Kitanaka and Kuchino, 1999; Denecker et al., 2001a; Kalai et al., 2002), possibly initiating inflammatory or autoimmune responses (Fadok et al., 2001a). Several studies demonstrated that necrosis occurs during embryonic development in a programmed, nonaccidental manner. Caspase-independent necrotic cell death of the interdigital cells can replace apoptosis and correctly shape the fingers of Apaf-1–deficient mice or mice receiving zVAD-fmk, suggesting that necrosis can function appropriately in a developmental context (Chautan et al., 1999). Necrosis also occurs in pathological conditions such as bacterial infection (Francois et al., 2000), inflammatory disease (Wang et al., 1996), brain ischemia (Beilharz et al., 1995), myocardial infarction, and Alzheimer's and Parkinson's diseases (Kitanaka and Kuchino, 1999). Moreover, caspase-independent necrotic cell death in T cells induced by Fas ligand is executed through a specific pathway requiring the presence of RIP1 (Holler et al., 2000). Down-modulation of RIP1 by geldanamycin prevents necrosis and results in caspase-dependent apoptosis in L929 cells treated with tumor necrosis factor (TNF) (Vanden Berghe et al., 2003), suggesting that the composition of the death receptor complex may be decisive in determining the type of cell death.
Uptake of necrotic cells was reported to induce maturation of dendritic cells (Sauter et al., 2000) and production of proinflammatory cytokines in phagocytes (Fadok et al., 2001a). Most studies on uptake of necrotic cells are based on model systems in which necrosis is caused by physical damage of the target cells, leading to rupture of the membrane and rapid release of the intracellular content. We developed a cellular system in which L929sAhFas cells die by apoptosis when stimulated with agonistic anti-Fas antibodies or soluble Fas ligand, and by necrosis when treated with TNF (Vercammen et al., 1997). Here, we used this cellular system to study the molecular mechanisms involved in the phagocytosis of necrotic cells in comparison with the mechanism of clearance of apoptotic cells.
MATERIALS AND METHODS
Cells
The L929sA murine fibrosarcoma cell line, selected for its sensitivity to the cytotoxic activity of TNF (Vanhaesebroeck et al., 1991), was stably transfected with the cDNAs encoding the human TNF receptor (Vercammen et al., 1995) and the human Fas receptor (Vercammen et al., 1997, 1998), to produce L929sAhR55 and L929sAhFas cells, respectively. L929rTA cells (Vanhoenacker et al., 1999) were stably cotransfected with three plasmids: pPHT, pTet-tTS (BD Biosciences Clontech, Palo Alto, CA), and pUHD10–3-FKBP2-link-FADD coding for hygromycin resistance, a tetracyclin-controlled transcriptional silencer (Freundlieb et al., 1999), and a FKBP2-link-FADD fusion protein, respectively.
Mf4/4 (Desmedt et al., 1998) and P388D1 are mouse macrophage cell lines and J774 is a mouse monocyte-derived macrophage cell line. Cells were grown in RPMI medium (Invitrogen, Eggenstein, Germany), supplemented with 10% fetal calf serum, penicillin (100 U/ml), glutamax I (200 μM), β-mercaptoethanol (2 × 10-5 mM), and sodium pyruvate (1 mM), and kept under lipopolysaccharide (LPS)-free conditions.
Antibodies, Cytokines, and Reagents
Recombinant murine TNF was produced in-house (Tavernier et al., 1987). The receptor-specific mutein of hTNF (R32WS86T) has been described previously (Van Ostade et al., 1993). Agonistic antihuman Fas antibody (IgG3, clone 2R2) was from BioCheck (Münster, Germany). Soluble FLAG-tagged Fas ligand (FasL) was produced and purified in-house, as described previously (Schneider et al., 1998). Recombinant annexin V (BD PharMingen, San Diego, CA) was used at 10 μg per 105 cells. Annexin V-fluorescein isothiocyanate (FITC) conjugate (BD PharMingen) was used at 1 μg/ml. Cell Tracker Green and Orange were from Molecular Probes (Eugene, OR). Fluorescent cell linkers PKH26 (Red) and PKH67 (Green) were from Sigma-Aldrich (St. Louis, MO). Propidium iodide (BD Biosciences, San Jose, CA) was used at 30 μM. Purified rat antimouse CD16/CD32 (Fcγ III/II receptor) IgG2b′κ antibody (BD PharMingen) was used at 5 μg/ml. zVAD-fmk was from Enzyme Systems Products (Livermore, CA). Doxycyclin (Duchefa Biochemicals, Haarlem, The Netherlands) was used at 1 μg/ml. The FKBP dimerizer AP1510 was kindly provided by ARIAD Pharmaceuticals (Cambridge, MA) and used at 1 μM (Amara et al., 1997). Phycoerythrin-conjugated F4/80 rat-antibody was from Serotec (Oxford, United Kingdom).
Induction of Apoptotic and Necrotic Cell Death
L929sAhR55 and L929sAhFas target cells were seeded at 2.5 × 105 cells/well in uncoated 24-well tissue culture plates (Sarstedt, Newton, NC). The next day, anti-Fas antibody (250 ng/ml), Fas ligand (150 ng/ml), or mTNF (10,000 IU/ml) were added to L929sAhFas cells, and hTNF R32WS86T (100 ng/ml) to L929sAhR55 cells. Cells were harvested at indicated times and put on ice before analysis with a FACSCalibur flow cytometer (BD Biosciences). Loss of cell membrane integrity as a measure of cell death was determined by propidium iodide (PI) fluorescence (excitation 535/emission 617). PS exposure was monitored by annexin V-FITC staining (excitation 494/emission 518) (BD PharMingen).
In Vitro Phagocytosis Assay
Target cells were stained with 5 μM Cell Tracker Green (excitation 492/emission 516) and seeded at 2.5 × 105 cells/well in uncoated 24-well suspension tissue culture plates. Macrophage-like cells were stained with 5 μM Cell Tracker Orange (excitation 540/emission 566) and seeded in adherent 24-well plates at 2.5 × 105 cells/well. Cells were cultured overnight at 37°C in 5% CO2. Target cells were induced to undergo apoptosis or necrosis and collected for coincubation with phagocytes at the indicated time points. For coincubation, 200 μl of a 2.5 × 106/ml suspension of target cells was added to each well, resulting in a 1:1 ratio of macrophage-like cells to target cells. Cells were cocultured at 37°C in 5% CO2 for the indicated times, after which the cells were detached from the plates with enzyme-free cell dissociation buffer (Invitrogen), washed, and resuspended in phosphate-buffered saline (PBS). Data acquisition was performed on a FACSCalibur flow cytometer, by using Cell Quest software (BD Biosciences). At each time point, 5000 cells were analyzed. Quantitative analysis was done using Flowjo software (Tree Star, Ashland, OR).
In Vivo Phagocytosis Assay
L929rTA and L929rTAFADD cells stained with 5 μM CTGr were treated with doxycyclin and AP1510, 10 and 5 h before injection, respectively. After washing in PBS, 2 × 106 target cells were injected into the peritoneum of each mouse. Mice were sacrificed 4 h later, and their peritoneal cells were recovered by flushing with 10 ml of PBS. The recovered cells were counted. The cell type composition was determined microscopically from cytospin slides stained with May-Grünwald/Giemsa (Sigma-Aldrich). Phagocytosis was determined by flow cytometry as described above, after the staining of the macrophage population with a phycoerythrin-conjugated F4/80 antibody (McKnight et al., 1996).
Transmission Electron Microscopy
Nonlabeled adherent cocultures of macrophages and target cells (prepared as described above) were fixed by immersion in 2% glutaraldehyde containing 1 mM CaCl2, buffered with 0.1 M Na-cacodylate (pH 7.4). After a rinse in 100 mM Na-cacodylate containing 7.5% sucrose, the cocultures were osmicated overnight in 1% OsO4 in the same buffer (without sucrose) and embedded in LX medium after dehydration in an ethanol ascending series (Ladd, Burlington, VT). Ultrathin 60-nm sections, mounted on Formvar-coated copper grids, were stained with uranyl acetate and lead citrate and examined with a JEOL 1200 EXII electron microscope.
RNase Protection Assay
L929sAhR55 and L929sAhFas target cells were seeded at 5 × 106 cells/plate in uncoated 9-cm-diameter tissue culture dishes (Bibby Sterilin, Staffordshire, United Kingdom). Mf4/4 cells were seeded at 5 × 106 in adherent 9-cm-diameter culture dishes (Nunclon, Roskilde, Denmark). The next day, L929sAhFas cells were stimulated with Fas ligand (150 ng/ml) for 1 h and L929sAhR55 cells with hTNF R32WS86T (100 ng/ml) for 14 h. Mf4/4 cells were left untreated or treated with 100 ng/ml LPS 2 h before addition of control or treated target cells. Target cells were added to the phagocytes and coincubated for 1 h and then washed away. Total RNA was prepared using RNeasy (QIAGEN, Westburg B.V., The Netherlands), 4 h later. Cytokine mRNA levels were measured by RNase protection assay by using the Riboquant multiprobe set (BD PharMingen). In brief, 15 μg of RNA per sample was hybridized overnight with a 32P-labeled RNA multiprobe template set (mck-3b; BD PharMingen). Single-stranded RNA and free probe were digested by RNase A and T1. Protected RNA was separated on a 6% denaturing polyacrylamide gel and visualized and quantified using PhosphorImager software (Molecular Dynamics, Sunnyvale, CA). Probes specific for mRNAs of the housekeeping genes L32 and GAPDH were included as negative controls. For quantification, cytokine values were expressed as a percentage of the mean values of L32 for each lane.
Interleukin (IL)-6 and TNF Bioactivity Measurement
IL-6 secreted in the incubation medium was quantified using a bioassay, viz., IL-6–dependent growth of 7TD1 cells (Poupart et al., 1987). TNF concentration was determined in a standardized cytotoxicity assay by using L929sA cells.
RESULTS
Flow Fluorocytometrical Analysis of Phagocytosis of Dying Cells
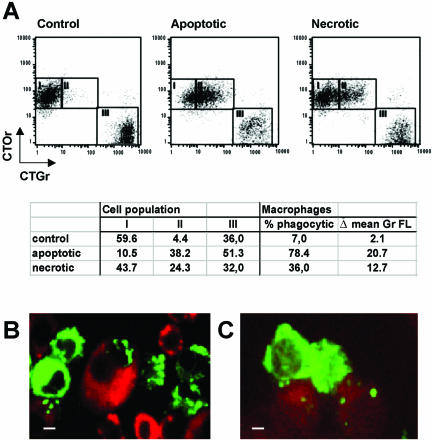
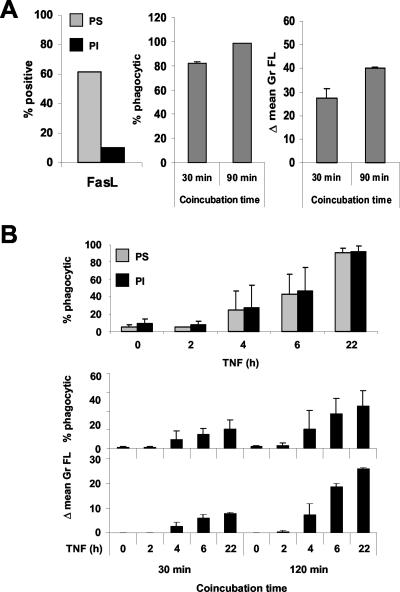
To quantify phagocytosis of apoptotic and necrotic cells by macrophages, we designed a two-parameter flow cytometry phagocytosis assay in which Cell Tracker Green (CTGr) labeled apoptotic, necrotic, or viable control L929sAhFas target cells were incubated at a ratio of 1:1 with a monolayer of Mf4/4 macrophages labeled with Cell Tracker Orange (CTOr). Target cells were labeled and then treated with agonistic anti-Fas antibodies to elicit apoptotic cell death or with TNF to initiate necrotic cell death. After 30 min of coincubation, macrophages and target cells were detached from the plates with enzyme-free dissociation buffer, a treatment that also detaches adhering target cells from the macrophages, as verified by light microscopy. The extent of phagocytosis was quantified by fluorescence activated cell sorter (FACS) analysis (Figure 1A). The percentage of double-stained macrophages (square II) out of the whole macrophage population (squares I + II) measures the fraction of the macrophage population involved in phagocytosis of target cells (% phagocytic). The difference in the mean green fluorescence values of the macrophage population before and after coincubationwith the CTGr-labeled target cells (Δ mean Gr FL) determines the amount of target cell material actually taken up by the macrophages.
Figure 1.
Mf4/4 macrophage-like cells take up apoptotic and necrotic L929sAhFas cells. (A) Dot plot representation of a flow fluorocytometrical analysis of CTOr-stained Mf4/4 cells after 30-min coincubation with CTGr-stained target cells treated as follows: untreated (control); 2 h, anti-Fas 250 ng/ml (apoptotic); and 18 h, TNF 104 IU/ml (necrotic). Mf4/4 cells accumulate in squares I (no uptake) and II (uptake) and free target cells in square III. The calculated percentage of double-positive Mf4/4 cells (% phagocytic) was used as a measure of the engagement of macrophages in phagocytosis, and the increase in the mean green fluorescence (Δ mean Gr FL) was used as an estimate of the amount cleared by the phagocytes. (B and C) Confocal microscopic visualization of uptake of apoptotic (B) and necrotic cells (C). Mf4/4 cells were labeled with PKH26 (red) and target cells with PKH67 (green). Note in B an engulfed apoptotic cell nucleus. Bars (B and C), 0.5 μm.
These results were confirmed by confocal laser scanning microscopy, by using cell linker dye PKH26 (red) for Mf4/4 cells, and PKH67 (green) for target cells (Figure 1, B and C). When apoptotic cells were coincubated with Mf4/4 cells, uptake consisted of apoptotic bodies of various sizes, up to several per phagocyte (Figure 1B). Occasionally, whole cells or parts containing the nucleus of a dying cell were engulfed (Figure 1B). Phagocytosis of necrotic target cells was limited to the engulfment of one or more small cytosolic particles per phagocyte (Figure 1C).
Phagocytosis of Apoptotic and Necrotic Cells Occurs through a PS-dependent Mechanism
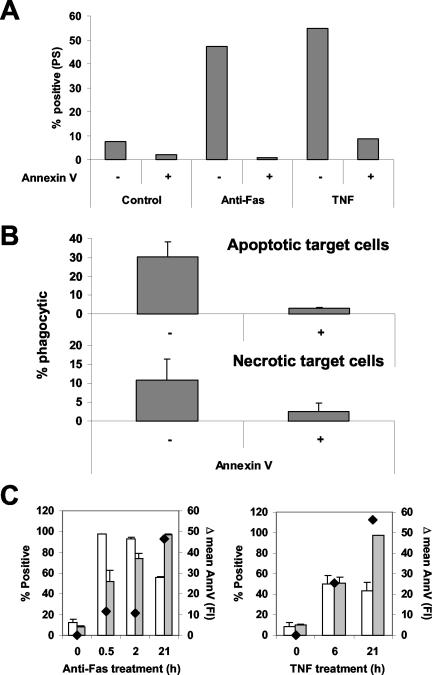
Apoptotic and necrotic cell death can be distinguished by the differential appearance of two characteristics, which are PS exposure, as measured by annexin V-FITC labeling, and membrane permeability, assessed by PI staining. In Fas-mediated apoptosis, PS exposure precedes loss of membrane integrity, whereas in TNF-induced necrosis both processes occur simultaneously (Denecker et al., 2001b). We determined in parallel the kinetics of these parameters on target cells and compared them with the extent of macrophage-mediated uptake after 30-min coincubation with apoptotic or necrotic cells. Macrophages specifically recognized dying cells, because no uptake of living control cells was observed (Figure 1A, 2). Uptake of apoptotic cells coincided with the rapid exposure of PS (Figure 2A). With necrotic cells, phagocytosis occurred concomitantly with PI and PS positivity (Figure 2B). These results demonstrate that phagocytosis of apoptotic and necrotic cells by macrophages, coincides with PS exposure. Therefore, we examined whether the recognition and uptake of both types of dying cells is PS dependent, by shielding the PS exposed on the surface of apoptotic, necrotic and viable control target cells with recombinant unlabeled annexin V. This treatment not only protected the cells from annexin V-FITC staining (Figure 3A) but also led to a drastic, nearly complete inhibition of phagocytosis of both apoptotic and necrotic cells (Figure 3B). These data demonstrate that a PS-dependent mechanism mediates the uptake of necrotic as well as apoptotic cells.
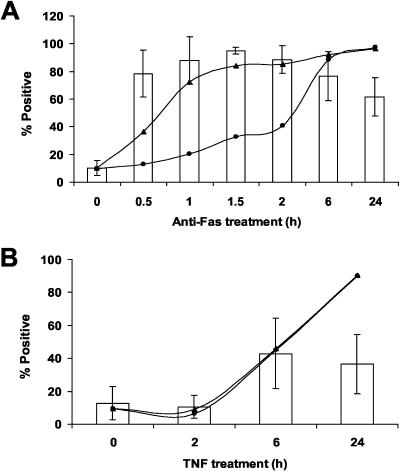
Figure 2.
Uptake of apoptotic and necrotic cells after PS exposure. Uptake of target cells stimulated with anti-Fas (250 ng/ml) (A) or TNF (10,000 IU/ml) (B) for the indicated time periods. Bars represent percentage of double-positive Mf4/4 cells (% phagocytic), triangles percentage PS positive, and circles percentage of PI positive cells as a measure of membrane permeability. Error bars represent the SD of at least three independent experiments.
Figure 3.
Pretreatment with recombinant annexin V inhibits the uptake of apoptotic and necrotic targets cells by Mf4/4 cells. Control, apoptotic (2 h, anti-Fas) and necrotic (20 h, TNF) L929hFas cells were treated with unlabeled recombinant annexin V (10 μg/105 cells) and stained with annexin V-FITC (A), or coincubated for 30 min with Mf4/4 cells (B). Annexin V staining and phagocytosis were analyzed by flow cytometry. (C) Although early apoptotic cells present a lower PS exposure level than necrotic or late apoptotic cells, as demonstrated by the relative mean increase in annexin V fluorescence with time [Δ mean Ann V (Fl)], they present better targets for phagocytosis. Empty bars represent percentage of double-positive Mf4/4 cells (% phagocytic), whereas gray bars represent the percentage of PS-positive target cells. Diamonds represent the Δ mean Ann V (Fl). Error bars represent the standard deviations of two independent experiments.
Phagocytosis of Necrotic Cells Is Less Efficient and Requires More Time
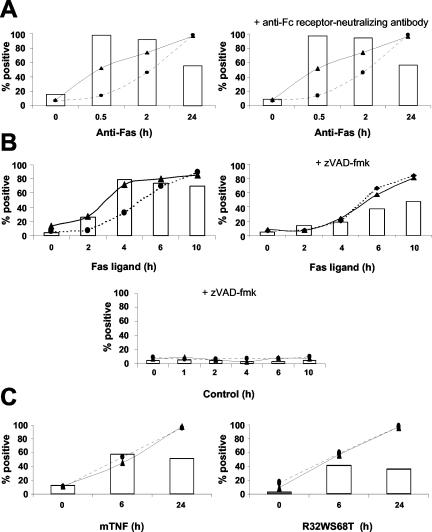
Although the levels of annexin V staining were higher with necrotic and late apoptotic cells, the best targets for phagocytosis were early apoptotic cells with a relatively low level of mean annexin V staining (Figure 3C). Indeed, exposure of macrophages for 30 min to early apoptotic cells was sufficient to induce phagocytic activity in most of the macrophages (Figure 2). However, only about one-half of the macrophages engaged in uptake of necrotic cells. To exclude the possibility that early apoptotic cells were more efficient than necrotic cells in inducing the phagocytic activity of macrophages due to Fc receptor anti-Fas antibody interaction, we compared the uptake of target cells by untreated Mf4/4 cells with others pretreated with a neutralizing rat anti-mouse Fc receptor antibody. Shielding the Fc receptor on the phagocytes had no effect on their ability to engulf anti-Fas–stimulated apoptotic cells (Figure 4A), suggesting that IgG3–Fc receptor interactions did not contribute to phagocytosis of the apoptotic cells. Similar results were obtained when apoptosis was induced by recombinant human FasL (Figure 4B), excluding the involvement of Fc receptors and effects dependent on antibody opsonization.
Figure 4.
Phagocytosis of L929sAhFas and L929sAhR55 cells induced to die by different stimuli, via Fas or TNF receptor pathway. CTGr-labeled target cells were coincubated with CTOr-labeled Mf4/4 cells for 30 min before analysis. Percentage of double-positive Mf4/4 cells (bars) was determined for uptake of apoptotic and necrotic targets. In parallel, nonlabeled target cells were double stained with annexin V-FITC and PI for assessment of PS exposure (triangles) and membrane permeability (circles), respectively. (A) Anti-Fas–treated L929sAhFas were coincubated with untreated Mf4/4 cells (left), or with Mf4/4 cells pretreated with an anti-Fc receptor neutralizing antibody (right). (B) L929sAhFas cells were untreated (left) or pretreated with 25 μM zVAD-fmk for 2 h (right) and then stimulated with Fas ligand for the indicated time before coincubation with Mf4/4 cells. Negative controls of L929sAhFas cells were treated with zVAD-fmk alone (bottom). (C) Necrotic cells were prepared from L929sAhFas cells (left) or L929sAhR55 cells (right) treated with mTNF and R32WS68T, respectively.
To exclude the possibility that murine TNF transferred from the necrotic cells affected the phagocytic activity of the macrophages, we tested uptake of necrotic cells induced to die by other stimuli. In L929sAFas cells, Fas-activation in the presence of the pan caspase inhibitor benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone (zVAD-fmk) results in necrosis (Vercammen et al., 1998). Comparison of the engagement of macrophages in phagocytosis of L929sAFas cells treated with FasL in the presence or absence of zVAD-fmk confirmed that phagocytosis of necrotic target cells is less efficient than phagocytosis of apoptotic cells (Figure 4B). Similar results were obtained with necrotic L929sAFas cells induced to die by wild-type human TNF, and necrotic L929sAhR55 cells expressing the human TNF-receptor 1 (R1) that were treated with R32SW68T, a human TNF mutant that solely activates human TNF-R1 (Loetscher et al., 1993) (Figure 4C). Moreover, in 16 independent experiments the efficiency of apoptotic cells in inducing phagocytosis decreased when the target cells lost their membrane integrity and became PI positive, a phenomenon designated secondary necrosis (Figure 2A). Together, these results exclude interference by ligand transfer and suggest that irrespective of the stimulus, the efficiency of uptake of necrotic cells was consistently lower than that of apoptotic cells. The onset of uptake under all of these conditions coincided with the exposure of PS. Similar results were obtained using two other murine macrophage cell lines (J774 and P388D1), extending the observation that macrophages internalize cellular particles of necrotic cells.
Next, we tested the effect of coincubation time on the efficiency of phagocytosis. Increasing the coincubation time from 30 to 90 min improved the uptake of early apoptotic cells without significantly increasing the number of Mf4/4 cells involved in phagocytosis, probably because most of them were already phagocytic after a short time of incubation (Figure 5A). In contrast, prolonging the coincubation time of necrotic cells with macrophages from 30 to 120 min increased both the number of Mf4/4 cells involved in phagocytosis and the amount of material cleared, indicating that uptake of necrotic particles is more difficult and requires more time (Figure 5B).
Figure 5.
Time kinetics of uptake of apoptotic and necrotic target cells. (A) Apoptotic target cells. L929hFas cells were stimulated with Fas ligand for 1 h and then coincubated with Mf4/4 cells for 30 or 90 min. (B) Necrotic target cells. L929hR55 cells were stimulated with R32WS68T (100 ng/ml) and coincubated with Mf4/4 cells for the indicated time periods and then phagocytosis was determined by flow cytometry. PI positivity and annexin V positivity were determined on the target cells just before coincubation. Error bars represent the SD of at least three independent experiments.
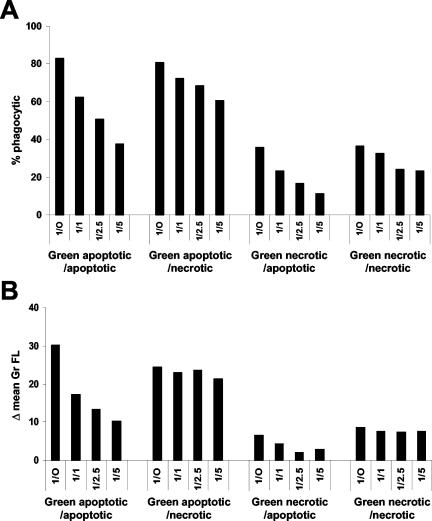
Next, we tested whether apoptotic and necrotic cells can compete with each other for uptake. We performed a phagocytosis assay using fixed amounts of labeled apoptotic or necrotic cells and increasing numbers of unlabeled apoptotic or necrotic competitors (Figure 6). Unlabeled apoptotic cells were able to compete with labeled apoptotic and necrotic cells in a dose-dependent manner (Figure 6). The effect was apparent both on the number of macrophages engaged in phagocytosis of labeled cells (Figure 6A) and on the amount of labeled material that was engulfed (Figure 6B). Conversely, unlabeled necrotic cells were poor competitors with both types of labeled target cells, decreasing the proportion of the macrophage population engaged in the phagocytosis of labeled cells only slightly (Figure 6A), and hardly affecting the amount of labeled material that was actually removed (Figure 6B). These results suggest that macrophages have a preference for clearing apoptotic compared with necrotic cells.
Figure 6.
Apoptotic cells are better competitors than necrotic cells for uptake of apoptotic or necrotic cells by Mf4/4 cells. Cell Tracker Green-labeled early apoptotic L929hFas cells treated for 1 h with Fas ligand, and necrotic L929hR55 cells treated for 14 h with R32WS86T, were incubated with Cell Tracker Orange-labeled Mf4/4 cells in the presence or absence of unlabeled competitor target cells. Labeled targets (2.5 × 105 cells/well) were mixed with unlabeled apoptotic or necrotic competitor cells at the indicated ratios and added to the macrophages. The extent of phagocytosis was determined after 30 min. (A) Percentage of double-positive Mf4/4 cells (% phagocytic). (B) The increase in the mean green fluorescence of the macrophage population (Δ mean Gr FL).
The Mechanisms of Phagocytosis of Apoptotic and Necrotic Cells Are Morphologically Different
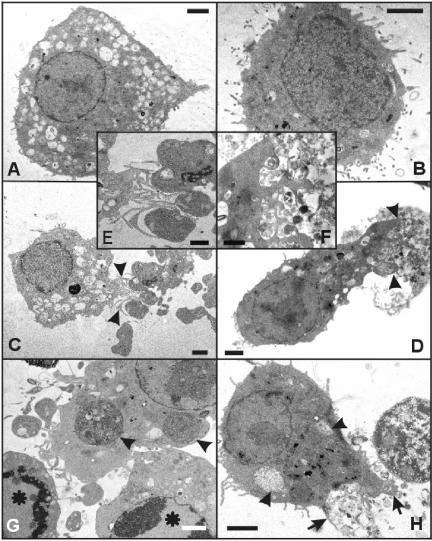
Our previous studies (Vercammen et al., 1998; Kalai et al., 2002; Vanden Berghe et al., 2003) and confocal microscopy analysis (Figure 1) showed that apoptotic L929 cells break down into many small apoptotic bodies, suggesting that the apoptotic process increases the number of easy to engulf targets. In contrast, necrotic cells swell and increase in size and may become harder to engulf. We used electron microscopy to examine and compare the subcellular structures involved in the phagocytic process of apoptotic and necrotic cells by macrophages (Figure 7). Although macrophages were previously reported to engulf small-sized, dying cells such as neutrophils or T cells as single entities (Fadok et al., 1998; Hirt et al., 2000; Cocco and Ucker, 2001), we did not observe uptake of whole necrotic and only rarely of whole apoptotic cells. This might be due to the fact that the target cells and Mf4/4 cells were of similar size (Figure 7, A and B) or due to the larger cytoplasm/nucleus size ratio of the target cells that led to more apoptotic body formation. Apoptotic cells showed a compacted nucleus, with dense chromatin distributed along the nuclear envelope, and condensed cytoplasm typically broken up into apoptotic bodies (Figure 7, C, E, and G). In contrast, necrotic cells contained a mottled-looking nucleus and very translucent cytoplasm, surrounded by an ill defined, damaged cell membrane (Figure 7, D, F, and H). Mf4/4 cells engaged in phagocytosis stretched out by moving most of their vacuole-containing cytoplasm toward the dying cells, in contrast to resting macrophages that showed a more symmetrical morphology. In dealing with apoptotic cells, macrophages formed thin protrusions that easily engulfed the well-defined, membrane-enclosed apoptotic bodies (Figure 7, C and E). Internalized apoptotic material showed up as dark, condensed areas within the lighter staining macrophage cytoplasm (Figure 7G). Phagocytes dealing with necrotic cells stretched out by sending thick protrusions into the swollen “ghost”-like structures of the target cells, grasping only small volumes of the cellular debris (Figure 7, D and F). On internalization, this necrotic material showed up as a lighter and probably less condensed material compared with the macrophage cytoplasm (Figure 7H). These results suggest that phagocytosis of necrotic and apoptotic cells occur by different mechanisms and that the former process may be more difficult.
Figure 7.
Electron microscopy micrographs of phagocytic uptake of apoptotic and necrotic L929sAhFas cell particles by Mf4/4 cells. (A) A control Mf4/4 macrophage-like cell with abundant cytoplasm containing well-developed lysosomal apparatus. (B) A control unstimulated L929sAhFas cell with well preserved cytoplasmic organelles, smoothly outlined nuclei containing hetero-chromatin, and numerous microvilli protruding from the entire surface. (C, E, and G) Uptake of early apoptotic L929sAhFas cells by Mf4/4 cells after 30 min of coculture. Target cells were treated with agonistic anti-Fas for 1 h before coculture. (D, F, and H) Uptake of necrotic L929sAhFas cells by Mf4/4 cells after 90 min of coculture. Target cells treated with TNF for 18 h before coculture. (C) Engulfment of apoptotic bodies by an Mf4/4 cell. (E) Enlargement of C, showing the protrusions of the phagocyte (arrowheads) toward the apoptotic bodies. (D) Uptake of necrotic material by an Mf4/4 cell (arrowheads). (F) Enlargement of D, showing a labyrinth of surface protrusions of the phagocyte surrounding disintegrated cytoplasm of a necrotic target cell. (G) An Mf4/4 cell with engulfed apoptotic bodies of different sizes (arrowheads), surrounded by two apoptotic cells (asterisks) with condensed chromatin at the margins of the nuclei (bottom). (H) An Mf4/4 cell containing engulfed necrotic particles (arrowheads) displaying surface protrusions (arrows) toward the cytoplasm of a disintegrating necrotic cell. Bars (A, B, C, D, G, and H), 1 μm; (E and F), 2 μm.
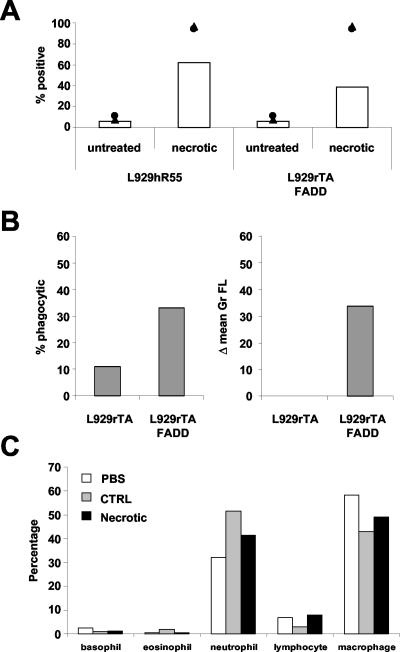
Phagocytosis of Necrotic Cells by Macrophages Occurs In Vitro and In Vivo and Does Not Stimulate Macrophages to Express Proinflammatory Cytokines
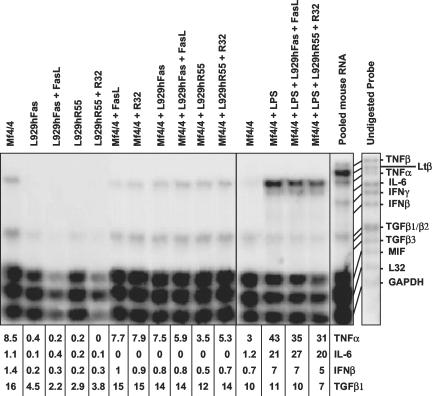
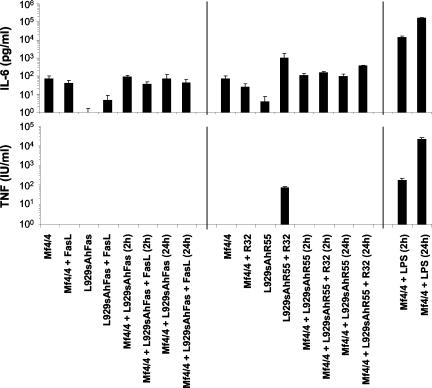
Overexpression of FADD in L929 cells leads to necrosis (Boone et al., 2000). To study phagocytosis of necrotic cells in vivo, while excluding effects due to transfer of the death-inducing stimulus, we used stably transfected L929rTA for inducible expression of a FKBP-FADD fusion protein (L929rTAFADD). Treatment of these cells with doxycyclin followed by the FKBP dimerizer AP1510 induces the expression and forced oligomerization of FADD and results in a typical necrotic cell death (Figure 8A). We compared the in vitro with the in vivo phagocytosis of necrotic L929rTAFADD cells by primary macrophages. For that purpose, we tested the uptake of the cells by Mf4/4 cells (Figure 8A). In parallel, we injected CTGr-labeled viable L929rTA or necrotic L929rTAFADD into the peritonea of mice, recovered the peritoneal cells after 4 h, stained the macrophage population with phycoerythrin-conjugated macrophage-specific antibody (McKnight et al., 1996), and determined their phagocytic efficiencies (Figure 8B). Phagocytosis of L929rTAFADD cells treated with doxycyclin and AP1510 by Mf4/4 cells was as efficient as that of R32WS86T-treated L929hR55 cells (Figure 8A). Similar results were obtained in vivo (Figure 8B), indicating that phagocytosis of necrotic cells by macrophages occurs to the same extent both in vivo and in vitro. Interestingly, analysis of the peritoneal cells by May Grünwald/Giemsa staining and microscopy demonstrated that the cell-type composition was not significantly affected by exposure to the control or necrotic cells (Figure 8C). These results suggest that exposure to necrotic cells for 4 h did not induce a strong proinflammatory response. Fadok and coworkers (Fadok et al., 1998, 2001a) suggested that in contrast to apoptotic cells, necrotic cells induce proinflammatory cytokine production in macrophages. Therefore, we examined the effects of apoptotic and necrotic cells on the transcriptional expression profile of several inflammatory cytokines in Mf4/4 macrophages by using an RNase protection assay. To assess the expression of cytokines by phagocytic macrophages, total RNA was extracted from Mf4/4 cells 4 h after their exposure for 1 h to control, apoptotic, or necrotic target cells. Mf4/4 macrophages alone expressed low levels of mRNAs for TNF-α and transforming growth factor (TGF)β1, and only traces of mRNAs of IL-6 and interferon-β (IFNβ) (Figure 9). No changes were observed in the expression of the mRNAs of the tested cytokines by mf4/4 cells, after coincubation of these macrophages with control, apoptotic, or necrotic target cells. Target cells on their own, with or without the apoptotic or necrotic treatments, expressed the mRNAs of TGFβ1, TNF-α, IL-6, or IFNβ. To assess the possibility that apoptotic or necrotic cells can modulate the levels of mRNA of preexisting proinflammatory cytokines in activated macrophages, we pretreated macrophages with LPS for 2 h before exposure to apoptotic or necrotic target cells. Total RNA was prepared 5 h after addition of the target cells and analyzed for the presence of cytokine mRNAs. On LPS-stimulation, Mf4/4 macrophages showed a marked increase in the expression of the mRNAs of TNF-α, IL-6, and IFNβ, indicating the reliability of this analytical method, and demonstrating that the macrophages were capable of producing these cytokines (Figure 9). Moreover, the levels of mRNAs for TGFβ1, TNF-α, IL-6, and IFNβ expressed by LPS-stimulated macrophages were not affected by uptake of either apoptotic or necrotic cells. Furthermore, no modulation of TNF-α or IL-6 activity was detected in supernatants of Mf4/4 cells, whether or not they were exposed to apoptotic or necrotic target cells. LPS stimulation strongly induced the expression of these cytokines (Figure 10). These results indicate that phagocytosis of apoptotic and necrotic L929 cells apparently does not modulate the expression pattern of proinflammatory cytokines by macrophages at the mRNA and the protein levels. Interestingly, pretreatment of macrophages with LPS for 2 h before exposure to target cells resulted in an increase in the percentage of the macrophage population involved in phagocytosis from 71 to 92% for apoptotic cells, and from 47 to 63% for necrotic cells, suggesting that a proinflammatory stimulus may prime the macrophages for phagocytosis of dying cells.
Figure 8.
Phagocytosis of necrotic cells by peritoneal macrophages in vivo. (A) CTOr-labeled Mf4/4 cells were coincubated with CTGr-labeled untreated or necrotic L929hR55 cells, or L929rTAFADD cells. Percentage of double-positive Mf4/4 cells (bars) was determined 4 h later. Necrosis in L929hR55 and L929rTAFADD cells was induced by R32WS86T and by doxycyclin + AP1510, respectively. PI (circles) and PS (triangles) positivity were determined on the target cells just before coincubation. (B) In parallel, CTGr-labeled viable L929rTA, or necrotic L929rTAFADD cells treated with doxycyclin + AP1510, were injected into the peritoneum of mice. Peritoneal cells were recovered 4 h later, and macrophages were stained with a phycoerythrin-conjugated F4/80 antibody; phagocytosis was determined by flow cytometry. (C) The peritoneal cell-type composition was determined microscopically following May-Grünwald/Giemsa (Sigma-Aldrich) staining.
Figure 9.
Analysis of the effects of uptake of apoptotic or necrotic cells on the expression of inflammatory cytokine mRNA by the phagocytes. Cytokine mRNA levels were measured by RNase protection assay by using a Riboquant multiprobe set (BD PharMingen). Mf4/4 cells were left unstimulated or were stimulated with LPS for 2 h and then either coincubated or not with untreated or FasL-treated apoptotic L929hFas cells, or with untreated or R32WS86T-treated necrotic L929hR55 cells. RNA was isolated 5 h later. Controls of untreated and FasL- or R32WS86T-treated target and Mf4/4 cells were also taken. A mouse pool RNA included in the kit as a positive control, and the undigested 32P-labeled RNA probe, are shown on the right. Relative quantification of intensities is expressed as percentage of L32 expression.
Figure 10.
Analysis of the effect of phagocytosis of apoptotic or necrotic cells on the expression of biologically active IL-6 and TNF. Mf4/4 cells were coincubated for the indicated time with untreated or FasL-treated apoptotic L929hFas cells, or with untreated or R32WS86T-treated necrotic L929hR55 cells. Incubation medium was collected 24 h later and the concentrations of biologically active IL-6 and TNF were determined. Positive controls of Mf4/4 cells treated for 2 and 24 h with LPS and negative controls of untreated and FasL- or R32WS86T-treated target, and Mf4/4 cells are also presented. Measurements are presented in logarithmic scale. Error bars represent the SD of at least three independent experiments.
DISCUSSION
Although the molecular mechanisms involved in phagocytosis of apoptotic cells are becoming better understood, little is known about the recognition and uptake of necrotically dying cells. Here, we used differentially labeled macrophages and target cells to study the uptake of apoptotic and necrotic cells in a quantitative flow cytometry phagocytosis assay. The L929 fibrosarcoma cellular system provided us with well-characterized models of apoptosis and necrosis mediated by a death domain receptor (Denecker et al., 2001b), distinct from cell death caused by physical damage such as rupture of the cell membrane by douncing, freeze thawing, or exposure to high temperature (Sauter et al., 2000; Cocco and Ucker, 2001; Fadok et al., 2001a).
Previous studies showed that PS is a prerequisite for the uptake of apoptotic cells by macrophages. PS exposed on the apoptotic cell membrane is recognized directly by the macrophage PS receptor (Fadok et al., 2000) or indirectly via binding of PS to a soluble intermediate (milk fat globule-epidermal growth factor-factor 8), which is secreted by macrophages, followed by recognition of the complex by the αvβ3 integrin on the phagocyte's surface (Hanayama et al., 2002). Our results demonstrate that both apoptotic and necrotic target cells are recognized and phagocytosed by macrophages, whereas viable control cells are not. Uptake of apoptotic and necrotic cells occurred irrespective of the stimulus inducing cell death. Phagocytosis of necrotic cells is a feature common to different macrophage cell lines and also occurs in vivo, as demonstrated in Figure 8. Apoptotic cells are taken up very efficiently as soon as PS exposure becomes evident, at a time when their membrane is still intact. Uptake of necrotic cells takes place when cells loose the integrity of the membrane and become PS positive. Moreover, recombinant annexin V can inhibit phagocytosis of both types of dying cells, suggesting that externalization of PS is a general signal indicating to phagocytes that a dying cell should be cleared, irrespective of the way the cell has died. Indeed, PS exposure is an old “eat me signal” also used for the recognition and uptake of aged erythrocytes (Schlegel and Williamson, 2001). Although the recognition of dying L929sA cells seems to be dependent on PS for both apoptotic and necrotic cells, the efficiency of uptake is higher for the former. Moreover, when given the choice, macrophages prefer to clear early apoptotic cells, as demonstrated by the competition experiment (Figure 6), even though these target cells present a lower mean annexin V staining level. The lower efficiency of uptake of necrotic cells might be due to physical constraints. It is possible that the PS exposed on necrotic and late apoptotic cells is less accessible to the macrophages. Moreover, although apoptotic cells fragment into many small-contained particles that are easy to recognize and engulf, necrotic cells swell and remain as large single entities for a long time. Similar observations were made in Caenorhabditis elegans, where necrotic corpses linger for much longer than apoptotic ones, probably due to their larger volume, although uptake of both types of dying cells requires the same engulfment genes (Chung et al., 2000). Indeed, electron microscopy analysis presented here suggests that the mechanism used by macrophages to engulf necrotic cells is morphologically different from that used for apoptotic cells. Macrophages that engulf necrotic cells protrude into the swollen ghost-like structures of the dying cells, grasping only small volumes of the cellular debris, whereas apoptotic cells are readily engulfed as contained distinct apoptotic bodies (Krysko et al., 2003).
Phagocytosis of apoptotic cells does not induce inflammation and is often referred to as a silent event (Fadok et al., 1998; Cocco and Ucker, 2001; Fadok et al., 2001a). A possible consequence of the greater difficulty apparently encountered by macrophages in clearing necrotic cells is spillage of the cellular contents of the dying cells, leading to more histotoxicity and development of proinflammatory (Haslett, 1992; Haslett et al., 1994; Wiegand et al., 2001; Medan et al., 2002) or autoimmune responses (Rovere et al., 2000; Chan et al., 2001; Magnus et al., 2001). Indeed, apoptotic neutrophils and PS-exposing membranes were reported to elicit an antiinflammatory effect, whereas incubation of macrophages with physically lysed neutrophils, but not with lysed lymphocytes, significantly stimulated the production of macrophage-inflammatory protein 2, IL-8, TNF-á, and IL-10 (Fadok et al., 2001a). However, the latter effect was attributed to the cleavage of PS-receptor on the surface of the macrophages by elastase, a protease released upon the lysis of neutrophils. The cleavage of PS-receptor probably also impairs the phagocytic capacity of the macrophages and may cause them some stress (Vandivier et al., 2002). Our results show that phagocytosis of apoptotic or necrotic cells by Mf4/4 macrophages does not induce the expression of IFNβ, TGFβ, TNF, or IL-6, neither at the mRNA nor at the protein level. On the other hand, exposure of the same macrophages to LPS strongly increased the expression of TNF and IL-6 and moderately increased the levels of IFNβ mRNA. Moreover, phagocytosis of apoptotic or necrotic L929 cells did not affect this LPS-induced response. Several reports are in agreement with these findings. Cocco and Ucker (2001) observed that heat-killed necrotic and late apoptotic thymoma and T-cell hybridoma target cells did not induce J774A.1 and RAW 264.7 macrophages to secrete TNF or IL-6. Furthermore, no signs of inflammation accompanied the necrotic interdigital cell death occurring in Apaf-1–deficient mice and further development proceeded normally (Chautan et al., 1999). Although inflammation often accompanies necrosis, the above-mentioned observations and our results indicate that this is not due to the induction of expression of proinflammatory cytokines in the macrophages clearing the dying cells. Therefore, it is conceivable that the release of cytokines or other factors from the necrotic cells themselves may be crucial for an inflammatory response. Additionally, our results demonstrate that the clearance of primary and probably also secondary necrotic cells is clearly less efficient and more difficult and time consuming than that of apoptotic cells. This process may cause the macrophages to remain at the same site longer, thus heightening the inflammatory state. These results suggest that prevention of necrosis and secondary necrosis, and promotion of apoptosis may allow a more rapid and efficient clearance of the dying cells and decrease the damage to the surrounding tissue both in injury and in antitumor cancer treatment.
Acknowledgments
We thank Ann Meeus, Wilma Burm, Dominique Jacobus, Hubert Stevens, and Wim Van Molle for excellent technical assistance and Alex Raeymaekers for preparation of TNF. J774 cells were kindly provided by Dr. E. Smits (DevGen N.V.). This work was supported by the Interuniversitaire Attractiepolen (IUAP-V), the Fonds voor Wetenschappelijk Onderzoek Vlaanderen (grants 31.5189.00 and 3G.0006.01), the EC-RTD (grant QLRT-CT-1999-00739), the RUG-cofinanciering EU project (011C0300), and GOA project (12050502). G.B. is a doctoral fellow with the Fond for Scientific Research-Flanders (FWO). D.V.K. is a doctoral fellow with the GOA project (120505502).
Abbreviations used: CTGr, Cell Tracker Green; CTOr, Cell Tracker Orange; FACS, fluorescence-activated cell sorter; FasL, Fas ligand; IFN, interferon; IL, interleukin; LPS, lipopolysaccharide; PI, propidium iodide; PS, phosphatidylserine; TGF-β, tumor growth factor-β; TNF, tumor necrosis factor; TNF-R1, TNF-receptor 1; zVAD-fmk, benzyloxycarbonyl-Val-Ala-Asp(OMe)-fluoromethylketone.
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.E03–09–0668. Article and publication date are available at www.molbiolcell.org/cgi/doi/10.1091/mbc.E03-09-0668.
References
- Amara, J.F., Clackson, T., Rivera, V.M., Guo, T., Keenan, T., Natesan, S., Pollock, R., Yang, W., Courage, N.L., Holt, D.A., and Gilman, M. (1997). A versatile synthetic dimerizer for the regulation of protein-protein interactions. Proc. Natl. Acad. Sci. USA 94, 10618-10623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beilharz, E.J., Williams, C.E., Dragunow, M., Sirimanne, E.S., and Gluckman, P.D. (1995). Mechanisms of delayed cell death following hypoxic-ischemic injury in the immature rat: evidence for apoptosis during selective neuronal loss. Brain Res. Mol. Brain Res. 29, 1-14. [DOI] [PubMed] [Google Scholar]
- Boone, E., Vanden Berghe, T., Van Loo, G., De Wilde, G., De Wael, N., Vercammen, D., Fiers, W., Haegeman, G., and Vandenabeele, P. (2000). Structure/function analysis of p55 tumor necrosis factor receptor and Fas-associated death domain. Effect on necrosis in L929sA cells. J. Biol. Chem. 275, 37596-37603. [DOI] [PubMed] [Google Scholar]
- Chan, A., Magnus, T., and Gold, R. (2001). Phagocytosis of apoptotic inflammatory cells by microglia and modulation by different cytokines: mechanism for removal of apoptotic cells in the inflamed nervous system. Glia 33, 87-95. [DOI] [PubMed] [Google Scholar]
- Chautan, M., Chazal, G., Cecconi, F., Gruss, P., and Golstein, P. (1999). Interdigital cell death can occur through a necrotic and caspase-independent pathway. Curr. Biol. 9, 967-970. [DOI] [PubMed] [Google Scholar]
- Chung, S., Gumienny, T.L., Hengartner, M.O., and Driscoll, M. (2000). A common set of engulfment genes mediates removal of both apoptotic and necrotic cell corpses in C. elegans. Nat. Cell. Biol. 2, 931-937. [DOI] [PubMed] [Google Scholar]
- Cocco, R.E., and Ucker, D.S. (2001). Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol. Biol. Cell 12, 919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecker, G., Dooms, H., Van Loo, G., Vercammen, D., Grooten, J., Fiers, W., Declercq, W., and Vandenabeele, P. (2000). Phosphatidyl serine exposure during apoptosis precedes release of cytochrome c and decrease in mitochondrial transmembrane potential. FEBS Lett. 465, 47-52. [DOI] [PubMed] [Google Scholar]
- Denecker, G., Vercammen, D., Declercq, W., and Vandenabeele, P. (2001a). Apoptotic and necrotic cell death induced by death domain receptors. Cell Mol. Life Sci. 58, 356-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denecker, G., et al. (2001b). Death receptor-induced apoptotic and necrotic cell death: differential role of caspases and mitochondria. Cell Death Differ. 8, 829-840. [DOI] [PubMed] [Google Scholar]
- Desmedt, M., Rottiers, P., Dooms, H., Fiers, W., and Grooten, J. (1998). Macrophages induce cellular immunity by activating Th1 cell responses and suppressing Th2 cell responses. J. Immunol. 160, 5300-5308. [PubMed] [Google Scholar]
- Earnshaw, W.C., Martins, L.M., and Kaufmann, S.H. (1999). Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68, 383-424. [DOI] [PubMed] [Google Scholar]
- Fadok, V.A., Bratton, D.L., Guthrie, L., and Henson, P.M. (2001a). Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J. Immunol. 166, 6847-6854. [DOI] [PubMed] [Google Scholar]
- Fadok, V.A., Bratton, D.L., and Henson, P.M. (2001b). Phagocyte receptors for apoptotic cells: recognition, uptake, and consequences. J. Clin. Investig. 108, 957-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok, V.A., Bratton, D.L., Konowal, A., Freed, P.W., Westcott, J.Y., and Henson, P.M. (1998). Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 101, 890-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadok, V.A., Bratton, D.L., Rose, D.M., Pearson, A., Ezekewitz, R.A., and Henson, P.M. (2000). A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85-90. [DOI] [PubMed] [Google Scholar]
- Francois, M., Le Cabec, V., Dupont, M.A., Sansonetti, P.J., and Maridonneau-Parini, I. (2000). Induction of necrosis in human neutrophils by Shigella flexneri requires type III secretion, IpaB and IpaC invasins, and actin polymerization. Infect. Immun. 68, 1289-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freundlieb, S., Schirra-Muller, C., and Bujard, H. (1999). A tetracycline controlled activation/repression system with increased potential for gene transfer into mammalian cells. J. Gene Med. 1, 4-12. [DOI] [PubMed] [Google Scholar]
- Hanayama, R., Tanaka, M., Miwa, K., Shinohara, A., Iwamatsu, A., and Nagata, S. (2002). Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182-187. [DOI] [PubMed] [Google Scholar]
- Haslett, C. (1992). Resolution of acute inflammation and the role of apoptosis in the tissue fate of granulocytes. Clin. Sci. 83, 639-648. [DOI] [PubMed] [Google Scholar]
- Haslett, C., Savill, J.S., Whyte, M.K., Stern, M., Dransfield, I., and Meagher, L.C. (1994). Granulocyte apoptosis and the control of inflammation. Phil. Trans. R. Soc. Lond. B. Biol. Sci. 345, 327-333. [DOI] [PubMed] [Google Scholar]
- Henson, P.M., Bratton, D.L., and Fadok, V.A. (2001). Apoptotic cell removal. Curr. Biol. 11, R795-R805. [DOI] [PubMed] [Google Scholar]
- Hirt, U.A., Gantner, F., and Leist, M. (2000). Phagocytosis of nonapoptotic cells dying by caspase-independent mechanisms. J. Immunol. 164, 6520-6529. [DOI] [PubMed] [Google Scholar]
- Holler, N., Zaru, R., Micheau, O., Thome, M., Attinger, A., Valitutti, S., Bodmer, J.L., Schneider, P., Seed, B., and Tschopp, J. (2000). Fas triggers an alternative, caspase-8-independent cell death pathway using the kinase RIP as effector molecule. Nat. Immunol. 1, 489-495. [DOI] [PubMed] [Google Scholar]
- Kalai, M., Van Loo, G., Vanden Berghe, T., Meeus, A., Burm, W., Saelens, X., and Vandenabeele, P. (2002). Tipping the balance between necrosis and apoptosis in human and murine cells treated with interferon and dsRNA. Cell Death Differ. 9, 981-994. [DOI] [PubMed] [Google Scholar]
- Kitanaka, C., and Kuchino, Y. (1999). Caspase-independent programmed cell death with necrotic morphology. Cell Death Differ. 6, 508-515. [DOI] [PubMed] [Google Scholar]
- Krysko, D.V., Brouckaert, G., Kalai, M., Vandenabeele, P., and D'Herde, K. (2003). Mechanisms of internalization of apoptotic and necrotic L929 cells by a macrophage cell line studied by electron microscopy. J. Morphol. 258, 336-345. [DOI] [PubMed] [Google Scholar]
- Lamkanfi, M., Declercq, W., Depuydt, B., Kalai, M., Saelens, X., and Vandenabeele, P. (2002a). The caspase family. In: Caspases: Their Role in Cell Death and Cell Survival, eds. M. Los and H. Walczak, Georgetown, TX: Landes Bioscience, Kluwer Academic Press, 1-40.
- Lamkanfi, M., Declercq, W., Kalai, M., Saelens, X., and Vandenabeele, P. (2002b). Alice in caspase land. A phylogenetic analysis of caspases from worm to man. Cell Death Differ. 9, 358-361. [DOI] [PubMed] [Google Scholar]
- Loetscher, H., Stueber, D., Banner, D., Mackay, F., and Lesslauer, W. (1993). Human tumor necrosis factor alpha (TNF alpha) mutants with exclusive specificity for the 55-kDa or 75-kDa TNF receptors. J. Biol. Chem. 268, 26350-26357. [PubMed] [Google Scholar]
- Magnus, T., Chan, A., Grauer, O., Toyka, K.V., and Gold, R. (2001). Microglial phagocytosis of apoptotic inflammatory T cells leads to down-regulation of microglial immune activation. J. Immunol. 167, 5004-5010. [DOI] [PubMed] [Google Scholar]
- Matsumura, H., Shimizu, Y., Ohsawa, Y., Kawahara, A., Uchiyama, Y., and Nagata, S. (2000). Necrotic death pathway in Fas receptor signaling. J. Cell Biol. 151, 1247-1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlroy, D., Tanaka, M., Sakahira, H., Fukuyama, H., Suzuki, M., Yamamura, K., Ohsawa, Y., Uchiyama, Y., and Nagata, S. (2000). An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 14, 549-558. [PMC free article] [PubMed] [Google Scholar]
- McKnight, A.J., Macfarlane, A.J., Dri, P., Turley, L., Willis, A.C., and Gordon, S. (1996). Molecular cloning of F4/80, a murine macrophage-restricted cell surface glycoprotein with homology to the G-protein-linked transmembrane 7 hormone receptor family. J. Biol. Chem. 271, 486-489. [DOI] [PubMed] [Google Scholar]
- Medan, D., Wang, L., Yang, X., Dokka, S., Castranova, V., and Rojanasakul, Y. (2002). Induction of neutrophil apoptosis and secondary necrosis during endotoxin-induced pulmonary inflammation in mice. J. Cell. Physiol. 191, 320-326. [DOI] [PubMed] [Google Scholar]
- Poupart, P., Vandenabeele, P., Cayphas, S., Van Snick, J., Haegeman, G., Kruys, V., Fiers, W., and Content, J. (1987). B cell growth modulating and differentiating activity of recombinant human 26-kd protein (BSF-2, HuIFN-beta 2, HPGF). EMBO J. 6, 1219-1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, Y., and Savill, J. (1998). Apoptosis: the importance of being eaten. Cell Death Differ. 5, 563-568. [DOI] [PubMed] [Google Scholar]
- Rovere, P., et al. (2000). The long pentraxin P.T.X3 binds to apoptotic cells and regulates their clearance by antigen-presenting dendritic cells. Blood 96, 4300-4306. [PubMed] [Google Scholar]
- Sauter, B., Albert, M.L., Francisco, L., Larsson, M., Somersan, S., and Bhardwaj, N. (2000). Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlegel, R.A., and Williamson, P. (2001). Phosphatidylserine, a death knell. Cell Death Differ. 8, 551-563. [DOI] [PubMed] [Google Scholar]
- Schneider, P., Holler, N., Bodmer, J.L., Hahne, M., Frei, K., Fontana, A., and Tschopp, J. (1998). Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J. Exp. Med. 187, 1205-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott, R.S., McMahon, E.J., Pop, S.M., Reap, E.A., Caricchio, R., Cohen, P.L., Earp, H.S., and Matsushima, G.K. (2001). Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207-211. [DOI] [PubMed] [Google Scholar]
- Tavernier, J., Franssen, L., Maremout, A., Van der Heyden, J., Mueller, J., Ruysschaert, M. R., Van Vliet, A., Bauden, R., and Fiers, W. (1987). Isolation and expression of the genes coding for mouse and human tumor necrosis factor (TNF) and biological properties of recombinant TNF. In: Molecular Cloning and Analysis of Lymphokines. Vol. 13, Lymphokines, eds. D.R. Webb, and D.V. Goeddel. Orlando, FL; Academic Press, 181-198. [Google Scholar]
- Van Ostade, X., Vandenabeele, P., Everaerdt, B., Loetscher, H., Gentz, R., Brockhaus, M., Lesslauer, W., Tavernier, J., Brouckaert, P., and Fiers, W. (1993). Human TNF mutants with selective activity on the p55 receptor. Nature 361, 266-269. [DOI] [PubMed] [Google Scholar]
- Vanden Berghe, T., Kalai, M., van Loo, G., Declercq, W., and Vandenabeele, P. (2003). Disruption of HSP90 function reverts tumor necrosis factor-induced necrosis to apoptosis. J. Biol. Chem. 278, 5622-5629. [DOI] [PubMed] [Google Scholar]
- Vandivier, R.W., Fadok, V.A., Hoffmann, P.R., Bratton, D.L., Penvari, C., Brown, K.K., Brain, J.D., Accurso, F.J., and Henson, P.M. (2002). Elastase-mediated phosphatidylserine receptor cleavage impairs apoptotic cell clearance in cystic fibrosis and bronchiectasis. J. Clin. Investig. 109, 661-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhaesebroeck, B., Van Bladel, S., Lenaerts, A., Suffys, P., Beyaert, R., Lucas, R., Van Roy, F., and Fiers, W. (1991). Two discrete types of Tumor Necrosis Factor-resistant cells derived from the same cell line. Cancer Res. 51, 2469-2477. [PubMed] [Google Scholar]
- Vanhoenacker, P., Gommeren, W., Luyten, W.H.M.L., Leysen, J.E., and Haegeman, G. (1999). Optimized expression of serotonin receptors in mammalian cells using inducible expression systems. Gene Ther. Mol. Biol. 3, 301-310. [Google Scholar]
- Vercammen, D., Brouckaert, G., Denecker, G., Van de Craen, M., Declercq, W., Fiers, W., and Vandenabeele, P. (1998). Dual signaling of the Fas receptor: initiation of both apoptotic and necrotic cell death pathways. J. Exp. Med. 188, 919-930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vercammen, D., Vandenabeele, P., Beyaert, R., Declercq, W., and Fiers, W. (1997). Tumour necrosis factor-induced necrosis versus anti-Fas-induced apoptosis in L929 cells. Cytokine 9, 801-808. [DOI] [PubMed] [Google Scholar]
- Vercammen, D., Vandenabeele, P., Declercq, W., Van de Craen, M., Grooten, J., and Fiers, W. (1995). Cytotoxicity in L929 murine fibrosarcoma cells after triggering of transfected human p75 tumour necrosis factor (TNF) receptor is mediated by endogenous murine TNF. Cytokine 7, 463-470. [DOI] [PubMed] [Google Scholar]
- Verhoven, B., Krahling, S., Schlegel, R.A., and Williamson, P. (1999). Regulation of phosphatidylserine exposure and phagocytosis of apoptotic T lymphocytes. Cell Death Differ. 6, 262-270. [DOI] [PubMed] [Google Scholar]
- Wang, J.H., Redmond, H.P., Watson, R.W., Duggan, S., McCarthy, J., Barry, M., and Bouchier-Hayes, D. (1996). Mechanisms involved in the induction of human endothelial cell necrosis. Cell. Immunol. 168, 91-99. [DOI] [PubMed] [Google Scholar]
- Wiegand, U.K., Corbach, S., Prescott, A.R., Savill, J., and Spruce, B.A. (2001). The trigger to cell death determines the efficiency with which dying cells are cleared by neighbours. Cell Death Differ. 8, 734-746. [DOI] [PubMed] [Google Scholar]