Abstract
Purpose.
We examined the biomechanical properties and correlation with the collagen solubility profile of the posterior sclera in a canine model of primary open-angle glaucoma caused by the G661R missense mutation in the ADAMTS10 gene.
Methods.
Scleral strips from ADAMTS10-mutant (affected) dogs and age-matched controls were collected. Viscoelastic properties (i.e., complex modulus and tan[δ]) were measured using dynamic mechanical analysis (DMA) with a 0.15% sinusoidal strain at different frequencies superimposed upon different preloads. A tensile ramp was performed following DMA. The collagen solubility profile was examined using a colorimetric hydroxyproline assay to determine the amount of soluble and insoluble collagen. The viscoelastic properties were compared between groups using linear mixed models for repeated measures at different preloads and frequencies. The correlation between the biomechanical properties and collagen content were evaluated using Pearson correlations.
Results.
Complex modulus and tan(δ) were significantly lower in the affected group (P < 0.001), and the differences were consistent at different preloads and frequencies. The B value from the tensile ramp test also was significantly lower in the affected group (P = 0.02). The insoluble collagen was significantly lower in the affected group (P < 0.05) and correlated positively with the complex modulus (R = 0.88, P < 0.005).
Conclusions.
An inherently weaker and biochemically distinct posterior sclera was observed in dogs with the G661R missense mutation in ADAMTS10 before clinical indications of optic nerve damage. It remains to be shown whether and how the altered scleral biomechanics may affect the rate of glaucoma progression following intraocular pressure elevation.
Keywords: sclera biomechanics, collagen, canine, glaucoma, ADAMTS10
Our study showed that the scleral biomechanical properties were distinct in ADAMTS10 mutant dogs and these properties were correlated with collagen solubility.
Introduction
The pathophysiology of glaucomatous vision loss involves a complex interplay of many factors. Although IOP as a primary risk factor for glaucoma has been validated extensively, it is well-known that the extent of optic nerve damage can vary substantially among individuals with similar IOPs. A wide range of other factors, such as genetics, extracellular matrix remodeling and mechanics, and ocular blood flow, as well as their interactions with IOP are being studied in humans and different animal models to identify new biomarkers and modifiable risk factors for glaucoma.1–8
Computational models have shown that the posterior sclera's geometric and mechanical properties have a significant influence on the stresses and strains “felt” by the optic nerve head (ONH), and thus may have an important role in glaucoma progression.9–12 Several studies have examined the biomechanical responses of the sclera during IOP elevations in experimental glaucoma models, as well as human cadaveric eyes.5,13,14 These studies suggested that glaucomatous eyes or eyes subject to chronic IOP elevations may exhibit altered sclera biomechanics. A recent study in mice with a collagen mutation suggested that a stiffer sclera might be associated with a delayed development of glaucoma.15 These studies point to the need to examine the mechanistic details of whether and how sclera biomechanical properties may interact with IOP elevations to modulate an individual's susceptibility to IOP damages during the course of glaucoma progression.
Primary open-angle glaucoma (POAG) in the Beagle dog is a well-established, spontaneous, large animal model.16 This nonsyndromic form of canine POAG is inherited as an autosomal recessive trait17 and has been linked to a variant (G661R missense mutation) of the ADAMTS10 gene.6 In humans, mutations in ADAMTS10 cause Weill-Marchesani syndrome, a connective tissue disorder that usually presents in childhood with short stature and/or ocular problems, including glaucoma.18 The ADAMTS10 protein is a member of the ADAMTS family of secreted metalloproteinases that contribute to the formation and turnover of the extracellular matrix (ECM).19–21 This protein has been shown to have a key role in the storage and activation of TGFβ, a known regulator of collagen turnover,20,22–25 suggesting ADAMTS10 mutations may lead to alterations in ECM composition, structure, and mechanical properties.
The primary goal of our study was to test the hypothesis that the biomechanical properties of the posterior sclera were altered in the ADAMTS10-mutant dogs before the detectable onset of glaucoma. Our study is an initial step in achieving our longer-term goal of understanding how scleral biomechanics may interact with IOP elevation during the development and progression of glaucoma. We tested scleral specimens temporal to the ONH from one eye of each homozygous ADAMTS10-mutant and normal, age-matched control dogs. Dynamic mechanical analysis (DMA) and tensile ramp test were conducted to examine the biomechanical properties of the sclera.26 The collagen solubility profile of the mechanically-tested tissue specimens then was examined using a colorimetric hydroxyproline assay27 to explore the underlying biochemical mechanisms of the observed mechanical data.
Methods
Animals
A colony of Beagle-derived mongrel dogs carrying the G661R ADAMTS10 mutation was developed and maintained at the University of Pennsylvania as a canine POAG model. All procedures in our study were approved by the University of Pennsylvania IACUC, and were done in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. All animals included in our study were born, raised, and maintained under the same environmental conditions.
To determine the genotype, blood samples were collected from all dogs, DNA extracted (QIAmp DNA Blood Mini Kit; Qiagen, Valencia, CA) and a 614 base pair (bp) fragment PCR amplified that included exon 17 of the ADAMTS10 gene with the G → A mutation (corresponding to the G661R mutation in the ADAMTS10 protein6) using these primers: 5′-ATTTGACAGCGTCCCCTTC-3′ (forward) and 5′-CACTTGTCCTCCCTCAGGTC-3′ (reverse). The PCR products were gel purified (QIAquick Gel Extraction Kit; Qiagen) and sequenced at the University of Pennsylvania DNA Sequencing Facility (ABI 3730XL and ABI 3100 Genetic Analyzers; Applied Biosystems, Foster City, CA) to determine if the dogs were homozygous affected, heterozygous carriers, or homozygous normal.
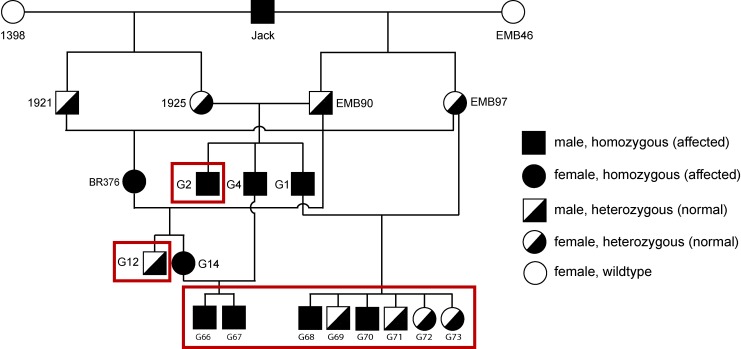
A partial pedigree of the canine POAG colony maintained in our laboratory is shown in Figure 1 to illustrate the genetic relationship of the experimental animals. Four affected dogs, homozygous for the G661R mutation in ADAMTS10, and between 5 and 7 months of age (G66, G67, G68, and G70), and 4 age-matched heterozygous normal control dogs (G69, G71, G72, and G73) were used for our study. At this age, these dogs are known not to have developed ocular hypertension yet.6,28 In addition to these eight young dogs, one older homozygous affected dog (38 months, G2) and one older heterozygous normal control dog (33 months, G12) also were tested.
Figure 1.
Partial pedigree of the canine POAG colony showing the ADAMTS10 genotypes. The animals used in our study are shown in boxes.
All the dogs underwent detailed ophthalmic examinations before euthanasia, including indirect ophthalmoscopy (Keeler All Pupil II; Keeler Instruments, Broomall, PA) with condensing lens (Pan Retinal 2.2 D; Volk Optical, Mentor, OH), slit-lamp biomicroscopy (Kowa SL-15; Kowa Optimed, Torrance, CA), and applanation tonometry (Tono-Pen Vet; Reichert, Depew, NY). The Tono-Pen was used for all IOP measurements in our study, which is the clinical standard for measuring IOP in dogs. Ocular surface anesthesia with 0.5% proparacaine ophthalmic solution was used before Tono-Pen measurement, and no sedation or general anesthesia was required. For the two older dogs, monthly diurnal IOPs also were measured from 4 to 6 months of age at 7 AM, 10 AM, and 1 PM on the measurement day in consideration of physiologic IOP variations throughout the day.
The right eye of each dog was recovered immediately after euthanasia, and the whole globes were shipped overnight to The Ohio State University in moist containers on ice. Fresh strips were excised from the posterior sclera and tested mechanically within 24 hours postmortem. The fellow left eye of four dogs (a young affected, a young control, an old affected, and an old control, as indicated in Table 1) was collected for histologic evaluation of the ONH. Both globes were fixed in Karnovsky's fixative (5% glutaraldehyde and 4% formaldehyde in 0.08 M sodium phosphate buffer), and the posterior segments were processed routinely for paraffin embedding. Vertical sections through the middle of the ONH were prepared and stained with hematoxylin and eosin (H&E).
Table 1. .
Demographic Information, Genotype, and IOP Before Euthanasia for Each Dog Used in Our Study
|
Dog ID |
Sex |
Eye |
Genotype |
Age, mo |
IOP at Cull, mm Hg |
| G66 | Male | Right | Affected | 7.0 | 17 |
| G67 | Male | Right | Affected | 7.0 | 17 |
| G68 | Male | Right | Affected | 5.6 | 16 |
| G70* | Male | Right | Affected | 5.6 | 14 |
| G2* | Male | Right | Affected | 38.4 | 17† |
| G69 | Male | Right | Carrier | 5.6 | 16 |
| G71* | Male | Right | Carrier | 5.6 | 13 |
| G72 | Female | Right | Carrier | 5.6 | 11 |
| G73 | Female | Right | Carrier | 5.6 | 14 |
| G12* | Male | Right | Carrier | 32.6 | 15† |
The fellow eye of the dog was collected for routine histology of the ONH.
The average diurnal IOP measured in 2 years before tissue collection was 22.0 mm Hg in G2 (range, 13.5–31.4 mm Hg), showing a moderate increase of average diurnal IOP, and 16.8 mm Hg in G12 (range, 10.8–22.2 mm Hg).
Mechanical Testing
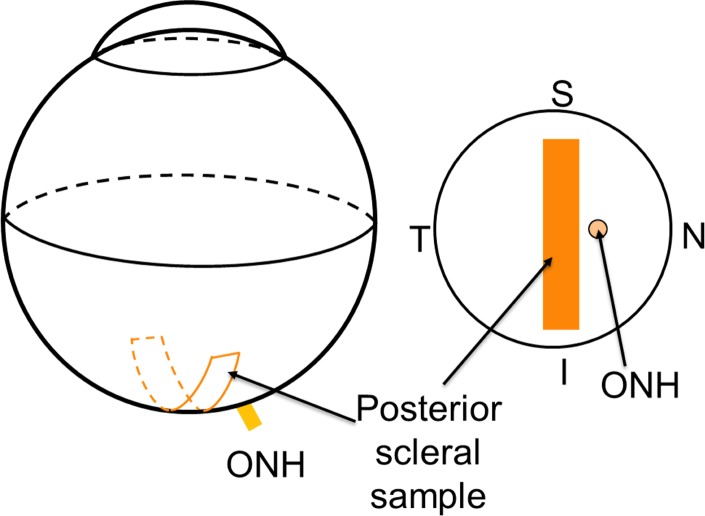
All measurements and mechanical testing were conducted by one operator (JRP) masked to the genotype of the specimens. The diameters of the eyes were measured with an electronic caliper before tissue dissection along the three main axes of the eye (i.e., the superior-inferior axis, optical axis, and nasal-temporal axis). The globes were cleaned carefully and a rectangular posterior strip adjacent to the ONH on the temporal side was dissected using a parallel blade excision device described in previous studies.26,29 A representation of the scleral cutting position is seen in Figure 2.
Figure 2.
A schematic of the excision location of the posterior scleral strip. The strip was cut adjacent to the temporal side of the ONH. S, superior; I, inferior; N, nasal; T, temporal.
The thickness and width of each strip were measured using high frequency B-mode ultrasound (Vevo660; VisualSonics, Toronto, Ontario, Canada) as described previously.26 Briefly, three thickness measurements and one width measurement were taken at each of three cross-sectional scans along the central gage length. The averages of the nine total thickness and three width measurements were used in further analysis. Before testing, the scleral strips were kept at 4°C and stored in PBS solution. All mechanical testing was performed within 6 hours of strip excision.
DMA is one of the standard methods to determine the viscoelastic properties of a material.30,31 A small, sinusoidal strain is applied to the specimen and the resultant sinusoidal stress response is observed. The ratios of the stress to strain amplitudes along with the phase difference between the stress response and the applied strain are used to calculate the viscoelastic properties, including complex modulus and tan(δ), assuming linear viscoelasticity. The complex modulus, which can be thought as the overall resistance to deformation under dynamic loading, has two components: the storage modulus (elastic component) and the loss modulus (viscous component). Tan(δ), which represents the damping ability of the tissue, is quantified by the ratio of the loss modulus to storage modulus.
In our study, DMA testing was performed using a Rheometrics System Analyzer (RSA III; TA Instruments, New Castle, DE) with a displacement resolution of 0.05 μm and a force resolution of 2 μN. Scleral strips were mounted carefully to ensure good alignment and prevent grip slippage. All samples were kept moist using a custom humidifying chamber and were tested at a temperature of approximately 37°C. The specimens were stretched from a relaxed state to a load between 0.001 and 0.002 N to flatten the curvature, and ensure full contact between sample and grips. Preconditioning was performed for 90 seconds using cyclic triangular waves at a frequency of 0.1 Hz to a peak load amplitude of 0.1 N. The tissue then was allowed to equilibrate in the moist environment for 5 minutes. The gage distance between the tissue grips was on average 10.9 mm according to the displacement readings from the RSA III device.
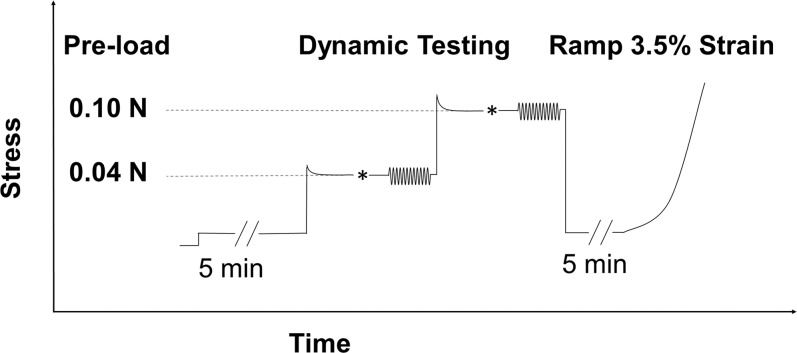
The DMA testing then was performed with 12 cycles of a sinusoidal strain input at each increasing angular frequency of 0.1, 0.5, 1.0, 3.0, 5.0, and 10.0 Hz. Preliminary DMA testing was first conducted at various strain amplitudes on a group of canine posterior scleral tissue that was not used in the data collection of the present study. The preliminary testing showed that strain amplitudes below 0.25% generated a linear tissue response in canine sclera. Therefore, a strain amplitude of 0.15% was chosen for our study. The evolution of the stress and strain over time was recorded for the last eight cycles for each frequency, with a tissue rest time of 90 seconds between each frequency. This dynamic protocol was performed in the same manner at the preload of 0.04 N followed by testing at the preload of 0.1 N. The tissue was first brought to a preload level approximately 35% above the desired preload, allowed to relax for 5 minutes, and then adjusted manually to achieve the desired preload level. Superimposing the dynamic loads to these two preloads was intended to approximate the dynamic component of IOP (such as the ocular pulse) superimposed on either a “normal” or “high” steady-state IOP. It is noted that the preloads were estimated according to the Laplace law, which does not take into account the nonlinear, heterogeneous, and viscoelastic properties of the tissue.
Following all DMA testing, each strip was brought to the initial preload, allowed to equilibrate for 5 minutes, and then underwent a tensile ramp up to 3.5% strain at a strain rate of 0.1%/s. An illustration of the testing protocol is presented in Figure 3.
Figure 3.
The DMA and tensile testing protocol for each specimen strip. *Indicated the small manual adjustments to fine tune the desired preload levels. Based on the predicted stress-relaxation that occurs in most soft tissue, the strip first was brought to a load 35% higher than the target preload (either 0.04 or 0.10 N) before the manual adjustment. This is indicated by the initial stress overshoot in the figure.
The stress/strain data obtained from each strip during the tensile ramp tests was fit to Fung's standard exponential model32:
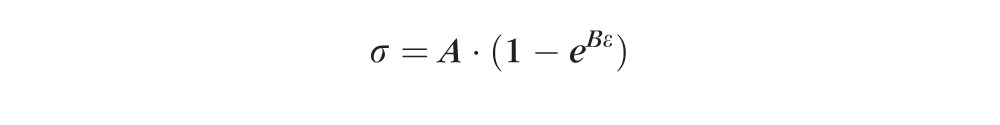 |
The constants A and B were fit using the Levenberg-Marquardt least squares method. The magnitude of A·B represents an initial elastic modulus of the tissue, while the B value represents the slope of change in the tissue's tangent elastic modulus with increasing stress.
Histology of Sclera
Following mechanical testing, the sclera specimens were flash frozen immediately in liquid nitrogen and stored for 4 weeks at −80°C. Each strip then was thawed at room temperature, and sectioned into portions for histology staining and analysis of collagen solubility. The central 4 mm were fixed using 4% paraformaldehyde and used for histology staining. The remaining tissue was refrozen and stored at −80°C before the hydroxyproline assay. The fixed tissue was embedded in paraffin so that sections could be made through the thickness perpendicular to the long axis of the strip. H&E staining, Masson's trichrome staining for collagen, and Verhoeff's staining for elastin were obtained from each tissue block. Histology images were assessed at ×100 and ×500 magnification levels. For each tissue section, one image of the central region of the section was examined at ×100 magnification, and additionally three images from three equally spaced regions across the entire length of the section were examined at ×500 magnification.
Biochemical Analysis of Scleral Collagen Content and Solubility Profile
The samples were thawed after storage at −80°C for 11 weeks for collagen content examination using a colorimetric hydroxyproline collagen assay.27,32 The wet weight of the scleral strips was measured by first immersing the specimens in 0.9% saline for 5 minutes and then blotting dry the surfaces. Each specimen was measured three times using this protocol and the average was taken for further analysis. This weighing procedure yielded repeatable measurements of the wet weight of sclera specimens. After weighing, the samples were minced using surgical micro scissors, placed in a 1.4 mL centrifuge tube, and the collagen extracted using a sequence of solvents. Neutral salt soluble collagen (NSC) was extracted first by adding 1.0 mL of 1.0 M NaCl with 0.1 mg/mL EDTA and 0.1 mg/mL phenylmethanesulfonylfluoride (PMSF) to the centrifuge tube, and shaking the mixture for 24 hours at 4°C. The following steps were used to collect the NSC: (1) The sample was centrifuged at 21,130g for 20 minutes at 4°C, (2) the supernatant was collected, (3) 500 μL of phosphate buffered saline (PBS) was added to resuspend the pellet, (4) the sample was centrifuged again for 10 minutes at 21,130g, and (5) the second supernatant was collected and added to the original supernatant. The remaining collagen pellet was suspended in a weak acetic acid (0.5 M) and stirred for 24 hours at 4°C to extract the acid soluble collagen (ASC). The pellet was suspended once more in 0.5 M acetic acid with 0.1 mg/mL pepsin and stirred for 24 hours at 4°C to extract the pepsin soluble collagen (PSC). The remaining collagen pellet was homogenized in 4 mL of distilled water and considered insoluble collagen (ISC). All collagen fractions, namely NSC, ASC, and PSC, were stored in their respective solvents at 4°C until further use.
Details of the hydroxyproline assay have been reported previously.27,32 Briefly, a small aliquot (10–30 μL) of each of the four sets of collagen (NSC, ASC, PSC, and ISC) extracted from each sclera specimen was hydrolyzed in a 4 N NaOH at 121°C for 30 minutes by autoclave. In addition to the tissue samples, four sets of hydroxyproline standards made from known increasing amounts of hydroxyproline solubilized in respective solvent also were hydrolyzed. The hydroxyproline in samples and standards then was oxidized with the addition of a chloramine-T solution followed by chromophore development after the addition of an Ehrlich's reagent and incubation at 65°C for 20 minutes. The absorbance of each sample was measured at 555 nm using a Beckman DU730 spectrophotometer (Beckman Coulter, Inc., Brea, CA) and the hydroxyproline content in each sample was calibrated against a fitted curve obtained from the measurements on the hydroxyproline standards. Collagen content then was calculated assuming the collagen contained approximately 14.0% hydroxyproline.27 This assay was conducted on the four groups of extracted collagens to calculate collagen content (micrograms of collagen per milligram of wet weight) of NSC, ASC, PSC, and ISC.
Statistical Analysis
Statistical analysis was performed using SAS 9.2 software package (SAS Institute, Inc., Cary, NC). The comparison between the two genotype groups (the affected and carriers) was performed on the eight young eyes (four from each group). The two older eyes (one from each group) were excluded from the statistical analysis to avoid the confounding effects of age. The difference of the dynamic mechanical properties between the affected and carrier groups was compared using linear mixed models for repeated measurements where preloads and frequencies (as well as their interactions with genotype) were considered as covariates. The properties from the tensile ramp tests (B and A·B) were compared using two-sample t-tests. Collagen contents between the four young affected and carrier dogs also were compared using two-sample t-tests. Correlation coefficients between collagen content and biomechanical properties, including complex modulus and tan(δ), were calculated using Pearson's correlations.
Results
Table 1 presents the genotype and IOP measured before euthanasia, along with demographic information for each dog. The mean IOP was 16.0 ± 1.4 mm Hg in the young affected group and 13.5 ± 2.1 mm Hg in the young carrier group (P = 0.09, two-sample t-test).
Ophthalmic examinations before euthanasia showed no indications of glaucomatous optic nerve damages in all the young dogs and the older carrier dog (G12). In two of the young affected animals (G66 and G70) small amounts of degenerated vitreous were found in the anterior chambers, indicating mild dysplasia of the lens zonules. Clinical signs of zonular dysplasia also were found in the older affected dog (G2) with more degenerated vitreous in the anterior chamber and subluxation of the lens (ectopia lentis). Furthermore, moderate atrophy of the ONH was seen in the older affected dog (G2).
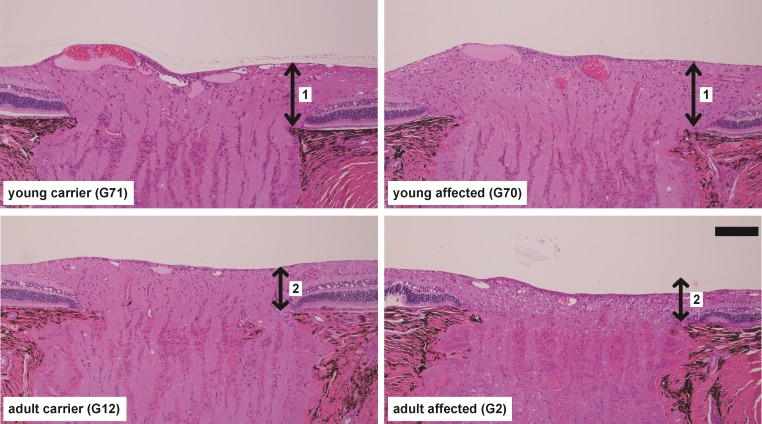
Histologic analysis of the ONH of two representative young dogs (littermate but different genotype) and the two older dogs are shown in Figure 4. Compared to the age-matched normal control eye (G71), there were no obvious indications of disease in the affected eye (G70) at 5.6 months of age (Fig. 4, top). The thicknesses of the prelaminar ONH were comparable (arrow pair “1” in Fig. 4). At approximately 3 years of age (Fig. 4, bottom), there was an approximately 30% loss of prelaminar ONH thickness (arrow pair “2” in Fig. 4) in the ADAMTS10-mutant (G2) compared to the age-matched normal control eye (G12).
Figure 4.
Vertical cross-sections through the central ONH of normal carriers and ADAMTS10-mutant dogs at two age groups. Up-down arrows indicate the thickness of the prelaminar ONH. H&E stain. Scale bar: 100 μm.
The mean posterior strip thickness for the eight young dogs was 0.63 ± 0.03 mm in the affected group and 0.59 ± 0.01 mm for the carrier group (P = 0.015, two-sample t-test). The posterior sclera in the older dogs was thinner than that in the young dogs (0.50 mm in the affected and 0.47 mm in the carrier). Axial length (AL), nasal-temporal (NT) diameter, and superior-inferior (SI) diameter were 21.1 ± 0.3, 21.4 ± 0.4, and 21.0 ± 0.8 mm, respectively, for the young affected group, and 20.1 ± 0.7, 21.1 ± 0.1, and 20.5 ± 0.4 mm, respectively, for the young carriers, respectively. The older dogs were approximately 2 mm longer in diameter in all three axes (AL 23.1 mm, NT 23.0 mm, SI 22.8 mm in the carrier; and AL 23.3 mm, NT 23.1 mm, SI 22.5 mm in the affected).
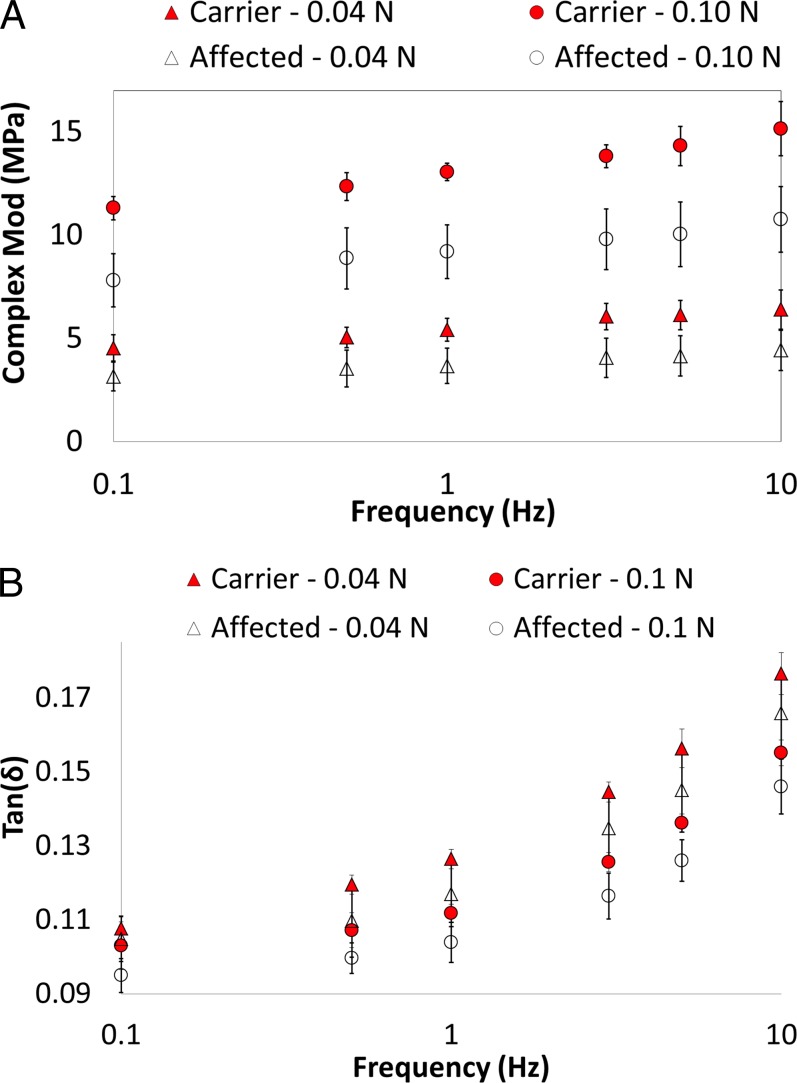
Figure 5 presents the complex modulus and tan(δ) for the two groups measured at two different preload levels and six different frequencies (only the eight young eyes were included in this analysis). The carriers had a higher complex modulus and a higher tan(δ) than the affected group (both P ≤ 0.001, linear mixed models). Preload and frequency had a statistically significant influence on the dynamic mechanical properties for both groups (all P < 0.001, linear mixed models). The complex modulus increased at the larger preload and at higher frequencies. The tan(δ) was greater at the lower preload and at higher frequencies. Complex modulus and tan(δ) increased at a similar rate in both groups as frequency increased (P = 0.26 and 0.27, respectively). The older carrier had a larger complex modulus, but a lower tan(δ) than the older affected dog.
Figure 5.
Complex modulus and tan(δ) for the four young affected and four young carrier dogs measured at two preloads and six frequencies. A statistically significant difference was found between the two groups for complex modulus and tan(δ), and the difference was consistent at different preloads and frequencies.
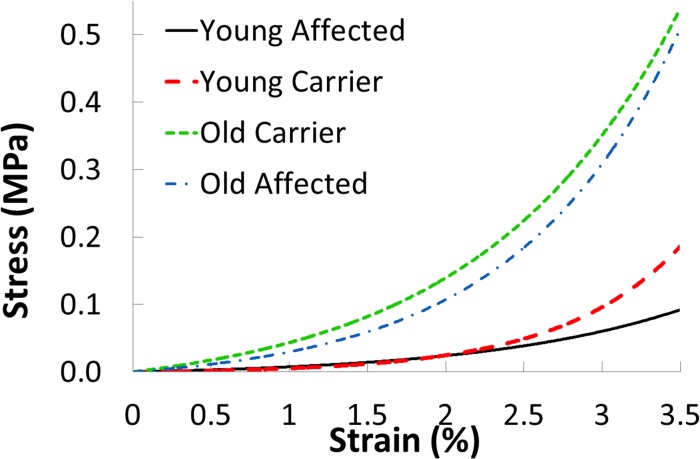
The stress-strain data of each scleral strip from the tensile ramp tests were fit to the standard exponential model to obtain the A and B values for that strip. All R2 values of the exponential fitting were greater than 0.98. The mean A·B value was 0.25 ± 0.18 MPa in the young carrier group and 0.56 ± 0.55 MPa in the young affected group (P = 0.32, two-sample t-test). The mean B value was 130.6 ± 6.7 in the young carrier group and 78.5 ± 30.4 in the young affected group (P = 0.02, two-sample t-test). Figure 6 shows the average response of the young affected and young carrier groups based on the fitted curves using the average A and B values of each group, as well as the fitted curves from the two older dogs.
Figure 6.
Exponential fitting of the stress-strain curves from the tensile ramp tests. The two older samples were noticeably stiffer than those from the young dogs.
Table 2 summarizes the scleral thickness and mechanical properties comparisons between the affected and carrier groups in the young dogs.
Table 2. .
Mean and Standard Deviation for Scleral Thickness and Mechanical Properties in the Affected and Carrier Groups (Data From the Older Dogs Were Not Included)
|
|
Thickness,* mm |
A·B, MPa |
B* |
Complex Modulus,† MPa |
tan(δ)† |
| Affected | 0.63 ± 0.03 | 0.56 ± 0.55 | 78.5 ± 30.4 | 3.66 ± 0.99 | 0.117 ± 0.010 |
| Carrier | 0.59 ± 0.01 | 0.25 ± 0.18 | 130.6 ± 6.8 | 5.41 ± 0.64 | 0.126 ± 0.003 |
P < 0.05.
P < 0.001.
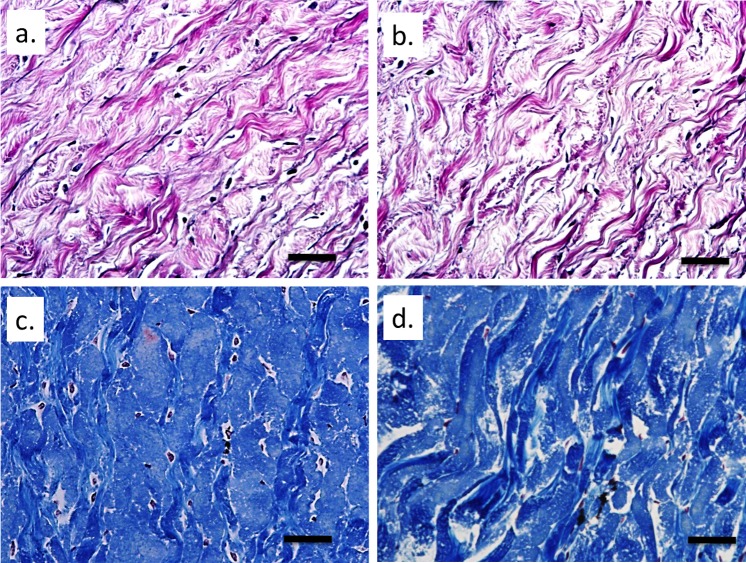
The H&E staining was used to ensure proper sectioning of the strips and that freezing did not produce any severe disruptions of the tissue microstructure. No gross disruptions of the tissue were noted, although some level of swelling may have occurred in all samples (Fig. 7). Verhoeff staining revealed minor elastin staining within the sclera and no apparent difference was found between the two groups (Figs. 8a, 8b). Masson's trichrome staining for collagen indicated some differences in collagen organization between the affected and carrier groups. More frequent and intense staining for contiguous collagen fiber bundles was observed in the carriers (Figs. 8c, 8d).
Figure 7.
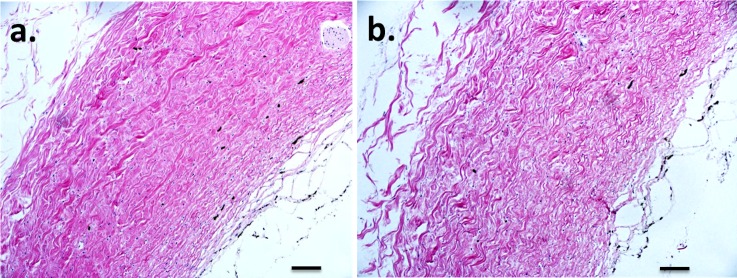
H&E staining of sclera specimens. (a) Affected. (b) Carrier. No large tissue disruptions were seen, but some swelling appeared to have occurred. Original magnification: ×100. Scale bar: 100 μm.
Figure 8.
Verhoeff staining for elastin: (a) affected and (b) carrier. Mason's trichrome staining for collagen: (c) affected and (d) carrier. The black stained elastin can be seen in small amounts dispersed within the sclera. Collagen fiber bundles appeared to be more strongly and evenly stained in the carriers. Original magnification: ×500. Scale bar: 100 μm.
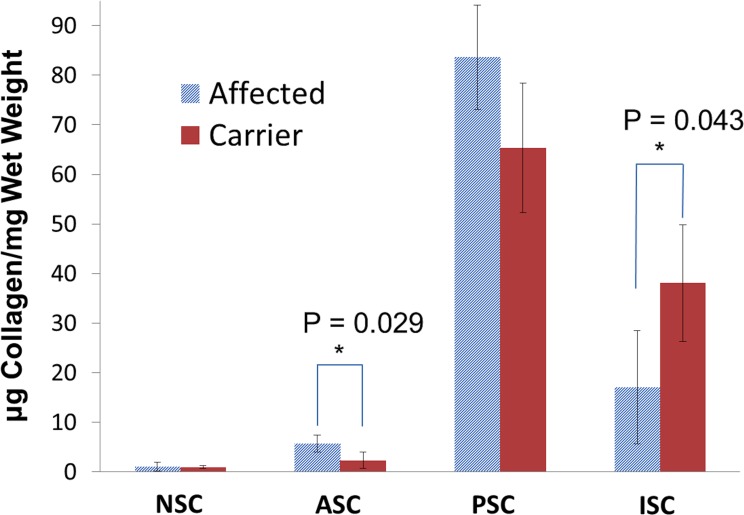
The mean amounts of NSC, ASC, PSC, and ISC collagen in the affected and carrier young dogs are summarized in Figure 9. The total amount of collagen between the groups showed no statistically significant difference (P = 0.824), with a mean total collagen of 107.5 ± 2.5 μg collagen/mg wet weight in the young affected group and 106.7 ± 5.7 μg collagen/mg wet weight in the young carriers. A general trend of increased soluble collagen (the sum of NSC, ASC, and PSC) and decreased ISC was seen in the young affected animals. Significant differences were found in the ASC (P = 0.029) and ISC (P = 0.043) content between the two groups.
Figure 9.
Mean μg collagen/mg wet weight for the NSC, ASC, PSC, and ISC of the young affected and young carrier groups. Error bars represent standard deviation of the means.
Table 3 presents the correlation coefficients with corresponding P values between the collagen content and the mechanical properties in the sclera of the young dogs, including the carriers and the affected group.
Table 3. .
Pearson Correlations and Corresponding P Values Between the Quasi-Static and Dynamic Mechanical Properties with ISC, PSC, ASC, NSC, and Total Soluble Collagen
|
|
A × B |
B |
tan(δ) 0.04N |
Complex Modulus 0.04
N |
tan(δ) 0.10N |
Complex Modulus 0.10
N |
||||||
|
R |
P
Value |
R |
P
Value |
R |
P
Value |
R |
P
Value |
R |
P
Value |
R |
P
Value |
|
| ISC | 0.094 | 0.826 | 0.722 | 0.043* | 0.642 | 0.086 | 0.878 | 0.004* | 0.603 | 0.113 | 0.88 | 0.004* |
| PSC | −0.018* | 0.965 | −0.588 | 0.125 | −0.539 | 0.168 | −0.809 | 0.015* | −0.503 | 0.204 | −0.814 | 0.014* |
| ASC | −0.077 | 0.855 | −0.854 | 0.007* | −0.795 | 0.018* | −0.802 | 0.017* | −0.775 | 0.030* | −0.874 | 0.005* |
| NSC | −0.732 | 0.039* | −0.503 | 0.204 | −0.66 | 0.075 | −0.504 | 0.203 | −0.695 | 0.056 | −0.388 | 0.342 |
| Total soluble | −0.055 | 0.897 | −0.659 | 0.076 | −0.613 | 0.106 | −0.846 | 0.008* | −0.577 | 0.135 | −0.856 | 0.007* |
, Pearson correlation coefficient.
P < 0.05.
Discussion
Our study investigated the biomechanical properties and collagen solubility of the posterior sclera in ADAMTS10-mutant dogs compared to normal controls. Our primary finding was that before clinically observable IOP elevations and optic nerve degeneration, the dynamic viscoelastic properties, including the complex modulus and tan(δ) were significantly lower in the young affected dogs as compared to the age-matched heterozygous normal control dogs. Biochemical analysis showed that the insoluble collagen was significantly lower in the posterior sclera of the affected dogs, and the amount of insoluble collagen was correlated positively with the complex modulus. In addition, tensile ramp tests revealed a significantly lower B value in the affected group, although the initial stiffness (A·B) was not different between the two groups.
The finding of an altered sclera in the ADAMTS10-mutant dogs before IOP elevations and optic neuropathy suggested that animal models of glaucoma, and potentially human patients with glaucoma, could have concomitant abnormalities in other structures, such as the sclera and/or ONH, even before clinical manifestation of the disease. These abnormalities may contribute to the susceptibility to IOP and could interact with an elevated IOP in a complex way affecting the course of glaucoma progression. This interaction represents a less understood aspect of glaucoma pathogenesis that must be studied in the future. Our study also highlights the complimentary importance of different glaucoma models. For example, experimental glaucoma models based on inducible, unilateral IOP elevations may harbor advantages in understanding the separate effects of an acute IOP elevation, while genetic mutation models could help study the complex structure/load interactions that underline some aspects of the pathogenesis in human glaucoma.
The significantly lower complex modulus in the posterior sclera of the affected dogs suggested a mechanically weaker sclera in these dogs with respect to dynamic forces, that is, lesser resistance to the deformation caused by dynamic loading. This remained true even if one considers “structural stiffness” by multiplying modulus with thickness33 and, thus, taking into account the slightly larger scleral thickness of the affected dogs. Tan(δ), a property indicating the tissue's ability to dampen dynamic forces, also was significantly lower in the affected sclera. Tan(δ) has been related to the movement of fluid in and out of tissue, and the internal friction caused by collagen molecules and fibrils sliding past one another during loading. The decreased tan(δ) combined with the decrease in complex modulus of the posterior sclera in the affected group suggested that this tissue has a decreased ability to dampen and resist dynamic insults. We have found this to be true across a range of frequencies, which are present in the dynamic forces the eye experiences regularly during physiologic disturbances, such as ocular pulse, blinking, ocular movements, posture change, and eye rubbing. Recent studies on humans34 and rhesus monkeys35 reported data upon long-term monitoring of IOP showing frequent and quick IOP fluctuations. Based on our estimate using spectral analysis of the reported dynamic IOP curves obtained from rhesus monkeys,35 the in vivo IOP could contain most of the frequencies tested in our study.
The tensile ramp tests, performed under quasi-static forces, revealed a lower B value in the affected group. The B value represents the rate of increase in tangent modulus as the stress increases, which is an indication of how much the tissue stiffens at higher loading. A lower B value means the tissue stiffens less when stresses increase. Interestingly, the initial stiffness (A·B), which represents the stiffness of the tissue at low stress/strain levels, was not different between the groups (Fig. 6). Our data suggested that the two groups started to differ appreciably in their responses at a strain level above 2.5%. Based on estimations using the Laplace law, and the average specimen thickness and curvature, this level of stress/strain corresponded to an IOP of approximately 45 mm Hg in the affected eyes. These results indicated mechanical weakness in the affected sclera at high IOPs (i.e., above 45 mm Hg).
Combining the dynamic mechanical tests and tensile ramp test data, our results suggested that the posterior sclera in dogs affected by the G661R-ADAMTS10 mutation was mechanically weaker than the carrier counterparts. This is an intriguing finding considering the current interest in understanding how sclera biomechanical properties, especially those of the posterior sclera, factor into glaucoma risk. Previous computational models have suggested that a weaker sclera would result in larger tensile strains in the ONH tissue under the same IOP because the larger stretch of the sclera forces the ONH to deform more anteriorly (i.e., being pulled taut).12 However, stiffer sclera likely results in more pronounced posterior bowing of the ONH due to the much higher compliance of the ONH in comparison with the stiff sclera.5,9 Therefore, it is unclear whether a mechanically weaker or stiffer sclera promotes glaucomatous damages. Our study revealed a much weaker sclera in the affected dogs relative to the carriers. It has been reported that the lamina cribrosa shows initial anterior displacement in the early stage of glaucomatous development in the affected dogs, which may result from the pulling of a weaker sclera.36,37
It also has been shown that the clinical signs of open-angle glaucoma similar to that of human POAG, including the loss of retinal ganglion cell axons, optic nerve cupping, and vision loss, are delayed until 2 to 5 years of age in the affected dogs, although they characteristically begin showing elevations of IOP between the ages of 8 and 16 months.36,38 Clinically, we have observed rather unusually slow glaucoma progression in some individual ADAMTS10-POAG–affected dogs that had elevated IOPs between 30 and 40 mm Hg for 1 to 2 years without significant optic nerve atrophy or retinal function loss. These observations suggested that it is important for future studies to determine whether a more compliant sclera, existing before ocular hypertension in this canine model, influences the eye's capability to handle chronic IOP increases. Besides static IOP, sclera biomechanics also may be important modulators of dynamic IOP (i.e., IOP fluctuations). Presumably, a more compliant sclera could better handle dynamic fluctuations in ocular volume and, thus, blunt out damaging IOP spikes on the ONH. Our laboratory has examined previously the correlation between short-term IOP elevations and corneoscleral (Tang et al., IOVS 2010;51:ARVO E-Abstract, 588) stiffness,39 showing that higher corneoscleral stiffness could result in significantly higher IOP peaks for the same intraocular volume change/displacement present during ocular pulse, blinking, eye movements, and postural changes.
The biochemical analysis provided additional insights into the mechanical testing results. It is interesting to note that the total collagen was similar between the two groups, but the fractions of insoluble and soluble collagens were different. The solubility of collagen is related in part to the degree and type of cross-linking between collagen molecules. The soluble collagens are in general less cross-linked, while the ISC is considered to be more extensively cross-linked. The percentage of ISC, thus, gives an indication of the degree of cross-linking within the tissue. Although an indirect approach, analysis of collagen solubility has been adopted frequently to measure the relative amounts of cross-linking in various types of tissue.40–46 We found in our study that the ISC amount was correlated positively with the complex modulus at both preloads (P = 0.004 and 0.004, respectively), as well as the B value (P = 0.043). The total soluble collagen amount was correlated negatively with the complex modulus at both preloads (P = 0.008 and 0.007, respectively). These results indicated that in the dog sclera, as in most fibrillar collagenous tissue, cross-linking has a major role in governing mechanical properties. The weaker mechanical properties (i.e., lower complex modulus and lower B value) of the affected canine sclera, thus, may be linked to the reduced inter- and intrafibril collagen cross-linking.
It has been found in rat tail tendons that during tensile stretch the collagen goes through a sequence of deformation steps that include a macroscopic uncrimping of collagen, followed by the removal of microscopic kinks in collagen molecules located within the gap regions of the fibril, and finally the gliding of collagen molecules past one another corresponding to the linear region in the stress-strain curve.47–49 Assuming the preloads of 0.04 and 0.10 N deformed the sclera strips past the initial crimp phase, the dynamic strain input would fall mostly on the region where the deformation was primarily due to collagen molecule sliding and on the macroscopic movement of fibrils through the embedding matrix. Decreased cross-linking between and within fibrils effectively would decrease the overall resistance of the tissue to dynamic deformation, thus decreasing the complex modulus in the affected canine sclera. This result was consistent with previous reports that the insoluble collagen (i.e., highly cross-linked collagen) was critical for resisting tensile strains, and correlated positively with fracture stress and tensile stiffness in rabbit Achilles tendon and rat aortic tissue.40,46
The decreased tan(δ) in the affected group appeared counterintuitive, because one would expect more sliding and energy dissipation within the fibrils relative to energy storage with decreased cross-linking. By monitoring the D-period in rat tail tendon collagen fibrils, it was found that a majority of the deformation (over 60%) in the linear region of the stress-strain curve took place outside the fibrils (i.e., the sliding of the fibrils against the embedding matrix).50 It also was found that the allocation of the deformation within or outside the fibrils was dependent on the degree of collagen cross-linking, and the percent deformation occurring outside the fibril increased in cross-link–deficient tissue at a low strain rate of 0.01%/s.50 This effect of cross-linking deficiency may partially explain the decreased mechanical damping properties (i.e., tan[δ]) in the affected canine sclera, because the decreased intrafibril sliding in the lesser cross-linked group effectively would reduce the relative amount of energy dissipated by the fibrils.
The measurements before euthanasia showed that all IOP values (11–17 mm Hg) were well within the normal range of IOPs seen in clinically healthy dogs,51–53 confirming that the young affected dogs still were normotensive. Although not statistically significant, the mean IOP at euthanasia was slightly higher in the young affected dogs compared to that in the young carrier dogs (16.0 ± 1.4 vs. 13.5 ± 2.1 mm Hg), prompting a consideration of whether this IOP could have confounded the outcome of the genotype comparisons reported in our study. Thus, the data were reanalyzed treating IOP at euthanasia as an additional covariate in the linear mixed models. Complex modulus and tan(δ) remained significantly different between the genotype groups (P = 0.0014 and 0.0095, respectively), confirming that the primary outcome of our study was not influenced by the effect of IOP. Nonetheless, a significant negative association was found between IOP at euthanasia and complex modulus (P = 0.0016), but no association existed between IOP and tan(δ) (P = 0.41). Pearson correlation was not significant between IOP and B (P = 0.07) or A·B (P = 0.78). Based on this analysis, IOP at euthanasia appeared to be a potential confounding factor in the comparison of complex modulus. Although limited by a small sample size and the one-time IOP measurement, these results suggested an intriguing possibility that a slight increase in IOP, even within the normal range, may have a biologic effect on some biomechanical characteristics of the sclera. Future studies with more comprehensive tonometry data and a larger sample size are needed to elucidate how IOP, within or outside the normal range, could affect scleral remodeling processes.
We also have examined two older dogs (one affected and one carrier), which showed marked increases in scleral mechanical stiffness compared to the young dogs (evident from the tensile ramp test results shown in Fig. 6, as well as the complex moduli values). The older carrier eye had a higher complex modulus than the older affected eye showing the same trend of genotype comparison in the younger eyes; however, the older carrier had a smaller tan(δ) and B value relative to the older affected dog showing the opposite trend of the genotype comparison in the younger eyes. These results may indicate that the effect of long-term IOP elevations influences the viscous properties of the sclera more so than the elastic properties that dominantly determine complex modulus in biologic tissues. However, having only one older eye for each group prevents any conclusive findings. It also is noted that our results were from the Beagle-derived mongrel dogs, which may differ from other species. Previous studies in monkey eyes have found that an increased IOP over an extended time may result in stiffening of the posterior sclera.13
There were several limitations in our study. First, the sample size (n = 4) for each group was small. However, the biomechanical contrast between the two groups was consistent with the biochemical contrast, and both comparisons reached statistical significance with the present sample size. The current results, thus, serve as a starting point for future mechanical studies on this canine glaucoma model. Second, IOP was measured only once in the eight young dogs. Previous reports have shown that the affected animals of this selected age range (5–7 months) have not yet developed ocular hypertension.6,28 Our monthly diurnal IOP data collected from a representative subgroup of the animals in this colony also validated that IOP was not significantly different between the affected dogs and controls of this age range. Future studies should characterize the detailed diurnal IOP curves of the studied animals to capture fully the dynamic IOP information. Third, the use of uniaxial tensile testing on excised strips has multiple drawbacks as noted in previous studies with excised corneoscleral tissue, including the deviation from the natural loading condition, which is multiaxial; lack of incorporation of natural curvature; severed collagen fibrils; and alignment of collagen in the loading direction, which all may alter the mechanical properties of the tissue.26,54,55 Thus, the mechanical properties reported in our study are most relevant for relative comparisons between the two groups. We recently have developed an inflation testing method based on high-resolution ultrasound,56 which will enable future mechanical testing on whole globes under physiologic loadings. Fourth, the hydroxyproline assay and histology staining were performed on tissue following mechanical testing and flash freezing. These steps most likely introduced a certain degree of collagen swelling in addition to degradation. However, careful analysis of the H&E stains for all specimens revealed that the collagen appeared to be intact, consistent between specimens, and comparable to previously reported H&E staining of sclera.57 Previous studies also found only mild changes in collagen content due to short freezing times of soft tissues.44,58 The hydration level of the sclera strips also was unknown, and could be greater than that of fresh, intact sclera due to the potential swelling during transport, mechanical testing, and freeze/thaw. This most likely decreased the magnitudes of μg collagen/mg wet weight for each strip, potentially making these data less comparable to the collagen content of fresh scleral tissue. However, all strips were put through the same mechanical testing, flash freezing, and weighing protocols, allowing for the relative comparison between groups and correlations with mechanical properties, as was the objective of our study. Fifth, the amount of insoluble collagen was a convenient but indirect way of measuring collagen cross-linking. Future analysis with methods, such as HPLC, will be necessary to measure directly the amount and types of cross-linking within the tissue.
In summary, our study identified a trend of weakened posterior scleral biomechanical properties and significantly altered collagen solubility in dogs with an ADAMTS10 mutation before clinical indications of ONH damage. The implications of the altered scleral biomechanical and biochemical properties, and their potential interactions with an elevated IOP during the course of glaucoma development and progression in this canine model warrant further investigations.
Acknowledgments
The authors thank Leandro Teixeira and Richard Dubielzig (University of Wisconsin) for their assistance with the optic nerve head histology, and Jessica Rowlan (University of Pennsylvania) for ADAMTS10 genotyping.
Supported by Grant NIHRO1EY020929 (JL and XP), The Ohio State University College of Medicine (JRP), University of Pennsylvania Research Foundation, and an unrestricted gift from Edward Sheppard and family (AMK).
Disclosure: J.R. Palko, None; S. Iwabe, None; X. Pan, None; G. Agarwal, None; A.M. Komáromy, None; J. Liu, None
References
- 1. Libby RT, Gould DB, Anderson MG, John SW. Complex genetics of glaucoma susceptibility. Annu Rev Genomics Hum Genet. 2005; 6: 15–44 [DOI] [PubMed] [Google Scholar]
- 2. Allingham RR, Liu Y, Rhee DJ. The genetics of primary open-angle glaucoma: a review. Exp Eye Res. 2009; 88: 837–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Roberts MD, Grau V, Grimm J, et al. Remodeling of the connective tissue microarchitecture of the lamina cribrosa in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2009; 50: 681–690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hernandez MR. The optic nerve head in glaucoma: role of astrocytes in tissue remodeling. Prog Ret Eye Res. 2000; 19: 297–321 [DOI] [PubMed] [Google Scholar]
- 5. Downs JC, Suh JKF, Thomas KA, Bellezza AJ, Hart RT, Burgoyne CF. Viscoelastic material properties of the peripapillary sclera in normal and early-glaucoma monkey eyes. Invest Ophthalmol Vis Sci. 2005; 46: 540–546 [DOI] [PubMed] [Google Scholar]
- 6. Kuchtey J, Olson LM, Rinkoski T, et al. Mapping of the disease locus and identification of ADAMTS10 as a candidate gene in a canine model of primary open angle glaucoma. PLoS Genet. 2011; 7: e1001306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Flammer J, Orgul S, Costa VP, et al. The impact of ocular blood flow in glaucoma. Prog Ret Eye Res. 2002; 21: 359–393 [DOI] [PubMed] [Google Scholar]
- 8. Kiel JW, van Heuven WA. Ocular perfusion pressure and choroidal blood flow in the rabbit. Invest Ophthalmol Vis Sci. 1995; 36: 579–585 [PubMed] [Google Scholar]
- 9. Bellezza AJ, Hart RT, Burgoyne CF. The optic nerve head as a biomechanical structure: Initial finite element modeling. Invest Ophthalmol Vis Sci. 2000; 41: 2991–3000 [PubMed] [Google Scholar]
- 10. Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part I: IOP-induced deformations and influence of geometry. Biomech Model Mechanobiol. 2009; 8: 85–98 [DOI] [PubMed] [Google Scholar]
- 11. Sigal IA, Flanagan JG, Tertinegg I, Ethier CR. Modeling individual-specific human optic nerve head biomechanics. Part II: influence of material properties. Biomech Model Mechanobiol. 2009; 8: 99–109 [DOI] [PubMed] [Google Scholar]
- 12. Sigal IA, Flanagan JG, Ethier CR. Factors influencing optic nerve head biomechanics. Invest Ophthalmol Vis Sci. 2005; 46: 4189–4199 [DOI] [PubMed] [Google Scholar]
- 13. Girard MJA, Suh JKF, Bottlang M, Burgoyne CF, Downs JC. Biomechanical changes in the sclera of monkey eyes exposed to chronic IOP elevations. Invest Ophthalmol Vis Sci. 2011; 52: 5656–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Coudrillier B, Tian J, Alexander S, Myers KM, Quigley HA, Nguyen TD. Biomechanics of the human posterior sclera: age- and glaucoma-related changes measured using inflation testing. Invest Ophthalmol Vis Sci. 2012; 53: 1714–1728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Steinhart MR, Cone FE, Nguyen C, et al. Mice with an induced mutation in collagen 8A2 develop larger eyes and are resistant to retinal ganglion cell damage in an experimental glaucoma model. Mol Vis. 2012; 18: 1093–1106 [PMC free article] [PubMed] [Google Scholar]
- 16. Gelatt KN, Brooks DE, Källberg ME. Veterinary Ophthalmology. 4th ed. Ames, Iowa: Blackwell Publishing; 2007. [Google Scholar]
- 17. Gelatt KN, Gum GG. Inheritance of primary glaucoma in the beagle. Am J Vet Res 1981; 42: 1691–1693 [PubMed] [Google Scholar]
- 18. Tsilou E, MacDonald IM. Weill-Marchesani Syndrome. In: Pagon RA, TD Bird, Dolan CR, Stephens K, Adam MP. eds GeneReviews. Seattle, WA: University of Washington, Seattle; 2007. [PubMed] [Google Scholar]
- 19. Le Goff C, Cormier-Daire V. The ADAMTS(L) family and human genetic disorders. Hum Mol Genet. 2011; 20: R163–R167 [DOI] [PubMed] [Google Scholar]
- 20. Kutz WE, Wang LW, Bader HL, et al. ADAMTS10 protein interacts with fibrillin-1 and promotes its deposition in extracellular matrix of cultured fibroblasts. J Biol Chem. 2011; 286: 17156–17167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hubmacher D, Apte SS. Genetic and functional linkage between ADAMTS superfamily proteins and fibrillin-1: a novel mechanism influencing microfibril assembly and function. Cell Mol Life Sci. 2011; 68: 3137–3148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ross R, Bornstein P. The elastic fiber. I. The separation and partial characterization of its macromolecular components. J Cell Biol. 1969; 40: 366–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Isogai Z, Ono RN, Ushiro S, et al. Latent transforming growth factor beta-binding protein 1 interacts with fibrillin and is a microfibril-associated protein. J Biol Chem. 2003; 278: 2750–2757 [DOI] [PubMed] [Google Scholar]
- 24. Rifkin DB. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005; 280: 7409–7412 [DOI] [PubMed] [Google Scholar]
- 25. Sakai LY, Keene DR, Engvall E. Fibrillin, a new 350-kD glycoprotein, is a component of extracellular microfibrils. J Cell Biol. 1986; 103: 2499–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Palko JR, Pan XL, Liu J. Dynamic testing of regional viscoelastic behavior of canine sclera. Exp Eye Res. 2011; 93: 825–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Reddy GK, Enwemeka CS. A simplified method for the analysis of hydroxyproline in biological tissues. Clin Biochem. 1996; 29: 225–229 [DOI] [PubMed] [Google Scholar]
- 28. Peiffer RL Jr, Gum GG, Grimson RC, Gelatt KN. Aqueous humor outflow in beagles with inherited glaucoma: constant pressure perfusion. Am J Vet Res 1980; 41: 1808–1813 [PubMed] [Google Scholar]
- 29. Tang J, Pan X, Weber PA, Liu J. Corneal modulus and IOP measurements in canine eyes using Goldmann applanation tonometry and Tono-pen. Invest Ophthalmol Vis Sci. 2011; 52: 7866–7871 [DOI] [PubMed] [Google Scholar]
- 30. Ferry J. Viscoelastic Properties of Polymer. New York, NY: John Wiley & Sons; 1980. [Google Scholar]
- 31. Fung Y. Biomechanics: Mechanical Properties of Living Tissues. New York, NY: Springer; 1993. [Google Scholar]
- 32. Mihai C, Iscru DF, Druhan LJ, Elton TS, Agarwal G. Discoidin domain receptor 2 inhibits fibrillogenesis of collagen type 1. J Mol Biol. 2006; 361: 864–876 [DOI] [PubMed] [Google Scholar]
- 33. Girard MJA, Suh JKF, Bottlang M, Burgoyne CF, Downs JC. Scleral biomechanics in the aging monkey eye. Invest Ophthalmol Vis Sci. 2009; 50: 5226–5237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mansouri K, Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: initial clinical experience in patients with open angle glaucoma. Brit J Ophthalmol. 2011; 95: 627–629 [DOI] [PubMed] [Google Scholar]
- 35. Downs JC, Burgoyne CF, Seigfreid WP, Reynaud JF, Strouthidis NG, Sallee V. 24-hour IOP telemetry in the nonhuman primate: implant system performance and initial characterization of IOP at multiple timescales. Invest Ophthalmol Vis Sci. 2011; 52: 7365–7375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Gelatt KN. Veterinary Ophthalmology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1999. :xv, 1544 [Google Scholar]
- 37. Brooks DE. Confocal scanning laser ophthalmoscopy. Trans Am Coll Vet Ophthalmol. 1996; 130 [Google Scholar]
- 38. Gelatt KN, Peiffer RL Jr, Gwin RM, Gum GG, Williams LW. Clinical manifestations of inherited glaucoma in the beagle. Invest Ophthalmol Vis Sci. 1977; 16: 1135–1142 [PubMed] [Google Scholar]
- 39. Liu J, He X. Corneal stiffness affects IOP elevation during rapid volume change in the eye. Invest Ophthalmol Vis Sci. 2009; 50: 2224–2229 [DOI] [PubMed] [Google Scholar]
- 40. Reddy GK. Cross-linking in collagen by nonenzymatic glycation increases the matrix stiffness in rabbit Achilles tendon. Exp Diabesity Res. 2004; 5: 143–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamauchi M, Woodley DT, Mechanic GL. Aging and cross-linking of skin collagen. Biochem Biophys Res Comm. 1988; 152: 898–903 [DOI] [PubMed] [Google Scholar]
- 42. Badenhorst D, Maseko M, Tsotetsi OJ, et al. Cross-linking influences the impact of quantitative changes in myocardial collagen on cardiac stiffness and remodelling in hypertension in rats. Cardiovasc Res. 2003; 57: 632–641 [DOI] [PubMed] [Google Scholar]
- 43. Woodiwiss AJ, Tsotetsi OJ, Sprott S, et al. Reduction in myocardial collagen cross-linking parallels left ventricular dilatation in rat models of systolic chamber dysfunction. Circulation. 2001; 103: 155–160 [DOI] [PubMed] [Google Scholar]
- 44. Chow MJ, Zhang Y. Changes in the mechanical and biochemical properties of aortic tissue due to cold storage. J Surg Res. 2010; 171: 434–442 [DOI] [PubMed] [Google Scholar]
- 45. Brownlee M, Vlassara H, Kooney A, Ulrich P, Cerami A. Aminoguanidine prevents diabetes-induced arterial wall protein cross-linking. Science. 1986; 232: 1629–1632 [DOI] [PubMed] [Google Scholar]
- 46. Reddy GK. AGE-related cross-linking of collagen is associated with aortic wall matrix stiffness in the pathogenesis of drug-induced diabetes in rats. Microvasc Res. 2004; 68: 132–142 [DOI] [PubMed] [Google Scholar]
- 47. Diamant J, Keller A, Baer E, Litt M, Arridge RG. Collagen; ultrastructure and its relation to mechanical properties as a function of ageing. Proc R Soc London B Biol Sci. 1972; 180: 293–315 [DOI] [PubMed] [Google Scholar]
- 48. Fratzl P, Misof K, Zizak I, Rapp G, Amenitsch H, Bernstorff S. Fibrillar structure and mechanical properties of collagen. J Struct Biol. 1998; 122: 119–122 [DOI] [PubMed] [Google Scholar]
- 49. Misof K, Rapp G, Fratzl P. A new molecular model for collagen elasticity based on synchrotron X-ray scattering evidence. Biophys J. 1997; 72: 1376–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Puxkandl R, Zizak I, Paris O, et al. Viscoelastic properties of collagen: synchrotron radiation investigations and structural model. Phil Trans R Soc Lond B Biol Sci. 2002; 357: 191–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Andrade SF, Palozzi RJ, Giuffrida R, de Campos RJ, Santos Gde C, Fukui RM. Comparison of intraocular pressure measurements between the Tono-Pen XL(R) and Perkins(R) applanation tonometers in dogs and cats. Vet Ophthalmol. 2012; 15 (Suppl 1): 14–20 [DOI] [PubMed] [Google Scholar]
- 52. Giannetto C, Piccione G, Giudice E. Daytime profile of the intraocular pressure and tear production in normal dog. Vet Ophthalmol. 2009; 12: 302–305 [DOI] [PubMed] [Google Scholar]
- 53. Greller AL, Hoffman AR, Liu C, et al. Effects of the topically applied calcium-channel blocker flunarizine on intraocular pressure in clinically normal dogs. Am J Vet Res 2008; 69: 273–278 [DOI] [PubMed] [Google Scholar]
- 54. Elsheikh A, Anderson K. Comparative study of corneal strip extensometry and inflation tests. J R Soc Interface. 2005; 2: 177–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hoeltzel DA, Altman P, Buzard K, Choe KI. Strip extensiometry for comparison of the mechanical response of bovine, rabbit, and human corneas. J Biomech Eng. 1992; 114: 202–215 [DOI] [PubMed] [Google Scholar]
- 56. Tang J, Liu J. Ultrasonic measurement of scleral cross-sectional strains during elevations of intraocular pressure: method validation and initial results in posterior porcine sclera. J Biomech Eng. 2012; 134: 091007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Curtin BJ. Physiopathologic aspects of scleral stress-strain. Trans Amer Ophthalmol Soc. 1969; 67: 417–461 [PMC free article] [PubMed] [Google Scholar]
- 58. Stemper BD, Yoganandan N, Stineman MR, Gennarelli TA, Baisden JL, Pintar FA. Mechanics of fresh, refrigerated, and frozen arterial tissue. J Surg Res. 2007; 139: 236–242 [DOI] [PubMed] [Google Scholar]