Key Points
High, but not low to moderate, HLA antibody levels are associated with platelet refractoriness.
Abstract
In the Trial to Reduce Alloimmunization to Platelets (TRAP) study, 101 of 530 participants became refractory to platelet transfusions without evidence of HLA or human platelet antigen (HPA) antibodies. We used a more sensitive bead-based assay to detect and quantify HLA antibodies and a qualitative solid-phase enzyme-linked immunosorbet assay for HPA to determine whether low-level antibodies could predict refractoriness in longitudinal panels from 170 lymphocytotoxicity assay (LCA)− and 20 LCA+ TRAP participants. All TRAP recipients who previously tested LCA+ were HLA antibody+, using the bead-based system. Levels of HLA or HPA antibodies did not predict refractoriness among LCA− recipients, although higher levels of HLA antibodies were associated with refractoriness among LCA+ recipients. These data demonstrate that weak to moderate HLA antibody levels detectable by modern binding assays are not associated with platelet refractoriness.
Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology.
Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 3299.
Disclosures
The authors, Associate Editor Mortimer Poncz, and CME questions author Charles P. Vega, Associate Professor and Residency Director, Department of Family Medicine, University of California-Irvine, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe alloimmunization due to HLA after platelet transfusion.
Analyze the significance of human platelet antigen (HPA) antibodies (Abs) in cases of alloimmunization after transfusion.
Evaluate the performance of newer tests for HLA Ab and HPA Ab.
Assess the role of HLA Ab and HPA Ab among patients refractory to treatment with platelet transfusions.
Release date: April 18, 2013; Expiration date: April 18, 2014
Introduction
Transfusion of blood and blood components exposes the recipient to a wide array of alloantigens expressed on the surface of donor WBCs, RBCs, and platelets. In response to this exposure, many transfusion recipients mount an immune response and become alloimmunized, resulting in antibody (Ab) generation against some of these alloantigens. With platelet transfusions, these responses are generally toward HLAs expressed on WBCs and platelets and/or other platelet antigens and can result in refractoriness to subsequent platelet transfusions.1,2
The generation of antibodies against HLA antigens is particularly common, with rates ranging from 7% to 55% after platelet transfusion, depending on study, patient population, and number and type of transfusions.1,3-9 These antibodies are usually detected within the first 2 weeks after exposure and can be either short-lived or persist long after transfusion.3,4,10-13 Leukoreduction of platelets has been shown in most studies to reduce the frequency of, but not eliminate, alloimmunization,3,5-8,14 although not necessarily in previously pregnant recipients.15 Rates are higher in previously pregnant women or those who have been transfused before.7,9,10,16
A number of methods have been used to measure HLA Abs. Originally, this was done using the lymphocytotoxicity assay (LCA), in which cells expressing the HLA protein of interest are incubated with the serum sample to be screened and lysis of these target cells is measured.17-19 More recently, several new assays have been developed including enzyme-linked immunosorbent assays (ELISAs), multianalyte bead-based assays, and flow cytometry assays.20-23 These systems are generally more sensitive than LCA, and several commercial kits are currently available.20,24-26
Antibodies against human platelet antigens (HPAs) can also be generated in response to platelet transfusion. These antigens appear to be less immunogenic than HLA antigens, resulting in a lower frequency of HPA alloimmunization, which ranges from 0% to 2%, depending on the patient population.27-30 These rates are higher in individuals who also have HLA Abs, with rates estimated to be between 9% and 25% among HLA alloimmunized recipients.27,31,32 Although rare, HPA Abs can cause refractoriness, even in the absence of HLA Abs or when HLA-matched platelets have been used.31,33,34
In the Trial to Reduce Alloimmunization to Platelets (TRAP) study, 17% to 21% of recipients of leukoreduced or ultraviolet-irradiated platelets and 45% of recipients of nonleukoreduced platelets developed new Abs against HLA antigens.17 Interestingly, of the 530 participants included in the study, 101 developed platelet refractoriness without evidence of HLA Abs, as measured by the LCA. This suggests either that refractoriness was being driven by other mechanisms in these individuals or that lower levels of Abs undetected by the LCA were driving the response. We measured the levels of anti-HLA Abs longitudinally in a subset of participants from the original TRAP study, using a newer, more-sensitive, complement-independent, bead-based assay to evaluate whether these patients were more likely to have low-level HLA Abs that were undetectable using the LCA. We also measured HPA Abs in these participants and quantified the duration and magnitude of HLA Ab responses in them.
Materials and methods
Participants and samples
From the TRAP study, 170 LCA− (70 clinically refractory [CR+] and 100 clinically nonrefractory [CR−]) and 20 LCA+ (10 CR+, 10 CR−) participants were selected.17 CR+ patients were those with a corrected count increment of less than 5000 after 2 sequential transfusions of ABO-compatible platelets, as described in the original TRAP analysis.17 LCA+ samples were included as positive controls for the detection of HLA Abs, and as many LCA− samples were included as were available. Participants with missing data were excluded, including 27 patients missing 1 or more corrected count increment data point, 3 participants who were refractory to only their last transfusion, and 7 patients who received at least a single non-ABO-compatible transfusion to which they were refractory. Of the 190 participants, 100 received leukoreduced platelets, 47 received UV-treated platelets, and 43 received untreated platelets. Longitudinal panels including pre- and posttransfusion points were tested in a blinded fashion. Samples were collected under institutional review board–approved protocols that included written informed consent, in accordance with the Declaration of Helsinki.
Anti-HLA Ab detection
Antibodies against class I and class II HLA antigens were measured in serum or plasma samples using the One Lambda (Canoga Park, CA) LabScreen mixed Luminex assay, run according to the manufacturer’s instructions. The results are reported as normalized background (NBG) ratios for each of 8 multiantigen beads, and the highest value for each sample was used. A NBG ratio greater than 10.8 for class I HLA antibodies and 6.9 for class II HLA antibodies was used as the cutoff for a positive result, based on previous work in nonalloexposed populations.35
Anti-HPA Ab detection
Antibodies against HPAs were detected in serum using the PAKPLUS qualitative solid-phase ELISA, manufactured by GTI Diagnostics (Waukesha, WI), run according to the manufacturer’s instructions. This assay detects antibodies against epitopes on the platelet glycoproteins IIb/IIIa, Ib/IX, Ia/IIa, and IV, and class I HLA. Absorbance values were normalized against those for negative controls run on the same plate by dividing the mean absorbance value of the duplicate wells by the mean absorbance value of the negative controls for the corresponding glycoprotein. Samples were considered positive for HPA Abs if any of the normalized values for platelet glycoproteins (excluding HLA) were greater than 2. Samples were run in duplicate and tested in a blinded fashion.
Statistical analysis
NBG ratios lower than 1 were assigned a value of 1. Unpaired t-tests were used for comparisons between 2 groups using GraphPad Prism (GraphPad Software Inc, La Jolla, CA). Differences in the frequency of new Ab development between 2 groups were evaluated using a χ2 test. Correlation between class I and class II Ab NBG ratios was calculated using GraphPad Prism. Receiver operating characteristic (ROC) analysis was used to evaluate the ability of class I and class II HLA Ab NBG ratios or HPA Ab normalized OD values to predict LCA responses and/or clinical refractoriness using R (R Foundation for Statistical Computing, Vienna, Austria).36 To evaluate the persistence of Ab responses, start times were normalized to the first point with an NBG ratio above the threshold of 10.8 for class I and 6.9 for class II, and time to loss below these thresholds was evaluated by Kaplan-Meier survival analysis using R. Differences in persistence of Abs between groups was examined by log-rank test using R.
Results
Sensitive detection of HLA Abs in TRAP participants
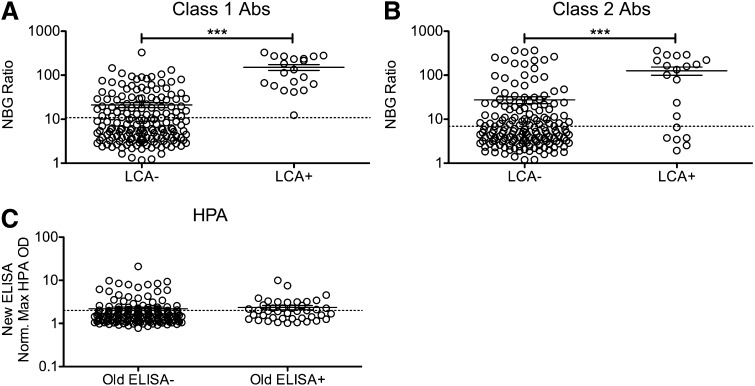
Serum samples of platelet transfusion recipients from the TRAP study were originally screened for HLA Abs using the LCA. We evaluated these same longitudinal samples for the presence of anti-HLA antibodies using a more-sensitive bead-based assay. One LCA−CR+ individual was dropped from the analysis because of test failure. To determine whether the LCA+ samples from the TRAP study had higher levels of measured HLA antibodies with the new multiplexing methods, peak NBG ratios (the highest detected value for each recipient) for class I and class II HLA Abs (Figure 1A-B) were compared between LCA− and LCA+ participants. LCA+ recipients had both significantly higher levels of both class I and class II HLA antibodies than LCA− recipients (P < .001) and significantly higher frequency of new antibodies (P < .001). All LCA+ participants had a peak NBG ratio that exceeded the cutoff for class I HLA antibodies, although only 70% of these were positive for class II antibodies, using the same criteria. Many of the LCA− recipients were positive for class I (31%) and class II (29%) HLA Abs, which is consistent with greater sensitivity with the newer assay.
Figure 1.
Increased antibody levels detected among LCA+ transfusion recipients. Longitudinal samples of platelet transfusion recipients from the TRAP study that had been previously screened for HLA antibodies using the LCA were screened for HLA antibodies using a multianalyte, bead-based fluorescent Ab detection assay. Peak class I (A) and class II (B) HLA Ab NBG ratios are plotted for LCA− and LCA+ blood recipients, and mean values were compared using a 1-tailed unpaired t-test. ***P < .001. The dashed line marks the cutoff used to distinguish positive and negative results. (C) Samples from platelet transfusion recipients from the TRAP study that had been previously screened for HPA antibodies using an ELISA were screened for HPA antibodies using a new ELISA. The maximum normalized OD value for antibodies against platelet antibodies (excluding HLA antibodies) were plotted for recipients that tested either ELISA− or ELISA+ with the original assay. The dashed line marks the cutoff used to distinguish positive and negative results. Error bars display mean and standard error.
To determine how well peak NBG ratios for class I and class II HLA Abs could predict LCA+ participants, ROC analysis was performed. Class I HLA NBG ratios predicted LCA+ samples very well, with an area under the curve (AUC) of 0.94 (see supplemental Figure 1A on the Blood website), whereas class II NBG ratios had a more moderate predictive ability with an AUC of 0.74 (supplemental Figure 1B). HPA antibodies were measured at a single time (4 weeks into study or nearest available time) for each of the included TRAP study transfusion recipients. OD values were normalized to controls, and the highest value for each sample was compared between recipients who had tested positive or negative for HPA antibodies by whole-platelet ELISA in the original study. There were no significant differences between these groups, although all levels were very low (Figure 1C). To assess the ability of the new test to predict a positive result in the original analysis, ROC analysis was performed. The normalized OD values had a slight predictive ability, with an AUC of 0.61 (supplemental Figure 1C).
Influence of moderate-strength HLA Abs and HPA Abs on platelet refractoriness
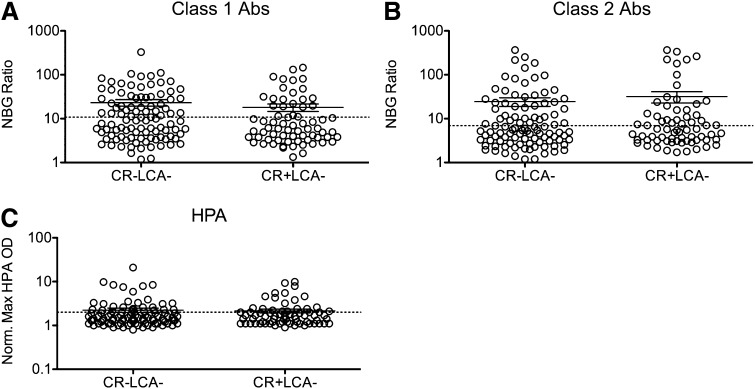
The majority of the transfusion recipients in the TRAP study who developed platelet refractoriness were negative for HLA Abs, as measured by LCA, which could be because of low assay sensitivity. To determine whether these participants have higher levels of HLA Abs than those who were CR−, peak NBG ratios for class I and class II HLA Abs (Figure 2A-B) were compared between CR− and CR+ LCA− participants. No significant differences were seen between these groups. To determine the ability of peak NBG ratios to predict CR, ROC analysis was performed. Neither class I nor class II Abs were able to predict CR (supplemental Figure 2A-B).
Figure 2.
Anti-HLA and anti-HPA Ab levels are not associated with refractoriness among LCA− recipients. Peak class I (A) and class II (B) HLA Ab NBG ratios and normalized maximum absorbance values for HPA Abs (C) were compared between CR− and CR+ LCA− recipients, and mean values were compared using a 1-tailed unpaired t-test. The dashed line marks the cutoff used to distinguish positive and negative results. Error bars display mean and standard error.
We next assessed whether other platelet antibodies were associated with CR and observed HPA Abs in 29% of LCA− CR− and 31% of LCA−CR+ recipients. Normalized absorbance was plotted for LCA− recipients, and no significant difference was seen between CR− and CR+ patients (Figure 2C). ROC analysis was performed, and normalized absorbance values for HPA Abs did not predict CR (supplemental Figure 2E), consistent with the original TRAP results.17
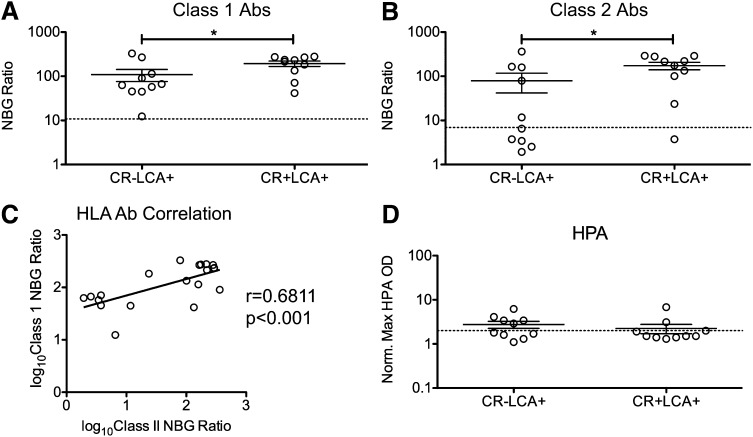
The majority of the LCA+ recipients in the TRAP study were not CR. To determine whether higher HLA Ab levels were associated with CR within the LCA+ group, peak NBG ratios for class I and class II HLA Abs (Figure 3A-B) were compared between CR− and CR+ LCA+ participants. Significantly higher NBG ratios were found in the CR+ group for both class I and class II HLA Abs. To determine the ability of peak NBG ratios to predict CR within the LCA+ participants, ROC analysis was performed. Both class I and class II Abs had moderate predictive ability for CR, with AUCs of 0.73 and 0.77, respectively (supplemental Figure 2C-D). As platelets express class I, but not class II, HLA, the association of higher levels of class II Abs with CR may be a result of an association between higher levels of class I Abs and higher levels of class II Abs. Consistent with such a relationship, a moderate correlation between the peak class I and class II HLA Ab NBG ratios was seen among the LCA+ recipients, with r = 0.6811 (Figure 3C). No significant differences were detected in HPA Abs between CR− and CR+ LCA+ recipients (Figure 3D; supplemental Figure 2D).
Figure 3.
Higher anti-HLA Ab NBG ratios are associated with refractoriness among LCA+ recipients. Peak class I (A) and class II (B) HLA Ab NBG ratios and normalized maximum absorbance values for HPA Abs (D) were compared between CR− and CR+ LCA+ recipients. The dashed lines mark the cutoff used to distinguish positive and negative results; mean values were compared using a 1-tailed unpaired t-test. *P < .05. (C) Class I HLA Ab NBG ratios were log10 transformed and plotted against class II HLA Ab NBG ratios, and correlation was evaluated. Error bars display mean and standard error.
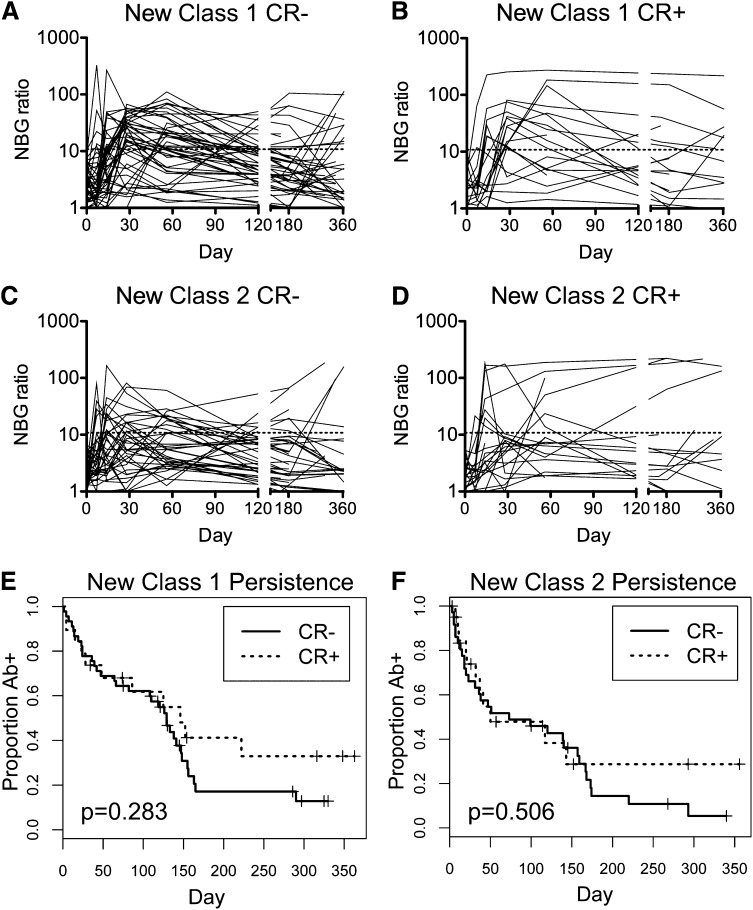
HLA Ab dynamics and persistence posttransfusion
Persistence of HLA Abs varies considerably among alloimmunized recipients. One possibility is that CR+ recipients may have more persistent HLA Ab responses. To evaluate the persistence of HLA Abs, class I (Figure 4A-B) and class II (Figure 4C-D) HLA Ab NBG ratios were plotted over time for CR− (Figure 4A,C) and CR+ (Figure 4B,D) recipients, excluding those positive for HLA Abs pretransfusion. Kaplan-Meier survival analysis was performed to assess the persistence of HLA Abs above the NBG threshold of 10.8 for class I and 6.9 for class II, and log-rank test was used to evaluate differences between CR− and CR+ recipients. No significant differences were seen in persistence of class I or class II HLA Abs based on clinical refractory status (Figure 4E-F).
Figure 4.
No significant differences in persistence of HLA antibodies between CR− and CR+ recipients. Longitudinal class I (A and B) and class II (C and D) HLA Ab NBG ratios were plotted over time for CR− (A and C) and CR+ recipients (B and D). The dashed line marks the cutoff used to distinguish positive and negative results. Kaplan-Meier survival analysis was used to evaluate the persistence of new class I (E) and class II (F) antibodies, with CR− and CR+ recipients compared using the log-rank test and associated P values reported.
Discussion
Transfusion of allogeneic platelets can result in the generation of antibodies against HLA or HPA, leaving recipients vulnerable to refractoriness to subsequent platelet transfusions. In the TRAP study, although HLA Abs were found to be linked to refractoriness, 19% of recipients were refractory without any detectable HLA Abs. We sought to determine whether this was the result of lower-level HLA Abs previously undetected by the relatively insensitive LCA used in the original analysis and found both that although the newer assays did detect antibodies among many recipients who were LCA−, these HLA Abs were not significantly higher among CR+ LCA− compared with CR− LCA− recipients, and that lower-level HLA Abs detected with the new assays did not predict refractoriness among LCA− recipients. HLA Abs detected using the bead-based assays were able to help distinguish between CR− and CR+ among the LCA+ recipients, with significantly higher levels of both class I and class II HLA Abs seen among the CR+ participants. These data show that only strong HLA Abs were associated with CR in the TRAP trial.
HPA Abs were also unable to account for the observed refractoriness within the LCA− recipients, as no differences were seen between CR− and CR+ patients within either the LCA− or LCA+ cohort. This is consistent with the original analysis of the TRAP samples.17 We did detect higher rates of HPA alloimmunization, with 31% of our samples positive for HPA Abs using the manufacturer’s cutoff values as opposed to the 8% seen in the original TRAP analysis. These higher levels may be a result of the relative sensitivity of the assays, although the whole-platelet ELISA used in the TRAP study is sensitive but not highly specific.17 When cutoffs of the ELISA used in the current study were increased from 2 times the background to 3 or 4 times background, the rates dropped to 16% and 9%, respectively.
The failure of low-level HLA Abs or HPA Abs to predict refractoriness among LCA− recipients suggests that other mechanisms are important in determining refractoriness. Although it is possible that additional antigens undetectable with our assays could be responsible for a portion of the LCA− refractory patients, these would be rare antigens and unlikely to account for many cases. Previous analysis of this patient cohort has shown an increased risk for refractoriness among men, women with 2 or more pregnancies, patients with fever or bleeding, and those given heparin. Products transfused were also shown to play a role, with a slight increase in risk for CR with use of γ-irradiated platelets and decreased risk associated with higher platelet dose or apheresis platelets.2 Other non-Ab driven causes of refractoriness can include splenomegaly, sepsis, disseminated intravascular coagulation, venoocclusive disease, and graft-vs-host disease.2,37,38
Although many LCA− recipients were positive for HLA Abs using the newer assay, data collected using the 2 different assays matched well, with significantly higher levels of class I and class II HLA Abs detected among the LCA+ recipients. All LCA+ recipients tested positive for class I HLA Abs using the bead-based assay, although 30% were negative for class II Abs. Furthermore, class I Ab NBG ratios predicted LCA status well. The increased frequency of antibodies detected using the newer bead-based assay suggests a much greater sensitivity of this assay compared with the LCA, but it may also be explained in part by the dependence of the LCA on complement activation. This helps explain why some of the LCA− participants have such high NBG ratios using this newer assay, yet this reactivity was not associated with clinical refractoriness.
Although the cohort of patients with acute myeloid leukemia used here is representative of many patients at risk for platelet refractoriness, it had some limitations. Other patient populations with different underlying health problems may have a different proportion of refractory cases driven by immune or nonimmune mechanisms, as these are influenced by various disease states. We also focused on the LCA− cases, and so chose only a small number of LCA+ control patients. In spite of this small sample, we were able to detect significant differences between the CR− and CR+ patients within the LCA+ group, although greater numbers might have strengthened this. Finally, the timing of sample collections differed somewhat between individuals, which may have limited our analysis of the kinetics of the response. It is also possible that individual peak responses were missed or underrepresented because of the timing of sample collection, although we would not expect this to differ between groups.
In summary, we hypothesized that previously undetected low-level HLA and/or HPA Abs would be found among the LCA− participants in the TRAP study and that these antibodies would be associated with refractoriness. We found that although we were able to detect these antibodies in a number of LCA− patients, these antibodies did not differ between refractory and nonrefractory patients among this population. We did, however, see higher levels of HLA Abs associated with refractoriness among the LCA+ patients. Together, these data suggest that high levels of HLA Abs are required for Ab-driven refractoriness.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Institutes of Health (grants R01HL-095470, R01HL-083388, and U01HL-42799). This manuscript was prepared using TRAP Research Materials obtained from the National Heart, Blood, and Lung Institute Biologic Specimen and Data Repository Information Coordinating Center.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.P.J. designed research; collected, analyzed, and interpreted data; performed statistical analysis; and wrote manuscript. X.D. performed statistical analysis. S.J.S. provided samples and designed research. D.B. designed research and provided statistical support. M.L. and J.W.H. collected data. M.P.B. and P.J.N. obtained samples, designed research, interpreted data, and edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Rachael P. Jackman, 270 Masonic Ave, San Francisco, CA 94118; email: rjackman@bloodsystems.org.
References
- 1.Howard JE, Perkins HA. The natural history of alloimmunization to platelets. Transfusion. 1978;18(4):496–503. doi: 10.1046/j.1537-2995.1978.18478251250.x. [DOI] [PubMed] [Google Scholar]
- 2.Slichter SJ, Davis K, Enright H, et al. Factors affecting posttransfusion platelet increments, platelet refractoriness, and platelet transfusion intervals in thrombocytopenic patients. Blood. 2005;105(10):4106–4114. doi: 10.1182/blood-2003-08-2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andreu G, Dewailly J, Leberre C, et al. Prevention of HLA immunization with leukocyte-poor packed red cells and platelet concentrates obtained by filtration. Blood. 1988;72(3):964–969. [PubMed] [Google Scholar]
- 4.Dutcher JP, Schiffer CA, Aisner J, et al. Alloimmunization following platelet transfusion: the absence of a dose-response relationship. Blood. 1981;57(3):395–398. [PubMed] [Google Scholar]
- 5.Fisher M, Chapman JR, Ting A, et al. Alloimmunisation to HLA antigens following transfusion with leucocyte-poor and purified platelet suspensions. Vox Sang. 1985;49(5):331–335. doi: 10.1111/j.1423-0410.1985.tb00807.x. [DOI] [PubMed] [Google Scholar]
- 6.Murphy MF, Metcalfe P, Thomas H, et al. Use of leucocyte-poor blood components and HLA-matched-platelet donors to prevent HLA alloimmunization. Br J Haematol. 1986;62(3):529–534. doi: 10.1111/j.1365-2141.1986.tb02965.x. [DOI] [PubMed] [Google Scholar]
- 7.Schiffer CA, Dutcher JP, Aisner J, et al. A randomized trial of leukocyte-depleted platelet transfusion to modify alloimmunization in patients with leukemia. Blood. 1983;62(4):815–820. [PubMed] [Google Scholar]
- 8.van Marwijk Kooy M, van Prooijen HC, Moes M, et al. Use of leukocyte-depleted platelet concentrates for the prevention of refractoriness and primary HLA alloimmunization: a prospective, randomized trial. Blood. 1991;77(1):201–205. [PubMed] [Google Scholar]
- 9.Karpinski M, Pochinco D, Dembinski I, et al. Leukocyte reduction of red blood cell transfusions does not decrease allosensitization rates in potential kidney transplant candidates. J Am Soc Nephrol. 2004;15(3):818–824. doi: 10.1097/01.asn.0000115399.80913.b1. [DOI] [PubMed] [Google Scholar]
- 10.Fauchet R, Genetet B, Gueguen M, et al. Transfusion therapy and HLA antibody response in patients undergoing open heart surgery. Transfusion. 1982;22(4):320–322. doi: 10.1046/j.1537-2995.1982.22482251219.x. [DOI] [PubMed] [Google Scholar]
- 11.Holohan TV, Terasaki PI, Deisseroth AB. Suppression of transfusion-related alloimmunization in intensively treated cancer patients. Blood. 1981;58(1):122–128. [PubMed] [Google Scholar]
- 12.Oksanen K, Kekomäki R, Ruutu T, et al. Prevention of alloimmunization in patients with acute leukemia by use of white cell-reduced blood components—a randomized trial. Transfusion. 1991;31(7):588–594. doi: 10.1046/j.1537-2995.1991.31791368333.x. [DOI] [PubMed] [Google Scholar]
- 13.Slichter SJ, Bolgiano D, Kao KJ, et al. Persistence of lymphocytotoxic antibodies in patients in the trial to reduce alloimmunization to platelets: implications for using modified blood products. Transfus Med Rev. 2011;25(2):102–110. doi: 10.1016/j.tmrv.2010.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sniecinski I, O’Donnell MR, Nowicki B, et al. Prevention of refractoriness and HLA-alloimmunization using filtered blood products. Blood. 1988;71(5):1402–1407. [PubMed] [Google Scholar]
- 15.Sintnicolaas K, van Marwijk Kooij M, van Prooijen HC, et al. Leukocyte depletion of random single-donor platelet transfusions does not prevent secondary human leukocyte antigen-alloimmunization and refractoriness: a randomized prospective study. Blood. 1995;85(3):824–828. [PubMed] [Google Scholar]
- 16.Payne R, Rolfs MR. Further observations on leukoagglutinin transfusion reactions in women: with special reference to leukoagglutinin transfusion reactions in women. Am J Med. 1960;29:449–458. doi: 10.1016/0002-9343(60)90041-3. [DOI] [PubMed] [Google Scholar]
- 17.The Trial to Reduce Alloimmunization to Platelets Study Group. Leukocyte reduction and ultraviolet B irradiation of platelets to prevent alloimmunization and refractoriness to platelet transfusions. N Engl J Med. 1997;337(26):1861–1869. doi: 10.1056/NEJM199712253372601. [DOI] [PubMed] [Google Scholar]
- 18.Phelan DL, Rodey GE, Anderson CB. The development and specificity of antiidiotypic antibodies in renal transplant recipients receiving single-donor blood transfusions. Transplantation. 1989;48(1):57–60. doi: 10.1097/00007890-198907000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Terasaki PI, McClelland JD. Microdroplet Assay of Human Serum Cytotoxins. Nature. 1964;204:998–1000. doi: 10.1038/204998b0. [DOI] [PubMed] [Google Scholar]
- 20.Carrick DM, Johnson B, Kleinman SH, et al. NHLBI Retrovirus Epidemiology Donor Study-II (REDS-II) Agreement among HLA antibody detection assays is higher in ever-pregnant donors and improved using a consensus cutoff. Transfusion. 2011;51(5):1105–1116. doi: 10.1111/j.1537-2995.2010.02938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pei R, Lee J, Chen T, et al. Flow cytometric detection of HLA antibodies using a spectrum of microbeads. Hum Immunol. 1999;60(12):1293–1302. doi: 10.1016/s0198-8859(99)00121-4. [DOI] [PubMed] [Google Scholar]
- 22.Uboldi de Capei M, Praticò L, Curtoni ES. Comparison of different techniques for detection of anti-HLA antibodies in sera from patients awaiting kidney transplantation. Eur J Immunogenet. 2002;29(5):379–382. doi: 10.1046/j.1365-2370.2002.00334.x. [DOI] [PubMed] [Google Scholar]
- 23.Wahrmann M, Exner M, Haidbauer B, et al. [C4d]FlowPRA screening—a specific assay for selective detection of complement-activating anti-HLA alloantibodies. Hum Immunol. 2005;66(5):526–534. doi: 10.1016/j.humimm.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 24.Fadeyi E, Adams S, Peterson B, et al. Analysis of a high-throughput HLA antibody screening assay for use with platelet donors. Transfusion. 2008;48(6):1174–1179. doi: 10.1111/j.1537-2995.2008.01684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lopes LB, Fabron-Jr A, Chiba AK, et al. Impact of using different laboratory assays to detect human leukocyte antigen antibodies in female blood donors. Transfusion. 2010;50(4):902–908. doi: 10.1111/j.1537-2995.2009.02523.x. [DOI] [PubMed] [Google Scholar]
- 26.Worthington JE, Robson AJ, Sheldon S, et al. A comparison of enzyme-linked immunoabsorbent assays and flow cytometry techniques for the detection of HLA specific antibodies. Hum Immunol. 2001;62(10):1178–1184. doi: 10.1016/s0198-8859(01)00282-8. [DOI] [PubMed] [Google Scholar]
- 27.Kickler T, Kennedy SD, Braine HG. Alloimmunization to platelet-specific antigens on glycoproteins IIb-IIIa and Ib/IX in multiply transfused thrombocytopenic patients. Transfusion. 1990;30(7):622–625. doi: 10.1046/j.1537-2995.1990.30790385520.x. [DOI] [PubMed] [Google Scholar]
- 28.Kiefel V, König C, Kroll H, et al. Platelet alloantibodies in transfused patients. Transfusion. 2001;41(6):766–770. doi: 10.1046/j.1537-2995.2001.41060766.x. [DOI] [PubMed] [Google Scholar]
- 29.Sanz C, Freire C, Alcorta I, et al. Platelet-specific antibodies in HLA-immunized patients receiving chronic platelet support. Transfusion. 2001;41(6):762–765. doi: 10.1046/j.1537-2995.2001.41060762.x. [DOI] [PubMed] [Google Scholar]
- 30.Kurz M, Knöbl P, Kalhs P, et al. Platelet-reactive HLA antibodies associated with low posttransfusion platelet increments:a comparison between the monoclonal antibody-specific immobilization of platelet antigens assay and the lymphocytotoxicity test. Transfusion. 2001;41(6):771–774. doi: 10.1046/j.1537-2995.2001.41060771.x. [DOI] [PubMed] [Google Scholar]
- 31.Novotny VM, van Doorn R, Witvliet MD, et al. Occurrence of allogeneic HLA and non-HLA antibodies after transfusion of prestorage filtered platelets and red blood cells: a prospective study. Blood. 1995;85(7):1736–1741. [PubMed] [Google Scholar]
- 32.Kekomäki S, Volin L, Koistinen P, et al. Successful treatment of platelet transfusion refractoriness: the use of platelet transfusions matched for both human leucocyte antigens (HLA) and human platelet alloantigens (HPA) in alloimmunized patients with leukaemia. Eur J Haematol. 1998;60(2):112–118. doi: 10.1111/j.1600-0609.1998.tb01007.x. [DOI] [PubMed] [Google Scholar]
- 33.Langenscheidt F, Kiefel V, Santoso S, et al. Platelet transfusion refractoriness associated with two rare platelet-specific alloantibodies (anti-Baka and anti-PlA2) and multiple HLA antibodies. Transfusion. 1988;28(6):597–600. doi: 10.1046/j.1537-2995.1988.28689059040.x. [DOI] [PubMed] [Google Scholar]
- 34.Pappalardo PA, Secord AR, Quitevis P, et al. Platelet transfusion refractoriness associated with HPA-1a (Pl(A1)) alloantibody without coexistent HLA antibodies successfully treated with antigen-negative platelet transfusions. Transfusion. 2001;41(8):984–987. doi: 10.1046/j.1537-2995.2001.41080984.x. [DOI] [PubMed] [Google Scholar]
- 35.Triulzi DJ, Kleinman S, Kakaiya RM, et al. The effect of previous pregnancy and transfusion on HLA alloimmunization in blood donors: implications for a transfusion-related acute lung injury risk reduction strategy. Transfusion. 2009;49(9):1825–1835. doi: 10.1111/j.1537-2995.2009.02206.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.R Development Core Team. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statisitical Computing; 2011. [Google Scholar]
- 37.Hod E, Schwartz J. Platelet transfusion refractoriness. Br J Haematol. 2008;142(3):348–360. doi: 10.1111/j.1365-2141.2008.07189.x. [DOI] [PubMed] [Google Scholar]
- 38.Ishida A, Handa M, Wakui M, et al. Clinical factors influencing posttransfusion platelet increment in patients undergoing hematopoietic progenitor cell transplantation—a prospective analysis. Transfusion. 1998;38(9):839–847. doi: 10.1046/j.1537-2995.1998.38998409004.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.