Background: NAADP is a messenger that links cell surface receptors to contraction in many cells, but its function is unknown in tracheal contraction.
Results: NAADP participates in contractions induced by a neurotransmitter receptor.
Conclusion: NAADP is a messenger that mediates tracheal contraction.
Significance: Inappropriate tracheal contractions underlie asthma, and NAADP represents a new physiological mediator and drug target.
Keywords: Calcium, Calcium Signaling, NAADP, Signal Transduction, Smooth Muscle, Ned-19, Second Messenger, Trachea
Abstract
Nicotinic acid adenine dinucleotide phosphate (NAADP) is increasingly being demonstrated to be involved in calcium signaling in many cell types and species. Although it has been shown to play a role in smooth muscle cell contraction in several tissues, nothing is known about its possible role in tracheal smooth muscle, a muscle type that is clinically relevant to asthma. To determine whether NAADP functions as a second messenger in tracheal smooth muscle contraction, we used the criteria set out by Sutherland for a molecule to be designated a second messenger. We report that NAADP satisfies all five criteria as follows. First, the NAADP antagonist Ned-19 inhibited contractions in tracheal rings and calcium increases in isolated smooth muscle cells induced by the muscarinic agonist carbachol. Second, NAADP increased cytosolic calcium in isolated cells when microinjected and was blocked by Ned-19. Third, tracheal homogenates could synthesize NAADP by base exchange from exogenous NADP and nicotinic acid and metabolize exogenous NAADP to nicotinic acid adenine dinucleotide by a 2′-phosphatase. Fourth, carbachol induced a rapid and transient increase in endogenous NAADP levels. Fifth, tracheal homogenates contained NAADP-binding sites of high affinity. Taken together, these data demonstrate that NAADP functions as a second messenger in tracheal smooth muscle, and therefore, steps in the NAADP signaling pathway might provide possible new drug targets.
Introduction
Airway diameter is dynamically regulated during normal physiological processes, but inappropriate narrowing caused by hyperresponsiveness results in asthma. Airway contractility is mediated by smooth muscle contraction, which is largely driven by increases in cytosolic calcium, as in other types of smooth muscle (1–6). Smooth muscle relaxation can occur through either a decrease in calcium or an increase in cyclic AMP (7). The G-protein-coupled receptors linked to cytosolic calcium and cyclic AMP have been intensively characterized not only for a better understanding of the basic biology but also because this biological knowledge can be exploited to provide pharmacological treatments (7–11).
Cytosolic calcium can increase through either release from intracellular stores or influx from the extracellular space (3, 12–16). Traditionally, focus has been on calcium stored in the sarcoplasmic reticulum (12, 13), which is released through channels, either inositol 1,4,5-trisphosphate (IP3)3 receptors activated by IP3 (12, 13) or ryanodine receptors activated by cyclic ADP-ribose (17–20). Cytosolic calcium can also increase in response to the second messenger nicotinic acid adenine dinucleotide phosphate (NAADP) (21–24). NAADP releases calcium from acidic endolysosomal stores (21–23, 25–28) by activation of two-pore channels (29–31). Since its discovery by Lee and colleagues (17, 22) almost 20 years ago, the emerging consensus is that NAADP is a widespread messenger that plays physiological roles in many mammalian systems (6, 21, 23, 24, 32–41).
In airway smooth muscle contraction, both IP3 and cyclic ADP-ribose and their signaling components have been reported and well characterized (3, 5, 6, 8, 12, 13, 34), but a role for NAADP in this muscle type has not been reported. One study did investigate a role for NAADP in tracheal smooth muscle but reported negative results (42). However, the authors based this on thio-NADP being an NAADP antagonist, which has been subsequently shown not to be the case (43), and a concentration of NAADP (100 μm) that is now known to be inhibitory due to a bell-shaped concentration-response curve for NAADP (21, 23, 24).
Sutherland and co-workers (44, 45) set out five criteria to establish that a given second messenger mediates the action of a particular hormone. We now provide evidence that NAADP satisfies all five of Sutherland's criteria, thereby demonstrating that NAADP mediates, at least in part, calcium increases and contractions in tracheal smooth muscle induced by muscarinic acetylcholine receptors.
EXPERIMENTAL PROCEDURES
Tissue Harvesting
Adult male Hartley guinea pigs (400–500 g) were killed humanely using cervical dislocation subsequent to stunning. Tracheas were excised aseptically and placed into Hanks' balanced salt solution (Sigma). All extraneous tissue was carefully removed.
Tracheal Contractions Measured by Wire Myography
Experiments were performed as described previously (46, 47) with minor modifications. Briefly, whole tracheas were cut into rings between two and three cartilages per ring. The segments were immersed in heated tissue baths (37 °C) containing 7 ml of Krebs solution. The solution was continuously equilibrated with 5% CO2 in O2 to maintain a pH of 7.4. Each tracheal segment was mounted on two L-shaped metal prongs, ensuring that the strip of smooth muscle was facing upwards. One prong was connected to a force-displacement transducer for continuous recording of isometric tension by Chart software (ADInstruments). Another prong was connected to a displacement device, allowing adjustment of the distance between the two parallel prongs. Following equilibration, a pre-tension of ∼5 millinewtons was applied to each segment and equilibrated for at least 30 min with washes every 10 min. Following equilibration, the maximum response was determined by addition of 100 μm carbachol. Tissue was then washed and incubated for 30 min with washes every 10 min and then for 1 h with either dimethyl sulfoxide or 10 μm Ned-19 (InterBioScreen). We then generated a cumulative concentration response to carbachol, followed by a single addition of 60 mm KCl.
Calcium Imaging
Tracheal smooth muscle cells were prepared using an explant technique and grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum, penicillin/streptomycin, and l-glutamine at 37 °C in a 5% CO2 incubator. Experiments were performed on cells passaged once. Cells were loaded with fura-2 via the acetoxymethyl ester (1 μm for 45 min at room temperature) and imaged on an inverted microscope using alternating 340 and 380 nm excitation and >510 nm emission (longpass) detected with a charged coupled device camera controlled with MetaFluor software. Cells were incubated with either dimethyl sulfoxide or 10 μm Ned-19 for 15 min before addition of 100 μm carbachol.
Microinjection of Messengers
Isolated airway smooth muscle cells from guinea pig were injected with either 5 μm NAADP or 5 μm IP3 (final concentration of ∼50 nm as 1% of the cellular volume was injected, estimated from the cell's dimensions). Injections were performed with Femtotips II and the InjectMan NI 2 and FemtoJet systems (Eppendorf AG). Pipettes were back-filled with an intracellular solution composed of 110 mm KCl, 10 mm NaCl, and 20 mm HEPES (pH 7.2) and supplemented with or without NAADP or IP3. The injection time was 0.5 s at 60 hectopascals with a compensation pressure of 20 hectopascals.
NAADP Synthesis and Metabolism
For synthesis, we incubated a tracheal homogenate (5%, w/v) with NADP (1 mm) and nicotinic acid (10 mm) at pH 5 (50 mm acetic acid) or pH 7 (20 mm HEPES). For metabolism, we incubated the homogenate with NAADP (100 μm) at pH 7.4 (20 mm HEPES). All reactions were conducted at 37 °C, stopped by immersion for 1 min in a 95 °C water bath, and centrifuged at 13,000 × g for 5 min to pellet denatured protein, and 100 μl of supernatant was then injected onto a Waters HPLC system. Compounds were separated by anion exchange using AG MP-1 resin (Bio-Rad) packed in a 1 × 10-cm column (Omnifit) using a concave up-gradient of trifluoroacetic acid as described previously (48) and monitored by absorbance at 254 nm. Compounds were identified by elution times of authentic samples.
NAADP Levels
Whole tracheas were cut into rings and placed into 200 μl of Hanks' balanced salt solution. Rings were incubated with carbachol for the desired times before addition of 200 μl of 0.75 m HClO4. Tracheal rings were sonicated and centrifuged to pellet denatured protein, and the supernatant was neutralized with an equal volume of 2 m KHCO3 as described (49). The levels of NAADP in each sample were determined as described previously (49). Briefly, NAADP standards or samples were incubated with sea urchin egg homogenate for 10 min. [32P]NAADP was added to each tube and incubated for an additional 10 min. Bound NAADP was filtered onto Whatman GF/B filter papers by washing using a Brandel cell harvester. Radioactivity was determined using a storage phosphor screen and Typhoon scanner (Amersham Biosciences).
NAADP Binding
Whole tracheas were chopped using a razor blade and homogenized using a Precellys 24 tissue homogenizer (Bertin Technologies). Specific binding was determined as described previously (49). Briefly, standards of NAADP were prepared in Hanks' balanced salt solution. Tracheal homogenate was then added to give a final protein concentration of ∼1 mg/ml. [32P]NAADP was synthesized from [32P]ATP (PerkinElmer Life Sciences) and added to give a final concentration of ∼ 0.2 nm. The tubes were then incubated for 1 h at room temperature, filtered using a Brandel cell harvester onto Whatman GF/B filter papers, and washed with ice-cold HEPES buffer (50 mm, pH 7.2). Radioactivity was determined using a storage phosphor screen and Typhoon scanner.
RESULTS AND DISCUSSION
To determine whether NAADP signaling plays a role in tracheal smooth muscle contraction, we determined if NAADP meets the five criteria suggested by Sutherland as necessary for a molecule to be designated a second messenger (44, 45). Specifically, first, antagonism of the action of the messenger blocks the effects of the extracellular messenger; second, a molecule applied intracellularly must mimic the effect of an extracellular stimulus; third, it can be synthesized and metabolized; fourth, its levels must be shown to change in response to a physiologically relevant stimulus; and fifth, specific intracellular binding sites must be present.
Sutherland's first criterion for a second messenger is that antagonism of the action of the messenger blocks the effects of the extracellular messenger (44, 45). To address this criterion, we determined the effect of the NAADP antagonist Ned-19 (50–52) on contraction of tracheal rings from guinea pig and calcium responses in isolated airway smooth muscle cells.
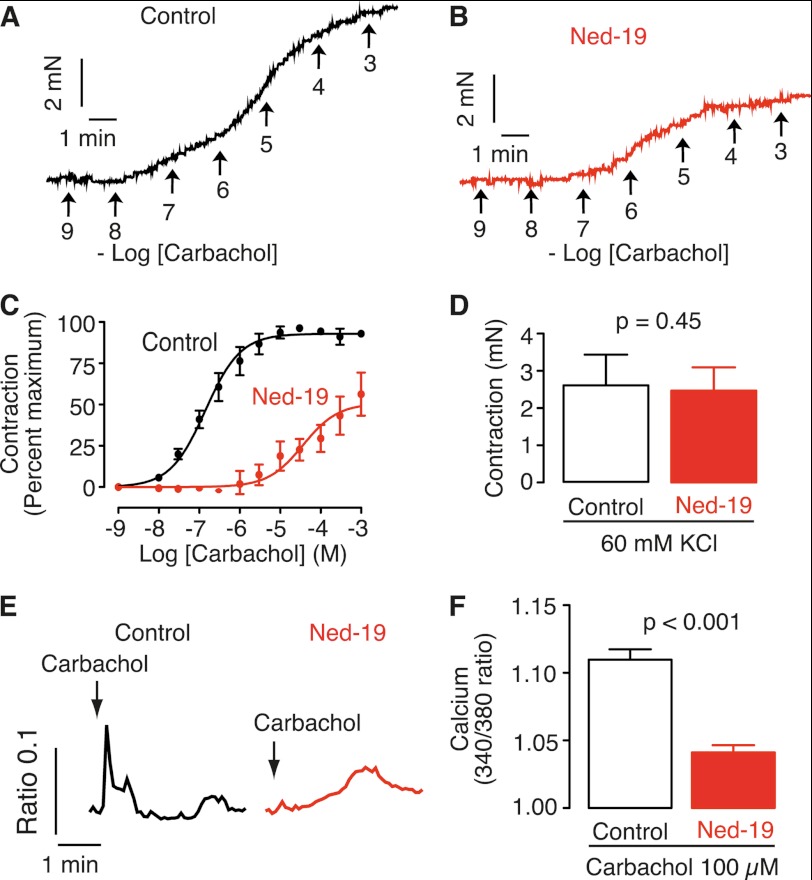
Tracheal smooth muscle has several G-protein-coupled receptors that mobilize calcium increases to induce contraction (3, 8). To determine whether the NAADP antagonist Ned-19 affects calcium increases stimulated by G-protein-coupled receptor signaling, we investigated the effect of Ned-19 on contractions of tracheal rings induced by carbachol. Carbachol is a hydrolysis-resistant agonist of the muscarinic acetylcholine receptor. In guinea pig tracheal smooth muscle, the M2 and M3 subtypes are equally present, and the M3 subtype mediates contraction (53). For these experiments, we employed cumulative concentration-response curves as commonly reported for such preparations (46, 47, 54). As reported (46, 47), increases in carbachol concentration led to increasing force of contraction until saturation of the response (Fig. 1A). The resulting concentration-response curve had an ED50 of ∼150 nm and a Hill coefficient of 0.9, indicating no cooperativity (Fig. 1C). Ned-19 (10 μm) reduced the maximum contraction by ∼50% and right-shifted the concentration-response curve to an ED50 of ∼30 μm and a Hill coefficient of 0.8 (Fig. 1, B and C). In these experiments, we used KCl to fully contract the tissue to demonstrate tissue viability at the end of the experiment, as described previously (46, 47). Importantly, Ned-19 did not affect KCl-induced contractions (Fig. 1D), which is consistent with the lack of effect of Ned-19 on KCl-induced responses in pancreatic beta cells (50) and rat uterine smooth muscle (32). The inhibition of the carbachol-induced contractions by Ned-19 is consistent with a role for NAADP in calcium mobilization and contraction in trachea. To demonstrate directly that Ned-19 affects NAADP-mediated contraction, we investigated the effect of Ned-19 on contractions mediated by NAADP acetoxymethyl ester, a cell-permeant form of NAADP (55). NAADP acetoxymethyl ester did not induce contractions in tracheal rings, possibly due to de-esterification in cell layers and not reaching the tracheal smooth muscle cells in high enough concentrations, which is a well known problem for molecules made membrane-permeant through esterification (56). Therefore, we addressed the question of Ned-19 action and selectivity in trachea by using isolated smooth muscle cells as described below.
FIGURE 1.
NAADP antagonist Ned-19 attenuates muscarinic signaling in trachea. A and B, effect of Ned-19 on carbachol-induced contraction of tracheal rings from guinea pig. Carbachol was added cumulatively to the concentrations indicated after a 1-h incubation with either 0.1% dimethyl sulfoxide (A) or 10 μm Ned-19 (B). pN, piconewtons. C, concentration-response curves for carbachol-induced contractions in the presence and absence of 10 μm Ned-19. Error bars represent the mean ± S.E. (n = 3). D, summary bar chart of the contractile response to 60 mm KCl. Error bars represent the mean ± S.E. (n = 5). Means were compared with an unpaired, one-tailed t test. E, effect of carbachol (100 μm) on cytosolic calcium over time in tracheal smooth muscle cells from guinea pig. Carbachol was added after a 15-min incubation with either 0.1% dimethyl sulfoxide (Control) or 10 μm Ned-19. F, summary bar chart of the increase in calcium. Error bars represent the mean ± S.E. (n = 15–20 cells). Means were compared with an unpaired, one-tailed t test between the control (n = 15) and Ned-19-treated (n = 20) cells.
Having established that Ned-19 blocks contractions of tracheal rings induced by carbachol, we next investigated whether Ned-19 also inhibits calcium increases induced by carbachol in isolated airway smooth muscle cells. Carbachol (100 μm) induced a peak and plateau increase in calcium (Fig. 1E). This response is typical for muscarinic acetylcholine receptor-induced calcium mobilization in tracheal smooth muscle (3, 57, 58). Ned-19 (10 μm) slowed the carbachol-induced rate of calcium increase and decreased its maximum amplitude (Fig. 1, E and F). This result is consistent with Ned-19 reducing the calcium response in other types of smooth muscle to agonists, such as oxytocin (32), endothelin-1, and norepinephrine (38), and to G-protein-coupled receptor signaling in other cell types, including thrombin and glycoprotein VI in platelets (59), ATP in astrocytes (60), histamine in endothelial cells (61), and insulin in Β-cells (62) and adipocytes (63). Combined, the above data support that an NAADP antagonist blocks action of the G-protein-coupled receptor-mediated responses of calcium increase and contraction, thereby supporting a role for NAADP in these processes.
Sutherland's second criterion for a second messenger is that, when applied intracellularly, it must mimic the effect of an extracellular stimulus. To address this criterion, we determined whether microinjection of NAADP increases cytosolic calcium in isolated airway smooth muscle cells. Microinjection of NAADP resulted in a rapid calcium increase (Fig. 2A). Preincubation (15 min) with 10 μm Ned-19 reduced the NAADP-induced calcium increase (Fig. 2, C and E). Microinjection of IP3 also resulted in a calcium increase (Fig. 2B), but this response was not blocked by preincubation with Ned-19 (Fig. 2, D and F). The ability of Ned-19 to attenuate NAADP but not IP3 shows selectivity and is consistent with the effect of Ned-19 in other cell types (32, 50, 64). These data demonstrate that Ned-19 is an antagonist of NAADP in tracheal smooth muscle cells. Additionally, the ability of NAADP to increase cytosolic calcium demonstrates that NAADP meets Sutherland's second criterion.
FIGURE 2.
Microinjected NAADP increases calcium and is antagonized selectively by Ned-19 in isolated tracheal smooth muscle cells. A–D, calcium responses of individual cells microinjected with NAADP (A and C) or IP3 (B and D) in the absence (A and B) or presence (C and D) of Ned-19 (10 μm). E and F, summary bar charts of the calcium responses induced by microinjection. Error bars represent the mean ± S.E. (n = 6), and p values were calculated by a two-tailed t test.
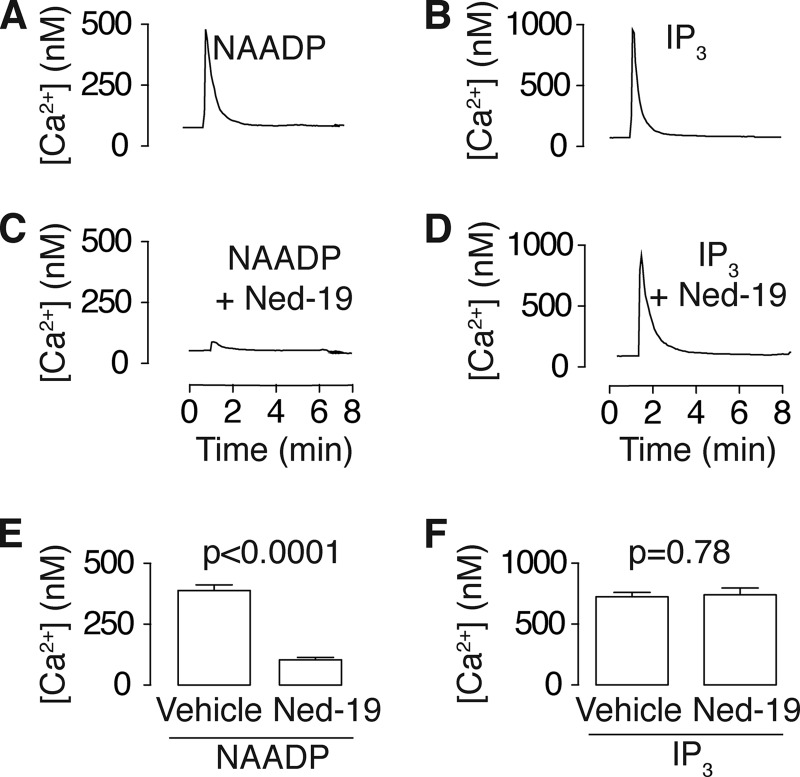
Sutherland's third criterion for a second messenger is that it can be synthesized and metabolized (44, 45). In other cell types and tissues, the consensus view is that NAADP is synthesized by base exchange (65, 66), whereby the nicotinamide group on NADP is swapped with nicotinic acid (Fig. 3A). To determine whether NAADP can be synthesized, we incubated tracheal homogenate with the substrates for NAADP synthesis via base exchange, NADP and nicotinic acid (39, 66). HPLC revealed a peak of NAADP that increased with time (Fig. 3B). Surprisingly, the reaction was as efficient at pH 7 as at pH 5 (Fig. 3C). Base exchange is catalyzed by a family of enzymes termed cyclases (as they are named for the synthesis of cyclic ADP-ribose (67)), of which CD38 is the prime candidate for mammalian synthesis (10, 41, 65, 66), but in certain tissues, CD38 is not required, and in others, alternative base exchange enzymes either exist (68) or compensate (41, 69). However, in mouse trachea, CD38 is the most likely candidate, as it accounts for all of the cyclase activity, and agonist-induced calcium transients are attenuated in CD38 knock-out mice (10, 65).
FIGURE 3.
NAADP is synthesized and metabolized by tracheal tissue. A, chemical structures and scheme showing NAADP synthesis via base exchange and metabolism by reported mechanisms. B and C, HPLC traces showing NAADP synthesis by base exchange using tracheal tissue homogenate incubated with NADP (1 mm) and nicotinic acid (NA; 10 mm) at pH 5 (B) and pH 7 (C). D, HPLC traces showing NAADP metabolism. NAADP (100 μm) was incubated with homogenate at pH 7. All reactions contained tracheal homogenate at 5% (w/v) and were incubated at 37 °C. Peaks were identified by the elution times of authentic samples. NiAM, nicotinamide; ADPR-P, ADP-ribose phosphate; NAAD, nicotinic acid adenine dinucleotide; Abs, absorbance.
As with IP3 (70), NAADP can be metabolized by several enzymes, which in vitro includes pyrophosphatase (71), alkaline phosphatase (71), CD38 (72), and glucose-6-phosphate dehydrogenase (73). To determine whether NAADP can be metabolized and to which product(s), we incubated NAADP (100 μm) with tracheal homogenate and monitored the reaction with HPLC. Over time, NAADP was converted primarily into nicotinic acid adenine dinucleotide (Fig. 3D). This result is consistent with previous reports implicating a 2′-phosphatase (74) that is likely alkaline phosphatase (75) as the physiologically relevant metabolic pathway. Taken together, these data demonstrate that NAADP is synthesized and metabolized by trachea, thereby fulfilling Sutherland's third criterion.
Sutherland's fourth criterion for a second messenger is that its level must change in response to a physiologically relevant stimulus (44, 45). The preceding data demonstrate that tracheal tissue has the enzymes required for NAADP synthesis and degradation; however, they do not address whether this occurs during physiological stimulation. To address Sutherland's fourth criterion, we measured endogenous NAADP levels during stimulation with carbachol. Carbachol (100 μm) induced a time-dependent increase in NAADP that peaked at 30 s and then returned to the base line (Fig. 4). This type of rapid and transient messenger increase is characteristic of NAADP and has been reported in several tissues and cells, including sperm (76), T-cells (77), beta cells (37), pancreatic acinar cells (40), heart cells (78) and smooth muscle cells (32, 79). To enable pooling of different experiments, we present the data normalized to the maximum absolute response with a given tissue on a given day. For comparison, phosphatidylinositol turnover in guinea pig tracheal rings induced by carbachol was reported to have an ED50 of 100 μm, with maximum stimulation phosphatidylinositol turnover approximately doubled (80). In absolute terms, resting levels varied dramatically from pmol/mg of protein to <1 fmol/mg of protein, roughly the sensitivity of our binding assay (49). These values are on the low end of the published range of 0.1–10 pmol/mg of protein. Older literature attributed carbachol-induced contractions entirely to changes in IP3 and inositol turnover in tracheal ring preparations (80, 81). More recently, cyclic ADP-ribose and CD38 have been shown to be involved in contraction (4, 10, 11, 82), but there are no reports of changes in cyclic ADP-ribose levels. We can now conclude that calcium increases and contractions are mediated not just by IP3 and cyclic ADP-ribose but also by NAADP. Taken together, the above data demonstrate physiologically relevant increases in NAADP and fulfill Sutherland's fourth criterion.
FIGURE 4.
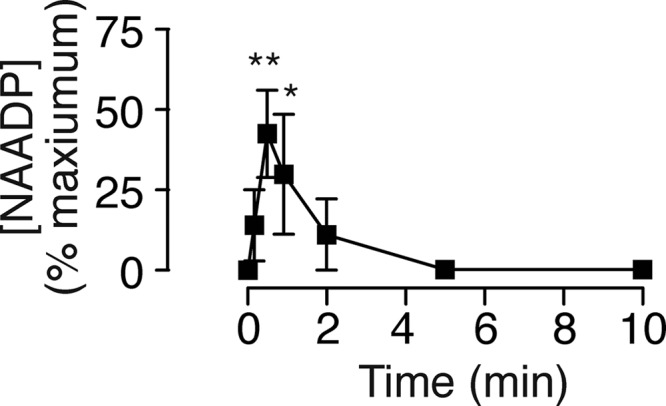
NAADP levels increase transiently in response to activation of muscarinic receptors. Shown are NAADP levels in guinea pig tracheal rings over time after exposure to the muscarinic agonist carbachol (100 μm). Error bars represent the mean ± S.E. (n = 3). *, p < 0.05; **, p < 0.01. Means were compared by analysis of variance (p = 0.03), followed by Dunnett's multiple comparisons test against time 0.
Sutherland's fifth criterion for a second messenger is that specific intracellular binding sites are present. To address this criterion, we used a competitive binding assay in which we incubated tissue homogenates with [32P]NAADP and competed with nonradioactive NAADP. The resulting concentration-displacement curve was best fit with two binding sites with affinities of ∼1 pm and 170 nm, with each contributing to about half of the total binding (Fig. 5). A two-site binding curve for NAADP is consistent with that obtained using HeLa cells expressing two-pore channels and endogenous receptors in mouse liver homogenates (29). The differences in affinity may simply reflect the different buffer conditions, as NAADP binding is acutely sensitive to the ionic composition of the buffer (83). Additionally, recent evidence demonstrates that the actual binding protein is not the two-pore channel itself but an accessory protein (84–86). The salient finding is that specific binding sites for NAADP are present, thereby fulfilling Sutherland's fifth criterion.
FIGURE 5.
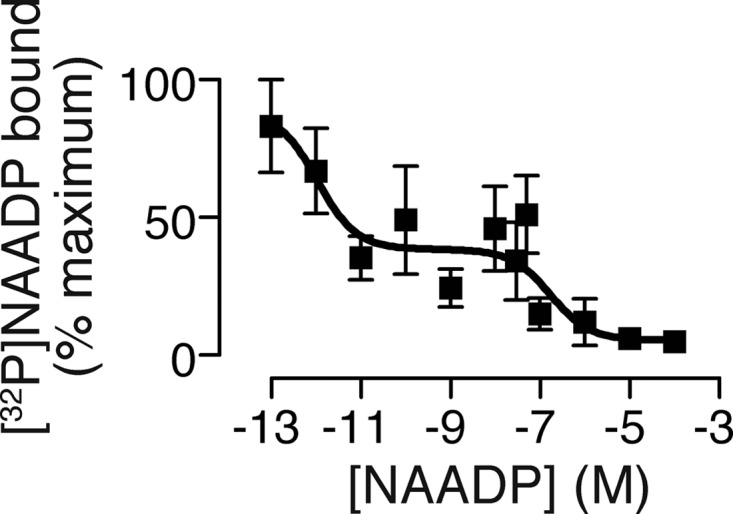
NAADP binds to guinea pig tracheal homogenate at specific high affinity sites. The graph shows a competitive binding curve for NAADP in guinea pig tracheal homogenate. The curve is derived from a two-site fit to the Hill equation, which yields Kd values of 1 pm and 172 nm (n = 6).
The exact details of how NAADP coordinates calcium increases with IP3 and cyclic ADP-ribose will require further study. However, the data we obtained for NAADP in tracheal tissue and smooth muscle cells are consistent with its reported mechanism of action in other cell types. The rapid and transient increase in NAADP upon muscarinic receptor stimulation fits with NAADP acting as a “trigger” for a larger global calcium release, with a small release of calcium from acidic organelles triggering a secondary larger increase from the sarcoplasmic reticulum (5, 18). Thus, the messengers cooperate under normal circumstances to elicit an agonist-induced calcium increase. The independent actions of the messengers can be revealed only by blocking one response or the other. Since it was first proposed (24), a trigger role for NAADP has been demonstrated in many cell types (87). The most direct evidence for this comes from experiments in which NAADP was either perfused into the cell through a patch pipette or photo-released with or without the IP3 antagonist heparin or xestospongin (29, 35, 88–90). NAADP can also function as a trigger through a two-pool mechanism, originally proposed by Berridge and Galione (91). In sea urchin eggs (92), arterial smooth muscle cells (90), and heart cells (93), NAADP shuttles calcium from endolysosomal stores into the endoplasmic reticulum, which then promotes release through IP3 or ryanodine receptors.
In summary, we have used Sutherland's five criteria for a second messenger as a template to demonstrate that NAADP plays a role in calcium increase and contraction in tracheal smooth muscle. We suggest that NAADP plays a parallel and complementary role to IP3 and cyclic ADP-ribose, enabling a more sensitive response to agonist stimulation. This emerging role for NAADP reveals new possibilities for physiological control and possible new drug targets for the treatment of asthma (8).
Acknowledgment
We thank Dr. Keith Brain for the loan of a myograph.
This work was supported by Asthma UK Grant 09/037.
- IP3
- inositol 1,4,5-trisphosphate
- NAADP
- nicotinic acid adenine dinucleotide phosphate.
REFERENCES
- 1. Gerthoffer W. T. (1991) Regulation of the contractile element of airway smooth muscle. Am. J. Physiol. 261, L15–L28 [DOI] [PubMed] [Google Scholar]
- 2. Hakonarson H., Grunstein M. M. (1998) Regulation of second messengers associated with airway smooth muscle contraction and relaxation. Am. J. Respir. Crit. Care Med. 158, S115–S122 [DOI] [PubMed] [Google Scholar]
- 3. Jude J. A., Wylam M. E., Walseth T. F., Kannan M. S. (2008) Calcium signaling in airway smooth muscle. Proc. Am. Thorac. Soc. 5, 15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Prakash Y. S., Kannan M. S., Walseth T. F., Sieck G. C. (1998) Role of cyclic ADP-ribose in the regulation of [Ca2+]i in porcine tracheal smooth muscle. Am. J. Physiol. 274, C1653–C1660 [DOI] [PubMed] [Google Scholar]
- 5. Sanderson M. J., Delmotte P., Bai Y., Perez-Zogbhi J. F. (2008) Regulation of airway smooth muscle cell contractility by Ca2+ signaling and sensitivity. Proc. Am. Thorac. Soc. 5, 23–31 [DOI] [PubMed] [Google Scholar]
- 6. Evans A. M. (2010) The role of intracellular ion channels in regulating cytoplasmic calcium in pulmonary arterial smooth muscle: which store and where? Adv. Exp. Med. Biol. 661, 57–76 [DOI] [PubMed] [Google Scholar]
- 7. Billington C. K., Hall I. P. (2012) Novel cAMP signalling paradigms: therapeutic implications for airway disease. Br. J. Pharmacol. 166, 401–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Siddiqui S., Redhu N. S., Ojo O. O., Liu B., Irechukwu N., Billington C., Janssen L., Moir L. M. (2013) Emerging airway smooth muscle targets to treat asthma. Pulm. Pharmacol. Ther. 26, 132–144 [DOI] [PubMed] [Google Scholar]
- 9. Deshpande D. A., Wang W. C. H., McIlmoyle E. L., Robinett K. S., Schillinger R. M., An S. S., Sham J. S. K., Liggett S. B. (2010) Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat. Med. 16, 1299–1304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Deshpande D. A., White T. A., Dogan S., Walseth T. F., Panettieri R. A., Kannan M. S. (2005) CD38/cyclic ADP-ribose signaling: role in the regulation of calcium homeostasis in airway smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L773–L788 [DOI] [PubMed] [Google Scholar]
- 11. White T. A., Kannan M. S., Walseth T. F. (2003) Intracellular calcium signaling through the cADPR pathway is agonist-specific in porcine airway smooth muscle. FASEB J. 17, 482–484 [DOI] [PubMed] [Google Scholar]
- 12. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 13. Berridge M. J., Lipp P., Bootman M. D. (2000) The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1, 11–21 [DOI] [PubMed] [Google Scholar]
- 14. Ito S., Kume H., Yamaki K., Katoh H., Honjo H., Kodama I., Hayashi H. (2002) Regulation of capacitative and noncapacitative receptor-operated Ca2+ entry by Rho kinase in tracheal smooth muscle. Am. J. Respir. Cell Mol. Biol. 26, 491–498 [DOI] [PubMed] [Google Scholar]
- 15. Sweeney M., McDaniel S. S., Platoshyn O., Zhang S., Yu Y., Lapp B. R., Zhao Y., Thistlethwaite P. A., Yuan J. X.-J. (2002) Role of capacitative Ca2+ entry in bronchial contraction and remodeling. J. Appl. Physiol. 92, 1594–1602 [DOI] [PubMed] [Google Scholar]
- 16. Tao F. C., Tolloczko B., Eidelman D. H., Martin J. G. (1999) Enhanced Ca2+ mobilization in airway smooth muscle contributes to airway hyperresponsiveness in an inbred strain of rat. Am. J. Respir. Crit. Care Med. 160, 446–453 [DOI] [PubMed] [Google Scholar]
- 17. Clapper D. L., Walseth T. F., Dargie P. J., Lee H. C. (1987) Pyridine nucleotide metabolites stimulate calcium release from sea urchin egg microsomes desensitized to inositol trisphosphate. J. Biol. Chem. 262, 9561–9568 [PubMed] [Google Scholar]
- 18. Lee H. C., Walseth T. F., Bratt G. T., Hayes R. N., Clapper D. L. (1989) Structural determination of a cyclic metabolite of NAD+ with intracellular Ca2+-mobilizing activity. J. Biol. Chem. 264, 1608–1615 [PubMed] [Google Scholar]
- 19. Galione A., Lee H. C., Busa W. B. (1991) Ca2+-induced Ca2+ release in sea urchin egg homogenates: modulation by cyclic ADP-ribose. Science 253, 1143–1146 [DOI] [PubMed] [Google Scholar]
- 20. Lee H. C. (2011) Cyclic ADP-ribose and NAADP: fraternal twin messengers for calcium signaling. Sci. China Life Sci. 54, 699–711 [DOI] [PubMed] [Google Scholar]
- 21. Lee H. C. (2005) Nicotinic acid adenine dinucleotide phosphate (NAADP)-mediated calcium signaling. J. Biol. Chem. 280, 33693–33696 [DOI] [PubMed] [Google Scholar]
- 22. Lee H. C., Aarhus R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 270, 2152–2157 [DOI] [PubMed] [Google Scholar]
- 23. Galione A., Parrington J., Funnell T. (2011) Physiological roles of NAADP-mediated Ca2+ signaling. Sci. China Life Sci. 54, 725–732 [DOI] [PubMed] [Google Scholar]
- 24. Cancela J. M., Churchill G. C., Galione A. (1999) Coordination of agonist-induced Ca2+-signalling patterns by NAADP in pancreatic acinar cells. Nature 398, 74–76 [DOI] [PubMed] [Google Scholar]
- 25. Genazzani A. A., Galione A. (1996) Nicotinic acid- adenine dinucleotide phosphate mobilizes Ca2+ from a thapsigargin-insensitive pool. Biochem. J. 315, 721–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee H. C. (2000) Multiple calcium stores: separate but interacting. Sci. STKE 2000, pe1. [DOI] [PubMed] [Google Scholar]
- 27. Patel S. (2004) NAADP-induced Ca2+ release–a new signalling pathway. Biol. Cell 96, 19–28 [DOI] [PubMed] [Google Scholar]
- 28. Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 29. Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K.-T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. (2009) The two-pore channel TPCN2 mediates NAADP-dependent Ca2+ release from lysosomal stores. Pflugers Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., Patel S. (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Aley P. K., Noh H. J., Gao X., Tica A. A., Brailoiu E., Churchill G. C. (2010) A functional role for nicotinic acid adenine dinucleotide phosphate in oxytocin-mediated contraction of uterine smooth muscle from rat. J. Pharmacol. Exp. Ther. 333, 726–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pandey V., Chuang C.-C., Lewis A. M., Aley P. K., Brailoiu E., Dun N. J., Churchill G. C., Patel S. (2009) Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem. J. 422, 503–512 [DOI] [PubMed] [Google Scholar]
- 34. Evans A. M., Wyatt C. N., Kinnear N. P., Clark J. H., Blanco E. A. (2005) Pyridine nucleotides and calcium signalling in arterial smooth muscle: from cell physiology to pharmacology. Pharmacol. Ther. 107, 286–313 [DOI] [PubMed] [Google Scholar]
- 35. Kinnear N. P., Wyatt C. N., Clark J. H., Calcraft P. J., Fleischer S., Jeyakumar L. H., Nixon G. F., Evans A. M. (2008) Lysosomes co-localize with ryanodine receptor subtype 3 to form a trigger zone for calcium signalling by NAADP in rat pulmonary arterial smooth muscle. Cell Calcium 44, 190–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berg I., Potter B. V., Mayr G. W., Guse A. H. (2000) Nicotinic acid adenine dinucleotide phosphate (NAADP+) is an essential regulator of T-lymphocyte Ca2+ signaling. J. Cell Biol. 150, 581–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Masgrau R., Churchill G. C., Morgan A. J., Ashcroft S. J. H., Galione A. (2003) NAADP: a new second messenger for glucose-induced Ca2+ responses in clonal pancreatic beta cells. Curr. Biol. 13, 247–251 [DOI] [PubMed] [Google Scholar]
- 38. Thai T. L., Churchill G. C., Arendshorst W. J. (2009) NAADP receptors mediate calcium signaling stimulated by endothelin-1 and norepinephrine in renal afferent arterioles. Am. J. Physiol. Renal Physiol. 297, F510–F516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vasudevan S. R., Lewis A. M., Chan J. W., Machin C. L., Sinha D., Galione A., Churchill G. C. (2010) The calcium-mobilizing messenger nicotinic acid adenine dinucleotide phosphate participates in sperm activation by mediating the acrosome reaction. J. Biol. Chem. 285, 18262–18269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yamasaki M., Thomas J. M., Churchill G. C., Garnham C., Lewis A. M., Cancela J.-M., Patel S., Galione A. (2005) Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ spiking in mouse pancreatic acinar cells. Curr. Biol. 15, 874–878 [DOI] [PubMed] [Google Scholar]
- 41. Cosker F., Cheviron N., Yamasaki M., Menteyne A., Lund F. E., Moutin M.-J., Galione A., Cancela J.-M. (2010) The ecto-enzyme CD38 is a nicotinic acid adenine dinucleotide phosphate (NAADP) synthase that couples receptor activation to Ca2+ mobilization from lysosomes in pancreatic acinar cells. J. Biol. Chem. 285, 38251–38259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Iizuka K., Yoshii A., Dobashi K., Horie T., Mori M., Nakazawa T. (1998) InsP3, but not novel Ca2+ releasers, contributes to agonist-initiated contraction in rabbit airway smooth muscle. J. Physiol. 511, 915–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dickey D. M., Aarhus R., Walseth T. F., Lee H. C. (1998) Thio-NADP is not an antagonist of NAADP. Cell Biochem. Biophys. 28, 63–73 [DOI] [PubMed] [Google Scholar]
- 44. Robison G. A., Butcher R. W., Sutherland E. W. (1971) Cyclic AMP, Academic Press, New York [Google Scholar]
- 45. Sutherland E. W., Robison G. A., Butcher R. W. (1968) Some aspects of the biological role of adenosine 3′,5′-monophosphate (cyclic AMP). Circulation 37, 279–306 [Google Scholar]
- 46. Chen H., Tliba O., Van Besien C. R., Panettieri R. A., Jr., Amrani Y. (2003) Selected contribution: TNF-α modulates murine tracheal rings responsiveness to G-protein-coupled receptor agonists and KCl. J. Appl. Physiol. 95, 864–872 [DOI] [PubMed] [Google Scholar]
- 47. Campos-Bedolla P., Montaño L. M., Flores-Soto E., Aguilar A., Puebla A. M., Lozoya X., Vargas M. H. (2005) Effect of Gnaphalium conoideum HBK on guinea pig airway smooth muscle: role of L-type Ca2+ channels. J. Ethnopharmacol. 97, 267–272 [DOI] [PubMed] [Google Scholar]
- 48. Axelson J. T., Bodley J. W., Walseth T. F. (1981) A volatile liquid chromatography system for nucleotides. Anal. Biochem. 116, 357–360 [DOI] [PubMed] [Google Scholar]
- 49. Lewis A. M., Masgrau R., Vasudevan S. R., Yamasaki M., O'Neill J. S., Garnham C., James K., Macdonald A., Ziegler M., Galione A., Churchill G. C. (2007) Refinement of a radioreceptor binding assay for nicotinic acid adenine dinucleotide phosphate. Anal. Biochem. 371, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Naylor E., Arredouani A., Vasudevan S. R., Lewis A. M., Parkesh R., Mizote A., Rosen D., Thomas J. M., Izumi M., Ganesan A., Galione A., Churchill G. C. (2009) Identification of a chemical probe for NAADP by virtual screening. Nat. Chem. Biol. 5, 220–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Pitt S. J., Funnell T. M., Sitsapesan M., Venturi E., Rietdorf K., Ruas M., Ganesan A., Gosain R., Churchill G. C., Zhu M. X., Parrington J., Galione A., Sitsapesan R. (2010) TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J. Biol. Chem. 285, 35039–35046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Rosen D., Lewis A. M., Mizote A., Thomas J. M., Aley P. K., Vasudevan S. R., Parkesh R., Galione A., Izumi M., Ganesan A., Churchill G. C. (2009) Analogues of the nicotinic acid adenine dinucleotide phosphate (NAADP) antagonist Ned-19 indicate two binding sites on the NAADP receptor. J. Biol. Chem. 284, 34930–34934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Eglen R. M., Hegde S. S., Watson N. (1996) Muscarinic receptor subtypes and smooth muscle function. Pharmacol. Rev. 48, 531–565 [PubMed] [Google Scholar]
- 54. Van Rossum J. M. (1963) Cumulative dose-response curves. II. Technique for the making of dose-response curves in isolated organs and the evaluation of drug parameters. Arch. Int. Pharmacodyn. Ther. 143, 299–330 [PubMed] [Google Scholar]
- 55. Parkesh R., Lewis A. M., Aley P. K., Arredouani A., Rossi S., Tavares R., Vasudevan S. R., Rosen D., Galione A., Dowden J., Churchill G. C. (2008) Cell-permeant NAADP: a novel chemical tool enabling the study of Ca2+ signalling in intact cells. Cell Calcium 43, 531–538 [DOI] [PubMed] [Google Scholar]
- 56. Schultz C. (2003) Prodrugs of biologically active phosphate esters. Bioorg. Med. Chem. 11, 885–898 [DOI] [PubMed] [Google Scholar]
- 57. Prakash Y. S., Pabelick C. M., Kannan M. S., Sieck G. C. (2000) Spatial and temporal aspects of ACh-induced [Ca2+]i oscillations in porcine tracheal smooth muscle. Cell Calcium 27, 153–162 [DOI] [PubMed] [Google Scholar]
- 58. Shieh C. C., Petrini M. F., Dwyer T. M., Farley J. M. (1991) Concentration dependence of acetylcholine-induced changes in calcium and tension in swine trachealis. J. Pharmacol. Exp. Ther. 256, 141–148 [PubMed] [Google Scholar]
- 59. Coxon C. H., Lewis A. M., Sadler A. J., Vasudevan S. R., Thomas A., Dundas K. A., Taylor L., Campbell R. D., Gibbins J. M., Churchill G. C., Tucker K. L. (2012) NAADP regulates human platelet function. Biochem. J. 441, 435–442 [DOI] [PubMed] [Google Scholar]
- 60. Barceló-Torns M., Lewis A. M., Gubern A., Barneda D., Bloor-Young D., Picatoste F., Churchill G. C., Claro E., Masgrau R. (2011) NAADP mediates ATP-induced Ca2+ signals in astrocytes. FEBS Lett. 585, 2300–2306 [DOI] [PubMed] [Google Scholar]
- 61. Esposito B., Gambara G., Lewis A. M., Palombi F., D'Alessio A., Taylor L. X., Genazzani A. A., Ziparo E., Galione A., Churchill G. C., Filippini A. (2011) NAADP links histamine H1 receptors to secretion of von Willebrand factor in human endothelial cells. Blood 117, 4968–4977 [DOI] [PubMed] [Google Scholar]
- 62. Alejandro E. U., Kalynyak T. B., Taghizadeh F., Gwiazda K. S., Rawstron E. K., Jacob K. J., Johnson J. D. (2010) Acute insulin signaling in pancreatic beta-cells is mediated by multiple Raf-1 dependent pathways. Endocrinology 151, 502–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Song E.-K., Lee Y.-R., Kim Y.-R., Yeom J.-H., Yoo C.-H., Kim H.-K., Park H.-M., Kang H.-S., Kim J.-S., Kim U.-H., Han M.-K. (2012) NAADP mediates insulin-stimulated glucose uptake and insulin sensitization by PPARγ in adipocytes. Cell Rep. 2, 1607–1619 [DOI] [PubMed] [Google Scholar]
- 64. Brailoiu G. C., Oprea T. I., Zhao P., Abood M. E., Brailoiu E. (2011) Intracellular cannabinoid type 1 (CB1) receptors are activated by anandamide. J. Biol. Chem. 286, 29166–29174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chini E. N., Chini C. C. S., Kato I., Takasawa S., Okamoto H. (2002) CD38 is the major enzyme responsible for synthesis of nicotinic acid adenine dinucleotide phosphate in mammalian tissues. Biochem. J 362, 125–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Aarhus R., Graeff R. M., Dickey D. M., Walseth T. F., Lee H. C. (1995) ADP-ribosyl cyclase and CD38 catalyze the synthesis of a calcium-mobilizing metabolite from NADP. J. Biol. Chem. 270, 30327–30333 [DOI] [PubMed] [Google Scholar]
- 67. Lee H. C., Aarhus R. (1991) ADP-ribosyl cyclase: an enzyme that cyclizes NAD+ into a calcium-mobilizing metabolite. Cell Regul. 2, 203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Soares S., Thompson M., White T., Isbell A., Yamasaki M., Prakash Y., Lund F. E., Galione A., Chini E. N. (2007) NAADP as a second messenger: neither CD38 nor base-exchange reaction is necessary for in vivo generation of NAADP in myometrial cells. Am. J. Physiol. Cell Physiol. 292, C227–C239 [DOI] [PubMed] [Google Scholar]
- 69. Palade P. (2007) The hunt for an alternate way to generate NAADP. Focus on “NAADP as a second messenger: neither CD38 nor base-exchange reaction is necessary for in vivo generation of NAADP in myometrial cells.” Am. J. Physiol. Cell Physiol. 292, C4–C7 [DOI] [PubMed] [Google Scholar]
- 70. Irvine R. F., Schell M. J. (2001) Back in the water: the return of the inositol phosphates. Nat. Rev. Mol. Cell Biol. 2, 327–338 [DOI] [PubMed] [Google Scholar]
- 71. Lee H. C., Aarhus R. (1997) Structural determinants of nicotinic acid adenine dinucleotide phosphate important for its calcium-mobilizing activity. J. Biol. Chem. 272, 20378–20383 [DOI] [PubMed] [Google Scholar]
- 72. Graeff R., Liu Q., Kriksunov I. A., Hao Q., Lee H. C. (2006) Acidic residues at the active sites of CD38 and ADP-ribosyl cyclase determine nicotinic acid adenine dinucleotide phosphate (NAADP) synthesis and hydrolysis activities. J. Biol. Chem. 281, 28951–28957 [DOI] [PubMed] [Google Scholar]
- 73. Billington R. A., Thuring J. W., Conway S. J., Packman L., Holmes A. B., Genazzani A. A. (2004) Production and characterization of reduced NAADP (nicotinic acid adenine dinucleotide phosphate). Biochem. J. 378, 275–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Berridge G., Cramer R., Galione A., Patel S. (2002) Metabolism of the novel Ca2+-mobilizing messenger nicotinic acid adenine dinucleotide phosphate via a 2′-specific Ca2+-dependent phosphatase. Biochem. J. 365, 295–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Schmid F., Fliegert R., Westphal T., Bauche A., Guse A. H. (2012) Nicotinic acid adenine dinucleotide phosphate (NAADP) degradation by alkaline phosphatase. J. Biol. Chem. 287, 32525–32534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Churchill G. C., O'Neill J. S., Masgrau R., Patel S., Thomas J. M., Genazzani A. A., Galione A. (2003) Sperm deliver a new second messenger: NAADP. Curr. Biol. 13, 125–128 [DOI] [PubMed] [Google Scholar]
- 77. Gasser A., Bruhn S., Guse A. H. (2006) Second messenger function of nicotinic acid adenine dinucleotide phosphate revealed by an improved enzymatic cycling assay. J. Biol. Chem. 281, 16906–16913 [DOI] [PubMed] [Google Scholar]
- 78. Lewis A. M., Aley P. K., Roomi A., Thomas J. M., Masgrau R., Garnham C., Shipman K., Paramore C., Bloor-Young D., Sanders L. E. L., Terrar D. A., Galione A., Churchill G. C. (2012) β-Adrenergic receptor signaling increases NAADP and cADPR levels in the heart. Biochem. Biophys. Res. Commun. 427, 326–329 [DOI] [PubMed] [Google Scholar]
- 79. Kinnear N. P., Boittin F.-X., Thomas J. M., Galione A., Evans A. M. (2004) Lysosome-sarcoplasmic reticulum junctions. A trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J. Biol. Chem. 279, 54319–54326 [DOI] [PubMed] [Google Scholar]
- 80. Robertson D. N., Coyle A. J., Rhoden K. J., Grandordy B., Page C. P., Barnes P. J. (1988) The effect of platelet-activating factor on histamine and muscarinic receptor function in guinea pig airways. Am. Rev. Respir. Dis. 137, 1317–1322 [DOI] [PubMed] [Google Scholar]
- 81. Roffel A. F., Meurs H., Elzinga C. R., Zaagsma J. (1990) Characterization of the muscarinic receptor subtype involved in phosphoinositide metabolism in bovine tracheal smooth muscle. Br. J. Pharmacol. 99, 293–296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Franco L., Bruzzone S., Song P., Guida L., Zocchi E., Walseth T. F., Crimi E., Usai C., De Flora A., Brusasco V. (2001) Extracellular cyclic ADP-ribose potentiates ACh-induced contraction in bovine tracheal smooth muscle. Am. J. Physiol. Lung Cell. Mol. Physiol. 280, L98–L106 [DOI] [PubMed] [Google Scholar]
- 83. Dickinson G. D., Patel S. (2003) Modulation of NAADP (nicotinic acid adenine dinucleotide phosphate) receptors by K+ ions: evidence for multiple NAADP receptor conformations. Biochem. J. 375, 805–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin-Moshier Y., Walseth T. F., Churamani D., Davidson S. M., Slama J. T., Hooper R., Brailoiu E., Patel S., Marchant J. S. (2012) Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 287, 2296–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Walseth T. (2012) Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP) proteins in sea urchin egg. J. Biol. Chem. 287, 2308–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Chuang K.-T. (2011) Characterization of Mouse Two-Pore Channels (TPCs) in NAADP-mediated Ca2+ signalling. Ph.D. thesis, University of Oxford [Google Scholar]
- 87. Guse A. H., Lee H. C. (2008) NAADP: a universal Ca2+ trigger. Sci. Signal. 1, re10. [DOI] [PubMed] [Google Scholar]
- 88. Churchill G. C., Galione A. (2000) Spatial control of Ca2+ signaling by nicotinic acid adenine dinucleotide phosphate diffusion and gradients. J. Biol. Chem. 275, 38687–38692 [DOI] [PubMed] [Google Scholar]
- 89. Santella L., Kyozuka K., Genazzani A. A., De Riso L., Carafoli E. (2000) Nicotinic acid adenine dinucleotide phosphate-induced Ca2+ release. Interactions among distinct Ca2+ mobilizing mechanisms in starfish oocytes. J. Biol. Chem. 275, 8301–8306 [DOI] [PubMed] [Google Scholar]
- 90. Boittin F.-X., Galione A., Evans A. M. (2002) Nicotinic acid adenine dinucleotide phosphate mediates Ca2+ signals and contraction in arterial smooth muscle via a two-pool mechanism. Circ. Res. 91, 1168–1175 [DOI] [PubMed] [Google Scholar]
- 91. Berridge M. J., Galione A. (1988) Cytosolic calcium oscillators. FASEB J. 2, 3074–3082 [DOI] [PubMed] [Google Scholar]
- 92. Churchill G. C., Galione A. (2001) NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 20, 2666–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Macgregor A., Yamasaki M., Rakovic S., Sanders L., Parkesh R., Churchill G. C., Galione A., Terrar D. A. (2007) NAADP controls cross-talk between distinct Ca2+ stores in the heart. J. Biol. Chem. 282, 15302–15311 [DOI] [PubMed] [Google Scholar]