Background: The mechanism by which the p75 neurotrophin receptor (p75NTR) and TrkA interact to enhance neurotrophin signaling is unknown.
Results: The p75NTR intracellular domain fragment, p75ICD, but not full-length p75NTR enhanced NGF binding to TrkA and neurite outgrowth.
Conclusion: The results suggest that p75ICD causes a conformational change within the extracellular domain of TrkA.
Significance: The findings challenge our current understanding of how p75NTR enhances neurotrophic activity.
Keywords: Intramembrane Proteolysis, Neurotrophins, Receptor Structure-function, RIP, Signaling, NGF, TrkA, p75 Neurotrophin Receptor
Abstract
Facilitation of nerve growth factor (NGF) signaling by the p75 neurotrophin receptor (p75NTR) is critical for neuronal survival and differentiation. However, the interaction between p75NTR and TrkA receptors required for this activity is not understood. Here, we report that a specific 29-amino acid peptide derived from the intracellular domain fragment of p75NTR interacts with and potentiates binding of NGF to TrkA-expressing cells, leading to increased neurite outgrowth in sympathetic neurons as a result of enhanced Erk1/2 and Akt signaling. An endogenous intracellular domain fragment of p75NTR (p75ICD) containing these 29 amino acids is produced by regulated proteolysis of the full-length receptor. We demonstrate that generation of this fragment is a requirement for p75NTR to facilitate TrkA signaling in neurons and propose that the juxtamembrane region of p75ICD acts to cause a conformational change within the extracellular domain of TrkA. This finding provides new insight into the mechanism by which p75NTR and TrkA interact to enhance neurotrophic signaling.
Introduction
Nerve growth factor (NGF)2 is crucial for neurite outgrowth, differentiation, and survival during development of both the central and peripheral nervous systems. NGF elicits its effects by binding two structurally unrelated receptors, the common p75 neurotrophin receptor (p75NTR) and the tropomyosin receptor kinase (Trk) family member TrkA (1). TrkA mediates the survival and neurite outgrowth-promoting effects of NGF during development (2–4), whereas p75NTR has been shown to promote apoptosis and neurite pruning following neurotrophin binding. Independently, p75NTR and TrkA have low-affinity binding rates for NGF (5, 6). However, p75NTR can increase the specificity and binding affinity of Trk receptors for their preferred neurotrophins, promoting neuronal survival during development (7–11).
Over the past two decades, a number of mechanistic models of the functional interactions between p75NTR and Trk receptors have been proposed (12), including (i) the formation of a classic 1:1 heterodimer complex with a 25-fold higher on-rate than that of the individual receptors (6, 13) and (ii) the ligand passing model, in which p75NTR first binds to NGF before releasing the ligand for TrkA to bind (12–14). However, no model is consistent with all the existing experimental data. The p75NTR-TrkA heterodimer model is not supported by structural data (15–17), and the ligand passing model, although consistent with the finding that a NGF mutant that cannot bind p75NTR has only low binding affinity (18), is inconsistent with the observation that the extracellular ligand-binding domain of p75NTR is not required to create high-affinity NGF binding sites (19, 20). Therefore, although a heteroreceptor complex may form, and ligand transfer from p75NTR to TrkA could occur, these mechanisms cannot be the sole basis for the formation of high-affinity binding sites.
p75NTR receptors lacking the ligand-binding domain are generated endogenously via proteolytic cleavage. An α-secretase removes the extracellular domain, leaving a membrane-bound carboxyterminal fragment (p75CTF), which is subsequently cleaved by γ-secretase, releasing the intracellular domain (p75ICD) into the cytoplasm (21, 22). It is now emerging that these p75NTR cleavage steps are important in mediating a number of the neurotrophin functions (23).
Trk-mediated signals have recently been reported to induce the generation of p75ICD, with this fragment being capable of promoting both TrkA- and TrkB-initiated trophic signaling (24–27). Furthermore, we have observed that a proximal-intracellular juxtamembrane 29-amino acid fragment of p75ICD, termed the “Chopper” domain, or a peptide comprising the Chopper fragment lacking a transmembrane linker (c29) inhibits neuronal death (28–30). Interestingly, removal of either the whole p75NTR intracellular domain or just the Chopper domain results in diminished interactions with Trk receptors and a reduced number of neurotrophin high-affinity binding sites (8, 19, 20). In this study, we investigated the mechanism of action of the c29 peptide, investigating whether this juxtamembrane fragment of p75NTR has neurotrophic properties.
EXPERIMENTAL PROCEDURES
Expression Constructs
The p75NTR, p75Δ-JUX, and p75ICD constructs have been described previously (29). YFP versions of p75NTR constructs were made as described in Ref. 27. The p75N-Gly TrkA and TrkAK538R constructs have been described previously (31), as has TrkP203A (32) The epidermal growth factor (EGF) receptor-GFP and control YFP constructs were generously provided by Rob Parton (The University of Queensland).
Peptide Synthesis
The 29-amino acid residue peptide of the juxtamembrane Chopper domain (29) (c29, KRWNSCKQNKQGANSRPVNQTPPPEGEKL) and a randomly scrambled version (SC, SKGQVCRNQPGQNKPEPANKSWKETPLRN) were synthesized as N-terminal fusions to a non-naturally occurring protein transduction domain peptide (YARAAARNARA) (33) using t-boc chemistry and then purified using reverse-phase HPLC by James I. Elliott (Yale University). No effects were seen in cells treated with the PTD alone or peptides without carrier. For pulldown experiments, the c29 peptide was labeled on the amino terminus with biotin via a six-carbon spacer. The biotinylated control peptide used for pulldowns mimics the p75NTR extracellular juxtamembrane domain LC1 (RGTTDNLIGGSC) and was manufactured by Auspep.
SCG Assays
For neurite outgrowth assays, mouse superior cervical ganglia (SCG) were dissected from c57Bl6J and c57Bl6J p75NTR(exonIII) knock-out (10) postnatal day 3–5 mouse pups, with approval from the institutional Animal Ethics Committee. SCG explants were cultured as described previously (34). Where indicated, explants were pre-incubated with 200 nm compound E (Calbiochem) and 1 μm c29 peptide or scrambled peptide before addition of 10 ng/ml NGF (Biosensis). Explants were fixed after 2 days in 4% paraformaldehyde containing PBS, stained with anti-β-III tubulin (Promega, G712A), and detected with Alexa Fluor 488 anti-mouse secondary antibodies. For neurite outgrowth experiments using dissociated SCG, cultures were prepared as reported previously (35) and then seeded in 24-well plates and treated as described. Neuritogenesis from individual cells/well was captured in 15-min intervals over 24 h using a Zeiss Axio Observer Z1 fitted with an Incubator XL S1 system. Neurite outgrowth analysis was performed with Imaris (Bitplane Scientific Software).
PC12 and HEK293 Cell Culture and Transfections
PC12 cells were cultured as described previously (36). p75NTR-deficient (p75KO) PC12 cells, stably transfected with shRNA against rat p75NTR targeting the 3′-UTR region and cultured under G418 selection, were kindly provided by Carlos Ibanez (Karolinska Institute). PC12 cells were transfected using the Neon Transfection System (Invitrogen) with one pulse of 1410 volts and a 30-ms pulse duration. For neurite outgrowth assays, cells were grown in the presence of 1, 10, or 100 ng/ml NGF, and 1 μm c29 or control peptide, 20 μm TAPI-2 (Calbiochem) or 200 nm compound E for 3 days, with fresh medium and NGF added on day 2. Cells were photographed live on an Olympus microscope (IX81) fitted with a CO2 atmospheric chamber. Measurements of neurite length (minimum 100 cells) were performed using Olympus Image analysis software analySIS FIVE. For cleavage assays, cells were serum starved for 4 h prior to the addition of cleavage inhibitors and 5 μm proteasome inhibitor clasto-lactacystin-β-lactone (Sigma) for 4 h, followed by cell lysis.
HEK293 fibroblast cells (transformed by sheared adenovirus type 5 DNA-HEK293AD cells) were cultured as described previously (37). HEK293 cells were transfected with constructs using FuGENE 6 (Roche Applied Science), harvested 48 h later, and used for flow cytometry experiments or lysed for immunoblotting.
Cell Lysis and Immunoblotting
Both PC12 and HEK293 cells were lysed for 20 min on ice (25). For immunoblotting, samples were separated by SDS-PAGE and transferred onto PVDF membranes and Western blotted using standard protocols. The following antibodies were used: rabbit anti-p75NTR (1:2000; Promega, catalog no. G323A, or 1:1000; Upstate, catalog no. 07-476), goat anti-p75NTR (1:1000; R&D Systems, catalog no. AF1157), rabbit anti-TrkA (1:500; Upstate, catalog no. 06-574), mouse anti-phospho-Erk1/2 (1:2000; Cell Signaling, catalog no. 9101S), rabbit anti-panErk (1:2000; Cell Signaling, catalog no. 9102), rabbit anti-phospho-Akt (1:2000; Cell Signaling; catalog no. 4060S), mouse anti-panAkt (1:2000; Cell Signaling, catalog no. 9272) and anti-β-III tubulin (Promega, catalog no. G712A). Immunoreactive bands were detected using Invitrogen anti-rabbit Alexa Fluor 680 (1:10,000) or anti-mouse Alexa Fluor 800 (1:50,000) secondary antibodies and imaged using an Odyssey Imaging System (LI-COR Biosciences). NIH ImageJ software was used for the quantification of Western blots.
Cross-linking, Pulldown, and Immunoprecipitation Assays
For peptide pulldown experiments, the biotinylated c29 or LC1 control peptides were incubated with Dynabeads MyOne streptavidin T1 (Invitrogen) overnight at 4 °C. Cell lysates were then added and incubated for 1 h at 4 °C, before being eluted by boiling in Laemmli sample buffer.
For p75NTR construct immunoprecipitations, transfected HEK293 cells were treated with NGF at 50 ng/ml for 10 min, harvested in ice-cold PBS, and lysed as described above. Lysates were precleared in 75 μl (1:2) Gammabind G-Sepharose beads (Amersham Biosciences) for 2 h at 4 °C and then incubated with 3–5 μg of mouse anti-GFP (Roche Applied Science; catalog no. 11814460001), rabbit anti-TrkA (Abcam, catalog no. ab8871), or rabbit anti-human p75NTR intracellular domain antibody (Promega, catalog no. G323A) for 2 h at 4 °C. Immunoprecipitation was performed by the addition of (1:2) Gammabind G-Sepharose beads incubated for 16 h at 4 °C. The immunoprecipitate was washed and eluted in 2× LDS sample buffer (Invitrogen).
For surface biotinylation and TrkA cross-linking experiments, PC12 cells were serum-starved prior to treatment with peptides and NGF for 1 h. Cellular proteins were then either biotinylated for 90 min with EZ-Link Sulfo-NHS-biotin (Thermo Scientific) or non-reducibly cross-linked with disuccinimidyl suberate (Pierce) on ice for 2 h according to the manufacturer's instructions. Lysates were immunoprecipitated overnight at 4 °C using Gammabind G-Sepharose beads coupled with anti-Trk (C-14) antibody (Santa Cruz Biotechnology, catalog no. SC11). Samples were washed and eluted by boiling samples in reducing buffer (20 mm dithioerthritol, 2.5% SDS). Lysates were precleared using blank Gammabind G-Sepharose beads before immunoprecipitation. For the detection of phospho-TrkA following immunoprecipitation with TrkA antibodies, rabbit anti-phosphotyrosine antibodies (1:1000; Promega, catalog no. V2171) were used.
NGF-FITC Conjugation
NGF was labeled with fluorescein isothiocyanate (FITC) via free amine groups with a resulting fluorophore/protein ratio of 1.8–2.5:1 per NGF molecule adapted from Refs. 38 and 39. 400 μg of βNGF in 200 μl of NaHCO3 buffer, pH 8.5, was mixed with 5–10 μl of 10 mg/ml FITC in 100 μl of dimethyl sulfoxide for 10 h at 4 °C. Excess FITC was removed by dialysis against 10 mm Tris, 150 mm NaCl, 0.2% acetic acid, pH 8.2, for 24 h at 4 °C. The degree of NGF-FITC labeling was determined by measuring absorbance at 280 nm and 495 nm according to the manufacturer's instructions. Only FITC-NGF with equivalent activity to unlabeled NGF in PC12 cell neurite outgrowth assays was used in our assays (supplemental Fig. 1).
Flow Cytometry, NGF, and Antibody Binding Assays
In studies investigating NGF binding, PC12 cells or transfected p75KO PC12 or HEK293 cells were serum-starved for 4 h, after which c29 or scrambled peptide was added to the cultures for 1 h. Cells were harvested and washed with DMEM prior to use. To determine real-time association rates for NGF-FITC, cells were kept on ice and analyzed for 30 s at an analysis rate of 200–300 events per second immediately prior to the addition of 26 nm NGF-FITC, with analysis continuing for a further 30 min. Binding kinetics were calculated from raw data files of 550,000–600,000 cells compressed to 50,000 binding events using FlowJo and MatLab software. For steady-state binding assays, cells were incubated for 1 h in 10 μm NGF-FITC and washed with ice-cold PBS before analysis. Where applicable, the following antibodies were used: goat anti-TrkA against the NGF binding site (1:500; R&D Systems, catalog no. AF1056), non-agonist rabbit anti-TrkA (1:500; Upstate, catalog no. 06-574), and the monoclonal anti-rat p75NTR (MC192; 1:1000) purified from hybridoma-conditioned supernatant and conjugated to FITC (Sigma-Aldrich). Fluorescence parameters of at least 20,000 events for PC12 cells and 100,000 events for HEK293 cells were acquired by flow cytometry list mode, and measurements were performed on a single-cell basis (with compensation for double-event counting). Dead cells and debris were gated from the analysis on the basis of propidium iodide forward scatter fluorescence and mean, median, and maximal fluorescence. Values of the gated cell populations were analyzed using FACS Diva software (BD Biosciences) and FACS Express Software (BD Biosciences). The background fluorescence of HEK293 cells represents non-transfected cells (± c29 or scrambled peptide treatment) treated with NGF-FITC. For PC12 cells, the control population was cells that had not been treated with NGF-FITC.
Statistical Analysis
For all tests, p ≤ 0.05 was considered to be statistically significant. In experiments with two matched observations, a paired t test analysis was used. In experiments with three or more matched groups, repeated measures one-way ANOVA with a Friedman test was applied. In ligand-binding experiments two-way ANOVA with Bonferroni post-test analysis was used. GraphPad Prism software (version 5.0c) was used for all analyses.
RESULTS
SCG Neurons Exhibit an Enhanced Response to NGF in the Presence of c29
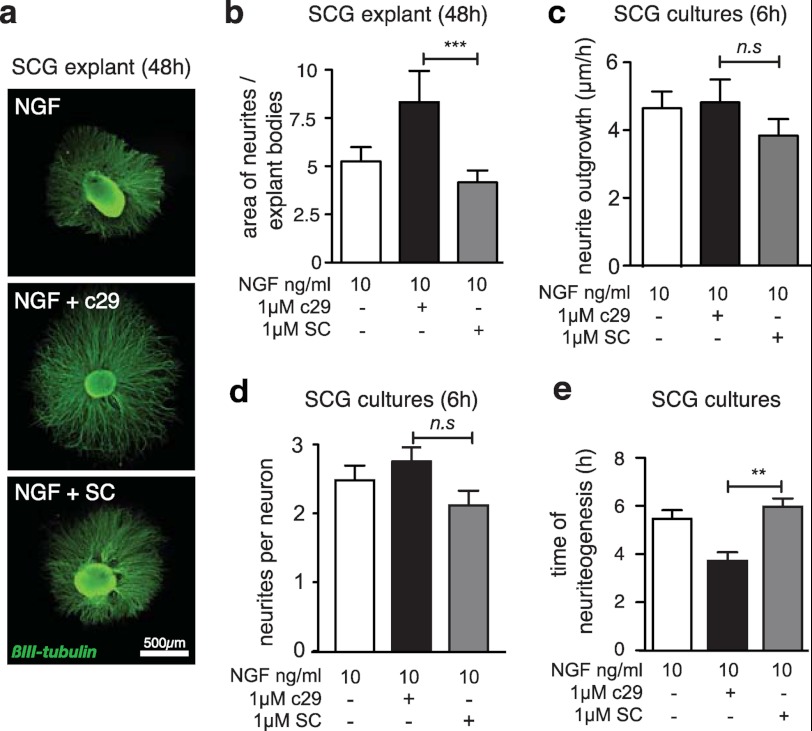
We have previously demonstrated that the c29 cell-permeable peptide, encompassing 29 amino acids of the juxtamembrane intracellular domain of p75NTR fused to a synthetic protein transduction domain peptide (see “Experimental Procedures”), can inhibit p75NTR-mediated cell death (29). As p75NTR has a well characterized role in the survival and differentiation of sympathetic neurons, we tested whether c29 is neurotrophic by using SCG explants, which selectively express p75NTR and TrkA, and require NGF for neurite outgrowth and survival. SCG explants isolated from newborn mice and cultured in the presence of 10 ng/ml NGF and 1 μm c29 displayed significantly enhanced neurite outgrowth compared with explants exposed to NGF alone or NGF and scrambled peptide controls (Fig. 1, a and b). To determine the reason for this effect, we examined neurite outgrowth of dissociated SCG cultures. Time-lapse microscopy revealed that the speed of axonal growth and the number of axonal branches per dissociated SCG neuron were not significantly different between treatments (Fig. 1, c and d); however, the presence of c29 significantly reduced the time required for dissociated SCG neurons to initiate NGF-mediated neuritogenesis (Fig. 1e).
FIGURE 1.
c29-treated SCG explants exhibit an enhanced response to NGF. a, representative photomicrographs of individual mouse SCG explants cultured in 10 ng/ml NGF and 1 μm of c29 or scrambled control peptide and stained for βIII-tubulin (scale bar, 500 μm), showing enhanced neurite outgrowth in the presence of c29 and NGF. b, quantification of axonal outgrowth from SCG explants cultured with NGF and c29 or scrambled control (SC) peptide. (≥8 explants were quantified per condition.) c, quantification of speed of neurite growth of dissociated SCG neurons during the first 6 h after plating in medium containing 10 ng/ml NGF and c29 or scrambled peptide. No significant differences were found between experimental conditions (n = 18 neurons per conditions). d, quantification of the number of neurites sprouting from dissociated SCG neurons from the time of plating in NGF and c29 or scrambled peptide. No significant differences were observed between treatments (n = 18 neurons per condition). e, quantification of the time it took for each dissociated SCG neuron to extend its first neurite following plating in NGF and c29 or scrambled peptide. SCG neurons in the presence of c29 and NGF initiated neuritogenesis 2 h earlier than neurons plated in NGF alone or NGF and scrambled peptide (n = 18 per condition) mean ± S.E.; **, p < 0.01; ***, p < 0.001; ANOVA).
PC12 Cells Exhibit an Enhanced Response to NGF in the Presence of c29
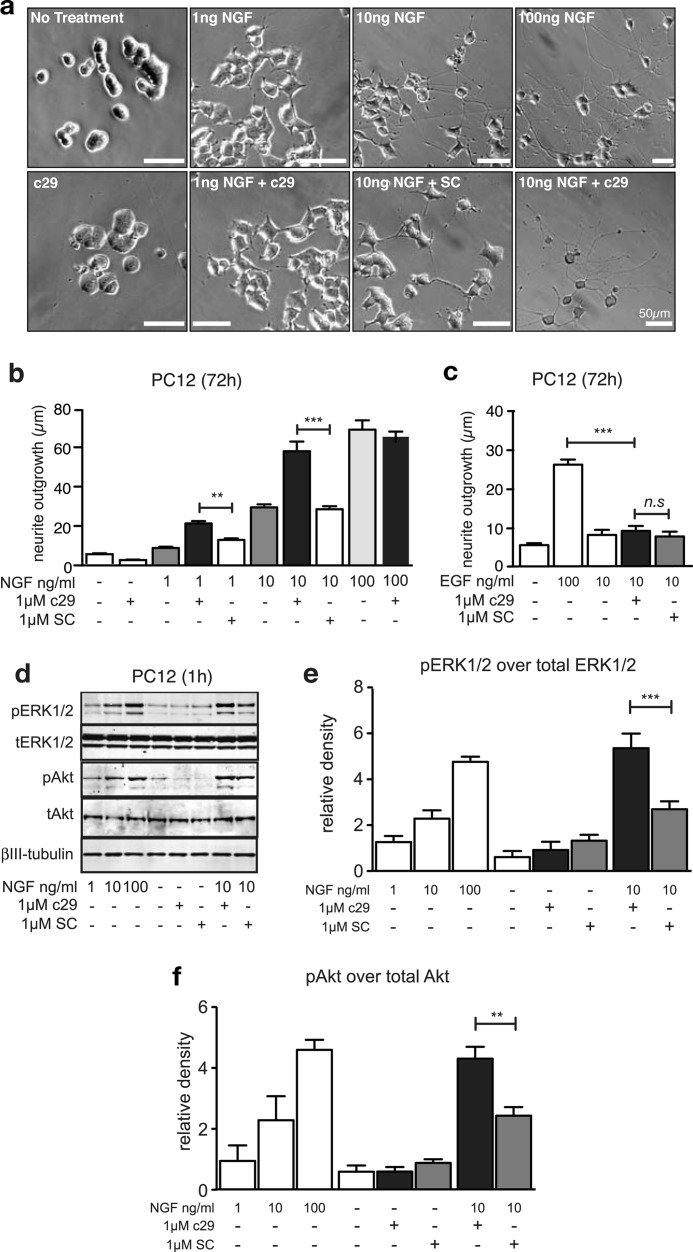
To understand the biochemical events underlying this effect, we analyzed neurite outgrowth in PC12 cells, which differentiate into a neuron-like phenotype when treated with NGF, and are commonly used to model sympathetic neurons (40). PC12 cells treated with c29 and low concentrations of NGF (1 ng/ml or 10 ng/ml) extended neurites that were significantly longer than those observed in cultures treated with equivalent concentrations of NGF, either with or without scrambled peptide (Fig. 2, a and b). Indeed, the neurite length of cells treated with 1 ng/ml or 10 ng/ml NGF in the presence of c29 was comparable with that of cells exposed to 10-fold higher NGF concentrations alone (Fig. 2b); however, c29 had no effect on neurite outgrowth of PC12 cells in the presence of saturating concentrations of 100 ng/ml NGF (Fig. 2b). c29 also had no effect on PC12 cell neurite outgrowth in the absence of NGF (Fig. 2, a and b) or in the presence of EGF (Fig. 2c), which also stimulates neurite outgrowth via endogenous EGF receptors when applied at high concentrations. This suggested that c29 had no intrinsic trophic effect (41).
FIGURE 2.
PC12 cells exhibit an enhanced neurite outgrowth and enhanced trophic signaling in the presence of c29. a, bright field micrographs of PC12 cells following differentiation in the presence of various NGF concentrations and treatment with c29 or scrambled control (SC) peptide (scale bars, 50 μm). Quantification of neurite length (median ± S.E.) of wild-type PC12 cells (b and c; n = 6 experiments, >50 cells per condition) 72 h after treatment with various concentrations of NGF (b and d) or EGF (c) and c29 and scrambled peptides are as indicated. Shown are representative Western blots (d) and quantification of immunopositive bands of total (tAkt and tERK) and phosphorylated forms of Erk1/2 (e) and Akt (f) in lysates of PC12 cells pretreated with c29 peptides and exposed to various concentrations of NGF for 1 h (n = 3 experiments, mean ± S.E.; **, p < 0.01; ***, p < 0.001; ANOVA).
NGF-dependent differentiation and neurite outgrowth in PC12 cells depends on increased and sustained activation of Erk1/2 by TrkA (2, 42, 43) and serine/threonine-specific protein kinase Akt (44). We therefore analyzed phosphorylated Erk1/2 and Akt by immunoblotting lysates from cells treated for 24 h with NGF, either alone or in combination with c29 or scrambled peptide. Addition of NGF to c29-treated cells produced a significant and dose-dependent increase in the levels of phosphorylated Erk1/2 (pErk1/2) (Fig. 2, d and e) and phosphorylated Akt (pAkt) (Fig. 2, d and f), relative to those in the control cultures. Total levels of Erk1/2 or Akt were unaffected by any treatment (Fig. 2d). In the absence of NGF, treatment of cells with c29 or scrambled peptide had no effect on pErk1/2 or pAkt signaling (Fig. 2, d–f), indicating that c29 did not independently stimulate either Erk1/2 or Akt activity. These increases in pErk1/2 and pAkt activation are consistent with and can account for the biological effects that we observed in the neurite outgrowth assays.
Endogenous p75NTR Is Not Required for c29 to Enhance NGF-stimulated Neurite Outgrowth
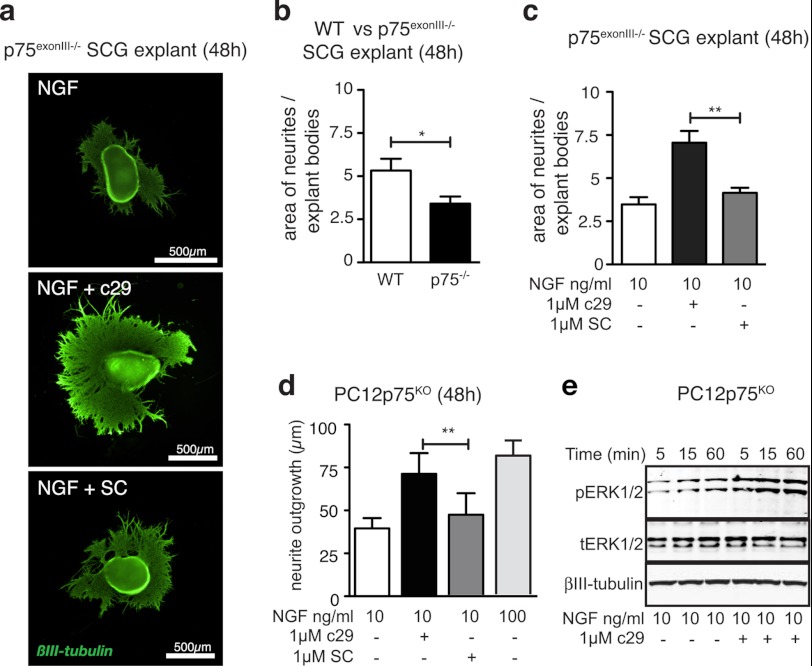
To determine whether c29 required endogenous p75NTR expression for its trophic actions, we treated SCG explants isolated from p75NTR-deficient mice with c29 and NGF (Fig. 3a). NGF-treated p75NTR-deficient ganglia had less neurite outgrowth over 48 h than NGF-treated ganglia from wild-type mice (Fig. 3b). However, c29 treatment enhanced the extent of outgrowth in p75NTR-deficient explants, restoring it to the level of NGF-stimulated outgrowth of p75NTR-deficient ganglia (Fig. 3, a and c).
FIGURE 3.
Endogenous p75NTR is not required for c29 to enhance NGF-stimulated neurite outgrowth. a, representative photomicrographs of individual mouse p75NTR(exonIII) knock-out SCG explants cultured in 10 ng/ml NGF and 1 μm of c29 or scrambled control peptide and stained for βIII-tubulin (scale bar = 500 μm) show enhanced neurite outgrowth in the presence of c29 and NGF. b, quantification of axonal outgrowth of wild-type and p75NTR(exonIII) knock-out mouse SCG explants cultured in 10 ng/ml NGF for 48 h. c, quantification of axonal outgrowth from p75NTR(exonIII) knock-out mouse SCG explants cultured with NGF and c29 or scrambled control (SC) peptide (five explants quantified per condition; mean ± S.E.). d, quantification of neurite length of p75KO PC12 cells (n = 4 experiments; median ± S.E.; >50 cells per condition) 72 h after treatment with 10 ng/ml concentrations of NGF, c29, or scrambled peptide as indicated. e, representative Western blots of immunopositive bands of total (tERK) and phosphorylated forms of Erk1/2 and Akt in lysates of p75KO PC12 cells pretreated with or without c29 peptides and exposed to 10 ng/ml NGF for various times as indicated (n = 3 experiments, mean ± S.E.;*, p < 0.05; **, p < 0.01; ANOVA).
Similarly, when PC12 cells lacking p75NTR (p75KO) were treated with c29 and NGF, the cultures displayed enhanced neurite outgrowth (Fig. 3d). Addition of NGF to c29-treated p75KO PC12 cultures also resulted in an early and enhanced activation of downstream Erk1/2 signaling compared with that in cultures treated with NGF alone (Fig. 3e). These results demonstrated that endogenous p75NTR is not required for c29 to promote neurotrophic functions.
Endogenous p75ICD Is Required for TrkA-mediated Neurite Outgrowth
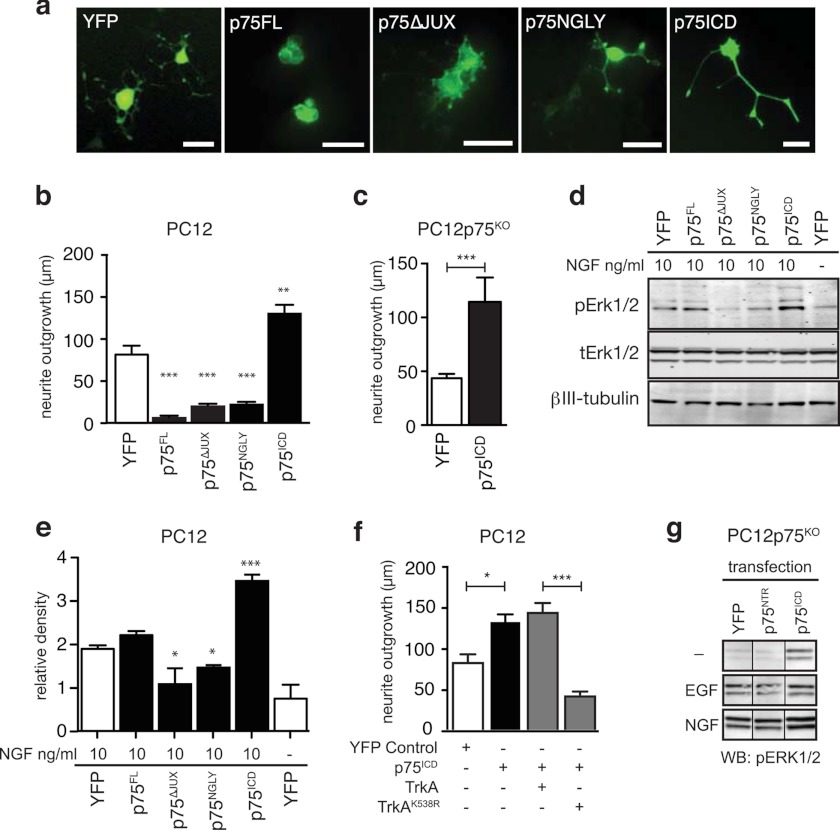
We next investigated whether the membrane-free p75ICD containing the c29 sequence (22, 26) had a similar neurotrophic effect to that of c29. NGF-treated wild-type (Fig. 4, a and b) and p75KO PC12 cells (Fig. 4c) transfected with p75ICD tagged with YFP had significantly longer neurites than control YFP-expressing cells (Fig. 4, a–c) and displayed enhanced Erk1/2 phosphorylation (Fig. 4, d and e).
FIGURE 4.
p75ICD but not full-length p75NTR promotes an enhanced response to NGF. a, photomicrographs of PC12 cells transfected with control YFP, full-length wild-type (p75FL), or mutant p75NTR-YFP plasmids 5 days after transfection and treatment with 100 ng/ml NGF. Scale bars are 50 μm. Shown is quantification of neurite length of wild-type (b) and p75KO (c) PC12 cells transfected with p75NTR variants 5 days after NGF treatment (n > 50 cells per condition from three experiments; median ± S.E.; **, p < 0.01; ***, p < 0.001; ANOVA, compared with YFP control). d, representative Western blots of pErk1/2 and total Erk1/2 (tErk) in lysates of p75NTR-transfected PC12 cells 24 h after treatment with NGF. e, quantification of Western blots for pErk1/2 relative to total Erk1/2 (tErk) in lysates of p75NTR-transfected PC12 cells 24 h after treatment with NGF (n = 3 experiments). f, quantification of neurite length of wild-type PC12 cells transfected with p75ICD and either wild-type TrkA or dominant-negative kinase-dead TrkAK538R 5 days after NGF treatment (n > 50 cells per condition, from three experiments; median ± S.E.; *, p < 0.05; ***, p < 0.001; ANOVA). g, representative Western blots of pErk1/2 in lysates of p75NTR-transfected p75KO PC12 cells 24 h after treatment with NGF or EGF. Although both NGF and EGF increase pErk1/2, p75ICD potentiated this effect in the presence of NGF but not EGF, whereas full-length p75NTR had no effect on pErk1/2 levels in any condition.
Because p75ICD has been reported to independently activate Erk1/2 signaling (45), we next determined whether the trophic effect of p75ICD expression required TrkA activity. Cells co-expressing p75ICD together with a dominant-negative TrkA construct containing a mutation in the kinase active site (TrkAK538R) had significantly shorter neurites than cells expressing p75ICD and wild-type TrkA (Fig. 4f). This indicated that TrkA kinase activity is required for the trophic effects of p75ICD. As with c29 treatment, PC12 cells transfected with a p75ICD construct displayed increased pErk1/2 signaling in response to NGF but not EGF treatment (Fig. 4g), suggesting that both the c29 peptide and p75ICD enhanced TrkA-kinase responsiveness to NGF.
To test whether other forms of p75NTR also had this neurotrophic effect, we used a number of p75NTR variant constructs. PC12 cells overexpressing full-length p75NTR (p75FL) or a protein mimicking p75CTF were subject to increased rates of cell death within 48 h of transfection (data not shown), as has been reported previously (29, 31, 46). However, cells overexpressing a non-cleavable variant of p75NTR (p75N-Gly) that prevents formation of both p75CTF and p75ICD or a variant with a deletion in the juxtamembrane region corresponding to c29 (p75Δ-JUX) remained viable. Cells expressing these variants, in contrast to those expressing p75ICD, had significantly reduced neurite outgrowth and Erk1/2 signaling when cultured in the presence of NGF (Fig. 4, a and b). This suggested that the neurotrophic effect of p75NTR was limited to the p75ICD fragment containing the c29 sequence.
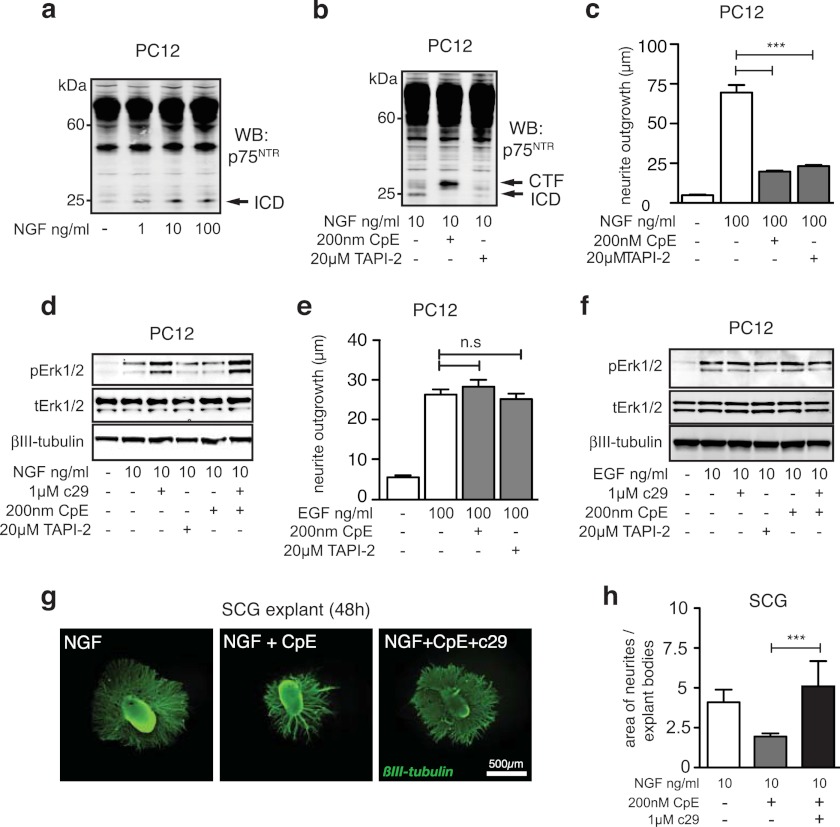
We next examined whether the generation of endogenous p75ICD fragments generated by intramembrane proteolysis of p75NTR was required for neurite outgrowth. NGF has previously been shown to induce cleavage of p75NTR to generate p75ICD (24, 25, 27). In agreement with these previous studies, treatment of PC12 cells with 1, 10, or 100 ng/ml NGF resulted in the dose-dependent generation of p75ICD (Fig. 5a). However, in the presence of inhibitors of p75NTR proteolysis (TAPI-2, a metalloprotease inhibitor, and compound E, a γ -secretase inhibitor), NGF-stimulated p75ICD generation was inhibited (Fig. 5b). These compounds also markedly reduced NGF-stimulated PC12 cell neurite outgrowth (Fig. 5c) and Erk1/2 activation (Fig. 5d). In contrast, neither the α- nor the γ- secretase inhibitor had any significant effect on EGF-induced neurite outgrowth (Fig. 5e) or signaling (Fig. 5f).
FIGURE 5.
Endogenous p75ICD generation is required for enhanced responses to NGF. Shown are Western blots of p75NTR fragments in lysates of PC12 cells treated with various concentrations of NGF (a) or 10 ng/ml NGF and the cleavage inhibitors TAPI-2 and compound E (CpE) (b). Shown is the quantification of neurite length of NGF- (c) and EGF-treated (e) PC12 cells cultured in the presence of TAPI-2 and compound E (median ± S.E., n = 4 experiments; ***, p < 0.001). Representative Western blot (WB) of phosphorylated and total Erk1/2 (tErk) in lysates of PC12 cells treated with cleavage inhibitors, c29, and either NGF (d) or EGF (f). g, representative photomicrographs of individual mouse SCG explants cultured in 10 ng/ml NGF, compound E, and 1 μm of c29 or scrambled peptide and stained for βIII-tubulin (scale bar, 500 μm). h, quantification of neurite outgrowth from SCG explants cultured with compound E and c29 (n = 6 explants per condition; mean ± S.E.; ***, p < 0.001; ANOVA). c29 treatment rescues the compound E-induced reduction in pErk1/2 and neurite outgrowth.
SCG explants treated with compound E in the presence of NGF also had clearly reduced neurite outgrowth compared with that observed in control cultures (Fig. 5, g and h). Importantly, the inhibitory effect of compound E on pErk1/2 production (Fig. 5f) and neurite growth of SCG explants was rescued by co-treatment with c29 (Fig. 5, g and h), mimicking the results obtained using p75NTR-deficient SCG explants (Fig. 3, a–c). These observations demonstrate that the γ-secretase cleavage of endogenous p75NTR specifically enhances TrkA-mediated neurite outgrowth, a function that can be mimicked by c29. Thus, these data are consistent with the idea that generation of p75ICD by p75NTR proteolysis is required for TrkA-mediated responses at low concentrations of NGF.
c29 Interacts with TrkA and Facilitates Increased NGF Binding
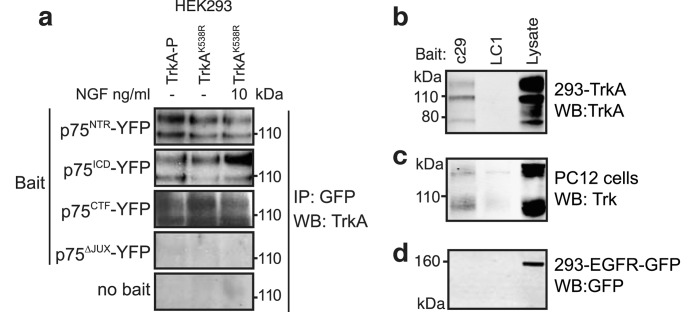
To understand the mechanism by which p75ICD and c29 facilitate TrkA signaling, we investigated whether a p75NTR-TrkA complex (47, 48) could be retained following p75NTR proteolysis. TrkA-p75NTR interactions were examined via co-immunoprecipitation following transfection of HEK293 cells. Full-length and truncated p75NTR constructs were transfected together with either kinase-active TrkA or kinase-inactive TrkAK538A. Both full-length p75NTR (Fig. 6a) and its C-terminal fragment (Fig. 6a) were able to co-immunoprecipitate TrkA and TrkAK538A, indicating that the interaction was not mediated via the extracellular domain of p75NTR. Consistent with this, neither TrkA activation nor dimerization by NGF significantly influenced the amount of TrkA that was co-immunoprecipitated with p75NTR (Fig. 6a). Importantly, p75ICD was also able to co-immunoprecipitate TrkA (Fig. 6a), whereas the p75Δ-JUX protein (lacking 33 amino acids of the intracellular juxtamembrane region) was not (Fig. 6a). Moreover, c29 but not a control peptide (LC1), was able to pull down overexpressed (Fig. 6b) as well as endogenously expressed TrkA (Fig. 6c). Neither peptide pulled down the EGF receptor (Fig. 6d). These results indicate that the 29-amino acid intracellular juxtamembrane sequence is sufficient and necessary for p75NTR to interact with TrkA.
FIGURE 6.
p75ICD and c29 interact with TrkA. a, co-immunoprecipitation (IP) of kinase-active TrkA or kinase-inactive TrkAK538A following precipitation by anti-YFP antibodies in lysates from cells co-expressing full-length p75NTR (p75FL-YFP), p75ICD-YFP, p75CTF-YFP, or p75NTR lacking the juxtamembrane c29 domain (p75Δ-JUX-YFP) proteins with or without NGF. Western blot (WB) of TrkA (b) or EGF receptor (EGFR-YFP) (d) from lysate of transfected HEK293 cells or PC12 cells (c) following pulldown with biotinylated c29 or LC1, a control biotinylated peptide (representative figure for n = 3).
Based on a report that the juxtamembrane domain of p75NTR is required for the generation of high-affinity NGF receptors (19), we next tested whether c29 was acting by affecting binding of NGF to TrkA. To investigate this, we used fluorescently labeled NGF (NGF-FITC) and flow cytometry analysis of ligand binding. The advantage of using flow cytometry, rather than the more traditional method based on radiolabeled NGF, is the ability to assess ligand binding in real time, and on a per cell as well as total population basis (49, 50).
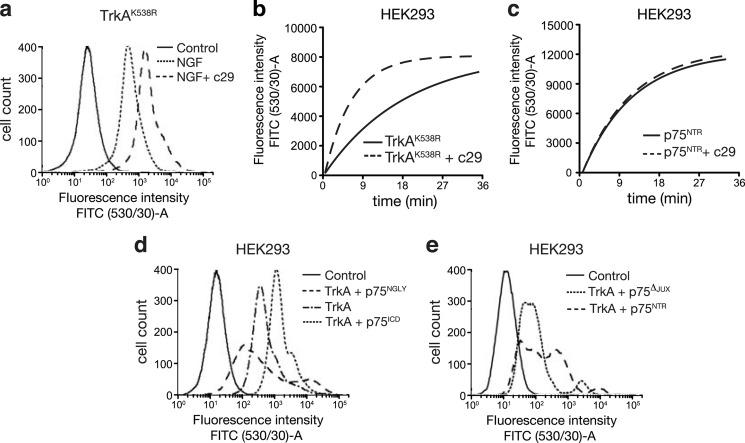
c29-treated HEK293 cells transfected with TrkAK538R and PC12 cells bound significantly more NGF per cell than untreated or scrambled peptide-treated cultures (Fig. 7a and supplemental Table 1). To determine whether this increase in NGF binding in the presence of c29 was due to an increased rate of association of NGF for its receptors, we measured the real-time binding rate of NGF in HEK293 cells expressing either TrkA or p75NTR. Cells expressing only TrkA (Fig. 7b), showed a 3-fold faster binding rate of NGF than untreated or scrambled peptide-treated controls, whereas NGF-FITC binding to cells expressing only p75NTR was unaffected by c29 treatment (Fig. 7c).
FIGURE 7.
p75ICD and c29 facilitate NGF binding to TrkA-expressing cells. a, flow cytometry histogram plots of TrkAK538R-transfected HEK293 cells treated for 1 h with saturating concentrations (10 μm) of NGF-FITC (steady-state binding) following preincubation with or without c29. (All plots are representative of four experiments.) b, plot of on-rate (the cell population mean fluorescence due to bound NGF-FITC) of TrkA-transfected HEK293 cells with or without c29 preincubation and treated at time = 0 with 26 nm NGF-FITC. c, plot of on-rate (association) of NGF-FITC-treated p75NTR-transfected HEK293 cells with or without c29 preincubation. Shown are flow cytometry histogram plots of HEK293 cells transfected with TrkA and either p75ICD or non-cleavable p75N-Gly (d) and TrkA and wild-type p75NTR or p75ΔJUX (e) treated for 1 h with NGF-FITC (steady-state binding). p75ICD expression enhanced NGF-FITC binding, whereas expression of either of the p75NTR variants inhibited NGF-FITC binding compared with TrkA alone (d) or TrkA and wild-type p75NTR (e) (n = 4 for all experiments; mean ± S.E.).
Cells expressing p75ICD together with TrkA also bound significantly more NGF-FITC (Fig. 7e). In contrast, cells expressing TrkA and either p75N-Gly (Fig. 7e) or p75Δ-JUX (Fig. 7f and supplemental Table 1) did not have significantly enhanced NGF binding, with a trend toward reduced NGF binding capacity despite increased numbers of NGF-binding receptors expressed by these cells compared with those transfected with TrkA alone. These results are consistent with our finding that cells treated with c29 and p75ICD display enhanced responses to low concentrations of NGF in neurite outgrowth and signaling assays, whereas full-length p75NTR failed to mediate these effects.
c29 Alters Ligand Accessibility Not TrkA Receptor Levels
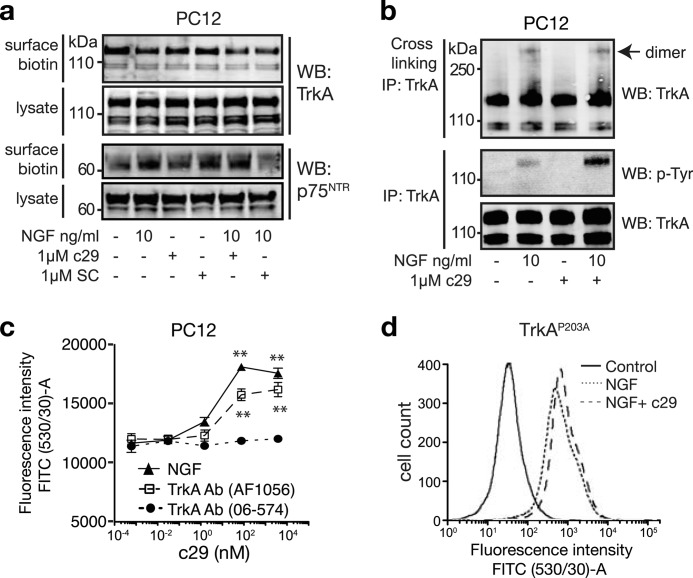
To determine whether c29 facilitates TrkA ligand binding and function by altering TrkA or p75NTR expression, we measured the cell surface expression of these receptors in PC12 cells. The presence of c29, scrambled peptide, or NGF had no effect on the levels of surface TrkA or p75NTR (Fig. 8a). Similarly, although NGF induced the formation of TrkA dimers, the number of TrkA dimers (basal or NGF-induced) was unchanged in the presence of c29, despite the markedly increased level of TrkA phosphorylation compared with that of cells treated with NGF alone (Fig. 8b).
FIGURE 8.
c29 modulates NGF-mediated TrkA phosphorylation, but not cell-surface receptor expression. a, Western blots (WB) of cell surface-biotinylated TrkA and p75NTR in lysates of PC12 cells treated with NGF and c29 peptides (representative of four experiments). b, Western blots of total and phosphorylated TrkA in PC12 cell lysates. Cells were treated to cross-link TrkA dimers following treatment with NGF and c29 as indicated (representative of four experiments). c, graph of mean population fluorescence of PC12 cells preincubated with various doses of c29 and then incubated with NGF-FITC or agonist (AF1056) or non-agonist (06-574) TrkA antibodies (Ab) precoupled to Alexa Fluor 488 secondary antibodies (n = 4 experiments, mean ± S.E.; **, p < 0.01; one-way ANOVA). d, flow cytometry histogram plot of HEK293 cells transfected with a constitutively active TrkAP203A mutant treated for 1 h with NGF-FITC in the presence or absence of c29 showing no significant effect of NGF-FITC binding in the presence of c29 (n = 4). IP, immunoprecipitation.
One mechanism by which receptor ligand-binding capacity can be increased without changing receptor numbers is through structural modulation of the ligand-binding site (32, 51). To investigate whether c29 might be causing a conformational change to facilitate the access of ligands to their binding site within the extracellular domain of TrkA, we compared the extent of binding of a TrkA antibody that targets the NGF ligand-binding site (AF1056), versus a non-modulatory TrkA antibody (06-574), to PC12 cells treated with c29. The binding of the antibody raised against the ligand-binding site of TrkA was significantly increased in a c29 dose-dependent manner, similar to the enhanced binding of NGF (Fig. 8c). In contrast, the binding of the non-modulatory TrkA antibody was unaffected by the presence of c29 (Fig. 8c). This supports the idea that c29 could facilitate the access of TrkA ligands to their binding site within the extracellular ligand-binding domain of TrkA.
Finally, we examined the ability of c29 to modulate NGF binding to a TrkA variant (TrkAP203A), which has a constitutively increased binding affinity for NGF due to a mutation within the extracellular flexible linker region (32). c29 had a negligible effect on the rate of NGF binding to HEK293 cells expressing TrkAP203A (Fig. 8d), suggesting that c29 mediates a conformational change within wild-type TrkA, an effect that is constrained in the TrkP203A mutant.
DISCUSSION
Here, we show that the juxtamembrane intracellular domain of p75NTR is required and sufficient to potentiate TrkA-mediated signaling and functional outcomes at nanomolar concentrations of neurotrophins. This domain mediates interactions between p75NTR and TrkA but does not associate with the EGF receptor. Moreover, it modulates the binding of NGF to TrkA, significantly increasing the amount of NGF (or an agonist ligand-mimicking TrkA antibody) bound to TrkA-expressing cells, with no change in the surface expression of either p75NTR or TrkA receptors. Together these results suggest that this small p75ICD juxtamembrane domain acts as a modulator of Trk receptor function.
c29 and p75ICD Potentiate Trk-mediated Trophic Signaling Pathways and Functions
Our previous work (29) as well as studies assessing the role of p75NTR in high-affinity NGF binding (19, 20) have implicated the proximal-intracellular juxtamembrane 29-amino acid fragment of p75ICD, termed the Chopper domain, in potentiating neurotrophic actions. Here, we established that a peptide comprising the Chopper fragment lacking a transmembrane linker (c29) enables PC12 cells and SCG neurons to respond to a 10-fold lower neurotrophin concentration in terms of neurite outgrowth and activation of trophic Erk1/2 and Akt signal cascades. A consistent finding from these results was that the trophic actions of c29 did not occur in the absence of Trk activation and did not require expression of full-length p75NTR. This strongly suggests that the effect of the peptide does not occur solely via inhibition of p75NTR death signaling cascades (52).
The generation of p75ICD has been reported as necessary for many functions of p75NTR (23), including activation of trophic signaling pathways (25, 26) and cell death in the absence of trophic support (53). In this and our previous studies (29–31), the p75ICD fragment did not mediate death. Rather, we have reported that the p75CTF promotes neuronal death (29, 31, 54, 55) and here have observed that p75ICD enhanced TrkA-dependent neurite outgrowth and Erk1/2 signaling. Importantly, in these assays, other forms of p75NTR did not have the same trophic effects as p75ICD. Non-cleavable p75N-Gly and p75Δ-JUX inhibited the neurotrophic effects of NGF, reducing rather than enhancing NGF binding to TrkA-expressing cells. Full-length p75NTR or p75CTF again promoted cell death in PC12 cells. Our findings suggest that the generation of a membrane-free intracellular domain fragment containing the c29 sequence is critical for endogenous p75NTR to mediate these trophic actions. Both cleavage inhibitors, which prevent the generation of p75ICD from endogenous p75NTR, and p75NTR gene deletion curtailed NGF-induced neurite outgrowth. These results, together with the rescue of neurite outgrowth by c29, illustrate that the production of this p75NTR fragment is required for the trophic response of the cells to NGF via TrkA.
c29 Alters the NGF Binding Affinity of TrkA-expressing Cells
Our experiments demonstrate that the c29 sequence is necessary for and sufficient to increase the amount and association rate of NGF binding to TrkA-expressing cells, with no measurable effect on NGF binding to p75NTR. Whether this observation represents high-affinity NGF receptor activity has not been addressed. Nonetheless, our results are consistent with previous reports demonstrating that the intracellular domain of p75NTR, in particular the c29-encompassing region of the intracellular domain, is required to reconstitute NGF binding sites with a faster association rate than that of cells expressing TrkA alone (19, 20). The p75NTR transmembrane domain was also shown to be necessary for high-affinity NGF binding as a chimeric p75NTR protein containing the EGF receptor transmembrane domain failed to generate high-affinity receptor sites (20). We recently reported that the p75NTR transmembrane sequence and structure have a significant effect on the rate of γ-secretase cleavage (27). Therefore, the p75NTR-EGF receptor chimeric protein may have failed to recapitulate high-affinity NGF binding sites because generation of the p75ICD fragment was hindered.
Our observation of enhanced NGF binding per cell following the application of c29 or overexpression of p75ICD is also consistent with the idea that the ratio of p75NTR to TrkA affects NGF binding affinity (19, 56) and that only a small fraction of surface Trk receptors participate in high-affinity binding (6). Indeed, our finding that c29 and expressed p75ICD could potentiate the neurotrophic activity in cells already expressing endogenous p75NTR indicates that the response of the cells was not yet maximal. A limiting step in the endogenous response to low neurotrophin levels may not be the ratio of Trk to p75NTR in itself but rather the amount of available p75ICD relative to TrkA. Jung et al. (21) concluded that p75ICD did not interact with TrkA as the majority of p75ICD generated following phorbolesters treatment of transfected cells was not found in the same size exclusion fractions as TrkA. However, the small proportion of p75ICD in the TrkA fraction observed in their experiments may be sufficient for enhanced function under physiological conditions with the rate or location of p75ICD generation being a limiting factor. An estimation of the amount of c29 within cells relative to the amount of p75ICD generated after phorbolesters treatment suggests that c29 would be present in cells at a much higher concentration than endogenous p75ICD (supplemental Fig. 2). Therefore, in our experiments, application of excess c29 or overexpression of p75ICD may have eliminated this barrier.
Inside-out Modulation of Trk by p75ICD?
Our results indicate that c29 and p75ICD can interact with TrkA but do not significantly affect surface receptor levels or dimerization of the receptors. Rather, our data are consistent with these fragments altering the ability of the extracellular domain of TrkA to interact with and bind its ligands. We speculate that this is by an inside-out structural modulation similar to that which occurs during activation of the integrin receptor (57).
Neurotrophins bind to the IgG-C2 domains of Trk receptors (16, 51). Structural and biochemical data indicate that ligand binding to TrkA at these sites can be allosterically modulated and facilitated by p75NTR through the additional surface exposure of the more N-terminally located IgG-C1/LRM domains (51). Although this may occur due to mutation of residues within these N-terminal domains, such as occurs in the TrkAP203A mutant used herein (32), the extracellular domain of wild-type TrkA is considered to be rigid. Thus, the structural change required to enable this modulation in vivo is probably mediated via the TrkA intracellular and/or transmembrane domains (16). We therefore propose that c29 and p75ICD act to cause and/or stabilize such a change, thereby resulting in the formation of receptors with enhanced ligand-binding abilities.
An in vivo trigger for such an inside-out structural change could be the release of p75NTR from its transmembrane domain following γ-cleavage generation of the p75ICD (24–26). The p75ICD juxtamembrane domain is considered to be structurally flexible (58), which means that it may form a different configuration when membrane-bound compared with its structure following γ-secretase cleavage. Structural change within the juxtamembrane region could, in turn, be permissive for altered association with the Trk receptor, thereby mediating allosteric modulation of the Trk receptor. Additional structural studies will be required to determine whether our proposed model is viable.
In summary, we have demonstrated that a specific fragment of p75NTR, p75ICD, can interact with TrkA and is required for its enhanced function. Furthermore, our results show that a c29 amino acid fragment of p75ICD is sufficient to increase the binding of NGF to TrkA-expressing cells, promote NGF trophic signaling, and enhance neurite outgrowth. Our work suggests that the interaction of this 29-amino acid region of p75ICD with TrkA triggers a conformational change within the extracellular domain of TrkA, resulting in increased receptor binding site availability and receptor activation. These results provide evidence for a new model of interaction between TrkA and the p75ICD fragment and identify the 29-amino acid functional moiety of this p75NTR fragment as being responsible for facilitating enhanced TrkA activation.
Supplementary Material
Acknowledgments
Flow cytometry experiments were performed and analyzed with the assistance of Geoff Osborne, VA Nink, Angelo Keramidas, and Christine Dixon. We thank Moses V Chao (Skirball Institute Biomolecular Medicine) for anti-p75NTR antibody 9992 and Rowan Tweedale for editorial assistance.
This work was supported by National Health and Medical Research Council of Australia (NHMRC) Project Grants 10012610 and 569507, NHMRC Career Development Fellowship 569601 (to E. J. C.), and Australian Research Council Grant LP110100403.

This article contains supplemental Table 1 and Figs. 1 and 2.
- NGF
- nerve growth factor
- p75NTR
- p75 neurotrophin receptor
- p75ICD
- intracellular domain fragment of p75NTR
- Trk
- tropomyosin receptor kinase
- p75CTF
- carboxyterminal fragment
- SCG
- superior cervical ganglia
- ANOVA
- analysis of variance.
REFERENCES
- 1. Reichardt L. F. (2006) Neurotrophin-regulated signalling pathways. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 361, 1545–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kaplan D. R., Stephens R. M. (1994) Neurotrophin signal transduction by the Trk receptor. J. Neurobiol. 25, 1404–1417 [DOI] [PubMed] [Google Scholar]
- 3. Kaplan D. R., Hempstead B. L., Martin-Zanca D., Chao M. V., Parada L. F. (1991) The trk proto-oncogene product: a signal transducing receptor for nerve growth factor. Science 252, 554–558 [DOI] [PubMed] [Google Scholar]
- 4. Friedman W. J., Greene L. A. (1999) Neurotrophin signaling via Trks and p75. Exp. Cell Res. 253, 131–142 [DOI] [PubMed] [Google Scholar]
- 5. Block T., Bothwell M. (1983) The nerve growth factor receptor on PC12 cells: interconversion between two forms with different binding properties. J. Neurochem. 40, 1654–1663 [DOI] [PubMed] [Google Scholar]
- 6. Hempstead B. L., Martin-Zanca D., Kaplan D. R., Parada L. F., Chao M. V. (1991) High-affinity NGF binding requires coexpression of the trk proto-oncogene and the low-affinity NGF receptor. Nature 350, 678–683 [DOI] [PubMed] [Google Scholar]
- 7. Davies A. M., Lee K. F., Jaenisch R. (1993) p75-deficient trigeminal sensory neurons have an altered response to NGF but not to other neurotrophins. Neuron 11, 565–574 [DOI] [PubMed] [Google Scholar]
- 8. Bibel M., Hoppe E., Barde Y. A. (1999) Biochemical and functional interactions between the neurotrophin receptors trk and p75NTR. EMBO J. 18, 616–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chao M. V., Hempstead B. L. (1995) p75 and Trk: a two-receptor system. Trends Neurosci. 18, 321–326 [PubMed] [Google Scholar]
- 10. Lee K. F., Li E., Huber L. J., Landis S. C., Sharpe A. H., Chao M. V., Jaenisch R. (1992) Targeted mutation of the gene encoding the low affinity NGF receptor p75 leads to deficits in the peripheral sensory nervous system. Cell 69, 737–749 [DOI] [PubMed] [Google Scholar]
- 11. Lee K. F., Davies A. M., Jaenisch R. (1994) p75-deficient embryonic dorsal root sensory and neonatal sympathetic neurons display a decreased sensitivity to NGF. Development 120, 1027–1033 [DOI] [PubMed] [Google Scholar]
- 12. Barker P. A. (2007) High Affinity Not in the Vicinity? Neuron 53, 1–4 [DOI] [PubMed] [Google Scholar]
- 13. Mahadeo D., Kaplan L., Chao M. V., Hempstead B. L. (1994) High affinity nerve growth factor binding displays a faster rate of association than p140trk binding. Implications for multi-subunit polypeptide receptors. J. Biol. Chem. 269, 6884–6891 [PubMed] [Google Scholar]
- 14. Barker P. A., Shooter E. M. (1994) Disruption of NGF binding to the low affinity neurotrophin receptor p75LNTR reduces NGF binding to TrkA on PC12 cells. Neuron 13, 203–215 [DOI] [PubMed] [Google Scholar]
- 15. Aurikko J. P., Ruotolo B. T., Grossmann J. G., Moncrieffe M. C., Stephens E., Leppänen V. M., Robinson C. V., Saarma M., Bradshaw R. A., Blundell T. L. (2005) Characterization of symmetric complexes of nerve growth factor and the ectodomain of the pan-neurotrophin receptor, p75NTR. J. Biol. Chem. 280, 33453–33460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wehrman T., He X., Raab B., Dukipatti A., Blau H., Garcia K. C. (2007) Structural and mechanistic insights into nerve growth factor interactions with the TrkA and p75 receptors. Neuron 53, 25–38 [DOI] [PubMed] [Google Scholar]
- 17. Gong Y., Cao P., Yu H. J., Jiang T. (2008) Crystal structure of the neurotrophin-3 and p75NTR symmetrical complex. Nature 454, 789–793 [DOI] [PubMed] [Google Scholar]
- 18. Rydén M., Hempstead B., Ibáñez C. F. (1997) Differential modulation of neuron survival during development by nerve growth factor binding to the p75 neurotrophin receptor. J. Biol. Chem. 272, 16322–16328 [DOI] [PubMed] [Google Scholar]
- 19. Hempstead B. L., Patil N., Thiel B., Chao M. V. (1990) Deletion of cytoplasmic sequences of the nerve growth factor receptor leads to loss of high affinity ligand binding. J. Biol. Chem. 265, 9595–9598 [PubMed] [Google Scholar]
- 20. Esposito D., Patel P., Stephens R. M., Perez P., Chao M. V., Kaplan D. R., Hempstead B. L. (2001) The cytoplasmic and transmembrane domains of the p75 and Trk A receptors regulate high affinity binding to nerve growth factor. J. Biol. Chem. 276, 32687–32695 [DOI] [PubMed] [Google Scholar]
- 21. Jung K. M., Tan S., Landman N., Petrova K., Murray S., Lewis R., Kim P. K., Kim D. S., Ryu S. H., Chao M. V., Kim T. W. (2003) Regulated intramembrane proteolysis of the p75 neurotrophin receptor modulates its association with the TrkA receptor. J. Biol. Chem. 278, 42161–42169 [DOI] [PubMed] [Google Scholar]
- 22. Kanning K. C., Hudson M., Amieux P. S., Wiley J. C., Bothwell M., Schecterson L. C. (2003) Proteolytic processing of the p75 neurotrophin receptor and two homologs generates C-terminal fragments with signaling capability. J. Neurosci. 23, 5425–5436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Skeldal S., Matusica D., Nykjaer A., Coulson E. J. (2011) Proteolytic processing of the p75 neurotrophin receptor: A prerequisite for signalling?: Neuronal life, growth and death signalling are crucially regulated by intra-membrane proteolysis and trafficking of p75NTR. Bioessays 33, 614–625 [DOI] [PubMed] [Google Scholar]
- 24. Urra S., Escudero C. A., Ramos P., Lisbona F., Allende E., Covarrubias P., Parraguez J. I., Zampieri N., Chao M. V., Annaert W., Bronfman F. C. (2007) TrkA receptor activation by nerve growth factor induces shedding of the p75 neurotrophin receptor followed by endosomal γ-secretase-mediated release of the p75 intracellular domain. J. Biol. Chem. 282, 7606–7615 [DOI] [PubMed] [Google Scholar]
- 25. Ceni C., Kommaddi R. P., Thomas R., Vereker E., Liu X., McPherson P. S., Ritter B., Barker P. A. (2010) The p75NTR intracellular domain generated by neurotrophin-induced receptor cleavage potentiates Trk signaling. J. Cell Sci. 123, 2299–2307 [DOI] [PubMed] [Google Scholar]
- 26. Kommaddi R. P., Thomas R., Ceni C., Daigneault K., Barker P. A. (2011) Trk-dependent ADAM17 activation facilitates neurotrophin survival signaling. FASEB J. 25, 2061–2070 [DOI] [PubMed] [Google Scholar]
- 27. Sykes A. M., Palstra N., Abankwa D., Hill J. M., Skeldal S., Matusica D., Venkatraman P., Hancock J. F., Coulson E. J. (2012) The effects of transmembrane sequence and dimerization on cleavage of the p75 neurotrophin receptor by γ-secretase. J. Biol. Chem. 287, 43810–43824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Coulson E. J., Reid K., Barrett G. L., Bartlett P. F. (1999) p75 neurotrophin receptor-mediated neuronal death is promoted by Bcl-2 and prevented by Bcl-xL. J. Biol. Chem. 274, 16387–16391 [DOI] [PubMed] [Google Scholar]
- 29. Coulson E. J., Reid K., Baca M., Shipham K. A., Hulett S. M., Kilpatrick T. J., Bartlett P. F. (2000) Chopper, a new death domain of the p75 neurotrophin receptor that mediates rapid neuronal cell death. J. Biol. Chem. 275, 30537–30545 [DOI] [PubMed] [Google Scholar]
- 30. Coulson E. J., Reid K., Shipham K. M., Morley S., Kilpatrick T. J., Bartlett P. F. (2004) The role of neurotransmission and the Chopper domain in p75 neurotrophin receptor death signaling. Prog. Brain Res. 146, 41–62 [DOI] [PubMed] [Google Scholar]
- 31. Underwood C. K., Reid K., May L. M., Bartlett P. F., Coulson E. J. (2008) Palmitoylation of the C-terminal fragment of p75NTR regulates death signaling and is required for subsequent cleavage by γ-secretase. Mol. Cell Neurosci. 37, 346–358 [DOI] [PubMed] [Google Scholar]
- 32. Arevalo J. C., Conde B., Hempstead B. I., Chao M. V., Martín-Zanca D., Pérez P. (2001) A novel mutation within the extracellular domain of TrkA causes constitutive receptor activation. Oncogene 20, 1229–1234 [DOI] [PubMed] [Google Scholar]
- 33. Ho A., Schwarze S. R., Mermelstein S. J., Waksman G., Dowdy S. F. (2001) Synthetic protein transduction domains: enhanced transduction potential in vitro and in vivo. Cancer Res. 61, 474–477 [PubMed] [Google Scholar]
- 34. Bodmer D., Levine-Wilkinson S., Richmond A., Hirsh S., Kuruvilla R. (2009) Wnt5a mediates nerve growth factor-dependent axonal branching and growth in developing sympathetic neurons. J. Neurosci. 29, 7569–7581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zareen N., Greene L. A. (2009) Protocol for culturing sympathetic neurons from rat superior cervical ganglia (SCG). J. Vis. Exp. 32, e988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Greene L. A., Tischler A. S. (1976) Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc. Natl. Acad. Sci. U.S.A. 73, 2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Shaw G., Morse S., Ararat M., Graham F. L. (2002) Preferential transformation of human neuronal cells by human adenoviruses and the origin of HEK 293 cells. FASEB J. 16, 869–871 [DOI] [PubMed] [Google Scholar]
- 38. Weible M. W., 2nd, Bartlett S. E., Reynolds A. J., Hendry I. A. (2001) Prolonged recycling of internalized neurotrophins in the nerve terminal. Cytometry 43, 182–188 [DOI] [PubMed] [Google Scholar]
- 39. Weible M. W., 2nd, Ozsarac N., Grimes M. L., Hendry I. A. (2004) Comparison of nerve terminal events in vivo effecting retrograde transport of vesicles containing neurotrophins or synaptic vesicle components. J. Neurosci. Res. 75, 771–781 [DOI] [PubMed] [Google Scholar]
- 40. Kaplan D. R., Miller F. D. (2000) Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 10, 381–391 [DOI] [PubMed] [Google Scholar]
- 41. Traverse S., Gomez N., Paterson H., Marshall C., Cohen P. (1992) Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 288, 351–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Vaudry D., Stork P. J., Lazarovici P., Eiden L. E. (2002) Signaling pathways for PC12 cell differentiation: making the right connections. Science 296, 1648–1649 [DOI] [PubMed] [Google Scholar]
- 43. Fukuda M., Gotoh Y., Tachibana T., Dell K., Hattori S., Yoneda Y., Nishida E. (1995) Induction of neurite outgrowth by MAP kinase in PC12 cells. Oncogene 11, 239–244 [PubMed] [Google Scholar]
- 44. Kim Y., Seger R., Suresh Babu C. V., Hwang S. Y., Yoo Y. S. (2004) A positive role of the PI3-K/Akt signaling pathway in PC12 cell differentiation. Mol. Cells 18, 353–359 [PubMed] [Google Scholar]
- 45. Barker P. A. (2004) p75NTR is positively promiscuous: novel partners and new insights. Neuron 42, 529–533 [DOI] [PubMed] [Google Scholar]
- 46. Barrett G. L., Georgiou A. (1996) The low-affinity nerve growth factor receptor p75NGFR mediates death of PC12 cells after nerve growth factor withdrawal. J. Neurosci. Res. 45, 117–128 [DOI] [PubMed] [Google Scholar]
- 47. Huber L. J., Chao M. V. (1995) A potential interaction of p75 and trkA NGF receptors revealed by affinity crosslinking and immunoprecipitation. J. Neurosci. Res. 40, 557–563 [DOI] [PubMed] [Google Scholar]
- 48. Wolf D. E., McKinnon C. A., Daou M. C., Stephens R. M., Kaplan D. R., Ross A. H. (1995) Interaction with TrkA immobilizes gp75 in the high affinity nerve growth factor receptor complex. J. Biol. Chem. 270, 2133–2138 [DOI] [PubMed] [Google Scholar]
- 49. Fay S. P., Posner R. G., Swann W. N., Sklar L. A. (1991) Real-time analysis of the assembly of ligand, receptor, and G protein by quantitative fluorescence flow cytometry. Biochemistry 30, 5066–5075 [DOI] [PubMed] [Google Scholar]
- 50. Sklar L. A., Edwards B. S., Graves S. W., Nolan J. P., Prossnitz E. R. (2002) Flow cytometric analysis of ligand-receptor interactions and molecular assemblies. Annu. Rev. Biophys. Biomol. Struct. 31, 97–119 [DOI] [PubMed] [Google Scholar]
- 51. Zaccaro M. C., Ivanisevic L., Perez P., Meakin S. O., Saragovi H. U. (2001) p75 Co-receptors regulate ligand-dependent and ligand-independent Trk receptor activation, in part by altering Trk docking subdomains. J. Biol. Chem. 276, 31023–31029 [DOI] [PubMed] [Google Scholar]
- 52. Roux P. P., Barker P. A. (2002) Neurotrophin signaling through the p75 neurotrophin receptor. Prog. Neurobiol. 67, 203–233 [DOI] [PubMed] [Google Scholar]
- 53. Kenchappa R. S., Zampieri N., Chao M. V., Barker P. A., Teng H. K., Hempstead B. L., Carter B. D. (2006) Ligand-dependent cleavage of the p75 neurotrophin receptor is necessary for NRIF nuclear translocation and apoptosis in sympathetic neurons. Neuron 50, 219–232 [DOI] [PubMed] [Google Scholar]
- 54. Sotthibundhu A., Sykes A. M., Fox B., Underwood C. K., Thangnipon W., Coulson E. J. (2008) β-amyloid1–42 induces neuronal death through the p75 neurotrophin receptor. J. Neurosci. 28, 3941–3946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Coulson E. J., May L. M., Osborne S. L., Reid K., Underwood C. K., Meunier F. A., Bartlett P. F., Sah P. (2008) p75 neurotrophin receptor mediates neuronal cell death by activating GIRK channels through phosphatidylinositol 4,5-bisphosphate. J. Neurosci. 28, 315–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Ibáñez C. F. (1994) Structure-function relationships in the neurotrophin family. J. Neurobiol. 25, 1349–1361 [DOI] [PubMed] [Google Scholar]
- 57. Margadant C., Monsuur H. N., Norman J. C., Sonnenberg A. (2011) Mechanisms of integrin activation and trafficking. Curr. Opin. Cell Biol. 23, 607–614 [DOI] [PubMed] [Google Scholar]
- 58. Liepinsh E., Ilag L. L., Otting G., Ibáñez C. F. (1997) NMR structure of the death domain of the p75 neurotrophin receptor. EMBO J. 16, 4999–5005 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.