Background: MutT proteins hydrolyze oxidatively damaged nucleotides.
Results: Mycobacterium tuberculosis MutT1 and Rv1700 cooperate to detoxify 8-oxo-dGTP to 8-oxo-dGMP and 8-oxo-GTP to 8-oxo-GMP and protect bacteria against oxidative stress.
Conclusion: MtuMutT1 and Rv1700 constitute a novel two-stage mechanism of 8-oxo-dGTP and 8-oxo-GTP detoxification.
Significance: Functional homolog of mycobacterial MutT, whose co-targeting may accentuate the impact of the known antibiotics, has been identified and characterized.
Keywords: DNA Enzymes, Enzyme Kinetics, Genetics, HPLC, Mass Spectrometry (MS), Oxidative Stress, Drug Target, MutT, Mutation Spectrum, Nudix Hydrolases
Abstract
Approximately one third of the world population is infected with Mycobacterium tuberculosis, the causative agent of tuberculosis. A better understanding of the pathogen biology is crucial to develop new tools/strategies to tackle its spread and treatment. In the host macrophages, the pathogen is exposed to reactive oxygen species, known to damage dGTP and GTP to 8-oxo-dGTP and 8-oxo-GTP, respectively. Incorporation of the damaged nucleotides in nucleic acids is detrimental to organisms. MutT proteins, belonging to a class of Nudix hydrolases, hydrolyze 8-oxo-G nucleoside triphosphates/diphosphates to the corresponding nucleoside monophosphates and sanitize the nucleotide pool. Mycobacteria possess several MutT proteins. However, a functional homolog of Escherichia coli MutT has not been identified. Here, we characterized MtuMutT1 and Rv1700 proteins of M. tuberculosis. Unlike other MutT proteins, MtuMutT1 converts 8-oxo-dGTP to 8-oxo-dGDP, and 8-oxo-GTP to 8-oxo-GDP. Rv1700 then converts them to the corresponding nucleoside monophosphates. This observation suggests the presence of a two-stage mechanism of 8-oxo-dGTP/8-oxo-GTP detoxification in mycobacteria. MtuMutT1 converts 8-oxo-dGTP to 8-oxo-dGDP with a Km of ∼50 μm and Vmax of ∼0.9 pmol/min per ng of protein, and Rv1700 converts 8-oxo-dGDP to 8-oxo-dGMP with a Km of ∼9.5 μm and Vmax of ∼0.04 pmol/min per ng of protein. Together, MtuMutT1 and Rv1700 offer maximal rescue to E. coli for its MutT deficiency by decreasing A to C mutations (a hallmark of MutT deficiency). We suggest that the concerted action of MtuMutT1 and Rv1700 plays a crucial role in survival of bacteria against oxidative stress.
Introduction
Mycobacterium tuberculosis causes tuberculosis, which is one of the most feared infectious diseases of humankind (1). The pathogen resides and multiplies within the host macrophages where it is subjected to the reactive oxygen species and reactive nitrogen intermediates released as part of the host's innate immune response (2). Because guanine and cytosine are highly susceptible to damage by reactive oxygen species and reactive nitrogen intermediates, the G+C richness of the M. tuberculosis genome makes it highly prone to these agents.
A common damage that occurs due to oxidative stress is the oxidation of guanine to 7,8-dihydro-8-oxoguanine (8-oxo-G),4 both in DNA and the free nucleotide pool. Although 8-oxo-G in DNA does not block replication of DNA, it induces base mismatches resulting in C to A mutations, and incorporation of 8-oxo-G against A in the template causes A to C mutations. On the other hand, incorporation of 8-oxo-G in RNA leads to mistranslation of proteins, which may lead to cell death (3–8). Like other organisms, mycobacteria possess an elaborate GO repair system consisting of Fpg (MutM), MutY, and MutT enzymes to deal with the oxidative threats to genomic integrity (9–11). MutT is a Nudix hydrolase protein characterized by a 23-amino acid conserved sequence motif named Nudix box, GX5EX7REUXEEXGU, where U is a bulky hydrophobic residue and X is any residue (12). In Escherichia coli, MutT hydrolyzes 8-oxo-dGTP and 8-oxo-dGDP to 8-oxo-dGMP. It also hydrolyzes 8-oxo-GTP and 8-oxo-GDP to 8-oxo-GMP. Thus, the MutT activities prevent genomic mutations and maintain the fidelity of protein synthesis under oxidative stress (13, 14). The Nudix box sequence motif is found in many proteins that hydrolyze nucleoside diphosphates linked to another moiety X, and because these proteins sanitize the nucleotide pool in various organisms, they are also considered housekeeping enzymes (12). In humans, biochemical activities of several Nudix box proteins (NUDTs) have been identified (6, 15–17). NUDT1 (MTH1) converts 8-oxo-dGTP to 8-oxo-dGMP but does not utilize 8-oxo-dGDP as substrate. NUDT15 (MTH2) is capable of hydrolyzing 8-oxo-dGTP and 8-oxo-dGDP to 8-oxo-dGMP although with lower activity. NUDT5 possesses both the ADPRase and 8-oxo-dGDPase activities. The latter activity results in hydrolysis of 8-oxo-dGDP to 8-oxo-dGMP. More recently, NUDT18 (renamed as MTH3) has been identified whose biochemical properties resemble that of NUDT5. However, unlike NUDT5, NUDT18 is more active under physiological conditions and has been proposed to be the major enzyme to sanitize 8-oxo-dGDP, 8-oxo-GDP, and the oxidized forms of ADP (such as 2-hydroxy-dADP and 8-hydroxy-dADP).
Bioinformatics analyses predicted the presence of nine proteins with Nudix hydrolase motif in mycobacteria (18, 19). Of these, four have been annotated as MutT homologs (MutT1, MutT2, MutT3, and MutT4). Here, we report the biochemical characterization and physiological role of MutT1 of M. tuberculosis (MtuMutT1) and another Nudix box protein, Rv1700. Our results suggest that in mycobacteria, the MtuMutT1 and Rv1700 function in concert to detoxify 8-oxo-dGTP to 8-oxo-dGMP and play an important role in supporting cellular growth under oxidative stress.
EXPERIMENTAL PROCEDURES
Plasmids, Bacterial Strains, and Media
Plasmids and bacterial strains used are listed in Table 1. dNTPs were purchased from Jena Bioscience. Unless specified otherwise, E. coli was grown in Luria-Bertani (LB) broth (Difco). Agar (1.5%) was added to the LB broth for growth on solid surface (LB-agar). Culture media were supplemented with ampicillin (Amp, 100 μg/ml), tetracycline (7.5 μg/ml), respectively, as required.
TABLE 1.
List of strains and plasmids
| Strain/plasmid | Details | Reference |
|---|---|---|
| E. coli MG1655 | An E. coli K strain, F− LAM− rph-1 | 31 |
| E. coli JW0097 | ΔmutT790::kan, LAM− rph-1 | 32 |
| E. coli ΔmutT | E. coli MG1655 containing ΔmutT::kan from E. coli JW0097 | This study |
| E. coli CC101 | F', ara-600, Δ(gpt-lac)5, λ−, relA1, spoT1, thiE1, F128 (used to screen for A→C or T→G mutation) | 22 |
| E. coli CC102 | F', ara-600, Δ(gpt-lac)5, λ−, relA1, spoT1, thiE1, F128 (used to screen for C→T or G→A mutation) | 22 |
| E. coli CC103 | F', ara-600, Δ(gpt-lac)5, λ−, relA1, spoT1, thiE1, F128 (used to screen for C→G or G→C mutation) | 22 |
| E. coli CC104 | F', ara-600, Δ(gpt-lac)5, λ−, relA1, spoT1, thiE1, F128 (used to screen for C→A or G→T mutation) | 22 |
| E. coli CC105 | F', ara-600, Δ(gpt-lac)5, λ−, relA1, spoT1, thiE1, F128 (used to screen for A→T or T→A mutation) | 22 |
| E. coli CC106 | F', ara-600, Δ(gpt-lac)5, λ−, relA1, spoT1, thiE1, F128 (used to screen for A→G or T→C mutation) | 22 |
| E. coli CC101–CC106 ΔmutT | E. coli CC101 to CC106 containing ΔmutT::kan allele from JW0097 | This study |
| pBAD-HisB | An expression vector containing arabinose-inducible promoter (ColE1 ori, AmpR) | Invitrogen |
| pTrcNdeHis | A derivative of pTrc99c vector having N-terminal His tag | 30 |
| pET14bMtuMutT1 | pET14b plasmid with MtuMutT1 cloned in its NdeI/ClaI site | This study |
| pBAD-MtuMutT1 | pTrcNdeHis plasmid with MtuMutT1 cloned in its NdeI/EcoRI site (from pET14b MtuMutT1) | This study |
| pACDH | A derivative of pACD plasmid with the ACYC origin of replication | 33 |
| pACDHMtuMutT1 | pACDH plasmid with MtuMutT1cloned in its NcoI/XmnI site (from pET14b MtuMutT1) | This study |
| pTrcRv1700 | pTrcNdeHis plasmid with Rv1700 cloned in its NdeI/HindIII site | This study |
Cloning of MtuMutT1
The open reading frame (ORF) sequence of MtuMutT1 (Rv2985) was retrieved from the M. tuberculosis database and PCR-amplified from M. tuberculosis H37Rv DNA using MtuMutT1 Fp (5′-ggagttcatatgtcgatccagaactcggtc-3′) and MtuMutT1 Rp (5′-atatgatcgattttaggcccgcacgttggcgg-3′) primers containing NdeI and ClaI sites, respectively. PCR was carried out using Dynazyme EXT DNA polymerase (Finnzymes). The reaction was heated to 94 °C for 4 min followed by 30 cycles of incubations at 94 °C for 1 min, 58 °C for 30 s, and 72 °C for 1 min, and then incubated at 72 °C for 10 min. The amplicon (957 bp) was digested with NdeI and ClaI and cloned into similarly digested pET14b (isolated from E. coli JM110 (dam− and dcm− strain)) to obtain pET14bMtuMutT1. The clone was confirmed by HincII digestion and DNA sequence analysis. MtuMutT1 was then subcloned from pET14bMtuMutT1 into pBAD-HisB by partially digesting with NcoI and EcoRI (due to the presence of an internal EcoRI site), taking along the His6 tag from pET14b vector.
Cloning of Rv1700
Rv1700 (ADPRase) ORF was amplified by PCR from M. tuberculosis H37Rv genomic DNA using Rv1700 Fp (5′-cgtcatatggctgagcatgatt-3′) and Rv1700 Rp (5′-tgcaagcttcttcatcgctcg 3′) primers containing NdeI and HindIII sites, respectively. PCR was carried out using the Phusion DNA polymerase by first heating the tube at 98 °C for 4 min followed by 36 cycles of incubations at 98 °C for 1 min, 63 °C for 30 s, and 72 °C for 45 s, and a final incubation of 10 min at 72 °C. The amplicon (637 bp) was eluted, ligated to pJET1.2 vector, and transformed into E. coli TG1 to obtain pJET Rv1700. The pJET Rv1700 was then digested with NdeI and HindIII, and the insert was ligated into similarly digested pTrcNdeHis to generate pTrcRv1700. The pTrcRv1700 was confirmed by restriction digestion with EcoRV and DNA sequencing.
Purification of MtuMutT1
A single colony of E. coli MG1655 ΔmutT/pBADMtuMutT1 was inoculated in 20 ml of LB broth containing Amp and grown for ∼4 h at 37 °C (A600 ∼0.4–0.6). This culture was diluted 1,000-fold to grow 6 liters of culture in LB broth containing Amp at 18 °C. When the culture reached an A600 of ∼0.6, expression of MtuMutT1 was induced with 0.02% arabinose, and the culture was further incubated for 3 h at 18 °C. The cells were harvested, washed, and resuspended in 50 ml of buffer A (20 mm Tris-HCl, pH 8.0, 500 mm NaCl, 10% glycerol (v/v), 2 mm β-mercaptoethanol) containing 20 mm imidazole, ultrasonicated, and centrifuged at 12,000 rpm for 1 h using AF-5004CA rotor in Kubota 3500. The supernatant was then centrifuged at 29,000 rpm for 2 h in (AvantiTM J-30I, JA 30.50 Ti). The supernatant was loaded onto a Ni-NTA column equilibrated with buffer A and eluted with buffer A containing 1 m imidazole. Fractions were analyzed on SDS-PAGE (15%), and those containing MtuMutT1 were pooled and precipitated with 80% ammonium sulfate. The precipitate was redissolved in buffer A, loaded on to Sephadex G-75, and eluted with buffer A. Fractions enriched for MtuMutT1 were pooled, reloaded onto a Ni-NTA column, and eluted with a 20 mm to 1 m gradient of imidazole in buffer B. Fractions containing MtuMutT1 were pooled, concentrated using Centricon (30-kDa cutoff), and dialyzed against 50% glycerol in buffer A at 4 °C.
Purification of Rv1700
The plasmid pTrcRv1700 was transformed into E. coli JW0097ΔmutT. A single colony was inoculated into 50 ml of LB broth (Amp), grown at 37 °C to A600 of ∼0.6, and subcultured into 4 liters of LB broth containing Amp, supplemented with 0.3 mm isopropyl 1-thio-β-d-galactopyranoside when the culture reached an A600 of ∼0.6, and allowed to grow for 2.5 h. The culture was harvested and the cells resuspended into buffer B (20 mm Tris-HCl, pH 8.0, 300 mm NaCl, 10% glycerol (v/v) and 2 mm β-mercaptoethanol) containing 10 mm imidazole. Cells were lysed by ultrasonication, processed for S100 extract preparation and purification from Ni-NTA column as above for MtuMutT1. The fractions containing Rv1700 protein were pooled, concentrated using Centricon tubes (30-kDa cutoff), and dialyzed against 50% glycerol in buffer B at 4 °C.
Activity Assays for MtuMutT1, Rv1700, and EcoMutT
Various NTPs and dNTPs (∼250 μm) were incubated with 100 ng of MtuMutT1, Rv1700, or EcoMutT in a 10-μl reaction containing 1× MutT buffer (20 mm Tris-HCl, pH 8.0, 8 mm MgCl2, 40 mm NaCl, 5 mm DTT, 2% glycerol) for 30 min at 37 °C (for kinetic analysis 8-oxo-dGTP was used as substrate at 12.5–125 μm concentrations, and the reaction time was 10 min). The reactions were stopped with 0.1% SDS and analyzed by HPLC (UltiMate 3000) using a DNAPac column (DNAPac PA200 analytical, 4 × 250 mm) using solvent system consisting of 25 mm Tris-HCl, pH 9.0, and 1 m lithium chloride gradient (0–40%) at a flow rate of 0.5 ml/min, for 25 min. For kinetic analysis of Rv1700, 8-oxo-dGDP was used at a 5–40 μm concentration, and the reactions were terminated after a 15-min incubation. The Km and Vmax were determined from Michaelis-Menten plots.
Mass Spectrometry Analysis
For MALDI-MS analysis of the reaction products, a 5-μl reaction consisting of 10 ng of purified protein (MtuMutT1, EcoMutT, or Rv1700) and 1.5 nmol of substrate (8-oxo-dGTP, 8-oxo-dGDP, or 8-oxo-GTP) was incubated at 37 °C overnight (except for the combined reaction of MtuMutT1 and Rv1700 which was done for 2 h) in a buffer containing 20 mm Tris-HCl, pH 7.5, 8 mm MgCl2, 5 mm DTT, 40 mm NaCl, and 2% glycerol, mixed with equal volume of α-cyanocinnamic acid (10 mg/ml in 50% acetonitrile + 0.1% trifluoroacetic acid) and spotted directly on MALDI plates. Spectra were acquired in positive ion mode using a BrukerDaltonics AutoFlex III SmartBeam mass spectrometer using a 337-nm laser source, and data were analyzed in FlexAnalysis software provided by the manufacturer. Reaction products were identified by comparison with masses of standards.
Survival Analysis
Various plasmid constructs were transformed into E. coli MG1655 and its ΔmutT derivative. Three independent colonies were inoculated in LB broth and grown to A600 of ∼0.6. Cells were harvested and washed with 0.1 mm phosphate-buffered saline, PBS (pH 7.0). The cells were resuspended in PBS at A600 ∼0.2. Different concentrations of H2O2 (0, 1, and 2 mm) were added to the cell suspensions and plated on LB-agar at 0, 1, 2, and 3 h of incubation at 37 °C to determine total viable counts as cfu. The log values of cfu were plotted. For treatment with acidified NaNO2, cells were washed with PBS adjusted to pH 5.5, and experiments were done as described above. NaNO2 concentrations of 0 and 7.5 mm were used.
Analysis of Mutation Spectrum
Various plasmid constructs were introduced into E. coli CC101-106 and their ΔmutT derivatives by transformation. In the case of E. coli CC101-106 strains, isolated colonies were streaked on LB-agar plates containing X-gal (50 μg/ml), and single white colonies were inoculated into 2 ml of LB broth and grown at 37 °C for 14 h. Aliquots (100 μl) of the 10−6 dilution of the cultures were plated on M9 minimal media agar containing 0.2% glucose for viable count determination, and 1-ml cultures were spun down and plated on minimal media agar containing 0.2% lactose to determine Lac+ revertants. Plates were incubated at 37 °C. The number of revertants and viable counts was enumerated after 24 and 72 h, respectively.
RESULTS
Purification and Activity Assays of MtuMutT1
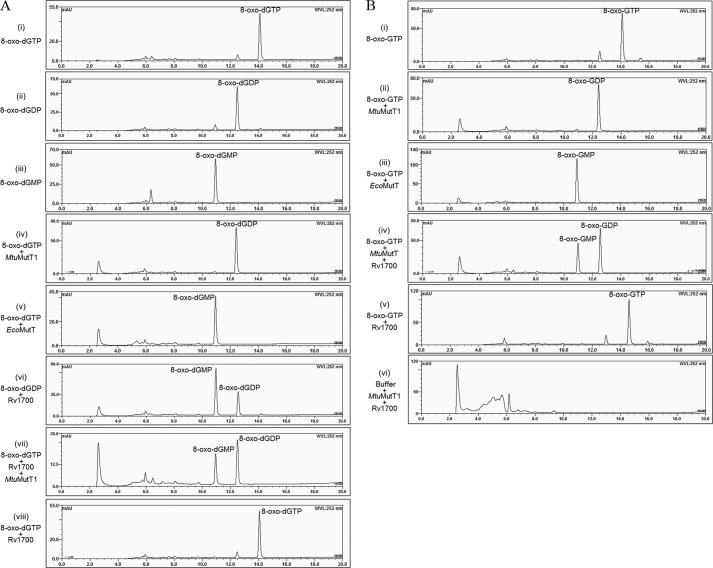
MtuMutT1 ORF was cloned in a T7 RNA polymerase based expression vector, pET14b, and checked for MtuMutT1 expression at different temperatures (37, 30, and 18 °C) as well as with different concentrations (0.3–0.6 mm) of isopropyl 1-thio-β-d-galactopyranoside. In these conditions, MtuMutT1 partitioned into insoluble fraction of the cell lysate. Subsequently, MtuMutT1 ORF was subcloned into pBADHisB, and expression of MtuMutT1 was checked at 37, 30, and 18 °C upon induction with arabinose (0.02%). Expression from the pBADMtuMutT1 at 18 °C yielded MtuMutT1 in S100 supernatant, which was subjected to purification by Ni-NTA and gel filtration chromatography. The gel filtration chromatography profile (Fig. 1A) showed that on the SDS-PAGE, MtuMutT1 migrated as a doublet. A second chromatography of the pooled fractions from gel filtration column on Ni-NTA column still retained the doublet character of MtuMutT1 on SDS-PAGE (Fig. 1B). MALDI-MS analysis showed that both bands represent MtuMutT1, suggesting that the MtuMutT1 was pure. Further, an analysis of the eluted proteins showed redistribution of the protein from each band into the upper and lower bands, suggesting that the doublet character of MtuMutT1 could be due to the presence of a faster migrating minor conformer (supplemental Fig. S1). Biochemical activity of MtuMutT1 was analyzed using dGTP, GTP, dATP, ATP, dCTP, CTP, 8-oxo-dATP, 8-oxo-dGTP, and 8-oxo-GTP. Among these, MtuMutT1 used 8-oxo-dGTP and 8-oxo-GTP as substrates. Unlike EcoMutT, which hydrolyzed 8-oxo-dGTP to 8-oxo-dGMP (Fig. 2A, compare panel v with the standards in panels i–iii), MtuMutT1 hydrolyzed it to 8-oxo-dGDP (compare panel iv with panels i–iii and v). Likewise, the retention time of the product of MtuMutT1 reaction on 8-oxo-GTP suggests that 8-oxo-GTP was also hydrolyzed to the corresponding nucleoside diphosphate (Fig. 2B, compare panel ii with panels i (an 8-oxo-GTP standard) and iii (8-oxo-GMP, a known reaction product of EcoMutT on 8-oxo-GTP)).
FIGURE 1.
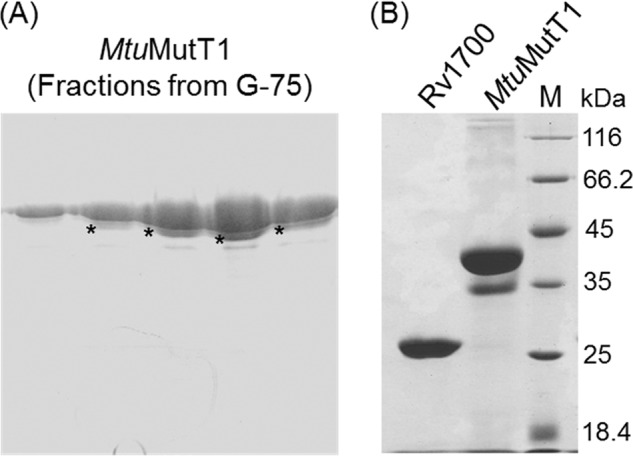
Analysis of N-terminally His-tagged MtuMutT1 and Rv1700 proteins on SDS-PAGE (15%). A, elution profile of MtuMutT1 from gel filtration column (following the first Ni-NTA column chromatography) showing the presence of a fast migrating form of MtuMutT1 indicated by asterisks (*). B, analysis of purified preparation of MtuMutT1 (from second Ni-NTA column, see “Results”) and Rv1700 on SDS-PAGE (15%). Molecular mass markers (in kDa) are as shown. Calculated molecular masses of MtuMutT1with and without a His tag are 36.8 and 34.78 kDa, respectively. Calculated molecular masses of Rv1700 with and without a His tag are 24.9 and 22.86 kDa, respectively.
FIGURE 2.
Activity assays of MtuMutT1, EcoMutT, and Rv1700 proteins on 8-oxo-dGTP/8-oxo-dGDP (A) and 8-oxo-GTP (B). Approximately 250 μm 8-oxo-G nucleotides were incubated with ∼100 ng of MtuMutT1, Rv1700, and EcoMutT, as indicated in a 10-μl reaction in 1×MutT buffer for 30 min at 37 °C. The reactions were stopped with 0.1% SDS and analyzed by HPLC using a DNAPac column with buffer A consisting of 25 mm Tris-HCl, pH 9.0, and 1 m lithium chloride as buffer B. Lithium chloride gradient (0–40%) was set at a flow rate of 0.5 ml/min for 25 min. Various panels are as indicated.
Purification and Activity Assays Using Rv1700
As the products of MtuMutT1-mediated reaction 8-oxo-dGDP and 8-oxo-GDP (from 8-oxo-dGTP and 8-oxo-GTP, respectively) can be converted back to 8-oxo-dGTP and 8-oxo-GTP (for example by nucleoside diphosphate kinase), they would still compromise the fidelity of DNA and RNA synthesis, respectively. Therefore, we explored if 8-oxo-dGDP and 8-oxo-GDP may be further converted to nucleoside monophosphates by another protein. Such Nudix box proteins (NUDT5, NUDT18 (MTH3)) have been described in human (20). Hence, we searched for a homolog of NUDT5/NUDT18 among the Nudix hydrolases in M. tuberculosis and identified Rv1700 as a candidate. The ORF of Rv1700 protein (annotated as ADPRase (21) was cloned into pTrcNdeHis expression vector, and the protein was purified to apparent homogeneity by Ni-NTA chromatography (Fig. 1B). Analysis of biochemical activity of this protein revealed that it indeed converted 8-oxo-dGDP to 8-oxo-dGMP (Fig. 2A, panel vi). In concert with MtuMutT1, it (Rv1700) converted the reaction intermediate of 8-oxo-dGDP to 8-oxo-dGMP (Fig. 2A, panel vii). However, it (Rv1700) did not hydrolyze 8-oxo-dGTP (Fig. 2A, panel viii). Likewise, Rv1700 converted 8-oxo-GDP to 8-oxo-GMP (Fig. 2B, panel iv) but did not act on 8-oxo-GTP (Fig. 2B, panel v). We may add that a peak of retention time ∼2.5 min and a general background in the initial stages of the elution seen in the enzyme reactions corresponded to UV-absorbing contaminants in the proteins plus buffer (Fig. 2B, panel vi). Further, the 8-oxo-dGTP and 8-oxo-GTP preparations contained detectable but minor peaks corresponding to 8-oxo-dGDP and 8-oxo-GDP, respectively. Because the area under these peaks did not change in Fig. 2A (panel viii) or Fig. 2B (panel v), the presence of these minor peaks in these panels was ignored.
Mass Spectrometric Analyses
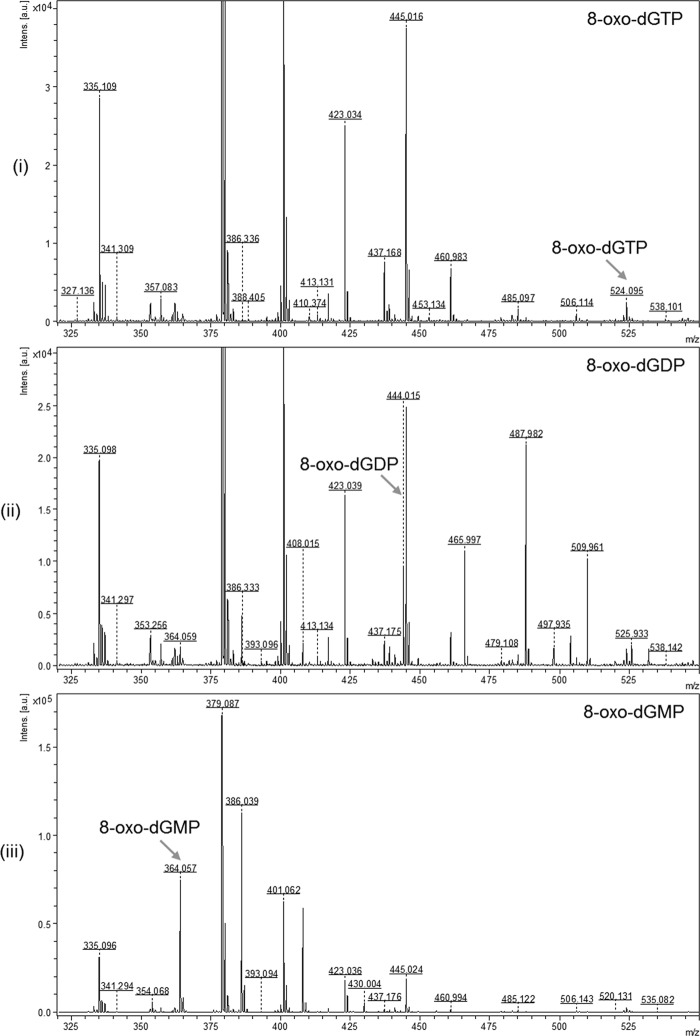
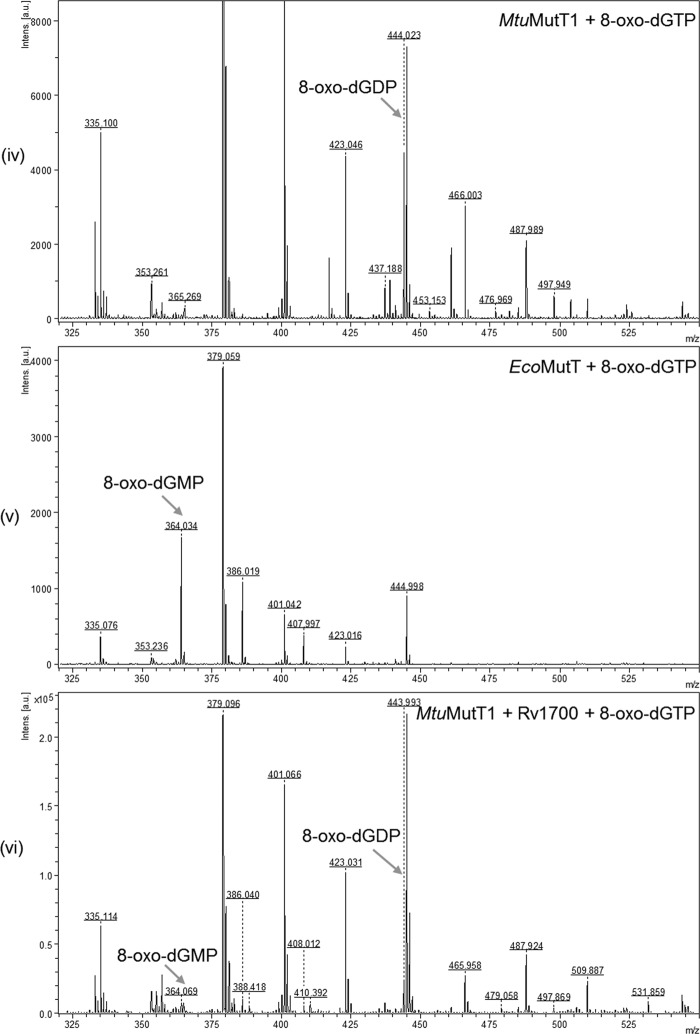
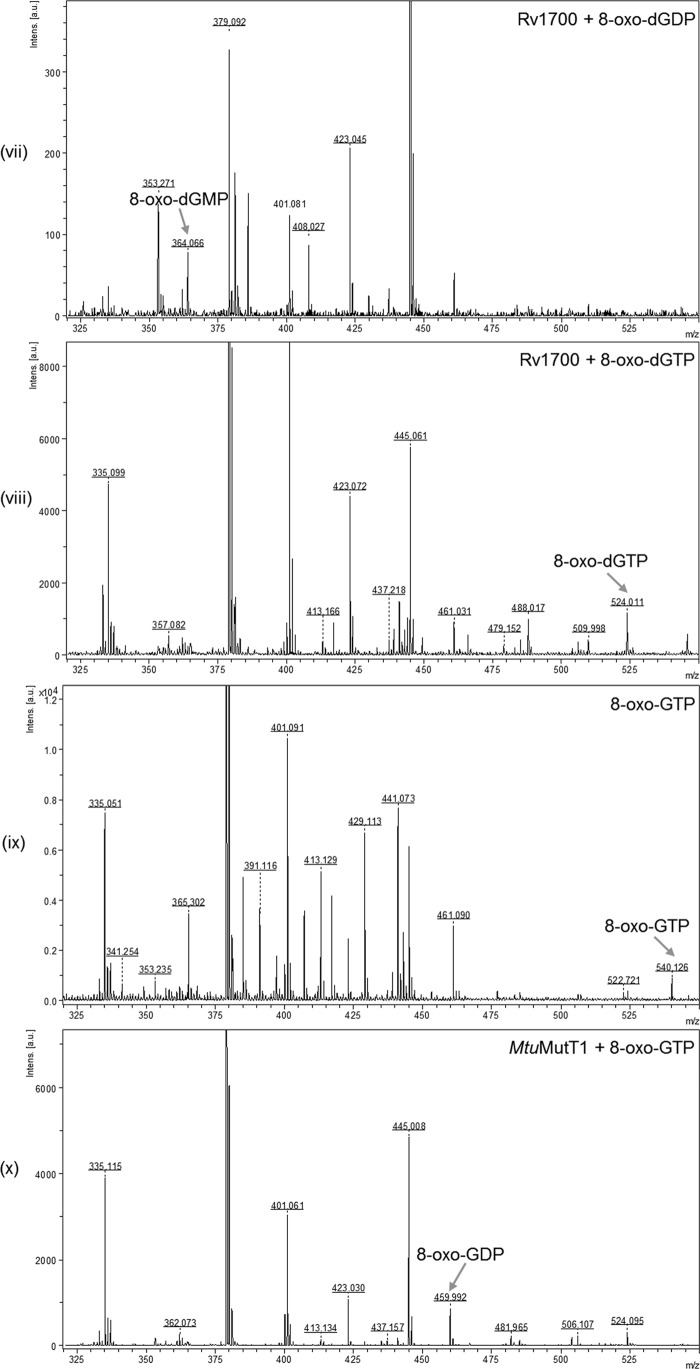
To validate further the nature of the products of 8-oxo-dGTP/8-oxo-GTP treatment with MtuMutT1/Rv1700, we analyzed the reaction products by MALDI-MS. Despite the fact that background peaks are typically present in the lower mass range due to ionization of matrix and low molecular mass impurities, the m/z signals of 524.095, 444.015, and 364.057 corresponding to the characteristic theoretical masses of 523.99, 444.02, and 364.06, respectively, for the standards of 8-oxo-dGTP, 8-oxo-dGDP, and 8-oxo-dGMP were distinctively obtained (Fig. 3, panels i–iii, as indicated by the arrows), making it possible to analyze the reaction products by this method. On treatment with MtuMutT1, 8-oxo-dGTP was converted into 8-oxo-dGDP (m/z peak of 444.023), and no peaks corresponding to 8-oxo-dGMP were seen (Fig. 3, panel iv). As a control, treatment of 8-oxo-dGTP with EcoMutT resulted in its conversion to 8-oxo-dGMP (m/z peak of 364.034) (Fig. 3, panel v). Likewise, the peak corresponding to 8-oxo-dGMP was also seen when 8-oxo-dGTP was treated simultaneously with MtuMutT1 and Rv1700 (m/z peak of 364.069, panel vi) or upon treatment of 8-oxo-dGDP with Rv1700 alone (m/z peak of 364.066, panel vii). Also, as expected, treatment of 8-oxo-dGTP (m/z peak of 524.011, panel viii) with Rv1700 resulted in production of neither 8-oxo-dGDP nor 8-oxo-dGMP. Because the MS/MS analysis of the product peaks agreed with the MS/MS analysis of the standards (supplemental Fig. S2, panels i–vi), the results shown in Fig. 3 (panels i–vii) further validate the observations made in Fig. 2A. Similar analyses of the reaction products of 8-oxo-GTP (theoretical mass 539.99) with MtuMutT1 and Rv1700 were complicated by the presence of matrix peaks (m/z 380.08) corresponding to the theoretical mass of 8-oxo-GMP (380.05) (supplemental Fig. S3). Nonetheless, consistent with the results shown in Fig. 2B, treatment of 8-oxo-GTP with MtuMutT1 resulted in the disappearance of an m/z peak of 540.126 seen in the 8-oxo-GTP standard and the appearance of a peak of m/z 459.992 corresponding to 8-oxo-GDP with a predicted mass of 460.02 (Fig. 3, panels ix and x). However, for the reason mentioned above, and for the lack of standards of 8-oxo-GDP and 8-oxo-GMP, the MS analyses of the reaction products of 8-oxo-GTP were not pursued any further.
FIGURE 3.
Mass spectrometric analysis of the 8-oxo-G nucleotides and the reaction products with MtuMutT1 and/or Rv1700 using MALDI-MS. Panels: i, 8-oxo-dGTP standard; ii, 8-oxo-dGDP standard; iii, 8-oxo-dGMP standard; iv, 8-oxo-dGTP treated with MtuMutT1; v, 8-oxo-dGTP treated with EcoMutT; vi, 8-oxo-dGTP treated with MtuMutT1 and Rv1700; vii, 8-oxo-dGDP treated with Rv1700; viii, 8-oxo-dGTP treated with Rv1700; ix, 8-oxo-GTP standard; and x, 8-oxo-GTP treated with MtuMutT1. The m/z values of the various peaks are as shown. Theoretical masses for singly charged peaks of 8-oxo-dGTP, 8-oxo-dGDP, 8-oxo-dGMP, 8-oxo-GTP, 8-oxo-GDP, and 8-oxo-GMP are 523.99, 444.02, 364.06, 539.99, 460.02, and 380.05 Da.
Analysis of Kinetic Parameters of Substrate Utilization by MtuMutT1 and Rv1700
The kinetics of 8-oxo-dGTP to 8-oxo-dGDP conversion by MtuMutT1 and 8-oxo-dGDP to 8-oxo-dGMP by Rv1700 were determined using HPLC (Fig. 4). MtuMutT1 converted 8-oxo-dGTP to 8-oxo-dGDP with a Km of approximately 50 μm and Vmax of 0.88 pmol/min per ng of protein. Likewise, Rv1700 converted 8-oxo-dGDP to 8-oxo-dGMP with a Km of approximately 9.5 μm and Vmax of 0.04 pmol/min per ng of protein at 37 °C.
FIGURE 4.
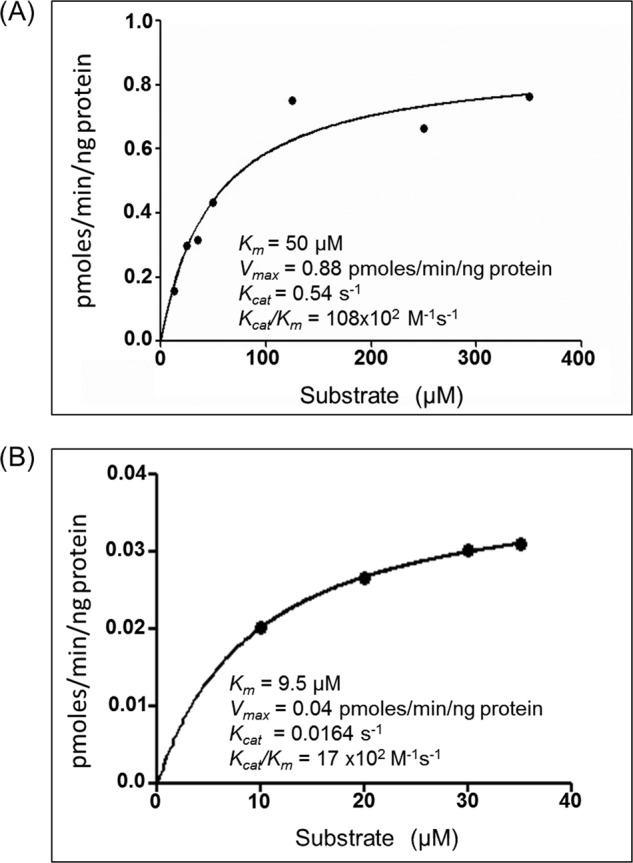
Michaelis-Menten plots of the kinetics of MtuMutT1 on 8-oxo-dGTP (A) and Rv1700 on 8-oxo-dGDP (B). Insets show the kinetic parameters calculated from the plot.
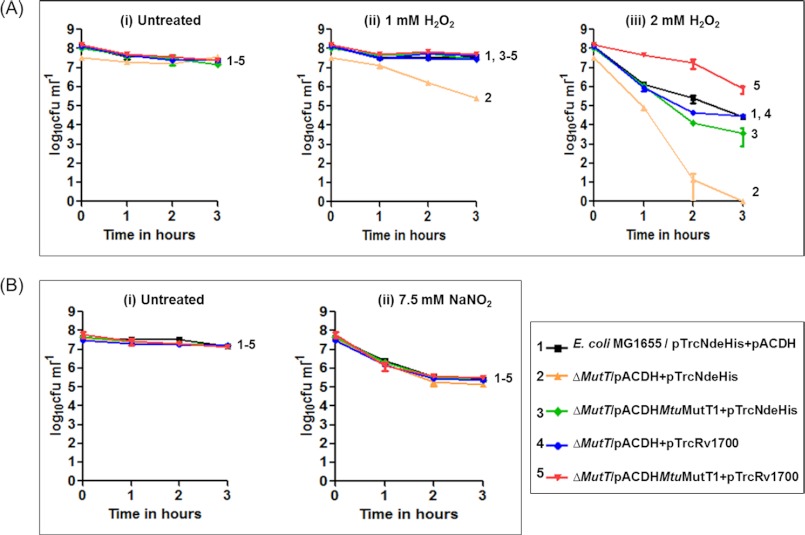
Effect of H2O2 and Acidified NaNO2 on Survival
One of the major sources of oxidative damage to nucleotide pool is reactive oxygen species. Hence, we treated various strains of E. coli with H2O2 to analyze the effect of the oxidative stress and the impact of MtuMutT1/Rv1700 on their survival. As a control (Fig. 5A, panel i), we observed no apparent differences in the survival of the untreated E. coli MG1655, and its ΔmutT derivatives harboring vectors alone (pACDH/pTrcNdeHis) or the expression constructs for the two proteins (pACDHMtuMutT1/pTrcRv1700). Because isopropyl 1-thio-β-d-galactopyranoside was not used, MtuMutT1 and Rv1700 were expressed at low levels (undetectable by Coomassie Blue staining following SDS-PAGE, supplemental Fig. S4). The survival of the ΔmutT strain harboring vectors alone (curve 2) was compromised upon treatment with 1 mm H2O2 and severely compromised upon treatment with 2 mm H2O2 for 3 h (panels ii and iii, respectively). When treated with 1 mm H2O2, the presence of MtuMutT1 and/or Rv1700 rescued strain survival (panel ii). In the experiment with 2 mm H2O2, the simultaneous presence of MtuMutT1 and Rv1700 best rescued the strain survival. In fact, their simultaneous presence conferred tolerance to H2O2 treatment in that the strain harboring the expression constructs for MtuMutT1 and Rv1700 showed better survival than even the parent strain (panel iii, compare curve 5 with curve 1). As shown in Fig. 5B, we did not observe any specific detrimental effect upon treatment of the ΔmutT strain with acidified NaNO2 even at a concentration as high as 7.5 mm. Survival of all strains was equally compromised (panels i and ii), suggesting that MutT deficiency did not cause any specific compromise of survival under the acidified NaNO2, nor did the presence of either or both of the proteins (MtuMutT1 and Rv1700) offer any advantage to the strains under the conditions used.
FIGURE 5.
Survival analysis of E. coli MG1655 and its ΔmutT::kan derivative harboring either vector alone or the expression constructs of MtuMutT1 and/or Rv1700 in the presence of different concentration of H2O2 (A) and acidified NaNO2 (B). Various symbols are as shown in the bottom right panel. Time in hours (x axis) indicates the time of exposure of the DNA-damaging agent prior to determination of viable counts.
Rescue of A to C Mutations in E. coli by MtuMutT1 and Rv1700
E. coli strains have been characterized which possess specific mutations at the active site Glu461 codon (GAG) of the lacZ gene to TAG, GGG, CAG, GCG, GTG, or AAG in strains CC101, CC102, CC103, CC104, CC105, and CC106, respectively, which fail to grow on minimal lactose plates (22). Conditions that result in increase in specific mutations, e.g. A to C (or T to G) in CC101, result in the appearance of isolated colonies on minimal lactose because of increased chances of TAG (CTA) to GAG (CTC) reversion of the lacZ mutation. Introduction of a knock-out allele of mutT (known to result in increase in A to C transversion mutations) in these strains results in a tremendous increase in the number of colonies of E. coli CC101 (but not in the case of other strains) coming up on minimal lactose medium. Plating of E. coli CC101ΔmutT on minimal lactose agar, therefore, allows one to investigate whether MtuMutT1 and Rv1700 decrease the frequency of appearance of these colonies on minimal lactose plates. As shown in Fig. 6, E. coli CC101ΔmutT resulted in 2905/108 colonies on minimal lactose plates (bar 2). Although there was a decrease in the frequency of colonies appearing in the presence of either MtuMutT1 or Rv1700 (bars 3 and 4), the best rescue was observed by their simultaneous presence (bar 5). As a control, when we checked for the impact of deletion of mutT (ΔmutT) in CC102-106 strains, there were no detectable increases in reversion of the mutant lacZ (data not shown).
FIGURE 6.
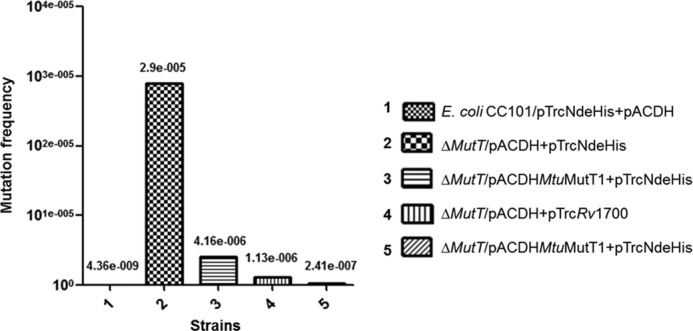
Mutation frequency analysis with respect to A to C (or T to G) using E. coli CC101 strain and its ΔmutT derivative harboring vector alone or the expression constructs of MtuMutT1 and/or Rv1700 by scoring for Lac+ revertants on minimal media containing lactose as carbon source. Mutation frequency was calculated by dividing the number of Lac+ revertants with the number of bacteria (colonies) on minimal glucose plate. The mean ± SD of (n = 9) independent colonies were calculated (shown above the bars) and plotted relative to E. coli CC101.
DISCUSSION
Recent studies in E. coli have highlighted the importance of MutT proteins in protection against oxidative stress leading to generation of oxidatively damaged forms of guanine in dGTP and GTP. In fact, treatment of bacteria even with antibiotics whose primary targets are known to be other essential cellular processes may cause toxicity via oxidative damage to GTP/dGTP and their misincorporation in DNA and RNA. Interestingly, MutT overexpression was found to rescue the DNA-damaging effects of the antibiotics such as β-lactams and quinolones (23). Thus, inhibiting MutT proteins in bacteria may provide with a strategy to accentuate the impact of the known antibiotics.
M. tuberculosis, the causative agent of tuberculosis, is a highly successful pathogen, which resides latently in nearly one third of the human population (1). Appearance of multidrug-resistant, extremely drug-resistant, and more recently of totally drug-resistant strains of M. tuberculosis has made the management of tuberculosis a serious health concern (24). The primary site of infection of M. tuberculosis is the alveolar macrophages (25). As a part of host's innate immune response, the macrophages respond by producing reactive oxygen species and reactive nitrogen intermediates (2). The oxidative damage causes accumulation of damaged guanine nucleotides such as 8-oxo-dGTP and 8-oxo-GTP. As the 8-oxo-dGTP is a potent mutagen (because of its ambiguous base pairing properties), the MutT proteins are likely to be crucial for M. tuberculosis survival in the host. As shown in Fig. 5, MutT deficiency in E. coli results in high susceptibility to oxidative stress; also, in mice, MutT deficiency has been reported to result in increased occurrence of tumors of various organs (26).
A functional homolog of E. coli MutT had not been characterized so far from M. tuberculosis. MtuMutT2 converts 8-oxo-dGTP to 8-oxo-GMP (27). However, it is known to be a better dCTPase (18). Moreover, it is kinetically very inefficient both as 8-oxo-dGTPase and dCTPase, and it does not rescue E. coli for its deficiency of MutT activity (27). Therefore, it appears that MtuMutT2 may not be a major player in vivo, at least not as an 8-oxo-dGTPase. Genetic studies have also shown that the MutT2 deficiency in M. tuberculosis as well as in Mycobacterium smegmatis causes an insignificant increase of ∼1.5-fold in mutation frequency, as assessed by appearance of RifR colonies (28).
In this study, we have shown that MtuMutT1 functions as an efficient 8-oxo-dGTPase. However, it has a novel activity of converting 8-oxo-dGTP to 8-oxo-dGDP. MutT proteins are commonly known to convert either a nucleoside triphosphate or nucleoside diphosphate to nucleoside monophosphate (see the Introduction). To convert 8-oxo-dGDP, the product of MtuMutT1, to 8-oxo-dGMP, the activity of another Nudix box hydrolase, Rv1700, is required (Figs. 2 and 3). Together, these two proteins provide maximum protection to the MutT-deficient E. coli against oxidative damage both for its survival as well as prevention of A to C mutations (Figs. 5 and 6). Rv1700 has earlier been shown to possess ADPRase activity (21). Our studies show that this protein has additional activity of converting 8-oxo-dGDP and 8-oxo-GDP to 8-oxo-dGMP and 8-oxo-GMP, respectively. It should also be said that our observations do not rule out the possibility of some of the uncharacterized MutT (MtuMutT3, MtuMutT4) or other Nudix box proteins carrying out the function of efficiently hydrolyzing 8-oxo-dGTP and 8-oxo-GTP directly to their corresponding monophosphates. However, the fact that of the four MutT proteins described in mycobacteria, deficiency of MutT1 has the maximum mutator phenotype of approximately 15-fold in mycobacteria (28) suggests that MutT1 carries out the major MutT function. Thus, it appears that even in M. tuberculosis cells, MutT1 initiates the major function of detoxifying oxidatively damaged guanine nucleoside triphosphates. Both MtuMutT1 and Rv1700 proteins are highly conserved among mycobacteria (supplemental Figs. S5 and S6). However, it is unclear what advantage such a two-stage mechanism of detoxifying oxidatively damaged 8-oxo-dGTP/8-oxo-GTP may offer. Based on the sequence comparisons, we identified that the mycobacterial MutT1 proteins have two domains. Of these, the N-terminal Nudix hydrolase domain corresponds to the single-domain E. coli MutT (29) whereas the C-terminal domain corresponds to a phosphatase domain belonging to the histidine phosphatase superfamily proteins (30). We are currently in the process of understanding the three-dimensional structure of M. smegmatis MutT1 using x-ray crystallography. Insights from these studies may provide us with a better understanding of the substrate specificity of mycobacterial MutT1.
Supplementary Material
Acknowledgments
We thank our laboratory colleagues for suggestions on the manuscript.
This work was supported by grants from the Department of Biotechnology, New Delhi, and the Council of Scientific and Industrial Research, New Delhi.

This article contains supplemental Figs. S1–S6.
- 8-oxo-G
- 7,8-dihydro-8-oxoguanine
- Amp
- ampicillin
- LB
- Luria-Bertani
- EcoMutT
- MutT of E. coli
- MtuMutT1
- MutT1 of M. tuberculosis
- Ni-NTA
- nickel-nitrilotriacetic acid
- NUDT
- Nudix box protein.
REFERENCES
- 1. Dye C., Scheele S., Dolin P., Pathania V., Raviglione M. C. (1999) Consensus statement. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. WHO Global Surveillance and Monitoring Project. JAMA 282, 677–686 [DOI] [PubMed] [Google Scholar]
- 2. Ehrt S., Schnappinger D. (2009) Mycobacterial survival strategies in the phagosome: defence against host stresses. Cell. Microbiol. 11, 1170–1178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fraga C. G., Shigenaga M. K., Park J. W., Degan P., Ames B. N. (1990) Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc. Natl. Acad. Sci. U.S.A. 87, 4533–4537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. David S. S., O'Shea V. L., Kundu S. (2007) Base-excision repair of oxidative DNA damage. Nature 447, 941–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsuzuki T., Egashira A., Kura S. (2001) Analysis of MTH1 gene function in mice with targeted mutagenesis. Mutat. Res. 477, 71–78 [DOI] [PubMed] [Google Scholar]
- 6. Yoshimura D., Sakumi K., Ohno M., Sakai Y., Furuichi M., Iwai S., Nakabeppu Y. (2003) An oxidized purine nucleoside triphosphatase, MTH1, suppresses cell death caused by oxidative stress. J. Biol. Chem. 278, 37965–37973 [DOI] [PubMed] [Google Scholar]
- 7. Sakumi K., Furuichi M., Tsuzuki T., Kakuma T., Kawabata S., Maki H., Sekiguchi M. (1993) Cloning and expression of cDNA for a human enzyme that hydrolyzes 8-oxo-dGTP, a mutagenic substrate for DNA synthesis. J. Biol. Chem. 268, 23524–23530 [PubMed] [Google Scholar]
- 8. Sakamoto K., Tominaga Y., Yamauchi K., Nakatsu Y., Sakumi K., Yoshiyama K., Egashira A., Kura S., Yao T., Tsuneyoshi M., Maki H., Nakabeppu Y., Tsuzuki T. (2007) MUTYH-null mice are susceptible to spontaneous and oxidative stress-induced intestinal tumorigenesis. Cancer Res. 67, 6599–6604 [DOI] [PubMed] [Google Scholar]
- 9. Kurthkoti K., Srinath T., Kumar P., Malshetty V. S., Sang P. B., Jain R., Manjunath R., Varshney U. (2010) A distinct physiological role of MutY in mutation prevention in mycobacteria. Microbiology 156, 88–93 [DOI] [PubMed] [Google Scholar]
- 10. Kurthkoti K., Kumar P., Jain R., Varshney U. (2008) Important role of the nucleotide excision repair pathway in Mycobacterium smegmatis in conferring protection against commonly encountered DNA-damaging agents. Microbiology 154, 2776–2785 [DOI] [PubMed] [Google Scholar]
- 11. Jain R., Kumar P., Varshney U. (2007) A distinct role of formamidopyrimidine DNA glycosylase (MutM) in down-regulation of accumulation of G, C mutations and protection against oxidative stress in mycobacteria. DNA Repair 6, 1774–1785 [DOI] [PubMed] [Google Scholar]
- 12. McLennan A. G. (2006) The Nudix hydrolase superfamily. Cell Mol. Life Sci. 63, 123–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maki H., Sekiguchi M. (1992) MutT protein specifically hydrolyses a potent mutagenic substrate for DNA synthesis. Nature 355, 273–275 [DOI] [PubMed] [Google Scholar]
- 14. Akiyama M., Maki H., Sekiguchi M., Horiuchi T. (1989) A specific role of MutT protein: to prevent dG·dA mispairing in DNA replication. Proc. Natl. Acad. Sci. U.S.A. 86, 3949–3952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mishima M., Sakai Y., Itoh N., Kamiya H., Furuichi M., Takahashi M., Yamagata Y., Iwai S., Nakabeppu Y., Shirakawa M. (2004) Structure of human MTH1, a Nudix family hydrolase that selectively degrades oxidized purine nucleoside triphosphates. J. Biol. Chem. 279, 33806–33815 [DOI] [PubMed] [Google Scholar]
- 16. Kakuma T., Nishida J., Tsuzuki T., Sekiguchi M. (1995) Mouse MTH1 protein with 8-oxo-7,8-dihydro-2′-deoxyguanosine 5′-triphosphatase activity that prevents transversion mutation: cDNA cloning and tissue distribution. J. Biol. Chem. 270, 25942–25948 [DOI] [PubMed] [Google Scholar]
- 17. Egashira A., Yamauchi K., Tsuzuki T. (2001) [MTH1 functions in the prevention of the spontaneous mutations]. Tanpakushitsu Kakusan Koso 46, 1162–1170 [PubMed] [Google Scholar]
- 18. Moreland N. J., Charlier C., Dingley A. J., Baker E. N., Lott J. S. (2009) Making sense of a missense mutation: characterization of MutT2, a Nudix hydrolase from Mycobacterium tuberculosis, and the G58R mutant encoded in W-Beijing strains of M. tuberculosis. Biochemistry 48, 699–708 [DOI] [PubMed] [Google Scholar]
- 19. Cole S. T., Brosch R., Parkhill J., Garnier T., Churcher C., Harris D., Gordon S. V., Eiglmeier K., Gas S., Barry C. E., 3rd, Tekaia F., Badcock K., Basham D., Brown D., Chillingworth T., Connor R., Davies R., Devlin K., Feltwell T., Gentles S., Hamlin N., Holroyd S., Hornsby T., Jagels K., Krogh A., McLean J., Moule S., Murphy L., Oliver K., Osborne J., Quail M. A., Rajandream M. A., Rogers J., Rutter S., Seeger K., Skelton J., Squares R., Squares S., Sulston J. E., Taylor K., Whitehead S., Barrell B. G. (1998) Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393, 537–544 [DOI] [PubMed] [Google Scholar]
- 20. Takagi Y., Setoyama D., Ito R., Kamiya H., Yamagata Y., Sekiguchi M. (2012) Human MTH3 (NUDT18) protein hydrolyzes oxidized forms of guanosine and deoxyguanosine diphosphates: comparison with MTH1 and MTH2. J. Biol. Chem. 287, 21541–21549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang L. W., Gabelli S. B., Cunningham J. E., O'Handley S. F., Amzel L. M. (2003) Structure and mechanism of MT-ADPRase, a Nudix hydrolase from Mycobacterium tuberculosis. Structure 11, 1015–1023 [DOI] [PubMed] [Google Scholar]
- 22. Cupples C. G., Miller J. H. (1989) A set of lacZ mutations in Escherichia coli that allow rapid detection of each of the six base substitutions. Proc. Natl. Acad. Sci. U.S.A. 86, 5345–5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Foti J. J., Devadoss B., Winkler J. A., Collins J. J., Walker G. C. (2012) Oxidation of the guanine nucleotide pool underlies cell death by bactericidal antibiotics. Science 336, 315–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Velayati A. A., Masjedi M. R., Farnia P., Tabarsi P., Ghanavi J., Ziazarifi A. H., Hoffner S. E. (2009) Emergence of new forms of totally drug-resistant tuberculosis bacilli: super extensively drug-resistant tuberculosis or totally drug-resistant strains in Iran. Chest 136, 420–425 [DOI] [PubMed] [Google Scholar]
- 25. González-Juarrero M., O'Sullivan M. P. (2011) Optimization of inhaled therapies for tuberculosis: the role of macrophages and dendritic cells. Tuberculosis 91, 86–92 [DOI] [PubMed] [Google Scholar]
- 26. Tsuzuki T., Nakatsu Y., Nakabeppu Y. (2007) Significance of error-avoiding mechanisms for oxidative DNA damage in carcinogenesis. Cancer Sci. 98, 465–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sang P. B., Varshney U. (2013) Biochemical properties of MutT2 proteins from Mycobacterium tuberculosis and M. smegmatis and their contrasting antimutator roles in Escherichia coli. J. Bacteriol. 195, 1552–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dos Vultos T., Blázquez J., Rauzier J., Matic I., Gicquel B. (2006) Identification of Nudix hydrolase family members with an antimutator role in Mycobacterium tuberculosis and Mycobacterium smegmatis. J. Bacteriol. 188, 3159–3161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Nakamura T., Meshitsuka S., Kitagawa S., Abe N., Yamada J., Ishino T., Nakano H., Tsuzuki T., Doi T., Kobayashi Y., Fujii S., Sekiguchi M., Yamagata Y. (2010) Structural and dynamic features of the MutT protein in the recognition of nucleotides with the mutagenic 8-oxoguanine base. J. Biol. Chem. 285, 444–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arif S. M., Patil A. G., Varshney U., Vijayan M. (2012) Crystallization and preliminary x-ray studies of MutT1 (MSMEG_2390) from Mycobacterium smegmatis. Acta Crystallogr. Sect. F Struct. Biol. Cryst. Commun. 68, 1214–1216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Blattner F. R., Plunkett G., 3rd, Bloch C. A., Perna N. T., Burland V., Riley M., Collado-Vides J., Glasner J. D., Rode C. K., Mayhew G. F., Gregor J., Davis N. W., Kirkpatrick H. A., Goeden M. A., Rose D. J., Mau B., Shao Y. (1997) The complete genome sequence of Escherichia coli K-12. Science 277, 1453–1462 [DOI] [PubMed] [Google Scholar]
- 32. Baba T., Ara T., Hasegawa M., Takai Y., Okumura Y., Baba M., Datsenko K. A., Tomita M., Wanner B. L., Mori H. (2006) Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2, 2006 0008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Rao A. R., Varshney U. (2001) Specific interaction between the ribosome recycling factor and the elongation factor G from Mycobacterium tuberculosis mediates peptidyl-tRNA release and ribosome recycling in Escherichia coli. EMBO J. 20, 2977–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.