Background: Loss of keratins 8 and 18 (K8/18) is a hallmark of epithelial-mesenchymal transition (EMT), but its role in tumor progression is unclear.
Results: Epithelial cancer cells depleted in K8/18 are more invasive and sensitive to cisplatin through the up-regulation of claudin1.
Conclusion: K8/18 loss promotes collective cancer cell migration without inducing EMT.
Significance: Cancer cell invasion can arise from K8/18 loss independently of EMT.
Keywords: Akt, Apoptosis, Cancer Biology, Cell Invasion, Cell Migration, Epithelial-Mesenchymal Transition, NF-κB (NF-KB), Phosphatidylinositol 3-Kinase, Claudin1, Keratins
Abstract
Keratins 8 and 18 (K8/18) are simple epithelial cell-specific intermediate filament proteins. Keratins are essential for tissue integrity and are involved in intracellular signaling pathways that regulate cell response to injuries, cell growth, and death. K8/18 expression is maintained during tumorigenesis; hence, they are used as a diagnostic marker in tumor pathology. In recent years, studies have provided evidence that keratins should be considered not only as markers but also as regulators of cancer cell signaling. The loss of K8/18 expression during epithelial-mesenchymal transition (EMT) is associated with metastasis and chemoresistance. In the present study, we investigated whether K8/18 expression plays an active role in EMT. We show that K8/18 stable knockdown using shRNA increased collective migration and invasiveness of epithelial cancer cells without modulating EMT markers. K8/18-depleted cells showed PI3K/Akt/NF-κB hyperactivation and increased MMP2 and MMP9 expression. K8/18 deletion also increased cisplatin-induced apoptosis. Increased Fas receptor membrane targeting suggests that apoptosis is enhanced via the extrinsic pathway. Interestingly, we identified the tight junction protein claudin1 as a regulator of these processes. This is the first indication that modulation of K8/18 expression can influence the phenotype of epithelial cancer cells at a transcriptional level and supports the hypothesis that keratins play an active role in cancer progression.
Introduction
Metastasis, the foremost cause of mortality in cancer patients, is a coordinated biological process involving multiple intracellular signaling pathways. In the early steps of metastasis, carcinoma cells undergo a “dedifferentiation” process, also called “epithelial-mesenchymal transition” (EMT),3 which is characterized by loss of cell polarity, alteration of cell junctions, and reorganization of cytoskeletal components. Activation of several transcription factors also promotes the degradation of extracellular matrix through expression of metalloproteinases (MMPs) and increases resistance to apoptosis. All together these changes enhance invasiveness and chemoresistance, making the EMT process a hallmark of tumor progression (1). Among all these phenotypic changes, the reduction or loss of keratin proteins is often considered as a histological and biochemical feature for tumor cells that are going through an EMT (2). Keratins are epithelial cell-specific intermediate filament (IF) proteins that are expressed in a tissue- and differentiation state-specific manner. Keratins 8 and 18 (K8/18) are typically co-expressed as the primary keratin pair in simple epithelial cells, and their expression is maintained during malignant transformation until the tumor becomes invasive. Their specific re-expression in tissue metastasis explains their use as a diagnostic marker in determining tumor origin (3). As part of the cytoskeleton, keratins are important for the mechanical stability and integrity of epithelial cells and tissues. Keratin filaments form a complex network that extends from the periphery of the nucleus to the plasma membrane where they associate with desmosomes and hemidesmosomes. IF proteins are not only structural but are now also considered as regulatory proteins (for a review, see Ref. 4). For instance, they regulate organelle positioning and protein targeting (5). Moreover, a number of keratins are involved in intracellular signaling pathways that regulate response to injuries (6, 7), protein synthesis (8, 9), the cell cycle (8, 10–12), cell death (13–16), and cancer progression (17–19). Thus, a concept emerges from these different studies that IFs should not be considered only as marker proteins in tumor cells but also as regulators of cancer cell signaling. For instance, our previous work highlighted a link between the oncogenic Akt isoforms and IF protein expression (20), and the PI3K/Akt pathway has been shown to play a major role in tumorigenesis, chemoresistance, and EMT (21–24). Class I PI3Ks are heterodimeric proteins composed of a regulatory subunit (p85) and a catalytic subunit (p110). Activated PI3K phosphorylates phosphoinositides at position 3 of the inositol ring to generate the lipid second messenger phosphatidylinositol 3,4,5-trisphosphate (PIP3), which promotes Akt activation (25). This process is antagonized by the tumor suppressor phosphatase and tensin homologue PTEN that hydrolyzes the PIP3 to generate phosphatidylinositol 4,5-bisphosphate (PIP2) (26). In epithelial polarized cells, PIP3 is specifically localized at the basolateral plasma membrane (27), whereas PTEN localizes to the apical plasma membrane where it enhances PIP2 levels (28). Considering that the leading edge of migrating cells has a composition similar to that of the basolateral surface of epithelia, PI3K/PTEN have been proposed to maintain the phosphoinositide gradient that establishes front-to-back polarity in invasive cancer cells (29, 30).
In the present study, we investigated the role of K8/18 loss in EMT. Because we have previously shown that Akt isoforms induce reorganization of the IF network (20), we analyzed the role of the K8/18 network in PI3K/Akt signaling in the context of cell motility, invasion, and cisplatin-induced apoptosis.
EXPERIMENTAL PROCEDURES
Cell Culture, Transfection, and Reagents
Human endometrial carcinoma KLE, human hepatocellular carcinoma HepG2, and human cervical carcinoma HeLa cell lines were purchased from American Type Culture Collection (Manassas, VA). Cells were maintained in DMEM (without HEPES for KLE) supplemented with 10% FBS (KLE and HepG2) or 2% bovine growth serum (HeLa) (Thermo Fisher Scientific, Waltham, MA) and 50 μg/ml gentamycin (Sigma). K8/18 stable knockdown was achieved using SureSilencing predesigned short hairpin RNA (shRNA) sequences against keratin 8, keratin 18, or scrambled negative control (NC) (Qiagen, Mississauga, Ontario, Canada). The cells were transfected with FuGENE 6 according to the manufacturer's protocol (Promega, Madison, WI), and G418 was applied for isolating resistant clones (Invivogen, San Diego, CA). Transient gene silencing was performed using 100 nm scrambled NC small interfering RNA (siRNA), claudin1 siRNA (oligo1 ID, 138564; oligo2 ID, 29051; Ambion, Invitrogen), or NF-κBp65 siRNA (Cell Signaling Technology, Danvers, MA) delivered in cells using TransIT-TKO reagent following the supplier's instructions (Mirus Bio LLC, Madison, WI). Transient transfection of claudin1 full-length cDNA was carried out using the pCMV-SPORT6 vector (Thermo Fisher Scientific). Modulation of the PI3K/Akt signaling pathway was done with 200 ng/ml recombinant IGF-1 (Sigma), 5–50 μm LY294002, or 0.2–1 μm wortmannin (Cell Signaling Technology). Cisplatin was purchased from Sigma. Cell proliferation was assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay as described (31) using Cell Proliferation kit I (Roche Diagnostics).
Subcellular Fractionation
The Subcellular Protein Fractionation kit (Thermo Fisher Scientific) was used according to the manufacturer's instructions.
Immunoblotting and Immunoprecipitation
Cell lysis, electrophoresis, and Western blotting were performed as described previously (31). All of the antibodies were from Cell Signaling Technology except for K8 (Troma1, Developmental Studies Hybridoma Bank, University of Iowa); K18 (L2A1) (32), which was a generous gift from Dr. M. Bishr Omary (University of Michigan); Akt3 (Millipore, Billerica, MA); horseradish peroxidase (HRP)-conjugated β-actin (Sigma); β-tubulin (Abcam Inc., Cambridge, MA); and HRP-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories, West Grove, PA). Akt isoforms, PI3Kp85, and claudin1 were immunoprecipitated following a procedure described previously (33).
Immunofluorescence
Cells were fixed with 4% paraformaldehyde and prepared as described (31) using rhodamine phalloidin conjugate (Invitrogen), mouse monoclonal anti-β-tubulin clone TUB2.1 (Sigma), rabbit monoclonal anti-NF-κBp65 (D14E12) (Cell Signaling Technology), rabbit anti-claudin1 antibody (Invitrogen), mouse monoclonal anti-phospho-Akt (Ser-473) clone 6F5 (Millipore), or isotypic control IgG (Santa Cruz Biotechnology). Cells were viewed under a Carl Zeiss Axio Observer Z1 microscope. Normalization of the claudin1 staining at the leading edge was performed as described previously (34). Fluorescence intensities of every pixel within three individual lines drawn across the cell membrane were divided by their average cytoplasmic fluorescence intensities using ImageJ software.
Wound Healing Assay
To evaluate cell motility, cells were grown to near confluence, and a wound was created with the blunt end of a tip. Images were captured at fixed time points between 0 and 48 h postwounding, and the pictures were analyzed using ImageJ software. The results were plotted as a percentage of wound closure compared with the area of the initial wound. Each experiment was performed in duplicates and repeated three times.
Matrigel Invasion Assay
Invasive properties of cells were measured using 2 mg/ml Matrigel-coated Transwell inserts (BD Biosciences). Invasive cells that had adhered to the porous insert were fixed in methanol, and nuclear staining was performed with Hoechst dye. Total fluorescence of each insert was quantified by densitometric analysis. Each experiment was performed in duplicates and repeated three times.
PI3K Assay
PI3K activity was assessed using a commercially available phosphatidylinositol 3-kinase ELISA kit (K-1000s, Echelon Biosciences, Salt Lake City, UT). The cell lysates were precleared, immunoprecipitated with PI3Kp85 antibody, and incubated with dioctanoyl-PIP2 substrate and reaction buffer. The amount of PIP3 formed from PIP2 by PI3K activity was detected using a competitive ELISA. Absorbance of the samples was measured at 450 nm, and PIP3 was quantified by comparison with a PIP3 standard curve carried out in parallel with the experimental samples and plotted on a log scale.
Conventional RT-PCR
Total RNA extraction, cDNA synthesis, and PCRs were performed as described previously (31). The conditions for PCRs are listed in Table 1. PCR products were analyzed by electrophoresis on 1–2% agarose gels in Tris borate-EDTA buffer.
TABLE 1.
Primers for conventional PCR amplification
| Gene | Forward primer | Reverse primer | Tm (°C) | Cycles | Product size |
|---|---|---|---|---|---|
| bp | |||||
| KRT19 | tttgagacggaacaggctct | gccatgacctcatattggct | 58 | 35 | 275 |
| KRT7 | caggatgtggtggaggactt | ttgctcatgtaggcagcatc | 58 | 35 | 116 |
| β-Actin | cctccctggagaagagcta | acgtcacacttcatgatgga | 60 | 25 | 348 |
| 18 S rRNA | tggtcgctcgctcctctccc | cagcgcccgtcggcatgtat | 60 | 25 | 70 |
| CLDN1 site a | tagtatccagactccagcgc | cgagaatgaagcccaacagc | 61 | 40 | 244 |
| CLDN1 site b | gtgagccgccctgaaacc | ggcgctggagtctggatac | 61 | 40 | 150 |
| COX2 | ggcaaagactgcgaagaaga | gggtaggctttgctgtctga | 58 | 40 | 426 |
| GAPDH | tactagcggttttacgggcg | tcgaacaggaggagcagagagcga | 59 | 40 | 166 |
Quantitative Real Time RT-PCR
RNA was extracted using an RNeasy Mini kit (Qiagen). Total RNA (1 μg) was subjected to reverse transcription using qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD). All samples were subjected to real time PCR analysis with SYBR Green Brilliant III Master Mix using an Mx3000P system (Agilent Technologies, Mississauga, Ontario, Canada). For each gene target, a standard curve was generated to determine the efficiency of the reaction, and the Pfaffl analysis method was used to measure the relative quantity of gene expression (35). Each real time PCR was performed in duplicates from at least three independent experiments. Human CLDN1 was amplified using sense primer 5′-ccctatgaccccagtcaatg-3′ and antisense primer 5′-acctcccagaaggcagaga-3′. For MMPs, expression was determined using sense primer 5′-atgccgcctttaactggag-3′ and antisense primer 5′-aagaagtagctgtgaccgcc-3′ for MMP2 and sense primer 5′-gcactgcaggatgtcatagg-3′ and antisense primer 5′-acgacgtcttccagtaccga-3′ for MMP9. 18 S rRNA was used as a reference gene based on its stable expression in all cell clones.
Chromatin immunoprecipitation (ChIP) assay
The ChIP assay was done using an EZ-ChIP kit (Millipore) according to the manufacturer's protocol. One-tenth of sonicated (10 × 12-s pulse) and precleared chromatin from 2.5 × 107 HepG2 shK8/18 or HeLa cells was used for each ChIP. Immunoprecipitations were performed at 4 °C overnight with 1 μg of NF-κBp65 antibody (Cell Signaling Technology), RNA polymerase II antibody (Millipore), or normal IgG (Santa Cruz Biotechnology). The supernatant from the above reaction lacking the primary antibody was saved as total input of chromatin and processed in the same way as the eluted immunoprecipitates, starting with the cross-linking reversal step. Inputs and immunoprecipitates were amplified using CLDN1 promoter-specific primers under conditions listed in Table 1. COX2 and GAPDH promoter-specific primers served as positive and negative controls, respectively (Table 1). PCR products were analyzed by electrophoresis on 2% agarose gels in Tris borate-EDTA buffer.
Luciferase Reporter Assay
Cells were transfected with NF-κB-Luc reporter plasmid (pGL4.32) and TK-hRLuc (pGL4.74) in a 10:1 ratio. After 24 h, the cells were transfected with NC or claudin1 siRNA for 24 h followed by the Dual-Luciferase reporter assay (Promega). Each experiment was repeated three times.
Determination of Apoptosis Level
The induction of apoptosis was determined by counting the apoptotic cells (irregular Hoechst nuclear staining with multiple bright specks of chromatin fragmentation and condensation) stained with Hoechst 33258 dye (Sigma) and by flow cytometer analysis of annexin V/propidium iodide staining as described previously (36).
Statistical Analysis
Experiments were repeated three times. Statistical analyses were carried out with GraphPad (La Jolla, CA) Prism software, version 3.03. Differences between experimental groups were determined using Student's t test. Statistical significance was accepted when the p value was <0.05.
RESULTS
Keratin 8 and 18 Knockdown Increases Epithelial Cancer Cell Motility and Invasion without Modulating EMT Markers
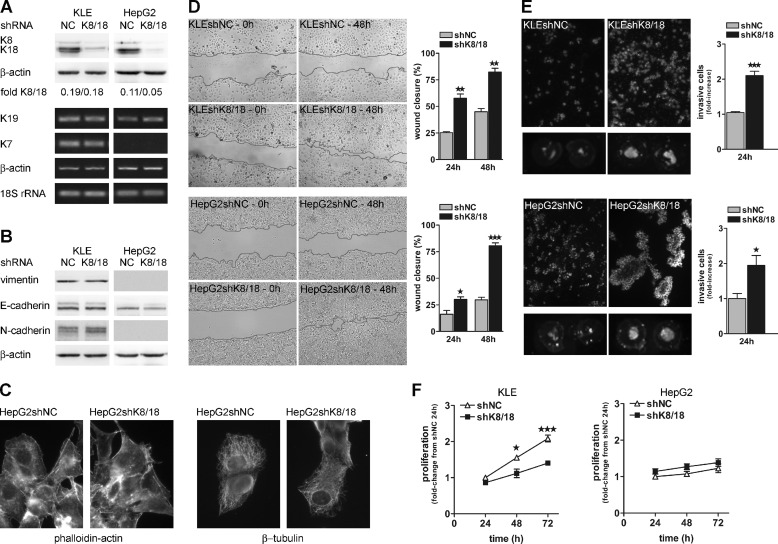
The conversion of epithelial cell into mesenchymal cell involves a change in the composition of IF proteins such that epithelial cells lose the expression of keratins and take on the expression of vimentin, a mesenchymal cell-specific IF protein (2). To better understand the role of the keratin cytoskeleton in EMT, we used an RNA interference approach targeted against K8/18 to mimic keratin loss during the EMT process. We used two epithelial carcinoma cell lines whose various differentiation states constitute an interesting experimental model: the HepG2 cell line obtained from well differentiated carcinoma (K8/18+; vimentin−) and the KLE cell line from a poorly differentiated carcinoma (K8/18+; vimentin+). To generate an effective monoclonal population of cells deficient in K8/18, we used shRNA constructs. We monitored the knockdown efficiency by analyzing K8 and K18 protein levels. We observed a decrease of 80% in KLE cells and of more than 90% in HepG2 cells when compared with negative control cells (shNC) expressing scrambled shRNA (Fig. 1A). Moreover, specificity of the knockdown was validated by analyzing the expression levels of other keratin proteins normally expressed in the KLE cell line. Keratin 7 and keratin 19 were not affected by K8/18 knockdown (Fig. 1A). As keratin loss is associated with EMT, first we determined whether K8/18 depletion induced expression of EMT markers. Western blot analysis showed that E-cadherin, N-cadherin, and vimentin levels were not modified in K8/18 knockdown cells (Fig. 1B). Moreover, there were no detectable levels of Snail and Slug transcription factors in both cell lines (data not shown). Immunofluorescence staining with anti-phalloidin and anti-β-tubulin antibodies also demonstrated that K8/18 knockdown did not affect cell morphology or cytoskeletal organization of actin and microtubule networks (Fig. 1C). However, using in vitro wound healing and Transwell invasion assays, we observed that K8/18 knockdown directly affected the motility and invasiveness of cancer cells. Indeed, K8/18-deficient cells closed the wound 2–3 times faster than the control cells (Fig. 1D). Moreover, K8/18 depletion significantly increased cell invasion through Matrigel of KLE (2.1 ± 0.12-fold, p < 0.0002) and HepG2 cells (1.95 ± 0.28-fold, p < 0.0247) (Fig. 1E). The increase in cell invasion was not due to an enhanced proliferation because results from 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assays revealed that stable knockdown of K8/18 had either no effect on or rather decreased cell viability (Fig. 1F).
FIGURE 1.
K8/18 loss promotes cancer cell motility and invasion without modulating epithelial phenotype. A, KLE and HepG2 cells were stably transfected with scrambled negative control (shNC) or keratin 8 and 18 (shK8/18) shRNA (as described under “Experimental Procedures”), and K8/18 levels were analyzed by Western blot analysis. β-Actin levels are included as a loading control. Silencing specificity was monitored by analysis of keratin 19 (K19) and keratin 7 (K7) mRNA levels. β-Actin and 18 S rRNA were used as reference genes. K8/18 knockdown does not affect EMT marker expression as shown by Western blot of vimentin, E-cadherin, and N-cadherin (B) or the organization of other cytoskeletal networks (C) as shown by immunofluorescence staining of phalloidin-actin and β-tubulin in HepG2 cells stably transfected with NC or K8/18 shRNA. D, wound healing assay. Stable shNC and shK8/18 KLE and HepG2 clones grown to subconfluence were scraped with the blunt end of a tip to make a cell-free area (0 h). The area was measured 24 and 48 h after wounding, and results are plotted as a percentage of wound closure (0 h = 100%). E, Matrigel invasion assay. Invasive shNC and shK8/18 KLE and HepG2 cells that had adhered to the Transwell insert were fixed and stained with Hoechst dye. Results show representative pictures of five randomly selected fields in the insert (magnification, ×100) as well as the total fluorescence of the insert (two Transwell inserts/experiment). Quantification of total fluorescence of each insert is shown as -fold change from shNC densitometric values. F, proliferation of KLE and HepG2 clones was assessed using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide proliferation assay. The results are presented as -fold change from absorbance values of shNC clones grown for 24 h. Error bars, S.E. All experiments were performed three times in duplicate (*, p < 0.05; **, p < 0.005; ***, p < 0.0005).
Keratin 8 and 18 Knockdown Improves PI3K/Akt Activation in Epithelial Cancer Cells
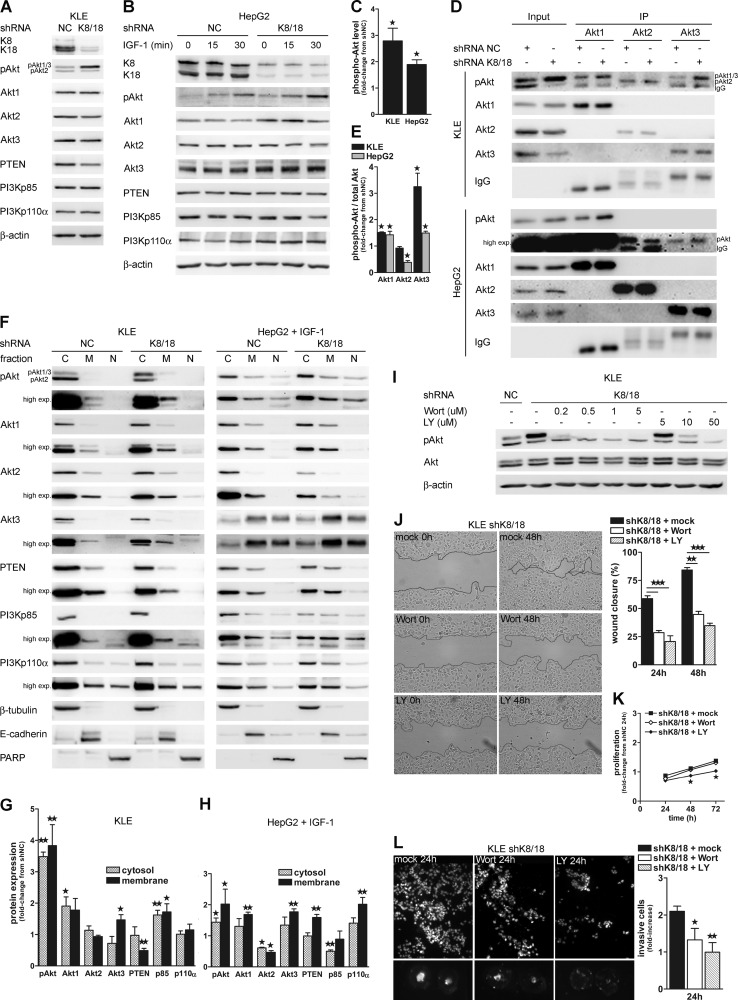
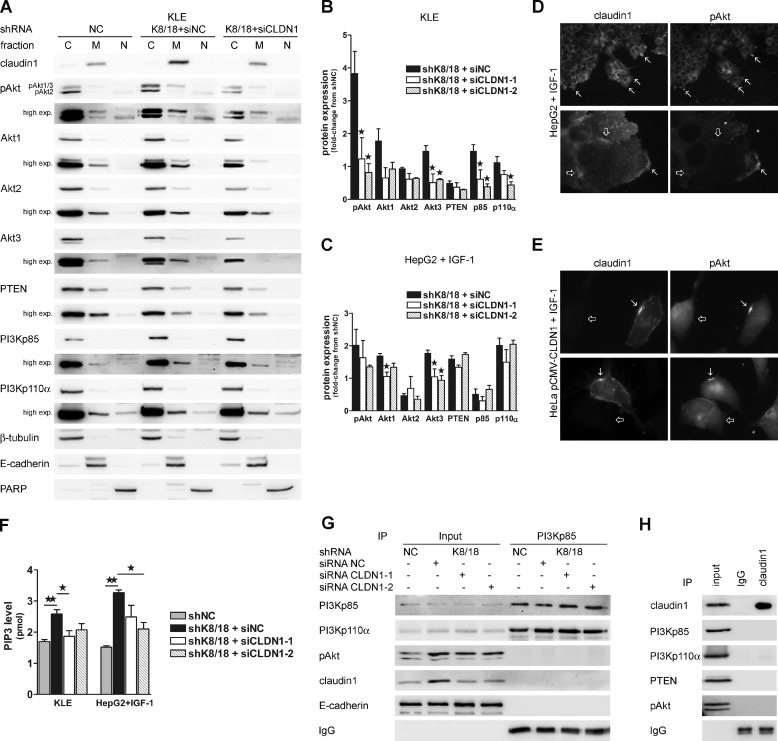
The PI3K/Akt pathway plays a pivotal role in cancer cell motility and invasion. We proceeded to an analysis of the expression levels of proteins involved in this pathway. KLE cells constitutively express the three Akt isoforms in their activated/phosphorylated form, making this cell line a very useful tool for the present study (33). The antibody against phospho-Akt recognizes two distinct bands on the blot; the upper band corresponds to phosphorylated Akt1 and Akt3, and the lower band corresponds to phosphorylated Akt2. This interpretation comes from a previous study in which we characterized the antibody against Akt phosphorylated on serine 473 (catalog number 9271, Cell Signaling Technology) (33). HepG2 cells also express Akt isoforms, but stimulation with IGF-1 is necessary to induce their activation/phosphorylation. Our results show that K8/18 depletion modestly affects Akt isoform protein levels. Western blot analysis revealed that the Akt1 level is similar, whereas Akt2 and Akt3 isoforms slightly decreased in KLE cells without K8/18 compared with the control cells (Fig. 2A). In HepG2shK8/18 cells, Akt1 and Akt3 were slightly increased compared with control cells, whereas the Akt2 level was not affected by K8/18 knockdown (Fig. 2B). Interestingly, K8/18 depletion induced a 2.8-fold increase (±0.48; p < 0.02) of Akt1 and/or Akt3 phosphorylation (upper band) in KLE cells (Fig. 2, A and C). Similarly, after 15 min of IGF-1 stimulation, the phospho-Akt level was 1.9-fold increased (±0.18; p < 0.0159) in HepG2shK8/18 cells compared with control cells (Fig. 2, B and C). To determine which Akt isoform was activated in K8/18 knockdown cells, we immunoprecipitated each Akt isoform from shNC and shK8/18 cell lysates and assessed their phosphorylation state by phospho-Akt Western blot (Fig. 2D). Akt3 was predominantly activated in KLEshK8/18 cells (3.25 ± 0.52-fold, p < 0.049) compared with the control cells. The Akt1 isoform was slightly more phosphorylated in K8/18-depleted cells (1.51 ± 0.025-fold, p < 0.0065), whereas the Akt2 isoform did not show any difference in its activation state (Fig. 2E). After stimulation with IGF-1, HepG2shK8/18 cells also showed an increase in Akt1 (1.427 ± 0.1334-fold, p < 0.0397) and Akt3 (1.496 ± 0.069-fold, p < 0.0021) phosphorylation compared with the control cells (Fig. 2, D and E), whereas Akt2 activation was decreased (0.3839 ± 0.07-fold, p < 0.0123). PTEN, which is a negative regulator of Akt phosphorylation, was decreased in KLEshK8/18 cells, whereas K8/18 knockdown did not affect the PTEN level in HepG2 cells (Fig. 2, A and B). No significant changes were observed in the total level of the PI3K p85 and p110a subunits in both cell lines (Fig. 2, A and B).
FIGURE 2.
K8/18 knockdown improves Akt1 and Akt3 membrane targeting and phosphorylation. Western blot analysis of PI3K/Akt pathway mediators in KLE shNC and shK8/18 clones (A) and HepG2 shNC and shK8/18 clones treated with 200 ng/ml IGF-1 for 0, 15, and 30 min after 24-h serum starvation (B). β-Actin levels are included as a loading control. C, phospho-Akt (pAkt) levels are shown as -fold change from densitometric values of the KLEshNC clone (upper band on the phospho-Akt blot) and HepG2shNC clone treated with IGF-1 for 15 min. D, the phosphorylation state of each Akt isoforms was monitored in shNC and shK8/18 cells by immunoprecipitation (IP) using anti-Akt1, anti-Akt2, or anti-Akt3 antibody followed by Western blot analysis with anti-phospho-Akt antibody. The protein content of whole cell lysates is shown (input). Quantification of the ratio of phospho-Akt to total Akt for each isoform immunoprecipitation was determined by densitometric analysis and is plotted as -fold change from shNC values (E). F, the impact of K8/18 knockdown on subcellular localization of PI3K/Akt pathway mediators in KLE and IGF-1-treated HepG2 cells was determined using cytosolic (C)/membrane (M)/nuclear (N) fractionation followed by Western blot analysis. β-Tubulin, E-cadherin, and PARP were used as controls for the purity of cytosolic, membrane, and nuclear fractions, respectively. Quantification of protein levels in cytosolic and membrane fractions of KLE shK8/18 (G) and IGF-1-treated HepG2 shK8/18 cells (H) was determined by densitometric analysis and is shown as -fold change from shNC for each fraction. I, dose response of KLE cells to PI3K inhibitor exposure (1 h) and the effect on cell proliferation (K), motility (J), and invasion through Matrigel (L). Results are shown as described in Fig. 1. Data represent the means of three independent experiments. Error bars, S.E. (*, p < 0.05; **, p < 0.005; ***, p < 0.0005). Wort, wortmannin; LY, LY294002; exp., exposure.
Membrane recruitment is an essential step in Akt signaling because its activating kinase, phosphoinositide-dependent kinase-1, is linked to PIP3 in the plasma membrane (37, 38). Therefore, we performed biochemical subcellular fractionation of cytosolic, membrane, and nuclear proteins from control and shK8/18 cell lysates to find out whether the keratin cytoskeleton could modulate the intracellular localization of PI3K/Akt pathway mediators. Western blots of β-tubulin (cytosolic), E-cadherin (membrane), and poly(ADP-ribose) polymerase (PARP) (nucleus) were used to validate the low cross-contamination among fractions. The level of phospho-Akt (upper band) was significantly increased in both cytosolic and membrane fractions of shK8/18 cells when compared with the control in both cell lines (Fig. 2, F–H). According to our immunoprecipitation results, Akt1 and Akt3 isoforms were significantly increased predominantly in the membrane pool, whereas Akt2 either did not change (KLE) or rather decreased (HepG2). The densitometric analysis also showed a significant decrease of PTEN at the membrane of KLEshK8/18 cells that is consistent with the diminution of the total level of PTEN (Fig. 2A). In contrast, the amount of PTEN in the membrane fraction of HepG2shK8/18 cells was increased compared with the control cells. The concomitant localization of PI3K/Akt and PTEN in the membrane extract supports the concept of a polarized delivery of intracellular signaling molecules during cell migration. The PI3Kp85 level was increased in cytoplasm and membrane of the KLEshK8/18 clone, whereas in HepG2shK8/18 cells, there was an up-regulation of p110a subunit at the membrane. Interestingly, we could detect expression of some mediators of the PI3K/Akt pathway in the nuclear fraction, particularly in HepG2 cells. The nuclear pool of Akt3 was increased in K8/18-depleted cells as was phospho-Akt and PI3Kp85 but to a lesser extent. The distribution of Akt isoforms in distinct subcellular locations has also been described by others (39). All together these results suggest that the absence of the K8/18 cytoskeleton facilitates and/or extends the activation of Akt1 and Akt3 isoforms by modulating their subcellular localization.
To determine whether PI3K/Akt activation is involved in the motility of K8/18 knockdown cells, we used pharmacological PI3K inhibitors and performed wound healing and invasion assays. As shown by dose-response results, treatment of KLEshK8/18 and IGF-1-treated HepG2shK8/18 cells with 0.5–1 μm wortmannin or 10 μm LY294002 reduced Akt phosphorylation (Fig. 2I and supplemental Fig. S1A), particularly of Akt1/3 isoforms (Fig. 2I, upper band). However, only wortmannin treatment did not affect cell proliferation for the entire duration of the experiments (Fig. 2K and supplemental Fig. S1C). Using these conditions, we observed that PI3K inhibition by both drugs significantly decreased cell motility (Fig. 2J and supplemental Fig. S1B) and invasion through Matrigel (Fig. 2L and supplemental Fig. S1D).
Keratin 8 and 18 Knockdown Increases NF-κB Transcriptional Activity through PI3K Pathway
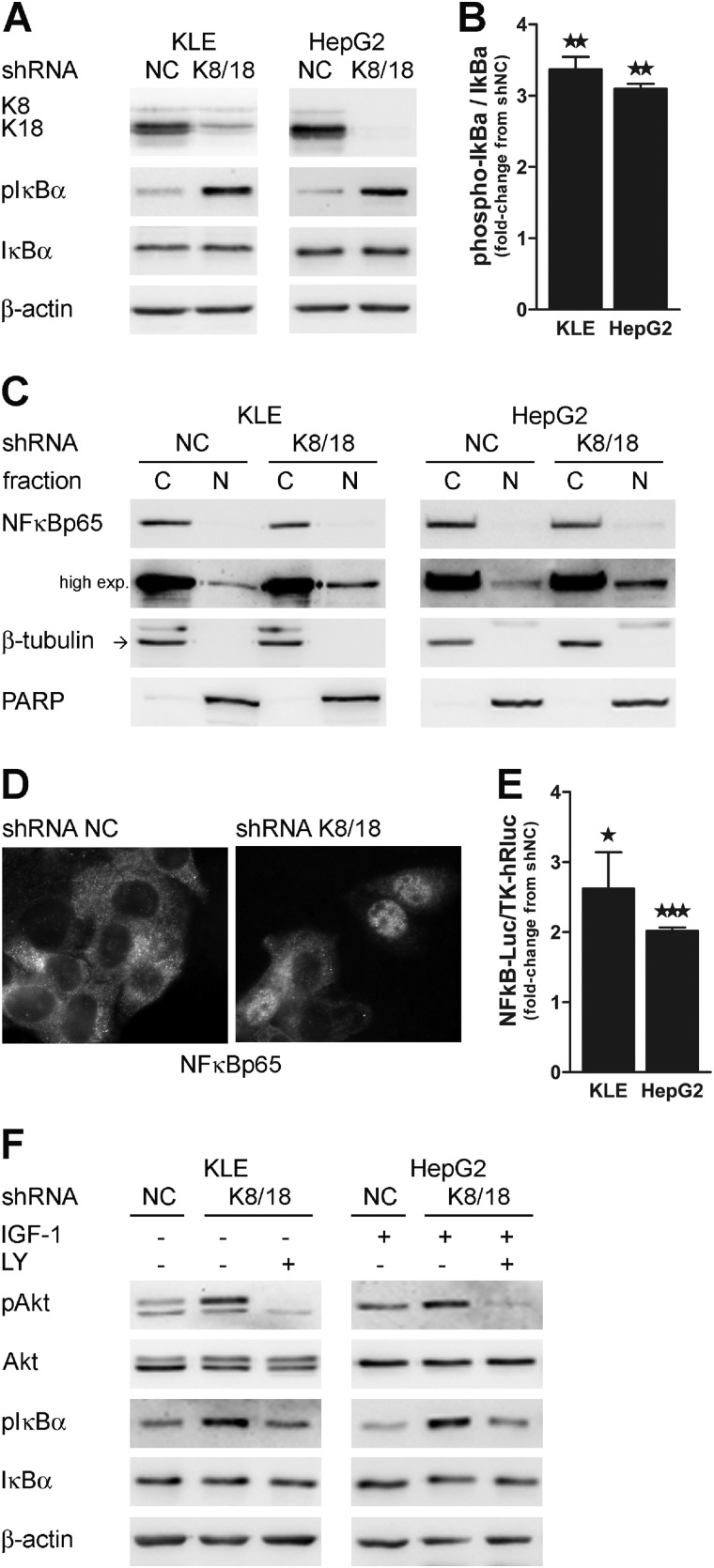
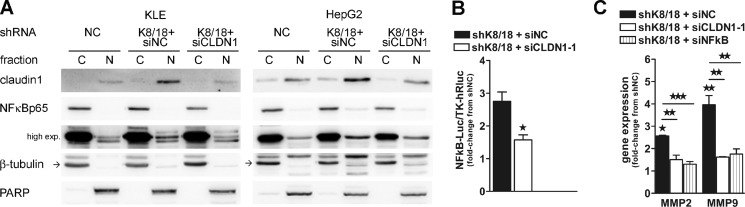
Several studies have indicated that transcription factor NF-κB activity is involved in both promoting and maintaining an invasive phenotype (40). Western blot analysis showed a 3.4-fold (±0.18; p < 0.0008) and a 3.1-fold increase (±0.07; p < 0.0011) of IκBα phosphorylation in K8/18-depleted KLE and HepG2 cells, respectively (Fig. 3, A and B). IκBα binds to and inhibits the transcriptional activity of NF-κB. Once phosphorylated, IκBα is degraded, and NF-κB is free to translocate from the cytoplasm to the nucleus where it binds to its response element on gene promoter regions and regulates a wide spectrum of gene expression. Thus, we verified the intracellular localization of NF-κBp65 by subcellular fractionation (Fig. 3C) and immunofluorescence staining (Fig. 3D). Both techniques revealed that there is an increase in nuclear NF-κBp65. Moreover, by using a luciferase reporter construct that contains five copies of an NF-κB response element, we found that there was a 2-fold increase in the NF-κBp65 transcriptional activity in K8/18 knockdown cells (2.6 ± 0.52-fold, p < 0.012 for KLE and 2.0 ± 0.048-fold, p < 0.0001 for HepG2) (Fig. 3E).
FIGURE 3.
K8/18 knockdown increases NF-κBp65 transcriptional activity through PI3K. Loss of K8/18 increases IκBα phosphorylation as shown by Western blot analysis (A) and densitometric quantification (B). β-Actin levels are shown as a loading control. The impact of K8/18 depletion on nuclear localization of NF-κBp65 was determined by cytosolic (C)/nuclear (N) fractionation (C) and immunofluorescence staining (D). β-Tubulin (cytosolic) and PARP (nuclear) were used to validate the low cross-contamination among fractions. E, NF-κB DNA binding was monitored by the Dual-Luciferase reporter assay. KLE and HepG2 clones were co-transfected with NF-κB-Luc reporter plasmid and TK-hRLuc for 24 h, and then reporter activity was measured and normalized to the Renilla luciferase activity used as an internal control. Each experiment was repeated three times in duplicate. F, inhibition of PI3K activity using LY294002 (LY) (50 μm; 1-h treatment) reduces the phosphorylation of IκBα as shown by Western blot analysis. Data represent the means of three independent experiments. Error bars, S.E. (*, p < 0.05; **, p < 0.005; ***, p < 0.0005). exp., exposure; pAkt, phospho-Akt; pIκBa, phospho-IκBa.
The PI3K/Akt pathway can induce IκBα degradation through phosphorylation of IκB kinases. Because we have previously shown that PI3K/Akt signaling is activated in our model, we used the PI3K inhibitor LY294002 to determine whether IκBα phosphorylation is dependent on PI3K activity. In both cell lines, inhibition of PI3K decreased IκBα phosphorylation to the basal level observed in control cells. These results suggest that K8/18 knockdown promotes PI3K/Akt/NFκB signaling pathway activation (Fig. 3F).
Keratin 8 and 18 Knockdown Increases Expression of the Tight Junction Protein Claudin1 through NF-κB Transcriptional Activity
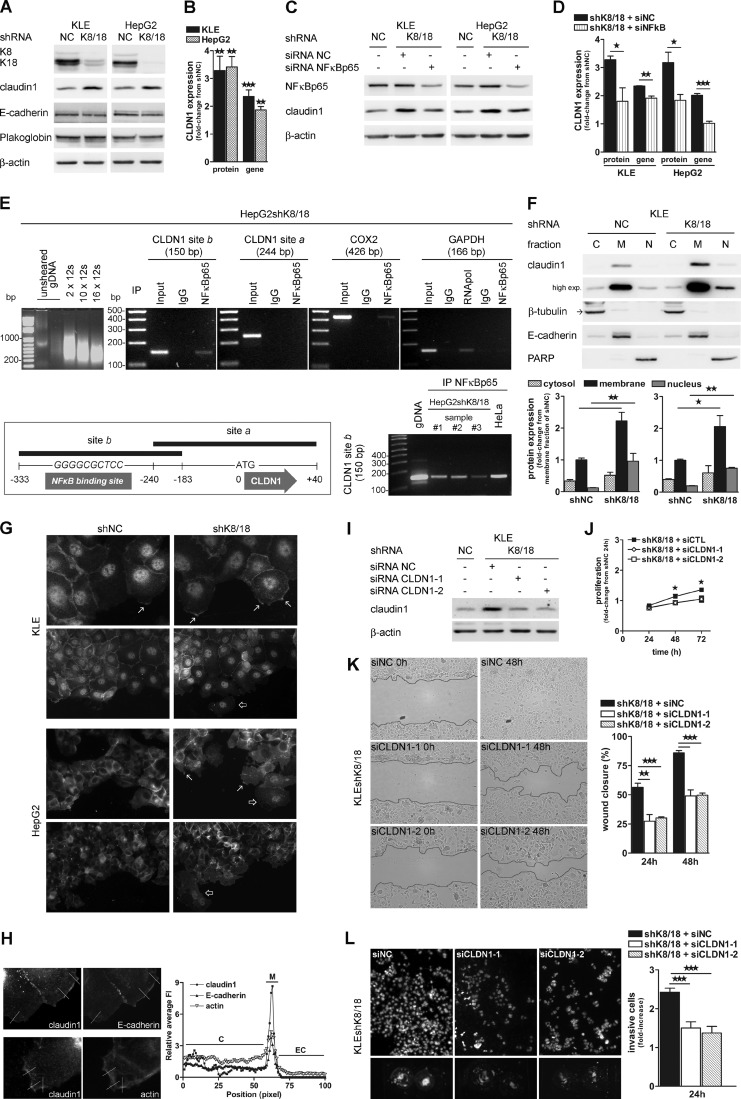
During EMT, cell junctions are altered either by a decrease in expression or a mislocalization of their components. We investigated expression and distribution of three major cell junction proteins: claudin1, E-cadherin, and plakoglobin, which are part of the tight junctions (TJs), adherens junctions, and desmosomes, respectively. Whereas no change was observed in the protein levels of E-cadherin and plakoglobin, the K8/18 knockdown induced a significant 3-fold increase of the claudin1 protein level in both cell lines (3.3 ± 0.51-fold, p < 0.0012 for KLE and 3.4 ± 0.37-fold, p < 0.0033 for HepG2) (Fig. 4, A and B). Quantitative real time PCR analysis showed that claudin1 gene expression was also significantly increased in cells without K8/18 cytoskeleton (2.35 ± 0.23-fold, p < 0.0004 for KLE and 1.87 ± 0.12-fold, p < 0.0023 for HepG2) (Fig. 4B). Given the increased NF-κBp65 transcriptional activity in K8/18-deficient cells, we examined whether this transcription factor could regulate claudin1 expression. For this purpose, we used siRNA targeted against NF-κBp65, which was decreased by 65 and 78% in KLE and HepG2shK8/18 cells, respectively. As shown in Fig. 4, C and D, NF-κBp65 silencing significantly decreased claudin1 protein (KLE, p < 0.0412; HepG2, p < 0.0184) and gene (KLE, p < 0.0059; HepG2, p < 0.0005) expression in both cell lines compared with cells transfected with siRNA. Based upon these findings, we further investigated whether NF-κBp65 could directly bind the CLDN1 promoter in vivo by performing a ChIP analysis (Fig. 4E). We immunoprecipitated NF-κBp65 cross-linked with sheared DNA from three different lysates of HepG2shK8/18 cells and one from HeLa cells. Then we have performed PCR in different regions of the CLDN1 promoter (site a and site b). Our results reveal that NF-κBp65 is linked to the CLDN1 promoter (site b, −333 to −183 bp relative to the translation start site) in all the samples. Binding of NF-κBp65 to COX2 promoter was used as a positive control for NF-κBp65 DNA binding. To validate the assay, simultaneous ChIP was performed with an antibody to RNA polymerase II, and DNA enrichment was monitored for the GAPDH gene. We confirmed these results by finding the well known NF-κB DNA binding motif (41) (5′-GGGRNNYYCC-3′ where R correspond to a purine, Y represents a pyrimidine, and N can be any base) in the site b region (−310 to −301 bp). For the first time, we identified NF-κBp65 as a functional regulator of claudin1 transcription, strongly supporting the role of claudin1 in cancer cell invasion and tumor progression.
FIGURE 4.
Claudin1 is up-regulated in K8/18-depleted cells and is involved in cell motility and invasiveness. K8/18 knockdown increases claudin1 protein (A and B) and gene (B) expression (quantitative PCR) in KLE and HepG2 cells but does not modulate other cell junction components as shown by Western blot analysis of E-cadherin and plakoglobin. NF-κBp65 silencing decreases claudin1 protein (C and D) and gene (D) expression in both cell lines. E, in vivo binding of NF-κBp65 to the CLDN1 promoter. The average size of cross-linked DNA after shearing is shown. The optimal sonication condition was 12 s for 10 times. CLDN1 (sites a and b), COX2, and GAPDH promoters resulting from the ChIP assay with IgG (negative control), NF-κBp65 antibody, RNA polymerase II antibody (negative and positive control), or input DNA (precleared chromatin; no antibody) in HepG2shK8/18 cells were amplified by PCR. A schematic representation of the NF-κBp65 binding site in the CLDN1 promoter (−310 to −301 bp) is shown. Site b (−333 to −183 bp) in CLDN1 promoter resulting from the ChIP assay with NF-κBp65 antibody in three different lysates of HepG2shK8/18 cells and one from HeLa cells was amplified by PCR. F, the effect of K8/18 loss on cellular localization of claudin1 was determined using cytosolic (C)/membrane (M)/nuclear (N) fractionation (C and D) followed by Western blot analysis. β-Tubulin, E-cadherin, and PARP were used as controls for the purity of cytosolic, membrane, and nuclear fractions, respectively. Quantification of claudin1 levels in each fraction of KLE shK8/18 and IGF-1-treated HepG2 shK8/18 cells (D) was determined by densitometric analysis and is shown as -fold change from shNC of the membrane fraction. G, claudin1 localization at the leading edge membrane (arrows) and in the cytosol/nucleus of migrating cells (unfilled arrows) was compared between shNC and shK8/18 cells in both cell lines using immunofluorescence. Magnification, ×400 and ×200. H, KLEshK8/18 cells were immunostained for claudin1 with E-cadherin or phalloidin-actin after wounding. The relative average of fluorescence intensities (FI; y axis) at the indicated lines (distance along the line from inside to outside of the cell; x axis) is shown. Fluorescence intensities are defined as (fluorescence intensities)/(mean cytoplasmic fluorescence intensities). C, cytoplasmic region; M, membrane region; EC, extracellular region. The role of claudin1 in cell motility and invasion was determined by transfection of KLEshK8/18 cells with NC siRNA (siNC) or with two claudin1 siRNAs (siCLDN1-1 and siCLDN1-2) for 24 h (I) followed by proliferation (J), wound healing (K), and Matrigel invasion assays (L). Results are shown as described in Fig. 1. Data represent the means of three independent experiments. Error bars, S.E. (*, p < 0.05; **, p < 0.005; ***, p < 0.0005). IP, immunoprecipitation; gDNA, genomic DNA; exp., exposure.
To determine whether the increase in claudin1 expression could influence its intracellular localization, we performed as described above a subcellular fractionation (Fig. 4F) and confirmed the results by immunofluorescence staining of claudin1 protein in KLE and HepG2 clones (Fig. 4G). Both techniques allowed us to assert that claudin1 is present in the three cellular fractions and that the distribution is not changed between control and K8/18 knockdown cells. However, a 2- and 3-fold increase of claudin1 was detected in the membrane and the nuclear fraction, respectively, in shK8/18 cells compared with control cells (Fig. 4F). Biochemical fractionation demonstrated that claudin1 localization is mostly at the membrane in both cell lines regardless of whether there is a K8/18 cytoskeleton. Because K8/18 knockdown cells showed increased motility, we analyzed claudin1 localization in the context of wound healing by immunofluorescence staining. In subconfluent sheet of cells, claudin1 showed an extensive plasma membrane staining. Interestingly, the migrating cells at the leading edge showed a more scattered membrane staining (Fig. 4G, arrows) and an intense cytoplasmic/nuclear localization of claudin1 (Fig. 4G, unfilled arrows). Follower cells demonstrated continuous membrane and moderate nuclear staining. To exclude the possibility that claudin1 staining at the leading edge was due to the thickening of the cell membrane, we compared claudin1 fluorescence intensity at the edge with E-cadherin and actin staining as controls. Cytoplasmic fluorescence was adjusted to the same level for the different staining. The ratio of the claudin1 signal at the edge versus the average cytoplasm signal was higher than that of E-cadherin or actin fluorescence, suggesting that the thickening of the cell at the leading edge contributed little to the claudin1 signal (Fig. 4H). Some studies have already shown that claudin1 expression is not exclusively localized at the TJs but can be distributed continuously along the plasma membrane (42, 43) as well as in the cytoplasm (44, 45) and in the nucleus (45, 46). Our data indicate that the cellular localization of claudin1 depends on the cell location relative to the wound, suggesting a role in cell motility.
Claudin1 Is Involved in Cell Motility and Invasion of K8/18-deficient Cells
Given the considerable increase in the expression level of claudin1, we evaluated whether this protein could play a role in the motility and invasion potential of K8/18-deficient cells. To this end, we used two different siRNAs targeted against claudin1 (siCLDN1-1 and siCLDN1-2), which was decreased by 71 and 75% in KLEshK8/18 (Fig. 4I) and 58 and 53% in HepG2shK8/18 cells (supplemental Fig. S2A). Both cell clones transfected with siCLDN1-1 or siCLDN1-2 were unable to close the wound after 48 h, whereas cells transfected with NC siRNA (siNC) showed almost complete wound healing after the same period of time (Fig. 4K and supplemental Fig. S2C). Claudin1 is also involved in the invasion potential of K8/18-depleted cells. Indeed, the results of invasion assays show that claudin1 inhibition by both oligonucleotides decreased the number of K8/18 knockdown cells able to pass through Matrigel (p < 0.0004) (Fig. 4L and supplemental Fig. S2D). However, there was a 20% decrease in KLEshK8/18 cell proliferation induced by claudin1 silencing as shown by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay (Fig. 4J), whereas no effect on HepG2 cell viability was observed after siCLDN1 transfection (supplemental Fig. S2B). Overall our results indicate that the absence of the K8/18 cytoskeleton enhances epithelial cancer cell motility and invasion through an up-regulation of claudin1 expression and its localization at the cell membrane and the nucleus.
Claudin1 Increases PI3K Activity and Phosphorylated Akt Localization at the Plasma Membrane in K8/18 Knockdown Cells
Because it is well known that the PI3K/Akt pathway regulates cell motility and invasion and because claudin1 is involved in these processes in our model, we wanted to determine whether claudin1 could play a role in the Akt hyperactivation observed in cells lacking K8/18. Western blot analysis of the membrane fraction of siCLDN1-1- or siCLDN1-2-transfected KLEshK8/18 and HepG2shK8/18 cells showed that claudin1 inhibition reduced membrane localization of phospho-Akt (upper band), Akt1, and Akt3 at the basal level in KLE cells (Fig. 5, A and B). These results are consistent with our preceding immunoprecipitation results in that Akt1 and Akt3 were the predominant activated isoforms in shK8/18 cells (Fig. 2E). Membrane targeting of PI3Kp85 was also decreased by claudin1 silencing in KLE clones (Fig. 5, A and B). However, claudin1 inhibition did not restore PTEN membrane localization (Fig. 5, A and B). Thus, the role of claudin1 in the PI3K/Akt/PTEN signaling axis seems to be more related to PI3K/Akt. The effect of claudin1 inhibition was quite similar in HepG2shK8/18 cells (Fig. 5C), although the Akt phosphorylation was decreased to a lesser extent. The lower efficiency of the siRNA transfection in HepG2 cells compared with KLE cells could explain the slight effect of siCLDN1 on cell motility, invasion, and Akt phosphorylation observed in HepG2 cells (supplemental Fig. S2).
FIGURE 5.
Claudin1 regulates membrane localization and activity of PI3K/Akt pathway mediators. A, the effect of claudin1 silencing on subcellular localization of PI3K/Akt pathway mediators in KLE cells was determined using cytosolic (C)/membrane (M)/nuclear (N) fractionation followed by Western blot analysis. β-Tubulin, E-cadherin, and PARP were used to validate low cross-contamination among fractions. Quantification of protein levels in the membrane fraction of KLE shK8/18 (B) and IGF-1-treated HepG2 shK8/18 cells (C) transfected with NC siRNA (siNC) or two claudin1 siRNAs (siCLDN1-1 and siCLDN1-2) was determined by densitometric analysis and is shown as -fold change from shNC. Double immunofluorescence staining for claudin1 and phospho-Akt in IGF-1-treated HepG2 shK8/18 cells (D) and HeLa cells transfected with pCMV-CLDN1 vector (E) shows that both proteins are localized in the same regions of the plasma membrane of migrating cells at the front (D and E, arrows), whereas no phospho-Akt staining is detected in cell-cell junction regions (D, unfilled arrows). Non-transfected HeLa cells show a diffuse cytoplasmic staining for phospho-Akt (E, unfilled arrows). Magnification, ×200 and ×1000. F, the effect of claudin1 silencing on PI3K activity was measured as PIP3 generated from immunoprecipitated PI3Kp85 in a kinase assay and assessed in a competitive ELISA. Western blot analyses of PI3Kp85 (G) and claudin1 (H) immunoprecipitates are shown. Data represent the means of three independent experiments. Error bars, S.E. (*, p < 0.05; **, p < 0.005). pAkt, phospho-Akt; exp., exposure; IP, immunoprecipitation.
Because claudin1 depletion affects phospho-Akt membrane localization, we analyzed whether there was a relationship between the localization of the two proteins during cell migration. We performed a double immunofluorescence staining of claudin1 and phospho-Akt in K8/18-depleted HepG2 cells during wound healing. Interestingly, the cells at the front of migration showed intense accumulation of phospho-Akt at specific regions of the leading edge membrane similar to claudin1 staining (Fig. 5D, arrows). Akt is known to localize at the moving front of the plasma membrane where it promotes lamellipodium formation (47, 48). The recruitment of claudin1 and phospho-Akt at the leading edge is a specific event because there was no staining for phospho-Akt at cell-cell junctions (Fig. 5D, unfilled arrows). To determine whether the concomitant membrane targeting of claudin1 and Akt could be a common feature in cell migration, we performed the double staining in subconfluent IGF-1-stimulated HeLa cells transfected with claudin1. As we observed in HepG2shK8/18 cells, claudin1 localized uniformly along the membrane with some punctate aggregates that were also intensively stained for phospho-Akt (Fig. 5E, arrows). Interestingly, in comparison with claudin1-expressing HeLa cells, non-transfected cells showed a diffuse cytoplasmic phospho-Akt staining (Fig. 5E, unfilled arrows). Based on these observations, we hypothesize that the enhanced membrane localization of claudin1 by K8/18 knockdown could increase Akt membrane recruitment to specific membrane domains and promote its activation.
Previous studies have shown that cell junction components could modulate PI3K/Akt signaling. For instance, occludin, a TJ partner of claudin1, has been shown to regulate cell migration by PI3K recruitment at the leading edge (34). E-cadherin also recruits PI3K during adherens junction formation (49, 50). Thus, it was tempting to speculate that claudin1 could increase phospho-Akt membrane recruitment by regulating PI3K activity. The effect of claudin1 silencing on PI3K activity was determined with a kinase assay and detected by an ELISA for PIP3 product. Our data show that K8/18 knockdown significantly increased PI3K kinase activity in a claudin1-dependent manner in both cell lines (Fig. 5F). The PI3Kp85 immunoprecipitates used for the kinase assay were blotted to validate the experiment and to identify protein interaction. The decrease of Akt phosphorylation in claudin1-depleted cells correlates with PIP3 levels detected. As expected, PI3Kp110α subunit was pulled down with PI3Kp85, but neither claudin1 nor E-cadherin interacted with the kinase. Immunoprecipitation of claudin1 confirmed that no interaction occurs between claudin1 and PI3K/Akt mediators in our models (Fig. 5G). Overall, these results highlight a role for claudin1 in the regulation of PI3K activity that is in agreement with the improved Akt activation observed in K8/18 knockdown cells.
Claudin1 Modulates Invasiveness of K8/18 Knockdown Cells by Increasing NF-κB-induced MMP2 and MMP9 Expression
Claudin1 is known to promote MMP2 activation by recruiting MT1-MMP at the cell surface and increasing their protein expression (44, 51, 52). Because NF-κB can directly bind the MMP9 promoter (53) and activate MMP2 (54) through MT1-MMP expression (55), we investigated whether the enhanced NF-κB transcriptional activity observed in K8/18-depleted cells could modulate cell invasiveness through MMP2 and MMP9 expression. We first validated that claudin1 expression is involved in NF-κB nuclear translocation and transcriptional activity of K8/18 knockdown cells. Indeed, claudin1 silencing decreased the level of nuclear NF-κBp65 in shK8/18 cells (Fig. 6A) and therefore decreased its DNA binding (Fig. 6B). Then we examined whether claudin1 and NF-κBp65 could effectively modulate the expression of the gelatinases MMP2 and MMP9 in our model. We performed quantitative real time PCR in HepG2shNC and HepG2shK8/18 cells transfected with NC siRNA, claudin1 siRNA, or NF-κBp65 siRNA. The results are shown as -fold change from shNC cells (Fig. 6C). Knockdown of K8/18 induced a 2.6-fold increase in MMP2 (±0.45; p < 0.0269) and a 3.9-fold increase in MMP9 (±0.41; p < 0.002) expression. Both claudin1 and NF-κBp65 siRNAs significantly blocked these increases and reduced MMP2 and MMP9 expression close to the level of shNC cells. These results demonstrate that K8/18 depletion promotes MMP expression through claudin1/NF-κBp65 activity. Because we identified NF-κBp65 as a transcriptional regulator of claudin1 expression (Fig. 4, C–E), altogether these results suggest a feedback loop process that could explain the overexpression of claudin1 in K8/18 knockdown cells.
FIGURE 6.
Claudin1 regulates NF-κBp65 transcriptional activity and MMP expression. A, the effect of claudin1 silencing on the subcellular localization of NF-κBp65 in KLE and IGF-1-treated HepG2 cells transfected with NC siRNA (siNC) or claudin1 siRNA (siCLDN1-1) was determined using cytosolic (C)/nuclear (N) fractionation followed by Western blot analysis. β-Tubulin and PARP were used to validate low cross-contamination among fractions. B, NF-κB DNA binding was monitored by the Dual-Luciferase reporter assay. KLE shK8/18 cells were co-transfected with NF-κB-Luc reporter plasmid and TK-hRLuc for 24 h followed by transfection with NC siRNA (siNC) or claudin1 siRNA (siCLDN1-1) for 24 h. Reporter activity was measured and normalized to the Renilla luciferase activity used as an internal control. Each experiment was repeated three times in duplicate. The role of claudin1 and NF-κBp65 in MMP expression was determined by transfection of HepG2shK8/18 cells with NC siRNA (siNC), claudin1 siRNA (siCLDN1-1), or NF-κBp65 siRNA (siNFκB) for 24 h followed by quantitative PCR analysis of MMP2 and MMP9 transcripts (C). Data represent the means of three independent experiments performed in duplicate. Error bars, S.E. (*, p < 0.05; **, p < 0.005; ***, p < 0.0005). Results are shown as -fold change from shNC. exp., exposure.
K8/18 Knockdown Increases Cisplatin Sensitivity
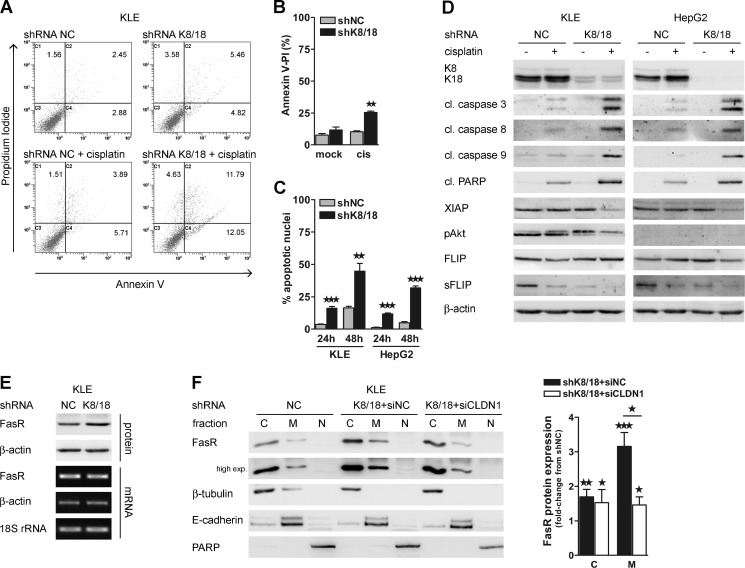
Emerging evidence suggests a role for EMT in chemotherapeutic drug resistance (56). Thus, we examined the effect of K8/18 knockdown in cisplatin-induced apoptosis. KLE and HepG2 clones were exposed to cisplatin (10 μm) for 24 h before analysis. K8/18 loss highly increased apoptosis in response to cisplatin as shown by annexin V/propidium iodide staining (Fig. 7, A and B), the percentage of apoptotic cells determined by irregular Hoechst 33258 dye staining (Fig. 7C), and an increase in cleavages of proapoptotic caspases 3, 8, and 9 and PARP (Fig. 7D). On the other hand, antiapoptotic proteins like X-linked inhibitor of apoptosis protein (XIAP) and phospho-Akt were decreased in KLEshK8/18 cells exposed to cisplatin compared with control cells under the same treatment (Fig. 7D). Similarly, XIAP was only decreased in HepG2shK8/18 cells treated with cisplatin. However, in the absence of serum starvation and IGF-1 stimulation, we could only detect a very weak signal of phospho-Akt in HepG2 cells. Interestingly, the short variant of FLICE-like inhibitory protein (sFLIP), which is an inhibitor of Fas death receptor signaling, was highly reduced in both K8/18-depleted cell lines under the control condition and cisplatin treatment (Fig. 7D). This raises the possibility that cisplatin-mediated apoptosis is enhanced by the Fas death receptor pathway in K8/18 knockdown cells. Thus, we investigated whether K8/18 loss affects the level and localization of the Fas receptor (FasR). Western blot and PCR analysis showed that there is an increase in the protein level of FasR between KLE shNC and shK8/18 cells (Fig. 7E). Subcellular fractionation of untreated KLE clones showed a highly significant increase of FasR at the membrane of K8/18-depleted cells (3.16 ± 0.4-fold, p < 0.0009) and in the cytosol to a lesser extent (1.62 ± 0.12-fold, p < 0.0012) (Fig. 7F). Importantly, in K8/18 knockdown cells, claudin1 silencing reduced the membrane FasR level exclusively (1.46 ± 0.23-fold, p < 0.0261) because siCLDN1 did not change the cytoplasmic FasR level (Fig. 7F). These results indicate that K8/18 loss sensitizes cells to apoptosis by down-regulating the antiapoptotic protein sFLIP and by increasing FasR at the membrane through a claudin1-dependent mechanism. Altogether these data confirm that K8/18 loss affects the epithelial cancer cell response to cisplatin in a different way than an EMT.
FIGURE 7.
K8/18 loss sensitizes cells to cisplatin-induced apoptosis. Control (shNC) and K8/18 knockdown (shK8/18) cells were mock-treated or treated with cisplatin (cis) (10 μm) for 24 h and then collected for flow cytometry using Alexa Fluor 488-annexin V/propidium iodide (PI) staining (A and B), for apoptotic cells counting using Hoechst staining (C), and for Western blot analysis (D). C1, C2, C3, and C4 represent quadrants for dead, late apoptotic, viable, and early apoptotic cells, respectively, and percentage of cells in each quadrant is indicated (A). A shows representative results of three independent experiments that are plotted in B. C, histogram showing the percentage of apoptotic cells in different groups using Hoechst staining. The impact of K8/18 knockdown on FasR expression (E) and localization (F) was determined by Western blot and conventional PCR (E) and by cytosolic (C)/membrane (M)/nuclear (N) fractionation followed by Western blot analysis (F). β-Actin and 18 S rRNA were used as loading controls. β-Tubulin, E-cadherin, and PARP were used to validate low cross-contamination among fractions. Quantification of FasR levels in cytosolic and membrane fractions of KLEshK8/18 transfected with NC siRNA (siNC) or claudin1 siRNA (siCLDN1-1) was determined by densitometric analysis and is shown as -fold change from shNC. Data represent the means of three independent experiments. Error bars, S.E. (*, p < 0.05; **, p < 0.005; ***, p < 0.0005). cl., cleaved; pAkt, phospho-Akt.
DISCUSSION
EMT is a phenotypic transformation of epithelial cancer cells that promotes their dedifferentiation and metastatic behavior. When tumor cells undergo EMT, the expression of keratin is down-regulated and replaced by the expression of vimentin, a mesenchymal IF protein. Although the change in IF protein composition is a useful biomarker of EMT, the role of the keratin network in tumor progression has only begun to be unraveled. For instance, studies have suggested that keratins contribute to the maintenance of epithelial phenotype and regression of malignancy. For example, forced expression of keratin 18 in a metastatic breast cancer cell line reduced the vimentin expression level, invasiveness in vitro, and metastatic spread in mice (18). In a similar way, stable silencing of vimentin in squamous carcinoma cells resulted in re-emergence of keratins and a concomitant reduction in cell invasiveness and tumorigenic potential (57). Conversely, suppression of both K8/18 in non-small cell lung cancer cell lines increased their invasiveness (58). In the present study, we have demonstrated that K8/18 constitutes a signaling platform capable of moderating invasion and cell death in tumor cells.
Our study establishes that K8/18 knockdown significantly increases epithelial cancer cell motility and invasiveness. This is associated with the overexpression of the TJ protein claudin1, which seems to be a key step in these processes. In normal epithelium, TJs maintain cell polarity by preventing the free diffusion of membrane lipids and proteins between apical and basolateral cellular poles and by regulating the paracellular permeability (59). Many tumors exhibit overexpressed TJ proteins, resulting in a deficient epithelial barrier function that increases access of nutrients and growth factors to cancer cells. Overexpression of claudin1 protein in cancer cell lines is associated with increased invasiveness and metastatic behavior (44, 46, 51, 52, 60–62). Moreover, some cancerous tissue and cell lines show mislocalized TJ proteins either in the cytoplasm or in the nucleus, suggesting that they can have more dynamic functions than simply being constituents of TJs (63). However, the molecular mechanisms involved in overexpression and/or mislocalization of claudin1 in cancer are poorly understood. PKC and PKA activity were shown to modulate claudin1 expression and intracellular localization, respectively (42, 44, 45). Endocytic recycling is also known to regulate internalization of TJ proteins (64). Our present study uncovers a functional role for NF-κBp65 in transcriptional regulation of claudin1 gene. Our ChIP assays in HepG2shK8/18 and HeLa cells demonstrate that it is a common transcriptional regulator that directly binds the CLDN1 promoter independently of K8/18 expression. As nuclear translocation and transcriptional activity of NF-κBp65 are increased by K8/18 loss, our findings provide the first evidence that claudin1 is a target of NF-κB, promoting cell motility and invasion. According to the EMT process, the keratin loss in our model supports the invasive behavior of cancer cells. A recent study also shows that depletion of keratins enhances keratinocyte migration through hemidesmosomes scattered along the basement membrane (65). In a similar way, we observed that in K8/18 knockdown cells claudin1 is localized specifically at the leading edge of migrating cells. Indeed, cells in front show an increase of claudin1 in the cytoplasm and nucleus with some punctate accumulation at the plasma membrane. Meanwhile, follower cells show an increase of claudin1 at the membrane, suggesting improved cell-cell cohesion in the rear moving sheet. Thus, the keratin network seems to be essential to prevent the aberrant expression and localization of cell junctions and thus to restrain cell motility. Moreover, claudin1 internalization is known to occur in moving cells during wound healing (66). This process could explain the predominant cytoplasmic/nuclear localization of claudin1 that we observed in front migrating K8/18 knockdown cells. However, further investigations are required to determine whether K8/18 loss improves claudin1 internalization. Despite the fact that there is a clear correlation between altered cell junctions and invasive behavior, the relationship between claudin1 and signaling pathways that promote cell invasion is only beginning to be understood. Extensive studies have correlated claudin1 overexpression with MT1-MMP level and MMP2 activity (44, 51, 52) as well as with β-catenin/Lef transcriptional activity (46). However, the significance of claudin1 nuclear localization in cell invasion is still ambiguous. For example, forced expression of claudin1 in the nucleus did not increase the invasion potential of melanoma cells compared with cells displaying a more cytoplasmic pattern (45). On the other hand, transcriptional regulation seems to be the major mechanism by which claudin1 promotes colon cancer cell invasion by repressing E-cadherin via ZEB1 activity (67). The results that we present in this study establish a functional role for claudin1 in the PI3K/Akt/NF-κB pathway. We show that both membrane and nuclear claudin1 enhances this intracellular signaling cascade to promote motility and invasiveness in K8/18-depleted epithelial cancer cells. K8/18 knockdown increases Akt1 and Akt3 membrane targeting and activation. The predominant activity of Akt1 and Akt3 in cell motility and invasion is in agreement with our recent study showing that Akt2 blocks cell motility of endometrial carcinoma cells, whereas Akt1 and Akt3 increase wound closure (68). Because claudin1 silencing decreases the presence of phospho-Akt1 and Akt3 at the membrane, we propose that the localized accumulation of claudin1 generates specific membrane regions suitable for PI3K/Akt activation. Indeed, we demonstrate that claudin1 increases PI3K activity and PIP3 production in K8/18-depleted cells in support of the improved Akt activation observed in these cells. However, we were unable to detect any interaction shown previously by others (34, 49, 50). Characterization of the link between claudin1 and PI3K activity will need further investigations. The absence of the keratin cytoskeleton also induces an increase of claudin1 in the nucleus. We found that in K8/18-depleted cells NF-κBp65 transcriptional activity was increased and induced MMP2 and MMP9 expression. It was already shown that K8/18 moderates TNFα-mediated NF-κB activity, thus providing resistance to the apoptotic effect of TNFα (15). Our data show that the presence of claudin1 is partially involved in the NF-κBp65 DNA binding and that claudin1 silencing strongly reduces MMP2 and MMP9 expression. The decrease in MMP2 and MMP9 is equivalent to that observed after NF-κB silencing. These results strongly suggest that nuclear claudin1 plays a role in MMP transcription. Because NF-κB regulates claudin1 transcription, these data reveal the existence of a positive feedback loop that could amplify cell motility and invasion.
Our current work also shows that K8/18 knockdown significantly increases epithelial cancer cell sensitivity to cisplatin. It is well known that keratins provide resistance to stress-induced apoptosis in hepatocytes (13, 14). Recent reports also suggested that K8/18 could mediate cisplatin resistance in different cancer cell lines (69, 70). Here we show that K8/18 depletion increases cisplatin-induced apoptosis by increasing the level of cleaved caspases 3, 8, and 9 and their target PARP. Although K8/18 knockdown improves Akt1 and Akt3 activation, the cells without K8/18 are more sensitive to the drug. In fact, cisplatin treatment induces a decrease of Akt phosphorylation and XIAP levels in K8/18-depleted cells only. Previous studies have already shown that cisplatin decreases Akt phosphorylation and XIAP levels in chemosensitive but not in chemoresistant cell lines (33, 71, 72). The present results suggest that K8/18 knockdown engages signaling processes similar to those involved in chemosensitivity. Moreover, our results show that K8/18 loss increases the membrane targeting of FasR and decreases the level of the inhibitory protein sFLIP. The role of the keratin cytoskeleton in the Fas death receptor pathway has already been shown in hepatocytes (14, 73). Interestingly, our results reveal that claudin1 is involved in the enhanced localization of FasR at the membrane. A recent report demonstrated that claudin mislocalization promotes the activation of the extrinsic apoptosis pathway through a direct interaction with components of the death-inducing signaling complex (74). Altogether these results suggest a dual role for claudin1 in invasiveness and sensitivity to apoptosis of K8/18-depleted cells.
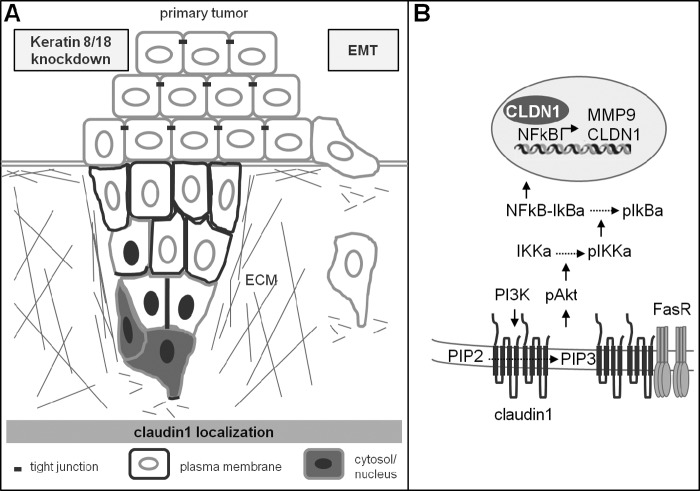
Cancer cells can migrate as single cells when undergoing EMT or can move collectively as an epithelial sheet. Collective migration requires maintenance of cell-cell contacts to provide pushing forces at the rear and to force protrusions to occur at the front, thus establishing a collective front-back polarity and directionality (75). Our results are in agreement with this concept in that at the leading edge of the migrating sheets K8/18 loss improves PI3K/Akt activation involved in the direction of the movement (76) and local matrix degradation (47, 77) through NF-κB activity. In the following cell rows, K8/18 knockdown increases the TJ protein claudin1 at the cell membrane, strengthening cell cohesion (78). This led to our proposal of a model in which K8/18 loss promotes collective migration of epithelial cancer cells in a different way than an EMT by increasing directional migration and intercellular cohesion (Fig. 8). Moreover, we show that K8/18 knockdown does not induce morphological changes or resistance to apoptosis associated with an EMT. Our results are consistent with the increasing evidence that cancer cells can become invasive without undergoing an EMT (79–81).
FIGURE 8.
Hypothetical model of epithelial cancer cell invasiveness and sensitivity mediated by K8/18 loss. K8/18 knockdown in epithelial cancer cells promotes collective migration and sensitivity to apoptosis in a different way than epithelial-mesenchymal transition. A, at the leading edge of the migrating sheets, K8/18 loss improves PI3K/Akt/NF-κB/MMP activation through an increase of claudin1 in specific regions of the plasma membrane and in the cytosol/nucleus, thus promoting the direction of the movement and local matrix degradation. In the following cell rows, K8/18 loss promotes cohesion between neighboring cells by increasing claudin1 at the plasma membrane. B, localized accumulation of claudin1 at the plasma membrane generates specific membrane regions suitable for PI3K/Akt activation, which leads to an increase of NF-κB nuclear translocation and transcriptional activity. NF-κB induces MMP2 and MMP9 expression as well as claudin1 expression, revealing a positive feedback process. K8/18 loss also increases FasR membrane targeting in a claudin1-dependent manner, thus enhancing cisplatin-induced apoptosis by the extrinsic pathway. ECM, extracellular matrix; IKK, IκB kinase; pAkt, phospho-Akt; pIκBa, phospho-IκBa.
In summary, our current findings demonstrate a functional role for the K8/18 cytoskeleton in the restraint of epithelial cancer cell motility, invasion, and cisplatin sensitivity. K8/18 loss enhances CLDN1 expression through NF-κB, activating the PI3K/Akt/NF-κB pathway and increasing FasR membrane targeting. To our knowledge, this is the first study to reveal that claudin1 is a target of NF-κB to promote cell motility and invasion. Our study strongly indicates that in cancer keratins should not be considered only as markers of differentiation but also as key regulators in cancer progression.
Supplementary Material
Acknowledgment
We thank Sophie Parent for guidance in the ChIP experimentations.
This work was supported in part by a research grant from the Natural Sciences and Engineering Research Council of Canada (to M. C.).

This article contains supplemental Figs. S1 and S2.
- EMT
- epithelial-mesenchymal transition
- MMP
- metalloproteinase
- IF
- intermediate filament
- K8
- keratin 8
- K18
- keratin 18
- PIP2
- phosphatidylinositol 4,5-bisphosphate
- PIP3
- phosphatidylinositol 3,4,5-trisphosphate
- PTEN
- phosphatase and tensin homolog deleted on chromosome 10
- PARP
- poly(ADP-ribose) polymerase
- IGF-1
- insulin-like growth factor-1
- NF-κB
- nuclear factor of κ light polypeptide gene enhancer in B-cells
- IκBα
- NF-κB inhibitor α
- TJ
- tight junction
- CLDN1
- claudin1
- XIAP
- X-linked inhibitor of apoptosis protein
- sFLIP
- short variant of FLICE-like inhibitory protein
- FLICE
- Fas-associated death domain-like interleukin-1b-converting enzyme
- FasR
- Fas receptor
- NC
- negative control.
REFERENCES
- 1. Kalluri R., Weinberg R. A. (2009) The basics of epithelial-mesenchymal transition. J. Clin. Investig. 119, 1420–1428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Thiery J. P., Sleeman J. P. (2006) Complex networks orchestrate epithelial-mesenchymal transitions. Nat. Rev. Mol. Cell Biol. 7, 131–142 [DOI] [PubMed] [Google Scholar]
- 3. Moll R., Divo M., Langbein L. (2008) The human keratins: biology and pathology. Histochem. Cell Biol. 129, 705–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eriksson J. E., Dechat T., Grin B., Helfand B., Mendez M., Pallari H. M., Goldman R. D. (2009) Introducing intermediate filaments: from discovery to disease. J. Clin. Investig. 119, 1763–1771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Toivola D. M., Tao G. Z., Habtezion A., Liao J., Omary M. B. (2005) Cellular integrity plus: organelle-related and protein-targeting functions of intermediate filaments. Trends Cell Biol. 15, 608–617 [DOI] [PubMed] [Google Scholar]
- 6. Ku N. O., Omary M. B. (2006) A disease- and phosphorylation-related nonmechanical function for keratin 8. J. Cell Biol. 174, 115–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zatloukal K., Stumptner C., Lehner M., Denk H., Baribault H., Eshkind L. G., Franke W. W. (2000) Cytokeratin 8 protects from hepatotoxicity, and its ratio to cytokeratin 18 determines the ability of hepatocytes to form Mallory bodies. Am. J. Pathol. 156, 1263–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kim S., Wong P., Coulombe P. A. (2006) A keratin cytoskeletal protein regulates protein synthesis and epithelial cell growth. Nature 441, 362–365 [DOI] [PubMed] [Google Scholar]
- 9. Kim S., Kellner J., Lee C. H., Coulombe P. A. (2007) Interaction between the keratin cytoskeleton and eEF1Bγ affects protein synthesis in epithelial cells. Nat. Struct. Mol. Biol. 14, 982–983 [DOI] [PubMed] [Google Scholar]
- 10. Galarneau L., Loranger A., Gilbert S., Marceau N. (2007) Keratins modulate hepatic cell adhesion, size and G1/S transition. Exp. Cell Res. 313, 179–194 [DOI] [PubMed] [Google Scholar]
- 11. Ku N. O., Michie S., Resurreccion E. Z., Broome R. L., Omary M. B. (2002) Keratin binding to 14-3-3 proteins modulates keratin filaments and hepatocyte mitotic progression. Proc. Natl. Acad. Sci. U.S.A. 99, 4373–4378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Toivola D. M., Nieminen M. I., Hesse M., He T., Baribault H., Magin T. M., Omary M. B., Eriksson J. E. (2001) Disturbances in hepatic cell-cycle regulation in mice with assembly-deficient keratins 8/18. Hepatology 34, 1174–1183 [DOI] [PubMed] [Google Scholar]
- 13. Inada H., Izawa I., Nishizawa M., Fujita E., Kiyono T., Takahashi T., Momoi T., Inagaki M. (2001) Keratin attenuates tumor necrosis factor-induced cytotoxicity through association with TRADD. J. Cell Biol. 155, 415–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gilbert S., Loranger A., Daigle N., Marceau N. (2001) Simple epithelium keratins 8 and 18 provide resistance to Fas-mediated apoptosis. The protection occurs through a receptor-targeting modulation. J. Cell Biol. 154, 763–773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Caulin C., Ware C. F., Magin T. M., Oshima R. G. (2000) Keratin-dependent, epithelial resistance to tumor necrosis factor-induced apoptosis. J. Cell Biol. 149, 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ku N. O., Soetikno R. M., Omary M. B. (2003) Keratin mutation in transgenic mice predisposes to Fas but not TNF-induced apoptosis and massive liver injury. Hepatology 37, 1006–1014 [DOI] [PubMed] [Google Scholar]
- 17. Long H. A., Boczonadi V., McInroy L., Goldberg M., Määttä A. (2006) Periplakin-dependent re-organisation of keratin cytoskeleton and loss of collective migration in keratin-8-downregulated epithelial sheets. J. Cell Sci. 119, 5147–5159 [DOI] [PubMed] [Google Scholar]
- 18. Bühler H., Schaller G. (2005) Transfection of keratin 18 gene in human breast cancer cells causes induction of adhesion proteins and dramatic regression of malignancy in vitro and in vivo. Mol. Cancer Res. 3, 365–371 [DOI] [PubMed] [Google Scholar]
- 19. Schaller G., Fuchs I., Pritze W., Ebert A., Herbst H., Pantel K., Weitzel H., Lengyel E. (1996) Elevated keratin 18 protein expression indicates a favorable prognosis in patients with breast cancer. Clin. Cancer Res. 2, 1879–1885 [PubMed] [Google Scholar]
- 20. Fortier A. M., Van Themsche C., Asselin E., Cadrin M. (2010) Akt isoforms regulate intermediate filament protein levels in epithelial carcinoma cells. FEBS Lett. 584, 984–988 [DOI] [PubMed] [Google Scholar]
- 21. Testa J. R., Bellacosa A. (2001) AKT plays a central role in tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 98, 10983–10985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Larue L., Bellacosa A. (2005) Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene 24, 7443–7454 [DOI] [PubMed] [Google Scholar]
- 23. Bakin A. V., Tomlinson A. K., Bhowmick N. A., Moses H. L., Arteaga C. L. (2000) Phosphatidylinositol 3-kinase function is required for transforming growth factor β-mediated epithelial to mesenchymal transition and cell migration. J. Biol. Chem. 275, 36803–36810 [DOI] [PubMed] [Google Scholar]
- 24. Fortier A. M., Asselin E., Cadrin M. (2011) Functional specificity of Akt isoforms in cancer progression. Biomol. Concepts 1–2, 1–11 [DOI] [PubMed] [Google Scholar]
- 25. Alessi D. R., James S. R., Downes C. P., Holmes A. B., Gaffney P. R., Reese C. B., Cohen P. (1997) Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 7, 261–269 [DOI] [PubMed] [Google Scholar]
- 26. Zhang S., Yu D. (2010) PI(3)king apart PTEN's role in cancer. Clin. Cancer Res. 16, 4325–4330 [DOI] [PubMed] [Google Scholar]
- 27. Gassama-Diagne A., Yu W., ter Beest M., Martin-Belmonte F., Kierbel A., Engel J., Mostov K. (2006) Phosphatidylinositol-3,4,5-trisphosphate regulates the formation of the basolateral plasma membrane in epithelial cells. Nat. Cell Biol. 8, 963–970 [DOI] [PubMed] [Google Scholar]
- 28. Martin-Belmonte F., Gassama A., Datta A., Yu W., Rescher U., Gerke V., Mostov K. (2007) PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell 128, 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Funamoto S., Meili R., Lee S., Parry L., Firtel R. A. (2002) Spatial and temporal regulation of 3-phosphoinositides by PI 3-kinase and PTEN mediates chemotaxis. Cell 109, 611–623 [DOI] [PubMed] [Google Scholar]
- 30. Iijima M., Devreotes P. (2002) Tumor suppressor PTEN mediates sensing of chemoattractant gradients. Cell 109, 599–610 [DOI] [PubMed] [Google Scholar]
- 31. Van Themsche C., Mathieu I., Parent S., Asselin E. (2007) Transforming growth factor-β3 increases the invasiveness of endometrial carcinoma cells through phosphatidylinositol 3-kinase-dependent up-regulation of X-linked inhibitor of apoptosis and protein kinase C-dependent induction of matrix metalloproteinase-9. J. Biol. Chem. 282, 4794–4802 [DOI] [PubMed] [Google Scholar]
- 32. Chou C. F., Omary M. B. (1993) Mitotic arrest-associated enhancement of O-linked glycosylation and phosphorylation of human keratins 8 and 18. J. Biol. Chem. 268, 4465–4472 [PubMed] [Google Scholar]
- 33. Gagnon V., Van Themsche C., Turner S., Leblanc V., Asselin E. (2008) Akt and XIAP regulate the sensitivity of human uterine cancer cells to cisplatin, doxorubicin and Taxol. Apoptosis 13, 259–271 [DOI] [PubMed] [Google Scholar]
- 34. Du D., Xu F., Yu L., Zhang C., Lu X., Yuan H., Huang Q., Zhang F., Bao H., Jia L., Wu X., Zhu X., Zhang X., Zhang Z., Chen Z. (2010) The tight junction protein, occludin, regulates the directional migration of epithelial cells. Dev. Cell 18, 52–63 [DOI] [PubMed] [Google Scholar]
- 35. Pfaffl M. W. (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29, e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chaudhry P., Singh M., Parent S., Asselin E. (2012) Prostate apoptosis response 4 (Par-4), a novel substrate of caspase-3 during apoptosis activation. Mol. Cell. Biol. 32, 826–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stokoe D., Stephens L. R., Copeland T., Gaffney P. R., Reese C. B., Painter G. F., Holmes A. B., McCormick F., Hawkins P. T. (1997) Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567–570 [DOI] [PubMed] [Google Scholar]
- 38. Currie R. A., Walker K. S., Gray A., Deak M., Casamayor A., Downes C. P., Cohen P., Alessi D. R., Lucocq J. (1999) Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 337, 575–583 [PMC free article] [PubMed] [Google Scholar]
- 39. Santi S. A., Lee H. (2010) The Akt isoforms are present at distinct subcellular locations. Am. J. Physiol. Cell Physiol. 298, C580–C591 [DOI] [PubMed] [Google Scholar]
- 40. Min C., Eddy S. F., Sherr D. H., Sonenshein G. E. (2008) NF-κB and epithelial to mesenchymal transition of cancer. J. Cell. Biochem. 104, 733–744 [DOI] [PubMed] [Google Scholar]
- 41. Inoue J., Gohda J., Akiyama T., Semba K. (2007) NF-κB activation in development and progression of cancer. Cancer Sci. 98, 268–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sjö A., Magnusson K. E., Peterson K. H. (2010) Protein kinase C activation has distinct effects on the localization, phosphorylation and detergent solubility of the claudin protein family in tight and leaky epithelial cells. J. Membr. Biol. 236, 181–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gregory M., Dufresne J., Hermo L., Cyr D. (2001) Claudin-1 is not restricted to tight junctions in the rat epididymis. Endocrinology 142, 854–863 [DOI] [PubMed] [Google Scholar]
- 44. Leotlela P. D., Wade M. S., Duray P. H., Rhode M. J., Brown H. F., Rosenthal D. T., Dissanayake S. K., Earley R., Indig F. E., Nickoloff B. J., Taub D. D., Kallioniemi O. P., Meltzer P., Morin P. J., Weeraratna A. T. (2007) Claudin-1 overexpression in melanoma is regulated by PKC and contributes to melanoma cell motility. Oncogene 26, 3846–3856 [DOI] [PubMed] [Google Scholar]
- 45. French A. D., Fiori J. L., Camilli T. C., Leotlela P. D., O'Connell M. P., Frank B. P., Subaran S., Indig F. E., Taub D. D., Weeraratna A. T. (2009) PKC and PKA phosphorylation affect the subcellular localization of claudin-1 in melanoma cells. Int. J. Med. Sci. 6, 93–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Dhawan P., Singh A. B., Deane N. G., No Y., Shiou S. R., Schmidt C., Neff J., Washington M. K., Beauchamp R. D. (2005) Claudin-1 regulates cellular transformation and metastatic behavior in colon cancer. J. Clin. Investig. 115, 1765–1776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kim D., Kim S., Koh H., Yoon S. O., Chung A. S., Cho K. S., Chung J. (2001) Akt/PKB promotes cancer cell invasion via increased motility and metalloproteinase production. FASEB J. 15, 1953–1962 [DOI] [PubMed] [Google Scholar]
- 48. Enomoto A., Murakami H., Asai N., Morone N., Watanabe T., Kawai K., Murakumo Y., Usukura J., Kaibuchi K., Takahashi M. (2005) Akt/PKB regulates actin organization and cell motility via Girdin/APE. Dev. Cell 9, 389–402 [DOI] [PubMed] [Google Scholar]
- 49. De Santis G., Miotti S., Mazzi M., Canevari S., Tomassetti A. (2009) E-cadherin directly contributes to PI3K/AKT activation by engaging the PI3K-p85 regulatory subunit to adherens junctions of ovarian carcinoma cells. Oncogene 28, 1206–1217 [DOI] [PubMed] [Google Scholar]
- 50. Kovacs E. M., Ali R. G., McCormack A. J., Yap A. S. (2002) E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277, 6708–6718 [DOI] [PubMed] [Google Scholar]
- 51. Miyamori H., Takino T., Kobayashi Y., Tokai H., Itoh Y., Seiki M., Sato H. (2001) Claudin promotes activation of pro-matrix metalloproteinase-2 mediated by membrane-type matrix metalloproteinases. J. Biol. Chem. 276, 28204–28211 [DOI] [PubMed] [Google Scholar]
- 52. Oku N., Sasabe E., Ueta E., Yamamoto T., Osaki T. (2006) Tight junction protein claudin-1 enhances the invasive activity of oral squamous cell carcinoma cells by promoting cleavage of laminin-5 γ2 chain via matrix metalloproteinase (MMP)-2 and membrane-type MMP-1. Cancer Res. 66, 5251–5257 [DOI] [PubMed] [Google Scholar]
- 53. Chakraborti S., Mandal M., Das S., Mandal A., Chakraborti T. (2003) Regulation of matrix metalloproteinases: an overview. Mol. Cell. Biochem. 253, 269–285 [DOI] [PubMed] [Google Scholar]
- 54. Lin M. L., Lu Y. C., Chung J. G., Wang S. G., Lin H. T., Kang S. E., Tang C. H., Ko J. L., Chen S. S. (2010) Down-regulation of MMP-2 through the p38 MAPK-NF-κB-dependent pathway by aloe-emodin leads to inhibition of nasopharyngeal carcinoma cell invasion. Mol. Carcinog. 49, 783–797 [DOI] [PubMed] [Google Scholar]
- 55. Han Y. P., Tuan T. L., Wu H., Hughes M., Garner W. L. (2001) TNF-α stimulates activation of pro-MMP2 in human skin through NF-κB mediated induction of MT1-MMP. J. Cell Sci. 114, 131–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Voulgari A., Pintzas A. (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim. Biophys. Acta 1796, 75–90 [DOI] [PubMed] [Google Scholar]
- 57. Paccione R. J., Miyazaki H., Patel V., Waseem A., Gutkind J. S., Zehner Z. E., Yeudall W. A. (2008) Keratin down-regulation in vimentin-positive cancer cells is reversible by vimentin RNA interference, which inhibits growth and motility. Mol. Cancer Ther. 7, 2894–2903 [DOI] [PubMed] [Google Scholar]
- 58. Kanaji N., Bandoh S., Ishii T., Fujita J., Ishida T., Matsunaga T., Kubo A. (2011) Cytokeratins negatively regulate the invasive potential of lung cancer cell lines. Oncol. Rep. 26, 763–768 [DOI] [PubMed] [Google Scholar]
- 59. Steed E., Balda M. S., Matter K. (2010) Dynamics and functions of tight junctions. Trends Cell Biol. 20, 142–149 [DOI] [PubMed] [Google Scholar]
- 60. Dos Reis P. P., Bharadwaj R. R., Machado J., Macmillan C., Pintilie M., Sukhai M. A., Perez-Ordonez B., Gullane P., Irish J., Kamel-Reid S. (2008) Claudin 1 overexpression increases invasion and is associated with aggressive histological features in oral squamous cell carcinoma. Cancer 113, 3169–3180 [DOI] [PubMed] [Google Scholar]
- 61. Myal Y., Leygue E., Blanchard A. A. (2010) Claudin 1 in breast tumorigenesis: revelation of a possible novel “claudin high” subset of breast cancers. J. Biomed. Biotechnol. 2010, 956897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yoon C. H., Kim M. J., Park M. J., Park I. C., Hwang S. G., An S., Choi Y. H., Yoon G., Lee S. J. (2010) Claudin-1 acts through c-Abl-protein kinase Cδ (PKCδ) signaling and has a causal role in the acquisition of invasive capacity in human liver cells. J. Biol. Chem. 285, 226–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Singh A. B., Sharma A., Dhawan P. (2010) Claudin family of proteins and cancer: an overview. J. Oncol. 2010, 541957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Dukes J. D., Fish L., Richardson J. D., Blaikley E., Burns S., Caunt C. J., Chalmers A. D., Whitley P. (2011) Functional ESCRT machinery is required for constitutive recycling of claudin-1 and maintenance of polarity in vertebrate epithelial cells. Mol. Biol. Cell 22, 3192–3205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Seltmann K., Roth W., Kröger C., Loschke F., Lederer M., Hüttelmaier S., Magin T. M. (2013) Keratins mediate localization of hemidesmosomes and repress cell motility. J. Invest. Dermatol. 133, 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Matsuda M., Kubo A., Furuse M., Tsukita S. (2004) A peculiar internalization of claudins, tight junction-specific adhesion molecules, during the intercellular movement of epithelial cells. J. Cell Sci. 117, 1247–1257 [DOI] [PubMed] [Google Scholar]
- 67. Singh A. B., Sharma A., Smith J. J., Krishnan M., Chen X., Eschrich S., Washington M. K., Yeatman T. J., Beauchamp R. D., Dhawan P. (2011) Claudin-1 up-regulates the repressor ZEB-1 to inhibit E-cadherin expression in colon cancer cells. Gastroenterology 141, 2140–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Girouard J., Lafleur M. J., Parent S., Leblanc V., Asselin E. (2013) Involvement of Akt isoforms in chemoresistance of endometrial carcinoma cells. Gynecol. Oncol. 128, 335–343 [DOI] [PubMed] [Google Scholar]
- 69. Sullivan B. T., Cherry J. A., Sakamoto H., Henkes L. E., Townson D. H., Rueda B. R. (2010) Cytokeratin 18 expression inhibits cytokine-induced death of cervical cancer cells. Int. J. Gynecol. Cancer 20, 1474–1481 [DOI] [PubMed] [Google Scholar]
- 70. Wang Y., He Q. Y., Tsao S. W., Cheung Y. H., Wong A., Chiu J. F. (2008) Cytokeratin 8 silencing in human nasopharyngeal carcinoma cells leads to cisplatin sensitization. Cancer Lett. 265, 188–196 [DOI] [PubMed] [Google Scholar]
- 71. Asselin E., Mills G. B., Tsang B. K. (2001) XIAP regulates Akt activity and caspase-3-dependent cleavage during cisplatin-induced apoptosis in human ovarian epithelial cancer cells. Cancer Res. 61, 1862–1868 [PubMed] [Google Scholar]
- 72. Amantana A., London C. A., Iversen P. L., Devi G. R. (2004) X-linked inhibitor of apoptosis protein inhibition induces apoptosis and enhances chemotherapy sensitivity in human prostate cancer cells. Mol. Cancer Ther. 3, 699–707 [PubMed] [Google Scholar]
- 73. Gilbert S., Loranger A., Marceau N. (2004) Keratins modulate c-Flip/extracellular signal-regulated kinase 1 and 2 antiapoptotic signaling in simple epithelial cells. Mol. Cell. Biol. 24, 7072–7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Beeman N., Webb P. G., Baumgartner H. K. (2012) Occludin is required for apoptosis when claudin-claudin interactions are disrupted. Cell Death Dis. 3, e273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Friedl P., Gilmour D. (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat. Rev. Mol. Cell Biol. 10, 445–457 [DOI] [PubMed] [Google Scholar]
- 76. Merlot S., Firtel R. A. (2003) Leading the way: directional sensing through phosphatidylinositol 3-kinase and other signaling pathways. J. Cell Sci. 116, 3471–3478 [DOI] [PubMed] [Google Scholar]
- 77. Park B. K., Zeng X., Glazer R. I. (2001) Akt1 induces extracellular matrix invasion and matrix metalloproteinase-2 activity in mouse mammary epithelial cells. Cancer Res. 61, 7647–7653 [PubMed] [Google Scholar]
- 78. Furuse M., Sasaki H., Fujimoto K., Tsukita S. (1998) A single gene product, claudin-1 or -2, reconstitutes tight junction strands and recruits occludin in fibroblasts. J. Cell Biol. 143, 391–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Li J., Lau G. K., Chen L., Dong S. S., Lan H. Y., Huang X. R., Li Y., Luk J. M., Yuan Y. F., Guan X. Y. (2011) Interleukin 17A promotes hepatocellular carcinoma metastasis via NF-κB induced matrix metalloproteinases 2 and 9 expression. PLoS One 6, e21816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Macpherson I. R., Hooper S., Serrels A., McGarry L., Ozanne B. W., Harrington K., Frame M. C., Sahai E., Brunton V. G. (2007) p120-catenin is required for the collective invasion of squamous cell carcinoma cells via a phosphorylation-independent mechanism. Oncogene 26, 5214–5228 [DOI] [PubMed] [Google Scholar]
- 81. Gaggioli C. (2008) Collective invasion of carcinoma cells: when the fibroblasts take the lead. Cell Adh. Migr. 2, 45–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.