Background: The molecular mechanisms underlining the immune regulatory property of mesenchymal stem cells (MSCs) are unknown.
Results: Inflammatory cytokine-induced miR-155 reverses the immunosuppressive capacity of MSCs through inhibiting iNOS expression.
Conclusion: The immune regulation-mediated by MSCs is tightly regulated by miR-155.
Significance: This study revealed a novel role of miR-155 in regulating the immune modulatory activities of MSCs.
Keywords: Immunosuppression, Mesenchymal Stem Cells, MicroRNA, Nitric Oxide, Nitric Oxide Synthase, TAB2, miR-155
Abstract
MSCs possess potent immunosuppressive capacity. We have reported that mouse MSCs inhibit T cell proliferation and function via nitric oxide. This immune regulatory capacity of MSCs is induced by the inflammatory cytokines IFNγ together with either TNFα or IL-1β. This effect of inflammatory cytokines on MSCs is extraordinary; logarithmic increases in the expression of iNOS and chemokines are often observed. To investigate the molecular mechanisms underlying this robust effect of cytokines, we examined the expression of microRNAs in MSCs before and after cytokine treatment. We found that miR-155 is most significantly up-regulated. Furthermore, our results showed that miR-155 inhibits the immunosuppressive capacity of MSCs by reducing iNOS expression. We further demonstrated that miR-155 targets TAK1-binding protein 2 (TAB2) to regulate iNOS expression. Additionally, knockdown of TAB2 reduced iNOS expression. In summary, our study demonstrated that miR-155 inhibits the immunosuppressive capacity of MSCs by reducing iNOS expression by targeting TAB2. Our data revealed a novel role of miR-155 in regulating the immune modulatory activities of MSCs.
Introduction
Mesenchymal stem cells (MSCs)4 exist in almost all tissues and have been isolated from bone marrow, adipose tissue, umbilical cord, and amniotic fluid and successfully expanded in vitro (1, 2). MSCs possess specific cell surface markers (variably express CD105, CD73, CD90, CD146, and nestin and lack CD45, CD34, CD14 or CD11b, CD79a or CD19, and MHC class II molecules), and differentiate into adipocytes, osteoblasts, and chondrocytes (3).
Since their initial isolation, MSCs have been investigated for their multipotent differentiation potential for regenerative medicine (4). Interestingly, recent studies have found that MSCs have potent immunosuppressive capacity and possess therapeutic potential for various inflammation-related diseases (5–7). These cells can effectively block T cell and B cell proliferation, NK cell cytotoxicity, and dendritic cell maturation. The mechanism of MSC-mediated immunosuppression is under intensive investigations, and various molecules including IDO, iNOS, PGE2, TGFβ, and PD-L1 have been shown to be responsible (8). Our previous studies have shown that mouse MSCs can potently inhibit T cell proliferation and function in an iNOS-dependent manner. Further studies revealed that the immunosuppressive property of MSCs is not innate but rather induced by inflammatory cytokines produced by inflammatory cells. In response to inflammatory cytokines, MSCs produce NO and chemokines, which act in concert to inhibit T cell proliferation and function (6, 9, 10).
Recent studies have shown that miRNAs are critical in the regulation of immune responses (11). These non-coding short RNAs regulate gene expression in a post-transcriptional manner by degradation of the target mRNA and/or inhibition of mRNA translation (12). miR-155 is encoded in bic, a non-coding transcript, and was found to play critical roles in cancer, immune response and hematopoiesis (12). miR-155 is induced via TLR in macrophages and dentritic cells and exerts profound effects on the activities of these cells (13–15). Most studies on miRNAs in MSCs have focused on their roles in differentiation, but whether miRNAs can regulate immunosuppressive capacity of MSCs is unknown (16).
In the present study, we found that, similar to iNOS, miR-155 is induced by inflammatory cytokines. Surprisingly, this miRNA reduces the immunosuppressive capacity of inflammatory cytokine-activated MSCs. Because NO is essential for the immunosuppressive capacity of MSCs, we examined whether miR-155 affects iNOS. Our results showed that miR-155 inhibited iNOS expression in cytokine-activated MSCs. This effect of miR-155 was not exerted directly on iNOS, but rather by targeting TAB2, a signaling protein connecting inflammatory cytokines and the NF-κB pathway (17). Therefore, our study revealed a novel role for miR-155 in regulating the immunosuppressive capacity of MSCs.
EXPERIMENTAL PROCEDURES
Cells
MSCs were generated from bone marrow of tibia and femur of 6–10-week-old mice. The cells were cultured in DMEM supplemented with 10% FBS, 2 mm glutamine, 100 units/ml penicillin, and 100 mg/ml streptomycin (Invitrogen). Non-adherent cells were removed after 24 h, and medium was replenished every 3 days.
Plasmids
The TAB2 3′-UTR was amplified with primers: acgctcgaggtgagataaaaggaaagctg and acggcggccgcctatgggataccacacag and was inserted between the XhoI and NotI of psiCheck2 to obtain plasmid psiCheck2-tab2–3′-UTR. The miR-155 target site in the TAB2 3′-UTR, UAAU, was mutated to AUUA with primers gcaatgccataaggtagaaagctaatatgaaatgaaactgtgcac and gtgcacagtttcatttcatattagctttctaccttatggcattgc to obtain plasmid psiCheck2-tab2–3′-UTR-mut. NF-κB reporter plasmid was purchased from Beyotime (Haimen, China).
Reagents
IFNγ, TNFα, IL-1α, IL-1β, anti-CD3, anti-CD28 were purchased from R&D Systems (Minneapolis, MN), Griess reagent and LPS were from Sigma-Aldrich, miR-155 mimics and inhibitor were from Genepharma (Shanghai, China), TAB2 siRNA was from Thermo Scientific (Lafayette, Colorado), Lipofectamine 2000 was from Invitrogen, Dual-Luciferase assay kit was from Promega (Madison, WI), and Pan T cell isolation kit II was from Miltenyi (Miltenyi Biotec).
Real-time PCR
Total RNA was extracted with TRIzol (Invitrogen) and reverse-transcribed into cDNA with the Taqman reverse transcription kit from ABI (Carlsbad, CA). cDNA was used as template in real-time PCR with SYBR Green reagent from Takara (Dalian, China) to determine specific gene expression. Primer sequences were as follows: GAPDH, cacattgggggtaggaacac (forward primer) and acccagaagactgtggatgg (reverse primer); TAB2, atttctggtctacgcaatcacat (forward primer) and gctgtgtgttaaagtcctgcttc (reverse primer); iNOS, cagctgggctgtacaaacctt (forward primer) and cattggaagtgaagcgtttcg (reverse primer); endothelial NOS, caacgctaccacgaggacatt (forward primer) and ctcctgcaaagaaaagctctgg (reverse primer); and neuronal NOS gggaaactctcggaggagga (forward primer) and tgagggtgaccccaaagatg (reverse primer). To determine specific miRNA expression, the Taqman miRNA reverse transcription kit and Taqman miRNA assay kit from ABI were used following the product instructions.
Griess Test
50 μl of culture supernatant and standards were transferred to a flat bottomed 96-well plate, and then 50 μl of Griess reagent was added. After incubation for 5 min in the dark at room temperature, the plate was read at 540 nm, and nitrate concentrations were calculated.
Transfection
MSCs were seeded the day before transfection in antibiotic-free medium. The next day, siRNA, miRNA mimics, or inhibitors were transfected with Lipofectamine 2000. For siRNA transfectants, cells were treated 48 h after transfection. For miRNA mimics and inhibitors, cells were treated 24 h after transfection.
Luciferase Reporter Assay
293T cells were seeded in a 24-well plate the day before transfection in antibiotic-free medium. Different amounts of miR-155 mimics were transfected with 100 ng of psiCheck2-tab2–3′-UTR or psiCheck2-tab2–3′-UTR-mut. 24 h later, cells were collected and measured with the Dual-Luciferase assay kit from Promega. For the NF-κB reporter assay, 100 nm TAB2 siRNA or control siRNA were transfected into MSCs together with NF-κB reporter plasmid. Twelve h later, MSCs were treated with IFNγ and TNFα for various times. Luciferase activity was determined as mentioned above.
Proliferation Assay
MSCs were plated in 96-well plates at different number per well. The next day, supernatant was discarded and 4 × 105 splenocytes were added per well with 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28. After 48 h, 1 μCi of [3H]methylthymidine was added per well. After another 6 h of incubation, cells were frozen and measured after being thawed.
Flow Cytometry
MSCs were transfected with Cy3-labeled control miRNA mimics. Twelve h later, the cells were harvested upon trypsinization and analyzed for Cy3 by flow cytometry using a FACS Calibur.
Statistical Analysis
Statistical significance was assessed by unpaired two-tailed Student's t test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
RESULTS
Inflammatory Cytokines Induce miR-155 in Mesenchymal Stem Cells
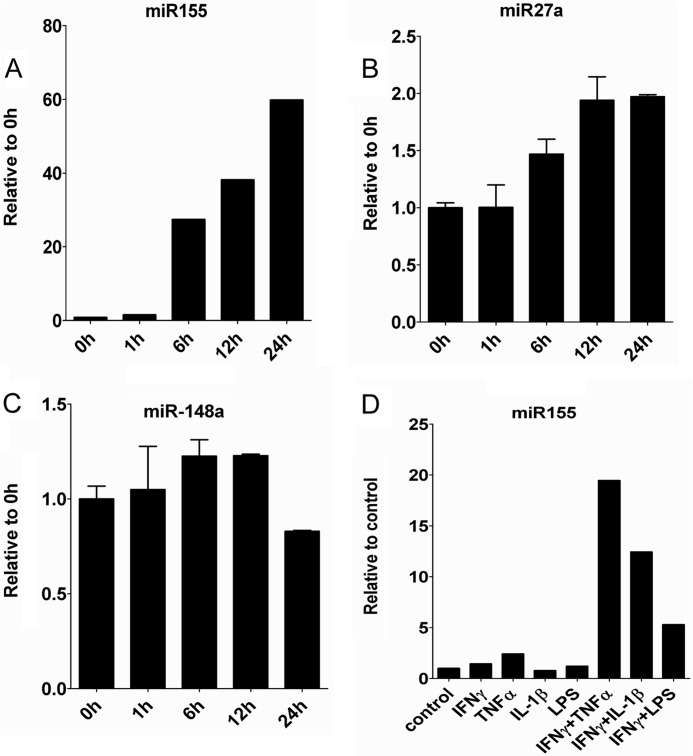
The immunosuppressive property of MSCs is not innate but is induced by the inflammatory cytokines IFNγ together with TFNα or IL-1β. This response of MSCs is extremely dramatic, with the induction of many genes at many thousand folds. To investigate the underlining molecular mechanism, we systemically analyzed miRNA expression in MSCs before and after IFNγ and TNFα treatment with microarrays. miRNAs with significant changes are listed in Table 1, and the three most significantly induced miRNAs were further analyzed with real-time PCR (Fig. 1, A–C). miR-155 exhibited the most significant increase in both microarray and real time PCR results. We therefore focused on miR-155 for further studies. Interestingly, similar to iNOS, miR-155 can also be induced by IFNγ in combination with TNFα, IL-1β, or LPS (Fig. 1D).
TABLE 1.
Differential miRNA expression in MSCs after inflammatory cytokine treatment
MSCs were treated with 10 ng/ml IFNγ and 10 ng/ml TNFα for 24 h. Total RNA was extracted with TRIzol. miRNA expression was analyzed by μParaflo® MicroRNA microarray assay (LC Sciences). (miRNAs shown are those with >2-fold change.)
| miRNA | Activated MSC/resting MSC (log2) |
|---|---|
| Mus musculus miR-155 | 6.45 |
| M. musculus miR-27a | −1.93 |
| M. musculus miR-148a | −1.73 |
| M. musculus miR-720 | 1.61 |
| M. musculus miR-1937a | 1.47 |
| M. musculus miR-762 | −1.38 |
| M. musculus miR-27b | −1.04 |
FIGURE 1.
Inflammatory cytokines induce miR-155 expression. MSCs were treated with 10 ng/ml IFNγ and 10 ng/ml TNFα for 1 h, 6 h, 12 h and 24 h, and cells were collected in TRIzol. miR-155 (A), miR-27a (B), and miR-148a (C) expression were determined with the Taqman quantitative PCR kit. D, miR-155 expression after treatment with different inflammatory cytokines for 24 h. miR-155 levels were determined as described in A.
miR-155 Negatively Regulates the Immunosuppressive Capacity of MSCs
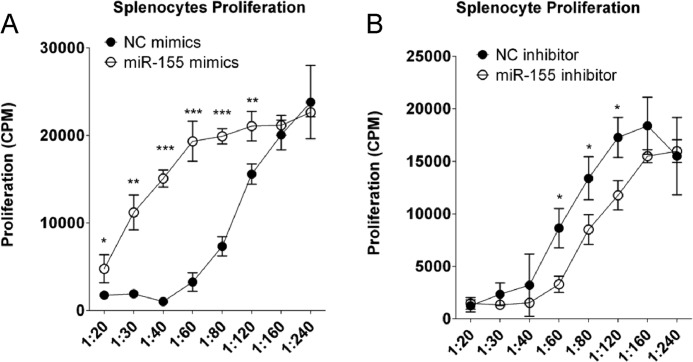
Because the immunosuppression of MSCs induced by IFNγ and TNFα was accompanied by miR-155 induction, we hypothesized that miR-155 is involved in regulating the immunosuppressive capacity of MSCs. We used T cell antigen receptor-activated T cells proliferation as a model of immune response and measured [3H]methylthymidine incorporation to determine T cell proliferation. This method can accurately reflect T cells proliferation as shown in supplemental Fig. 1, and MSCs directly inhibited T cell proliferation as shown in supplemental Fig. 2. To test this hypothesis, miR-155 mimics or inhibitor was transfected into MSCs, and the transfected cells were tested for their ability to suppress T cell antigen receptor-activated splenocytes proliferation. miR-155 mimics unexpectedly inhibited MSC-mediated immunosuppression (Fig. 2A). By contrast, miR-155 inhibitor enhanced the immunosuppressive capacity of MSCs (Fig. 2B). These results showed that miR-155 negatively regulates MSC-mediated immunosuppression.
FIGURE 2.
miR-155 negatively regulates the immunosuppression of MSCs. MSCs were transfected with 80 nm negative control (NC) or miR-155 mimics (A) or inhibitor (B). After 24 h, transfected cells were harvested upon trypsinization and seeded into 96-well plates at different cell numbers per well. After 24 h, supernatant was discarded, and 4 × 105 splenocytes were added per well with 1 μg/ml anti-CD3 and 1 μg/ml anti-CD28. After 48 h, 1 μCi of [3H]methylthymidine was added to each well. After another 6 h, cells were frozen, thawed, and harvested. The radioactivity of incorporated [3H]thymidine was measured by scintillation.
miR-155 Negatively Regulates iNOS Expression in Inflammatory Cytokine-stimulated MSCs
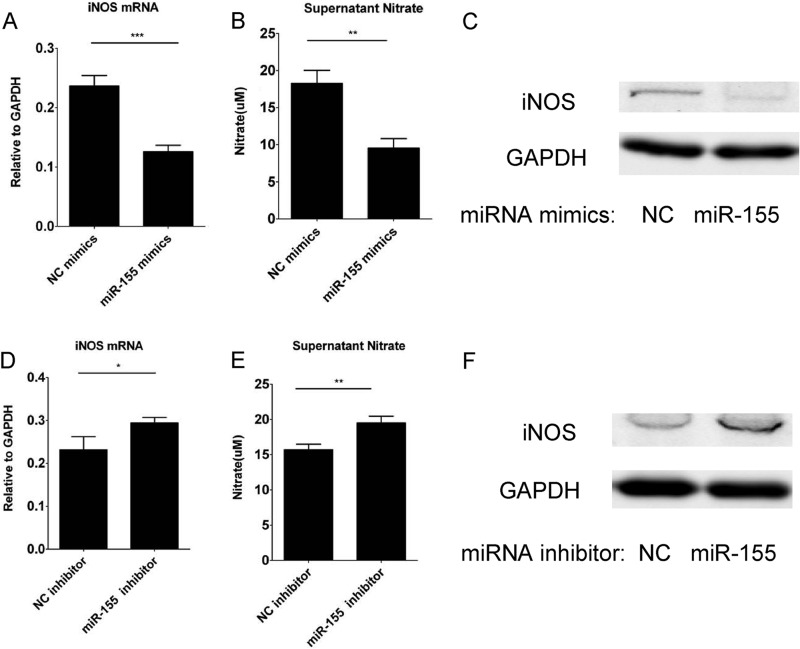
Our previous studies demonstrated that iNOS is essential for MSC-induced immunosuppression (6), and the same result was also shown in supplemental Fig. 4C. To examine whether the effect of miR-155 on immunosuppression of MSCs is exerted through regulating iNOS expression, we transfected MSCs with miR-155 mimics and inhibitors and treated the transfected cells with IFNγ and TNFα. The transfection efficiency was >90% as shown in supplemental Fig. 3. As mouse MSCs express only iNOS, but not neuronal NOS or endothelial NOS, supernatant nitrate was a measure of iNOS expression level (supplemental Fig. 4, A and B). The supernatant nitrate concentration, iNOS mRNA and iNOS protein expression was examined by Griess reagents, real-time PCR, and Western blotting analysis. Supernatant nitrate, iNOS mRNA, and protein were all reduced dramatically by miR-155 mimics transfection (Fig. 3, A–C) and enhanced by miR-155 inhibitor transfection (Fig. 3, D–F). Therefore, miR-155 inhibits the immunosuppressive capacity of MSCs by reducing iNOS expression and thereby NO release.
FIGURE 3.
miR-155 negatively regulates iNOS expression in MSCs. MSCs were transfected with 80 nm negative control (NC) or miR-155 mimics (A–C) or inhibitors (D–F). After 24 h, medium was discarded, and fresh medium either lacking (control) or containing 10 ng/ml IFNγ and 10 ng/ml TNFα was added. The level of nitrate in the supernatant (B and E), iNOS mRNA (A and D), and protein were determined 24 h later by Griess test, real-time PCR, and Western blotting analysis, respectively.
miR-155 Regulates iNOS by Targeting TAB2
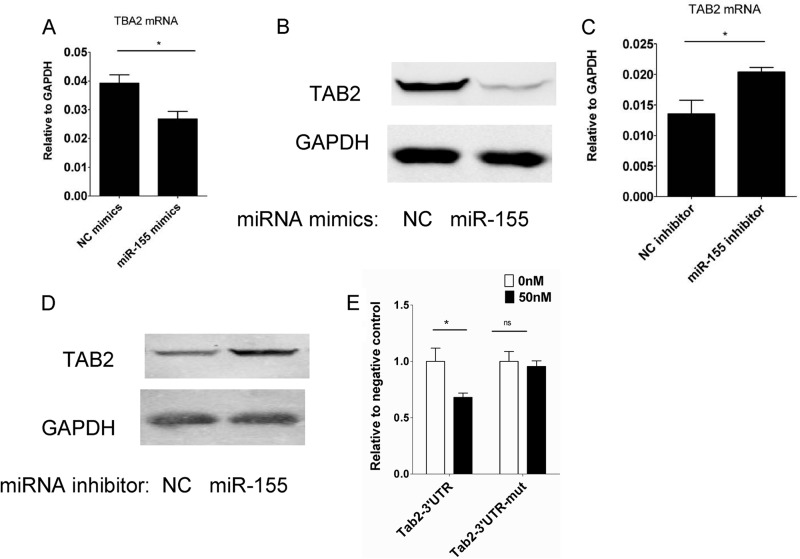
miRNAs function by decreasing the expression level of targeting genes. A search for potential targets of miR-155 in MSCs with Target Scan (18), combined with a literature search (14, 19), revealed that TAB2 is a potential target of miR-155. TAB2 is a well characterized signaling protein in the NF-κB pathway. To verify whether TAB2 is indeed a target of miR-155 in MSCs, TAB2 expression was examined in cells transfected with miR-155 mimics or inhibitor. TAB2 mRNA and protein expression level were reduced by miR-155 mimics (Fig. 4A and B). However, miR-155 inhibitor transfection increased TAB2 expression at both mRNA and protein levels (Fig. 4, C and D). Therefore, miR-155 negatively regulates TAB2 expression in MSCs. To test whether miR-155 directly targets TAB2, a TAB2 3′-UTR reporter plasmid was constructed and transfected together with miR-155 mimics into 293T cells (Fig. 4E). miR-155 reduced the reporter expression in the wild-type plasmid transfected cells but not in cells transfected with the mutant reporter plasmid. These results strongly suggested that miR-155 decreases TAB2 expression in MSCs by directly targeting TAB2 mRNA.
FIGURE 4.
TAB2 is a miR-155 target in MSCs. MSCs were transfected with 80 nm negative control (NC) or miR-155 mimics (A and B) or inhibitors (C and D). After 24 h, medium was discarded, and fresh medium containing 10 ng/ml IFNγ and 10 ng/ml TNFα was added. TAB2 mRNA and protein levels were determined 24 h later by real-time PCR and Western blotting analysis, respectively. E, psiCheck2-TAB2–3′-UTR or miR-155 target sequence mutant plasmid, psiCheck2-TAB2–3′-UTR-mut, was transfected with internal control plasmid pRL-TK and either 0 or 50 nm miR-155 mimics into 293T cells. Cells were collected and measured 24 h later with the Dual-Luciferase assay kit.
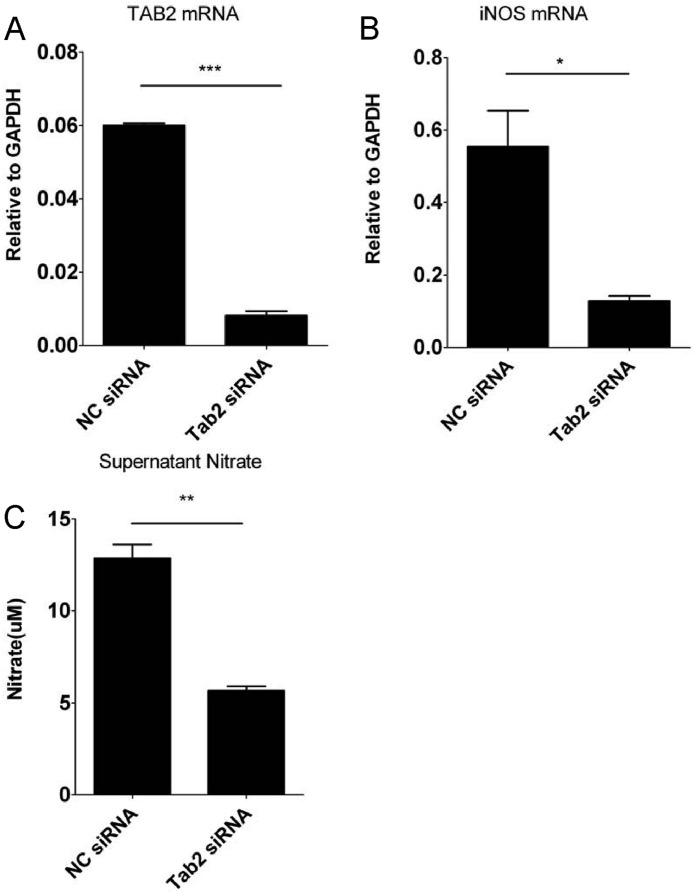
TAB2 Knockdown Reduces iNOS Expression in MSCs
We then tested whether TAB2 can regulate iNOS expression in MSCs. TAB2 siRNA or control siRNA was transfected into MSCs and TAB2 mRNA level was determined by real-time PCR. TAB2 mRNA was reduced ∼85% by siRNA against TAB2 compared with control siRNA (Fig. 5A). When the transfected cells were treated with IFNγ and TNFα, supernatant nitrate and iNOS mRNA levels were both significantly reduced in TAB2 siRNA-transfected cells (Fig. 5, B and C). To test whether the effect of TBA2 was exerted through NF-κB, we transfected MSCs with NF-κB reporter plasmid with TAB2 siRNA or control siRNA. As shown in supplemental Fig. 5, when TAB2 was knocked down, NF-κB activity was significantly decreased. Thus, we concluded that miR-155 decreases iNOS expression by targeting TAB2.
FIGURE 5.
TAB2 knockdown reduces iNOS expression in MSCs. A, MSCs were transfected with 200 nm negative control (NC) or TAB2 siRNAs. After 48 h, TAB2 mRNA levels were determined by real-time PCR. These cells were treated with 10 ng/ml IFNγ and 10 ng/ml TNFα for 24 h, and iNOS mRNA levels (B) and supernatant nitrate (C) were determined by real-time PCR and Griess test, respectively.
DISCUSSION
Mesenchymal stem cells have potent immunosuppressive effects on T cells, B cells, dentritic cells, and other inflammatory cells (8), but the detailed mechanisms are still not completely understood. Based on their immunosuppressive properties, MSCs have been used to treat various inflammatory diseases, resulting in promising results in some animal disease models. However, a better understanding of the mechanisms of immunosuppression is urgently needed to guide future clinical use (21, 22). Recent studies have found that miR-155 plays an important role in malignancies and immune responses (12). For example, macrophages and dentritic cells express miR-155 after TLR activation, which affects subsequent signaling by targeting TAB2 and SHIP1 (13–15). Several miRNAs, including miR-138, miR-335, miR-21, and miR-204 regulate differentiation of MSCs (16, 23–25). However, whether miRNAs play a role in the immunosuppression of MSCs is still unknown.
Our previous studies found that the immunosuppressive effect of MSCs was induced by inflammatory cytokines and the key effector molecule was iNOS in mouse MSCs (6, 20). Here, we found that miR-155 undergoes the most significant change after inflammatory cytokines treatment and negatively regulates the immunosuppressive capacity of MSCs by inhibiting iNOS expression. This effect of miR-155 was not exerted directly on iNOS, but rather by targeting TAB2, a signaling molecule connecting inflammatory cytokines and the NF-κB pathway. To our knowledge, this is the first time that miR-155 is shown to regulate the immunosuppressive capacity of MSCs. In inflammatory conditions, mouse MSCs express high levels of iNOS, which inhibits immune cell proliferation and function. However, excessive NO production also induces cell death of MSCs (unpublished observation). Interestingly, MSCs express miR-155 in inflammatory conditions and miR-155 negatively regulates iNOS expression. Through this mechanism, miR-155 may play a protective role for MSCs in inflammatory situations.
We and others have shown that MSCs have therapeutic effect in many inflammation related diseases. Here, we show that miR-155 can regulate the immunosuppressive capacity of MSCs. Further studies will test whether manipulating miR-155 levels can provide better therapeutic effects to treat immune disorders in preclinical and clinical settings. It is also important to decipher the role of miR-155 in endogenous MSCs in vivo.
Supplementary Material
This work was supported by grants from the Ministry of Science and Technology of China (2010CB945600 and 2011DFA30630), Scientific Innovation Project of the Chinese Academy of Science (XDA 01040107 and XDA 01040110), the National Science and Technology Project of China (31010103908 and 81273316), Shanghai Municipal Key Projects of Basic Research (12JC1409200), Shanghai Municipal Natural Science Foundation (12ZR1452600), the Knowledge Innovation Program of Shanghai Institutes for Biological Sciences, and the Chinese Academy of Sciences (2012KIP202).

This article contains supplemental Figs. 1–5.
- MSC
- bone marrow-derived mesenchymal stem cell
- TAB2
- TAK1-binding protein 2
- iNOS
- inducible NOS
- miRNA
- microRNA.
REFERENCES
- 1. Pittenger M. F., Mackay A. M., Beck S. C., Jaiswal R. K., Douglas R., Mosca J. D., Moorman M. A., Simonetti D. W., Craig S., Marshak D. R. (1999) Multilineage potential of adult human mesenchymal stem cells. Science 284, 143–147 [DOI] [PubMed] [Google Scholar]
- 2. Jiang Y., Jahagirdar B. N., Reinhardt R. L., Schwartz R. E., Keene C. D., Ortiz-Gonzalez X. R., Reyes M., Lenvik T., Lund T., Blackstad M., Du J., Aldrich S., Lisberg A., Low W. C., Largaespada D. A., Verfaillie C. M. (2002) Pluripotency of mesenchymal stem cells derived from adult marrow. Nature 418, 41–49 [DOI] [PubMed] [Google Scholar]
- 3. Horwitz E. M., Le Blanc K., Dominici M., Mueller I., Slaper-Cortenbach I., Marini F. C., Deans R. J., Krause D. S., Keating A. (2005) Clarification of the nomenclature for MSC: The International Society for Cellular Therapy position statement. Cytotherapy 7, 393–395 [DOI] [PubMed] [Google Scholar]
- 4. Caplan A. I. (2007) Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 213, 341–347 [DOI] [PubMed] [Google Scholar]
- 5. Le Blanc K., Rasmusson I., Sundberg B., Götherström C., Hassan M., Uzunel M., Ringdén O. (2004) Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 363, 1439–1441 [DOI] [PubMed] [Google Scholar]
- 6. Ren G., Zhang L., Zhao X., Xu G., Zhang Y., Roberts A. I., Zhao R. C., Shi Y. (2008) Mesenchymal stem cell-mediated immunosuppression occurs via concerted action of chemokines and nitric oxide. Cell Stem Cell 2, 141–150 [DOI] [PubMed] [Google Scholar]
- 7. Shi Y., Hu G., Su J., Li W., Chen Q., Shou P., Xu C., Chen X., Huang Y., Zhu Z., Huang X., Han X., Xie N., Ren G. (2010) Mesenchymal stem cells: a new strategy for immunosuppression and tissue repair. Cell Res. 20, 510–518 [DOI] [PubMed] [Google Scholar]
- 8. Shi Y., Su J., Roberts A. I., Shou P., Rabson A. B., Ren G. (2012) How mesenchymal stem cells interact with tissue immune responses. Trends Immunol. 33, 136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ren G., Zhao X., Zhang L., Zhang J., L'Huillier A., Ling W., Roberts A. I., Le A. D., Shi S., Shao C., Shi Y. Inflammatory cytokine-induced intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in mesenchymal stem cells are critical for immunosuppression. J. Immunol. 184, 2321–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Xu G., Zhang L., Ren G., Yuan Z., Zhang Y., Zhao R. C., Shi Y. (2007) Immunosuppressive properties of cloned bone marrow mesenchymal stem cells. Cell Res. 17, 240–248 [DOI] [PubMed] [Google Scholar]
- 11. O'Connell R. M., Rao D. S., Chaudhuri A. A., Baltimore D. (2010) Physiological and pathological roles for microRNAs in the immune system. Nat. Rev. Immunol. 10, 111–122 [DOI] [PubMed] [Google Scholar]
- 12. Baltimore D., Boldin M. P., O'Connell R. M., Rao D. S., Taganov K. D. (2008) MicroRNAs: new regulators of immune cell development and function. Nat. Immunol. 9, 839–845 [DOI] [PubMed] [Google Scholar]
- 13. O'Connell R. M., Taganov K. D., Boldin M. P., Cheng G., Baltimore D. (2007) MicroRNA-155 is induced during the macrophage inflammatory response. Proc. Natl. Acad. Sci. U.S.A. 104, 1604–1609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ceppi M., Pereira P. M., Dunand-Sauthier I., Barras E., Reith W., Santos M. A., Pierre P. (2009) MicroRNA-155 modulates the interleukin-1 signaling pathway in activated human monocyte-derived dendritic cells. Proc. Natl. Acad. Sci. U.S.A. 106, 2735–2740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Connell R. M., Chaudhuri A. A., Rao D. S., Baltimore D. (2009) Inositol phosphatase SHIP1 is a primary target of miR-155. Proc. Natl. Acad. Sci. U.S.A. 106, 7113–7118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Eskildsen T., Taipaleenmäki H., Stenvang J., Abdallah B. M., Ditzel N., Nossent A. Y., Bak M., Kauppinen S., Kassem M. (2011) MicroRNA-138 regulates osteogenic differentiation of human stromal (mesenchymal) stem cells in vivo. Proc. Natl. Acad. Sci. U.S.A. 108, 6139–6144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanayama A., Seth R. B., Sun L., Ea C. K., Hong M., Shaito A., Chiu Y. H., Deng L., Chen Z. J. (2004) TAB2 and TAB3 activate the NF-κB pathway through binding to polyubiquitin chains. Mol. Cell 15, 535–548 [DOI] [PubMed] [Google Scholar]
- 18. Lewis B. P., Shih I. H., Jones-Rhoades M. W., Bartel D. P., Burge C. B. (2003) Prediction of mammalian microRNA targets. Cell 115, 787–798 [DOI] [PubMed] [Google Scholar]
- 19. Zhou H., Huang X., Cui H., Luo X., Tang Y., Chen S., Wu L., Shen N. (2010) miR-155 and its star-form partner miR-155* cooperatively regulate type I interferon production by human plasmacytoid dendritic cells. Blood 116, 5885–5894 [DOI] [PubMed] [Google Scholar]
- 20. Ren G., Su J., Zhang L., Zhao X., Ling W., L'huillie A., Zhang J., Lu Y., Roberts A. I., Ji W., Zhang H., Rabson A. B., Shi Y. (2009) Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells 27, 1954–1962 [DOI] [PubMed] [Google Scholar]
- 21. Parekkadan B., Milwid J. M. (2010) Mesenchymal stem cells as therapeutics. Annu. Rev. Biomed. Eng. 12, 87–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Singer N. G., Caplan A. I. (2011) Mesenchymal stem cells: mechanisms of inflammation. Annu. Rev. Pathol. 6, 457–478 [DOI] [PubMed] [Google Scholar]
- 23. Huang J., Zhao L., Xing L., Chen D. (2010) MicroRNA-204 regulates Runx2 protein expression and mesenchymal progenitor cell differentiation. Stem Cells 28, 357–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lakshmipathy U., Hart R. P. (2008) Concise review: MicroRNA expression in multipotent mesenchymal stromal cells. Stem Cells 26, 356–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim Y. J., Hwang S. J., Bae Y. C., Jung J. S. (2009) MiR-21 regulates adipogenic differentiation through the modulation of TGF-β signaling in mesenchymal stem cells derived from human adipose tissue. Stem Cells 27, 3093–3102 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.