Background: STIM1-operated Orai1 channels mediate Ca2+ entry for T cell activation, but the gating mechanism remains elusive.
Results: The C and N termini of Orai1 have differential roles in STIM1-triggered channel activation.
Conclusion: The binding between STIM1 and the Orai1 C terminus docks STIM1 onto the Orai1 N terminus, leading to channel activation.
Significance: This stepwise gating mechanism contributes to ER-PM crosstalk for Ca2+ entry, a fundamental process in cell biology.
Keywords: Calcium Channels, Calcium Signaling, Calcium Transport, Gating, Ion Channels, CRAC Channel, Orai1, STIM1, Gating Mechanism, Store-operated Ca2+ Entry
Abstract
The entry of extracellular Ca2+, which is mediated by Ca2+ release-activated Ca2+ (CRAC) channels, is essential for T cell activation and the normal functioning of other immune cells. Although the molecular components of CRAC channels, the Orai1 pore-forming subunit and the STIM1-activating subunit have been recently identified, the gating mechanism by which Orai1 channels conduct Ca2+ entry upon Orai1-STIM1 interaction following Ca2+ store release remains elusive. Herein, we show that C-terminal truncations or point mutations prevented Orai1 from binding to STIM1 and subsequent channel opening. In contrast, an Orai1 mutant with an N-terminal truncation interacted with but failed to be activated by STIM1. Moreover, Orai1 channels with C-terminal disruption, but not N-terminal truncation, could be gated by fused functional domains of STIM1. Interestingly, the channel activities of Orai1 mutants carrying either an N-terminal or a C-terminal truncation were restored by a methionine mutation at the putative gating hinge, the conserved Gly-98 site in the first transmembrane segment (TM1) of Orai1. Collectively, these results support a stepwise gating mechanism of STIM1-operated Orai1 channels; the initial binding between STIM1 and the C terminus of Orai1 docks STIM1 onto the N terminus of Orai1 to initiate conformational changes of the pore-lining TM1 helix of Orai1, leading to the opening of the channel.
Introduction
The first observation that severe combined immunodeficiency (SCID) syndrome can be related to defective calcium release-activated calcium (CRAC)2 channels was made in 1996 and linked this condition to a failure of T cell activation and cytokine production (1). This T cell dysfunction was attributed to diminished store-operated Ca2+ entry through CRAC channels (2), which appears to influence Ca2+-dependent gene expression via the calcineurin/nuclear factor of activated T-cells (NFAT) pathway (3–5) and is the result of a point mutation (R91W) within the Orai1/CRACM1 gene coding for the pore-forming subunit of CRAC channels in the plasma membrane (PM) (6–9).
Endogenous CRAC channels in T lymphocytes and mast cells bear unique biophysical properties, including high Ca2+ selectivity, low unitary conductance, voltage-independent slow gating kinetics upon Ca2+ release from the endoplasmic reticulum (ER), and Ca2+-dependent inactivation through various mechanisms (10–12). Following Ca2+ store (ER) depletion, STIM1, an ER Ca2+ sensor with a single transmembrane (TM) segment, migrates to ER-PM junctions and activates CRAC channels for Ca2+ entry (13–17). Mutations (e.g. E136X) in the human STIM1 gene can also lead to SCID (18). The functional roles of the STIM1 and Orai1/CRACM1 proteins in and beyond the immune system have been progressively unveiled via gene silencing and knock-out techniques applied in cultured cells, tissues, and animal models (19–21). STIM2, the other human STIM molecule, may be involved in the regulation of the basal cytosolic Ca2+ level (22). The biophysical properties of Ca2+ channels formed by overexpressed Orai2/CRACM2 or Orai3/CRACM3, the other two human Orai proteins, are similar to that of Orai1 channels (23). However, their physiological functions have not been systematically characterized.
The full-length human Orai1 protein (301 amino acid residues) contains four TM helices and cytosolic N and C termini (6, 24, 25). Recent structural studies revealed that the Drosophila CRAC channel is composed of six Orai subunits (26). Based on site-directed mutagenesis studies, a conserved glutamate residue, Glu-106, in the first extracellular loop of Orai1 is critical for Ca2+ selectivity (7–9). Intracellular Ca2+ store depletion has been shown to strengthen the dynamic interaction between the STIM and Orai proteins for channel activation (7). STIM1 was suggested to form oligomers when Ca2+ is dissociated from its N-terminal EF-hand motif located in the ER lumen (27–29). STIM1 then directly binds to Orai1 through its cytosolic C terminus (30, 31). A small Orai1-binding region (called the CRAC activation domain, CAD (30), STIM1 Orai-activating region, SOAR (32), or Orai1-activating small fragment, OASF (33)) was identified in the STIM1 C terminus by mutagenesis studies. The molecular structure of SOAR was recently determined by x-ray crystallography studies (PDB ID codes 3TEQ (hSOAR) and 3TER (ceSTIM1-CCR)) (34). Removing the entire N or C terminus of Orai1 impairs channel function, and both fragments appear to interact with STIM1 despite a much lower binding affinity of the Orai1 N terminus to STIM1 (30–32, 35–37). However, the mechanisms by which the STIM1-Orai1 interaction triggers channel opening and the detailed contributions of the individual cytosolic regions of Orai1 to channel gating are still unclear. In the present study, we differentiated the contributions and roles of the N and C termini of Orai1 in channel activation and demonstrated the dominance of a methionine mutation at a conserved glycine site (i.e. the putative gating hinge) for channel opening with either full-length or truncated Orai1. Based on these results, we propose a three-step gating model for STIM1-operated Orai1 channels.
EXPERIMENTAL PROCEDURES
Molecular Cloning and Mutagenesis
The generation of pcDNA3/humanSTIM1, pcDNA5/Myc-humanOrai1, and enhanced green fluorescent protein (eGFP)-tagged human Orai1 was described previously (15, 38, 39). mCherry-tagged CAD (residues 342–448 of full-length human STIM1) was created by ligating the PCR-amplified CAD fragment into the XhoI and KpnI sites of the pmCherry-C1 vector (Clontech). HumanOrai1-SS-eGFP was received as a gift from Dr. T. Xu, Dr. P. Xu, and Dr. M. Prakriya. pcDNA3.1/Drosophila Orai (dOrai) was created by subcloning Drosophila Orai from the pAc5.1/V5-His B vector to the pcDNA3.1 (+) vector (25). Fifty human Orai1 mutants, seven Orai1-SS-eGFP mutants, and one dOrai mutant were tested in this study; the cloning protocols are summarized in the supplemental Experimental Procedures.
Cells
Human embryonic kidney (HEK) 293A cells obtained from Invitrogen were maintained in Dulbecco's modified Eagle's medium (Lonza) supplemented with 10% fetal bovine serum (Omega Scientific) and 2 mm l-glutamine (Sigma). HEK293A cells were transfected using Lipofectamine 2000 (Invitrogen) and used after 24 h for patch clamp electrophysiology, [Ca2+]i imaging, or confocal imaging.
Whole-cell Recording
Whole-cell recordings were performed using transfected HEK293A cells as described previously (38, 40), using the procedures and solutions described in the supplemental Experimental Procedures (supplemental Table S1). Data were analyzed with OriginPro 8 software (OriginLab) and are expressed as means ± S.E. (supplemental Table S2).
Single-cell [Ca2+]i Imaging
Ratiometric [Ca2+]i imaging was performed on an IX-81 microscope (Olympus)-based system as described previously (38, 40). HEK293A cells were incubated with 1 μm Fura-2 AM in the culture medium at 37 °C for 30 min. Transfected cells were identified by the presence of fused or co-expressed eGFP or maxGFP (Lonza). Semrock filters were used to minimize contamination of the Fura-2 fluorescence by the GFP fluorescence. Data were analyzed with MetaFluor software (Universal Imaging) and OriginPro 8 software (OriginLab) and are expressed as means ± S.E. (supplemental Table S2).
RESULTS
Orai1 Mutants with C-terminal Truncations Fail to Recruit the CAD Domain to the Peripheral Regions of the PM
To elucidate how STIM1 interacts with Orai1 for CRAC channel activation and to determine the role of the individual intracellular termini of Orai1 in this process, a series of Orai1 truncations and Orai1 point mutants was systematically generated (Fig. 1). First, the C terminus of Orai1 was incrementally removed up to the predicted membrane boundary of the TM4 segment. The eGFP-tagged Orai1-ΔC1-(1–276) and Orai1-ΔC2-(1–266) truncations, in which a predicted coiled-coil domain (36) was deleted, were well expressed in the peripheral regions of the PM of HEK293A cells (supplemental Fig. S1A). However, the majority of eGFP-Orai1-ΔC3-(1–256) failed to incorporate into the PM (supplemental Fig. S1A), most likely due to protein misfolding. In parallel, the L273S point mutation was introduced into the C terminus of eGFP-Orai1. The residue equivalent to this leucine in the Drosophila Orai proteins was recently shown to contribute to a hydrophobic patch, packing an antiparallel coiled-coil motif between the C termini of two Drosophila Orai subunits in a hexamer (26). The serine substitution at the 273 site was reported to prevent the STIM1-Orai1 interaction, possibly by disrupting the short coiled-coil motif within the C terminus of Orai1 (36). In the present study, we showed that HEK293A cells overexpressing STIM1 plus Orai1-L273S exhibited diminished store-operated Ca2+ entry as compared with control cells (Fig. 2, B and D). In another set of eGFP-tagged Orai1 truncations, the N termini were progressively truncated. Orai1-ΔN1-(74–301) lacked the first 73 nonconserved residues, and Orai1-ΔN2-(81–301) bore part of the putative interface for a second physical contact with STIM1. Both were dominantly expressed in the peripheral regions of the PM (supplemental Fig. S1A). However, eGFP-Orai1-ΔN3-(88–301) was not stably presented on or near the PM (supplemental Fig. S1A). Thus, Orai1-ΔC3 and Orai1-ΔN3 were not followed for further study.
FIGURE 1.
Schematic diagram of the Orai1 mutants with truncations or point mutations described in the present study.
FIGURE 2.
Both intracellular termini of Orai1 are indispensable for STIM1-mediated channel activation. A, confocal images of HEK293A cells transfected with mCherry-CAD (red) alone or in combination with full-length eGFP-Orai1-E106A (106A, 301 residues, green) or truncated eGFP-Orai1-E106A: ΔC1 (1–276), ΔC2 (1–266), or ΔN2 (81–301) are presented. The CAD of STIM1 was not recruited to the peripheral regions of the PM by the Orai1-E106A-ΔC1 or ΔC2 mutants. Scale bar, 10 μm. B and C, representative intracellular free Ca2+ traces ([Ca2+]i, mean ± S.E.) show TG-triggered store-operated Ca2+ entry in HEK293A cells transfected with (i) STIM1 + eGFP (n = 14 cells); (ii) STIM1 + eGFP-Orai1 (positive control; n = 15); STIM1 + eGFP-Orai1 mutants: (iii) E106A (negative control; n = 14); (iv) L273S (n = 15); (v) ΔC1 (n = 10); (vi) ΔC2 (n = 9); (vii) ΔN1 (n = 5); and (viii) ΔN2 (n = 6), respectively. TG (2 μm) was used to deplete the Ca2+ store. Top bars and vertical lines on the x axis indicate the time of solution exchange (supplemental Table S1). D, averaged peak [Ca2+]i values coupled to TG-dependent Ca2+ influx for the nine groups of cells (from left to right, n = 56, 88, 24, 15, 15, 15, 16, 42, and 38 cells) represented in B and C. N/A, not available.
To evaluate the ability of the individual Orai1 mutants to interact with STIM1, the mutants were co-expressed with mCherry-tagged SOAR/CAD fragments of STIM1 (30, 32) in HEK293A cells. Instead of using full-length STIM1 or the entire cytosolic portion of STIM1 (38, 41), SOAR/CAD was selected in the present study for its higher binding affinity to Orai1 (30, 32). Confocal imaging showed that CAD was evenly distributed in the cytosol when expressed alone (Fig. 2A). However, CAD co-localized with the Orai1 channels, surrounding the peripheral regions of the PM, when co-expressed with wild-type (WT) Orai1. It is worth noting that many transfected cells did not survive, presumably due to uncontrollable Ca2+ entry resulting from the CAD-Orai1 interaction and persistent CRAC channel activation. Nevertheless, cell survival was improved by introducing the E106A point mutation to Orai1, leading to nonconducting channels with an altered selectivity filter. The majority of CAD molecules were associated with Orai1-E106A along the peripheral regions of the PM, without any detectable change in cell morphology (Fig. 2A). Interestingly, CAD was not coupled to C-terminally truncated Oria1 (Orai1-E106A-ΔC1 or Orai1-E106A-ΔC2; Fig. 2A) but was able to co-localize with N-terminally truncated Orai1 (Orai1-E106A-ΔN2; Fig. 2A). This observation is consistent with several recent studies (30, 32, 35, 36).
Both Termini of Orai1 Are Essential for Channel Activation upon Store Depletion
In a series of parallel studies, the original set of Orai1 mutants without the E106A mutation was co-expressed with full-length STIM1 in HEK293A cells for functional analysis. Thapsigargin (TG), a sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) pump inhibitor, was applied to passively deplete the Ca2+ store for CRAC channel activation, and the intracellular Ca2+ mobilization associated with TG-triggered store-operated Ca2+ entry was recorded at the single-cell level. In contrast to the confocal imaging data described above, mutations or truncations at either terminus of Orai1 markedly reduced channel activity (Fig. 2, B–D). This result is consistent with the conclusions from a few recent studies (30, 32, 35, 36), suggesting that both cytosolic regions of Orai1 are essential for channel function. To rule out the possibility that the fusion of eGFP to Orai1-ΔN2 prevented the contact between STIM1 and the remaining N terminus of Orai1-ΔN2 and thereby silenced the channel, another series of studies was performed in a set of Orai1 proteins with a simple Myc tag for identification. The Myc-Orai1-ΔN2 mutant co-expressed with STIM1 also failed to respond to store depletion induced by TG (supplemental Fig. S1, B and C). On the other hand, eGFP-Orai1-ΔN1 exhibited similar channel activities as compared with the full-length WT channels (Fig. 2, C and D). However, a reduction of channel activities in two Orai1 truncations similar to our eGFP-Orai1-ΔN1 has been reported (32, 35). This discrepancy may be due to the differences in tags/tag locations of these constructs.
Together, the results from this set of experiments suggest that the C terminus of Orai1 is the key element responsible for the initial engagement between STIM1 and Orai1. It appears that this step may stabilize the STIM1-Orai1 binding complex and allow the subsequent physical contact between STIM1 and the N terminus of Orai1 because STIM1 and the N terminus of Orai1, as measured by in vitro pulldown assays, have a very low binding affinity (30, 31). The binding may not occur in vivo unless STIM1 is adjacent to the Orai1 N terminus. In other words, the requirement for the C terminus in Orai1 channel activation may be substituted by an alternative mechanism in which STIM1 is physically placed proximal to the N terminus of Orai1 to facilitate their communication.
Differential Roles of the Intracellular Domains of Orai1 in Channel Gating
To test the above hypothesis, Orai1-SS-eGFP, which contains two tandem STIM1 cytoplasmic activation domains (residues 336–485, designated as the S domain) tethered to the C terminus of Orai1, was utilized (42, 43). This fusion protein, as reported previously (42, 43), is constitutively active and conducts a Ca2+ current with similar biophysical properties as that of endogenous CRAC channels (Fig. 3, A and C–E). Mutations in both termini of Orai1 were individually introduced into Orai1-SS-eGFP, and the corresponding channel mutants were examined for their function via [Ca2+]i monitoring and whole-cell current recording. Full-length wild-type Orai1-SS-eGFP served as the positive control, and Orai1-E106A-SS-eGFP was used as the negative control; Orai1-E106A-SS-eGFP carries an alanine substitution at the site of the channel selectivity filter, preventing Ca2+ entry (Fig. 3, A and C). Consistent with our hypothesis, the L273S mutation in the C terminus did not prevent spontaneous channel activity, as evident by the high resting [Ca2+]i levels (Fig. 3, B and C) and the large whole-cell currents that emerged from the break-in time point (Fig. 3I). To exclude the possibility that a single mutation is not sufficient to fully eliminate the binding between the C terminus of Orai1 and the linked tandem SS domain, a C-terminal truncation was generated. The resulting Orai1-ΔC1-SS-eGFP proteins retained spontaneous channel activity, which was inhibited by CRAC channel blockers, 10 μm BTP2 (44) or Gd3+ (13) (Fig. 3, B, C, and I). Orai1-ΔN1-SS-eGFP, which contains an N-terminal truncation (residues 1–73 of Orai1), also showed similar spontaneous channel activity (Fig. 3C). However, Orai1-ΔN2-SS-eGFP (truncation of residues 1–80) lost its ability to conduct Ca2+ (Fig. 3, B and C). Moreover, this N-terminal truncation did not affect the distribution of the mutant to the peripheral regions of the PM (Fig. 3F) as compared with Orai1-SS-eGFP (supplemental Fig. S2). Although a spontaneous current was detected by patch clamp recording in cells overexpressing Orai1-ΔN2-SS-eGFP (Fig. 3, G and H), the overall current level was significantly reduced as compared with that of full-length Orai1-SS-eGFP (Fig. 3I). Interestingly, the ion substitution experiments, in which choline replaced Na+, suggested that the observed residual current was primarily carried by Na+ (Fig. 3, G and H). Two Orai1-SS-eGFP mutants with double truncations at both termini of Orai1 were also tested. Although Orai1-ΔN1-ΔC1-SS-eGFP showed strong spontaneous channel activity (Fig. 3C), Orai1-ΔN2-ΔC1-SS-eGFP did not (Fig. 3, C and I). In conclusion, linking the functional S domain of STIM1 to Orai1 bypasses the requirement for the C terminus but not the N terminus of Orai1 in channel gating.
FIGURE 3.
Tethering the functional domains of STIM1 to Orai1 bypasses the requirement for the C terminus, but not the N terminus, of Orai1 for channel activation. A and B, representative averaged [Ca2+]i traces from HEK293A cells expressing eGFP (n = 14), full-length (FL) Orai1-SS-eGFP (n = 3), Orai1-E106A-SS-eGFP (n = 15), Orai1-L273S-SS-eGFP (n = 10), Orai1-ΔC1-SS-eGFP (n = 8), or Orai1-ΔN2-SS-eGFP (n = 16) are shown. Solution exchanges are indicated. Disruption of the N terminus (ΔN2), but not the C terminus (L273S or ΔC1), of Orai1-SS-eGFP abolished spontaneous Ca2+ entry. C, the summary of averaged resting [Ca2+]i levels from cells expressing eGFP (37 cells) or Orai1-SS-eGFP proteins: full length (22 cells), E106A (74 cells), L273S (29 cells), ΔC1 (26 cells), ΔN1 (18 cells), ΔN2 (69 cells), ΔN1-ΔC1 (20 cells), and ΔN2-ΔC1 (77 cells). D, representative time course of inward Orai1-SS-eGFP currents, measured at −110 mV, is shown. E, corresponding I-V curves are presented for the time points indicated in D. Ch: choline. F, Orai1-ΔN2-SS-eGFP is mainly expressed in the peripheral regions of the PM. Scale bar, 10 μm. G and H, representative time course and I-V relationship of Orai1-ΔN2-SS-eGFP currents are shown. Traces in E and H are leak-subtracted; the observed residual current after 10 μm Gd3+ (CRAC channel blocker) treatment was considered as the leak. I, break-in inward current densities (picoamperes/picofarads (pA/pF)) were averaged from cells transfected with wild-type Orai1-SS-eGFP (full-length, 9 cells), Orai1-E106A-SS-eGFP (7 cells), Orai1-L273S-SS-eGFP (4 cells), Orai1-ΔC1-SS-eGFP (7 cells), Orai1-ΔN2-SS-eGFP (10 cells), or Orai1-ΔN2-ΔC1-SS-eGFP (7 cells). *, p < 0.01 in comparison with full-length Orai1-SS-eGFP.
Orai1-G98M, Which Contains a Methionine Substitution at the Putative Gating Hinge Site, Forms Constitutively Active Channels
Based on our recent findings from the gain-of-function Orai1 mutants, Orai1-G98D and Orai1-G98P, we speculated that the Gly-98 site in the middle of the Orai1 TM1 segment serves as a gating hinge, controlling the opening of the channel after the contact between STIM1 and Orai1 (40). However, we also found that G98D mutants exhibited cellular toxicity, which is most likely attributed to the misfolding of Orai1-G98D and ER stress in association with the destabilization of its transmembrane helices by the negatively charged aspartate (Fig. 4, A–C). To address this issue, the original glycine residue was replaced with each of the other 19 amino acid residues. Eleven individual substitutions at the 98 site, all with considerably elongated side chains, were able to constitutively open the corresponding channel mutants (Fig. 4, E–G). Correlating the results of these G98X mutants with the structural differences in the side chains and backbones of their 98 sites, our current data suggest that long side chains extruding from the 98 site are likely to add certain tension, pushing the pore-lining TM1 into an “open” configuration in the absence of STIM1. Among the mutants, the Orai1-G98M mutant mediated the most store-independent Ca2+ entry (Figs. 4G and 5B) and spontaneous current without the co-expression of STIM1 (Fig. 5, C and D). Similar to Orai1-G98D and Orai1-G98P, the current conducted by Orai1-G98M was nonselective and was blocked by Gd3+ (Fig. 5, C and D). However, unlike Orai1-G98D, the Orai1-G98M protein was well tolerated in transfected cells and predominantly distributed to the peripheral regions of the PM (Figs. 4D and 5, E and F). Therefore, the G98M mutation was utilized to unveil the function of the individual Orai1 cytosolic domains in channel gating in the following studies.
FIGURE 4.
Characterization of the Orai1-G98X mutants. A, prediction of TM helices in human Orai1 proteins. B, probabilities of the original glycine residue and the individual amino acid substitutions at the Gly-98 site of Orai1 to form transmembrane helices. C and D, prediction of TM helices in Orai1-G98D (C) and Orai1-G98M (D). E and F, representative [Ca2+]i recordings show STIM1-independent spontaneous Ca2+ entry in HEK cells overexpressing Orai1-G98L (n = 8), Orai1-G98F (n = 9), Orai1-G98E (n = 3), or Orai1-G98Y (n = 12), but not in cells overexpressing Orai1-G98R (n = 9). 2-APB, 2-aminoethyl diphenylborinate. G, bar graph summarizes the averaged resting [Ca2+]i levels of cells expressing WT GFP-Orai1 or various Orai1-G98X mutants without the co-expression of STIM1 (from left to right, n = 58, 7, 11, 13, 14, 15, 15, 22, 28, 3, 2, 3, 2, 57, 3, 8, 27, 15, 8, and 16 cells). *, p < 0.01 in comparison with WT GFP-Orai1.
FIGURE 5.
G98M is a novel gain-of-function Orai1 mutation. A and B, representative [Ca2+]i responses from HEK293A cells expressing wild-type Orai1 (A, Orai1-WT, n = 5) or the Orai1-G98M mutant (B, n = 10) are presented. Gd3+-sensitive, spontaneous Ca2+ entry through Orai1-G98M channels is shown. C and D, representative time course and corresponding I-V curves of STIM1-independent, nonselective cationic currents mediated by Orai1-G98M channels are presented. The red trace in D shows currents from a representative control cell expressing WT eGFP-Orai1 alone, which was recorded under the same conditions as G98M (the black trace). Ch: choline. E and F, fluorescent images (green + differential interference contrast) of HEK cells expressing eGFP-Orai1-G98M (E) or eGFP-Orai1-G98D (F) indicate that the Orai1-G98M proteins are less toxic to host cells than the Orai1-G98D proteins. Scale bar, 10 μm.
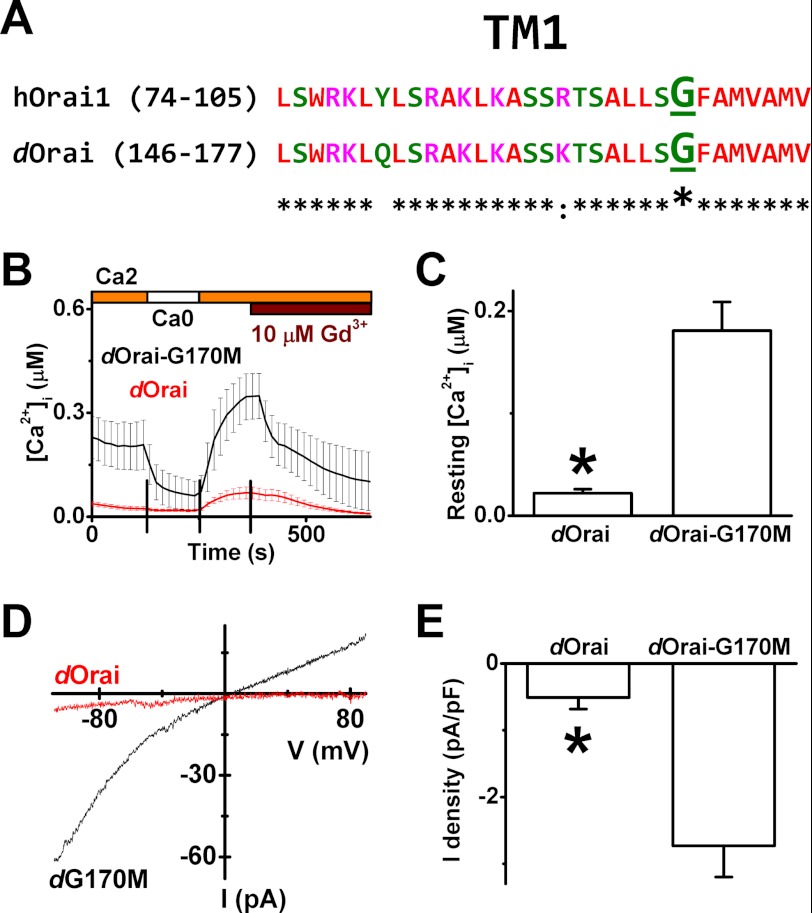
Endogenous CRAC channels formed by Orai1 and dOrai proteins in human T cells and Drosophila S2 cells, respectively, share very similar electrophysiological properties (25, 45). If the Gly-98 residue represents the gating hinge for Orai1 channel activation, the equivalent glycine in the Drosophila Orai proteins (Gly-170, Fig. 6A) should display channel-gating properties as well. To test this glycine hinge hypothesis, the Drosophila Orai-G170M mutant was generated and expressed in HEK293A cells. As expected, dOrai-G170M channels conducted spontaneous Ca2+ entry as well as unprompted current in a Gd3+ (10 μm)-sensitive manner (Fig. 6, B--E) as compared with the wild-type dOrai channels, which showed nondetectable channel activity (Fig. 6, B–E).
FIGURE 6.
Drosophila Orai-G170M channels spontaneously conduct Ca2+ influx in HEK293A cells. A, sequence alignment of the TM1 regions of the human Orai1 and Drosophila Orai proteins. B, resting [Ca2+]i levels in HEK cells co-expressing maxGFP and Drosophila Orai (n = 15) or in cells co-expressing maxGFP and dOrai-G170M (n = 5) are shown. C, averaged resting [Ca2+]i levels of cells overexpressing maxGFP plus WT dOrai (25 cells) or dOrai-G170M (27 cells) are summarized. D, representative I-V curves from cells expressing dOrai (the red trace) or dOrai-G170M (the black trace) are shown. E, comparison of the normalized break-in inward currents (−110 mV) through WT (7 cells) and dOrai-G170M mutant channels (10 cells). *, p < 0.01. pA/pF, picoamperes/picofarads.
G98M Can Gate Truncated Orai1 Channels without the Reputed STIM1-binding Sites
We speculated that STIM1 triggers conformational changes in the pore-lining TM1 above the N terminus of Orai1 for channel activation after its recruitment by the Orai1 C terminus and binding to the N terminus. Therefore, a study with gain-of-function mutations at the gating hinge, which force TM1 into an open configuration, was conducted to circumvent the requirement for STIM1 or STIM1-binding sites within both termini of Orai1 for channel gating. In this regard, an additional G98M or G98D mutation was introduced into individual Orai1 mutants (summarized in Fig. 1), and the channel activities of the resulting mutants were examined. These extra gain-of-function mutations spontaneously activated Orai1-L273S, Orai1-ΔC1, Orai1-ΔC2, Orai1-ΔN1, and Orai1-ΔN2 channels (Fig. 7 and supplemental Fig. S3) by restoring their functions without the need of the interaction with STIM1 (Fig. 8). Furthermore, Orai1-ΔN1-ΔC2-(74–266) and Orai1-ΔN2-ΔC2-(81–266), with and without the G98M mutation, were generated, and their corresponding channel activities were also analyzed. Although Orai1-ΔN1-ΔC2 and Orai1-ΔN2-ΔC2 had no channel activity when expressed alone, both G98M-ΔN1-ΔC2 and G98M-ΔN2-ΔC2 presented spontaneous channel activity that was similar to that observed with full-length G98M (Fig. 9). As a control, Orai1-ΔN2-ΔC2 was co-expressed with STIM1 and showed no significant channel activity upon store depletion (Fig. 2D). These studies reveal Orai1-ΔN2-ΔC2 as the possible minimal structure required for the functional display of Orai1 channels independent of STIM1.
FIGURE 7.
G98M restores the channel activity of Orai1 truncated at the N or C terminus. A and B, averaged cytosolic Ca2+ concentrations from HEK cells expressing: (A) Orai1-ΔC2 (n = 11) or Orai1-G98M-ΔC2 (n = 8) or (B) Orai1-ΔN2 (n = 8) or G98M-ΔN2 (n = 7). C, the bar graph presents the resting [Ca2+]i levels in cells transfected with five pairs of Orai1 cDNA constructs, with or without the G98M mutation: L273S (31 and 59 cells), ΔC1 (27 and 46 cells), ΔC2 (25 and 70 cells), ΔN1 (22 and 28 cells), and ΔN2 (36 and 46 cells), respectively. D and E, representative store-independent currents conducted by exogenous Orai1-ΔC2, G98M-ΔC2, Orai1-ΔN2, or G98M-ΔN2 channels without the co-expression of STIM1 are shown. F, comparison of the normalized break-in current amplitude at −110 mV (picoamperes/picofarads (pA/pF)) from the wild type and a series of mutated Orai1 channels to the Orai1-G98M channels with corresponding mutations: full-length (FL, 13 and 19 cells), L273S (6 and 5 cells), ΔC2 (6 and 19 cells), and ΔN2 (6 and 7 cells), respectively. All current traces in this figure are leak-subtracted; the currents after 10 μm Gd3+ treatment were considered as the leak. *, p < 0.01.
FIGURE 8.
CAD does not co-localize with the Orai1-G98M-ΔC1 or ΔC2 mutants. Confocal images show the expression of mCherry-CAD (red) alone or in combination with full-length eGFP-Orai1-G98M, eGFP-Orai1-G98M-ΔC1, or ΔC2 (green) in HEK cells. Scale bar, 10 μm. N.A., not available.
FIGURE 9.
G98M restores the channel activity of Orai1 truncated at both the N and the C termini. A, representative [Ca2+]i traces recorded from cells transfected with WT Orai1-ΔN2-ΔC2 (n = 10) or Orai1-G98M-ΔN2-ΔC2 (n = 8) are shown. B, summary of resting [Ca2+]i levels of HEK cells expressing WT Orai1-ΔN1-ΔC2 or Orai1-ΔN2-ΔC2 (red columns, 17 and 65 cells, respectively) or the corresponding Orai1-G98M truncations (black columns, 25 and 52 cells, respectively). C, overexpression of Orai1-G98M-ΔN2-ΔC2 elicits a preactivated current (the black trace), whereas Orai1-ΔN2-ΔC2 channels carry a negligible spontaneous current (the red trace). D, averaged current densities (−110 mV, break-in) from cells overexpressing WT Orai1-ΔN2-ΔC2 (n = 5) or Orai1-G98M-ΔN2-ΔC2 (n = 6) are presented. *, p < 0.01. pA/pF, picoamperes/picofarads.
DISCUSSION
An understanding of the molecular mechanisms behind ion channel gating provides decisive information that can explain the complexity of ion channel function at the cellular and system levels. The present study aims to advance our knowledge on the gating mechanism of CRAC channel, a type of voltage-independent, highly Ca2+-selective channel with low single-channel conductance, which is predominant in most nonexcitable cells and plays indispensable roles in Ca2+ signal transduction (11). Accumulating evidence suggests that CRAC channels are also crucial in refilling the Ca2+ store/sarcoplasmic reticulum in skeletal muscles during contraction (46), regulating the proliferation and migration of smooth muscle cells (47–50), triggering the hypertrophic growth of cardiomyocytes (51, 52), and modulating Ca2+ homeostasis in neurons (53, 54).
Mammalian immune cells exert their biological functions through surface receptor activation by utilizing elevated intracellular Ca2+ conducted by CRAC channels (55). This sustained Ca2+ signal redirects the cellular behavior and reprograms the global transcription network of immune cells for activation, proliferation, and cytokine secretion (21, 55). In 1993, Zweifach and Lewis (56) demonstrated that endogenous CRAC channels in T lymphocytes are activated by the depletion of intracellular Ca2+ stores. Recently, a series of studies suggested that the direct physical interaction between the CRAC pore-forming subunits (e.g. Orai1) and an ER Ca2+ sensor (STIM1) is the molecular basis for the “store-dependent operation” of Ca2+ influx (7, 29–33, 35, 36, 57). This classifies CRAC channels as ligand-gated ion channels, which have a relatively large protein ligand (STIM1) as compared with the neurotransmitters, cyclic nucleotides, and ATP that are present in other types of ligand-gated ion channels. Thus, it is essential to understand the nature of the interaction between STIM1 and Orai1 and how this interaction triggers the opening of Orai1 channels. In the present study, we propose a three-step gating model to elucidate the unique gating mechanism of CRAC channels upon the STIM1-Orai1 interaction (Fig. 10).
FIGURE 10.
Schematic gating model for Orai1 channels upon STIM1 activation. A, two subunits of an Orai1 channel are shown for clarity. The Glu-106, Gly-98, and Arg-91 sites are indicated. Prior to store depletion, STIM1 molecules are not engaged with Orai1, and the channel remains closed. SAM, sterile α-motif. B, the three sequential steps of STIM1-operated channel activation are highlighted in green. Upon Ca2+ release from the ER, the C terminus of Orai1 directly binds to STIM1 (Step 1) and docks STIM1 onto the N terminus of Orai1 (Step 2), which leads to the conformational change of the pore-lining TM1 of Orai1 (Step 3) to gate the channel for Ca2+ entry. This model does not imply a particular stoichiometry between STIM1 and Orai1 in this gating process.
In Step 1, STIM1 interacts with the C terminus of Orai1 following Ca2+ release from the ER. The importance of this first step, the initial binding between STIM1 and the C terminus of Orai1, is supported by two lines of evidence. First, truncations (ΔC1 or ΔC2) or a point mutation (L273S) in the C terminus of Orai1 prevents Orai1 gating upon TG-triggered store depletion (Fig. 2, B and D), presumably by disrupting its interaction with the SOAR/CAD region of STIM1 (Fig. 2A), and second, these mutants are able to form functional channels with an additional gain-of-function mutation at the putative gating hinge (Fig. 7). The fact that the currents through Orai1-ΔC2-G98M channels (Fig. 7D) are indistinguishable from the currents mediated by full-length Orai1-G98M channels (Fig. 5D) indicates that removing the C terminus of Orai1 does not significantly influence the overall structure of the channels. We speculate that the C terminus of Orai1 serves as a molecular post to tie the accessible STIM1 molecules to the Orai1 channels upon store depletion and to position STIM1 proximal to the pore region of the Orai1 channels.
In Step 2, after binding to the Orai1 C terminus, STIM1 is in proximity to the N terminus of Orai1, allowing for its subsequent interaction. Initially, the functional data from Orai1 mutants carrying N-terminal truncations indicated that the deletion of this region leads to diminished channel function (32, 35–37). Subsequently, the results from in vitro co-immunoprecipitation and pulldown assays between the C terminus of STIM1 and Orai1 N-terminal fragments suggested their physical interaction (30, 31). However, the functional role of this interaction remained unclear. In the present study, our patch clamp and Ca2+ imaging data revealed that the Orai1 and Orai1-SS-eGFP mutants that lacked the putative interface for the physical contact between the Orai1 N terminus and STIM1 were defective (Fig. 2, C and D and Fig. 3), similar to the Orai1 mutants with disrupted C termini. In contrast, the Orai1-L273S-SS-eGFP and Orai1-ΔC1-SS-eGFP mutants retained their channel functions (Fig. 3). Together, these results indicate that the interaction between STIM1 and the N terminus of Orai1 is functionally vital for channel gating after the initial binding between STIM1 and the C terminus of Orai1. It appears that this second binding event has a very low affinity (30, 31). Thus, the first binding event may stabilize the STIM1-Orai1 complex and provide an opportunity for STIM1 to make contact with the N terminus of Orai1. This finding explains the critical role of the C terminus of Orai1 in channel activation. Steps 1 and 2 may occur simultaneously, but the functional integrity of the Orai1 N terminus does not rely on the presence of an intact C terminus.
In Step 3, the binding of STIM1 to the Orai1 N terminus triggers conformational changes of the pore-lining TM1 of Orai1, subsequently converting the channels from the closed state to the open state. We previously suggested the presence of a glycine gating hinge in Orai1 channels: the Gly-98 site lying in the middle of the TM1 helix (40). Note that the Gly-98 site of Orai1 is located directly above its N terminus. Mutants with point mutations at this putative gating hinge (e.g. G98D and G98M) are constitutively active (Figs. 4 and 5). Although C- and N-terminal Orai1 truncation mutants are deficient with respect to normal channel activities (Fig. 2), the additional gain-of-function mutation at the 98 site reopens the channels and bypasses the requirement for either terminus (Fig. 7). This set of data demonstrates that the channel-gating defects of the Orai1 truncation mutants are not due to the lack of a functional pore but result from the inability to form a proper interaction with STIM1. This evidence supports the dual-interaction model between STIM1 and Orai1 proposed above and also indicates that the assembly of Orai1 channels does not necessarily require the cytosolic domains of Orai1.
Collectively, based on the findings from our laboratory and others (30–32, 35, 36, 40), we suggest that the interaction of STIM1 and Orai1 is initiated between STIM1 and the C terminus of Orai1 (Step 1) followed by a second low affinity contact between STIM1 and the N terminus of Orai1 (Step 2), leading to channel activation (Step 3). Our data also indicate that both termini of Orai1 are necessary for STIM1-initiated gating but are not critical for channel assembly and opening. Because STIM1 has been suggested to modulate the ion selectivity of CRAC channels, it is possible that after STIM1 binds to the N terminus of Orai1, it pulls TM1 from the cytosolic side and reduces the “diameter” of the glutamate ring formed at the Glu-106 site to confer Ca2+ selectivity (43).
A very recent study has described the crystal structure of modified Drosophila Orai proteins (residues 132–341 of Orai, termed Oraicryst) as hexamers in the absence of Stim molecules (26). The observed structure, which is apparently in the closed conformation, shows that the C termini of Orai form dimerized helices and are not in contact with the N termini, which is consistent with a bridging role for STIM1 in linking the N and C termini of Orai1 for channel gating. Our Drosophila Orai-G170M mutant (Fig. 6) may serve as a useful model for the open conformation. In parallel, the Orai1-ΔN2-SS-eGFP and Orai1-ΔC1-SS-eGFP mutants (Fig. 3) may serve as templates to mimic the closed and open states of wild-type Orai1 channels in association with STIM1, respectively.
Supplementary Material
Acknowledgments
We thank Dr. M. D. Cahalan for providing laboratory facilities for the initial screen of Orai1-G98X mutants; T. Xu, P. Xu, and M. Prakriya for the gift of the full-length Orai1-SS-eGFP construct; and A. Webb for technical support with confocal imaging. Confocal microscopy was performed at the Integrated Microscopy and Imaging Laboratory, Texas A&M Health Science Center.
This work was supported by the 2012 Texas A&M-Weizmann Program (to S. L. Z. and X. P.), the Research Grants Program Grant 110598, Scott & White Memorial Hospital (to S. L. Z.), the Kruse Family Centennial Chair Fund, Scott & White Memorial Hospital (to L. K.), the National Basic Research Program of China (973 Program; Grant 2010CB833702 to J. H.), and National Natural Science Foundation of China (NSFC) Grant 31000515 (to C. H.).

This article contains supplemental Experimental Procedures, Tables S1 and S2, and Figs. S1–S3.
- CRAC channel
- Ca2+ release-activated Ca2+ channel
- STIM1
- stromal interaction molecule 1
- TM
- transmembrane segment
- PM
- plasma membrane
- ER
- endoplasmic reticulum
- CAD
- CRAC activation domain
- SOAR
- STIM1 Orai-activating region
- TG
- thapsigargin
- eGFP
- enhanced green fluorescent protein
- dOrai
- Drosophila Orai.
REFERENCES
- 1. Feske S., Müller J. M., Graf D., Kroczek R. A., Dräger R., Niemeyer C., Baeuerle P. A., Peter H. H., Schlesier M. (1996) Severe combined immunodeficiency due to defective binding of the nuclear factor of activated T cells in T lymphocytes of two male siblings. Eur. J. Immunol. 26, 2119–2126 [DOI] [PubMed] [Google Scholar]
- 2. Feske S., Prakriya M., Rao A., Lewis R. S. (2005) A severe defect in CRAC Ca2+ channel activation and altered K+ channel gating in T cells from immunodeficient patients. J. Exp. Med. 202, 651–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Negulescu P. A., Shastri N., Cahalan M. D. (1994) Intracellular calcium dependence of gene expression in single T lymphocytes. Proc. Natl. Acad. Sci. U.S.A. 91, 2873–2877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dolmetsch R. E., Lewis R. S., Goodnow C. C., Healy J. I. (1997) Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature 386, 855–858 [DOI] [PubMed] [Google Scholar]
- 5. Feske S., Giltnane J., Dolmetsch R., Staudt L. M., Rao A. (2001) Gene regulation mediated by calcium signals in T lymphocytes. Nat. Immunol. 2, 316–324 [DOI] [PubMed] [Google Scholar]
- 6. Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. (2006) A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature 441, 179–185 [DOI] [PubMed] [Google Scholar]
- 7. Yeromin A. V., Zhang S. L., Jiang W., Yu Y., Safrina O., Cahalan M. D. (2006) Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature 443, 226–229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Prakriya M., Feske S., Gwack Y., Srikanth S., Rao A., Hogan P. G. (2006) Orai1 is an essential pore subunit of the CRAC channel. Nature 443, 230–233 [DOI] [PubMed] [Google Scholar]
- 9. Vig M., Beck A., Billingsley J. M., Lis A., Parvez S., Peinelt C., Koomoa D. L., Soboloff J., Gill D. L., Fleig A., Kinet J. P., Penner R. (2006) CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 16, 2073–2079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lewis R. S., Cahalan M. D. (1989) Mitogen-induced oscillations of cytosolic Ca2+ and transmembrane Ca2+ current in human leukemic T cells. Cell Regul. 1, 99–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Parekh A. B., Putney J. W., Jr. (2005) Store-operated calcium channels. Physiol. Rev. 85, 757–810 [DOI] [PubMed] [Google Scholar]
- 12. Hoth M., Penner R. (1992) Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 355, 353–356 [DOI] [PubMed] [Google Scholar]
- 13. Zhang S. L., Yu Y., Roos J., Kozak J. A., Deerinck T. J., Ellisman M. H., Stauderman K. A., Cahalan M. D. (2005) STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature 437, 902–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liou J., Kim M. L., Heo W. D., Jones J. T., Myers J. W., Ferrell J. E., Jr., Meyer T. (2005) STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr. Biol. 15, 1235–1241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Roos J., DiGregorio P. J., Yeromin A. V., Ohlsen K., Lioudyno M., Zhang S., Safrina O., Kozak J. A., Wagner S. L., Cahalan M. D., Veliçelebi G., Stauderman K. A. (2005) STIM1, an essential and conserved component of store-operated Ca2+ channel function. J. Cell Biol. 169, 435–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wu M. M., Buchanan J., Luik R. M., Lewis R. S. (2006) Ca2+ store depletion causes STIM1 to accumulate in ER regions closely associated with the plasma membrane. J. Cell Biol. 174, 803–813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Luik R. M., Wu M. M., Buchanan J., Lewis R. S. (2006) The elementary unit of store-operated Ca2+ entry: local activation of CRAC channels by STIM1 at ER-plasma membrane junctions. J. Cell Biol. 174, 815–825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Picard C., McCarl C. A., Papolos A., Khalil S., Lüthy K., Hivroz C., LeDeist F., Rieux-Laucat F., Rechavi G., Rao A., Fischer A., Feske S. (2009) STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N. Engl. J. Med. 360, 1971–1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Parekh A. B. (2010) Store-operated CRAC channels: function in health and disease. Nat. Rev. Drug Discov. 9, 399–410 [DOI] [PubMed] [Google Scholar]
- 20. Beech D. J. (2012) Orai1 calcium channels in the vasculature. Pflugers Arch. 463, 635–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Feske S. (2009) ORAI1 and STIM1 deficiency in human and mice: roles of store-operated Ca2+ entry in the immune system and beyond. Immunol. Rev. 231, 189–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brandman O., Liou J., Park W. S., Meyer T. (2007) STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca2+ levels. Cell 131, 1327–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lis A., Peinelt C., Beck A., Parvez S., Monteilh-Zoller M., Fleig A., Penner R. (2007) CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr. Biol. 17, 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vig M., Peinelt C., Beck A., Koomoa D. L., Rabah D., Koblan-Huberson M., Kraft S., Turner H., Fleig A., Penner R., Kinet J. P. (2006) CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science 312, 1220–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang S. L., Yeromin A. V., Zhang X. H., Yu Y., Safrina O., Penna A., Roos J., Stauderman K. A., Cahalan M. D. (2006) Genome-wide RNAi screen of Ca2+ influx identifies genes that regulate Ca2+ release-activated Ca2+ channel activity. Proc. Natl. Acad. Sci. U.S.A. 103, 9357–9362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hou X., Pedi L., Diver M. M., Long S. B. (2012) Crystal structure of the calcium release-activated calcium channel Orai. Science 338, 1308–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stathopulos P. B., Li G. Y., Plevin M. J., Ames J. B., Ikura M. (2006) Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 281, 35855–35862 [DOI] [PubMed] [Google Scholar]
- 28. Stathopulos P. B., Zheng L., Li G. Y., Plevin M. J., Ikura M. (2008) Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell 135, 110–122 [DOI] [PubMed] [Google Scholar]
- 29. Luik R. M., Wang B., Prakriya M., Wu M. M., Lewis R. S. (2008) Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature 454, 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Park C. Y., Hoover P. J., Mullins F. M., Bachhawat P., Covington E. D., Raunser S., Walz T., Garcia K. C., Dolmetsch R. E., Lewis R. S. (2009) STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell 136, 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou Y., Meraner P., Kwon H. T., Machnes D., Oh-hora M., Zimmer J., Huang Y., Stura A., Rao A., Hogan P. G. (2010) STIM1 gates the store-operated calcium channel ORAI1 in vitro. Nat. Struct. Mol. Biol. 17, 112–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yuan J. P., Zeng W., Dorwart M. R., Choi Y. J., Worley P. F., Muallem S. (2009) SOAR and the polybasic STIM1 domains gate and regulate Orai channels. Nat. Cell Biol. 11, 337–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Muik M., Fahrner M., Derler I., Schindl R., Bergsmann J., Frischauf I., Groschner K., Romanin C. (2009) A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J. Biol. Chem. 284, 8421–8426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yang X., Jin H., Cai X., Li S., Shen Y. (2012) Structural and mechanistic insights into the activation of stromal interaction molecule 1 (STIM1). Proc. Natl. Acad. Sci. U.S.A. 109, 5657–5662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Li Z., Lu J., Xu P., Xie X., Chen L., Xu T. (2007) Mapping the interacting domains of STIM1 and Orai1 in Ca2+ release-activated Ca2+ channel activation. J. Biol. Chem. 282, 29448–29456 [DOI] [PubMed] [Google Scholar]
- 36. Muik M., Frischauf I., Derler I., Fahrner M., Bergsmann J., Eder P., Schindl R., Hesch C., Polzinger B., Fritsch R., Kahr H., Madl J., Gruber H., Groschner K., Romanin C. (2008) Dynamic coupling of the putative coiled-coil domain of ORAI1 with STIM1 mediates ORAI1 channel activation. J. Biol. Chem. 283, 8014–8022 [DOI] [PubMed] [Google Scholar]
- 37. Lis A., Zierler S., Peinelt C., Fleig A., Penner R. (2010) A single lysine in the N-terminal region of store-operated channels is critical for STIM1-mediated gating. J. Gen. Physiol. 136, 673–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zhang S. L., Kozak J. A., Jiang W., Yeromin A. V., Chen J., Yu Y., Penna A., Shen W., Chi V., Cahalan M. D. (2008) Store-dependent and -independent modes regulating Ca2+ release-activated Ca2+ channel activity of human Orai1 and Orai3. J. Biol. Chem. 283, 17662–17671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lioudyno M. I., Kozak J. A., Penna A., Safrina O., Zhang S. L., Sen D., Roos J., Stauderman K. A., Cahalan M. D. (2008) Orai1 and STIM1 move to the immunological synapse and are up-regulated during T cell activation. Proc. Natl. Acad. Sci. U.S.A. 105, 2011–2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang S. L., Yeromin A. V., Hu J., Amcheslavsky A., Zheng H., Cahalan M. D. (2011) Mutations in Orai1 transmembrane segment 1 cause STIM1-independent activation of Orai1 channels at glycine 98 and channel closure at arginine 91. Proc. Natl. Acad. Sci. U.S.A. 108, 17838–17843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Huang G. N., Zeng W., Kim J. Y., Yuan J. P., Han L., Muallem S., Worley P. F. (2006) STIM1 carboxyl-terminus activates native SOC, Icrac, and TRPC1 channels. Nat. Cell Biol. 8, 1003–1010 [DOI] [PubMed] [Google Scholar]
- 42. Li Z., Liu L., Deng Y., Ji W., Du W., Xu P., Chen L., Xu T. (2011) Graded activation of CRAC channel by binding of different numbers of STIM1 to Orai1 subunits. Cell Res. 21, 305–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McNally B. A., Somasundaram A., Yamashita M., Prakriya M. (2012) Gated regulation of CRAC channel ion selectivity by STIM1. Nature 482, 241–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zitt C., Strauss B., Schwarz E. C., Spaeth N., Rast G., Hatzelmann A., Hoth M. (2004) Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 279, 12427–12437 [DOI] [PubMed] [Google Scholar]
- 45. Yeromin A. V., Roos J., Stauderman K. A., Cahalan M. D. (2004) A store-operated calcium channel in Drosophila S2 cells. J. Gen. Physiol. 123, 167–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Stiber J., Hawkins A., Zhang Z. S., Wang S., Burch J., Graham V., Ward C. C., Seth M., Finch E., Malouf N., Williams R. S., Eu J. P., Rosenberg P. (2008) STIM1 signalling controls store-operated calcium entry required for development and contractile function in skeletal muscle. Nat. Cell Biol. 10, 688–697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Potier M., Gonzalez J. C., Motiani R. K., Abdullaev I. F., Bisaillon J. M., Singer H. A., Trebak M. (2009) Evidence for STIM1- and Orai1-dependent store-operated calcium influx through ICRAC in vascular smooth muscle cells: role in proliferation and migration. FASEB J. 23, 2425–2437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Wang Y., Deng X., Mancarella S., Hendron E., Eguchi S., Soboloff J., Tang X. D., Gill D. L. (2010) The calcium store sensor, STIM1, reciprocally controls Orai and CaV1.2 channels. Science 330, 105–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Li J., McKeown L., Ojelabi O., Stacey M., Foster R., O'Regan D., Porter K. E., Beech D. J. (2011) Nanomolar potency and selectivity of a Ca2+ release-activated Ca2+ channel inhibitor against store-operated Ca2+ entry and migration of vascular smooth muscle cells. Br. J. Pharmacol. 164, 382–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mancarella S., Potireddy S., Wang Y., Gao H., Gandhirajan R. K., Autieri M., Scalia R., Cheng Z., Wang H., Madesh M., Houser S. R., Gill D. L. (2013) Targeted STIM deletion impairs calcium homeostasis, NFAT activation, and growth of smooth muscle. FASEB J. 27, 893–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Voelkers M., Salz M., Herzog N., Frank D., Dolatabadi N., Frey N., Gude N., Friedrich O., Koch W. J., Katus H. A., Sussman M. A., Most P. (2010) Orai1 and Stim1 regulate normal and hypertrophic growth in cardiomyocytes. J Mol. Cell. Cardiol. 48, 1329–1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hulot J. S., Fauconnier J., Ramanujam D., Chaanine A., Aubart F., Sassi Y., Merkle S., Cazorla O., Ouillé A., Dupuis M., Hadri L., Jeong D., Mühlstedt S., Schmitt J., Braun A., Bénard L., Saliba Y., Laggerbauer B., Nieswandt B., Lacampagne A., Hajjar R. J., Lompré A. M., Engelhardt S. (2011) Critical role for stromal interaction molecule 1 in cardiac hypertrophy. Circulation 124, 796–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Park C. Y., Shcheglovitov A., Dolmetsch R. (2010) The CRAC channel activator STIM1 binds and inhibits L-type voltage-gated calcium channels. Science 330, 101–105 [DOI] [PubMed] [Google Scholar]
- 54. Gemes G., Bangaru M. L., Wu H. E., Tang Q., Weihrauch D., Koopmeiners A. S., Cruikshank J. M., Kwok W. M., Hogan Q. H. (2011) Store-operated Ca2+ entry in sensory neurons: functional role and the effect of painful nerve injury. J. Neurosci. 31, 3536–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hogan P. G., Lewis R. S., Rao A. (2010) Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 28, 491–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zweifach A., Lewis R. S. (1993) Mitogen-regulated Ca2+ current of T lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. U.S.A. 90, 6295–6299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Smyth J. T., Petranka J. G., Boyles R. R., DeHaven W. I., Fukushima M., Johnson K. L., Williams J. G., Putney J. W., Jr. (2009) Phosphorylation of STIM1 underlies suppression of store-operated calcium entry during mitosis. Nat. Cell Biol. 11, 1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.