Abstract
Indispensable for shortening treatment of drug-susceptible tuberculosis (TB), pyrazinamide (PZA, Z) is also essential in the treatment of multidrug-resistant (MDR)-TB. While resistance to PZA in MDR-TB is associated with poor treatment outcome, bacillary susceptibility to PZA along with the use of fluoroquinolone (FQ) and second-line injectable drugs (SLIDs) may predict improved treatment success in MDR-TB. Despite a high prevalence of PZA resistance among MDR-TB patients (10%–85%), PZA susceptibility testing is seldom performed because of technical challenges. To improve treatment of MDR-TB, we propose to: (i) classify MDR-TB into PZA-susceptible MDR-TB (ZS-MDR-TB) and PZA-resistant MDR-TB (ZR-MDR-TB); (ii) use molecular tests such as DNA sequencing (pncA, gyrA, rrs, etc.) to rapidly identify ZS-MDR-TB versus ZR-MDR-TB and susceptibility profile for FQ and SLID; (iii) refrain from using PZA in ZR-MDR-TB; and (iv) explore the feasibility of shortening the treatment duration of ZS-MDR-TB with a regimen comprising PZA plus at least two bactericidal agents especially new agents like TMC207 or PA-824 or delamanid which the bacilli are susceptible to, with one or two other agents. These measures may potentially shorten therapy, save costs, and reduce side effects of MDR-TB treatment.
Keywords: MDR-TB, pncA, pyrazinamide, susceptibility testing, therapy, tuberculosis
Drug-resistant tuberculosis (TB), especially multidrug-resistant TB (MDR-TB), defined by bacillary resistance to at least isoniazid (INH) and rifampin (RIF), and extensively drug-resistant TB (XDR-TB), poses an increasing challenge for TB control.1 XDR-TB refers to MDR-TB with additional bacillary resistance to fluoroquinolones (FQs) and one or more of the three second-line injectable drugs (SLIDs)—kanamycin, amikacin and capreomycin. WHO estimates that 500 000 MDR-TB cases occur every year.1 Treatment of MDR-TB is difficult with an average cure rate of only around 62% in the best clinics.2 In addition, the recommended treatment duration of MDR-TB, which is at least 18–24 months, is expensive and toxic in a substantial proportion of patients.
Unique role of PZA in the treatment of TB and MDR-TB
Pyrazinamide (PZA) plays a unique role in modern TB chemotherapy.3 Inclusion of PZA enables considerable shortening of the treatment period from the previously 9–12 months to 6 months, thus the drug plays a pivotal role in the current short-course chemotherapy for drug susceptible TB.4 The powerful sterilizing activity of PZA is due to its ability to kill a population of persister tubercle bacilli that are not killed by other TB drugs.4 Studies in the mouse model of TB showed that substitution of PZA, but not INH and RIF, invariably led to poorer treatment outcomes.5,6,7 Furthermore, the synergistic activity of PZA with newly developed agents such as the diarylquinoline bedaquiline suggests that the use of PZA in regimens including novel agents could improve efficacy substantially, if the organism retains susceptibility to PZA.8,9
Feasibility of establishing a simple and shortened treatment regimen for PZA-susceptible MDR-TB
There is fairly good evidence from animal and human studies that the treatment duration of ZS-MDR-TB can be shortened to a minimum of 9 months with a regimen comprising PZA accompanied by two bactericidal drugs. McCune et al.10,11 demonstrated in the mouse model that murine TB could be better sterilized with PZA plus a companion drug, especially a bactericidal one. In the treatment of drug-susceptible TB, the 2-year relapse rates of 9-month regimens comprising streptomycin, INH and PZA given daily or intermittently were only 5%–6%.12,13 A small retrospective study suggested that inclusion of PZA in the treatment regimen was associated with a favorable outcome.14 A recent observational study among second-line treatment-naive MDR-TB patients suggested that the treatment duration of MDR-TB could be shortened to a minimum of 9 months with a gatifloxacin-based regimen that contained PZA and clofazimine throughout with kanamycin, high-dose INH and prothionamide given for at least 4 months in the initial phase.15 Although the impressive treatment outcome was partially attributed to clofazimine by conjecture,15 interpretation of findings could have been confounded by PZA susceptibility, which was not checked and might be present in at least 31% of the study sample according to a systematic review.16 The feasibility of shortening TB treatment for selected MDR-TB patients was further corroborated by a recent report.17 Subsequent retrospective analysis with the same updated dataset suggests that PZA use with in vitro activity alongside later-generation FQs and SLID may considerably increase the proportion with three-month sputum culture conversion, and marginally increase that with two-year treatment success. (Chang KC et al., unpublished). However, the above results are preliminary and future prospective studies are required to assess the possibility that PZA, alongside two or three bactericidal agents might improve the treatment of ZS-MDR-TB.
Problem with PZA susceptibility testing: phenotypic tests versus molecular tests
Despite the potential importance of PZA resistance in MDR-TB treatment outcome,14,18 standard phenotypic PZA susceptibility testing is seldom performed owing to technical challenges.3 This also explains why PZA resistance data are generally unavailable in TB drug resistance surveys. However, a number of studies have demonstrated a high prevalence of PZA resistance among MDR-TB patients in different localities, ranging from 10% in Papua New Guinea19 and 25% in Turkey,20 to 49% in Thailand,21 50% in Central Africa,22 52% in South Africa,23 53% in Japan,24 55% in Taiwan,25 77% in Pakistan26 and 85% in South Korea27 and in India.28 Bacillary resistance to PZA is generally higher in XDR-TB than MDR-TB cases, ranging from 72% in Chongqing, China (Zhang WH, unpublished), 86% in South Korea,27 to 93% in FQ-resistant pre-XDR-TB in Cambodia.29 The reason for the high PZA resistance rates in many high-burden areas may be partly related to widespread use of PZA in retreatment regimens without drug susceptibility guidance or maybe false resistance. It is also possible that some of the above studies that reported very high PZA resistance among MDR-TB overestimated the PZA resistance frequency due to false resistance. However, the XDR-TB studies that reported very high PZA resistance were all based on molecular test of pncA sequencing rather than conventional PZA susceptibility testing.
There are different methods for PZA susceptibility testing such as Lowenstein–Jensen medium and 7H10/11 agar at pH 5.5, BACTEC 460 and MGIT 960 or BacT/ALERT systems at pH 6.0.30 However, PZA susceptibility testing is prone to errors,3,31,32 which arise from: (i) acidity of the medium required for PZA activity inhibits the growth of Mycobacterium tuberculosis—about 20%–25% of clinical isolates do not grow on acidic 7H10 plates (pH 5.5), and even with pH 6.0 in BACTEC 460 liquid medium, 3.5% of the strains did not grow;33 and (ii) use of too large an inoculum (over 107 bacilli/ml) leads to increase in medium pH, which then inactivates PZA.34 In a recent study, the MGIT 960 PZA susceptibility testing method was found to be even less reliable than the radioactive BACTEC 460 method giving rise to more false resistant results, presumably due to the larger inoculum used in the MGIT method. The authors suggested retesting of PZA-resistant strains by the ‘gold standard' BACTEC 460 method and pncA sequencing of PZA-resistant strains identified by the MGIT method.32 Automated PZA susceptibility testing methods, including the BACTEC 460 method, are not exempt from false resistance owing to the use of either a lower resistance breakpoint (100 µg/ml) or an inadvertently large inoculum. According to the Henderson–Hasselbalch equation, the minimum inhibitory concentration (MIC) cutoff for PZA resistance should be at least 156 µg/ml,34 rather than 100 µg/ml, which is the currently used breakpoint for PZA resistance in MGIT 960 or BACTEC 460.
To circumvent the above problems, use of nicotinamide at high concentrations (0.5–2 mg/ml) at neutral pH has been proposed as a surrogate method for PZA susceptibility testing in acidic Lowenstein–Jensen medium,3,35,36 with promising results.36,37,38 The nicotinamide test can be used potentially as an inexpensive alternative for PZA susceptibility testing in clinical microbiology laboratories, but it has a long (several weeks) turnaround time. The PZase enzyme test (the Wayne test), using PZase as a surrogate of PZA susceptibility39 may also give rise to false resistance, due to the need for a sufficiently large inoculum that inevitably increases its turnaround time.
Mutation in the pncA gene encoding PZase40 is the major mechanism for PZA resistance in M. tuberculosis.40,41,42 Although a lower percentage of pncA mutations in PZA-resistant strains, i.e. 64%43 and 72%,44 has been reported, these studies did not retest PZA-resistant strains without pncA mutations to rule out false resistance. Because of the problem of PZA susceptibility testing discussed above, the lower percentage of PZA-resistant strains, i.e., 64%43 and 72%,44 with pncA mutations is most likely due to false resistance, lack of vigorous retesting to rule out false resistance, the low resistance breakpoint (100 µg/ml PZA in BACTEC or MGIT) used, or the small number of strains analyzed in the study.43 A recent systematic review with meta-analysis showed no significant difference between pncA sequencing and the Wayne PZase test by sensitivity and specificity in detecting PZA resistance,16 indicating good correlation between pncA mutations and lack of PZase activity and PZA resistance. In analysis of PZA-resistant strains, pncA mutations were found in an average of 87% of PZA-resistant strains16 and sometimes in as high as 99% of PZA-resistant strains.45 However, some studies suggest that a few PZA susceptible strains have pncA mutations that do not appear to alter the PZase enzyme activity,46,47,48 indicating that false resistance can potentially occur by the sequencing approach. In addition, a few PZ-resistant strains with no PZase activity did not have pncA mutations,42 indicating a potential regulatory gene of pncA that may have acquired a mutation. A few genuine low level PZA-resistant strains do not have pncA or rpsA mutations.3,41,49 However, the above three situations are rare24,41,50 and do not pose a significant problem for use of pncA sequencing for rapid detection of PZA susceptibility or resistance. Nevertheless, it would be of interest to develop a database of rare mutations that are not associated with PZA resistance to guide clinical treatment. In view of the good correlation of pncA mutations and PZA resistance.41,42,45 the extremely diverse pncA mutations that are impossible to be included in current molecular tests such as MTBDRplus (Hain Lifescience) and GeneXpert (Cepheid) and new advances in sequencing technology and increasing affordability of DNA sequencing, we propose pncA sequencing as the best available molecular test for rapid PZA susceptibility testing. Although various molecular tests such as PCR single stranded conformation polymorphism (PCR-SSCP),41 microarray,51 expression of PncA protein followed by PZase activity testing,52 and line-probe assay53 have been used to detect pncA mutations in PZA-resistant strains, these tests are generally more onerous and expensive than pncA sequencing. As phenotypic PZA susceptibility testing is prone to false resistance, pncA sequencing can be more sensitive and specific than the BACTEC 460 or MGIT 960 method. Indeed, a recent study showed a disturbingly low sensitivity of MGIT 960 PZA susceptibility testing in comparison with the molecular test owing to a high false resistance rate of 68%.54 Clinical studies comparing these two tests and the molecular test with treatment outcome are needed.
Proposition
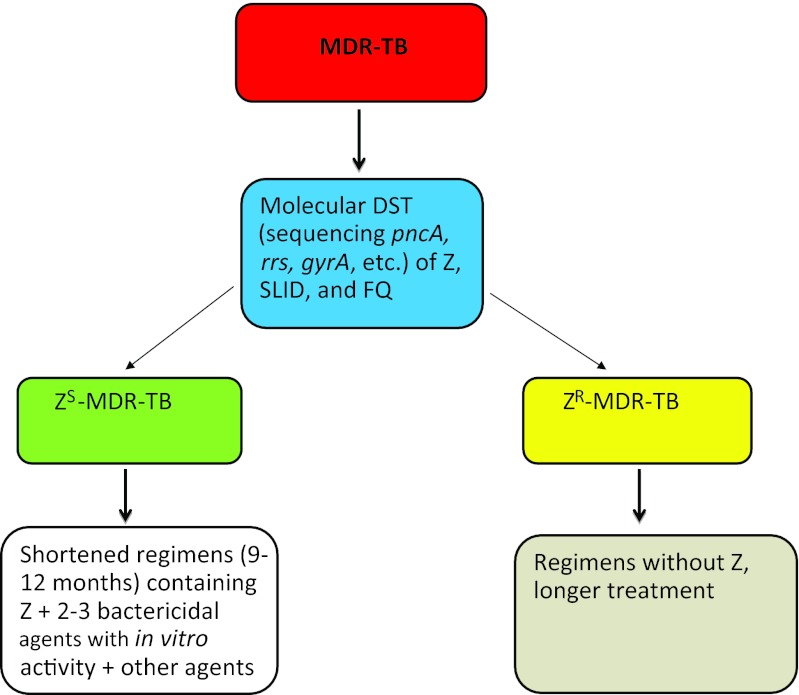
In the area of drug-resistant TB, emphasis has previously been focused on INH and RIF resistance as in MDR-TB. In addition, in the management of MDR-TB, attention has been focused on the use of FQs and SLID. However, in view of the potentially important role of PZA in treatment outcome of MDR-TB, its unique sterilizing activity, and a considerable proportion of MDR-TB strains that are susceptible to PZA (about 50% resistance on average), we propose to classify MDR-TB based on PZA susceptibility into ZS-MDR-TB and PZA-resistant MDR-TB (ZR-MDR-TB) (Figure 1). This classification may allow ZS-MDR-TB treatment to be shortened without compromising cure rates and also will improve evaluation of treatment outcomes of novel regimens in observational studies. Because of the good correlation between pncA mutations and PZA resistance, we further propose to use molecular tests such as sequencing of pncA (and FQ and SLID mutations, e.g., gyrA, rrs) to rapidly identify ZS-MDR-TB and ZR-MDR-TB with backup phenotypic tests to guide therapy. Moreover, we propose to sequence the pncA gene for all drug-resistant TB, including MDR/XDR-TB, even INH- or RIF-resistant TB. As PZA may cause hepatotoxicity,55 it may be prudent to omit PZA in the treatment of ZR-MDR-TB. Finally, with the implication of PZA susceptibility on treatment outcome of MDR-TB in human studies and its superior sterilizing activity, we suggest actively exploring a simple and shortened treatment regimen for ZS-MDR-TB (possibly 9–15 months) comprising PZA plus at least two bactericidal agents including new agents like TMC2079 or PA-82456 or delamanid57 as companion drugs, with one or two other agents. The above measures may potentially help to shorten therapy, protect against development of resistance to PZA, reduce costs and ameliorate side effects in MDR-TB treatment. Future clinical studies are needed to validate these propositions for better MDR-TB treatment.
Figure 1.
Classification of MDR-TB into PZA-susceptible and PZA-resistant MDR-TB and the potential to shorten the treatment of PZA-susceptible MDR-TB. DST, drug susceptibility test; Z, PZA.
References
- WHO WHO report 2010: Global Tuberculosis Control. 2010.
- Anti-tuberculosis Drug Resistance in the World, Report No, 4. 2008. Available at http://www.who.int/tb/publications/2008/en/index.html (accessed 13 March 2012).
- Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- Mitchison DA. The action of antituberculosis drugs in short course chemotherapy. Tubercle. 1985;66:219–225. doi: 10.1016/0041-3879(85)90040-6. [DOI] [PubMed] [Google Scholar]
- Nuermberger E, Tyagi S, Tasneen R, et al. Powerful bactericidal and sterilizing activity of a regimen containing PA-824, moxifloxacin, and pyrazinamide in a murine model of tuberculosis. Antimicrob Agents Chemother. 2008;52:1522–1524. doi: 10.1128/AAC.00074-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal IM, Zhang M, Williams KN, et al. Daily dosing of rifapentine cures tuberculosis in three months or less in the murine model. PLoS Med. 2007;4:e344. doi: 10.1371/journal.pmed.0040344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andries K, Verhasselt P, Guillemont J, et al. A diarylquinoline drug active on the ATP synthase of Mycobacterium tuberculosis. . Science. 2005;307:223–227. doi: 10.1126/science.1106753. [DOI] [PubMed] [Google Scholar]
- Ibrahim M, Andries K, Lounis N, et al. Synergistic activity of R207910 combined with pyrazinamide against murine tuberculosis. Antimicrob Agents Chemother. 2007;51:1011–1015. doi: 10.1128/AAC.00898-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diacon AH, Pym A, Grobusch M, et al. The diarylquinoline TMC207 for multidrug-resistant tuberculosis. N Engl J Med. 2009;360:2397–2405. doi: 10.1056/NEJMoa0808427. [DOI] [PubMed] [Google Scholar]
- McCune RM, Jr, McDermott W, Tompsett R. The fate of Mycobacterium tuberculosis in mouse tissues as determined by the microbial enumeration technique. II. The conversion of tuberculous infection to the latent state by the administration of pyrazinamide and a companion drug. J Exp Med. 1956;104:763–802. doi: 10.1084/jem.104.5.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune RM, Feldmann FM, Lambert HP, McDermott W. Microbial persistence. I. The capacity of tubercle bacilli to survive sterilization in mouse tissues. J Exp Med. 1966;123:445–468. doi: 10.1084/jem.123.3.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong Kong TB Treatment Service/British Medical Research Council Controlled trial of 8-month and 9-month regimens of daily and intermittent streptomycin plus isoniazid plus pyrazinamide for pulmonary tuberculosis in Hong Kong. Tubercle. 1975;56:81–96. doi: 10.1016/0041-3879(75)90020-3. [DOI] [PubMed] [Google Scholar]
- Hong Kong TB Treatment Service/British Medical Research Council Controlled trial of 6-month and 9-month regimens of daily and intermittent streptomycin plus isoniazid plus pyrazinamide for pulmonary tuberculosis in Hong Kong. The results up to 30 months. Am Rev Respir Dis. 1977;115:727–735. doi: 10.1164/arrd.1977.115.5.727. [DOI] [PubMed] [Google Scholar]
- Mitnick C, Bayona J, Palacios E, et al. Community-based therapy for multidrug-resistant tuberculosis in Lima, Peru. N Engl J Med. 2003;348:119–128. doi: 10.1056/NEJMoa022928. [DOI] [PubMed] [Google Scholar]
- van Deun A, Maug AK, Salim MA, et al. Short, highly effective and inexpensive standardized treatment of multidrug-resistant tuberculosis. Am J Respir Crit Care Med. 2010;182:684–692. doi: 10.1164/rccm.201001-0077OC. [DOI] [PubMed] [Google Scholar]
- Chang KC, Yew WW, Zhang Y. Pyrazinamide susceptibility testing in Mycobacterium tuberculosis: a systematic review with meta-analyses. Antimicrob Agents Chemother. 2011;55:4499–4505. doi: 10.1128/AAC.00630-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung E, Yew W, Leung C, Leung W, Tam C. Shorter treatment duration for selected patients with multidrug-resistant tuberculosis. Eur Respir J. 2011;38:227–230. doi: 10.1183/09031936.00186310. [DOI] [PubMed] [Google Scholar]
- Migliori GB, Besozzi G, Girardi E, et al. Clinical and operational value of the extensively drug-resistant tuberculosis definition. Eur Respir J. 2007;30:623–626. doi: 10.1183/09031936.00077307. [DOI] [PubMed] [Google Scholar]
- Simpson G, Coulter C, Weston J, et al. Resistance patterns of multidrug-resistant tuberculosis in Western Province, Papua New Guinea. Int J Tuberc Lung Dis. 2011;15:551–552. doi: 10.5588/ijtld.10.0347. [DOI] [PubMed] [Google Scholar]
- Senol G, Coskun M, Gunduz AT, Bicmen C, Gayaf M, Ozsoz A.[Investigation of pyrazinamide resistance in multidrug-resistant tuberculosis cases in Hospital of Pulmonary Diseases, Izmir, Turkey] Mikrobiyoloji Bulteni 200842591–597.Turkish. [PubMed] [Google Scholar]
- Jonmalung J, Prammananan T, Leechawengwongs M, Chaiprasert A. Surveillance of pyrazinamide susceptibility among multidrug-resistant Mycobacterium tuberculosis isolates from Siriraj Hospital, Thailand. BMC Microbiol. 2010;10:223. doi: 10.1186/1471-2180-10-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minime-Lingoupou F, Pierre-Audigier C, Kassa-Kelembho E, et al. Rapid identification of multidrug-resistant tuberculosis isolates in treatment failure or relapse patients in Bangui, Central African Republic. Int J Tuberc Lung Dis. 2010;14:782–785. [PubMed] [Google Scholar]
- Louw GE, Warren RM, Donald PR, et al. Frequency and implications of pyrazinamide resistance in managing previously treated tuberculosis patients. Int J Tuberc Lung Dis. 2006;10:802–807. [PubMed] [Google Scholar]
- Ando H, Mitarai S, Kondo Y, et al. Pyrazinamide resistance in multidrug-resistant Mycobacterium tuberculosis isolates in Japan. Clin Microbiol Infect. 2010;16:1164–1168. doi: 10.1111/j.1469-0691.2009.03078.x. [DOI] [PubMed] [Google Scholar]
- Chiu YC, Huang SF, Yu KW, Lee YC, Feng JY, Su WJ. Characteristics of pncA mutations in multidrug-resistant tuberculosis in Taiwan. BMC Infect Dis. 2011;11:240. doi: 10.1186/1471-2334-11-240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao NA, Irfan M, Soomro MM, Mehfooz Z. Drug resistance pattern in multidrug resistance pulmonary tuberculosis patients. J Coll Physicians Surg Pak. 2010;20:262–265. [PubMed] [Google Scholar]
- Kim HJ, Kwak HK, Lee J, et al. Patterns of pncA mutations in drug-resistant Mycobacterium tuberculosis isolated from patients in South Korea. Int J Tuberc Lung Dis. 2012;16:98–103. doi: 10.5588/ijtld.10.0739. [DOI] [PubMed] [Google Scholar]
- Shenai S, Rodrigues C, Sadani M, Sukhadia N, Mehta A. Comparison of phenotypic and genotypic methods for pyrazinamide susceptibility testing. Indian J Tuberc. 2009;56:82–90. [PubMed] [Google Scholar]
- Pierre-Audigier C, Surcouf C, Cadet-Daniel V, et al. Fluoroquinolone and pyrazinamide resistance in multidrug-resistant tuberculosis. Int J Tuberc Lung Dis. 2012;16:221–223. i–ii. doi: 10.5588/ijtld.11.0266. [DOI] [PubMed] [Google Scholar]
- Aragon LM, Garrigo M, Moreno C, Espanol M, Coll P. Evaluation of the BacT/ALERT PZA kit in comparison with the BACTEC 460TB PZA for testing Mycobacterium tuberculosis susceptibility to pyrazinamide. J Antimicrob Chemother. 2007;60:655–657. doi: 10.1093/jac/dkm252. [DOI] [PubMed] [Google Scholar]
- Hewlett D, Jr, Horn DL, Alfalla C. Drug-resistant tuberculosis: inconsistent results of pyrazinamide susceptibility testing. JAMA. 1995;273:916–917. [PubMed] [Google Scholar]
- Chedore P, Bertucci L, Wolfe J, Sharma M, Jamieson F. Potential for erroneous results indicating resistance when using the Bactec MGIT 960 system for testing susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol. 2010;48:300–301. doi: 10.1128/JCM.01775-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Thibert L, Desjardins F, Siddiqi SH, Dascal A. Testing of susceptibility of Mycobacterium tuberculosis to pyrazinamide: comparison of Bactec method with pyrazinamidase assay. J Clin Microbiol. 1995;33:2468–2470. doi: 10.1128/jcm.33.9.2468-2470.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Permar S, Sun Z. Conditions that may affect the results of susceptibility testing of Mycobacterium tuberculosis to pyrazinamide. J Med Microbiol. 2002;51:42–49. doi: 10.1099/0022-1317-51-1-42. [DOI] [PubMed] [Google Scholar]
- Brander E. A simple way of detecting pyrazinamide resistance. Tubercle. 1972;53:128–131. doi: 10.1016/0041-3879(72)90029-3. [DOI] [PubMed] [Google Scholar]
- Martin A, Takiff H, Vandamme P, Swings J, Palomino JC, Portaels F. A new rapid and simple colorimetric method to detect pyrazinamide resistance in Mycobacterium tuberculosis using nicotinamide. J Antimicrob Chemother. 2006;58:327–331. doi: 10.1093/jac/dkl231. [DOI] [PubMed] [Google Scholar]
- Mirabal NC, Yzquierdo SL, Lemus D, et al. Evaluation of colorimetric methods using nicotinamide for rapid detection of pyrazinamide resistance in Mycobacterium tuberculosis. . J Clin Microbiol. 2010;48:2729–2733. doi: 10.1128/JCM.00311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A, Cubillos-Ruiz A, von Groll A, del Portillo P, Portaels F, Palomino JC. Nitrate reductase assay for the rapid detection of pyrazinamide resistance in Mycobacterium tuberculosis using nicotinamide. J Antimicrob Chemother. 2008;61:123–127. doi: 10.1093/jac/dkm418. [DOI] [PubMed] [Google Scholar]
- McClatchy JK, Tsang AY, Cernich MS. Use of pyrazinamidase activity on Mycobacterium tuberculosis as a rapid method for determination of pyrazinamide susceptibility. Antimicrob Agents Chemother. 1981;20:556–557. doi: 10.1128/aac.20.4.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- Scorpio A, Lindholm-Levy P, Heifets L, et al. Characterization of pncA mutations in pyrazinamide-resistant Mycobacterium tuberculosis. . Antimicrob Agents Chemother. 1997;41:540–543. doi: 10.1128/aac.41.3.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng SJ, Thibert L, Sanchez T, Heifets L, Zhang Y. pncA mutations as a major mechanism of pyrazinamide resistance in Mycobacterium tuberculosis: spread of a monoresistant strain in Quebec, Canada. Antimicrob Agents Chemother. 2000;44:528–532. doi: 10.1128/aac.44.3.528-532.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TS, Lee SS, Tu HZ, et al. Correlation between pyrazinamide activity and pncA mutations in Mycobacterium tuberculosis isolates in Taiwan. Antimicrobial Agents and Chemotherapy. 2003;47:3672–3673. doi: 10.1128/AAC.47.11.3672-3673.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreevatsan S, Pan X, Zhang Y, Kreiswirth BN, Musser JM. Mutations associated with pyrazinamide resistance in pncA of Mycobacterium tuberculosis complex organisms. Antimicrob Agents Chemother. 1997;41:636–640. doi: 10.1128/aac.41.3.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somoskovi A, Dormandy J, Parsons LM, et al. Sequencing of the pncA gene in members of the Mycobacterium tuberculosis complex has important diagnostic applications: identification of a species-specific pncA mutation in ‘Mycobacterium canettii' and the reliable and rapid predictor of pyrazinamide resistance. J Clin Microbiol. 2007;45:595–599. doi: 10.1128/JCM.01454-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Deng JY, Bi LJ, et al. Characterization of Mycobacterium tuberculosis nicotinamidase/pyrazinamidase. Febs J. 2008;275:753–762. doi: 10.1111/j.1742-4658.2007.06241.x. [DOI] [PubMed] [Google Scholar]
- Sheen P, Ferrer P, Gilman RH, et al. Effect of pyrazinamidase activity on pyrazinamide resistance in Mycobacterium tuberculosis. Tuberculosis (Edinb) 2009;89:109–113. doi: 10.1016/j.tube.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre N, Callebaut I, Frenois F, Jarlier V, Sougakoff W. Study of the structure-activity relationships for the pyrazinamidase (PncA) from Mycobacterium tuberculosis. . Biochem J. 2001;353 Pt 3:453–458. doi: 10.1042/0264-6021:3530453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi W, Zhang X, Jiang X, et al. Pyrazinamide inhibits trans-translation in Mycobacterium tuberculosis. . Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jureen P, Werngren J, Toro JC, Hoffner S. Pyrazinamide resistance and pncA gene mutations in Mycobacterium tuberculosis. . Antimicrobial Agents and Chemotherapy. 2008;52:1852–1854. doi: 10.1128/AAC.00110-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade MM, Volokhov D, Peredelchuk M, Chizhikov V, Zhang Y. Accurate mapping of mutations of pyrazinamide-resistant Mycobacterium tuberculosis strains with a scanning-frame oligonucleotide microarray. Diagn Microbiol Infect Dis. 2004;49:89–97. doi: 10.1016/j.diagmicrobio.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Zhou M, Geng X, Chen J, et al. Rapid colorimetric testing for pyrazinamide susceptibility of M. tuberculosis by a PCR-based in-vitro synthesized pyrazinamidase method. PLoS One. 2011;6:e27654. doi: 10.1371/journal.pone.0027654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiguchi J, Nakamura T, Miyoshi-Akiyama T, et al. Development and evaluation of a line probe assay for rapid identification of pncA mutations in pyrazinamide-resistant mycobacterium tuberculosis strains. J Clin Microbiol. 2007;45:2802–2807. doi: 10.1128/JCM.00352-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons SO, van Ingen J, van der Laan T, et al. Validation of pncA gene sequencing in combination with the MGIT method to test susceptibility of Mycobacterium tuberculosis to pyrazinamide. J Clin Microbiol. 2012;50:428–434. doi: 10.1128/JCM.05435-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang KC, Leung CC, Yew WW, Lau TY, Tam CM. Hepatotoxicity of pyrazinamide: cohort and case–control analyses. Am J Respir Crit Care Med. 2008;177:1391–1396. doi: 10.1164/rccm.200802-355OC. [DOI] [PubMed] [Google Scholar]
- Stover CK, Warrener P, VanDevanter DR, et al. A small-molecule nitroimidazopyran drug candidate for the treatment of tuberculosis. Nature. 2000;405:962–966. doi: 10.1038/35016103. [DOI] [PubMed] [Google Scholar]
- Gler MT, Skripconoka V, Sanchez-Garavito E, et al. Delamanid for multidrug-resistant pulmonary tuberculosis. N Engl J Med. 2012;366:2151–2160. doi: 10.1056/NEJMoa1112433. [DOI] [PubMed] [Google Scholar]