Abstract
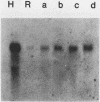
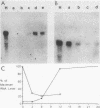
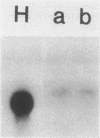
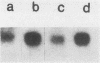
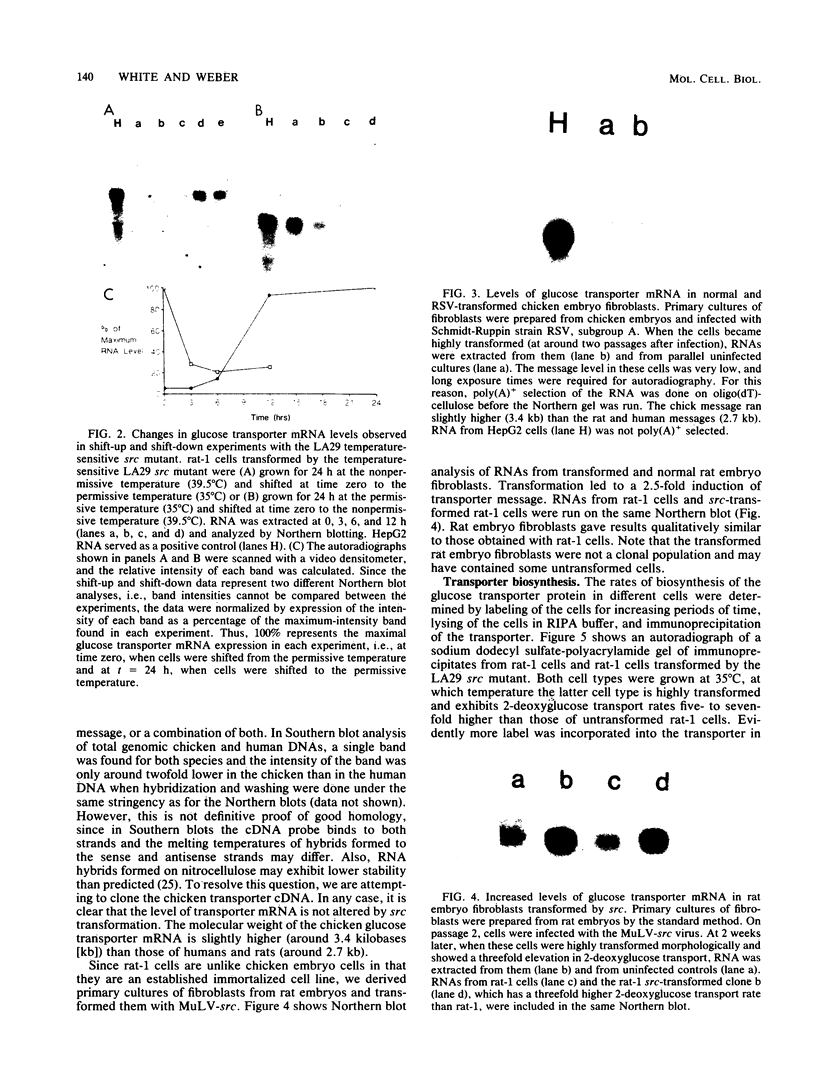
Transformation of both rat and chicken fibroblasts by the src oncogene leads to a four- to fivefold increase in the rate of glucose transport and in the level of the glucose transporter protein. We have previously shown that, with chicken embryo fibroblasts, transformation leads to a reduction in the rate of degradation of the transporter, with little or no increase in the rate of its biosynthesis. We now show that, with the rat-1 cell line, the opposite result was obtained. src-induced transformation led to an increase in transporter biosynthesis, with little effect on turnover. A src-induced increase in transporter mRNA entirely accounted for the increase in biosynthesis of the protein. By contrast, in chicken embryo fibroblasts, the level of transporter mRNA was low and was not induced to rise by src transformation. Thus, src induced an increase in the level of the glucose transport protein by fundamentally different mechanisms in chicken embryo fibroblasts and rat-1 cells. To test whether this difference was due to rat-1 cells being an immortalized cell line, we measured transporter mRNA levels in primary fibroblast cultures from rat embryos and in parallel cultures transformed by src. Transporter mRNA was inducible by src in these cells. Thus, the difference in mRNA inducibility between chicken and rat cells is not due to immortalization.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S. M., Scolnick E. M. Construction and isolation of a transforming murine retrovirus containing the src gene of Rous sarcoma virus. J Virol. 1983 May;46(2):594–605. doi: 10.1128/jvi.46.2.594-605.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum M. J., Haspel H. C., Rosen O. M. Transformation of rat fibroblasts by FSV rapidly increases glucose transporter gene transcription. Science. 1987 Mar 20;235(4795):1495–1498. doi: 10.1126/science.3029870. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson E., Erikson R. L. The specific interaction of the Rous sarcoma virus transforming protein, pp60src, with two cellular proteins. Cell. 1981 Aug;25(2):363–372. doi: 10.1016/0092-8674(81)90055-6. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Ciaraldi T. P., Horuk R., Matthaei S. Biochemical and functional characterization of the rat liver glucose-transport system. Comparisons with the adipocyte glucose-transport system. Biochem J. 1986 Nov 15;240(1):115–123. doi: 10.1042/bj2400115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Bishop J. M. Transit of pp60v-src to the plasma membrane. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7117–7121. doi: 10.1073/pnas.79.23.7117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker S., Lipmann F. Transport of D-glucose by membrane vesicles from normal and avian sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5358–5361. doi: 10.1073/pnas.78.9.5358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flier J. S., Mueckler M. M., Usher P., Lodish H. F. Elevated levels of glucose transport and transporter messenger RNA are induced by ras or src oncogenes. Science. 1987 Mar 20;235(4795):1492–1495. doi: 10.1126/science.3103217. [DOI] [PubMed] [Google Scholar]
- Gamou S., Shimizu N. Change in metabolic turnover is an alternate mechanism increasing cell surface epidermal growth factor receptor levels in tumor cells. J Biol Chem. 1987 May 15;262(14):6708–6713. [PubMed] [Google Scholar]
- Haspel H. C., Birnbaum M. J., Wilk E. W., Rosen O. M. Biosynthetic precursors and in vitro translation products of the glucose transporter of human hepatocarcinoma cells, human fibroblasts, and murine preadipocytes. J Biol Chem. 1985 Jun 25;260(12):7219–7225. [PubMed] [Google Scholar]
- Hatanaka M., Hanafusa H. Analysis of a functional change in membrane in the process of cell transformation by Rous sarcoma virus; alteration in the characteristics of sugar transport. Virology. 1970 Aug;41(4):647–652. doi: 10.1016/0042-6822(70)90429-0. [DOI] [PubMed] [Google Scholar]
- Horuk R., Matthaei S., Olefsky J. M., Baly D. L., Cushman S. W., Simpson I. A. Biochemical and functional heterogeneity of rat adipocyte glucose transporters. J Biol Chem. 1986 Feb 5;261(4):1823–1828. [PubMed] [Google Scholar]
- Kawai S., Hanafusa H. The effects of reciprocal changes in temperature on the transformed state of cells infected with a rous sarcoma virus mutant. Virology. 1971 Nov;46(2):470–479. doi: 10.1016/0042-6822(71)90047-x. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Regulation of sugar transport in chick embryo fibroblasts and in fibroblasts transformed by a temperature-sensitive mutant of the Rous sarcoma virus. J Cell Physiol. 1976 Dec;89(4):723–728. doi: 10.1002/jcp.1040890432. [DOI] [PubMed] [Google Scholar]
- Kletzien R. F., Perdue J. F. Sugar transport in chick embryo fibroblasts. II. Alterations in transport following transformation by a temperature-sensitive mutant of the Rous sarcoma virus. J Biol Chem. 1974 Jun 10;249(11):3375–3382. [PubMed] [Google Scholar]
- Lang D. R., Weber M. J. Increased membrane transport of 2-deoxyglucose and 3-O-methylglucose is an early event in the transformation of chick embryo fibroblasts by Rous sarcoma virus. J Cell Physiol. 1978 Mar;94(3):315–319. doi: 10.1002/jcp.1040940309. [DOI] [PubMed] [Google Scholar]
- Lanks K. W., Kasambalides E. J., Chinkers M., Brugge J. S. A major cytoplasmic glucose-regulated protein is associated with the Rous sarcoma virus pp60src protein. J Biol Chem. 1982 Aug 10;257(15):8604–8607. [PubMed] [Google Scholar]
- Mueckler M., Caruso C., Baldwin S. A., Panico M., Blench I., Morris H. R., Allard W. J., Lienhard G. E., Lodish H. F. Sequence and structure of a human glucose transporter. Science. 1985 Sep 6;229(4717):941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- Salter D. W., Baldwin S. A., Lienhard G. E., Weber M. J. Proteins antigenically related to the human erythrocyte glucose transporter in normal and Rous sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1540–1544. doi: 10.1073/pnas.79.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter D. W., Weber M. J. Glucose-specific cytochalasin B binding is increased in chicken embryo fibroblasts transformed by Rous sarcoma virus. J Biol Chem. 1979 May 10;254(9):3554–3561. [PubMed] [Google Scholar]
- Shawver L. K., Olson S. A., White M. K., Weber M. J. Degradation and biosynthesis of the glucose transporter protein in chicken embryo fibroblasts transformed by the src oncogene. Mol Cell Biol. 1987 Jun;7(6):2112–2118. doi: 10.1128/mcb.7.6.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker A. W., Enrietto P. J., Wyke J. A. Functional domains of the pp60v-src protein as revealed by analysis of temperature-sensitive Rous sarcoma virus mutants. Mol Cell Biol. 1984 Aug;4(8):1508–1514. doi: 10.1128/mcb.4.8.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venuta S., Rubin H. Sugar transport in normal and Rous sarcoma virus-transformed chick-embryo fibroblasts. Proc Natl Acad Sci U S A. 1973 Mar;70(3):653–657. doi: 10.1073/pnas.70.3.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber M. J. Hexose transport in normal and in Rous sarcoma virus-transformed cells. J Biol Chem. 1973 May 10;248(9):2978–2983. [PubMed] [Google Scholar]
- White M. K., Bramwell M. E., Harris H. Hexose transport in hybrids between malignant and normal cells. Nature. 1981 Nov 19;294(5838):232–235. doi: 10.1038/294232a0. [DOI] [PubMed] [Google Scholar]
- White M. K., Bramwell M. E., Harris H. Kinetic parameters of hexose transport in hybrids between malignant and nonmalignant cells. J Cell Sci. 1983 Jul;62:49–80. doi: 10.1242/jcs.62.1.49. [DOI] [PubMed] [Google Scholar]