Abstract
Insomnia encompasses a difficulty in falling asleep (sleep-onset insomnia) and/or a difficulty in staying asleep (SMI). Several selective serotonin-2A (5-HT2A) receptor antagonists have been in development as potential treatments for SMI. However, none have shown a sufficiently robust benefit-to-risk ratio, and none have reached market approval. We review the role of the 5-HT2A mechanism in sleep, the preclinical and clinical data supporting a role for 5-HT2A receptor antagonism in improving sleep maintenance, and the status of 5-HT2A receptor antagonists in clinical development. Overall, the polysomnography data strongly support an increase in slow-wave sleep and a decrease in waking after sleep onset following treatment with 5-HT2A receptor antagonists, although it has been more difficult to show subjective improvements in sleep with these agents. The incidence and prevalence of SMI, whether primary or secondary to psychiatric, neurologic, or other medical conditions, will increase as our population ages. There will be an increased need for safe and efficacious treatments of insomnia characterized by difficulty maintaining sleep, and there remains much promise for 5-HT2A receptor antagonism to play a role in these future treatments.
Keywords: 5-HT2A, insomnia, serotonin, slow-wave sleep, wake after sleep onset
Introduction
Insomnia is defined as a complaint of poor quality sleep that is often associated with daytime fatigue, irritability, cognitive dysfunction, and general malaise.1 Insomnia encompasses both a difficulty in falling asleep (sleep-onset insomnia) and/or a difficulty staying asleep (sleep-maintenance insomnia, SMI), both of which lead to nonrefreshing sleep. The DSM-IV-TR (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition Text Revision)2 defines primary insomnia as a complaint of difficulty initiating sleep or maintaining sleep or of nonrestorative sleep of at least one month’s duration and which causes significant distress or functional impairment, and is not secondary to another condition. The prevalence of primary insomnia is 1%–10% in the adult population, and up to 25% in the elderly.2 In contrast, insomnia may be a feature of many other conditions and disease states (secondary insomnia). For example, insomnia related to other mental disorders is quite common, and can be reported as comorbid with major depressive disorder, dysthymic disorder, bipolar mood disorder, generalized anxiety disorder, schizophrenia, and others. Sleep disorders can also be secondary to general medical conditions, including neurologic disorders, cerebrovascular disease, endocrine dysfunction, viral or bacterial infections, pulmonary disease and coughing, and pain disorders. Insomnia, whether primary or secondary, and other sleep complaints increase with age and may affect up to 50% of the elderly.3,4 As the population of older adults increases, so does the need for effective insomnia treatments.
Cognitive behavioral therapy and pharmacotherapy, either alone or combined, are effective treatments to reduce insomnia.5 Cognitive behavioral therapy may be more effective overall than pharmacotherapy for the treatment of insomnia,6 although pharmacotherapy has a more immediate onset of effect.7 For persistent insomnia, the addition of medication to cognitive behavioral therapy can improve the acute response, although cognitive behavioral therapy appears to be better than pharmacotherapy at sustaining improvements in sleep over time.5,8 Current pharmacotherapy for the treatment of primary insomnia, characterized by difficulty falling asleep, includes benzodiazepines (such as triazolam and temazepam), nonbenzodiazepine modulators of gamma aminobutyric acid-A (GABA-A) receptors (such as zolpidem, eszopiclone, and zaleplon), or melatonin receptor agonists (ramelteon). Sedating antidepressants also are often prescribed off-label and used to improve symptoms of insomnia.9,10 Two drugs, extended-release zolpidem and eszopiclone, are currently approved in the US for the treatment of both sleep-onset insomnia and SMI.
Development of a drug that selectively targets sleep maintenance by decreasing the duration of wake after sleep onset (WASO) is greatly needed. The ideal sleep maintenance drug would also increase total sleep time without psychomotor impairment, abuse potential, and cognitive impairment. Many people have no trouble falling asleep, but wake in the middle of the night unable to fall back asleep or suffer from early morning wakefulness. For example, the overall prevalence of insomnia characterized by difficulty maintaining sleep is about 23%, whereas the prevalence of insomnia characterized by difficulty initiating sleep is only about 11%.11 Patients with SMI only require a selective treatment that increases deep sleep, decreases the number of awakenings during the night, decreases the time spent awake during the night, and improves the quality of sleep so that they awaken feeling refreshed and cognitively alert. Drugs like eszopiclone may help people stay asleep during the night, but may require higher doses than those that help people with sleep-onset insomnia to fall asleep.12 Moreover, while extended-release zolpidem may improve sleep early in the night, it can cause rebound early morning wakefulness (as indicated in its prescribing information package insert) and may not be appropriate for all patients. Non-GABAergic mechanisms have therefore been explored to provide a therapeutic alternative for selectively improving sleep maintenance. Low-dose doxepin, an antidepressant drug acting primarily as a histamine H1 antagonist at low doses, has shown efficacy in the treatment of insomnia,13 and is the first drug approved by the Food and Drug Administration (FDA) for the selective treatment of SMI. However, better treatments for SMI are needed, with more robust efficacy and fewer side effects.
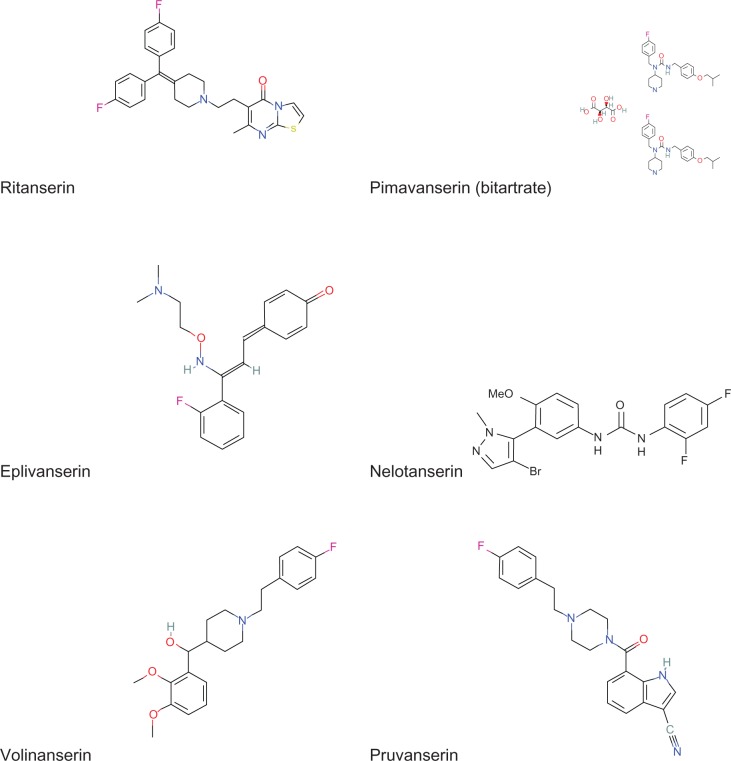
The present review focuses on the development of serotonin (5-HT) receptor 2A subtype (5-HT2A) antagonists for the selective treatment of SMI. A number of drugs with this mechanism have been evaluated for effects on sleep in clinical trials. A list of these drugs is provided in Table 1 and their chemical structures are shown in the Figure 1. Many 5-HT2A receptor antagonists are not silent or neutral antagonists, but rather exhibit intrinsic activity as inverse agonists (ie, activity in the opposite direction to receptor agonists).14,15 This inverse agonist activity essentially attenuates the basal constitutive signaling activity of 5-HT2A receptors, in contrast with neutral antagonists that exhibit no negative intrinsic efficacy, but just occupy the receptor and block agonist-induced responses. However, because all inverse agonists can also block agonist-induced responses (ie, act as antagonists) and not all 5-HT2A receptor antagonists have been evaluated in functional assays to determine intrinsic activity, both inverse agonists and antagonists will be referred to as antagonists in the present paper. Moreover, not all 5-HT2A receptor antagonists discussed are selective for 5-HT2A receptors. Selectivity for key compounds and implications for interpreting results will be discussed. However, selectivity data themselves have to be interpreted with some caution because the affinity for a given receptor can vary with assay conditions. This has been shown to be the case for a variety of compounds tested for 5-HT2A receptor affinity, with different potency and selectivity values for functional cell-based assays, membrane binding assays, and whole cell binding assays.14 Selectivity data were also reported differently, based on binding in receptor-enriched tissues, before receptor subtypes were cloned, and are dependent on relatively more or less selective radioligands. Subsequently, assays in cloned rat or cloned human receptor subtypes provided additional information regarding subtype selectivity.
Table 1.
Relative binding affinities for selective 5-HT2A receptor antagonists. Potency values are expressed as pKi, Ki (nM) or IC50 (nM) as noted
| 5-HT2A | 5-HT2C | Development status | |
|---|---|---|---|
| Ritanserin | pKi 10.08a | pKi 9.36a | Discontinued |
| pKi 8.88b | pKi 7.61b | ||
| Eplivanserin | pKi 10.34a | pKi 8.87a | Discontinued |
| pKi 9.40b | pKi 6.13b | ||
| Volinanserin | pKi 10.52a | pKi 8.42a | Discontinued |
| pKi 9.76b | pKi 6.35b | ||
| IC50 0.3c | |||
| IC50 0.39d | IC50 36d | ||
| Pimavanserin | pKi 9.3a | pKi 8.80a | Phase III clinical development for indications other than insomnia |
| pKi 9.7b | pKi 8.00b | ||
| Nelotanserin | Ki 2.3c | Ki 221c | Discontinued |
| Ki 0.4d | Ki 106d | ||
| Pruvanserin | IC50 1.0c | Discontinued | |
| IC50 0.35d | IC50 1334d |
Figure 1.
Chemical structures of 5-HT2A receptor antagonists.
Measurement of sleep parameters
Sleep architecture can be objectively quantified using polysomnography (PSG), electroencephalography (EEG), electro-oculography, and electromyography. These measures richly define the latency to and duration of each sleep stage and the number of cycles through the various stages of sleep that occur during the night. A scoring system for standardizing the measurement of sleep stages in humans has been developed, used extensively,16 and recently modified.17 Sleep consists of paradoxical sleep or rapid-eye movement (REM) sleep and nonrapid eye movement (non-REM) sleep. Non-REM sleep is divided into stages 1 and 2, and slow-wave sleep (SWS). Sleep architecture changes with age, with some diseases/disorders, and with some drug treatment.
Although sleep stages can be measured using PSG in the laboratory, it is really the subjective complaints of difficulty in falling asleep, difficulty in maintaining sleep, and/or nonrefreshing or nonrestorative sleep that are associated with the significant distress or functional impairment that defines insomnia.2 Thus, subjective measures are an important determinant of sleep quality but are difficult to measure systematically.
Localization and pharmacology of serotonin 5-HT2A receptors
Serotonin has long been known to play a role in sleep.18 Serotonin receptors have been divided into many subclasses with different localization and function.19 The broad molecular biology and pharmacology of serotonin 5-HT2A receptors have been critically reviewed elsewhere.20 Of particular relevance to sleep, the 5-HT2A receptor subtype has been suggested to play an essential role in maintaining the reciprocal inhibitory interactions between acetylcholine- and serotonin-containing neurons that mediate the sleep-wake cycle.21 The population of serotonergic neurons is diverse, with complex electrophysiologic differentiating features contributing to the sleep-wake cycle, including the existence of serotonergic neurons that exhibit slow clock-like activity that may be mediated in part by G protein-coupled cortical 5-HT2A receptors.22 Tritiated eplivanserin-specific binding in the rodent brain was predominant in the prefrontal cortex, parietal cortex, occipital cortex, and striatum, with less specific binding in the hypothalamus, thalamus, hippocampus, and medulla oblongata, and no specific binding detected in the cerebellum.23 Anatomic distribution of 5-HT2A receptors, especially in neocortical areas in the primate brain, was confirmed using tritiated volinanserin and mRNA detected by in situ hybridization.24 Using 5-HT2 immunoreactivity, the distribution of 5-HT2 receptors in the rat brain appears in high concentration in the basal forebrain, olfactory bulb, layer II of the pyriform cortex, neostriatum, dorsal hippocampus, and the pedunculopontine and laterodorsal tegmental nuclei in the brainstem, with more sparse and even distribution in cerebral cortex.2 Based on the distribution, localization, and morphology of the 5-HT2-immunoreactive neurons, Morliak et al hypothesized that cortical GABAergic interneurons or cholinergic neurons express 5-HT2 receptors.25 Subsequent electrophysiologic data confirmed that serotonin increases GABA-evoked inhibitory activity, an effect blocked by 5-HT2A receptor antagonism,26,27 and that 5-HT2A receptors mediate activity of interneurons in the piriform cortex in brain slices.28 It is perhaps through the modulation of interneurons that 5-HT2A receptors drive the balance between sleep and wakefulness. Consistent with this hypothesis, a 5-HT2 receptor agonist, 1-(2,5-dimethoxy-4-iodophenyl)-2-aminopropane (DOI), significantly increases wakefulness and reduces slow-wave sleep, effects that can be blocked by a 5-HT2A receptor antagonist.29
5-HT2A receptor antagonists
Many 5-HT2A receptor antagonists have been studied for their effects on sleep in animals and humans. Historically, one of the first compounds to be studied was ritanserin (6-[2-[4-[bis(4-fluorophenyl)methylene]-1-piperidinyl] ethyl]-7-methyl-5H-thiazolo[3,2-a]pyrimidinin-5-one), first reported as a 5-HT2 receptor antagonist with a mean IC50 value of 0.9 nM.30 Leysen et al reported that ritanserin had lesser, but measurable, affinity for histamine H1 receptors (IC50 35 nM), dopamine D2 receptors (IC50 70 nM), and adrenergic alpha1 and alpha2 receptors (IC50 97 nM and 150 nM, respectively). Later experiments revealed that ritanserin was relatively selective for 5-HT2A over 5-HT2C receptor subtypes14 (see Table 1). An extensive amount of preclinical and clinical data is available for ritanserin. These data provided an initial starting point for understanding the role of 5-HT2A receptors in sleep, as described below. However, the clinical development of ritanserin was discontinued.
Eplivanserin (Ciltyri®, SR 46349B; trans, 4-[(3Z) 3-(2-dimethylamino-ethyl) oxyimino-3 (2-fluorophenyl) propen-1-yl] phenol hemifumarate), was previously in development by Sanofi-aventis which coined the term “ASTAR” (Antagonist of Serotonin Two A Receptors), in an effort to educate the public regarding this new mechanism of action for sleep aids. Also originally designated as a 5-HT2 receptor antagonist,31 eplivanserin is even more selective than ritanserin for 5-HT2A over 5-HT2C14 (Table 1). Eplivanserin was reviewed by the FDA as a potential treatment for patients with chronic insomnia, but the FDA requested additional information regarding benefit-risk (sanofi-aventis press release, September 16, 2009) and development of the drug has been discontinued (sanofi-aventis press release, December 21, 2009).
Other selective 5-HT2A receptor antagonists have been evaluated in clinical trials, such as volinanserin (M100907; (+/−) 2,3dimethoxyphenyl-1-[2-(4-piperidine)-methanol), pruvanserin (EMD 281014; 7-[4-[2-(4-fluorophenyl)ethyl]piperazine-1-carbonyl]-1H-indole-3-carbonitrile), nelotanserin (APD125; 1-[3-(4-bromo-1-methyl-1H-pyrazol-5-yl)-4-methoxyphenyl]-3-(2,4-difluorophenyl)urea), and pimavanserin (ACP-103; N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N’-(4-(2-methylpropyl oxy[phenylmethyl]carbamide), but none have reached market approval. Volinanserin, another selective 5-HT2A receptor antagonist in development by Sanofi-aventis, was discontinued in development (source: www.clinicaltrials.org). Pruvanserin development was also discontinued by Eli Lilly,32 although they are continuing development of a drug with a dual mechanism of action, ie, histamine H1 antagonism and 5-HT2A antagonism (HY10275). However, very little has been reported on the clinical effects of either compound. Along the same lines, esmirtazapine (ORG 50081; (S)-1,2,3,4,10,14b-hexahydro-2-methylpyrazino(2,1-a)pyrido(2,3-c)(2)benzazepine) combines 5-HT2A receptor antagonism with H1 receptor antagonism, and is in Phase III clinical development for insomnia, as well as for hot flashes associated with menopause. Again, little information regarding the clinical effects of esmirtazapine on sleep is available. Nelotanserin, a selective 5-HT2A receptor inverse agonist, has also been discontinued (Arena Pharmaceuticals press release, December 9, 2008). Pimavanserin is in Phase III clinical development for the treatment of psychosis associated with Parkinson’s disease, but is not being pursued for the treatment of sleep disorders after initial promising results in healthy volunteers (source: http://www.acadia-pharm.com/).
Additional selective 5-HT2A receptor antagonists have been tested in animal models, but have not advanced to the clinic for the treatment of insomnia. Fananserin (RP 62203; 2-(3-(4-(p-Fluorophenyl)-1-piperazinyl)propyl)-2-H-naphth(1,8-cd)isothiazole 1,1-dioxide) is an example of a 5-HT2A receptor antagonist with dopamine D4 receptor antagonism which was evaluated in animal models for effects on sleep, but pursued in the clinic (and later discontinued) as an antipsychotic drug.33 Other drugs with mixed pharmacology including 5-HT2A receptor antagonism are also marketed, including atypical antipsychotic drugs and some antidepressant drugs. Some of these are prescribed off-label to improve sleep, but none have FDA approval for the treatment of insomnia.
5-HT2A receptor antagonists and sleep in animal models
Ritanserin increases slow-wave activity and non-REM sleep in rats while decreasing REM sleep.34–37 Interestingly, ritanserin has the opposite effect, decreasing slow-wave sleep in cats.38 The increase in slow-wave sleep caused by 5-HT2 antagonists in rats appears to be more predictive of effects in humans than are the cat data (see below). Other 5-HT2 receptor antagonists, such as Fananserin, pruvanserin, and nelotanserin, have shown increases in non-REM or slow-wave sleep in rats,29,39,40 and eplivanserin and pruvanserin also show decreases in REM sleep in rats.29,31 The data with nelotanserin further suggest that 5-HT2A receptor antagonism or inverse agonism is associated with increased sleep consolidation, as evidenced by a statistically significant increase in non-REM bout duration and a statistically significant decrease in the number of non-REM bouts in rats.39 Nelotanserin also produced dose-dependent increases in delta power, a correlate of increased SWS. Importantly, repeated dose administration of nelotanserin did not result in the development of tolerance, and there was no evidence of rebound insomnia after cessation of dose administration of nelotanserin.3 Taken together, these data strongly support a role for 5-HT2A in mediating sleep in animals.
A specific role for 5-HT2A receptors in sleep was confirmed in 5-HT2A receptor knock-out mice; increases in non-REM sleep induced by subtype-selective 5-HT2A receptor antagonists, such as volinanserin, in wild-type mice were absent in 5-HT2A receptor knock-out mice.41 Although others have suggested a role for 5-HT2C,42 independent confirmation of the increase of non-REM sleep in rats by the highly selective 5-HT2A receptor antagonist, volinanserin, has been reported, providing supporting evidence for the role for 5-HT2A in mediating non-REM sleep.43 Moreover, increases in slow-wave sleep induced by the 5-HT2A/2C receptor agonist, DOI, are blocked by a selective 5-HT2A receptor antagonist, but not by a selective 5-HT2C receptor antagonist.29
Clinical efficacy of 5-HT2A receptor antagonists
Ritanserin was the first selective 5-HT2 receptor antagonist to be evaluated for effects on sleep in humans.44,45 An acute dose of ritanserin 10 mg increased slow-wave sleep (Stage 3 F[3,24] = 14.12, P < 0.01; Stage 4 F[3,24] = 10.67, P < 0.01) in healthy volunteers.45 The increase in slow-wave sleep occurred at the expense of decreased Stage 2 sleep (F[3,24] = 18.25, P < 0.01) and decreased REM sleep (F[3,24] = 4.10, P < 0.05).45 These data were in contrast with the benzodiazepine control, nitrazepam, which decreased deeper Stage 4 sleep and increased lighter Stage 2 sleep.45 The effects of ritanserin on increasing slow-wave sleep appear to be dose-related, with the effects increasing across a dose range from 1 mg to 10 mg.46,47 Ritanserin increased slow-wave sleep by an average of 53 minutes, 82 minutes, 101 minutes, and 136.5 minutes at doses of 1, 3, 10, and 30 mg (F[5,54] = 26.75, P < 0.001).48 There appears to be no tolerance to the sleep-enhancing effects of ritanserin after repeated administration.44,48 The early studies lacked the statistical power to detect significant differences in subjective effects, although the effects measured by PSG were robust, even with such small sample sizes (n = 444 and n = 945). Interestingly, Idzikowski et al demonstrated that morning administration of ritanserin showed larger increases in SWS during the night (increase of about 94 minutes on average) than when ritanserin was administered just before bedtime (increase of about 52 minutes on average).45 There was no psychomotor sedation during the day after morning administration of ritanserin, suggesting that ritanserin was acting via mechanisms that selectively mediate SWS with no effect on sleep initiation.
A single oral dose of eplivanserin 1 mg significantly incre ased slow-wave sleep by an average of about 61 minutes (F[1,8] = 58.1, P < 0.01) and decreased Stage 2 sleep by about 34 minutes (F[1,8] = 6.4, P = 0.04) in healthy volunteers (n = 10).49 Although eplivanserin decreased the duration of WASO by an average of 9.5 minutes, the study was not sufficiently powered to be able to detect a statistically significant difference (F[1,8] = 1.2, P = 0.31). Eplivanserin did not significantly alter latency to sleep or latency to REM sleep, consistent with selective improvements in sleep maintenance. Although the efficacy of eplivanserin in a patient population has not been reported in a peer-reviewed journal, sanofi-aventis summarized the Phase III efficacy data used to support their New Drug Application (NDA) in a 2007 financial earnings summary report dated February 12, 2008. This report stated that eplivanserin significantly reduced patient-reported (subjective) WASO and patient-reported number of nocturnal awakenings in patients with primary insomnia. As mentioned above, the FDA reviewed the NDA of eplivanserin, but requested additional information regarding benefit-risk. Further details were not disclosed, but the development of eplivanserin was soon discontinued. Among 5-HT2A receptor antagonists, eplivanserin was the most advanced compound in development. It is unclear what the confounding risks of eplivanserin are, but efficacy data from eplivanserin and other 5-HT2A receptor antagonists appear to support the involvement of the 5-HT2A receptor mechanism in maintaining sleep.
Nelotanserin, another selective 5-HT2A receptor inverse agonist, at doses of 10 mg or 40 mg significantly decreased WASO by about 15 minutes on average (P < 0.001) after acute administration, but less robustly after one week of repeated administration (P = 0.01 for 10 mg and P = 0.2 for 40 mg).50 The number of arousals decreased from an average of about 47 at baseline to about 39 after acute administration (P < 0.0001) and remained significantly decreased at about 40 (P < 0.0001) after repeated administration of nelotanserin 40 mg in patients with primary insomnia.50 Similarly, the number of awakenings decreased from about 10 at baseline to about seven or eight after acute (P < 0.0001) or repeated (P < 0.0001) administration of nelotanserin 40 mg, respectively.50 These effects were associated with significant increases in slow-wave sleep (about 3% change from baseline and about 7% change from placebo at 10 mg, P < 0.0001, and about 4% change from baseline and about 8% change from placebo at 40 mg, P < 0.0001) in patients50 and nearly doubling slow-wave sleep (P < 0.01) in healthy volunteers.39 However, nelotanserin showed no dose-response relationship across a wide range of doses (10–40 mg)39 and failed to show efficacy in a larger Phase IIB trial (Arena Pharmaceuticals press release, December 9, 2008). In the earlier trial, although nelotanserin significantly improved some subjective measures of sleep after acute administration, including decreased subjective number of awakenings (P < 0.0002), increased subjective total sleep time (P = 0.01), and improved sleep quality (P = 0.01), the magnitude of these changes was small (from an average of 3.0 subjective awakenings at baseline to an average of 2.3 after 40 mg nelotanserin; from an average of 4.96 hours of subjective total sleep time at baseline to 5.81 hours after 40 mg; from an average of 3.5 on the sleep quality index at baseline to an average of 2.7 after 40 mg), and did not maintain statistical significance after repeated administration.50
Pimavanserin, a selective 5-HT2A receptor inverse agonist in late-stage clinical development for the treatment of psychosis associated with Parkinson’s disease, increased slow-wave sleep (nearly doubled after acute or two-week repeated administration, P < 0.001) in older adult healthy volunteers,51,52 but has not been evaluated in patients with insomnia. Consistent with the nonpsychomotor sedating mechanism, neither pimavanserin nor nelotanserin exhibited next-day cognitive deficits.39,50–52
Hypnion reported that HY10275, a dual-acting H1 receptor antagonist and 5-HT2A receptor antagonist, improves WASO in patients with transient insomnia (Hypnion press release, January 5, 2007) before the company was acquired by Eli Lilly in April 2007. Since then, Lilly has discontinued clinical development of pruvanserin, its selective 5-HT2A receptor antagonist,6 and continues development of HY10275, but no clinical data have been published in peer-reviewed journals on either compound. Volinanserin was originally in clinical development for the treatment of schizophrenia, and subsequently as a treatment for insomnia. Although clinical trials in patients with insomnia for volinanserin have been posted on www.clinicaltrials.org, no clinical data on volinanserin’s effects on sleep in humans have been published.
In the published literature taken together, the PSG data strongly suggest that 5-HT2A receptor antagonism increases slow-wave sleep without psychomotor sedation and without effect on the initiation of sleep. The question arises as to the impact of increasing slow-wave sleep on the quality of sleep. It has been suggested that increasing deep, slow-wave sleep results in fewer awakenings during the night and reduced duration of wakefulness during the night (ie, when people do wake up, they fall back asleep quickly). However, increased slow-wave sleep does not necessarily correlate with subjective reports of deeper sleep or sleep quality, at least for drugs with other mechanisms, such as tiagabine.53 In fact, the recommended practices for the evaluation of chronic insomnia include primarily subjective reports and detailed sleep histories, rather than PSG evaluation.1 Therefore, drug development efforts need to target improvements in subjective sleep. There are limited data for 5-HT2A receptor antagonists in studies sufficiently statistically powered in order to be able to measure subjective improvements in sleep. However, data suggest that 5-HT2A receptor antagonism can improve subjective sleep.54 Moreover, 5-HT2A receptor antagonists may improve clinical symptoms of depression, anxiety, and/or psychosis in specific patient populations.55,56 These data suggest a potentially unique clinical opportunity for this neurochemical mechanism, as discussed in more detail below.
Ritanserin increases slow-wave sleep (nearly doubled from an average of 69 minutes at baseline to an average of 129 after ritanserin, F[3,24] = 17.9, P < 0.0001) and improves subjective quality of sleep (F[4,32] = 4.48, P = 0.006) in middle-aged poor sleepers.57 Similar findings (an average of 133 minutes of slow-wave sleep after ritanserin compared with placebo at an average of 77 minutes, P < 0.05) were documented in young poor sleepers.58 Ritanserin also has demonstrated benefit in patients with narcolepsy.54 Although ritanserin did not affect the number of sleep attacks compared with placebo in an adjunctive study in patients with narcolepsy, treatment with ritanserin for four weeks did significantly increase slow-wave sleep (average decrease of 0.8 minutes from baseline for placebo compared with an average increase of 9.3 minutes after ritanserin 5 mg [P = 0.001] compared with an average increase of 10 minutes after ritanserin 10 mg [P = 0.001]) and increased self-reports of feeling refreshed (average rating for placebo of 1.3 compared with 0.9 after ritanserin 5 mg [P = 0.019] or compared with 1.0 after ritanserin 10 mg [P = 0.021]).54
Even though ritanserin did not increase total sleep time in healthy volunteers (only increased SWS with a commensurate decrease in Stage 2 sleep), ritanserin could significantly increase total sleep time in patients exhibiting a deficit in sleep. For example, in abstinent alcoholics with dysthymia and exhibiting less than six hours sleep/night at baseline, ritanserin treatment for 2–4 weeks significantly increased total sleep time (placebo at second week showed no change in total sleep time, compared with an average 47 minute increase from baseline with ritanserin [P < 0.05]; placebo at the fourth week showed an average five-minute increase from baseline in total sleep time, compared with an average 48-minute increase with ritanserin [P < 0.05]).55 WASO was also reduced (P < 0.05) after only four weeks of treatment with ritanserin, and sleep efficiency improved (P < 0.05) after two or four weeks in these patients.55 Importantly, symptoms of depression and anxiety were also significantly reduced by ritanserin; Hamilton Depression Scale scores were reduced by greater than 50% (P = 0.01) and Hamilton Anxiety Scale scores were similarly reduced (P = 0.01).55
Some psychiatric disorders are associated with disrupted sleep patterns. Ritanserin increases slow-wave sleep in patients with generalized anxiety disorder59 and in patients with depression,60 with a similar magnitude of effect as that seen in healthy volunteers. However, one study demonstrated that the increase was predominantly in Stage 3 sleep with no significant effect on Stage 4 sleep in depressed patients, whereas healthy volunteers show an increase in both Stage 3 and Stage 4 sleep, and combined Stage 3 + Stage 4, defined as SWS.61
Despite some differences in response to 5-HT2 antagonism between healthy subjects and patient populations, 5-HT2 receptor antagonists have been shown to normalize sleep disturbances characterized by fragmented sleep and frequent awakenings. For example, in dysthymic patients with fragmented superficial sleep, higher Stage 2 sleep and lower slow-wave sleep, ritanserin (10 mg once daily in the morning for four weeks) significantly increased the proportion of time spent in slow-wave sleep compared with placebo in a double-blind study.62 Ritanserin also increased the number of transitions from lighter sleep stages to deeper, slow-wave sleep. Although a relationship between clinical improvement in dysthymic symptoms as a consequence of increased slow-wave sleep has not been determined, depressed patients may benefit from normalized sleep.
Looking at the clinical data for the 5-HT2A receptor antagonists, there is considerable evidence to support a role for 5-HT2A receptor antagonism in the treatment of insomnia characterized by difficulty maintaining sleep. In general, 5-HT2A receptor antagonists increase deep slow-wave sleep, decrease WASO, and decrease the frequency of arousals and awakenings during the night (Table 2). Together, these data suggest improved sleep maintenance and improved sleep efficiency, and potential benefit in special psychiatric populations. 5-HT2A receptor antagonists are generally well tolerated. A summary of common side effects is provided in Table 2, and a more detailed discussion of safety and tolerability is described below.
Table 2.
Positive effects on sleep and side effects common to 5-HT2A receptor antagonists
| 5-HT2A receptor antagonism
| |
|---|---|
| Positive effects on sleep | Side effects |
| Increased deep slow wave sleep | Dizziness/hypotension |
| Decreased wake after sleep onset | Fatigue |
| Decreased number of arousals/awakenings | Headache |
| Improved sleep maintenance | Nausea/vomiting |
| Improved sleep efficiency | Constipation |
Safety and tolerability
As mentioned above, 5-HT2 antagonism does not cause psychomotor sedation or motor incoordination. Unlike benzodiazepines that impair psychomotor function and memory retention, ritanserin has little effect on measures of psychomotor and cognitive function.63 Withdrawal symptoms from benzodiazepines and GABA-A mediated drugs can be a safety issue and can require slow tapering from the drug when discontinuing. Treatment with 5-HT2 receptor antagonists does not appear to be associated with adverse effects upon withdrawal.64 Moreover, there is no evidence of tolerance to 5-HT2 receptor antagonists44,48 and, therefore, no need for progressively increased doses to maintain efficacy over time with repeated administration.
The only adverse events reported with four weeks of ritanserin 5 mg/day in patients with narcolepsy were headache and pre-existing sleep/wake problems.54 In abstinent alcoholics with dysthymia, side effects with ritanserin 10 mg/day reported included numbness to touch, impaired coordination, constipation, dry mouth, dizziness, and increased appetite, but each side effect was only reported in one subject.55 In a study investigating the effects of ritanserin for the treatment of schizophrenia in an open-label setting, the most frequent adverse events that were not present at baseline included palpitations, drowsiness, micturition disturbances, headache, and pruritus.65 In a large (n = 423) Phase III clinical trial evaluating the efficacy of ritanserin in the treatment of alcohol dependency, the most frequent adverse events were fatigue, headache, rhinitis, constipation, and insomnia.66 A dose-related statistically significant prolongation of the QTc interval was observed in this study. It is likely that this safety issue contributed to the discontinuation of development of ritanserin, although it is also likely that this was a compound-specific safety issue and not a “class effect”, because it has not been reported as a safety concern for other 5-HT2 receptor antagonists.
The dose-limiting side effect of pimavanserin in healthy volunteers was nausea and vomiting.67 Another common side effect of pimavanserin was postural dizziness.67 Dizziness, nausea, and vomiting were also side effects observed with nelotanserin in patients with insomnia.50 Fatigue and headache were also more common with nelotanserin treatment than with placebo.51
Fananserin was first identified as a 5-HT2 receptor antagonist68 and later identified as having potent 5-HT2A and dopamine D4 receptor affinity.33 It was evaluated clinically as an antipsychotic,69 but failed to show efficacy for the treatment of schizophrenia and, therefore, was never pursued for the treatment of sleep disorders. In patients with schizophrenia, the dose-limiting side effect of fananserin was hypotension.70
The most extensive and relevant safety data were probably collected during the extensive development programs for eplivanserin and volinanserin that reached Phase III clinical development for the treatment of insomnia. Those data, however, are not publically available.
Patient perspectives
An insomnia treatment that focuses on maintaining normal restorative sleep during the night and feeling rested and alert upon awakening and throughout the day remains an important unmet medical need. Many patients with insomnia have no trouble falling asleep, but awaken during the night unable to fall back asleep. A great many of these patients do not want or need a sedating drug that can be associated with a multitude of side effects, such as motor slowing (that can contribute to falls), cognitive dulling, and next-day hangover effect. The risk associated with abuse and dependence is an additional cause for concern. A large number of patients with SMI likely remain untreated due to the desire to avoid the side effects associated with sleep-inducing drugs.
On the other hand, it may be the lack of a sedating discriminative stimulus that has hampered the ability of 5-HT2A receptor antagonists to achieve the kinds of profound subjective improvements in sleep, as their effects on EEG sleep patterns would suggest. In other words, patients who are used to taking a sleep aid that makes them feel drowsy or sedated may not think that they are receiving a beneficial effect on sleep when given a 5-HT2 antagonist that lacks these effects. This could confound subjective reports of sleep habits. If this is the case, dual-acting drugs for sleep initiation and sleep maintenance may fare better in larger clinical trials than selective sleep-maintenance drugs. Still, these kinds of drugs do not selectively target the need for improved sleep maintenance without additional pharmacology and the accompanying additional safety risks associated with off-target drug interactions. Alternatively, it may be simply that the right 5-HT2A receptor antagonist with sufficient efficacy at safe doses has yet to be developed. It remains an enigma that 5-HT2A receptor antagonists have such profound effects on sleep, yet so many drugs have failed in larger efficacy trials or have failed to show a sufficiently robust benefit to risk ratio in clinical trials.
Future treatment options
The selective treatment of SMI remains a goal yet to be achieved by 5-HT2A receptor antagonists. The currently available 5-HT2A receptor antagonists fail to show robust efficacy in large clinical trials. This may arise from an inherent limit of the impact of blocking this receptor on sleep mechanisms, problems with the drugs such as poor pharmacokinetics, dose-limiting side effects, insensitive clinical measurement approaches, or a combination of these factors. Better and different drugs are clearly needed. One approach that has been taken is to develop compounds opportunistically that possess 5-HT2A receptor antagonism combined with other receptor interactions. The focus to date has been on developing compounds that combine sleep-onset with sleep-maintenance properties, such as the H1 antagonist/5-HT2A antagonist approach. However, this approach may be misguided. As noted above, many patients with insomnia do not need nor want the troubling side effects of a hypnotic agent. A better approach might be to search for drugs with receptor interactions that act synergistically to maintain sleep but do not possess hypnotic effects. We have recently studied a unique compound, ITI-007, for its ability to improve sleep selectively in patients with SMI. At low doses, ITI-007 acts primarily as a serotonin 5-HT2A receptor antagonist. At higher doses, ITI-007 also acts as a dopamine D2 receptor partial agonist presynaptically, as an antagonist postsynaptically, and as a serotonin transporter inhibitor. In our study, ITI-007 decreased WASO and increased SWS in patients with insomnia in a dose-dependent manner.71 ITI-007 promotes natural, restorative sleep over the course of the night, with significant increases in SWS early in the night and significant increases in Stage 2 sleep late in the night and towards morning, with no early morning wakefulness and no impairment of next-day functioning. These data suggest that this compound may have improved efficacy for treating sleep maintenance insomnia and that the additional pharmacology beyond 5-HT2A receptor antagonism may be beneficial. The magnitude of these effects increased with dose, with dramatic improvements seen at the highest tested dose (10 mg). At this dose, 5-HT2A receptors are fully saturated and interactions with dopamine receptors and the serotonin transporter begin to emerge. It is possible, therefore, that ITI-007 robustly maintains sleep above and beyond that provided by 5-HT2A receptor antagonism through these additional target interactions. At these doses, ITI-007 does not induce sleep or impair next-day cognitive performance. These findings give hope that, as we better understand the neurobiologic underpinnings of sleep maintenance problems, we may be able to develop more targeted drugs with combination mechanisms that will maintain natural sleep patterns without inducing unwanted off-target side effects.
Conclusions
The incidence and prevalence of SMI will increase as our population ages. There will be an increased need for safe, efficacious, and selective treatments of insomnia characterized by difficulty maintaining sleep. Moreover, many psychiatric, neurologic, and other medical conditions are associated with SMI that remains largely untreated. Although there is much promise for selective 5-HT2A receptor antagonists to provide selective therapy for SMI, none have yet achieved the robust efficacy required for marketing approval. The corporate reasons for discontinuation of development of any given drug are not always transparent. However, it appears that many 5-HT2A receptor antagonists have lacked a sufficient benefit to risk ratio, whether it be due to lack of efficacy or safety concerns. Safety concerns, as discussed above, do not appear to be necessarily target-mediated. This would suggest that 5-HT2A receptor antagonism remains a viable mechanism for the treatment of SMI. In addition to an improved safety profile, challenges in development that need to be overcome include clinical trial designs able to measure more effectively the subjective sleep maintenance and improvement of quality of life that may be associated with improved sleep maintenance. Now that low-dose doxepin has been approved by the FDA for the selective treatment of SMI, the regulatory pathway has been established. Whether a selective 5-HT2A receptor antagonist can meet or beat the bar that has been set is yet to be seen.
By better understanding the neurobiologic basis of SMI, better drugs may be discovered. Still, a lot can be learned from dissecting the actions of nonselective drugs that also possess activity as 5-HT2A receptor antagonists. Atypical antipsychotics are a rich source for this latter endeavor. Data from clinical trials support the improvement of sleep by these drugs in special populations. For example, ziprasidone increases slow-wave sleep and improves sleep continuity in healthy volunteers,72 olanzapine increases slow-wave sleep and improves sleep continuity in healthy volunteers and in patients with depression,73,74 and risperidone75 and paliperidone76 improve sleep in patients with schizophrenia. While these drugs are unlikely to be used to treat SMI in the wider sense, understanding their impact on fundamental sleep mechanism may be revealing and may provide a direction for future drug development approaches. Yet, there remains a need for the development of better drugs for the treatment of insomnia characterized by difficulty maintaining sleep. Antagonists of the 5-HT2A receptors may play an important role in the development of improved treatment of these kinds of sleep disorders.
Acknowledgments
The authors would like to thank Drs S Mates and P Greengard for their helpful comments.
Footnotes
Disclosure
KEV is a paid employee of Intra-Cellular Therapies, Inc. and is a former paid employee of ACADIA Pharmaceuticals Inc. RED is a paid consultant to Intra-Cellular Therapies, Inc., a former paid consultant and former paid employee of ACADIA Pharmaceuticals Inc. Intra-Cellular Therapies, Inc., and ACADIA Pharmaceuticals are developing investigational new drugs.
References
- 1.Chesson A, Jr, Hartse K, Anderson WM, et al. Practice parameters for the evaluation of chronic insomnia. Sleep. 2000;23:1–5. [PubMed] [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. Fourth Edition. Text Revision (DSM-IV-TR) American Psychiatric Association; Arlington, VA: 2000. [Google Scholar]
- 3.Ancoli-Israel S. Sleep and its disorders in aging populations. Sleep Med. 2009;10:S7–S11. doi: 10.1016/j.sleep.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 4.Vitiello MV. Sleep in normal aging. Sleep Med Clin. 2006;1:191–196. doi: 10.1016/j.jsmc.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: A randomized controlled trial. JAMA. 1999;281:991–999. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 6.Jacobs GD, Pace-Schott EF, Stickgold R, Otto MW. Cognitive behavior therapy and pharmacotherapy for insomnia: A randomized controlled trial and direct comparison. Arch Intern Med. 2004;164:1888–1896. doi: 10.1001/archinte.164.17.1888. [DOI] [PubMed] [Google Scholar]
- 7.McClusky HY, Milby JB, Switzer PK, Williams V, Wooten V. Efficacy of behavioral versus triazolam treatment in persistent sleep-onset insomnia. Am J Psychiatry. 1991;148:121–126. doi: 10.1176/ajp.148.1.121. [DOI] [PubMed] [Google Scholar]
- 8.Morin CM, Vallières A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA. 2009;301:2005–2015. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buscemi N, Vandermeer B, Friesen C, et al. The efficacy and safety of drug treatments for chronic insomnia in adults: A meta-analysis of RCTs. J Gen Intern Med. 2007;22:1335–1350. doi: 10.1007/s11606-007-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walsh JK. Pharmacologic management of insomnia. J Clin Psychiatry. 2004;65(Suppl 16):41–45. [PubMed] [Google Scholar]
- 11.Ohayon MM, Reynolds CF., 3rd Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD) Sleep Med. 2009;10:952–960. doi: 10.1016/j.sleep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zammit GK, McNabb LJ, Caron J, Amato DA, Roth T. Efficacy and safety of eszopiclone across 6 weeks of treatment for primary insomnia. Curr Med Res Opin. 2004;20:1979–1991. doi: 10.1185/174234304x15174. [DOI] [PubMed] [Google Scholar]
- 13.Scharf M, Rogowski R, Hull S, et al. Efficacy and safety of doxepin 1 mg, 3 mg, and 6 mg in elderly patients with primary insomnia: A randomized, double-blind, placebo-controlled crossover study. J Clin Psychiatry. 2008;69:1557–1564. doi: 10.4088/jcp.v69n1005. [DOI] [PubMed] [Google Scholar]
- 14.Vanover KE, Weiner DM, Makhay M, et al. Pharmacological and behavioral profile of N-(4-fluoropheylmethyl)-N-(1-methylpiperidine-4-yl)-N’-(4-(2-methylpropyloxy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine2A receptor inverse agonist. J Pharmacol Exp Ther. 2006;317:910–918. doi: 10.1124/jpet.105.097006. [DOI] [PubMed] [Google Scholar]
- 15.Weiner DM, Burstein ES, Nash N, et al. 5-Hydroxytryptamine2A receptor inverse agonists as antipsychotics. J Pharmacol Exp Ther. 2001;299:268–276. [PubMed] [Google Scholar]
- 16.Rechtschaffen A, Kales AA. A Manual of Standardized Terminology Techniques and Scoring System for Sleep Stage of Human Subjects. Washington, DC: National Institutes of Health, Government Printing Office; 1968. Publication 204. [Google Scholar]
- 17.Silber MH, Ancoli-Israel S, Bonnet MH, et al. The visual scoring of sleep in adults. J Clin Sleep Med. 2007;3(2):121–131. [PubMed] [Google Scholar]
- 18.Jouvet M. The role of monamine and acetylcholine-containing neurons in the regulation of the sleep-wake cycle. Ergebn Physiol. 1972;64:166–307. doi: 10.1007/3-540-05462-6_2. [DOI] [PubMed] [Google Scholar]
- 19.Hoyer D, Clarke DE, Fozard JR, et al. International Union of Pharmacology classification of receptors for 5-hydroxytryptamine (serotonin) Pharmacol Rev. 1994;46:157–203. [PubMed] [Google Scholar]
- 20.Roth BL, Berry SA, Kroeze WK, Willins DL, Kristiansen K. Serotonin 5-HT2A receptors: Molecular biology and mechanisms of regulation. Crit Rev Neurobiol. 1998;12:319–338. doi: 10.1615/critrevneurobiol.v12.i4.30. [DOI] [PubMed] [Google Scholar]
- 21.Fay R, Kubin L. 5-HT(2A) receptor-like protein is present in small neurons located in rat mesopontine cholinergic nuclei, but absent from cholinergic neurons. Neurosci Lett. 2001;314:77–81. doi: 10.1016/s0304-3940(01)02208-x. [DOI] [PubMed] [Google Scholar]
- 22.Kocsis B, Varga V, Dahan L, Sik A. Serotonergic neuron diversity: Identification of raphe neurons with discharges time-locked to the hippocampal theta rhythm. Proc Natl Acad Sci USA. 2006;103:1059–1064. doi: 10.1073/pnas.0508360103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rinaldi-Carmona M, Congy C, Pointeau P, Vidal H, Breliere JC, Le Fur G. Identifiation of binding sites for SR 46349B, A 5-hydroxytryptamine2 receptor antagonist, in rodent brain. Life Sci. 1994;54:119–127. doi: 10.1016/0024-3205(94)00782-9. [DOI] [PubMed] [Google Scholar]
- 24.López-Giménez JF, Vilaró MT, Palacios JM, Mengod G. Mapping of 5-HT2A receptors and their mRNA in monkey brain: [3H]MDL100,907 autoradiography and in situ hybridization studies. J Comp Neurol. 2001;429:571–589. doi: 10.1002/1096-9861(20010122)429:4<571::aid-cne5>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 25.Morilak DA, Garlow SJ, Ciaranello RD. Immunocytochemical localization and description of neurons expressing serotonin2 receptors in the rat brain. Neuroscience. 1993;54:701–717. doi: 10.1016/0306-4522(93)90241-7. [DOI] [PubMed] [Google Scholar]
- 26.Piguet P, Galvan M. Transient and long-lasting actions of 5-HT on rat dentate gyrus neurones in vitro. J Physiol. 1994;481:629–639. doi: 10.1113/jphysiol.1994.sp020469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shen RY, Andrade R. 5-Hydroxytryptamine2 receptor facilitates GABAergic neurotransmission in rat hippocampus. J Pharmacol Exp Ther. 1998;285:805–812. [PubMed] [Google Scholar]
- 28.Marek GJ, Aghajanian GK. Excitation of interneurons in piriform cortex by 5-hydroxytryptamine: Blockade by MDL 100,907, a highly selective 5-HT2A receptor antagonist. Eur J Pharmacol. 1994;259:137–141. doi: 10.1016/0014-2999(94)90502-9. [DOI] [PubMed] [Google Scholar]
- 29.Monti JM, Jantos H. Effects of the serotonin 5-HT2A/2C receptor agonist DOI and of the selective 5-HT2A or 5-HT2C receptor antagonists EMD 281014 and SB-243213, respectively, on sleep and waking in the rat. Eur J Pharmacol. 2006;553:163–170. doi: 10.1016/j.ejphar.2006.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Leysen JE, Gommeren W, Van Gompel P, Wynants J, Janssen PFM, Laduron PM. Receptor-binding properties in vitro and in vivo of ritanserin: A very potent and long acting serotonin-S2 antagonist. Mol Pharmacol. 1985;27:600–611. [PubMed] [Google Scholar]
- 31.Rinaldi-Carmona M, Congy C, Santucci V, et al. Biochemical and pharmacological properties of SR 46349B, a new potent and selective 5-hydroxytryptamine2 receptor antagonist. J Pharmacol Exp Ther. 1992;262:759–768. [PubMed] [Google Scholar]
- 32.Ebert B. Discontinued drugs 2007: Central and peripheral nervous system drugs. Expert Opin Invest Drugs. 2009;18:109–123. doi: 10.1517/13543780802687371. [DOI] [PubMed] [Google Scholar]
- 33.Heuillet E, Petitet F, Mignani S, et al. The naphtosultam derivative RP 62203 (fananserin) has high affinity for the dopamine D4 receptor. Eur J Pharmacol. 1996;24:229–233. doi: 10.1016/s0014-2999(96)00554-7. [DOI] [PubMed] [Google Scholar]
- 34.Borbély AA, Trachsel L, Tobler I. Effect of ritanserin on sleep stages and sleep EEG in the rat. Eur J Pharmacol. 1988;156:275–278. doi: 10.1016/0014-2999(88)90332-9. [DOI] [PubMed] [Google Scholar]
- 35.Dugovic C, Wauquier A. 5-HT2 receptors could be primarily involved in the regulation of slow-wave sleep in the rat. Eur J Pharmacol. 1987;137:145–146. doi: 10.1016/0014-2999(87)90196-8. [DOI] [PubMed] [Google Scholar]
- 36.Dugovic C, Wauquier A, Leysen JE, Marrannes R, Janssen PA. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology. 1989;97:436–442. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- 37.Silhol S, Glin L, Gottesmann C. Study of the 5-HT2 antagonist ritanserin on sleep-walking cycle in the rat. Pharmacol Biochem Behav. 1992;41:241–243. doi: 10.1016/0091-3057(92)90091-s. [DOI] [PubMed] [Google Scholar]
- 38.Sommerfelt L, Ursin R. The 5-HT2 antagonist ritanserin decreases sleep in cats. Sleep. 1993;16:15–22. doi: 10.1093/sleep/16.1.15. [DOI] [PubMed] [Google Scholar]
- 39.Al-Shamma HA, Anderson C, Chuang E, et al. Nelotanserin, a novel selective human 5-hydroxytryptamine2A inverse agonist for the treatment of insomnia. J Pharmacol Exp Ther. 2010;332:281–290. doi: 10.1124/jpet.109.160994. [DOI] [PubMed] [Google Scholar]
- 40.Stutzmann JM, Eon B, Lucas M, Blanchard JC, Laduron PM. RP 62203, a 5-hydroxytryptamine2 antagonist, enhances deep NREM sleep in rats. Sleep. 1992;15:119–124. doi: 10.1093/sleep/15.2.119. [DOI] [PubMed] [Google Scholar]
- 41.Popa D, Lena C, Fabre V, et al. Contribution of 5-HT2 receptor subtypes to sleep-wakefulness and respiratory control, and functional adaptations in knock-out mice lacking 5-HT2A receptors. J Neurosci. 2005;25:11231–11238. doi: 10.1523/JNEUROSCI.1724-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sharpley AL, Elliott JM, Attenburrow MJ, Cowen PJ. Slow wave sleep in humans: Role of 5-HT2A and 5-HT2C receptors. Neuropharmacology. 1994;33:467–471. doi: 10.1016/0028-3908(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 43.Morairty SR, Hedley L, Flores J, Martin R, Kilduff TS. Selective 5HT2A and 5HT6 receptor antagonists promote sleep in rats. Sleep. 2008;31:34–44. doi: 10.1093/sleep/31.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Declerck AC, Wauquier A, Van Der Ham-Veltman PHM, Gelders Y. Increase in slow-wave sleep in humans with the serotonin-S2 antagonist ritanserin. The first exploratory polysomnographic sleep study. Curr Ther Res. 1987;41:427–432. [Google Scholar]
- 45.Idzikowski C, Mills FJ, Glennard R. 5-Hydroxytryptamine-2 antagonist increases human slow wave sleep. Brain Res. 1986;16:164–168. doi: 10.1016/0006-8993(86)90299-4. [DOI] [PubMed] [Google Scholar]
- 46.Idzikowski C, Mills FJ, James J. A dose-response study examining the effects of ritanserin on human slow wave sleep. Br J Clin Pharmacol. 1991;31:193–196. doi: 10.1111/j.1365-2125.1991.tb05514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharpley AL, Solomon RA, Fernando AI, da Roza Davis JM, Cowen PJ. Dose-related effects of selective 5-HT2 receptor antagonists on slow wave sleep in humans. Psychopharmacology. 1990;101:568–569. doi: 10.1007/BF02244239. [DOI] [PubMed] [Google Scholar]
- 48.Idzikowski C, Cowen PJ, Nutt D, Mills FJ. The effects of chronic ritanserin treatment on sleep and the neuroendocrine response to L-tryptophan. Psychopharmacology. 1987;93:416–420. doi: 10.1007/BF00207228. [DOI] [PubMed] [Google Scholar]
- 49.Landolt H-P, Meier V, Burgess HJ, et al. Serotonin-2 receptors and human sleep: Effect of a selective antagonist on EEG power spectra. Neuropsychopharmacology. 1999;21:455–466. doi: 10.1016/S0893-133X(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 50.Rosenberg R, Seiden DJ, Hull SG, et al. APD125, a selective serotonin 5-HT(2A) receptor inverse agonist, significantly improves sleep maintenance in primary insomnia. Sleep. 2008;31:1663–1671. doi: 10.1093/sleep/31.12.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Abbas A, Roth BL. Pimavanserin tartrate: A 5-HT2A inverse agonist with potential for treating various neuropsychiatric disorders. Expert Opin Pharmacother. 2008;9:3251–3259. doi: 10.1517/14656560802532707. [DOI] [PubMed] [Google Scholar]
- 52.Ancoli-Israel S, Vanover KE, Weiner DM, Davis RE, van Kammen DP. Pimavanserin tartrate, a 5-HT2Areceptor inverse agonist, increases slow wave sleep as measured by polysomnography in healthy adult volunteers. Submitted. [DOI] [PMC free article] [PubMed]
- 53.Walsh JK, Perlis M, Rosenthal M, Krystal A, Jiang J, Roth T. Tiagabine increases slow-wave sleep in a dose-dependent fashion without affecting traditional efficacy measures in adults with primary insomnia. J Clin Sleep Med. 2006;2:35–41. [PubMed] [Google Scholar]
- 54.Lammers GJ, Arends J, Declerck AC, Kamphuisen HAC, Schouwink G, Troost J. Ritanserin, a 5-HT2 receptor blocker, as add-on treatment in narcolepsy. Sleep. 1991;14:130–132. [PubMed] [Google Scholar]
- 55.Monti JM, Alterwain P, Estévez F, et al. The effects of ritanserin on mood and sleep in abstinent alcoholic patients. Sleep. 1993;16:647–654. doi: 10.1093/sleep/16.7.647. [DOI] [PubMed] [Google Scholar]
- 56.Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta-Trial Study Group Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. Am J Psychiatry. 2004;161:975–984. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- 57.Adam K, Oswald I. Effects of repeated ritanserin on middle-aged poor sleepers. Psychopharmacology. 1989;99:219–221. doi: 10.1007/BF00442811. [DOI] [PubMed] [Google Scholar]
- 58.Viola AU, Brandenberger G, Toussaint M, Bouhours P, Macher JP, Luthringer R. Ritanserin, a serotonin-2 receptor antagonist, improves ultradian sleep rhythmicity in young poor sleepers. Clin Neurophys. 2002;113:429–434. doi: 10.1016/s1388-2457(02)00014-7. [DOI] [PubMed] [Google Scholar]
- 59.da Roza Davis JM, Sharpley AL, Cowen PJ. Slow wave sleep and 5-HT2 receptor sensitivity in generalised anxiety disorder: A pilot study with ritanserin. Psychopharmacology. 1992;108:387–960. doi: 10.1007/BF02245128. [DOI] [PubMed] [Google Scholar]
- 60.da Roza Davis JM, Sharpley AL, Solomon RA, Cowen PJ. Sleep and 5-HT2 receptor sensitivity in recovered depressed patients. J Affect Disord. 1992;24:177–181. doi: 10.1016/0165-0327(92)90065-e. [DOI] [PubMed] [Google Scholar]
- 61.Staner L, Kempenaers C, Simonnet MP, Fransolet L, Mendlewicz J. 5-HT2 receptor antagonism and slow-wave sleep in major depression. Acta Psychiatr Scand. 1992;86:133–137. doi: 10.1111/j.1600-0447.1992.tb03241.x. [DOI] [PubMed] [Google Scholar]
- 62.Paiva T, Arriaga F, Wauquier A, Lara E, Largo R, Leitao JN. Effects of ritanserin on sleep disturbances of dysthymic patients. Psychopharmacology. 1988;96:395–399. doi: 10.1007/BF00216069. [DOI] [PubMed] [Google Scholar]
- 63.Danjou P, Warot D, Hergueta T, Lacomblez L, Bouhours P, Puech AJ. Comparative study of the psychomotor and antistress effects of ritanserin, alprazolam and diazepam in healthy subjects: Some trait anxiety-independent responses. Int Clin Psychopharmacol. 1992;7:73–79. [PubMed] [Google Scholar]
- 64.Kamali F, Stansfield SC, Ashton CH, Hammond GL, Emanuel MB, Rawlins MD. Absence of withdrawal effects of ritanserin following chronic dosing in healthy volunteers. Psychopharmacology. 1993;108:213–217. doi: 10.1007/BF02245310. [DOI] [PubMed] [Google Scholar]
- 65.Wiesel FA, Nordström AL, Farde L, Eriksson B. An open clinical and biochemical study of ritanserin in acute patients with schizophrenia. Psychopharmacology. 1994;114:31–38. doi: 10.1007/BF02245441. [DOI] [PubMed] [Google Scholar]
- 66.Johnson BA, Jasinski DR, Galloway GP, et al. Ritanserin in the treatment of alcohol dependence – a multi-center clinical trial. Psychopharmacology. 1996;128:206–215. doi: 10.1007/s002130050126. [DOI] [PubMed] [Google Scholar]
- 67.Vanover KE, Robbins-Weilert D, Wilbraham DG, et al. Pharmacokinetics, tolerability, and safety of ACP-103 following single or multiple oral dose administration in healthy volunteers. J Clin Pharmacol. 2007;47:704–714. doi: 10.1177/0091270007299431. [DOI] [PubMed] [Google Scholar]
- 68.Malleron JL, Comte MT, Gueremy C, et al. Naphthosultam derivatives: A new class of potent and selective 5-HT2 antagonists. J Med Chem. 1991;34:2477–2483. doi: 10.1021/jm00112a025. [DOI] [PubMed] [Google Scholar]
- 69.Truffinet P, Tamminga CA, Fabre LF, Meltzer HY, Rivière ME, Papillon-Downey C. Placebo-controlled study of the D4/5-HT2A antagonist fanaserin in the treatment of schizophrenia. Am J Psychiatry. 1999;156:419–425. doi: 10.1176/ajp.156.3.419. [DOI] [PubMed] [Google Scholar]
- 70.Sramek JJ, Kirkesseli S, Paccaly-Moulin A, et al. A bridging study of fananserin in schizophrenic patients. Psychopharmacol Bull. 1998;34:811–818. [PubMed] [Google Scholar]
- 71.Vanover KE, Staner L, Luthringer R, Mates S, Davis RE. ITI-007/ITI-722: A new approach for the treatment of sleep maintenance insomnia and sleep disorders associated with psychiatric and neurological diseases. Sleep. 2009;32:A284. [Google Scholar]
- 72.Cohrs S, Meier A, Neumann AC, Jordan W, Rüther E, Rodenbeck A. Improved sleep continuity and increased slow wave sleep and REM latency during ziprasidone treatment: A randomized, controlled, crossover trial of 12 healthy male subjects. J Clin Psychiatry. 2005;66:989–996. doi: 10.4088/jcp.v66n0805. [DOI] [PubMed] [Google Scholar]
- 73.Sharpley AL, Vassallo CM, Cowen PJ. Olanzapine increases slow-wave sleep: Evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry. 2000;47:468–470. doi: 10.1016/s0006-3223(99)00273-5. [DOI] [PubMed] [Google Scholar]
- 74.Sharpley AL, Attenburrow ME, Hafizi S, Cowen PJ. Olanzapine increases slow wave sleep and sleep continuity in SSRI-resistant depressed patients. J Clin Psychiatry. 2005;66:450–454. doi: 10.4088/jcp.v66n0407. [DOI] [PubMed] [Google Scholar]
- 75.Dursun SM, Patel JK, Burke JG, Reveley MA. Effects of typical antipsychotic drugs and risperidone on the quality of sleep in patients with schizophrenia: A pilot study. J Psychiatry Neurosci. 1999;24:333–337. [PMC free article] [PubMed] [Google Scholar]
- 76.Luthringer R, Staner L, Noel N, et al. A double-blind, placebo-controlled, randomized study evaluating the effect of paliperidone extended-release tablets on sleep architecture in patients with schizophrenia. Int Clin Psychopharmacol. 2007;22:299–308. doi: 10.1097/YIC.0b013e3281c55f4f. [DOI] [PubMed] [Google Scholar]
- 77.Bartoszyk GD, van Amsterdam C, Böttcher H, Seyfried CA. EMD 281014, a new selective serotonin 5-HT2A receptor antagonist. Eur J Pharmacol. 2003;25:229–230. doi: 10.1016/s0014-2999(03)01992-7. [DOI] [PubMed] [Google Scholar]