Abstract
Like the other hepatitis viruses, hepatitis E virus (HEV) has been difficult to study because of limitations in cell culture systems and small animal models. Much of what we know has come from epidemiological studies in developing countries and, more recently, in industrialized countries. However, the epidemiology is very different in these two settings: hepatitis E in developing countries is epidemic as well as sporadic, principally water-borne, most likely to cause disease in older children and young adults and relatively severe, especially in pregnant women; in industrialized countries the disease is sporadic, principally food-borne, most common in the elderly and probably associated with mostly inapparent infections. These differences are believed to be genotypically determined. To examine the biological parameters of hepatitis E, we have studied HEV infections in nonhuman primates, which are surrogates of man. Infections with HEV genotypes 1–3 were compared in rhesus and cynomolgus macaques and chimpanzees. In general, the biological characteristics of the different HEV genotypes mirrored their epidemiological characteristics.
Keywords: HEV, host range, nonhuman primates, virulence, genotypes
Introduction
Hepatitis E is a major cause of clinical acute hepatitis among adults in many developing countries.1,2,3 In such settings, it is responsible for water-borne outbreaks (some quite large), as well as sporadic disease. This hepatitis is caused principally by hepatitis E virus (HEV) belonging to genotypes 1 and 2. In contrast, until recently, hepatitis E was rarely diagnosed in industrialized countries. However, a growing number of cases are being diagnosed, principally from European countries, but also from Great Britain, the USA and Japan.4,5,6 Most of these cases are thought to be food-borne and caused by HEV genotypes 3 and 4.7,8 Since genotypes 3 and 4 infect principally swine as well as humans (but also certain other species of domestic and wild animals, including boar, wild deer, cattle and sheep), autochthonous hepatitis caused by these genotypes in industrialized countries is thought to be zoonotic in nature and transmitted to humans principally via ingestion of undercooked pork.
There are other differences in the epidemiology of HEV infection between developing and industrialized countries: the seroprevalence of anti-HEV seldom exceeds 40% in developing countries, even though there is significant disease, and a seroprevalence of 20% is not uncommon in industrialized countries where hepatitis E is uncommon.9,10,11 Furthermore, the highest clinical attack rate in developing countries is among older children and young adults, whereas the average age of hepatitis E cases in industrialized countries is greater than 50 years and a number of cases have been reported in individuals who are immunocompromised.1,2 Finally, hepatitis E is associated with a high mortality among pregnant women in certain developing countries but not in industrialized countries.2,3,12,13 These observations suggest that HEV in developing countries may be more virulent and likely to be shed at a higher titer than the strains circulating in industrialized countries.
The above hypotheses have been based upon distinct epidemiological patterns of infection but limited direct measurements. The purpose of this report is to examine HEV in animal models with the goal of quantifying some of the viral characteristics that might lead to the observed epidemiology.
Materials and methods
Laboratory animals
Transmission studies were carried out in rhesus monkeys (Macaca mulatta), cynomolgus monkeys (M. cynomolgus) and chimpanzees (Pan troglodytes) that were bred and raised in captivity. The animals were housed and maintained at Bioqual, Inc. (Rockville, MD, USA) or at the NIH Animal Facility (Poolsville, MD, USA) (both are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, International), under conditions that met or exceeded all the requirements for the care and housing of the relevant species. All protocols were approved by the institutional animal care and use committees of the respective facilities. This study reports results from different studies that were carried out over a span of a number of years for specific purposes but the general design, the methodology and the staff performing the procedures were essentially the same. Thus, a large body of data about hepatitis E in laboratory animal models is brought together to look for biological counterparts of epidemiological associations. Blood samples and needle liver biopsies were obtained weekly and feces were collected daily to three times a week. Serum samples were tested for liver enzyme levels by standard methods (AniLytics, Inc., Gaithersburg, MD, USA). Biopsy tissue was fixed in formalin, embedded, sectioned and stained with hematoxylin and eosin (American Histo Labs, Inc., Gaithersburg, MD, USA) and read under code by one of us (S. Govindarajan). Samples were scored for liver pathology on a scale of zero to four-plus.
HEV inocula
Four strains of HEV were used in this study. These were: the Sar-55 strain (genotype 1), recovered from an epidemic of hepatitis E in a military college in Sargodha, Pakistan in 1987;14 the Mex-14 strain (genotype 2), recovered from an epidemic in Telixtac, Mexico in 1986, and kindly supplied by K. McCaustland (Centers for Disease Control, USA);15 the US-2 strain (genotype 3) recovered from a sporadic case of autochthonous hepatitis in Memphis, TN, USA, in the 1990s and kindly supplied by Isa Mushawar (Abbott Laboratories);16 and the Meng strain (genotype 3), recovered from domestic swine in the midwestern USA in 1997.17 The viruses were contained in 10% suspensions of original human feces (Sar-55, Mex-14), second passage cynomolgus monkey feces (US-2) and original pig feces (Meng), respectively. The viruses had not been passed in cell culture or otherwise passed in animals.
Serological tests
One hundred-fold dilutions of serum samples were tested for anti-HEV of the IgG and IgM isotypes as reported previously, substituting species-specific secondary antibodies where necessary.18,19 Titers of antibody were determined by testing 10-fold dilutions of sera. The highest dilution exceeding the cutoff value of optical density was taken as the end point titer of the serum.
Results
Relative susceptibility of three primate species to infection with a human HEV strain
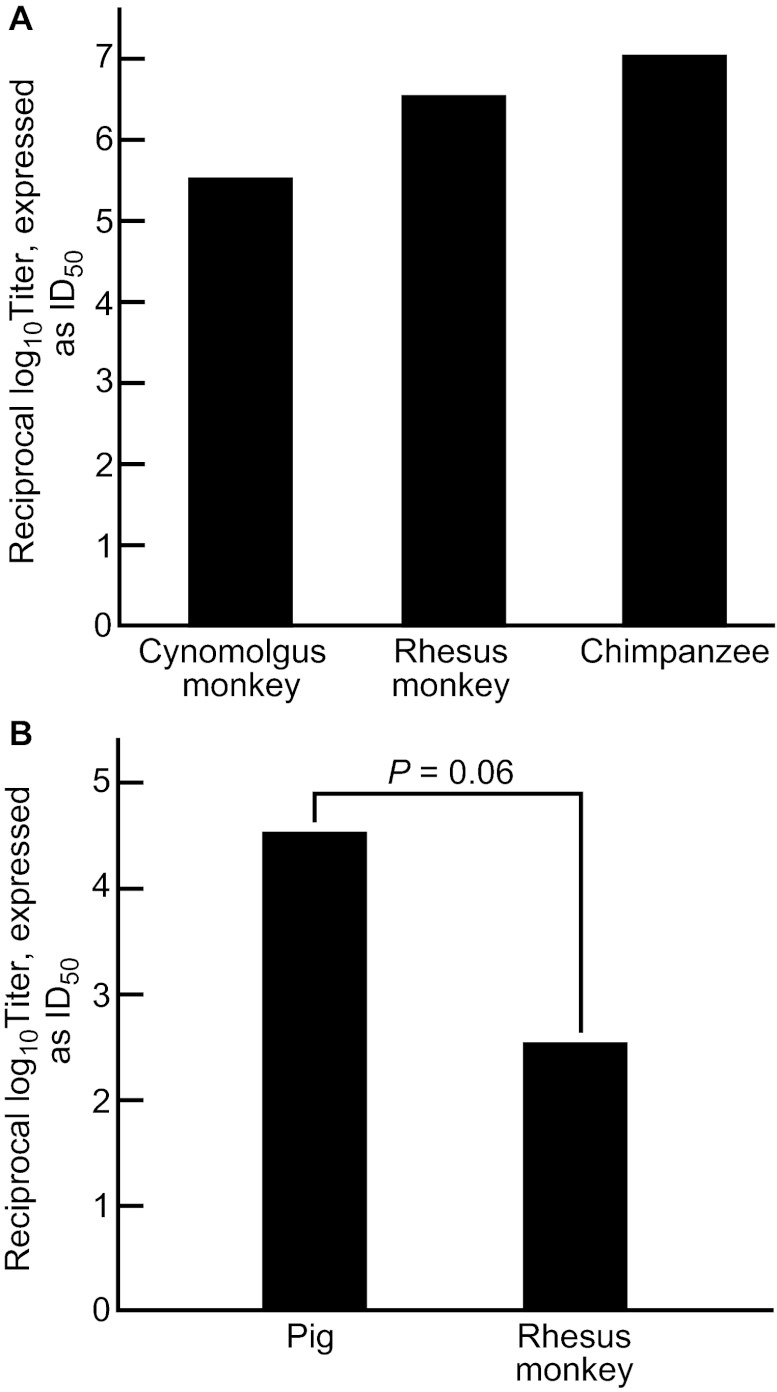
To determine the relative ability to infect cynomolgus monkeys, rhesus monkeys and chimpanzees with HEV, a reverse titration of a challenge pool of the genotype 1 Sar-55 strain of HEV was performed. All three species of primates were highly susceptible to infection, with 50% infectious dose (ID50) titers, respectively, of 105.5, 106.5 and 107.0 (Figure 1A). However, the number of animals available for these reverse titrations was not sufficient for statistical analysis.
Figure 1.
(A) The infectivity titer of a standard pool of human-derived genotype 1 HEV in three species of nonhuman primate. The data were obtained by reverse titration in order to limit the number of animals used. The differences in titer were not statistically significant. (B) The infectivity titer of a standard pool of pig-derived genotype 3 HEV in rhesus monkeys and pigs. The differences in titer approached statistical significance.
Relative susceptibility of primates and pigs to infection with a porcine HEV strain
We determined the infectivity titer of the genotype 3 Meng strain by reverse titration in pigs and rhesus monkeys. The inoculum consisted of dilutions of the original pig feces from which the virus was recovered. As seen in Figure 1B, pigs were more sensitive to infection than were rhesus monkeys.
Relative susceptibility of three primate species to HEV-related disease
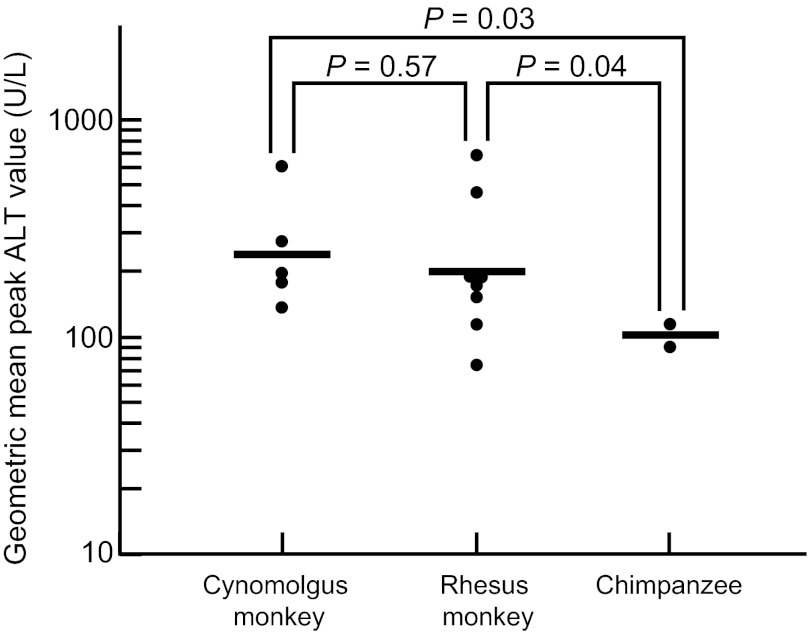
To determine which species was the most sensitive for responding with disease, as measured by liver enzyme levels, eight rhesus monkeys, five cynomolgus monkeys and two chimpanzees were inoculated intravenously with the same dose (105.5 rhesus monkey ID50 of the HEV Sar-55 challenge pool) and monitored for liver enzyme elevations. As seen in Figure 2, cynomolgus monkeys recorded the highest geometric mean peak alanine aminotransferase (ALT) value (242 U/L), followed by the rhesus monkeys (196 U/L) and the chimpanzees (101 U/L). The upper limit of normal for ALT values in these species is 31 U/L. The geometric mean peak ALT values in chimpanzees were significantly lower than those in the macaque species. Similar results were obtained with a second pool of the Sar-55 strain (data not shown). In a separate study, we compared peak ALT values in male and female rhesus monkeys infected with the same dose of the Sar-55 challenge pool. There was no significant difference in the responses (data not shown).
Figure 2.
Severity of hepatitis, as measured by peak ALT levels in three species of nonhuman primate inoculated intravenously with the same high dose (105.5 ID50) of a genotype 1 HEV strain. The infection was associated with higher peak ALT levels in the two macaque species than in chimpanzees. The upper limit of normal for these species is 31 U/L.
Effect of magnitude of challenge dose on magnitude of ALT response
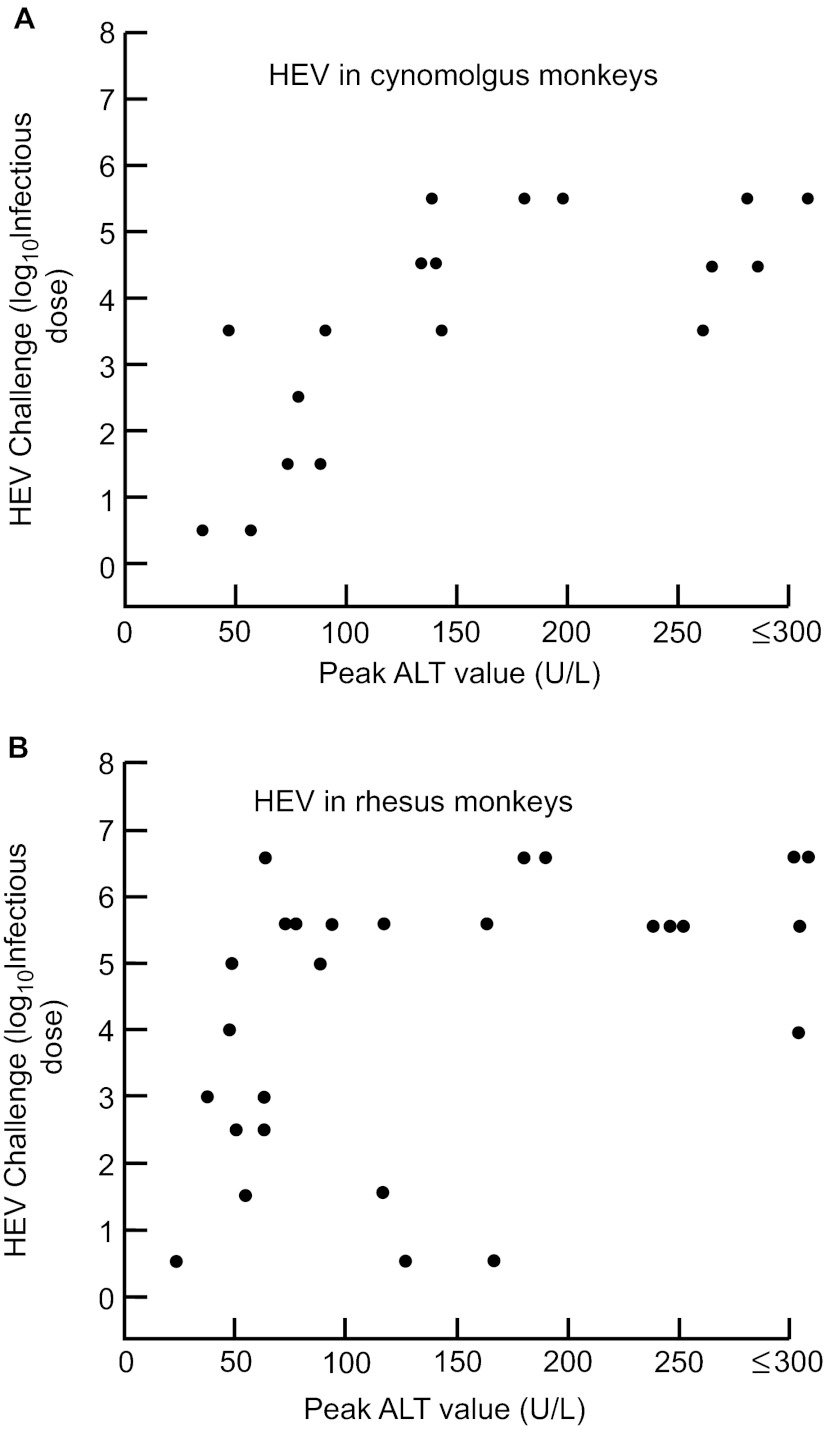
We have reported previously that there was no relationship between the size of the challenge dose and the clinical response (as measured by peak ALT levels) in tamarins to hepatitis A virus (HAV)20 or in chimpanzees to hepatitis B virus.21 To determine if that was true for HEV in macaques, we compiled data from past experiments in cynomolgus and rhesus monkeys and plotted the peak ALT value observed as a function of the challenge dose. As seen in Figure 3A, higher doses of challenge virus resulted in higher peak ALT values in cynomolgus monkeys. The relationship between ALT and virus challenge dose was similar in rhesus monkeys (Figure 3B).
Figure 3.
Relationship between intravenous challenge dose of genotype 1 HEV and the clinical response, as measured by peak ALT levels in two macaque species. The infections were relatively attenuated following challenge doses of 103 or less in both species. (A) cynomolgus monkeys; (B) rhesus monkeys.
Relationship between magnitude of challenge dose and markers of infection
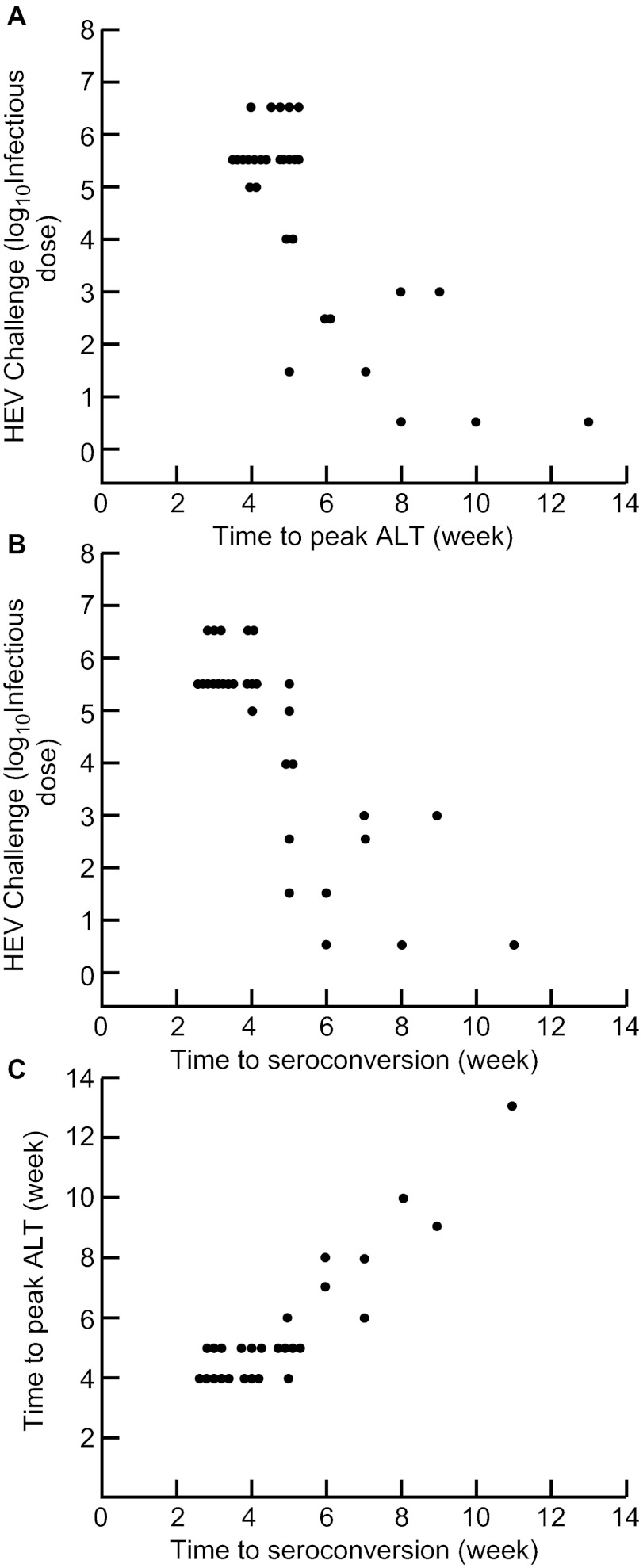
In previous experiments in tamarins, we demonstrated that the challenge dose of HAV was inversely related to the incubation period for peak ALT and seroconversion.20 To determine if this was also true for HEV in cynomolgus monkeys, we examined previous data on various challenge doses of genotype 1 HEV and its effect on incubation period to ALT peak and seroconversion. Both interval to ALT peak (Figure 4A) and interval to seroconversion (Figure 4B) were inversely related to the dose of virus administered, but seroconversion occurred slightly earlier on average. Thus, there was a direct relationship between week of peak ALT and week of seroconversion (Figure 4C), with the seroconversion occurring, on average, one half week earlier.
Figure 4.
Relationship between intravenous challenge dose of genotype 1 HEV and interval to markers of infection in rhesus monkeys. There was an inverse relationship between the challenge dose and interval to (A) peak ALT and (B) seroconversion. (C) Interval to peak ALT paralleled the interval to seroconversion, with the latter occurring, on average, one half week earlier.
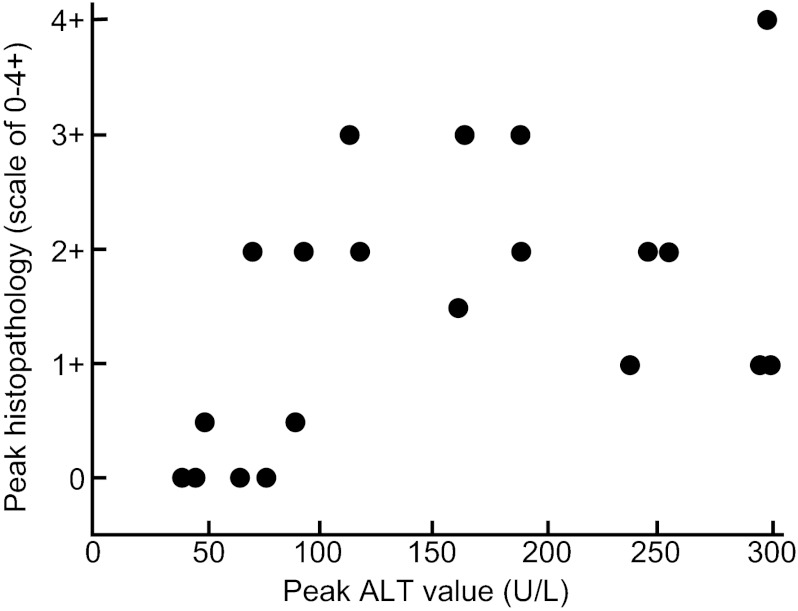
Relationship between peak hepatic histopathological scores and peak ALT values
It is generally assumed that ALT levels reflect pathological changes in the liver. However, there is not always a good correlation between these two markers of disease. We determined the relationship between histopathological changes (read under code) and peak ALT values in rhesus monkeys in this study. As anticipated, there was a direct but weak relationship between the magnitude of histopathological changes on a scale of 0–4+ and peak ALT values (Figure 5). The Spearman's correlation coefficient was 0.54, with a P value of 0.02, indicative of a weak but statistically significant positive correlation.
Figure 5.
Comparison of magnitude of histological versus biochemical evidence of hepatitis in rhesus monkeys inoculated intravenously with a genotype 1 (SAR-55) HEV strain. There was a weak but significant positive correlation (Spearman's correlation coefficient was 0.54; P=0.02).
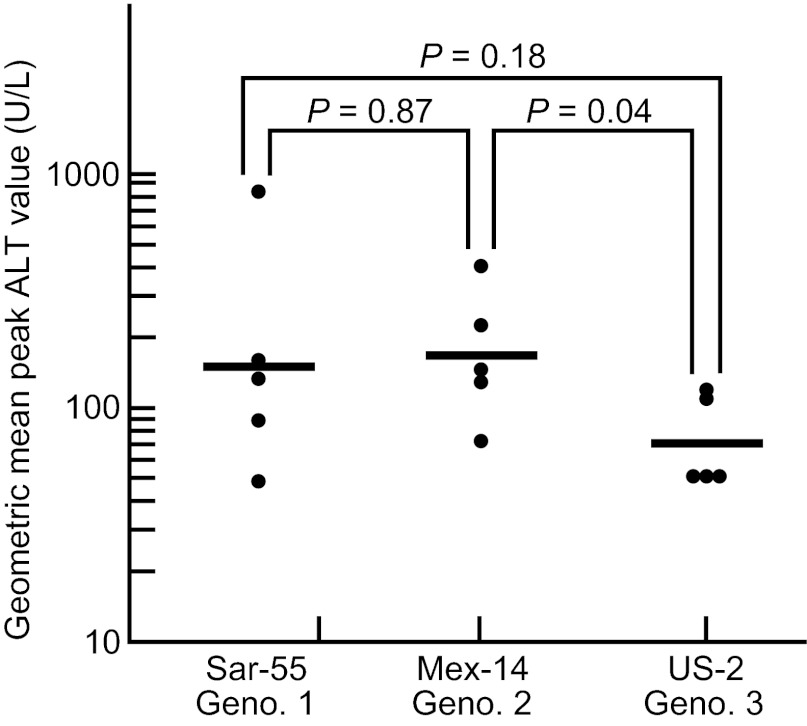
Comparative virulence of HEV genotypes
As noted, there is considerable epidemiological evidence that genotypes 3 and 4 may be less virulent for humans than genotypes 1 or 2.1 Certainly, genotype 3 causes little disease in its presumed natural host, swine.22,23 To compare the relative virulence of genotypes 1, 2 and 3 at a dose sufficiently high to cause disease, we intravenously inoculated five rhesus monkeys each with 104.0 50% monkey infectious dose (MID50) of the Sar-55, Mex-14 and US-2 strains of HEV (we did not have a pool of genotype 4 of sufficient titer to include it in this study). Both the Sar-55 and Mex-14 pools came directly from humans and the US-2 pool had been passaged only two times in cynomolgus monkeys. As seen in Figure 6, the highest geometric mean peak ALT value was caused by the genotype 2 virus (165), followed by the genotype 1 strain (150). In monkeys infected with the genotype 3 strain, the geometric mean peak ALT was 67, only slightly higher than baseline.
Figure 6.
Comparison of virulence, as measured by mean peak ALT levels of genotype 1, genotype 2 and genotype 3 HEV strains administered intravenously at a high dose (104.0 MID50) to rhesus monkeys. The genotype 3 strain was significantly less virulent than the genotype 2 strain; the difference between the genotype 3 and genotype 1 strains approached significance.
Reinfection with HEV in previously infected rhesus monkeys
Two infant rhesus monkeys were inoculated intravenously with 105.5 MID50 of the Sar-55 strain of HEV shortly after birth in 1994. Rhesus 394 was the offspring of a mother with pre-existing anti-HEV and had maternal antibody with a titer of 1/100. Rhesus 396 was the offspring of a seronegative mother and did not have detectable antibody at the time of inoculation. Rhesus 394 was infected as measured by the loss of detectable maternal antibody at 4 weeks post-inoculation, followed 2 weeks later by the development of a high level of anti-HEV. The animal was not viremic and did not have an ALT elevation (Figure 7). Rhesus 396 also seroconverted but did become viremic and did have a low-level ALT elevation 6 weeks post-inoculation, at the time of seroconversion (Figure 7). At the conclusion of this experiment, the animals were returned to the holding facility. Three years and seven years later, respectively, the animals were inadvertently selected for other HEV experiments. At that time, they were both seronegative (titer of <1/100). Rhesus 394 was inoculated intravenously with approximately 105.0 MID50 of the genotype 3 US-2 strain of HEV. The animal did not have an anamnestic response, but did seroconvert again four weeks after inoculation, at the same time as a mild elevation in ALT (Figure 7). Viremia was not detected. Rhesus 396 was part of a reverse titration of a genotype 1 strain (F23) recovered from a patient in Morocco.24 Following intravenous inoculation with a suspension of feces, the animal did not have an anamnestic response, but did seroconvert 7 weeks after inoculation (Figure 7). This animal had both viremia and a significant ALT elevation, at the time of seroconversion. Rhesus 394 was negative for IgM anti-HEV during both infections. Rhesus 396 was transiently IgM positive during the first infection, but was not tested during the second infection (Figure 7).
Figure 7.
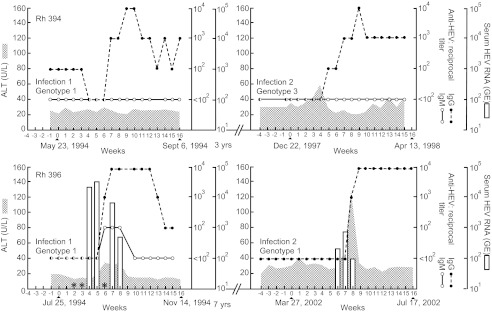
A second HEV infection in rhesus monkeys infected with HEV previously. Both animals were seronegative at the time of the second infection. Rhesus 394 was infected with a genotype 1 HEV and, 3 years late, with a genotype 3 strain. Viremia was not detected during either infection. Rhesus 396 was infected with a genotype 1 strain and, 7 years later, with a different genotype 1 strain. Viremia was detected during both infections (starred sera were not available for testing).
Discussion
In general, studies of HEV in nonhuman primates have yielded results that are consistent with current concepts of HEV infection in humans, based on epidemiological data. Chimpanzees were slightly more susceptible to infection with a human genotype 1 HEV strain than two species of macaques, suggesting that the virus may have adapted to infection in hominids (Figure 1A). Similarly, an inoculum of swine genotype 3 HEV strain had a higher infectivity titer in pigs than in rhesus monkeys, a surrogate of man, when a pool of the virus was titrated in both species (Figure 1B). A lower virus titer in primates could result in reduced transmission of virus during infections and consequent restricted transmission to contacts, consistent with epidemiological observations of limited person-to-person transmission of HEV in humans.3,25
A human strain of HEV was more virulent, as measured by liver enzyme elevations, in macaques than in chimpanzees, providing additional evidence that human HEV strains may have adapted to life in humans (Figure 2). In contrast, there was little difference in the degree of virulence when tested in rhesus versus cynomolgus monkeys.
We have performed a number of transmission studies of HEV in macaques in past years and this has permitted us to examine the effect of the size of the challenge dose on the clinical response, as measured by liver enzyme elevations. Unlike the response to HAV and hepatitis B virus, the response to HEV in macaques is very dependent upon the dose, with little evidence of hepatitis from doses below 103.0 infectious doses, administered intravenously (Figure 1A and 1B). These data were obtained with a human virus but, if they also apply to swine viruses, the combination of lower infectivity and lower virulence might explain the relatively low attack rate and high apparent subclinical infection rate of HEV in industrialized countries.
The incubation period of HEV (6–8 weeks) is generally considered to be somewhat longer than that of HAV (4–6 weeks), but it is not clear whether this represents a difference in replication rate or a difference in infecting dose of virus. When we plotted the challenge dose of virus against the interval to peak liver enzyme elevations, we found an inverse relationship (Figure 4A), similar to what we had found previously in studies of HAV in tamarins and chimpanzees20 and of hepatitis B virus in chimpanzees.21 Similarly, there was an inverse relationship between challenge dose and interval to seroconversion (Figure 4B), which was approximately one half week earlier than peak ALT elevations (Figure 1C). Importantly, the incubation period (the mean interval from exposure to average of seroconversion and ALT elevation) was exactly the same (4.25 weeks) when exactly the same challenge dose (105.0 ID50) of HAV and HEV were administered to pairs of tamarins and rhesus monkeys, respectively20 (and Figure 4A and 4B). Thus, the difference in incubation periods for hepatitis A and hepatitis E may be the result of smaller infecting doses of HEV and this also has implications for whether an infection will be clinical or subclinical. It is also important to point out that the close relationship between interval to seroconversion and interval to peak ALT elevation (Figure 4C) is also additional evidence that HEV is a non-cytopathogenic virus and that the pathogenesis of hepatitis E is likely the result of the host's immune response to infection. This is further borne out by the relationship between peak ALT values and the interval to most severe histopathology (Figure 5).
With this information in hand, we examined the relative virulence of human genotypes 1 and 2 and swine genotype 3 in rhesus monkeys, using a challenge dose, 104.0 ID 50, which has usually been associated with a clinical response in nonhuman primates. Swine genotype 3 was significantly less virulent than the two human genotypes (Figure 6), consistent with epidemiological evidence for an apparent high subclinical infection rate in normal humans and a predilection for clinical disease to occur in the elderly and the immunocompromised.1,4,10,11 Although we could not test genotype 4 for relative virulence, data from China suggest that it may also be relatively attenuated.26
Age-dependent patterns of antibody to HEV have been difficult to understand because they are quite different from patterns observed for most enterically-transmitted viruses: in developing countries, such viruses infect virtually 100% of the population by age 5–10 years.9 In contrast, serological evidence of HEV infection in the very young is rare in such countries. It has been suggested that HEV may infect early in life, but serological evidence is not long-lived, and there is some evidence for this.27 We have been able to demonstrate in two young rhesus monkeys the complete loss of serological evidence for, and immunological memory of, a prior HEV infection (Figure 7). If this proved to be relatively common in populations of developing countries, the unusual serological patterns observed would be more easily understood. The finding of complete loss of adaptive immunity would also be consistent with other observations, obtained by microarray analysis of HEV infections in chimpanzees, that the adaptive immune response to hepatitis E is the weakest of any of the human hepatitis viruses (unpublished data).
In summary, we have demonstrated, in nonhuman primates, a number of biological characteristics of human and swine HEV strains that are consistent with our epidemiological understanding of HEV infections in humans. Both biology and epidemiology point to two separate entities, a water-borne, moderately severe infection, often epidemic and typically in older children and young adults, caused by genotypes 1 and 2; and a food-borne, relatively less severe sporadic infection of older individuals and the immunologically compromised, by genotype 3 and probably genotype 4. They appear to comprise two separate diseases, even though they represent one serotype and can be prevented by one vaccine.
Acknowledgments
The authors are grateful for the excellent statistical support of Jing Qin, Biostatistics Research Branch, National Institute of Allergy and Infectious Diseases, National Institutes of Health. This study was supported by the intramural research program of the National Institute of Allergy and Infectious Diseases, National Institutes of Health. The authors have nothing to declare.
References
- Purcell RH, Emerson SU. Hepatitis E: an emerging awareness of an old disease. J Hepatol. 2008;48:494–503. doi: 10.1016/j.jhep.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Kamar N, Bendall R, Legrand-Abravanel F, et al. Hepatitis E. Lancet. 2012;379:2477–2488. doi: 10.1016/S0140-6736(11)61849-7. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH, Nelson KE, Purcell RH. Hepatitis E. N Engl J Med. 2012;367:1237–1244. doi: 10.1056/NEJMra1204512. [DOI] [PubMed] [Google Scholar]
- Dalton HR, Stableforth W, Thurairajah P, et al. Authtochthonous hepatitis E in Southwest England: natural history, complications and seasonal variation, and hepatitis E virus IgG seroprevalence in blood donors, the elderly and patients with chronic liver disease. Eur J Gastroenterol Hepatol. 2008;20:784–790. doi: 10.1097/MEG.0b013e3282f5195a. [DOI] [PubMed] [Google Scholar]
- Davern TJ, Chalasani N, Fontana RJ, et al. Acute hepatitis E infection accounts for some cases of suspected drug-induced liver injury. Gastroenterology. 2011;141:1665–1672. doi: 10.1053/j.gastro.2011.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sainokami S, Abe K, Kumagai I, et al. Epidemiological and clinical study of sporadic acute hepatitis E caused by indigenous strains of hepatitis E virus in Japan compared with acute hepatitis A. J Gastroenterol. 2004;39:640–648. doi: 10.1007/s00535-003-1359-5. [DOI] [PubMed] [Google Scholar]
- Meng XJ. From barnyard to food table: the omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23–30. doi: 10.1016/j.virusres.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell RH, Emerson SU. Hidden danger: the raw facts about hepatitis E. J Infect Dis. 2010;202:819–821. doi: 10.1086/655900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arankalle VA, Tsarev SA, Chadha MS, et al. Age-specific prevalence of antibodies to hepatitis A and E viruses in Pune, India, 1982 and 1992. J Infect Dis. 1995;171:447–450. doi: 10.1093/infdis/171.2.447. [DOI] [PubMed] [Google Scholar]
- Kuniholm MH, Purcell RH, McQuillan GM, Engle RE, Wasley A, Nelson KE. Epidemiology of hepatitis E virus in the United States: results from the Third National Health and Nutrition Examination Survey, 1988–1994. J Infect Dis. 2009;200:48–56. doi: 10.1086/599319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendall R, Ellis V, Ijaz S, Ali R, Dalton H. A comparison of two commercially available anti-HEV IgG kits and a re-evaluation of anti-HEV IgG seroprevalence data in developed countries. J Med Virol. 2010;82:799–805. doi: 10.1002/jmv.21656. [DOI] [PubMed] [Google Scholar]
- Viswanathan R. Epidemiology. Indian J Med Res. 1957;45:1–29. [PubMed] [Google Scholar]
- Khuroo MS, Teli MR, Skidmore S, Sofi MA, Khuroo MI. Incidence and severity of viral hepatitis in pregnancy. Am J Med. 1981;70:252–255. doi: 10.1016/0002-9343(81)90758-0. [DOI] [PubMed] [Google Scholar]
- Ticehurst J, Popkin TJ, Bryan JP, et al. Association of hepatitis E virus with an outbreak of hepatitis in Pakistan: serologic responses and pattern of virus excretion. J Med Virol. 1992;36:84–92. doi: 10.1002/jmv.1890360205. [DOI] [PubMed] [Google Scholar]
- Velazquez O, Stetler HC, Avila C, et al. Epidemic transmission of enterically transmitted non-A, non-B hepatitis in Mexico, 1986–1987. JAMA. 1990;263:3281–3285. [PubMed] [Google Scholar]
- Erker JC, Desai Sm, Schlauder GG, et al. A hepatitis E variant from the United States: molecular characterization and transmission in cynomolgus macaques. J Gen Virol. 1999;80:681–690. doi: 10.1099/0022-1317-80-3-681. [DOI] [PubMed] [Google Scholar]
- Meng XJ, Purcell RH, Halbur PG, et al. A novel virus in swine is closely related to the human hepatitis E virus. Proc Natl Acad Sci USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsarev SA, Tsareva TS, Emerson SU, et al. ELISA for antibody to hepatitis E virus (HEV) based on complete open-reading frame-2 protein expressed in insect cells: identification of HEV infection in primates. J Infect Dis. 1993;168:369–378. doi: 10.1093/infdis/168.2.369. [DOI] [PubMed] [Google Scholar]
- Engle RE, Yu C, Emerson SU, Meng XJ, Purcell RH. Hepatitis E virus (HEV) capsid antigens derived from viruses of human and swine origin are equally efficient for detecting anti-HEV by enzyme immunoassay. J Clin Microb. 2002;40:4576–4580. doi: 10.1128/JCM.40.12.4576-4580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell RH, Wong DC, Shapiro M. Relative infectivity of hepatitis E virus by the oral and intravenous routes in 2 species of nonhuman primates. J Infect Dis. 2002;185:1668–1671. doi: 10.1086/340520. [DOI] [PubMed] [Google Scholar]
- Barker LF, Maynard JE, Purcell RH, et al. Hepatitis B virus infection in chimpanzees: titration of subtypes. J Infect Dis. 1975;132:451–458. doi: 10.1093/infdis/132.4.451. [DOI] [PubMed] [Google Scholar]
- Halbur PG, Kasorandorkbua C, Gilbert C, et al. Comparative pathogenesis of infection of pigs with hepatitis E viruses recovered from a pig and human. J Clin Microbiol. 2001;39:918–923. doi: 10.1128/JCM.39.3.918-923.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martelli F, Toma S, Di Bartolo I, et al. Detection of hepatitis E virus (HEV) in Italian pigs displaying different pathological lesions. Res Vet Sci. 2010;88:492–496. doi: 10.1016/j.rvsc.2009.12.011. [DOI] [PubMed] [Google Scholar]
- Chatterjee R, Tsarev S, Pillot J, Coursaget P, Emerson SU, Purcell RH. African strains of hepatitis E virus that are distinct from Asian strains. J Med Virol. 1997;53:139–144. [PubMed] [Google Scholar]
- Khuroo MS. Discovery of hepatitis E: the epidemic of non-A, non-B hepatitis 30 years down the memory lane. Virus Res. 2011;161:3–14. doi: 10.1016/j.virusres.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Zhu FC, Zhang J, Zhang XF, et al. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: a large-scale, randomized, double-blind placebo-controlled, phase 3 trial. Lancet. 2010;376:895–902. doi: 10.1016/S0140-6736(10)61030-6. [DOI] [PubMed] [Google Scholar]
- Goldsmith R, Yarbough PO, Reyes GR, et al. Enzyme-linked immunosorbent assay for diagnosis of acute sporadic hepatitis E in Egyptian children. Lancet. 1992;339:328–331. doi: 10.1016/0140-6736(92)91647-q. [DOI] [PubMed] [Google Scholar]