Abstract
Analysis of microbial epidemics has been revolutionized by whole-genome sequencing. We recently sequenced the genomes of 601 type emm59 Group A Streptococcus (GAS) organisms responsible for an ongoing epidemic of invasive infections in Canada and some of the United States. The epidemic has been caused by the emergence of a genetically distinct, hypervirulent clone that has genetically diversified. The ease of obtaining genomic data contrasts with the relatively difficult task of translating them into insightful epidemiological information. Here, we sequenced the genomes of 90 additional invasive Canadian emm59 GAS organisms, including 80 isolated recently in 2010–2011. We used an improved bioinformatics pipeline designed to rapidly process and analyze whole-genome data and integrate strain metadata. We discovered that emm59 GAS organisms are undergoing continued multiclonal evolutionary expansion. Previously identified geographic patterns of strain dissemination are being diluted as mixing of subclones over time and space occurs. Our integrated data analysis strategy permits prompt and accurate mapping of the dissemination of bacterial organisms in an epidemic wave, permitting rapid generation of hypotheses that inform public health and virulence studies.
Keywords: bacterial epidemics, Canada, group A Streptococcus, invasive disease, United States, whole-genome sequencing
Introduction
The recent unparalleled discriminatory power afforded by whole-genome sequencing has proven decisive for understanding aspects of bacterial outbreaks, including cholera,1,2 tuberculosis,3 Listeria monocytogenes,4 Escherichia coli O1045,6 and Group A Streptococcus (GAS also known as streptococcus pyogenes).7,8,9 Decreasing costs, enhanced data output and improved protocols now make this technology the method of choice for some aspects of public health microbiology.10,11,12 The ease of obtaining genome data permits the study of the evolutionary events contributing to bacterial clone emergence, strain differentiation, and epidemics at the full-chromosomal level in large samples.13 Toward this end, we have used whole-genome sequencing of GAS as a model to understand the fine-structure and molecular architecture of epidemics occurring over many years.7,8
GAS is a human-specific pathogen that causes diseases ranging in severity from uncomplicated pharyngitis to life-threatening necrotizing fasciitis.14 GAS strains are classified based on the aminoterminal sequence of the M protein, a polymorphic cell-surface adhesin and anti-phagocytic factor encoded by the emm gene.15,16,17 Previously, we have used deep-genome sequencing data for 95 emm3 GAS strains integrated with epidemiological information to resolve the key molecular features of three successive epidemics caused by emm3 GAS in Ontario, Canada, over a period of 16 years.7 This comparative pathogenomic analysis delineated the fine-structure molecular population genetics of these epidemics and defined relationships among the invasive strains responsible for the three epidemics.
More recently, using animal infection models and whole-genome sequencing of 601 emm59 GAS strains, we unambiguously demonstrated that a recently emerged, hypervirulent clone was responsible for a large epidemic of invasive GAS disease that began in 2006 in Western Canada and caused more than 500 invasive cases in 4 years. The epidemic wave extended rapidly to 11 Canadian provinces and territories and to three of the United States.8,18 The epidemic clone was distantly related to historic emm59 GAS strains, and also differed from emm59 GAS organisms isolated in other countries.8,19 Our genomic investigation precisely informed us about the population genomic landscape of the emm59 GAS epidemic, and permitted us to delineate patterns of geographic dissemination of strains in widely-dispersed areas. We also discovered that, as a population, the emm59 epidemic isolates have accumulated relatively few genetic polymorphisms over the years since sharing a common ancestor. One hypothesis to explain this finding is that over the course of the four years represented by the emm59 strain sample used in that previous study, sufficient time has not elapsed to produce considerable genomic differentiation. Extending the genomic analysis by incorporating whole-genome data for epidemic strains more recently isolated will help to differentiate between this and other hypotheses.
Despite the ability to sequence bacterial genomes rapidly, translating the whole-genome data into insightful epidemiological findings remains relatively time consuming and requires intensive logistical and computing resources.13 In addition, incorporating critical strain or patient metadata into the analysis, and displaying the data in a more visual, intuitive and integrative fashion is challenging. Collectively, these problems hinder the clinical and public health responses to natural and accidental outbreaks, and deliberate release of infectious agents. Thus, methods to rapidly translate genome data into accurate, epidemiologically relevant information in a short period of time are needed. Here, we have attempted to address these limitations by use of whole-genome data from additional strains recovered from the ongoing emm59 GAS epidemic. Whole-genome data for 90 additional emm59 GAS strains isolated in Canada were generated and integrated into an improved pipeline for data processing and visualization. We show here that pertinent epidemiological information can be obtained rapidly and economically in meaningful clinical and public health situations.
Materials and methods
Bacterial strains
The strain collection includes 691 emm59 strains of different origins. 601 of these strains have been described in detail recently.8 The additional 90 strains (Table 1) were all isolated in Canada from patients with invasive GAS infections. One of these strains was isolated in 1993 in Ontario, and is thus far the oldest identified Canadian emm59 GAS organism. Nine strains were isolated during 2006–2009, the period of time covered by our previous report on the emm59 epidemic,8 but had not been included in the previous study. The vast majority (n=80) of the newly analyzed strains were isolated in 2010 and 2011.
Table 1. Type emm59 GAS strains incorporated in this study.
| ID | Strain name | Isolation date | Province | Source |
|---|---|---|---|---|
| 1 | MGAS23789 | Oct-10 | Alberta | Neck tissue |
| 2 | MGAS23790 | Jan-10 | Alberta | Blood |
| 3 | MGAS23791 | Jul-11 | Alberta | Bursa Lt olecranon |
| 4 | MGAS23792 | Mar-11 | Alberta | Knee fluid |
| 5 | MGAS23793 | Jul-10 | Alberta | Synovial elbow |
| 6 | MGAS23794 | Feb-10 | Alberta | Blood |
| 7 | MGAS23795 | Apr-11 | Alberta | Blood |
| 8 | MGAS23796 | Mar-11 | Alberta | Blood |
| 9 | MGAS23798 | Mar-10 | Alberta | Blood |
| 10 | MGAS23800 | Aug-10 | Alberta | Blood |
| 11 | MGAS23801 | Apr-10 | Alberta | Knee fluid |
| 12 | MGAS23802 | Apr-10 | Alberta | Blood |
| 13 | MGAS23803 | Dec-10 | Alberta | Blood |
| 14 | MGAS23805 | Apr-10 | Alberta | Blood |
| 15 | MGAS23806 | Jul-10 | Alberta | Leg Rt |
| 16 | MGAS23808 | Nov-10 | Alberta | Blood |
| 17 | MGAS23809 | Mar-11 | Alberta | Blood |
| 18 | MGAS23810 | Mar-11 | Alberta | Blood |
| 19 | MGAS23811 | Nov-10 | Alberta | Blood peripheral |
| 20 | MGAS23812 | May-11 | Alberta | Blood |
| 21 | MGAS23813 | Feb-10 | Alberta | Abscess chest wall |
| 22 | MGAS23814 | Feb-11 | Alberta | Blood |
| 23 | MGAS23815 | Nov-10 | Alberta | Blood |
| 24 | MGAS23816 | Nov-10 | Alberta | Tissue axilla abscess |
| 25 | MGAS23817 | Jun-10 | Alberta | Abscess neck |
| 26 | MGAS23818 | Nov-10 | Alberta | Blood |
| 27 | MGAS23819 | Nov-10 | Alberta | Blood |
| 28 | MGAS23787 | Jan-10 | British Columbia | Synovial fluid |
| 29 | MGAS23797 | Dec-09 | British Columbia | Blood |
| 30 | MGAS23799 | Mar-10 | British Columbia | Peritoneal |
| 31 | MGAS24513 | Jun-10 | British Columbia | Blood |
| 32 | MGAS24520 | Oct-10 | British Columbia | Undetermined sterile site |
| 33 | MGAS24522 | Nov-10 | British Columbia | Blood |
| 34 | MGAS24527 | Dec-10 | British Columbia | Undetermined sterile site |
| 35 | MGAS24530 | Feb-11 | British Columbia | Blood |
| 36 | MGAS24533 | Jun-11 | British Columbia | Blood |
| 37 | MGAS24535 | Jul-11 | British Columbia | Synovial fluid |
| 38 | MGAS24537 | Aug-11 | British Columbia | Blood |
| 39 | MGAS24541 | Aug-11 | British Columbia | Synovial fluid |
| 40 | MGAS24542 | Aug-11 | British Columbia | Blood |
| 41 | MGAS24509 | Apr-10 | Manitoba | Undetermined sterile site |
| 42 | MGAS24512 | Jun-10 | Manitoba | Not given |
| 43 | MGAS24525 | Dec-10 | Manitoba | Undetermined sterile site |
| 44 | MGAS24538 | Aug-11 | Manitoba | Abscess |
| 45 | MGAS24540 | Aug-11 | Manitoba | Synovial fluid |
| 46 | MGAS25517 | Sep-10 | Manitoba | Blood |
| 47 | MGAS24516 | Aug-10 | Northwest Territories | Blood |
| 48 | MGAS24510 | May-10 | Nunavut | Blood |
| 49 | MGAS23788 | Jan-10 | Ontario | Blood |
| 50 | MGAS23804 | Feb-10 | Ontario | Fluid Rt knee |
| 51 | MGAS24220 | Dec-93 | Ontario | Undetermined sterile site |
| 52 | MGAS24223 | Mar-06 | Ontario | Undetermined sterile site |
| 53 | MGAS24224 | Jun-06 | Ontario | Undetermined sterile site |
| 54 | MGAS24225 | Dec-06 | Ontario | Undetermined sterile site |
| 55 | MGAS24226 | May-08 | Ontario | Undetermined sterile site |
| 56 | MGAS24227 | Oct-08 | Ontario | Undetermined sterile site |
| 57 | MGAS24229 | Mar-09 | Ontario | Undetermined sterile site |
| 58 | MGAS24230 | Dec-09 | Ontario | Undetermined sterile site |
| 59 | MGAS24232 | Feb-10 | Ontario | Undetermined sterile site |
| 60 | MGAS24233 | Jul-10 | Ontario | Undetermined sterile site |
| 61 | MGAS24234 | Aug-10 | Ontario | Undetermined sterile site |
| 62 | MGAS24235 | Jul-10 | Ontario | Undetermined sterile site |
| 63 | MGAS24236 | Nov-10 | Ontario | Undetermined sterile site |
| 64 | MGAS24237 | May-11 | Ontario | Undetermined sterile site |
| 65 | MGAS24511 | May-10 | Ontario | Not given |
| 66 | MGAS24514 | Jul-10 | Ontario | Blood |
| 67 | MGAS24529 | Feb-11 | Ontario | Blood |
| 68 | MGAS24544 | Sep-11 | Ontario | Blood |
| 69 | MGAS24545 | Sep-11 | Ontario | Blood |
| 70 | MGAS24547 | Oct-11 | Ontario | Synovial fluid |
| 71 | MGAS23524 | Nov-10 | Quebec | Synovial fluid |
| 72 | MGAS23786 | Jan-10 | Quebec | Blood |
| 73 | MGAS23807 | Dec-09 | Quebec | Blood |
| 74 | MGAS24507 | Mar-10 | Quebec | Blood |
| 75 | MGAS24508 | Feb-10 | Quebec | Blood |
| 76 | MGAS24518 | Oct-10 | Quebec | Blood |
| 77 | MGAS24523 | Nov-10 | Quebec | Blood |
| 78 | MGAS24526 | Dec-10 | Quebec | Blood |
| 79 | MGAS24528 | Dec-10 | Quebec | Blood |
| 80 | MGAS24531 | Mar-11 | Quebec | Blood |
| 81 | MGAS24532 | Mar-11 | Quebec | Synovial fluid |
| 82 | MGAS24534 | Jun-11 | Quebec | Undetermined sterile site |
| 83 | MGAS24536 | Aug-11 | Quebec | Blood |
| 84 | MGAS24543 | Aug-11 | Quebec | Synovial fluid |
| 85 | MGAS24546 | Oct-11 | Quebec | Blood |
| 86 | MGAS24548 | Oct-11 | Quebec | Blood |
| 87 | MGAS24505 | Jan-10 | Saskatchewan | Blood |
| 88 | MGAS24506 | Mar-10 | Saskatchewan | Blood |
| 89 | MGAS24515 | Aug-10 | Saskatchewan | Blood |
| 90 | MGAS24519 | Oct-10 | Saskatchewan | Blood |
Abbreviations: Lt, left; Rt, right.
Whole-genome sequencing
DNA was extracted from each of the 90 emm59 GAS isolates and prepared for multiplexed (barcoded) sequencing as previously described.9 Genome sequence data were obtained using an Illumina HiSeq2000 instrument according to the manufacturer's instructions.
Bioinformatic analysis
Bioinformatic analysis was performed using a customized version of the Galaxy Suite20,21,22 running on the Amazon cloud. Namely, we created a pipeline composed of Tagdust,23 FastQC (http://www.bioinformatics.bbsrc.ac.uk/projects/fastqc/) and FastX (http://hannonlab.cshl.edu/fastx_toolkit/) toolkits, and used it to parse multiplexed sequencing reads, remove barcode information and perform run quality control analyses. Polymorphism discovery was performed using Variant Ascertainment Algorithm.24 Reads were aligned to the emm59 GAS strain MGAS15252 (GenBank accession number CP003116) reference genome using the Mosaik assembler (http://bioinformatics.bc.edu/marthlab/Mosaik). This strain was isolated in Canada in 2008 and its genome sequenced to closure previously; thus becoming the de facto reference genome for type emm59 GAS epidemic strains.8 Unaligned reads were placed into contigs using the Velvet de novo assembler.25 Contigs greater than 100 nucleotides in length were then used to search the NCBI non-redundant database using BLAST.26
Phylogenetic analysis
A matrix file containing the genotype of all strains at each polymorphic locus was created from the Variant Ascertainment Algorithm polymorphism output data using a custom script. Insertions and deletions (indels) were not considered for phylogenetic analysis. Single-nucleotide polymorphisms (SNPs) were concatenated in order of occurrence relative to the reference genome and converted to a multiFASTA sequence. ClustalW27 was used to align and generate a guide tree for the FASTA sequences. Neighbor-joining phylogenetic trees followed by bootstrap analysis of 1000 replicates were created using SplitsTree28 and graphically edited with TreeDyn.29 The Neighbor-joining phylogenetic tree for Canadian epidemic strains isolated 2006–2011 was exported to Path-O-Gen v1.3 (http://tree.bio.ed.ac.uk/software/pathogen) and a linear regression plot for isolation date versus root-to-tip distance was generated as described by Mutreja et al.30
Data visualizations
Visualizations were created using the resources offered by cBio Inc.'s Pathogen Annotated Tracking Resource Network located at http://www.patrn.net/. Illustrations were created using TIBCO Spotfire and Adobe Illustrator.
Results
Invasive infections caused by emm59 GAS remain abundant in Canada
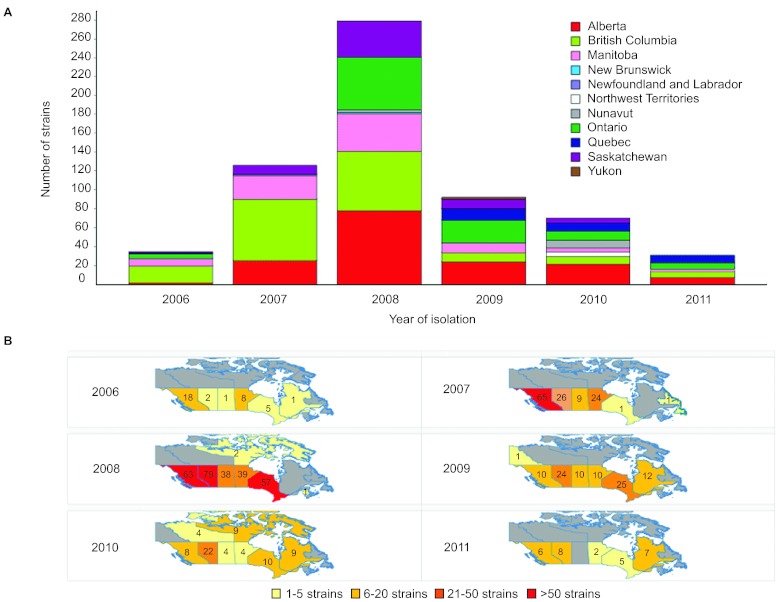
We reported previously that emm59 GAS strains were almost non-existent in Canada before 2006, but caused more than 500 invasive infections countrywide from that date until early 2010.8,18 The epidemic reached its peak in 2008, after which the number of cases began to decline (Figure 1A). However, emm59 GAS strains continued to cause invasive infections, with 56 newly identified cases in 2010 (total of 70 cases for 2010), and 32 cases in 2011. Since surveillance for GAS is passive in Canada, the actual number of emm59 GAS invasive cases is likely to have been higher in the period under investigation. During 2010 and 2011, cases occurred in vastly dispersed geographical areas covering most of the country (Figure 1B).
Figure 1.
Number of emm59 GAS recorded cases in Canada and their geographic distribution since the beginning of the epidemic. (A) The epidemic began in year 2006 and reached its peak in 2008. By that year, cases of invasive disease were recorded over the vast majority of the country. The number of cases started to decline since 2009. However, in years 2010 and 2011, strains could still be recovered in relatively large numbers. (B) Temporospatial display of strain origin. Provinces in which emm59 GAS strains were isolated are color coded based on the number of strain isolated each year. Numbers indicate the exact number of strains isolated in a particular year in each province.
The oldest Canadian emm59 GAS strain identified is not the immediate ancestor of the epidemic emm59 clone
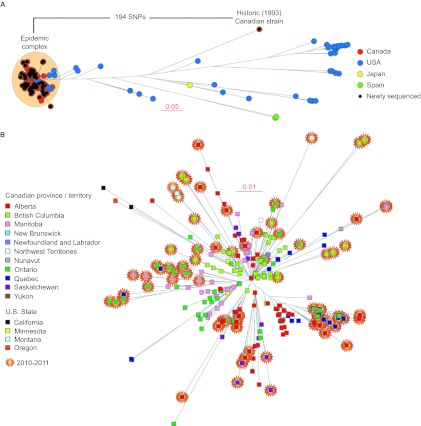
Our previous genome investigation of emm59 GAS strains recovered in Canada unambiguously demonstrated that regardless of geographic location or year of isolation, epidemic Canadian emm59 strains have descended from a recent, single ancestral cell that underwent rapid geographic dissemination.8 The last common ancestor remains unidentified, but it was remarkable that the genomes of the epidemic strains and those of the only seven emm59 strains isolated in Canada before the epidemic began (1997–2004) were closely related.8 We sequenced here the genome of an emm59 strain isolated in Canada in 1993, thus far the oldest Canadian emm59 organism identified. The genome sequence data show that this strain is genetically distantly related to the epidemic strains and thus is not the recent ancestor. Indeed, compared to reference epidemic strain MGAS15252, the 1993 emm59 GAS strain had 194 SNPs and 20 indels in the core genome (i.e., the ∼1670 kbp portion of the genome lacking mobile genetic elements that is conserved in gene content across all sequenced emm GAS types31). Phylogenetic analysis using SNP data for the core genomes found that this historic Canadian isolate also is not closely related to non-epidemic emm59 GAS organisms isolated in other geographic locations such as the United States, Japan or Spain (Figure 2A).
Figure 2.
(A) Inferred genetic relationships among strains based on 1443 concatenated SNP loci identified by whole-genome sequencing. Newly sequenced strains all cluster within the epidemic clonal complex, with the exception of a Canadian emm59 GAS strain isolated in 1993. This strain is also genetically distant from invasive strains from Spain, Japan and the United States. Scale bar represents genetic distance. (B) Magnification of the epidemic complex part of the tree, showing the geographical area of isolation (Canadian province or US state). Strains recovered during years 2010–2011 are highlighted. In general, these more recent strains are found in the outermost part of every tree branch.
Comparative genome sequencing of new Canadian epidemic emm59 strains
The 89 additional emm59 GAS Canadian organisms differed from the reference strain, on average, by 10 SNPs and 3.4 indels (median 12 SNPs, 3 indels). The notable exception was strain MGAS24525, isolated in Manitoba in 2010, which had 465 SNPs and 71 indels relative to the reference strain. However, the vast majority of these polymorphisms mapped to mobile genetic elements, namely, prophages present in the reference sequence. Indeed, compared with the core genome of the reference strain, the number of polymorphisms was reduced to only 30 SNPs and 5 indels. Moreover, 15 of these 30 SNPs mapped to sequences in close vicinity of prophage boundaries. These results suggest that strain MGAS24525 is a bona fide emm59 GAS epidemic strain that has very recently acquired a different prophage. Phylogenetic analysis using the whole-genome data showed that the Canadian emm59 GAS organisms clustered tightly in the epidemic complex (Figure 2A).
Epidemic Canadian emm59 GAS strains continue to undergo clonal expansion
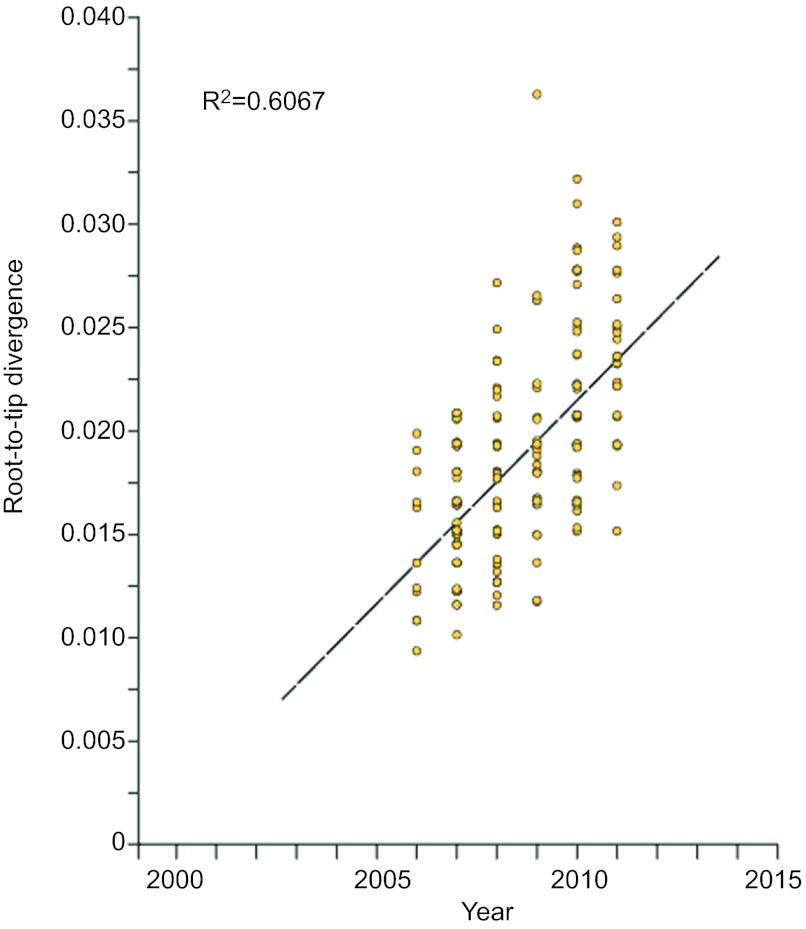
One of the findings of our previous work was that, as a population, the emm59 epidemic isolates have accumulated relatively few genetic polymorphisms over the years since sharing a last common ancestor. One hypothesis to explain that finding is that over the course of the 4 years represented by the emm59 strains used in the previous study, sufficient time has not elapsed to produce considerable genomic differentiation. Adding to the analysis data for 80 additional strains isolated in 2010–2011 provided us with the ability to test the hypothesis over a period of time of almost 6 years. Phylogenetic analysis of strains isolated during 2010–2011 found that these recent strains were located, in general, toward the tip of multiple branches of the phylogenetic tree, implying that they have accumulated additional SNPs compared to emm59 GAS organisms isolated previously (Figure 2B). When we divided the strain collection based on isolation year and calculated the average number of SNPs separating the epidemic strains isolated in a given year from the reference strain, we noticed an increase in the number of polymorphisms differentiating the epidemic strains from the reference strain over time. For example, epidemic strains recovered in 2006 differed from the reference genome, on average, by 7.6 SNPs, whereas strains isolated in 2011 differed, on average, by 13.1 SNPs. To test the hypothesis in more detail, we performed a linear regression analysis on all epidemic strains isolated since 2006 to calculate the rate of SNP accumulation on the basis of the date of isolation and the root-to-tip distance. Consistent with the hypothesis, linear regression analysis showed a steady rate of SNP accumulation (R2=0.6067; Figure 3) in the core genome.
Figure 3.
Linear regression plot showing the correlation (R2) between the root-to-tip distance (y-axis) and the date of the epidemic strains (x-axis).
Use of whole-genome data to study temporospatial spread of emm59 GAS subclones
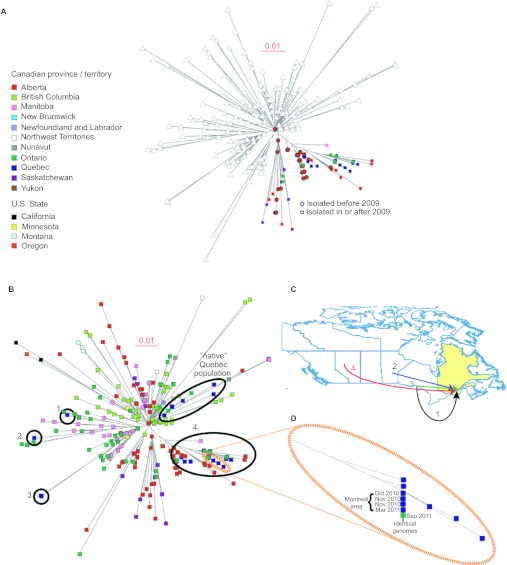
Among the critical questions that whole-genome sequencing and subsequent phylogenetic analyses can address is whether an apparent epidemic represents the emergence of a single distinct genotype, or, instead, it is caused by multiple clones that emerged simultaneously. In our initial investigation, we unambiguously demonstrated the emm59 GAS epidemic was caused by the emergence of a distinct genetic clone.8 The extensive whole-genome data also permitted us to delineate clear patterns of geographic dissemination of derivative subclones.8 We inferred that these geographic patterns were the result of the rapid spread of subclones in distinct geographic regions of the country. We hypothesized that the geographical patterns will begin to be obscured over time as a result of geographic mixing of subclones linked to human travel. Figure 4A clearly exemplifies how whole-genome data support this hypothesis: a subclone that before 2009 was composed only of strains isolated in the provinces of Alberta and British Columbia has expanded to four other Canadian provinces and territories. Mixing of the subclones is also clearly revealed among strains isolated in Quebec. Type emm59 GAS had been isolated in Quebec in 1996 and 2004, particularly in northern areas of the province. Quebec interrupted the provincial GAS surveillance program during 2005–2009. We discovered that none of the 28 emm59 GAS invasive cases reported in the province since 2009 was caused by direct lineal descendants of the strains originally identified in Quebec. Rather, they were caused by strains of genetically divergent emm59 subclones isolated first in other parts of Canada and subsequently introduced into the province on at least four different occasions (Figure 4B and 4C). Notably, we identified five strains whose genomes were essentially identical. Four of these strains were isolated from patients receiving care in the Montreal area, whereas the fifth was from a patient in Ontario. This is incontrovertible evidence that the strains have shared a very recent common ancestor and, also, most likely represents that the patients infected by these strains were connected to one another, either directly or via an unknown third-party donor. Temporal analysis revealed that the Ontario case was the last to be isolated (Figure 4D), indicating a reversal of the west to east route of transmission identified in our previous study.8
Figure 4.
(A) Geographic patterns of strains dissemination are diluted as the epidemic progresses over time. The highlighted areas of the tree exemplifies how specific subclones, previously restricted to specific geographical areas of Canada before 2009, are now found over vast regions of the country. Triangles represent emm59 GAS strains not considered for the analysis. (B) Epidemic strains isolated in the province of Quebec since 2009 were introduced into the province in at least five different occasions. Strains from Quebec are depicted in blue and their different locations in the phylogenetic tree highlighted by the black ellipses. With the exception of a ‘native' population of strains isolated before 2006, all cases in the province were the result of the introduction of multiple different emm59 GAS subclones active in other parts of Canada. (C) Introduction of the different genotypes into Quebec can be precisely mapped back to the origin strain. (D) Magnification of a region of the phylogenetic depicting a subclone composed of five strains of identical genome sequence which caused disease in the Montreal area, but was later isolated in the province of Ontario.
Convergent evolution of polymorphisms in virulence genes
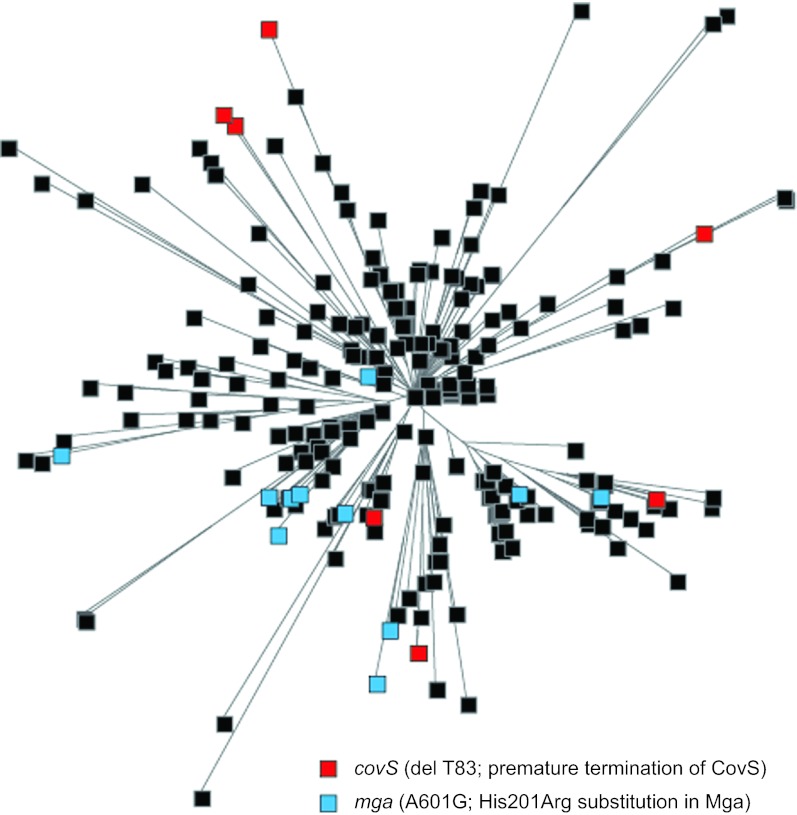
The radial structure of the epidemic phylogenetic tree (Figure 2B) suggested that most of the SNPs identified in our strain collection arose once and were transmitted vertically by descent. Polymorphisms that arise independently multiple times are of interest since they may represent genetic changes that are under evolutionary selection because they confer an advantage to the organism. We identified several examples of these polymorphisms, including a single nucleotide deletion in a homopolymeric tract of 7 Ts, predicted to result in premature termination of translation of CovS, the sensor kinase of the two-component regulatory system CovRS. This component regulatory system influences the expression of a large regulon that includes many virulence genes.32 CovR acts primarily as a transcriptional repressor of virulence genes. Inactivation of covR or covS has been reported to increase GAS virulence in strains of several different emm types.32 The distribution of this deletion-mutation strongly suggests that it evolved independently on least six different occasions in emm59 GAS strains (Figure 5). We also identified multiple different non-synonymous SNPs in the mga gene which encodes a stand alone global regulator of GAS virulence. Mga is important at several steps of the pathogenesis of the GAS infection, and is required for full GAS virulence in a skin model of infection.33,34 The phylogenetic distribution of one of these SNPs (resulting in a His201Arg amino-acid replacement) strongly suggests that it arose independently at least six times (Figure 5).
Figure 5.
Different polymorphisms arose multiple, independent times. Polymorphism analysis identified that several strains in different branches of the phylogenetic tree present either a nucleotide deletion (del T83) resulting in premature termination of the translation of the global regulator covS (indicated in red) and/or a SNP (A601C) resulting in a nonsynonymous amino acid replacement (His201Arg) in the stand alone regulator Mga (indicated in light blue). These polymorphisms could not have been inherited by descent. Their multiple, independent acquisition within the host suggests that they confer a selective advantage.
Discussion
Our study supports the idea that performing whole-genome sequencing and data interpretation rapidly can have a major impact on the study and management of epidemics. New and improved sequencing systems permit genome sequence data to be generated economically for hundreds of strains in as little as a week. A time frame as short as 1.5 days has been reported for obtaining high-quality genome data for a small number of strains.35 However, data analysis continues to be a key bottleneck in genome sequence-based investigations.
During an epidemic, irrespective of whether it is natural or caused by the accidental or deliberate release of a pathogenic organism, there is substantial pressure to obtain high-quality genome and linked epidemiologic information very rapidly. It is therefore important to identify exactly what is being traced at as high resolution as possible. Important questions to be addressed are: Is this a clonal outbreak or are several pathogen genetic backgrounds involved? What is the nature and extent of organism evolution during the course of the epidemic? Here, using an improved data analysis pipeline, we have been able to accurately identify polymorphisms for 90 strains in less than 24 h after obtaining the raw data from the sequencing instrument. As importantly, we were able to integrate new and preexisting data along with geographical and temporal strain metadata to rapidly generate phylogenetic trees and other visualizations that translated the data into intuitive and insightful epidemiological information. By applying this analysis, we were able to differentiate among emm59 GAS subclones causing disease in different and geographically dispersed parts of Canada, and obtain new information about how these clones are expanding and mixing over time.
Two other important issues when studying an epidemic are identifying the origin of the organism(s) causing it and the ability to exclude or confirm genetic modifications. By limiting our analysis to the province of Quebec, we demonstrated that whole-genome sequencing, phylogenetic and temporospatial analysis were able to assign the different cases reported in the province to at least five different subclones of emm59 GAS. Moreover, we unambiguously demonstrated transmission of a particular subclone which has been active for months in the Montreal area to the province of Ontario. This type of crucial information is important for providing input on public health maneuvers, for understanding strain spreading and for inferring whether an epidemic is naturally caused or potentially the result of deliberate release of a pathogenic organism.
Whole-genome sequencing of large numbers of strains causing epidemics has also proven useful to characterize many biological properties of the organisms under investigation and is revolutionizing both virulence factor discovery and characterization and our understanding of bacterial pathogenesis.36,37,38 In our analysis of the emm59 GAS epidemic in Canada, we rapidly identified several polymorphisms that arose multiple, independent times in the strains studied. Among others, we discovered such polymorphisms in global regulators of GAS virulence, such as a nucleotide deletion in the covS gene and a SNP resulting in a non-synonymous amino-acid change at position 201 of the predicted translated sequence of the standalone regulator Mga. Selection within the host of these polymorphisms may denote that they are of importance for the pathogenesis of infection. Although evaluating the potential involvement of these naturally occurring polymorphisms in GAS virulence requires further time and effort, it is worth noting that whole-genome sequencing analysis can lead to the quick generation of research hypothesis bearing on pathogenesis even during the initial analysis of epidemics.
In this study, we limited our analysis to genome data and temporospatial isolation information for bacterial strains. It would be possible, however, to rapidly build a well-curated database integrating other metadata such as, for example, disease manifestation and patient information, including patient genome data. Such efforts are currently underway in some clinical settings.13 Whole-genome sequencing and integrative metadata analysis holds the promise of monitoring the spread and evolution of pathogens in real time, facilitating management of disease and patient treatment and continuing to provide leads for future research aimed at deciphering the virulence arsenal of pathogenic bacteria. The strategy we used here is a step forward in the route toward automation of whole-genome sequencing data analysis and provides a framework upon which to build more intuitive, rapid and precise analytic tools.
Acknowledgments
We thank the following hospitals or provincial public health laboratories for contributing GAS isolates to this study: British Columbia Centre for Disease Control, Vancouver, British Columbia, Canada; Saskatchewan Disease Control Laboratory, Regina, Saskatchewan, Canada; Cadham Provincial Laboratory, Winnipeg, Manitoba, Canada; Public Health Ontario Laboratories, Toronto, Ontario, Canada; Laboratoire de santé publique du Québec, Ste-Anne-de-Bellevue, Quebec, Canada; Stanton Territorial Hospital Laboratory, Yellowknife, Northwest Territories, Canada. We thank Concepcion C. Cantu from the Methodist Hospital Research Institute for technical help. This work was supported by The Methodist Hospital. Nahuel Fittipaldi is funded in part by a Postdoctoral Fellowship granted by the Canadian Institutes of Health Research, Ottawa, Ontario, Canada.
References
- Dasgupta A, Banerjee R, Das S, Basak S. Evolutionary perspective on the origin of Haitian cholera outbreak strain. J Biomol Struct Dyn. 2012;30:338–346. doi: 10.1080/07391102.2012.680033. [DOI] [PubMed] [Google Scholar]
- Chin CS, Sorenson J, Harris JB, et al. The origin of the Haitian cholera outbreak strain. N Engl J Med. 2011;364:33–42. doi: 10.1056/NEJMoa1012928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardy JL, Johnston JC, Ho Sui SJ, et al. Whole-genome sequencing and social-network analysis of a tuberculosis outbreak. N Engl J Med. 2011;364:730–739. doi: 10.1056/NEJMoa1003176. [DOI] [PubMed] [Google Scholar]
- Gilmour MW, Graham M, van Domselaar G, et al. High-throughput genome sequencing of two Listeria monocytogenes clinical isolates during a large foodborne outbreak. BMC Genomics. 2010;11:120. doi: 10.1186/1471-2164-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasko DA, Webster DR, Sahl JW, et al. Origins of the E. coli strain causing an outbreak of hemolytic-uremic syndrome in Germany. N Engl J Med. 2011;365:709–717. doi: 10.1056/NEJMoa1106920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde H, Qin J, Cui Y, et al. Open-source genomic analysis of Shiga-toxin-producing E. coli O104:H4. N Engl J Med. 2011;365:718–724. doi: 10.1056/NEJMoa1107643. [DOI] [PubMed] [Google Scholar]
- Beres SB, Carroll RK, Shea PR, et al. Molecular complexity of successive bacterial epidemics deconvoluted by comparative pathogenomics. Proc Natl Acad Sci USA. 2010;107:4371–4376. doi: 10.1073/pnas.0911295107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fittipaldi N, Beres SB, Olsen RJ, et al. Full-genome dissection of an epidemic of severe invasive disease caused by a hypervirulent, recently emerged clone of group A Streptococcus. . Am J Pathol. 2012;180:1522–1534. doi: 10.1016/j.ajpath.2011.12.037. [DOI] [PubMed] [Google Scholar]
- Shea PR, Beres SB, Flores AR, et al. Distinct signatures of diversifying selection revealed by genome analysis of respiratory tract and invasive bacterial populations. Proc Natl Acad Sci USA. 2011;108:5039–5044. doi: 10.1073/pnas.1016282108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loman NJ, Constantinidou C, Chan JZ, et al. High-throughput bacterial genome sequencing: an embarrassment of choice, a world of opportunity. Nat Rev Microbiol. 2012;10:599–606. doi: 10.1038/nrmicro2850. [DOI] [PubMed] [Google Scholar]
- Otto TD. Real-time sequencing. Nat Rev Microbiol. 2011;9:633. doi: 10.1038/nrmicro2638. [DOI] [PubMed] [Google Scholar]
- Baldry S. Attack of the clones. Nat Rev Microbiol. 2010;8:390. doi: 10.1038/nrmicro2369. [DOI] [PubMed] [Google Scholar]
- Olsen RJ, Long SW, Musser JM. Bacterial genomics in infectious disease and the clinical pathology laboratory. Arch Pathol Lab Med. 2012;136:1414–1422. doi: 10.5858/arpa.2012-0025-RA. [DOI] [PubMed] [Google Scholar]
- Olsen RJ, Shelburne SA, Musser JM. Molecular mechanisms underlying group A streptococcal pathogenesis. Cell Microbiol. 2009;11:1–12. doi: 10.1111/j.1462-5822.2008.01225.x. [DOI] [PubMed] [Google Scholar]
- Lancefield RC. The antigenic complex of Streptococcus haemolyticus : I. Demonstration of a type-specific substance in extracts of Streptococcus haemolyticus. . J Exp Med. 1928;47:91–103. doi: 10.1084/jem.47.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott JR, Pulliam WM, Hollingshead SK, Fischetti VA. Relationship of M protein genes in group A streptococci. Proc Natl Acad Sci USA. 1985;82:1822–1826. doi: 10.1073/pnas.82.6.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manjula BN, Acharya AS, Fairwell T, Fischetti VA. Antigenic domains of the streptococcal Pep M5 protein. Localization of epitopes crossreactive with type 6 M protein and identification of a hypervariable region of the M molecule. J Exp Med. 1986;163:129–138. doi: 10.1084/jem.163.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrrell GJ, Lovgren M, St Jean T, et al. Epidemic of group A Streptococcus M/emm59 causing invasive disease in Canada. Clin Infect Dis. 2010;51:1290–1297. doi: 10.1086/657068. [DOI] [PubMed] [Google Scholar]
- Fittipaldi N, Olsen RJ, Beres SB, van Beneden C, Musser JM. Genomic analysis of emm59 group A Streptococcus invasive strains, United States. Emerg Infect Dis. 2012;18:650–652. doi: 10.3201/eid1804.111803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenberg D, von Kuster G, Coraor N, et al. Galaxy: a web-based genome analysis tool for experimentalists Curr Protoc Mol Biol 2010Chapter19Unit 19.10.1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giardine B, Riemer C, Hardison RC, et al. Galaxy: a platform for interactive large-scale genome analysis. Genome Res. 2005;15:1451–1455. doi: 10.1101/gr.4086505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goecks J, Nekrutenko A, Taylor J. Galaxy: a comprehensive approach for supporting accessible, reproducible, and transparent computational research in the life sciences. Genome Biol. 2010;11:R86. doi: 10.1186/gb-2010-11-8-r86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassmann T, Hayashizaki Y, Daub CO. TagDust—a program to eliminate artifacts from next generation sequencing data. Bioinformatics. 2009;25:2839–2840. doi: 10.1093/bioinformatics/btp527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusbaum C, Ohsumi TK, Gomez J, et al. Sensitive, specific polymorphism discovery in bacteria using massively parallel sequencing. Nat Methods. 2009;6:67–69. doi: 10.1038/nmeth.1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zerbino DR, Birney E. Velvet: algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huson DH, Bryant D. Application of phylogenetic networks in evolutionary studies. Mol Biol Evol. 2006;23:254–267. doi: 10.1093/molbev/msj030. [DOI] [PubMed] [Google Scholar]
- Chevenet F, Brun C, Banuls AL, Jacq B, Christen R. TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics. 2006;7:439. doi: 10.1186/1471-2105-7-439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mutreja A, Kim DW, Thomson NR, et al. Evidence for several waves of global transmission in the seventh cholera pandemic. Nature. 2011;477:462–465. doi: 10.1038/nature10392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beres SB, Musser JM. Contribution of exogenous genetic elements to the group A Streptococcus metagenome. PLoS ONE. 2007;2:e800. doi: 10.1371/journal.pone.0000800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchward G. The two faces of Janus: virulence gene regulation by CovR/S in group A streptococci. Mol Microbiol. 2007;64:34–41. doi: 10.1111/j.1365-2958.2007.05649.x. [DOI] [PubMed] [Google Scholar]
- Luo F, Lizano S, Banik S, Zhang H, Bessen DE. Role of Mga in group A streptococcal infection at the skin epithelium. Microb Pathog. 2008;45:217–224. doi: 10.1016/j.micpath.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hondorp ER, McIver KS. The Mga virulence regulon: infection where the grass is greener. Mol Microbiol. 2007;66:1056–1065. doi: 10.1111/j.1365-2958.2007.06006.x. [DOI] [PubMed] [Google Scholar]
- Koser CU, Holden MT, Ellington MJ, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366:2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades K. Genomics of epidemic pathogens. Clin Microbiol Infect. 2012;18:213–217. doi: 10.1111/j.1469-0691.2012.03781.x. [DOI] [PubMed] [Google Scholar]
- Dettman JR, Rodrigue N, Melnyk AH, Wong A, Bailey SF, Kassen R. Evolutionary insight from whole-genome sequencing of experimentally evolved microbes. Mol Ecol. 2012;21:2058–2077. doi: 10.1111/j.1365-294X.2012.05484.x. [DOI] [PubMed] [Google Scholar]
- Musser JM, Shelburne SA., 3rd A decade of molecular pathogenomic analysis of group A Streptococcus. . J Clin Invest. 2009;119:2455–2463. doi: 10.1172/JCI38095. [DOI] [PMC free article] [PubMed] [Google Scholar]