Abstract
Background
Night-to-night variability in sleep of children with attention deficit hyperactivity disorder (ADHD) may be a mediator of behavioral phenotype. We examined the potential association between alertness, sleep, and eating behaviors in children with ADHD and comorbid problems.
Methods
Sleep was monitored by actigraphy for 7 days. Questionnaires were used to assess sleep complaints, habits and food patterns by parental report, and sleep complaints and sleepiness by child report.
Results
The group comprised 18 children, including 15 boys, aged 9.4 ± 1.7 years, 88.9% Caucasian, who took one or multiple medications. Children slept on average for 6 hours and 58 minutes with a variability of 1 hour 3 minutes relative to the mean, and their sleepiness scores were highly variable from day to day. Most children had a normal body mass index (BMI). Sleepiness and BMI were associated with sleep schedules and food patterns, such that they accounted for 76% of variance, predominantly by the association of BMI with mean wake after sleep onset and by bedtime sleepiness, with wake after sleep onset variability. Similarly, 97% of variance was shared with eating behaviors, such as desserts and snacks, and fast food meals were associated with morning sleepiness.
Conclusion
Disrupted sleep and sleepiness appears to favor unhealthy food patterns and may place children with ADHD at increased risk for obesity.
Keywords: sleep, child, attention deficit hyperactivity disorder, actigraphy
Introduction
The association between attention deficit hyperactivity disorder (ADHD) and sleep was initially formulated in 1957,1 and generated substantial debate, which persists until today. In the past, sleep problems were included among the diagnostic criteria for ADHD. However, while sleep issues have now been removed from the diagnostic criteria, the interdependencies of sleep and ADHD are currently perceived as highly complex phenomena that have not been adequately elucidated. Indeed, sleep quantity as well as quality may affect or be affected by such ADHD symptomatology, and indeed, complaints expressed by either child or caregiver, such as difficulty in falling asleep, maintaining sleep, and somnolence, are extremely frequent.2–5
ADHD symptoms are primarily treated with medication, ie, methylphenidate and amphetamines. Stimulants have been associated with either no changes or with increased sleep complaints in children with ADHD,4 whereas treatment of underlying primary sleep disorders (ie, disordered breathing, periodic leg movement syndrome, and restless legs syndrome) have led to improved neurobehavioral functioning.6 In fact, because sleep problems are likely to coexist,7 both clinicians and researchers need to be very attentive to this overlap when facing ADHD symptomatology.
ADHD comorbidity is frequent, with 59%–87% of children diagnosed with ADHD presenting at least one comorbidity, and 20% having three or more comorbid conditions or symptoms. The latter include learning disabilities (15%–25%), language disorders (30%–35%), social adaptive dysfunction (eg, 10% autistic spectrum disorders), emotional lability (eg, 15%–20% mood disorder, 20%–25% anxiety disorder), motor coordination and balance deficits (60%),8 obesity,9,10 and sleep problems.11,12
The issue of obesity and ADHD has recently gained attention, whereby children with ADHD appear to be at increased risk for obesity.10,13–16 Although the increased prevalence of both obesity and ADHD are indisputable, these two conditions also share the frequent presence of excessive daytime sleepiness. Sleepiness may result from erratic highly variable sleep-wake patterns, as well as from sleep disorders. For instance, fragmented sleep, which is characteristic of children with sleep-disordered breathing, leads to altered behavior patterns that promote obesity, ie, eating less healthy food, and reducing the likelihood of engaging in organized sports.17 Furthermore, the hypoarousal state that is present in both obese children and ADHD children may predispose them towards “hyperstimulatory behaviors” in the form of nutritional or sensory content (eg, television watching, excessive eating).13 Night-to-night variability in the sleep patterns of children with ADHD18 has been suggested as a potential mediator of ADHD-associated symptomatology, and partial sleep deprivation could also exacerbate or contribute to both sleepiness and behaviors. Cortese et al14,15 went on to hypothesize that the association between alertness and feeding may potentially share pathways involving the hypocretin/orexin system located in the hypothalamus. We recently found that variable and short sleep were associated with increased obesity risk.19 Therefore, the present study was conducted as a pilot exploration of the hypothesis that sleepiness of ADHD children may be associated with weight, sleep, and food consumption patterns.
Methods
This study was approved by the University of Illinois at Chicago human research committee, and informed consent was obtained from the legal caregiver of each participant, with assent being obtained from children older than 7 years of age (University of Illinois at Chicago approval number 2009-0397). During the period from June 10 to August 22, 2010, 34 children aged 6–13 years were enrolled in the Camp Star summer treatment program20,21 and 24 consented to participate in the current study. Camp Star program is an intensive program for school-aged children with ADHD and associated social and behavioral difficulties. It offers these children with psychopathological disorders in excess of 280 hours of behavioral and social therapy over a 6-week span. The program combines a token economy behavior modification point system, daily report cards and timeout procedures with academic remediation, problem-solving and social skill training, sports skill training, and preparation in coping with frustration. This is accomplished through a 1:2 child to counselor ratio. Parents can join weekly training sessions in parenting skills specific to children with ADHD and comorbidities. The parentally reported diagnoses were confirmed by the Camp Star professional team. Exclusion criteria assessed during the screening process were cognitive impairment (intelligent quotient <55) and children with severe aggressive or violent behaviors. A clinical description of the sample prior to Camp Star involves the Conner’s Parent Rating Scale Global Index Total of 70.43 ± 10.02, pretreatment Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) total score of 72.71 ± 9.55, Weiss Functional Impairment Rating Scale of 1.27 ± 0.47, Social Responsiveness Scale of 70.69 ± 11.36. The diagnostic inclusion criterion for the sleep study was being a camper at the summer treatment program under the supervision of the clinical director. Participation in the sleep study was voluntary. The pilot data were collected during the mid-camp phase, and no sleep training was provided. Camp started at 8:30 am and ended at 4:00 pm.
Children agreed to wear the Actiwatch AW2 (Philips/Respironics, Bend, OR) which weighs 16 g with band and measures 43 × 23 × 10 mm for 7 consecutive days.22,23 Each child continuously wore the Actiwatch device on their non-dominant wrist. A concomittant sleep log was provided to the parent to mark bedtime/risetime, awakenings, and other night-time events. In addition, several questionnaires were used; on the one hand, such a multimethod and multitrait approach gives a more complete perspective of the sleep complaints, and on the other hand, this was applied because a lack of good sleep tools exists.24,25 As a result, self-reported sleepiness at bedtime, at the start of the camp day (8:30 am) and at the end of each day (3:40 pm) was collected from each child using the pictorial sleepiness scale.26 The pictorial sleepiness scale avoids semantic or geometric elements by using a Thurstone scaling procedure to transform rankings of 5 faces, ie, minimum (0) and maximum (5.58). The scale measures sleepiness, uncontaminated by pain, anger, or happiness. Children needed to select the face that represents their current sleepiness state. Children also filled out the Child Self-Report Sleep Questionnaire, a self-report tool developed by Owens et al.27,28 It comprises 26 questions rated on a three-point scale (rarely, sometimes, usually) reflecting the recording period. Children’s weight and height was assessed at camp to calculate body mass index (BMI) and derive corresponding z scores (by Centers for Disease Control 2000 growth standards www.cdc.gov/growthcharts).
Parents filled out questionnaires assessing sleep habits, sleep problems, and food and activity patterns. In addition to demographic information and the medical history of the child, the Gozal Pediatric Sleep Questionnaire,29,30 which includes questions on the child’s difficulty initiating sleep, restless sleep, enuresis, apnea, cyanosis during sleep, snoring and, if so, the severity of the snoring, was also administered. The responses are graded as “never”, “rarely” (once per week), “occasionally” (twice per week), “frequently” (3–4 times per week) and “almost always” (>4 times per week) by the parent. The Children’s Sleep Hygiene Scale (CSHS)31,32 is a parental tool and an important predictor of sleep quality. The 22 items form six subscales measuring activities surrounding sleep of children, ie, physiological, cognitive, emotional, environmental, bedtime routine and sleep stability, and are rated on a six-point Likert scale (never to always). The CSHS has an internal consistency of 0.76. The Bedtime Routines Questionnaire (BRQ) is a 31-item paper-and-pencil five-point Likert type, parent-report measure of children’s bedtime routines which has demonstrated a solid factor structure, adequate internal consistency, and fair validity coefficients in 2–8-year-old children.33 The Pediatric Food Frequency and Physical Activity Questionnaire17 allows for screening of overall eating and drinking patterns encompassing a one-week timeframe. The food intake patterns at home, school, and elsewhere can be assessed. Physically activity patterns for the preceding week were similarly assessed yet since all children were at camp, hence physically active, we discarded this part of the questionnaire. Measurements were performed in the child’s natural environment, ie, no restrictions on habits were implemented.
Statistical analysis
Statistical analyses were performed using Statistica version 9.0. (StatSoft Inc, Tulsa, OK). Sleep complaints reported by child and caregiver were recorded (often, sometimes and never a problem) and only sleep complaints with a >30% endorsement rate for “sometimes” or “often” are reported. Descriptive and correlational analyses will be presented whilst predictors of sleep schedules were analysed via regression analyses (standardized betas are printed). To assess the association between sleepiness and BMI on the one hand with the sleep, sleep hygiene and routine, as well as food patterns on the other, canonical correlation analyses were performed. This statistical technique needs to be interpreted as “multiple multiple” correlations whereby sets of variables are associated. Their association is commonly expressed in terms of shared variance, and can be detailed by the individual correlations (moderate correlations and above are reported).
Results
Sleep was successfully monitored by actigraphy for 7 days in 18 children (15 boys, aged 9.4 ± 1.7 years, 88.9% Caucasian, 11.1% mixed ethnicity) at mid-duration of the summer camp program. Two Actiwatch recordings were corrupted, two Actiwatches were damaged, and two children discontinued wearing the watch. All children were taking one or multiple medications, ie, 50% stimulants, 28.6% stimulants combined with antihypertensive drugs, 14.3% mood stabilizers or antidepressants, and 7.1% other nonstimulant medication. Categorized on parent-reported diagnosis, the group comprised seven ADHD, eight ADHD with comorbidity, and three children with cognitive disorders not otherwise specified, nonverbal learning disability, and autism. The children had a mean BMI z score of -0.10 (standard deviation 1.49) Fifteen children were of normal weight, whereas two were overweight and one was obese.
Sleep schedules
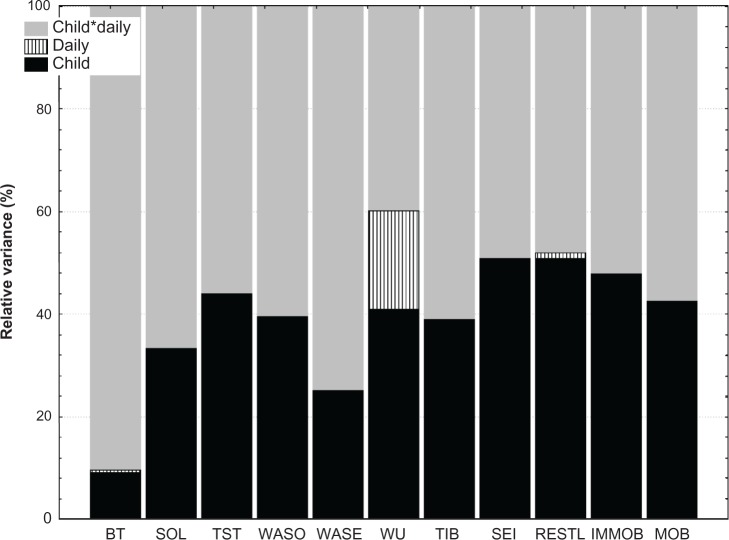
During the study, the children slept for an average of 6 hours and 58 minutes with a variability of one hour 3 minutes relative to the mean (Figure 1). On average, the children were awake for about 2 hours per night, with a variability of one hour. Sleep patterns showed 33% restlessness (ie, the sum of the percentage mobile and the percentage immobile bouts of <one minute in duration to the number of immobility bouts for the given interval), with no differences between weekdays and weekend. These were “typical” nights as reported by 66.7% of the parents. Because the cohort was a heterogeneous group, we performed secondary analyses to explore the variance in sleep schedules (Figure 2). A proportion of total variance in the sleep schedule parameters was due to differences between the children (Figure 2, shown in black), yet no variation could be ascribed to the diagnostic category. Alternatively, substantial variance was due to daily differences within each child (Figure 2, shown in gray). In other words, bedtime did not differ greatly in the sample (or between the children, 9% variance), but daily differences within each child were apparent (90% variance, Figure 2). About 40% of the variance in wakeup time, time in bed, sleep onset latency, wake after sleep onset (WASO), and wake after sleep end was ascribable to differences between children, but could also be ascribed to daily differences within each child (Figure 2, shown in grey). Part of the variance in wakeup time was solely due to the day of the week (bars, ie, 19%). In general, 50% or more of the variance in the sleep schedule was due to daily differences within the same child.
Figure 1.
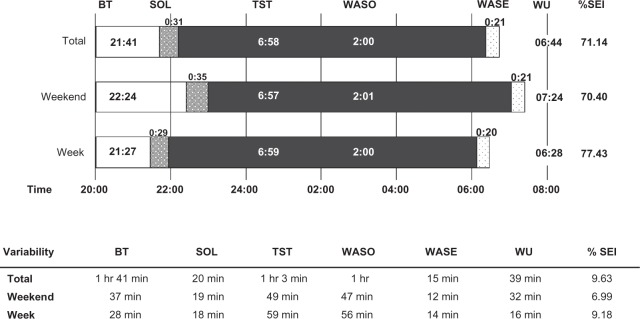
Sleep schedules, mean (hours:minutes) and variability relative to mean.
Abbreviations: BT, Bedtime; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset time; WASE, wakefulness after sleep end; WU, wake-up time; TIB, time in bed; SEI, % sleep efficiency index.
Figure 2.
Variance in sleep schedules in children with attention deficit hyperactivity disorder and comorbid problems.
Notes: Child: % Variance ascribed to child, Daily: % Variance ascribed to day, and Child*Daily: % Variance ascribed to child per day.
Abbreviations: BT, Bedtime; SOL, sleep onset latency; TST, total sleep time; WASO, wake after sleep onset time; WASE, wakefulness after sleep end; WU, wake-up time; TIB, time in bed; SEI, sleep efficiency index; RESTL, restlessness; IMMOB, immobility; MOB, mobility.
Sleep hygiene and bedtime routines
Given the substantial variability in sleep patterns, we tested if children’s sleep schedules were predicted by family sleep hygiene practices (CSHS) and bedtime routines (BRQ). The average total sleep hygiene score was 4.5 ± 0.5 (range 3.2–5.2). On the BRQ, the average subscale scores were for consistency (10 items) 40.2 ± 6.2, reactivity (5 items) 10.1 ± 5.2, and activities (16 items) 50.8 ± 6.8 (adaptive 37.8 ± 5 and maladaptive 13.1 ± 4). When compared with the values in the BRQ paper,33 our sample had significantly fewer activities [t(17) = −4.0, P < 0.001 and t(17) = −3.6, P < 0.01, respectively].
Better physiological hygiene (CSHS) such as drinking fewer caffeinated drinks or other liquids, feeling less hungry, reduced rough playing (β 0.8, P = 0.01), and good bedtime reactivity (BRQ, ie, the same order, place, person, and activity as bedtime routine; β 0.9, P = 0.03) significantly predicted longer mean time in bed. Additionally, the time in bed was less variable when adaptive activities (BRQ) such as bedtime hugs, being tucked in, brushing teeth, putting on pajamas, were present (β −0.8, P = 0.03). Physiological and environmental hygiene (ie, no noise, light, comfortable room, and bed CSHS) was beneficial towards mean sleep onset latency (ie, β −0.9, P = 0.04 and β 0.6, P = 0.01, respectively). Finally, performing adaptive activities decreased the variability in restlessness during sleep or sleep fragmentation (β −1.1, P = 0.01).
Sleep complaints
From parental reports, falling asleep was often a problem for 61% of the children, whereas in 39% it sometimes was a problem during the week of recording; 22% and 56% had problems often or sometimes with awakenings, respectively. Parental reports further indicated that 22% (often) and 61% (sometimes) of the children were somnolent. Snoring was highly prevalent (50% often and 44% sometimes). Enuresis was also very frequent (ie, 28% often and 28% sometimes), while restless sleep occurred often in 33% and sometimes in 56% of the children.
Children’s reporting of sleep complaints corroborated the difficulty in falling asleep (coefficient of concordance 0.40, P < 0.05; 50% often, 39% sometimes). Notwithstanding, the children also reported awakenings (39% often and 44% sometimes) and somnolence (28% often and 50% sometimes).
Food and drinking patterns
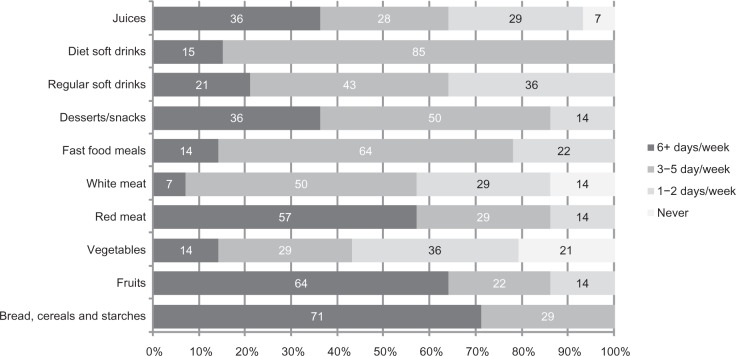
Daily food patterns (Figure 3) were characterized by bread, cereals, and starches (71%), red meat (57%), fruits (64%), desserts and snacks (36%), and juices (36%). Less frequent food patterns were white meat (50%), fast food meals (64%), and soft drinks.
Figure 3.
Food patterns (%) in children with attention deficit hyperactivity disorder and comorbid problems.
Note: Fox example: 71% of sample ate bread, cereals and starches 6+ days per week.
Sleepiness
Overall, during the week of recording, children at bedtime had an average score on the Sleepiness Scale of 2.87 ± 1.6 (0–5.58). Self-reported sleepiness at bedtime varied in nearly equal amounts between children (54.1% variance) as within any given child per day (variance 45.2%, variance ascribed to day 0.7%). However, more daily variation was seen within each child in the morning (64% variance) and the evening (65.6% variance), which concurs with the hypoarousal hypothesis (ie, the remaining percentage of variance can be attributed to the child). Average sleepiness at 8:30 am was 1.56 ± 1.1 and at 3:40 pm was 1.81 ± 1.35. Children became significantly more sleepy towards the end of the day [F(3,14) = 25.3, P = 0.00001].
Self-reported sleepiness at bedtime was unrelated to any of the sleep schedule parameters, except for Monday, ie, sleepiness was positively related to the sleep efficiency index (Spearman r = 0.67, P<0.01), to total sleep time (Spearman r = 0.53, P < 0.05) and inversely to restlessness (Spearman r = −0.54, P < 0.05). Children’s sleepiness at the beginning of the camp day was unrelated to their sleep schedules. However, sleepiness at the end of the day was correlated with sleep onset latency, sleep duration, immobile time, and wakeup time. More specifically, shorter mean sleep onset latency (Spearman r = −0.48, P < 0.05) and less variability (Spearman r = −0.47, P < 0.05) were associated with more sleepiness at 3:40 pm. The more variable total sleep time (Spearman r = 0.51, P < 0.05), wakeup time (Spearman r = 0.48, P < 0.05) and immobile time (Spearman r = 0.54, P < 0.05), the more sleepy the child felt at the end of the camp day.
Associations: sleepiness, sleep, and food patterns
Shared variance was assessed between the “sleepiness-BMI” set, ie, at bedtime, 8:30 am and 3:40 pm, and was also separately assessed with other sets of variables. No associations were found for the bedtime routines subscales set [Chi²(20) = 28.3, P = 0.10] and sleep hygiene subscales sets [Chi²(24) = 26.1, P = 0.35]. Nevertheless, the sleepiness-BMI set was associated with the following sleep schedules (ie, mean and variability per parameter) and food patterns sets. The sleepiness-BMI set shared 76% of variance with mean WASO and WASO variability [Chi²(8) = 17.3, P = 0.02]. More specifically, this shared variance can be attributed to the strong association between BMI and mean WASO (r = 0.53, or correlation of these variables in the sleepiness-BMI set and WASO set) and WASO variability being moderately associated with bedtime sleepiness (r = −0.35). No other associations with the sleepiness-BMI set were found regarding sleep pattern parameters: sleep onset latency set, Chi²(8) = 13.2, P = 0.11; total sleep time set, Chi²(8) = 7.3, P = 0.51; wake after sleep end set, Chi²(8) = 5.9, P = 0.66; time in bed set, Chi²(8) = 6.1, P = 0.63; sleep efficiency index set, Chi²(8) = 14.2, P = 0.08; and restlessness set, Chi²(8) = 6.6, P = 0.58. Furthermore, parental report of sleep problems as discussed above showed no association [Chi²(18) = 6.4, P = 0.99].
Regarding food patterns, the sleepiness-BMI set shared 97% of variance with the food patterns set [Chi²(28) = 41.7, P = 0.03]. Namely, desserts/snacks (r = 0.49) and fast food (r = 0.48) were associated with increased sleepiness at the start of the day (8:30 am). Sleepiness at the end of the day (3:40 pm) was inversely associated with red meat (r = −0.52), white meat (r = −0.39), and vegetables (r = −0.33) but positively associated with breads, cereals and starches (r = 0.31). Sleepiness at bedtime was inversely associated with red meat (r = −0.35). BMI was only moderately associated with desserts/snacks (r = 0.35), red meat (r = 0.33), and vegetables (r = 0.31), and the remainder were small (r < 0.3). No associations were found for drinks [Chi²(12) = 17.3, P = 0.14].
Discussion
This study provides preliminary evidence that even if children with psychopathology are of normal weight, their disrupted sleep and sleepiness appears to favor unhealthy food consumption patterns. Therefore, daytime somnolence may place children with ADHD at increased risk for obesity. These findings extend those published in the literature by providing further evidence on the night-to-night sleep variability and day-to-day hypoarousal state of children with ADHD and comorbidities.
Before discussing our findings in greater detail, we would like to address some methodological limitations. This study comprises only a small and heterogeneous group of children with psychopathological problems in terms of diagnosis and medical treatment, and its results should therefore be interpreted as pilot indicators. Even though recordings of sleep were performed during the summer break, the start and end times in camp were designed to mimic school schedules. At this stage, we were unable to include a control group, but comparisons of questionnaire scores with similar samples obtained in other cohorts are suggestive of a typical sample of children with primarily ADHD and comorbidities. This is also corroborated by the sleep complaints and sleep pattern findings. However, we have no baseline measurements, and parents and children were highly motivated and the only source of information, such that longer and/or repeated recording periods might have been advisable.
Previous studies have found a hypoarousal state in children with ADHD. For instance, excessive motor activity has been suggested as a strategy used to stay awake13,34 and because alternations in sleep patterns of children with ADHD were comparable with those found in narcolepsy, motor hyperactivity could indeed be perceived as a strategy to counteract somnolence.35,36 More recently, children with ADHD were shown to display significantly reduced sleep duration and an increased rate of stage shifts.37 Further, a lower cyclic alternating pattern rate and a lower number of cyclic alternating pattern sequences or reduced nonrapid eye movement instability emerged, and is highly suggestive of the presence of an hypoarousal state.37 Given that forms of stimulation-seeking behavior have been linked to the hypothalamo-pituitary-adrenal axis and to autonomic nervous system dysfunction, and given that these systems have been associated with biorhythms, a variety of physiological mechanisms, including sleep alertness, feeding behaviors, and endocrine and autonomic functions are potentially affected. Alternatively, we have previously shown that in typically developing children, the combination of shorter sleep duration and more variable sleep patterns was associated with increased obesity risk.19
Our multiple sleepiness assessments support the suggestion of disturbed biorhythms, because findings based on self-report at bedtime and at 8:30 am and 3:40 pm on a camp day indicated that these children were somnolent. More specifically, a substantial day-to-day variability was found within each child, irrespective of diagnosis. Variable sleep has been hypothesized to be a potential mediator that plays a role in eliciting ADHD-like phenotypes, and potentially contributes to or exacerbates their state. In our sample, bedtime across seven recording days showed a variability of nearly 2 hours, independent of day of the week, while only wakeup time was affected by day of the week. Indeed, our results further corroborate the hypothesized night-to-night variability; ie, across 5 days of monitoring sleep onset latency, total sleep time and time in bed were found to vary more in children with ADHD when compared with a control group.18 Gruber et al18 also found that children with ADHD slept on average 8 hours per night, as measured by actigraphy. Our sample slept on average for 7 hours and spent about 2 hours awake per night, indicating a substantial reduction in sleep efficiency. Also, sleep duration and wake after sleep onset varied within one hour during the period of our recordings. Sleep onset latency was about 30 minutes, with a variability of approximately 20 minutes, and not surprisingly, such findings are corroborated by the prevalence of subjective complaints on difficulties in falling asleep by both child and caregiver. Reduced sleep quantity and more disrupted sleep, as well as reduced sleep quality, have been commonly found and behavioral management strategies or pharmacological treatments have been suggested.5,38,39 In practice, good physiological and environmental hygiene might reduce such complaints, and likewise, adaptive activities and explicit routines favorably impacted the time spent in bed, and hence the sleep of children with psychopathologies. In other words, our findings indicate a need for good sleep-wake rhythmicity in children with ADHD and comorbid disorders. Although counterintuitive, several hypotheses with respect to the association of ADHD and obesity have been disputed. These include ADHD case reports suggestive of improving bulimic symptoms with stimulants, insufficient dopamine-related natural reward leading to the use of “unnatural” immediate rewards, eg, inappropriate eating,40,41 and abnormalities in the basal ganglia and cerebellum suggested in ADHD42 which may share the attention-dependent neuronal processes that are disrupted by short sleep.43 Further, it is now quite well established that sleep plays a vital role in brain maturation, somatic growth, information processing, memory consolidation, learning, and other important cognitive functions. As hypothesized by Cortese et al,14,15 the hypocretin/orexin pathways, which are involved in several homeostatic functions including sleep and food intake could be operationally pertinent. Such hypotheses potentially address a metalayer of the hypoarousal state, but its exploration is beyond the scope of the current study. Independently, we have now found preliminary evidence supporting the association between sleepiness, sleep, and eating behaviors in children with ADHD. Indeed, disrupted sleep (ie, mean wake after sleep onset and its variability, respectively) was associated with the child’s weight and bedtime sleepiness. Associations between food consumption preferences and morning sleepiness emerged as well. In this context, conditions for development of iron deficiency may be favored and then in turn contribute to the phenotype,44 and dietary patterns may become slanted towards less healthy nutrients45 among children with ADHD. Future research in children with psychopathologies such as ADHD should further refine our understanding of the association between day-to-day sleepiness, night-to-night sleep variability, and food patterns.
Acknowledgments
This study was supported by the Comer Children’s Hospital Golf Classic Research Award and by the University of Illinois at Chicago Center for Clinical and Translational Science, award number UL1RR029879 from the National Center For Research Resources.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Laufer MW, Denhoff E. Hyperkinetic behavior syndrome in children. J Pediatr. 1957;50(4):463–474. doi: 10.1016/s0022-3476(57)80257-1. [DOI] [PubMed] [Google Scholar]
- 2.Small A, Hibi S, Feinberg I. Effects of dextroamphetamine sulfate on EEG sleep patterns of hyperactive children. Arch Gen Psychiatry. 1971;25:369–380. doi: 10.1001/archpsyc.1971.01750160081015. [DOI] [PubMed] [Google Scholar]
- 3.Cortese S, Faraone SV, Konofal E, Lecendreux M. Sleep in children with attention-deficit/hyperactivity disorder: meta-analysis of subjective and objective studies. J Am Acad Child Adolesc Psychiatry. 2009;48:894–908. doi: 10.1097/CHI.0b013e3181ac09c9. [DOI] [PubMed] [Google Scholar]
- 4.Cohen-Zion M, Ancoli-Israel S. Sleep in children with attention-deficit hyperactivity disorder (ADHD): A review of naturalistic and stimulant intervention studies. Sleep Med Rev. 2004;8:379–402. doi: 10.1016/j.smrv.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Lecendreux M, Cortese S. Sleep problems associated with ADHD: A review of current therapeutic options and recommendations for the future. Expert Rev Neurother. 2007;7:1799–1806. doi: 10.1586/14737175.7.12.1799. [DOI] [PubMed] [Google Scholar]
- 6.Picchietti DL, Underwood DJ, Farris WA, et al. Further studies on periodic limb movement disorder and restless legs syndrome in children with attention-deficit hyperactivity disorder. Mov Disord. 1999;14:1000–1007. doi: 10.1002/1531-8257(199911)14:6<1000::aid-mds1014>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 7.Spruyt K, O’Brien LM, Cluydts R, Verleye GB, Ferri R. Odds, prevalence and predictors of sleep problems in school-age normal children. J Sleep Res. 2005;14:163–176. doi: 10.1111/j.1365-2869.2005.00458.x. [DOI] [PubMed] [Google Scholar]
- 8.Kadesjo B, Gillberg C. The comorbidity of ADHD in the general population of Swedish school-age children. J Child Psychol Psychiatry. 2001;42:487–492. [PubMed] [Google Scholar]
- 9.Waring ME, Lapane KL. Overweight in children and adolescents in relation to attention-deficit/hyperactivity disorder: results from a national sample. Pediatrics. 2008;122:e1–e6. doi: 10.1542/peds.2007-1955. [DOI] [PubMed] [Google Scholar]
- 10.Cortese S, Konofal E, Bernardina BD, Mouren MC, Lecendreux M.Does excessive daytime sleepiness contribute to explaining the association between obesity and ADHD symptoms? Med Hypotheses 20087012–16. [DOI] [PubMed] [Google Scholar]
- 11.Sadeh A, Pergamin L, Bar-Haim Y. Sleep in children with attention-deficit hyperactivity disorder: a meta-analysis of polysomnographic studies. Sleep Med Rev. 2006;10:381–398. doi: 10.1016/j.smrv.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Kirov R, Kinkelbur J, Banaschewski T, Rothenberger A. Sleep patterns in children with attention-deficit/hyperactivity disorder, tic disorder, and comorbidity. J Child Psychol Psychiatry. 2007;48:561–570. doi: 10.1111/j.1469-7610.2007.01729.x. [DOI] [PubMed] [Google Scholar]
- 13.Bazar KA, Yun AJ, Lee PY, Daniel SM, Doux JD. Obesity and ADHD may represent different manifestations of a common environmental oversampling syndrome: A model for revealing mechanistic overlap among cognitive, metabolic, and inflammatory disorders. Med Hypotheses. 2005;66:263–269. doi: 10.1016/j.mehy.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 14.Cortese S, Konofal E, Lecendreux M.Alertness and feeding behaviors in ADHD: Does the hypocretin/orexin system play a role? Med Hypotheses 200871770–775. [DOI] [PubMed] [Google Scholar]
- 15.Cortese S, Maffeis C, Konofal E, et al. Parent reports of sleep/alertness problems and ADHD symptoms in a sample of obese adolescents. J Psychosom Res. 2007;63:587–590. doi: 10.1016/j.jpsychores.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 16.Davis C, Patte K, Levitan RD, et al. A psycho-genetic study of associations between the symptoms of binge eating disorder and those of attention deficit (hyperactivity) disorder. J Psychiatr Res. 2009;43:687–696. doi: 10.1016/j.jpsychires.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Spruyt K, Sans Capdevila O, Serpero LD, Kheirandish-Gozal L, Gozal D. Dietary and physical activity patterns in children with obstructive sleep apnea. J Pediatr. 2010;156:724–730. doi: 10.1016/j.jpeds.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Gruber R, Sadeh A, Raviv A. Instability of sleep patterns in children with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2000;39:495–501. doi: 10.1097/00004583-200004000-00019. [DOI] [PubMed] [Google Scholar]
- 19.Spruyt K, Molfese DL, Gozal D. Sleep duration, sleep regularity, body weight, and metabolic homeostasis in school-aged children. Pediatrics. 2011;127:e345–e352. doi: 10.1542/peds.2010-0497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pelham WE, Hoza B, Pillow DR, et al. Effects of methylphenidate and expectancy on children with ADHD: behavior, academic performance, and attributions in a summer treatment program and regular classroom settings. J Consult Clin Psychol. 2002;70:320–335. [PubMed] [Google Scholar]
- 21.Pelham WE, Gnagy EM, Greiner AR, et al. Behavioral versus behavioral and pharmacological treatment in ADHD children attending a summer treatment program. J Abnorm Child Psychol. 2000;28:507–525. doi: 10.1023/a:1005127030251. [DOI] [PubMed] [Google Scholar]
- 22.Acebo C, LeBourgeois MK. Actigraphy. Respir Care Clin N Am. 2006;12:23–30. doi: 10.1016/j.rcc.2005.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Morgenthaler TI, Lee-Chiong T, Alessi C, et al. Practice parameters for the clinical evaluation and treatment of circadian rhythm sleep disorders: An American Academy of Sleep Medicine report. Sleep. 2007;30:1445–1459. doi: 10.1093/sleep/30.11.1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spruyt K, Gozal D. Development of pediatric sleep questionnaires as diagnostic or epidemiological tools: a brief review of dos and don’ts. Sleep Med Rev. 2011;15:7–17. doi: 10.1016/j.smrv.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spruyt K, Gozal D. Pediatric sleep questionnaires as diagnostic or epidemiological tools: a review of currently available instruments. Sleep Med Rev. 2011;15:19–32. doi: 10.1016/j.smrv.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maldonado CC, Bentley AJ, Mitchell D. A pictorial sleepiness scale based on cartoon faces. Sleep. 2004;27:541–548. doi: 10.1093/sleep/27.3.541. [DOI] [PubMed] [Google Scholar]
- 27.Owens JA, Spirito A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;23:1043–1051. [PubMed] [Google Scholar]
- 28.Owens JA, Maxim R, Nobile C, McGuinn M, Msall M. Parental and self-report of sleep in children with attention-deficit/hyperactivity disorder. Arch Pediatr Adolesc Med. 2000;154:549–555. doi: 10.1001/archpedi.154.6.549. [DOI] [PubMed] [Google Scholar]
- 29.Gozal D. Sleep-disordered breathing and school performance in children. Pediatrics. 1998;102(3 Part 1):616–620. doi: 10.1542/peds.102.3.616. [DOI] [PubMed] [Google Scholar]
- 30.Montgomery-Downs HE, O’Brien LM, Holbrook CR, Gozal D. Snoring and sleep-disordered breathing in young children: Subjective and objective correlates. Sleep. 2004;27:87–94. doi: 10.1093/sleep/27.1.87. [DOI] [PubMed] [Google Scholar]
- 31.Witcher L. Sleep Hygiene and Problem Behaviors in Snoring Children. (Psych D) Louisville, KY: Spalding University; 2009. [Google Scholar]
- 32.LeBourgeois MK, Giannotti F, Cortesi F, et al. Sleep hygiene and sleep quality in Italian and American adolescents. Ann N Y Acad Sci. 2004;1021:325–354. doi: 10.1196/annals.1308.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Henderson JA, Jordan SS. Development and preliminary evaluation of the bedtime routines questionnaire. J Psychopathol Behav Assess. 2009;32(2):1–10. [Google Scholar]
- 34.Weinberg WA, Harper CR. Vigilance and its disorders. Neurol Clin. 1993;11:59–78. [PubMed] [Google Scholar]
- 35.Ramos Platon MJ, Vela Bueno A, Espinar Sierra J, Kales S. Hypnopolygraphic alterations in Attention deficit Disorder (ADD) children. Int J Neurosci. 1990;53:87–101. doi: 10.3109/00207459008986591. [DOI] [PubMed] [Google Scholar]
- 36.Lecendreux M, Konofal E, Bouvard M, Falissard B, Mouren-Simeoni MC. Sleep and alertness in children with ADHD. J Child Psychol Psychiatry. 2000;41:803–812. [PubMed] [Google Scholar]
- 37.Miano S, Donfrancesco R, Bruni O, et al. NREM sleep instability is reduced in children with attention-deficit/hyperactivity disorder. Sleep. 2006;29:797–803. [PubMed] [Google Scholar]
- 38.Owens J, Sangal RB, Sutton VK, Bakken R, Allen AJ, Kelsey D. Subjective and objective measures of sleep in children with attention-deficit/hyperactivity disorder. Sleep Med. 2009;10:446–456. doi: 10.1016/j.sleep.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 39.Schroeder VM, Kelley ML. Associations between family environment, parenting practices, and executive functioning of children with and without ADHD. J Child Fam Stud. 2009;18:227–235. [Google Scholar]
- 40.Blum K, Braverman ER, Holder JM, et al. Reward deficiency syndrome: a biogenetic model for the diagnosis and treatment of impulsive, addictive, and compulsive behaviors. J Psychoactive Drugs. 2000;32( Suppl):1–112. doi: 10.1080/02791072.2000.10736099. [DOI] [PubMed] [Google Scholar]
- 41.Comings DE, Blum K. Reward deficiency syndrome: genetic aspects of behavioral disorders. Prog Brain Res. 2000;126:325–341. doi: 10.1016/S0079-6123(00)26022-6. [DOI] [PubMed] [Google Scholar]
- 42.Mahone EM, Wodka EL. The neurobiological profile of girls with ADHD. Dev Disabil Res Rev. 2008;14:276–284. doi: 10.1002/ddrr.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gumenyuk V, Roth T, Korzyukov O, Jefferson C, Bowyer S, Drake CL. Habitual short sleep impacts frontal switch mechanism in attention to novelty. Sleep. 2011;34:1659–1670. doi: 10.5665/sleep.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konofal E, Cortese S, Lecendreux M, Arnulf I, Mouren MC. Effectiveness of iron supplementation in a young child with attention-deficit/hyperactivity disorder. Pediatrics. 2005;116(5):e732–e734. doi: 10.1542/peds.2005-0715. [DOI] [PubMed] [Google Scholar]
- 45.Howard AL, Robinson M, Smith GJ, Ambrosini GL, Piek JP, Oddy WH. ADHD is associated with a ‘western’ dietary pattern in adolescents. J Atten Disord. 2011;15:403–411. doi: 10.1177/1087054710365990. [DOI] [PubMed] [Google Scholar]