Abstract
Purpose
Pediatric, clinical, and research data suggest that insufficient sleep causes tiredness and daytime difficulties in terms of attention-focusing, learning, and impulse modulation in children with attention deficit hyperactivity disorder (ADHD) or in those with ADHD and primary sleep disorders. The aim of the present study was to examine whether sleep duration was associated with ADHD-like symptoms in healthy, well-developing school-aged children.
Patients and methods
Thirty-five healthy children (20 boys, 15 girls), aged 7–11 years participated in the present study. Each child wore an actigraphic device on their nondominant wrist for two nights prior to use of polysomnography to assess their typical sleep periods. On the third night, sleep was recorded via ambulatory assessment of sleep architecture in the child’s natural sleep environment employing portable polysomnography equipment. Teachers were asked to report symptoms of inattention and hyperactivity/impulsivity on the revised Conners Teacher Rating Scale.
Results
Shorter sleep duration was associated with higher levels of teacher-reported ADHD-like symptoms in the domains of cognitive problems and inattention. No significant association between sleep duration and hyperactivity symptoms was evident.
Conclusion
Short sleep duration was found to be related to teacher-derived reports of ADHD-like symptoms of inattention and cognitive functioning in healthy children.
Keywords: ADHD-like symptoms, sleep duration, inattention, hyperactivity, impulsivity, healthy school-aged children
Introduction
A considerable proportion of elementary school-aged children sleep for less than the recommended 10–11 hours.1 For example, a study conducted in 2004 found that 43% of boys aged 10–11 years slept for less than 9 hours per night.2 Decreases in sleep time combined with increasingly delayed bedtimes suggest that sleep restriction is emerging as a preadolescent problem. A poll conducted by the National Sleep Foundation found that adolescents (6th–12th grade) averaged 0.5–2 hours less than the recommended amount of sleep each night.3 This finding is a major problem, given the negative impact of restricted sleep on the mental and physical health of children and adolescents.
Mounting evidence indicates that sleep has beneficial effects on learning, memory, attention, emotional regulation, and academic success. Conversely, fatigue and insufficient sleep can negatively affect academic performance, self-regulation, and attention, all of which are necessary for success in school (for a review see Gruber et al).4 The aspects of human behavior most affected by fatigue and insufficient sleep are the executive functions, learning, and memory; these are also the key functional domains required for academic success. Sleep loss impairs performance on tasks requiring abstract thinking, creativity, integration, and planning,5 and is associated with a decrease in the efficiency of learning and memory.6–13
Furthermore, pediatric, clinical, and research data suggest that insufficient sleep causes tiredness and daytime difficulties in terms of attention-focusing and impulse modulation.14–17 In 1991, Dahl et al18 observed that such difficulties were very similar to the core symptoms of attention deficit hyperactivity disorder (ADHD), the most commonly diagnosed neuropsychological disorder in children. Subsequently, several researchers have studied the association between sleep and neurobehavioral functioning in children with ADHD with or without sleep-disordered breathing and in children with both ADHD and restless leg syndrome/periodic leg movement disorder.14,19–22 The cited studies consistently demonstrated that, in such populations, sleep disruption was associated with hyperactivity and inattention.22
Although the cited works provide convincing evidence that sleep and attention interact in children with ADHD, or in those with ADHD and any or all of sleep apnea, sleep-disordered breathing, restless leg syndrome, and periodic leg movement disorder, it is not clear whether this is true of typically developing children that do not suffer from any such problems. The few survey- or actigraphy-based studies that have examined the association between short sleep duration and ADHD-like symptoms in typically developing children have yielded conflicting results.23–28 Inconsistent data were evident in both types of studies, with some reports finding associations between short sleep duration and inattention23,24,26,29,30 and hyperactivity,25,27,28 whereas others did not.23–25,31,32 Reasons for such inconsistencies could be related to methodological differences in the way that sleep was measured. Polysomnography (PSG) was not employed, and no study employed an objective measure of breathing symptoms, and no prior study has measured restless leg syndrome or periodic leg movement disorder using either objective or subjective measures. Breathing symptoms, restless leg syndrome, and periodic leg movement disorder are common conditions, but relatively underdiagnosed in pediatric populations33 and have been frequently associated with inattention and hyperactivity.34–38
Therefore, the presence of such comorbidities may have confounded prior study results, contributing to inconsistent findings. It is therefore impossible to draw firm conclusions in terms of an association between short sleep duration and symptoms of inattention and hyperactivity/impulsivity in healthy children based on the data of the cited works. This is important because if short sleep duration is indeed associated with ADHD-like symptoms in typically developing children who do not have primary sleep disorders, identification of such an association would support the use of interventions aimed at reducing sleep deprivation. These interventions could therefore help otherwise healthy children to fulfill their potential.
The goal of the present study was to determine whether shorter sleep duration was related to symptoms of inattention and hyperactivity/impulsivity, which are commonly used in the diagnosis of ADHD, in typically developing children. It was hypothesized that shorter sleep duration would be associated with higher teacher ratings of inattention and hyperactivity/impulsivity.
Material and methods
Participants
Thirty-five children (20 boys, 15 girls) aged 7–11 years (mean 8.60 years; standard deviation [SD] 1.12 years) participated in the present study. Psychiatric status was assessed using the Diagnostic Interview Schedule for Children;39 which was administered to parents. Medical information was obtained via a detailed health screening form. Participants were excluded if they had an intelligence quotient (IQ) <;80 (measured using the Wechsler Intelligence Scale for Children, 4th edition40), or any medical or psychiatric conditions. In addition, children with a saturation nadir lower than 90%, with paradoxical breathing, or with periodic limb movements associated with five or more leg movements per hour of sleep were excluded from the study.
Participants were recruited from elementary schools located in districts of middle socioeconomic status. The study was approved by the Research Ethics Board of the Douglas Mental Health University Institute. All enrolled children completed the study. Each child received compensation of CAD75. Parents signed consent forms permitting research team members to contact teachers. In addition, parents sent teachers information on the study, and informed them that team members would be in contact seeking completion of questionnaires. All teachers were contacted by a research assistant and subsequently completed the revised Conners Teacher Rating Scale (CTRS-R).41
Procedure
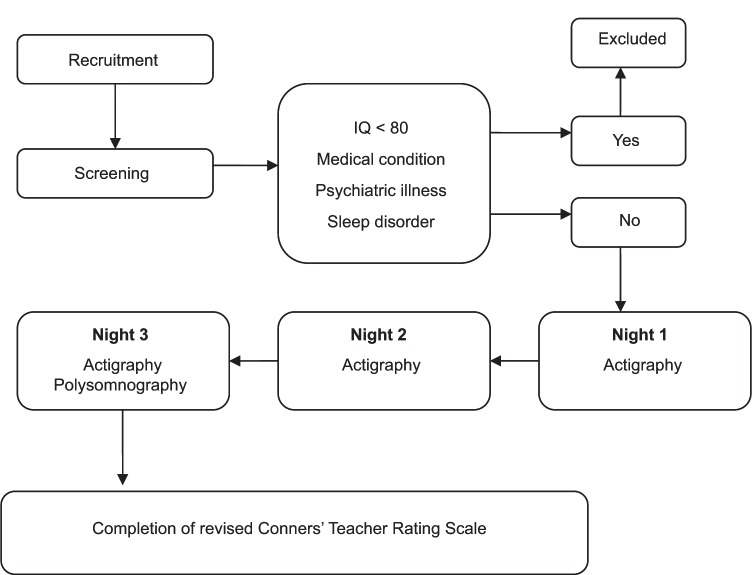
Children were first screened for eligibility. During initial contact, over-the-phone assessment was conducted using the Pediatric Sleep Questionnaire42 which assesses sleep-disordered breathing, snoring, and sleepiness. In addition, the Chervin and Hedger tool,43 which investigates leg restlessness, experience of growing pains in bed, insomnia, and morning headache, was administered to exclude those with restless leg syndrome. Children scoring 0.33 or higher (ie, replying positively to 33% or more of the 22 questions of either scale) were excluded. Health-related conditions and use of medication were assessed using a health screening form that included a detailed list of questions about each child’s health status. Children who passed over-the-phone screening next visited the laboratory, where both IQ and potential psychological diagnoses were evaluated in a quiet room. After initial screening, children meeting inclusion criteria were invited to participate in the study and their parents received a package that included a sleep assessment battery, a demographic questionnaire, and a consent form. Sleep assessment included use of the morningness–eveningness questionnaire,44 actigraphy, and a sleep log. Parents were asked to complete and return the questionnaire. Teachers were requested to complete the CTRS-R41 based on the week of sleep evaluation. Each child wore an actigraphic device on their nondominant wrist for a period of two nights prior to use of PSG, to assess their usual sleep period. On the third night, sleep was recorded using ambulatory assessment of sleep architecture employing portable PSG equipment. On the scheduled night, a sleep technician arrived at each child’s home 1.5 hours prior to habitual bedtime and connected the sleep recording apparatus. Recording commenced at the child’s habitual bedtime. Sleep pattern and architecture were recorded in the natural home environment because such data afford greater ecological validity than do records logged in sleep laboratories.45–47 For a visual representation of the study design, please see Figure 1.
Figure 1.
Study design.
Measures
Questionnaires/scales for neurobehavioral assessment
ADHD-like symptoms were evaluated using the CTRS-R, a well-validated and reliable instrument used to screen for ADHD in children.41 The CTRS-R has internal consistency ratings of 0.86–0.95 and high test-retest reliability.41 Teachers rated several behaviors on a scale from zero (not true at all) to three (very true), yielding four indices; data from the hyperactivity-impulsivity and cognitive problems/inattention domains were focused on.
The Full Scale IQ score of the Wechsler Intelligence Scale for Children, 4th edition40 was used to estimate general cognitive functioning.
Sleep assessment PSG
An in-home recording procedure was used to assess child sleep architecture, to allow children to sleep in their natural environment. PSG recordings were performed in the children’s homes using a digital ambulatory sleep recorder (Vitaport-3; TEMEC Instruments BV, Kerkrade, the Netherlands), measuring electroencephalography, submental electromyography, electrooculography, and finger pulse oximetry. Electroencephalography electrodes were placed bilaterally along the anteroposterior axes at locations F3, F4, C3, C4, P3, P4, O1, and O2. To assess respiration, two respiratory belts, measuring both chest and abdominal movement, were fitted to detect hypopnea and apneas, respectively, and pulse oximetry was used to measure oxygen saturation. The decision not to use cannula was based on the fact that, whereas the electrooculography, electromyography, and electroencephalography electrodes could be easily twisted together and attached to the recording equipment located close to the child’s head, the use of nasal cannula would interfere with the child’s sleep and would impact the ecological validity of the study.
Electromyography leg electrodes were used to identify leg-movements. Sleep stages were scored visually onscreen (LUNA; Stellate Systems, Montreal, Canada) using the C3 derivation (referential derivation: linked ears) according to standard criteria,48 but employing 20-second epochs.
Various PSG sleep measures were analyzed, but, in the present study, sleep duration was focused on.
Actigraphic measurements
Actigraphs (Actiwatch® 64; Mini Mitter Company Inc, Bend, OR) were used to assess sleep patterns in the natural home environment. These computerized wristwatch-like devices collect data generated by movement. Their use is minimally invasive and the devices therefore allow sleep to be reliably recorded without interfering with family routine. Each actigraphic sleep interval was manually marked with sleep log bedtime and rising time. For each 1-minute epoch, a total activity count was computed. If a threshold value was attained, then the epoch was considered to be wakefulness. If the value fell below that threshold, then the epoch was considered to be sleep. Actigraphic data were analyzed using Actiwatch 64 sleep software (Mini Mitter). The actigraphic parameter of interest was sleep period, representing the amount of time between sleep commencement and wakening. Sleep start and sleep end were automatically determined as the first and last 5-minute periods, respectively, in which no more than a single epoch was scored as mobile.
Circadian preference measure
The Children’s Morningness–Eveningness Preferences Scale,49 a ten-item multiple-choice scale adapted from the Horne–Ostberg morningness–eveningness questionnaire,44 was used to determine circadian preference. The scale ranges from ten (extreme evening preference) to 42 (extreme morning preference).
General evaluation and confounders
To characterize the profiles of the children and reconfirm that the psychological profiles of the study children were within the normal range, the Child Behavior Checklist was used, a frequently utilized dimensional measure of child psychopathology.50,51 Numerous studies have confirmed the stability of the instrument in terms of psychometric properties; the test shows good reliability and validity in both clinical and nonclinical populations.52,53
Body mass index was calculated by dividing weight in kilograms by height in meters squared.
Statistical analyses
Descriptive statistics on demographics, physical, and intellectual characteristics of participants were computed. To determine whether sleep duration on the PSG night was similar to those on the weeknights preceding PSG, Student’s t-test for related samples was used to compare the mean time in bed obtained in the two consecutive nights prior to PSG to the time spent in bed in the PSG night. Multiple linear regression analyses, adjusted for age, IQ, body mass index, and circadian tendency, were used to assess the strength of potential relationships between teacher reports of inattention/cognitive problems or hyperactivity (dependent variables) and total sleep duration as measured by PSG (independent variable). All analyses were performed using IBM SPSS (v 15.0 for Windows; SPSS Inc, Chicago, IL). P <; 0.05 was considered significant.
Results
Demographic and sleep characteristics of all enrolled participants are presented in Table 1. The age range was 7–11 years (mean 8.60 years, SD 1.12 years). Twenty (57%) of the children were male. Caucasian children represented 68.6% of enrolled participants, 2.9% had an African-American background, 8.6% were Asian, and 8.6% of mixed race. The average total score on the Child Behavior Checklist50–53 was 49.77 (SD 9.2), thus confirming that all participants were within the normal range (T score <; 60) on all subscales pertaining to child behavior. All of the participants who were included in the study were below the cutoff score on the Pediatric Sleep Questionnaire,42 had no indication of desaturation, and no paradoxical breathing at the thoracic and abdominal channels (Table 1).
Table 1.
Sleep, demographic, and clinical characteristics (frequency or mean ± standard deviation) of the sample (n = 35)
| Variable | Mean ± SD (or frequency) | |
|---|---|---|
| Sleep characteristics | ||
| Actigraphy | ||
| Sleep period (first two nights) | 539.64 (minutes) ± 59.96 | |
| Sleep period (third night) | 533.54 (minutes) ± 59.65 | |
| PSG | ||
| Sleep duration (third night, PSG data) | 523 (minutes) ± 54.69 | |
| Stage 1% | 3.45 ± 1.80 | |
| Stage 2% | 43.56 ± 7.03 | |
| Stage 3% | 13.19 ± 3.70 | |
| Stage 4% | 21.66 ± 5.54 | |
| REM % | 18.14 ± 3.28 | |
| Sleep efficiency | 95.88 ± .302 | |
| Oxygen saturation (minimum) | 94.3 ± 1.7 | |
| Oxygen saturation (mean) | 97.8 ± .62 | |
| PLM index | 1.83 ± 1.42 | |
| MES chronotype score | 31.06 ± 3.75 | |
| Demographic characteristics | ||
| Gender (male/female) | 20/15 | |
| Age | 8.6 ± 1.12 | |
| BMI | 18.44 ± 4.05 | |
| Ethnic background | ||
| Caucasian | 24 | |
| African | 1 | |
| Asian | 3 | |
| Multiethnic | 3 | |
| Unknown | 4 | |
| Clinical characteristics | ||
| IQ | 104.54 ± 17.99 | |
| CBCL score | 49.77 ± 9.2 | |
| CTRS-R cognitive problems/inattention | 48.46 ± 5.65 | |
| T score | ||
| CTRS-R hyperactivity-impulsivity T score | 48.32 ± 6.04 | |
Abbreviations: BMI, body mass index; CBCL, Child Behavior Checklist; CTRS-R, revised Conners Teacher Rating Scale; IQ, intelligence quotient; MES, Morningness– Eveningness Preferences Scale; PLM, periodic limb movement; PSG, polysomnography; REM, rapid eye movement; SD, standard deviation.
The average time slept in bed on the first two nights of actigraphic measurement was 539.64 minutes (SD 59.96 minutes) and 533.54 minutes (SD 59.65 minutes) on the PSG night. Sleep duration measured by PSG was 528 minutes (SD 54.69 minutes). No gender or racial difference was evident in average sleep period or average sleep duration.
No significant difference between the sleep period in the two nights preceding PSG evaluation and the sleep period of the PSG night measured by PSG (t [27] = −0.16; P >; 0.05) was evident. In addition, significant correlation between sleep period, as measured by actigraphy on the PSG night, and sleep duration measured by PSG was found (r = 0.74, P <; 0.005).
To explore whether sleep duration contributed to ADHD-like symptoms in healthy well-developing children, multiple linear regression analyses were performed (Table 2). Multiple linear regression analysis revealed that the addition of sleep duration, percentage of sleep stages, and sleep efficacy to the model significantly increased the R2 value, contributing 27% to the explained variance (R2 = 0.65; P <; 0.05) (∆R2 = 0.27) (Fchange [1,29] = 3; P <; 0.05) after controlling for body mass index, circadian tendency, and IQ in predicting cognitive problems/inattention. Sleep duration was found to be a significant predictor in the model predicting scores on teacher-reported cognitive problems and inattention; individuals with shorter sleep duration received higher scores on this scale.
Table 2.
Results of regression analyses studying main effects of sleep duration on cognitive problems/inattention and hyperactivity-impulsivity on the revised Conners Teacher Rating Scale
| Measure | Cognitive problems/inattention
|
Hyperactivity-impulsivity
|
|---|---|---|
| β | β | |
| Control (model 1) | ||
| Age | −0.22 | −0.12 |
| BMI | −0.02 | −0.21 |
| MES score | 0.20 | 0.20 |
| WISC-IV IQ score | −0.43* | −0.29 |
| Gender | 0.22 | 0.18 |
| Total R2 (adjusted) | 0.28* | 0.10 |
| ∆R2 | 0.38* | 0.25 |
| Main effect (model 2) | ||
| Age | −0.25 | −0.04 |
| BMI | −0.034 | −0.40 |
| MES score | 0.23 | 0.35 |
| WISC-IV IQ score | −0.31 | −0.34 |
| Gender | 0.11 | 0.17 |
| Sleep duration | −0.49* | −0.47 |
| Stage 1% | 0.29 | 0.08 |
| Stage 2% | 0.08 | 0.01 |
| Stage 3% | −0.03 | 0.35 |
| Stage 4% | 0.03 | −0.05 |
| REM % | 0.09 | 0.58* |
| Sleep efficiency | 0.15 | 0.05 |
| Total R2 (adjusted) | 0.49* | 0.17 |
| ∆R2 | 0.27* | 0.23 |
Notes:
P ≤ 0.05; β represents standardized partial regression coefficients; R2 values refer to the variation accounted for by the model; ΔR2 refers to R2 change.
Abbreviations: BMI, body mass index; IQ, intelligence quotient; MES, Morningness–Eveningness Preferences Scale; REM, rapid eye movement; WISC-IV, Wechsler Intelligence Scale for Children, 4th edition.
Sleep variables measured by PSG did not contribute significantly to the prediction of teacher-derived data on hyperactivity.
Discussion
The aim of the present study was to explore whether objective measures of sleep duration are associated with teacher ratings of inattention and hyperactivity/impulsivity in healthy, well-developing preadolescent school-aged children who do not suffer from sleep-disordered breathing, restless leg syndrome, or periodic leg movement disorder. The results showed that shorter sleep duration was associated with higher levels of teacher ratings of cognitive problems and inattention. Specifically, short sleep duration, objectively measured using PSG, was significantly related to teacher reports of difficulties in the realms of learning or memory, in organizational skills, and the ability to be attentive when required. However, no significant association between sleep duration and hyperactivity symptoms was evident.
Several previous reports have found that short sleep duration was related to poorer academic performance when measures such as the Standard Achievement Test scores or school grades were employed (for reviews, see Gruber et al,4 Dewald et al,54 and Wolfson and Carskadon55,56). The present findings add to such data by demonstrating a strong association between sleep duration and the day-to-day functioning of children in the school environment, as reported by te achers, who were blind to the sleep status of participants. Both lines of investigation converge to show that shorter sleep is associated with the manifestation of inattention and poorer school-related outcomes. However, the mechanisms underlying these associations remain unclear, which is an important question that needs to be addressed in future research.
The findings are consistent with previous data from studies conducted in healthy adults, which found that deficits resulting from sleep deprivation resemble those seen in patients with prefrontal cortex damage.57 The prefrontal cortex plays a significant role in executive functions,58,59 and sleep loss preferentially impairs functions governed by the prefrontal cortex. Considering that a deficit in executive functions and prefrontal cortex activity is a core feature associated with ADHD,60–64 it is not surprising that short sleep duration in healthy children is associated with ADHD-like symptoms. However, because the present study was correlational in nature, the causes and effects were unable to be determined.
In contrast to the conclusions of previous reports,27,28 short sleep duration was not associated with the manifestation of higher levels of hyperactivity in the present sample of typically developing, non-ADHD school-aged children. Although this is not consistent with previous suggestions regarding the potential impact of sleep deprivation on self-regulation,65–67 the data are in line with those of recent studies showing that different functional domains are differentially affected by sleep deprivation.68 The variations in the association between sleep duration and ADHD-like symptoms emphasize the need to better explore these relationships, as well as to better understand the underlying mechanisms.68
Sleep duration contributed 27% to the explained variance in inattention and cognitive problems even after IQ was considered. If sleep duration is so significantly associated with the manifestation of such ADHD-like symptoms in otherwise healthy children, it is possible that increasing sleep duration might offer an effective and inexpensive opportunity to optimize the school functioning of healthy children. Sleep does not cost money and does not have any negative side effects. A potential practical path to this end is to incorporate sleep education into the practice of health care providers and pediatricians, and into the health curricula of elementary schools, similar to Cain et al’s efforts at establishing motivational school-based interventions.69 In addition, it is critical to educate parents, students, educators, and clinicians on the importance of sleep and to develop tools aimed at preventing sleep deprivation.
The present study extends prior research in various ways. First, sleep was objectively assessed and an ecologically-based measure of ADHD-like symptoms at school was employed. In addition, healthy school-aged children were studied, excluding those with symptoms of primary sleep disorders or ADHD. Further, sleep parameters were measured at home, increasing the ecological validity of the results.
Limitations
Some limitations of this work are apparent. First, the participants were below the cutoff score on the Pediatric Sleep Questionnaire, had no indication of desaturation, and no paradoxical breathing at the thoracic and abdominal channels; however, the presence of sleep-disordered breathing cannot be completely excluded because of the lack of nasal cannula or thermistor. Second, even if a statistical power analysis indicated that the sample size was sufficient for detection of significant effects, the sample size was still relatively small and the results are thus preliminary in nature.
The data support an association between sleep duration and school cognitive performance; however, the mechanisms and potential causes and effects of such association require further exploration. Although sleep duration explained 27% of variance, there are, clearly, additional variables that affect inattention and cognition in healthy children. Future studies are needed to further identify such variables.
Sleep is also affected by the physical and emotional environment of the child/adolescent. Exposure to light or to an uncomfortable temperature in the evening, a high level of stimulation around bedtime, and a noisy environment have all been shown to compromise the ability to obtain sufficient sleep. In addition to physical features, the emotional environment, affected principally by family interactions (eg, marital conflict), also has a significant impact on sleep processes.70 In the present study, interference with regular sleep habits and the sleep environment was minimized by using at-home measurement of sleep parameters. However, environmental factors that might impact child sleep were not able to be identified. Future studies should further delimit the physical and environmental factors contributing to shortening of preadolescent sleep duration.
Conclusion
The findings suggest that short sleep duration is related to teacher-derived reports of ADHD-like symptoms of inattention and cognitive functioning in healthy children. The negative impact of sleep deprivation emphasizes the need to provide children and their parents with education on healthy sleep and tools that assist in achieving such sleep.
Acknowledgments
This study was supported by grants to Dr Gruber from the Canadian Institutes of Health Research (CIHR; grant number 153139) and the Fonds de la recherche en santé du Québec (FRSQ; grant number 10091).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Heussler HS. Common causes of sleep disruption and daytime sleepiness: childhood sleep disorders II. Med J Aust. 2005;182(9):484–489. doi: 10.5694/j.1326-5377.2005.tb06793.x. [DOI] [PubMed] [Google Scholar]
- 2.Spilsbury JC, Storfer-Isser A, Drotar D, et al. Sleep behavior in an urban US sample of school-aged children. Arch Pediatr Adolesc Med. 2004;158(10):988–994. doi: 10.1001/archpedi.158.10.988. [DOI] [PubMed] [Google Scholar]
- 3.National Sleep Foundation 2006 teens and sleep: sleep in America poll 2006Available from: http://www.sleepfoundation.org/article/sleep-america-polls/2006-teens-and-sleepAccessed July 20, 2010
- 4.Gruber R, Wiebe ST, Wells SA, Cassoff J, Monson E. Sleep and academic success: mechanisms, empirical evidence, and interventional strategies. Adolesc Med State Art Rev. 2010;21(3):522–541. [PubMed] [Google Scholar]
- 5.Dahl RE. The regulation of sleep and arousal: development and psychopathology. Dev Psychopathol. 1996;8(1):3–27. [Google Scholar]
- 6.Curcio G, Ferrara M, De Gennaro L. Sleep loss, learning capacity and academic performance. Sleep Med Rev. 2006;10(5):323–337. doi: 10.1016/j.smrv.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 7.Maquet P, Laureys S, Peigneux P, et al. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3(8):831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 8.Marshall L, Born J. The contribution of sleep to hippocampus-dependent memory consolidation. Trends Cogn Sci. 2007;11(10):442–450. doi: 10.1016/j.tics.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 9.Ofen N, Kao YC, Sokol-Hessner P, Kim H, Whitfield-Gabrieli S, Gabrieli JD. Development of the declarative memory system in the human brain. Nat Neurosci. 2007;10(9):1198–1205. doi: 10.1038/nn1950. [DOI] [PubMed] [Google Scholar]
- 10.Ribeiro S, Nicolelis MA. Reverberation, storage, and postsynaptic propagation of memories during sleep. Learn Mem. 2004;11(6):686–696. doi: 10.1101/lm.75604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schabus M, Gruber G, Parapatics S, et al. Sleep spindles and their significance for declarative memory consolidation. Sleep. 2004;27(8):1479–1485. doi: 10.1093/sleep/27.7.1479. [DOI] [PubMed] [Google Scholar]
- 12.Stickgold R. Sleep-dependent memory consolidation. Nature. 2005;437(7063):1272–1278. doi: 10.1038/nature04286. [DOI] [PubMed] [Google Scholar]
- 13.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265(5172):676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 14.Chervin RD, Dillon JE, Bassetti C, Ganoczy DA, Pituch KJ. Symptoms of sleep disorders, inattention, and hyperactivity in children. Sleep. 1997;20(12):1185–1192. doi: 10.1093/sleep/20.12.1185. [DOI] [PubMed] [Google Scholar]
- 15.Corkum P, Moldofsky H, Hogg-Johnson S, Humphries T, Tannock R. Sleep problems in children with attention-deficit/hyperactivity disorder: impact of subtype, comorbidity, and stimulant medication. J Am Acad Child Adolesc Psychiatry. 1999;38(10):1285–1293. doi: 10.1097/00004583-199910000-00018. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien LM, Mervis CB, Holbrook CR, et al. Neurobehavioral correlates of sleep-disordered breathing in children. J Sleep Res. 2004;13(2):165–172. doi: 10.1111/j.1365-2869.2004.00395.x. [DOI] [PubMed] [Google Scholar]
- 17.Stein MA. Unravelling sleep problems in treated and untreated children with ADHD. J Child Adolesc Psychopharmacol. 1999;9(3):157–168. doi: 10.1089/cap.1999.9.157. [DOI] [PubMed] [Google Scholar]
- 18.Dahl RE, Pelham WE, Wierson M. The role of sleep disturbances in attention deficit disorder symptoms: a case study. J Pediatr Psychol. 1991;16(2):229–239. doi: 10.1093/jpepsy/16.2.229. [DOI] [PubMed] [Google Scholar]
- 19.Archbold KH, Giordani B, Ruzicka DL, Chervin RD. Cognitive executive dysfunction in children with mild sleep-disordered breathing. Biol Res Nurs. 2004;5(3):168–176. doi: 10.1177/1099800403260261. [DOI] [PubMed] [Google Scholar]
- 20.Bass JL, Corwin M, Gozal D, et al. The effect of chronic or intermittent hypoxia on cognition in childhood: a review of the evidence. Pediatrics. 2004;114(3):805–816. doi: 10.1542/peds.2004-0227. [DOI] [PubMed] [Google Scholar]
- 21.Beebe DW, Gozal D. Obstructive sleep apnea and the prefrontal cortex: towards a comprehensive model linking nocturnal upper airway obstruction to daytime cognitive and behavioral deficits. J Sleep Res. 2002;11(1):1–16. doi: 10.1046/j.1365-2869.2002.00289.x. [DOI] [PubMed] [Google Scholar]
- 22.Melendres MC, Lutz JM, Rubin ED, Marcus CL. Daytime sleepiness and hyperactivity in children with suspected sleep-disordered breathing. Pediatrics. 2004;114(3):768–775. doi: 10.1542/peds.2004-0730. [DOI] [PubMed] [Google Scholar]
- 23.Fallone G, Acebo C, Arnedt JT, Seifer R, Carskadon MA. Effects of acute sleep restriction on behavior, sustained attention, and response inhibition in children. Percept Mot Skills. 2001;93(1):213–229. doi: 10.2466/pms.2001.93.1.213. [DOI] [PubMed] [Google Scholar]
- 24.Fallone G, Acebo C, Seifer R, Carskadon MA. Experimental restriction of sleep opportunity in children: effects on teacher ratings. Sleep. 2005;28(12):1561–1567. doi: 10.1093/sleep/28.12.1561. [DOI] [PubMed] [Google Scholar]
- 25.Gau SS, Kessler RC, Tseng WL. Association between sleep problems and symptoms of attention-deficit/hyperactivity disorder in young adults. Sleep. 2007;30(2):195–201. doi: 10.1093/sleep/30.2.195. [DOI] [PubMed] [Google Scholar]
- 26.Paavonen EJ, Porkka-Heiskanen T, Lahikainen AR. Sleep quality, duration and behavioral symptoms among 5–6-year-old children. Eur Child Adolesc Psychiatry. 2009;18(12):747–754. doi: 10.1007/s00787-009-0033-8. [DOI] [PubMed] [Google Scholar]
- 27.Paavonen EJ, Raikkonen K, Lahti J, et al. Short sleep duration and behavioral symptoms of attention-deficit/hyperactivity disorder in healthy 7- to 8-year-old children. Pediatrics. 2009;123(5):e857–e864. doi: 10.1542/peds.2008-2164. [DOI] [PubMed] [Google Scholar]
- 28.Touchette É, Petit D, Séguin JR, Boivin M, Tremblay RE, Montplaisir JY. Associations between sleep duration patterns and behavioral/cognitive functioning at school entry. Sleep. 2007;30(9):1213–1219. doi: 10.1093/sleep/30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aronen ET, Paavonen EJ, Fjallberg M, Soininen M, Torronen J. Sleep and psychiatric symptoms in school-age children. J Am Acad Child Adolesc Psychiatry. 2000;39(4):502–508. doi: 10.1097/00004583-200004000-00020. [DOI] [PubMed] [Google Scholar]
- 30.Epstein R, Chillag N, Lavie P. Starting times of school: effects on daytime functioning of fifth-grade children in Israel. Sleep. 1998;21(3):250–256. doi: 10.1093/sleep/21.3.250. [DOI] [PubMed] [Google Scholar]
- 31.Nixon GM, Thompson JM, Han DY, et al. Short sleep duration in middle childhood: risk factors and consequences. Sleep. 2008;31(1):71–78. doi: 10.1093/sleep/31.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Trabelsi S, Guillot S, Ritacco H, Boue F, Langevin D. Nano structures of colloidal complexes formed in oppositely charged polyelectrolyte/surfactant dilute aqueous solutions. Eur Phys J E Soft Matter. 2007;23(3):305–311. doi: 10.1140/epje/i2006-10192-y. [DOI] [PubMed] [Google Scholar]
- 33.Lumeng JC, Chervin RD. Epidemiology of pediatric obstructive sleep apnea. Proc Am Thorac Soc. 2008;5(2):242–252. doi: 10.1513/pats.200708-135MG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beebe DW. Neurobehavioral morbidity associated with disordered breathing during sleep in children: a comprehensive review. Sleep. 2006;29(9):1115–1134. doi: 10.1093/sleep/29.9.1115. [DOI] [PubMed] [Google Scholar]
- 35.Chervin RD, Ruzicka DL, Giordani BJ, et al. Sleep-disordered breathing, behavior, and cognition in children before and after adenotonsillectomy. Pediatrics. 2006;117(4):e769–e778. doi: 10.1542/peds.2005-1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cortese S, Konofal E, Yateman N, Mouren MC, Lecendreux M. Sleep and alertness in children with attention-deficit/hyperactivity disorder: a systematic review of the literature. Sleep. 2006;29(4):504–511. [PubMed] [Google Scholar]
- 37.Gozal D, Kheirandish-Gozal L. Neurocognitive and behavioral morbidity in children with sleep disorders. Curr Opin Pulm Med. 2007;13(6):505–509. doi: 10.1097/MCP.0b013e3282ef6880. [DOI] [PubMed] [Google Scholar]
- 38.Walters AS, Mandelbaum DE, Lewin DS, Kugler S, England SJ, Miller M. Dopaminergic therapy in children with restless legs/periodic limb movements in sleep and ADHD. Pediatr Neurol. 2000;22(3):182–186. doi: 10.1016/s0887-8994(99)00152-6. [DOI] [PubMed] [Google Scholar]
- 39.Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39(1):28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- 40.Wechsler D. The Wechsler Intelligence Scale for Children. 4th ed. London: Pearson Assessment; 2004. [Google Scholar]
- 41.Conners CK, Sitarenios G, Parker JD, Epstein JN. Revision and restandardization of the Conners Teacher Rating Scale (CTRS-R): factor structure, reliability, and criterion validity. J Abnorm Child Psychol. 1998;26(4):279–291. doi: 10.1023/a:1022606501530. [DOI] [PubMed] [Google Scholar]
- 42.Chervin RD, Hedger K, Dillon JE, Pituch KJ. Pediatric Sleep Questionnaire (PSQ): validity and reliability of scales for sleep-disordered breathing, snoring, sleepiness, and behavioral problems. Sleep Med. 2000;1(1):21–32. doi: 10.1016/s1389-9457(99)00009-x. [DOI] [PubMed] [Google Scholar]
- 43.Chervin RD, Hedger KM. Clinical prediction of periodic leg movements during sleep in children. Sleep Med. 2001;2(6):501–510. doi: 10.1016/s1389-9457(01)00069-7. [DOI] [PubMed] [Google Scholar]
- 44.Horne JA, Ostberg O. A self-assessment questionnaire to de termine morningness-eveningness in human circadian rhythms. Int J Chronobiol. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 45.Iber C, Redline S, Kaplan Gilpin AM, et al. Polysomnography performed in the unattended home versus the attended laboratory setting – Sleep Heart Health Study methodology. Sleep. 2004;27(3):536–540. doi: 10.1093/sleep/27.3.536. [DOI] [PubMed] [Google Scholar]
- 46.Quan SF, Griswold ME, Iber C, et al. Short-term variability of respiration and sleep during unattended nonlaboratory polysomnography – the Sleep Heart Health Study. Sleep. 2002;25(8):843–849. [PubMed] [Google Scholar]
- 47.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20(12):1077–1085. [PubMed] [Google Scholar]
- 48.Rechtschaffen A, Kales A. A Manual of Standardized Terminology, Techniques and Scoring System of Sleep Scoring Stages of Human Subjects. Washington, DC: Public Health Service, US Government Printing Office; 1968. [Google Scholar]
- 49.Carskadon MA, Feifer R, Acebo C. Reliability of six scales in a sleep questionnaire for adolescents. Sleep Res. 1991;20:421. [Google Scholar]
- 50.Achenbach TM, Howell CT, Quay HC, Conners CK. National survey of problems and competencies among four- to sixteen-year-olds: parents’ reports for normative and clinical samples. Monogr Soc Res Child Dev. 1991;56(3):1–131. [PubMed] [Google Scholar]
- 51.Bird HR. Epidemiology of childhood disorders in a cross-cultural context. J Child Psychol Psychiatry. 1996;37(1):35–49. doi: 10.1111/j.1469-7610.1996.tb01379.x. [DOI] [PubMed] [Google Scholar]
- 52.Jensen PS, Watanabe HK, Richters JE, et al. Scales, diagnoses, and child psychopathology: II. Comparing the CBCL and the DISC against external validators. J Abnorm Child Psychol. 1996;24(2):151–168. doi: 10.1007/BF01441482. [DOI] [PubMed] [Google Scholar]
- 53.Schmeck K, Poustka F, Dopfner M, et al. Discriminant validity of the child behaviour checklist CBCL-4/18 in German samples. Eur Child Adolesc Psychiatry. 2001;10(4):240–247. doi: 10.1007/s007870170013. [DOI] [PubMed] [Google Scholar]
- 54.Dewald JF, Meijer AM, Oort FJ, Kerkhof GA, Bogels SM. The influence of sleep quality, sleep duration and sleepiness on school performance in children and adolescents: a meta-analytic review. Sleep Med Rev. 2010;14(3):179–189. doi: 10.1016/j.smrv.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 55.Wolfson AR, Carskadon MA. Understanding adolescents’ sleep pa tterns and school performance: a critical appraisal. Sleep Med Rev. 2003;7(6):491–506. doi: 10.1016/s1087-0792(03)90003-7. [DOI] [PubMed] [Google Scholar]
- 56.Wolfson AR, Carskadon MA. Sleep schedules and daytime functioning in adolescents. Child Dev. 1998;69(4):875–887. [PubMed] [Google Scholar]
- 57.Horne JA. Human sleep, sleep loss and behaviour. Implications for the prefrontal cortex and psychiatric disorder. Br J Psychiatry. 1993;162:413–419. doi: 10.1192/bjp.162.3.413. [DOI] [PubMed] [Google Scholar]
- 58.Kane MJ, Engle RW. The role of prefrontal cortex in working-memory capacity, executive attention, and general fluid intelligence: an individual-differences perspective. Psychon Bull Rev. 2002;9(4):637–671. doi: 10.3758/bf03196323. [DOI] [PubMed] [Google Scholar]
- 59.Robbins TW. Dissociating executive functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351(1346):1463–1470. doi: 10.1098/rstb.1996.0131. [DOI] [PubMed] [Google Scholar]
- 60.Arnsten AF. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs. 2009;23(Suppl 1):33–41. doi: 10.2165/00023210-200923000-00005. [DOI] [PubMed] [Google Scholar]
- 61.Barkley RA. Genetics of childhood disorders: XVII. ADHD, Part 1: the executive functions and ADHD. J Am Acad Child Adolesc Psychiatry. 2000;39(8):1064–1068. doi: 10.1097/00004583-200008000-00025. [DOI] [PubMed] [Google Scholar]
- 62.Berlin L, Bohlin G, Nyberg L, Janols LO. How well do measures of inhibition and other executive functions discriminate between children with ADHD and controls? Child Neuropsychol. 2004;10(1):1–13. doi: 10.1076/chin.10.1.1.26243. [DOI] [PubMed] [Google Scholar]
- 63.Brennan AR, Arnsten AF. Neuronal mechanisms underlying attention deficit hyperactivity disorder: the influence of arousal on prefrontal cortical function. Ann N Y Acad Sci. 2008;1129:236–245. doi: 10.1196/annals.1417.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wodka EL, Mostofsky SH, Prahme C, et al. Process ex amination of executive function in ADHD: sex and subtype effects. Clin Neuropsychol. 2008;22(5):826–841. doi: 10.1080/13854040701563583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dahl RE. Sleeplessness and aggression in youth. J Adolesc Health. 2006;38(6):641–642. doi: 10.1016/j.jadohealth.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 66.Ireland JL, Culpin V. The relationship between sleeping problems and aggression, anger, and impulsivity in a population of juvenile and young offenders. J Adolesc Health. 2006;38(6):649–655. doi: 10.1016/j.jadohealth.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 67.Umlauf MG, Bolland JM, Lian BE. Sleep disturbance and risk behaviors among inner-city African-American adolescents. J Urban Health. 2011;88(6):1130–1142. doi: 10.1007/s11524-011-9591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tucker AM, Whitney P, Belenky G, Hinson JM, Van Dongen HP. Effects of sleep deprivation on dissociated components of executive functioning. Sleep. 2010;33(1):47–57. doi: 10.1093/sleep/33.1.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cain N, Gradisar M, Moseley L. A motivational school-based intervention for adolescent sleep problems. Sleep Med. 2011;12(3):246–251. doi: 10.1016/j.sleep.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 70.El-Sheikh M. Sleep and Development: Familial and Socio-cultural Considerations. New York: Oxford University Press; 2011. [Google Scholar]