Abstract
Background
The purpose of this cross-sectional study was to test the hypothesis that serum vitamin D levels are abnormally low in sleep clinic patients admitting to chronic nonspecific musculoskeletal pain and to assess the associated risk factors. A secondary purpose was to identify a clinical biomarker for vitamin D deficiency.
Methods
We enrolled 153 consecutive patients who admitted to the presence of chronic nonspecific musculoskeletal pain during a comprehensive sleep evaluation at a specialist sleep medicine clinic within an academic center. Venous blood sampling was performed for determination of serum 25-hydroxyvitamin D. Risk factors for vitamin D deficiency (25-hydroxyvitamin D < 20 ng/mL) were identified by odds ratios. Receiver-operating characteristic curve analysis was performed with 10-fold cross-validation to identify a biomarker for vitamin D deficiency calculated by linear discriminant analysis.
Results
The mean serum 25-hydroxyvitamin D level was 19.8 ± 11.1, with 54% of the study population having vitamin D deficiency. This mean 25-hydroxyvitamin D level was lower than that observed historically in healthy controls, and was either similar or lower than in all but one representative historical cohort formed on the basis of chronic nonspecific musculoskeletal pain. Risk factors for vitamin D deficiency were black ethnicity, age < 60 years, and obesity. These risk factors were identified both in the entire cohort and separately in subgroups with and without obstructive sleep apnea. The biomarker (based on race, age, and body mass index) had a sensitivity and specificity for predicting vitamin D deficiency of 0.73 and 0.74, respectively.
Conclusion
Vitamin D deficiency was prevalent in patients with sleep disorders and chronic nonspecific musculoskeletal pain on evaluation in a sleep medicine clinic. Vitamin D deficiency was reliably estimated in the study population using a biomarker derived from common demographic characteristics.
Keywords: vitamin D, biomarker, pain, sleep, linear discriminant analysis, receiver-operating analysis
Introduction
Vitamin D is a fat-soluble secosteroid prohormone ingested in the diet and produced in the skin following exposure to ultraviolet rays in sunlight, and conversion to active forms of vitamin D occurs in the liver and kidneys.1 The metabolic processes regulated by vitamin D include serum calcium and phosphate homeostasis, bone remodeling, neuromuscular function, inflammation, and transcription of proteins involved in cell growth and apoptosis.2 Nonspecific musculoskeletal pain and weakness are prominent features of many diseases linked to vitamin D deficiency.3–6 Vitamin D deficiency may increase the risk of developing obstructive sleep apnea mediated by inflammatory rhinitis7,8 and/or tonsillar hypertrophy.9,10 Vitamin D deficiency may also contribute to the development of daytime sleepiness via central inflammatory mediators.11,12
Patients seeking care in a sleep medicine clinic typically complain of sleep disruption, fatigue, and sleepiness, sometimes in association with chronic nonspecific musculoskeletal pain. Low levels of serum vitamin D have been reported in patients who report such pain,4,13 but it is not clear if these data apply to patients whose pain is discovered incidentally during evaluation for another complaint. We were interested in the possibility that the presence of pain might indicate low vitamin D levels contributing to poor sleep quality and/or daytime symptoms of impairment in patients undergoing evaluation for a suspected sleep disorder.
Traditional risk factors for low vitamin D include race (with darker skin tone conferring higher risk), obesity, and advancing age.1 Our primary aim was to test the hypothesis that subnormal levels of vitamin D exist in patients with sleep disorders who admit to chronic nonspecific musculoskeletal pain, and to evaluate whether predictive risk factors were present. Our secondary aim was to formulate a biomarker for vitamin D deficiency based on the risk factors observed.
Materials and methods
Patients
Between April, 2008 and October, 2010, consecutive patients seen during routine consultation visits at an academic sleep medicine clinic were interviewed within the context of a full sleep history and underwent a physical examination. The patients also completed a questionnaire screening for the presence of various symptoms potentially linked to sleep disruption, including the presence of moderate to severe musculoskeletal pain contributing to sleep disruption or daytime discomfort, or both. All questionnaire responses were subsequently reviewed and verified in a face-to-face interview by a physician board-certified in internal medicine and sleep medicine (DM). For some patients, pain symptoms were initially denied, but were disclosed after specific questioning about regions of the body where pain might be experienced (eg, “Do you have pain in your back, legs, or arms that you notice during sleep?”). Patients who admitted to the presence of functionally significant pain and agreed to undergo venous blood sampling for 25-hydroxyvitamin D (n = 153) were included in the analysis (Table 1). All research-related procedures were approved by the institutional review board for human research at our institution.
Table 1.
Characteristics of cohort (n = 153)
| Age (years) | 50.9 ± 13.7 |
| Gender (%) | |
| Male | 23.5 |
| Female | 76.5 |
| Race (%) | |
| African-American | 35.3 |
| Caucasian/Hispanic* | 64.7 |
| Body mass index (kg/m2) | 36.1 ± 9.8 |
| ≥30 | 72 |
| <30 | 28 |
| Sleep diagnosis (%) | |
| Obstructive sleep apnea | 77 |
| Hypersomnia | 8.5 |
| Insomnia | 15 |
| Restless legs syndrome | 28 |
| Other | 6.5 |
Notes: Values are presented as the mean ± standard deviation;
Hispanic subjects n = 2.
Serum measurements
Serum 25-hydroxyvitamin D levels were determined by immunoassay according to the specifications of the manufacturer (DiaSorin Liason, Saluggia, Italy). A 25-hydroxyvitamin D level below 20 ng/mL was considered to indicate vitamin D deficiency.1
Study design and statistical analysis
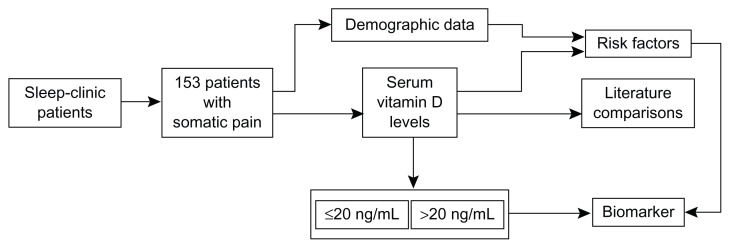
The study design is shown in Figure 1. The unpaired t-test was used to compare mean 25-hydroxyvitamin D levels, with reported representative mean values for normal subjects and for groups formed on the basis of a primary complaint of somatic pain. The relationship between vitamin D deficiency and known risk factors was evaluated by computing odds ratios and 95% confidence limits. The risk factors considered were race (black versus white/Hispanic), obesity (body mass index ≤ 30 kg/m2 versus >30 kg/m2), age (≤60 years versus >60 years), gender, and season (blood draw months during summer [May-June-July] versus winter [November-December-January]). Mean 25-hydroxyvitamin D values were analyzed for the entire cohort and according to significant risk factors.
Figure 1.
Study flow chart.
A biomarker function for predicting vitamin D deficiency was derived using linear discriminant analysis.14 Its predictive accuracy was assessed by calculating sensitivity, specificity, and area under the receiver-operator characteristics curve (AUROC).15 The likelihood that the results would be able to be generalized was estimated by computing a biomarker function using data from 90% of the patients and evaluating it using data from the remaining 10% of patients. The procedure was repeated ten times using different groups of ten patients to evaluate the predictive accuracy. Overall accuracy was estimated by averaging the results of the ten validation procedures.
Results
The mean 25-hydroxyvitamin D level in the study population was 19.8 ± 11.1 ng/mL, with 54% of the patients having vitamin D deficiency (25-hydroxyvitamin D level < 20 ng/mL). This 25-hydroxyvitamin D level was significantly lower than that commonly seen in clinically normal populations, and the prevalence of vitamin D deficiency was consistently higher (Table 2). Compared with the results of published studies involving patients for whom pain was a primary complaint, the mean level in our cohort was either significantly lower or statistically indistinguishable, with only one exception (Table 2).
Table 2.
Comparison of serum levels of 25-hydroxyvitamin D in our study group with those published for normal subjects and those with somatic pain
| n | 25-hydroxy vitamin D (ng/mL) | Percent deficient | Reference |
|---|---|---|---|
| 153 | 19.8 ± 11.1 | 54% | This study |
| Clinically normal subjects | |||
| 493 | *34.2 ± 13.8 | NR | 19 |
| 212 | *23.3 ± 8.4 | 40% | 20 (Winter) |
| 99 | *26.8 ± 10.3 | 20% | 20 (Summer) |
| 165 | *30 ± 10 | 30% | 21 (Winter) |
| 142 | *35 ± 10 | 12% | 21 (Summer) |
| 202 | *33.1 ± 28.4 | 36% | 22 |
| 13,369 | *24 ± 1 | 36% | 23 |
| Patients with pain | |||
| 128 | *28.6 ± 13.2 | NR | 19 |
| 150 | *12.1 ± 5.8 | 93% | 24 |
| 267 | *28.7 ± 11.6 | 26% | 25 |
| 276 | 23.8 ± 29.1 | 63% | 22 |
| 25 | 20.6 ± 6.8 | NR | 26 |
| 572 | 18.8 ± 1.0 | 58% | 27 |
| 263 | *23.9 ± 11.8 | 26%‡ | 28 |
Notes: Data are presented as the mean ± standard deviation. Vitamin D deficiency was defined as a serum concentration of 25-hydroxyvitamin D < 20 ng/mL except for ‡which indicates ≤ 15 ng/mL.
P < 0.05, t-test.
Abbreviation: NR, not reported.
The risk for vitamin D deficiency was 50 times higher in black than in white/Hispanic subjects, and an increased risk was also seen in subjects who were obese or younger than 60 years (Table 3). Increased risk was not observed in association with gender or winter months. In subjects from whom blood was collected during the summer months, 23 of 41 (56%) had vitamin D deficiency compared with 18 of 31 subjects (58%) from whom blood was collected during the winter months. Mean 25-hydroxyvitamin D levels in patients with and without obstructive sleep apnea were not significantly different (Table 4). Risk factors for vitamin D deficiency (Table 3) were generally found to be predictive in patients with and without obstructive sleep apnea (Table 4).
Table 3.
Association between known risk factors and vitamin D deficiency (25-hydroxyvitamin D < 20 ng/mL) in the study group
| Factor | Odds ratio (95% CI) |
|---|---|
| Race (African-American/Caucasian or Hispanic) | 50.8* (15.3–168.4) |
| Obesity (BMI > 30 kg/m2) | 2.87* (1.4–5.8) |
| Age (<60 years) | 2.5* (1.2–5.1) |
| Gender | 1.2 (0.6–2.4) |
| Season (winter/summer) | 1.2 (0.6–7.5) |
Note:
P < 0.05, Chi-square test.
Abbreviations: BMI, body mass index; CI, confidence interval.
Table 4.
Vitamin D levels and diagnosis stratified by ethnicity, obesity, and age
| Black | White | |
|---|---|---|
| OSA | *13.2 ± 7.4 (43) | 22.7 ± 10.9 (75) |
| Non-OSA | *15.0 ± 9.1 (11) | 25.3 ± 12.4 (24) |
| BMI > 30 kg/m2 | BMI ≤ 30 kg/m2 | |
| OSA | 18.3 ± 10.3 (88) | 22.1 ± 11.6 (30) |
| Non-OSA | *15.8 ± 10.5 (16) | 27.3 ± 11.5 (19) |
| Age < 60 years | Age ≥ 60 years | |
| OSA | *16.4 ± 8.3 (80) | 25.2 ± 12.7 (38) |
| Non-OSA | *20.0 ± 11.4 (31) | 38.1 ± 6.6 (4) |
| Entire cohort | ||
| OSA | 19.2 ± 10.7 (118) | |
| Non-OSA | 22.1 ± 12.3 (35) |
Notes: Number of patients shown in parentheses; data are presented as the mean ± standard deviation; vitamin D levels are presented as ng/mL.
P < 0.05.
Abbreviations: BMI, body mass index; OSA, obstructive sleep apnea.
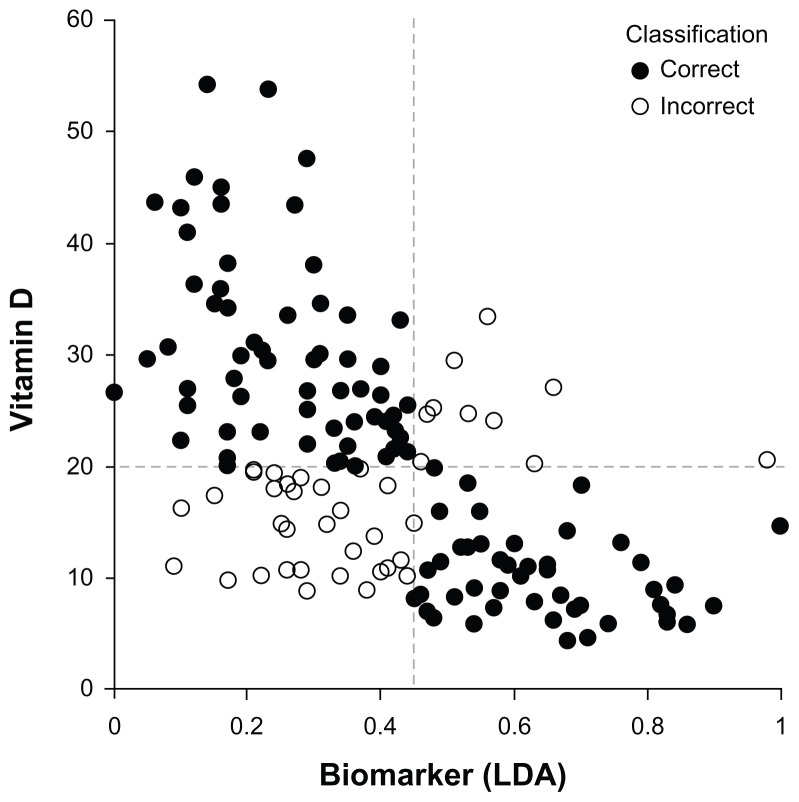
The biomarker function formed on the basis of the risk factors found to be associated with vitamin D deficiency was B(x) = 0.207x1 + 0.011x2 – 0.007x3 + 0.092, where x1 is race (1 = white/Hispanic, 2 = black), x2 is body mass index (kg/m2), and x3 is age (years). The AUROC was 0.78, and the mean AUROC for ten 90:10 cross-validation determinations was 0.75 ± 0.02. At a biomarker threshold value of 0.45, the sensitivity and specificity were 0.73 and 0.74, respectively. The performance of the biomarker function in classifying each patient in the cohort is shown in Figure 2.
Figure 2.
Correlation between vitamin D and biomarker by LDA based on age, race, and body mass index. Pearson correlation coefficient r = 0.505.
Abbreviation: LSA, linear discriminant analysis.
25-Hydroxyvitamin D levels were not significantly different between patients with and without obstructive sleep apnea. The biomarker function was found to be applicable for both groups (Table 4).
Discussion
Vitamin D is an ubiquitous metabolic regulator, and sleep is a physiologic process that can be impaired as a consequence of dysregulation of many different metabolic processes. We found that the presence of nonspecific functionally significant pain (revealed on direct questioning, but not necessarily mentioned a priori by the patient) is a reliable marker for the presence of low vitamin D in patients undergoing specialist evaluation for a suspected sleep disorder.
Although low 25-hydroxyvitamin D has been previously reported in patients with complaints of chronic pain, patients in the cohort described in our study were incidentally discovered to have pain symptoms during consultation for another reason. In some cases, pain symptoms were disclosed only after specific questioning. Nevertheless, the 25-hydroxyvitamin D values found mirror the low values reported in cohorts defined on the basis of pain as a chief complaint, suggesting that 25-hydroxyvitamin D may play a role in the pathophysiology of diseases typically classified as sleep disorders. One possibility is that chronic nonspecific musculoskeletal pain contributes to subjective sleep disturbances and/or a magnification of perceived daytime impairment, and both symptoms frequently lead to specialist evaluation for a suspected sleep disorder.
Vitamin D deficiency has the biologic potential to contribute mechanistically to obstructive sleep apnea via myopathy,16 facilitating development of chronic rhinitis,7,8 or contributing to tonsillar hypertrophy.10 In our cohort, mean 25-hydroxyvitamin D values were not significantly different between those with and without obstructive sleep apnea (P = 0.17), and the risk factors identified for vitamin D deficiency were generally valid for both groups (Table 4). We have previously described a patient presenting with a syndrome clinically indistinguishable from idiopathic central nervous system hypersomnia whose excessive daytime sleepiness symptoms resolved following treatment of severe vitamin D deficiency,17 and we have also recently reported a relationship between Epworth Sleepiness Scale scores and 25-hydroxyvitamin D levels.18 Taken together, the evidence supports the notion that the presence of nonspecific pain in a patient with a suspected sleep disorder may be predictive of vitamin D deficiency, a problem which could act as a cofactor for perceived symptoms of a broad array of sleep disorders.
Although the present evidence does not warrant routine testing of all sleep clinic patients for vitamin D deficiency, a biomarker that reliably predicts vitamin D deficiency might form a useful basis for selecting patients for testing, ultimately leading to improved diagnosis and treatment. In the present study, the vitamin D status of a considerable number of our patients (vitamin D deficiency present or absent) was correctly identified based solely on standard demographic data (Figure 2). The presence or absence of obstructive sleep apnea did not appreciably alter the predictive value of the biomarker (Table 4).
An unexpected finding in this cohort was that younger age appeared to confer a higher risk for vitamin D deficiency. Typically, older age is associated with decreased ability of the skin to produce active vitamin D. Our current data do not provide a satisfactory explanation for this paradox. Age-dependent sun exposure habits, use of supplements, and use of medications (such as anticonvulsants or steroids) are known to decrease circulating 25-hydroxyvitamin D, and may be responsible. It is also possible that the nonspecific physical symptoms associated with vitamin D deficiency prompt younger patients to seek specialist care for sleep disorders. Further research is needed to clarify the relative contribution of each of these factors.
It might be argued that a potential limitation of this study was the absence of a control group without chronic nonspecific musculoskeletal pain. Because our main purpose was to compare our cohort with a healthy adult population and with populations formed on the basis of musculoskeletal pain as a chief complaint, we consider that use of historical controls was reasonable and clinically relevant for comparison purposes. Notwithstanding the putative limitation of historical control groups, the consistency and size of the observed differences (Table 2) indicate that the vitamin D levels found in our study were unusually low. The reliability of this inference is further supported by our finding that two risk factors strongly associated with vitamin D deficiency, ie, race and obesity, were also associated with vitamin D deficiency in the cohort (Table 2).
Conclusion
Sleep clinic patients who incidentally disclosed chronic nonspecific musculoskeletal pain during specialist evaluation at our sleep medicine clinic had significantly lower serum levels of 25-hydroxyvitamin D and a higher prevalence of vitamin D deficiency than historical controls without pain, and had 25-hydroxyvitamin D levels and vitamin D deficiency rates similar to patient groups defined on the basis of chronic pain as a presenting complaint. The likelihood of vitamin D deficiency in this cohort was estimated reliably using a biomarker derived from common demographic data. Chronic nonspecific musculoskeletal pain may be a symptom of hypovitaminosis D.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. doi: 10.4065/81.3.353. [DOI] [PubMed] [Google Scholar]
- 3.Glerup H, Mikkelsen K, Poulsen L, et al. Hypovitaminosis D myopathy without biochemical signs of osteomalacic bone involvement. Calcif Tissue Int. 2000;66(6):419–424. doi: 10.1007/s002230010085. [DOI] [PubMed] [Google Scholar]
- 4.Heidari B, Shirvani JS, Firouzjahi A, Heidari P, Hajian-Tilaki KO. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010;13(4):340–346. doi: 10.1111/j.1756-185X.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 5.Holick MF. Vitamin D deficiency: what a pain it is. Mayo Clin Proc. 2003;78(12):1457–1459. doi: 10.4065/78.12.1457. [DOI] [PubMed] [Google Scholar]
- 6.Russell JA. Osteomalacic myopathy. Muscle Nerve. 1994;17(6):578–580. doi: 10.1002/mus.880170603. [DOI] [PubMed] [Google Scholar]
- 7.Wjst M, Hypponen E. Vitamin D serum levels and allergic rhinitis. Allergy. 2007;62(9):1085–1086. doi: 10.1111/j.1398-9995.2007.01437.x. [DOI] [PubMed] [Google Scholar]
- 8.Abuzeid W, Akbar N, Zacharek M. Vitamin D and chronic rhinitis. Curr Opin Allergy Clin Immunol. 2012;12(1):13–17. doi: 10.1097/ACI.0b013e32834eccdb. [DOI] [PubMed] [Google Scholar]
- 9.Grant WB. Tonsillectomy may be an indicator of low vitamin D status, a risk factor for cancer later in life. Cancer Causes Control. 2009;20(7):1235–1236. doi: 10.1007/s10552-009-9333-z. [DOI] [PubMed] [Google Scholar]
- 10.Reid D, Morton R, Salkeld L, Bartley J. Vitamin D and tonsil disease – preliminary observations. Int J Pediatr Otorhinolaryngol. 2011;75(2):261–264. doi: 10.1016/j.ijporl.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 11.McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin D deficiency. J Clin Sleep Med. 2010;6(6):605–608. [PMC free article] [PubMed] [Google Scholar]
- 12.Hoeck AD, Pall ML. Will vitamin D supplementation ameliorate diseases characterized by chronic inflammation and fatigue? Med Hypotheses. 2011;76(2):208–213. doi: 10.1016/j.mehy.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with persistent, nonspecific musculoskeletal pain. Mayo Clin Proc. 2003;78(12):1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 14.Krzanowkski WJ. Principles of Multivariate Analysis: A User’s Perspective Revised Edition. New York, NY: Oxford University Press; 2000. [Google Scholar]
- 15.Zhou X-H, Obuchowski NA, McClish DK. Statistical Methods in Diagnostic Medicine. New York, NY: Wiley & Sons; 2002. [Google Scholar]
- 16.Prabhala A, Garg R, Dandona P. Severe myopathy associated with vitamin D deficiency in western New York. Arch Intern Med. 2000;160(8):1199–1203. doi: 10.1001/archinte.160.8.1199. [DOI] [PubMed] [Google Scholar]
- 17.McCarty DE. Resolution of hypersomnia following identification and treatment of vitamin D deficiency. J Clin Sleep Med. 2010;6(6):605–608. [PMC free article] [PubMed] [Google Scholar]
- 18.McCarty DE, Reddy A, Keigley Q, Kim PY, Marino AA. Vitamin D, race, and excessive daytime sleepiness. J Clin Sleep Med. 2012;8(6):693–697. doi: 10.5664/jcsm.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed W, Khan N, Glueck CJ, et al. Low serum 25 (OH) vitamin D levels (<32 ng/mL) are associated with reversible myositis-myalgia in statin-treated patients. Transl Res. 2009;153(1):11–16. doi: 10.1016/j.trsl.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 20.Levis S, Gomez A, Jimenez C, et al. Vitamin D deficiency and seasonal variation in an adult South Florida population. J Clin Endocrinol Metab. 2005;90(3):1557–1562. doi: 10.1210/jc.2004-0746. [DOI] [PubMed] [Google Scholar]
- 21.Tangpricha V, Pearce EN, Chen TC, Holick MF. Vitamin D insufficiency among free-living healthy young adults. Am J Med. 2002;112(8):659–662. doi: 10.1016/s0002-9343(02)01091-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heidari B, Shirvani JS, Firouzjahi A, Heidari P, Hajian-Tilaki KO. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010;13(4):340–346. doi: 10.1111/j.1756-185X.2010.01561.x. [DOI] [PubMed] [Google Scholar]
- 23.Ginde AA, Liu MC, Camargo CA., Jr Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Arch Intern Med. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plotnikoff GA, Quigley JM. Prevalence of severe hypovitaminosis D in patients with presistent, nonspecific musculoskeletal pain. Mayo Clinic Proc. 2003;78(12):1463–1470. doi: 10.4065/78.12.1463. [DOI] [PubMed] [Google Scholar]
- 25.Turner MK, Hooten WM, Schmidt JE, Kerkvliet JL, Townsend CO, Bruce BK. Prevalence and clinical correlates of vitamin D inadequacy among patients with chronic pain. Pain Med. 2008;9(8):979–984. doi: 10.1111/j.1526-4637.2008.00415.x. [DOI] [PubMed] [Google Scholar]
- 26.Huisman AM, White KP, Algra A, et al. Vitamin D levels in women with systemic lupus erythematosus and fibromyalgia. J Rheumatol. 2001;28(11):2535–2539. [PubMed] [Google Scholar]
- 27.Knutsen KV, Brekke M, Gjelstad S, Lagerløv P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: a cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand J Prim Health Care. 2010;28(3):166–171. doi: 10.3109/02813432.2010.505407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McBeth J, Pye SR, O’Neill TW, et al. Musculoskeletal pain is associated with very low levels of vitamin D in men: results from the European Male Ageing Study. Ann Rheum Dis. 2010;69(8):1448–1452. doi: 10.1136/ard.2009.116053. [DOI] [PubMed] [Google Scholar]