Abstract
Background
Chitosan and chitosan derivatives have been proposed as alternative and biocompatible cationic polymers for nonviral gene delivery. However, the low transfection efficiency and low specificity of chitosan is an aspect of this approach that must be addressed prior to any clinical application. In the present study, folated poly(ethylene glycol)-chitosan-graft-polyethylenimine (FPCP) was investigated as a potential folate receptor-overexpressed cancer cell targeting gene carrier.
Methods
The FPCP copolymer was synthesized in two steps. In the first step, folate-PEG was synthesized by an amide formation reaction between the activated carboxyl groups of folic acid and the amine groups of bifunctional poly(ethylene glycol) (PEG). In the second step, FPCP was synthesized by an amide formation reaction between the activated carboxyl groups of folate-PEG and amine groups of CHI-g-polyethyleneimine (PEI). The composition of FPCP was characterized by 1H nuclear magnetic resonance.
Results:
FPCP showed low cytotoxicity in various cell lines, and FPCP-DNA complexes showed good cancer cell specificity as well as good transfection efficiency in the presence of serum. Further, FPCP-Pdcd4 complexes reduced tumor numbers and progression more effectively than PEI 25 kDa in H-ras12V liver cancer mice after intravenous administration.
Conclusion
Our data suggest that FPCP, which has improved transfection efficiency and cancer cell specificity, may be useful in gene therapy for liver cancer.
Keywords: liver cancer, targeted gene therapy, folated poly(ethylene glycol)-chitosan-graft-polyethylenimine, safety, efficiency
Introduction
Liver gene therapy is being developed as an alternative to orthotopic liver transplantation, which is an effective therapy for many liver diseases, including liver cancer.1 The naked genetic therapeutic method is vulnerable to enzymatic degradation, rapid clearance by renal filtration, and poor cellular uptake, as well as nontargeting, and successful gene therapy relies mainly on the development of safe and effective gene vector systems. Two key vectors, both viral and nonviral, have been utilized in gene therapy. While viral vectors have high transfection efficiency over a wide range of cell targets, they have major limitations, including inflammatory responses and oncogenic effects.2,3 Hence, there is a need for development of safe and efficient nonviral vectors as an alternative to viral vectors.
Of the nonviral vectors studied to date, chitosan and chitosan derivatives have been proposed as being suitable because of their cationic charge, biodegradability, and biocompatible properties.4,5 However, this system is significantly limited by the low transfection efficiency and low cell specificity of chitosans.6 To address these limitations, several ligands, such as chitosan modified by folate,6,7 transferrin,8 mannose,9,10 and galactose,11,12 have been designed and evaluated for receptor-mediated endocytotic gene delivery. Of these, folate chitosans have been reported as cancer cell-targeting gene carriers because of specific ligand-receptor interactions between folate moieties and the folate receptor, which are absent in most normal tissues at a high frequency, but are amplified in a variety of human cancers, including liver cancer.13,14
In a previous study, we prepared chitosan-graft-polyethyleneimine (CHI-g-PEI) as a gene carrier,15 which showed low cytotoxicity and high transfection efficiency, but also had limited cell specificity. Therefore, in this study, we prepared folate-PEG-chitosan-graft-low molecular weight PEI (FPCP) to achieve better cancer cell specificity. In general, PEG (poly[ethylene glycol]) facilitates the formation of polyplexes with improved solubility, diminished aggregation, lower cytotoxicity, and possibly decreased opsonization with serum proteins in the bloodstream.16 Therefore, we expected that surface modification of the polymer by PEG would enhance interactions the between polyplexes and target organs because of its hydrophilic properties. The physicochemical properties of FPCP-DNA complexes were analyzed, and their cytotoxicity, cancer cell specificity, and serum stability were also characterized. In vivo green fluorescent protein (GFP) gene expression and therapeutic efficiency using programmed cell death protein 4, a protein translation inhibitor that prevents tumorigenesis and tumor progression,17 were also investigated in liver tumor-bearing H-ras12V mice.
Materials and methods
Materials
Folic acid, chitosan (low molecular weight, deacetylation 85%), and branched PEI 25 kDa were obtained from Sigma-Aldrich (St Louis, MO, USA). A heterobifunctional PEG derivative (NH2-PEG-COOH, molecular weight 5000 Da, PA-050HC) was purchased from NOF Cooperation (Tokyo, Japan). Branched PEI 1800 Da was purchased from Wako (Osaka, Japan). A pGL3 expression vector (5.3 kb) containing a luciferase gene and driven by an SV40 promoter and enhancer was obtained from Promega (Madison, WI, USA). A pcDNA3.1/CT-GFP (6.1 kb) plasmid was purchased from Invitrogen (Carlsbad, CA, USA). Plasmids were propagated in Escherichia coli, extracted using the alkali lysis technique and purified using a QIAGEN kit (Chatsworth, CA, USA). All other chemicals were at least reagent grade. The programmed cell death protein 4 antibody was obtained from Cell Signaling Technology (Beverly, MA, USA). BAX, BAD, vascular endothelial growth factor, and actin antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
Preparation and characterization of FPCP copolymer
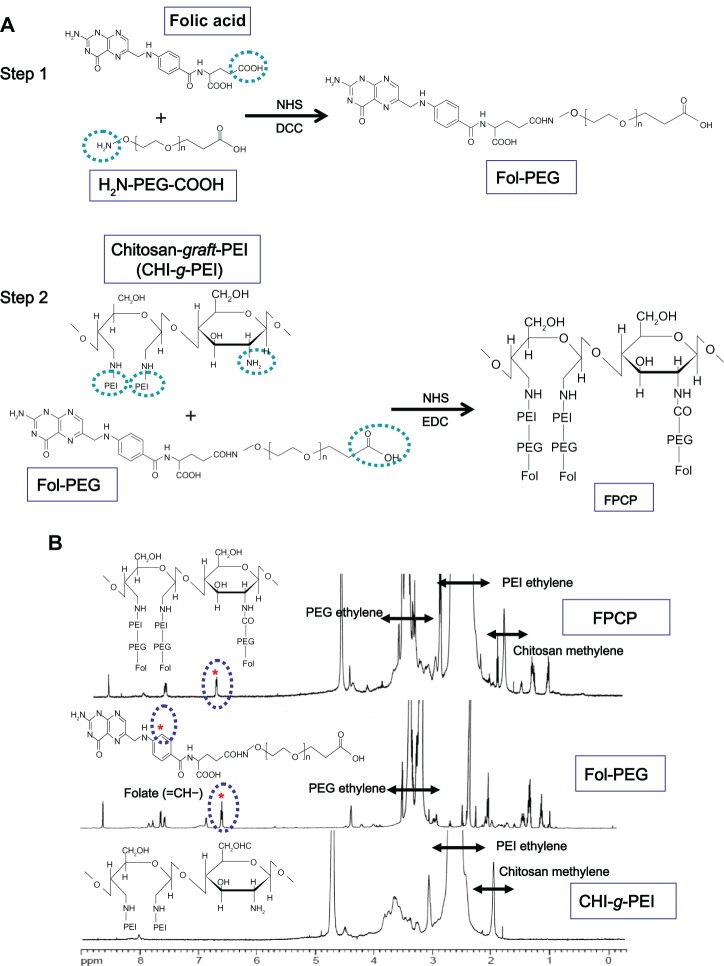
The FPCP copolymer was synthesized in two steps. In the first step, folate-PEG was synthesized by an amide formation reaction between the activated carboxyl groups of folic acid and the amine groups of bifunctional PEG.18 Briefly, folic acid (1.6 mmol) dissolved in 25 mL of dimethylsulfoxide was activated with a mixture of N-hydroxysuccinimide (3.2 mmol) and dicyclohexylcarbodiimide (3.2 mmol). Overnight activation of folic acid using excess dicyclohexyl-carbodiimide and N-hydroxysuccinimide in dimethylsulfoxide yielded dicyclohexyl urea. After activating the carboxyl groups, 0.2 mmol of PEG was added. The reaction was run for 24 hours at room temperature. The resulting product was purified by dialysis (molecular weight 3500) against dimethylsulfoxide for two days and distilled water for three days, followed by lyophilization. CHI-g-PEI was synthesized as previously described.15
In the second step, FPCP was synthesized via an amide formation reaction between the activated carboxyl groups of folate-PEG and the amine groups of CHI-g-PEI. Folate-PEG (0.1 mmol) dissolved in 10 mL of MES buffer solution (0.2 M, pH 5.5) was activated with a mixture of N-hydroxysuccinimide (0.2 mmol) and N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC, 0.2 mmol). After activating the carboxyl groups for 15 minutes, 1 mmol of CHI-g-PEI was added. The reaction was allowed to run 12 hours at 4°C, followed by an additional 12 hours at room temperature. The resulting product was purified by dialysis (molecular weight 12,000) against distilled water for two days at 4°C. The copolymer was then lyophilized. The reaction scheme is shown in Figure 1A. The composition of the prepared FPCP copolymer was estimated by measuring 1H nuclear magnetic resonance (1H NMR, AVANCE™ 600 FT-NMR, Bruker, Ettlingen, Germany).
Figure 1.
(A) Proposed reaction scheme for synthesis of FPCP. (B) Representative 1H nuclear magnetic resonance spectra for FPCP in D2O: δ = 6.8 ppm (=CH–, folic acid), δ = 3.6−3.1 ppm (–CH2CH2O–, PEG ethylene), δ = 2.8−2.2 ppm (–NHCH2CH2–, PEI ethylene), and δ = 2.0−1.9 ppm (–CH3, chitosan methylene).
Abbreviations: NHS, N-hydroxysuccinimide; DCC, dicyclohexylcarbodiimide; EDC, N-(3-dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride; PEI, polyethylenimine; FPCP, flolated poly(ethylene glycol)-chitosan-graft-polyethylenimine; Fol, folate; PEG, polyethylene glycol.
Preparation and characterization of FPCP-DNA complexes
The FPCP-DNA complexes were freshly prepared before use by combining equal volumes of a DNA solution and a FPCP copolymer solution, gently vortexing the solution, and incubating at room temperature for 30 minutes. The N/P of the FPCP-DNA complexes was expressed as the ratio of moles of amine groups in the copolymer to moles of phosphate groups in DNA.
Characterization of the FPCP-DNA complexes was performed using methods previously reported.15 Briefly, the DNA condensation and protection ability of the copolymer was confirmed by electrophoresis. DNA retardation was observed by irradiation with ultraviolet light and analyzed with Cam2com software. The morphology of the FPCP-DNA complexes was observed using energy-filtering transmission electron microscopy (LIBRA 120, Carl Zeiss, Oberkochen, Germany). A dynamic light scattering spectrophotometer (ELS8000, Otsuka Electronics, Osaka, Japan) at 90° and 20° scattering angles was used to measure the particle size and surface charge of the polyplexes at various weight ratios.
Cell lines, cell culture, and cell viability assays
HepG2 (human hepatoblastoma cells, ATCC, Manassas, VA, USA), A549 (human lung carcinoma, ATCC), and HeLa (human cervix epithelial carcinoma, ATCC) cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco-BRL, Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine serum, streptomycin 100 μg/mL, and penicillin 100 U/mL. KB (human epidermal carcinoma cells, ATCC) and WI-38 (human lung fibroblast cells, ATCC) were cultured in RPMI 1640 (Gibco-BRL, Invitrogen). KB cells overexpressing the folate receptor (KB/F) culture media were exchanged with RPMI 1640 without folate (Gibco BRL, 27016) for two weeks before transfection to induce overexpression of the folate receptor.18,19 Cells were incubated at 37°C in a humidified 5% CO2 atmosphere, and split using Trypsin/EDTA medium prior to reaching confluence.
In vitro cytotoxicity tests were performed using the Cell Titer 96® AQueous One Solution Cell Proliferation Assay (Promega).15 Briefly, HepG2, A549, HeLa, KB, and WI-38 cells were seeded in 96-well plates at an initial density of 1 × 104 cells/well in 0.2 mL of growth medium and incubated for 18 hours prior to addition of the filtered polymers. Growth medium was replaced by fresh serum-free medium containing various amounts of polymers. After incubation for a further 24 hours, the medium was exchanged for growth medium containing 20 μL of Cell Titer 96 AQueous One Solution Reagent. After further incubation for three hours, absorbance was measured at 570 nm using an enzyme-linked immunosorbent assay plate reader (GLR 1000, Genelabs Diagnostics, Singapore Science Park, Singapore).
Transfection efficiency, buffering capacity, competition, and serum effect
The in vitro transfection efficiency of FPCP was evaluated in KB/F cells. Cells were seeded in 24-well plates at an initial density of 1.5 × 105 cells/well in 1 mL of growth medium. After 18 hours of incubation (to reach 70% confluence at the time of transfection), the medium was replaced with serum-free (or containing 10% fetal bovine serum) medium plus polymer/pGL3 (1 μg) complexes at various N/P ratios (or at a functional N/P ratio), and the cultures were incubated for a further four hours. The medium was then exchanged for fresh medium containing 10% fetal bovine serum, and the cultures were incubated for a further 24 hours at 37°C. Luciferase assays were performed according to the protocol suggested by the manufacturer. Each transfection was performed in triplicate, and transfection efficiency was expressed in relative light units.
To determine the buffering capacity of FPCP, the cells were treated with bafilomycin A1, a specific inhibitor of vacuolar type H+ ATPase, during transfection. Next, 200 nM of bafilomycin A1 diluted in dimethylsulfoxide were put into 24-well plates. After 10 minutes of incubation, transfection solutions were added to the wells for four hours. The cells were then incubated in growth medium for 24 hours, and then checked by luciferase assay.
The competition studies were cross-checked by comparison of the transfection efficiencies for KB/F (cells overexpressing the folate receptor) and KB cells and by pretreatment with free folate (600 μM)20 as a competitor for 10 minutes before treatment with the polyplex.
In vivo transfection efficiency studies
The in vivo experiments were approved by the Animal Care and Use Committee at Seoul National University. Twenty-week-old male H-ras12V transgenic mice21 were maintained at 23°C ± 2°C and a relative humidity of 50% ± 20% on a 12-hour light-dark cycle.
FPCP transfection efficiency was determined by intravenous injection of 30 μg of GFP plasmid in 200 μL. The animals were sacrificed 48 hours after injection, and their livers were isolated and fixed in ice-cold 4% paraformaldehyde and sucrose solution. Tissue was fixed at room temperature and embedded in Tissue-Tek OCT (Sakura, Torrance, CA, USA). Tissue cryosections (10 μm) were cut with a microtome (Leica, Nussloch, Germany) and mounted on slides for analysis. The slides were evaluated for the GFP signal using a Zeiss LSM510 confocal microscope. The livers were collected and fixed in 10% neutral buffered formalin for histopathological examination. For histological analysis, liver sections were stained with hematoxylin and eosin.
In vivo therapeutic efficiency studies
The H-ras 12V mice with liver cancer were randomly divided into four treatment groups containing four mice each, ie, control, Pdcd4, FPCP-Pdcd4, and PEI 25 kDa-Pdcd4. The mice were intravenously injected with 30 μg of the therapeutic Pdcd4 gene twice per week for four weeks with or without the carrier. The same volume of phosphate-buffered solution was intravenously injected into mice in the control group. At the end of the experiment, the mice were sacrificed the livers were collected and the numbers and sizes of tumors on the surface were evaluated. The livers were homogenized using 2.5 × Passive Lysis Buffer (Promega), then centrifuged at 13,000 rpm and 4°C. The protein concentration from the homogenized liver samples was evaluated using the Bio-Rad Protein Assay reagent (Bio-Rad, Hercules, CA, USA). Western blotting was performed following a procedure described previously22 Bands were detected using a luminescent image analyzer LAS-3000 (Fujifilm, Tokyo, Japan) and quantified using Multi Gauge version 2.02 software (Fujifilm).
Statistical analysis
All values are presented as the mean ± standard deviation. The statistical significance of differences between the groups was determined using the unpaired t-test. P values < 0.05 were considered to be statistically significant, P < 0.01 was highly significant, and P < 0.001 was more significant when compared with corresponding values.
Results and discussion
Synthesis and characterization of FPCP copolymer
We were successful in synthesizing the FPCP copolymer, as shown in Figure 1A. The composition of the synthesized copolymer was analyzed by 1H NMR (Figure 1B). The chemical composition of the folate groups in FPCP was determined to be 5.3 mol% by assigning the protons of ethylene in PEG, PEI, and folic acid, respectively. The proton peaks of folate (=CH–) appeared at 6.8 ppm, PEG (–CH2–) at 3.6–3.1 ppm, PEI (–NHCH2CH–) at 3.3–2.5 ppm, and chitosan (–CH3–) at 2.0–1.8 ppm in the FPCP, indicating that folate-PEG was grafted to the CHI-g-PEI chain.
In a similar fashion, synthesis of non-folate targeted PEG-CHI-g-PEI was performed using N-hydroxysuccinimide and EDC as activating agents. Methoxy-PEG-sulfosuccinimide was activated using N-hydroxysuccinimide and EDC at room temperature and conjugated to CHI-g-PEI through samide linkage. The successful synthesis of PEG-CHI-g-PEI was also confirmed by 1H NMR for its composition (data not shown).
Characterization of FPCP-DNA complexes
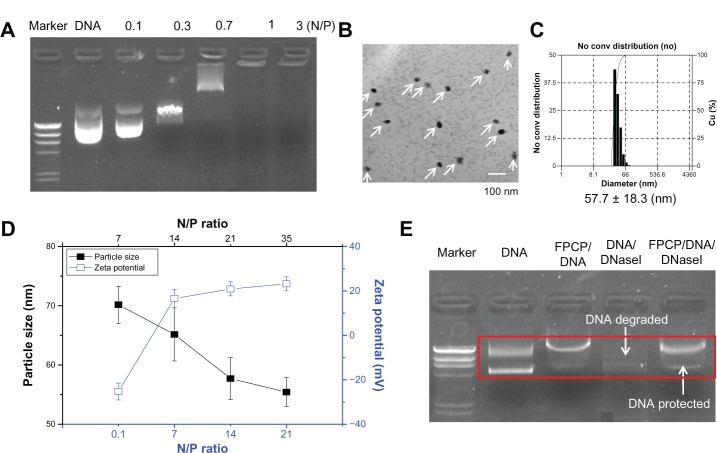
DNA condensation is one of the prerequisites for a successful polymeric gene carrier. Cationic polymers with high positive charge densities can bind negatively charged DNA effectively via electrostatic interactions.23 The ability of FPCP to condense with DNA was evaluated using an agarose gel electrophoresis shift assay. As shown in Figure 2A, migration of DNA was completely retarded when the N/P ratio was one. DNA bands disappeared as the N/P ratio increased, indicating that more stable DNA complexes were formed with increasing amounts of polymer. Figure 2B shows morphology representative of the FPCP-DNA complexes, indicating well formed spherical shapes with a compact structure. Particle size is a particularly important factor that influences the access and passage of complexes at the targeted site.12,15 As shown in Figure 2C, all complexes measured less than 75 nm, and the particle sizes tended to decrease with an increase in the N/P ratio (from 7 to 35). The relatively homogenous size distributions of the complexes, as measured by dynamic light scattering, were unimodal (Figure 2D). A positive surface charge is necessary for binding to anionic cell surfaces, which in turn facilitates uptake into the cell.24 As shown in Figure 2C, the zeta potentials of the FPCP-DNA complexes rapidly increased to positive values with increasing N/P ratios. FPCP also protected DNA from degradation by the DNase I enzyme (Figure 2E). Together, these results suggest that FPCP can efficiently transfer DNA to cells after complexation.
Figure 2.
Characterizations of FPCP-DNA complexes. (A) Agarose gel electrophoresis of FPCP-DNA complexes at various N/P ratios. (B) Energy-filtering transmission electron microscopy images of FPCP-DNA complexes at an N/P ratio of 21. Scale bar 100 nm. (C) Size distribution of complexes prepared at an N/P ratio of 21 (n = 3, error bars represent standard deviation). (D) Particle sizes and charges on copolymer-DNA complexes at various N/P ratios. (E) Protection and release assay.
Notes: Two units of DNase I or phosphate-buffered solution were added to the polyplex solution with an N/P ratio of 5 or to 0.1 μg of naked plasmid DNA, and inactivated with 4 μL of EDTA (250 mM). After treatment with 1% sodium dodecyl sulfate, the final samples were incubated for 2 hours, and electrophoresis was performed in 1% agarose gel with TAE running buffer for one hour at 50 V.
Abbreviation: FPCP, folated poly(ethylene glycol)-chitosan-graft-polyethylenimine; N/P, the ratio of moles of the amine groups of polymer to moles of phosphates of DNA; conv, conversion; Cu (%), accumulation (%).
Cytotoxicity of FPCP
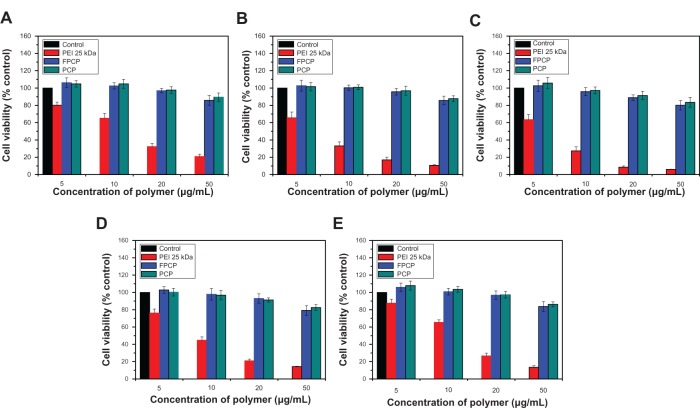
The optimal polycationic polymer for a gene delivery carrier should combine high transfection efficiency with low cytotoxicity. To investigate the cytotoxicity of the FPCP copolymer, cell viability was assayed at various concentrations of the copolymer using KB, A549, HepG2, HeLa, and WI-38 cell lines (Figure 3A–E). It has already been reported that free polymers are more toxic to cells compared with the polyplexes prepared from them.25 The viability of control cells decreased dramatically with increasing concentrations of PEI 25 kDa, and their relative viability was less than 20% at a polymer concentration of 50 μg/mL. Further, the FPCP copolymer exhibited low levels of cytotoxicity, with approximately 80% of cells being viable up to a polymer concentration of 50 μg/mL in the five different cell lines, indicating that the FPCP polymer is minimally toxic in gene delivery.
Figure 3.
Cytotoxicity of the FPCP copolymer at various concentrations in different cell lines. (A) KB, (B) A549, (C) HepG2, (D) HeLa, and (E) WI-38.
Note: n = 3, error bars represent standard deviation.
Abbreviations: PEI, polyethylenimine; FPCP, folated poly(ethylene glycol)-chitosan-graft-polyethylenimine; PCP, poly(ethylene glycol)-chitosan-graft-polyethylenimine; kDa, kilodalton.
Although PEI is one of the most widely used gene carriers because of its efficient proton sponge effect within sendosomes, its use has been limited because of its in vitro as well as in vivo cytotoxicity.26 The cytotoxicity of PEI is dependent on its molecular weight, with a lower molecular weight PEI having less cytotoxicity.24 On the other hand, the lower cytotoxicity of CHI-g-PEI compared with PEI 25 kDa has already been demonstrated in a previous study.15 PEG is known to help reduce the cytotoxicity of PEI by reducing the number of PEI amino groups.27 Cationic polymers with high charge densities have strong lytic and toxic properties, and a reduction in charge density is reported to result in less cytotoxicity.28 The grafted PEG reduces the surface charge of the polyplexes as a result of the brush effect, and diminished aggregation results in reduce cytotoxicity. Further, Luo et al recently reported that PEGylation succeeded in reducing the zeta potential of the complexes, leading to reduced cytotoxicity.29 Finally, it is reasonable to assume that FPCP was less cytotoxic than PEI 25 kDa because of the hydrophilic PEG groups and the minimal cytotoxicity of the CHI-g-PEI groups. In our study, we confirmed that cell viability was not affected by the folate moiety after covalent linkage with PEG (Figure 3A–E).
Transfection efficiency in vitro
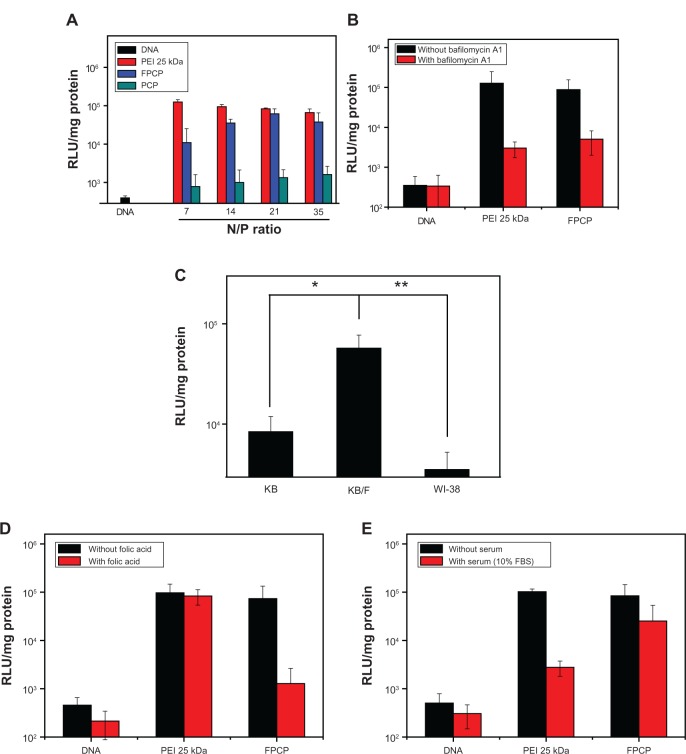
Luciferase activity was measured in a transfected KB/F cell line to investigate the transduction efficiency of the FPCP copolymer. To determine the optimal N/P ratio of FPCP-DNA complexes for transfection, KB/F cells were transfected with complexes prepared at different N/P ratios, as shown in Figure 4A. The transfection efficiency of the copolymer increased with an increasing N/P ratio, whereas the transfection efficiency of PEI 25 kDa alone was decreased because of the enhanced cytotoxicity. The maximal transfection efficiency of FPCP-DNA was observed at an N/P ratio of 21, and tended to decrease with further increases of the N/P ratio. As shown in Figure 4B, the transfection efficiency of the FPCP-DNA complexes was markedly decreased after treatment with bafilomycin A1, likewise with PEI 25 kDa, suggesting involvement of the proton sponge effect in PEI-mediated transfection. The transfection efficiency of the FPCP-DNA complexes in KB/F, KB, and WI-38 cells was also compared in order to demonstrate the effect of folate on receptor-mediated gene transfer, as shown in Figure 4C. The FPCP-DNA complexes showed significantly greater transfection efficiency compared with KB/F (overexpressing folate receptors), but not KB (not overexpressing folate receptors) cells as well as normal WI-38 (human lung fibroblast) cells, indicating that the folate ligand on FPCP plays a significant role in folate receptor recognition and enhanced transfection efficiency in KB/F cells. The transfection efficiency of FPCP/DNA complexes was greatly reduced in the presence of an excess of free folate (600 μM), indicating that the FPCP-DNA complexes were absorbed via receptor-mediated endocytosis (Figure 4D).
Figure 4.
(A) Transfection efficiency of FPCP-DNA complexes in KB/F cells. (B) Effect of bafilomycin A1 on gene transfection. (C) Competitive assay using KB/F cells (overexpressing folate receptors), KB cells (not overexpressing folate receptors) and normal WI-38 (human lung fibroblast) cells using the luciferase assay. (D) Competitive assay for FPCP-DNA complexes prepared at a charge ratio of 21 in the presence of folate (600 μM) and the functional folate group of FPCP for folate receptor sites (n = 3, error bars represent standard deviation). (E) Effect of serum on gene transfection efficiency.
Notes: KB/F cells were incubated in the absence or presence of 10% serum with the copolymer-DNA complex (n = 3, error bars represent standard deviation).
Abbreviations: PEI, polyethylenimine; FPCP, folated poly(ethylene glycol)-chitosan-graft-polyethylenimine; PCP, poly(ethylene glycol)-chitosan-graft-polyethylenimine; kDa, kilodalton; RLU, relative light unit; FBS, fetal bovine serum.
It has been reported that one of the practical problems for in vivo gene delivery is that gene expression is inhibited by serum; therefore, development of gene delivery systems that are stable even in serum is very important for improvement of gene therapy using nonviral vectors.30 We investigated the effect of serum on the transfection efficiency of the FPCP-DNA complexes. As shown in Figure 4E, the transfection efficiency of the PEI-DNA complexes was markedly decreased in the presence of 10% serum, whereas that of the FPCP-DNA complexes did not change substantially. It is well documented that PEGylation effectively decreases the polyplex surface charge, and that salt-induced or serum-induced aggregation extends lifetime in the circulation.31,32 In addition, the transfection efficiency of the chitosan-plasmid complex did not change substantially in the presence of serum.33 Therefore, it is thought that the minimal change in transfection efficiency of the FPCP-DNA complexes in the presence of serum is due to the hydrophilic group of PEG and chitosan.
Tumor targeting and therapeutic studies in vivo
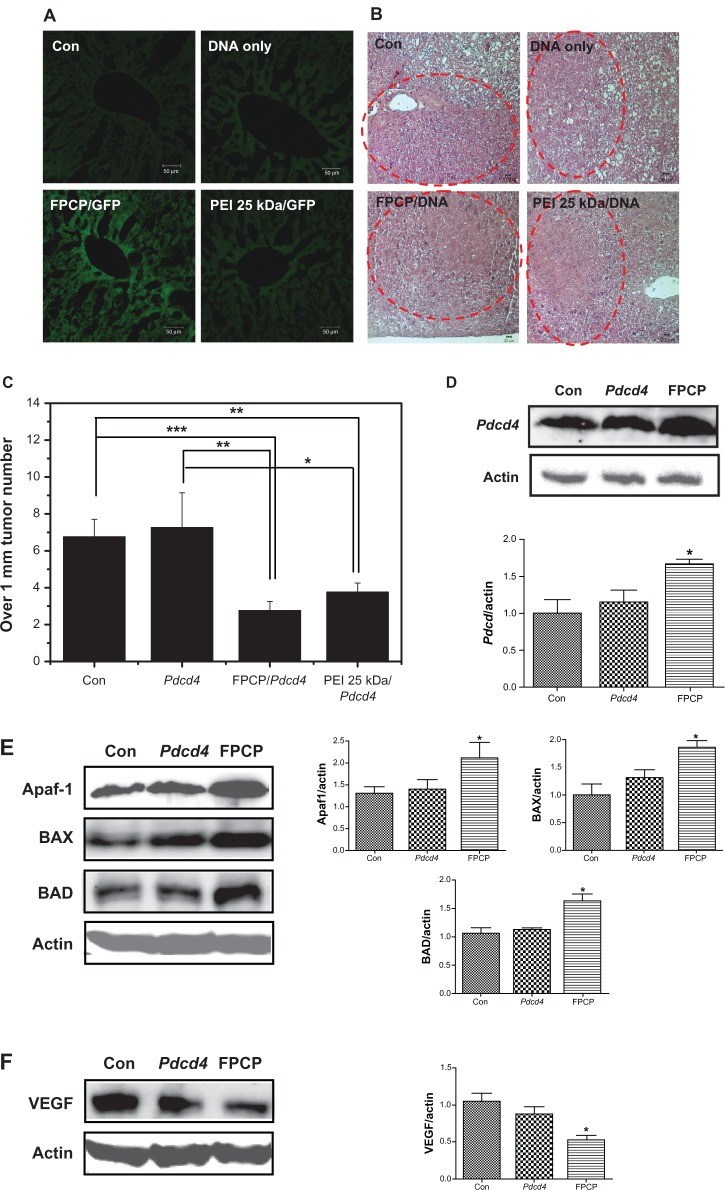
The liver is an attractive target tissue for gene therapy because of its large size and metabolic capacity. The liver is also an attractive organ for in vivo gene therapy because hepatocytes are readily accessible via the blood stream.1 To confirm whether FPCP was a more efficient gene carrier for targeting cancer cells in vivo compared with PEI 25 kDa, GFP expression in H-ras12V mice with liver cancer was observed after intravenous administration of GFP only, PEI-GFP, and FPCP-GFP complexes, as shown in Figure 5A. The FPCP-GFP complexes showed high levels of GFP expression in liver cancer tissues compared with GFP only and PEI-GFP, indicating that it may be an efficient gene carrier for targeting liver cancer. As shown in Figure 5B, the hematoxylin and eosin staining data clearly show the tumor regions in liver tissue from an H-ras12V mouse liver cancer model.
Figure 5.
In vivo transfection efficiency studies(A and B): (A) In vivo green fluorescent protein expression analysis in H-ras12V mice following intravenous administration (scale bar = 50 μm). FPCP/GFP could target to tumor tissue in vivo and highly expressed green fluorescent protein in liver tumor. (B) Observation of tumor tissue by liver histopathology study(hematoxylin and eosin staining; scale bar = 20 μm). Forming the tumor tissue was confirmed by H&E staining in every groups. In vivo therapeautic efficiency studies(C–F): (C) Tumor size over 1 mm, tumor numbers (n = 4, *P < 0.05; **P < 0.01; ***P < 0.001). Intravenous injection of FPCP-Pdcd4 significantly inhibited the number of liver tumors. (D) Western blot analysis of programmed cell death protein 4 protein expression in the liver (left). Bands of interest were further analyzed by densitometry (right). (E) Western blot analysis of apoptosis-regulating proteins, Apaf-1, BAX, and BAD (left), and bands of interest were further analyzed by densitometry (right). (F) Western blot analysis of VEGF (left), and bands of interest were further analyzed by densitometry (right, n = 4, *P < 0.05).
Abbreviations: PEI, polyethylenimine; FPCP, folated poly(ethylene glycol)-chitosan-graft-polyethylenimine; Con, controls; GFP, green fluorescent protein; VEGF, vascular endothelial growth factor; BAX, Bcl-2-associated X protein; BAD, Bcl-2-associated death promoter; Apaf-1, apoptotic peptidase activating factor 1; Pdcd4, programmed cell death 4; kDa, kilodalton.
Programmed cell death 4, also known as apoptosis, plays a fundamental role in many biological processes, including embryogenesis, normal turnover, and immune homeostasis.34 Therefore, the Pdcd4 gene was used in this study as a therapeutic gene to confirm further the efficiency of FPCP. As shown in Figure 5C, tumor numbers in the livers of H-ras12V mice showed a significant decrease in FPCP-Pdcd4 compared with nontreated controls and groups treated with Pdcd4 only. However, there was no significant difference in tumor numbers between FPCP-Pdcd4 and PEI 25 kDa-Pdcd4. Protein levels in total liver lysates were then evaluated by Western blot analysis. Significant overexpression of programmed cell death protein 4 in the group injected with FPCP-Pdcd4 was observed in comparison with that seen in the nontreated control group and the group treated with Pdcd4 only, indicating that FPCP has the potential to be an efficient gene carrier for targeting liver cancer (Figure 5D). Also, as shown in Figure 5E and F, the apoptosis-regulating proteins, including Apaf-1, BAX, and BAD, showed significant increases, whereas angiogenesis markers, such as vascular endothelial growth factor, showed significant decreases in the FPCP-Pdcd4 group compared with the group treated with Pdcd4 only. These results support the likelihood of FPCP-Pdcd4 having a role in tumor regression.
Conclusion
We successfully prepared and evaluated a novel FPCP copolymer as a new liver cancer targeted gene carrier. FPCP has an enhanced ability to form complexes with DNA and has physicochemical properties suitable for use as a gene delivery system. This copolymer had little cytotoxicity and showed high tumor cell specificity in vitro as well as in vivo when compared with PEI 25 kDa. Therefore, we conclude that FPCP has the potential to be a safe and efficient gene carrier.
Acknowledgments
HLJ was supported by the Specially Appointed Professors in Jiangsu Province Programs. Part of this work was supported by a grant from the Korea Research Foundation (2012M3A9C4048819) under the auspices of the Ministry of Education, Science and Technology. MHC was also partially supported by the Research Institute for Veterinary Science, Seoul National University.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Nguyen TH, Ferry N. Liver gene therapy: advances and hurdles. Gene Ther. 2004;11(Suppl 1):S76–S84. doi: 10.1038/sj.gt.3302373. [DOI] [PubMed] [Google Scholar]
- 2.Huang PI, Lo WL, Cherng JY, Chien Y, Chiou GY, Chiou SH. Nonviral delivery of RNA interference targeting cancer cells in cancer gene therapy. Curr Gene Ther. 2012;12(4):275–284. doi: 10.2174/156652312802083576. [DOI] [PubMed] [Google Scholar]
- 3.Rekha MR, Sharma CP. Polymers for gene delivery: current status and future perspectives. Recent Pat DNA Gene Seq. 2012;6(2):98–107. doi: 10.2174/187221512801327389. [DOI] [PubMed] [Google Scholar]
- 4.Saranya N, Moorthi A, Saravanan S, Devi MP, Selvamurugan N. Chitosan and its derivatives for gene delivery. Int J Biol Macromol. 2011;48(2):234–238. doi: 10.1016/j.ijbiomac.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 5.Mao S, Sun W, Kissel T. Chitosan-based formulations for delivery of DNA and siRNA. Adv Drug Deliv Rev. 2010;62(1):12–27. doi: 10.1016/j.addr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 6.Mansouri S, Cuie Y, Winnik F, et al. Characterization of folate-chitosan-DNA nanoparticles for gene therapy. Biomaterials. 2006;27(9):2060–2065. doi: 10.1016/j.biomaterials.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 7.Chan P, Kurisawa M, Chung JE, Yang YY. Synthesis and characterization of chitosan-g-poly(ethylene glycol)-folate as a non-viral carrier for tumor-targeted gene delivery. Biomaterials. 2007;28(3):540–549. doi: 10.1016/j.biomaterials.2006.08.046. [DOI] [PubMed] [Google Scholar]
- 8.Mao HQ, Roy K, Troung-Le VL, et al. Chitosan-DNA nanoparticles as gene carriers: synthesis, characterization and transfection efficiency. J Control Release. 2001;70(3):399–421. doi: 10.1016/s0168-3659(00)00361-8. [DOI] [PubMed] [Google Scholar]
- 9.Kim TH, Jin H, Kim HW, Cho MH, Cho CS. Mannosylated chitosan nanoparticle-based cytokine gene therapy suppressed cancer growth in BALB/c mice bearing CT-26 carcinoma cells. Mol Cancer Ther. 2006;5(7):1723–1732. doi: 10.1158/1535-7163.MCT-05-0540. [DOI] [PubMed] [Google Scholar]
- 10.Jiang HL, Kim YK, Arote R, et al. Mannosylated chitosan-graft-polyethylenimine as a gene carrier for Raw 264.7 cell targeting. Int J Pharm. 2009;375(1–2):133–139. doi: 10.1016/j.ijpharm.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 11.Kim TH, Park IK, Nah JW, Choi YJ, Cho CS. Galactosylated chitosan/DNA nanoparticles prepared using water-soluble chitosan as a gene carrier. Biomaterials. 2004;25(17):3783–3792. doi: 10.1016/j.biomaterials.2003.10.063. [DOI] [PubMed] [Google Scholar]
- 12.Jiang HL, Kwon JT, Kim EM, et al. Galactosylated poly(ethylene glycol)-chitosan-graft-polyethylenimine as a gene carrier for hepatocyte-targeting. J Control Release. 2008;131(2):150–157. doi: 10.1016/j.jconrel.2008.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Zhao XB, Lee RJ. Tumor-selective targeted delivery of genes and antisense oligodeoxyribonucleotides via the folate receptor. Adv Drug Deliv Rev. 2004;56(8):1193–1204. doi: 10.1016/j.addr.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Hong G, Yuan R, Liang B, Shen J, Yang X, Shuai X. Folate-functionalized polymeric micelle as hepatic carcinoma-targeted, MRI-ultrasensitive delivery system of antitumor drugs. Biomed Microdevices. 2008;10(5):693–700. doi: 10.1007/s10544-008-9180-9. [DOI] [PubMed] [Google Scholar]
- 15.Jiang HL, Kim YK, Arote R, et al. Chitosan-graft-polyethylenimine as a gene carrier. J Control Release. 2007;117(2):273–280. doi: 10.1016/j.jconrel.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Kim EM, Jeong HJ, Park IK, et al. Asialoglycoprotein receptor targeted gene delivery using galactosylated polyethylenimine-graft-poly(ethylene glycol): in vitro and in vivo studies. J Control Release. 2005;108(2–3):557–567. doi: 10.1016/j.jconrel.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 17.Jin H, Kim TH, Hwang SK, et al. Aerosol delivery of urocanic acid-modified chitosan/programmed cell death 4 complex regulated apoptosis, cell cycle, and angiogenesis in lungs of K-ras null mice. Mol Cancer Ther. 2006;5(4):1041–1049. doi: 10.1158/1535-7163.MCT-05-0433. [DOI] [PubMed] [Google Scholar]
- 18.Kim YK, Choi JY, Yoo MK, et al. Receptor-mediated gene delivery by folate-PEG-baculovirus in vitro. J Biotechnol. 2007;131(3):353–361. doi: 10.1016/j.jbiotec.2007.07.938. [DOI] [PubMed] [Google Scholar]
- 19.McHugh M, Cheng YC. Demonstration of a high affinity folate binder in human cell membranes and its characterization in cultured human KB cells. J Biol Chem. 1979;254(22):11312–11318. [PubMed] [Google Scholar]
- 20.Arote RB, Hwang SK, Lim HT, et al. The therapeutic efficiency of FP-PEA/TAM67 gene complexes via folate receptor-mediated endocytosis in a xenograft mice model. Biomaterials. 2010;31(8):2435–2445. doi: 10.1016/j.biomaterials.2009.11.106. [DOI] [PubMed] [Google Scholar]
- 21.Wang AG, Moon HB, Lee MR, et al. Gender-dependent hepatic alterations in H-ras12V transgenic mice. J Hepatol. 2005;43(5):836–844. doi: 10.1016/j.jhep.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 22.Hwang SK, Jin H, Kwon JT, et al. Aerosol-delivered programmed cell death 4 enhanced apoptosis, controlled cell cycle and suppressed AP-1 activity in the lungs of AP-1 luciferase reporter mice. Gene Ther. 2007;14(18):1353–1361. doi: 10.1038/sj.gt.3302983. [DOI] [PubMed] [Google Scholar]
- 23.De Smedt SC, Demeester J, Hennink WE. Cationic polymer based gene delivery systems. Pharm Res. 2000;17(2):113–126. doi: 10.1023/a:1007548826495. [DOI] [PubMed] [Google Scholar]
- 24.Kunath K, von Harpe A, Fischer D, et al. Low-molecular-weight polyethylenimine as a non-viral vector for DNA delivery: comparison of physicochemical properties, transfection efficiency and in vivo distribution with high-molecular-weight polyethylenimine. J Control Release. 2003;89(1):113–125. doi: 10.1016/s0168-3659(03)00076-2. [DOI] [PubMed] [Google Scholar]
- 25.Dubruel P, Dekie L, Christiaens B, et al. Poly-l-glutamic acid derivatives as multifunctional vectors for gene delivery. Part B. Biological evaluation. Biomacromolecules. 2003;4(5):1177–1183. doi: 10.1021/bm034015b. [DOI] [PubMed] [Google Scholar]
- 26.Namgung R, Kim J, Singha K, Kim CH, Kim WJ. Synergistic effect of low cytotoxic linear polyethylenimine and multiarm polyethylene glycol: study of physicochemical properties and in vitro gene transfection. Mol Pharm. 2009;6(6):1826–1735. doi: 10.1021/mp900096u. [DOI] [PubMed] [Google Scholar]
- 27.Zhang X, Pan SR, Hu HM, et al. Poly(ethylene glycol)-block-polyethylenimine copolymers as carriers for gene delivery: effects of PEG molecular weight and PEGylation degree. J Biomed Mater Res A. 2008;84(3):795–804. doi: 10.1002/jbm.a.31343. [DOI] [PubMed] [Google Scholar]
- 28.Schipper NG, Varum KM, Artursson P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm Res. 1996;13(11):1686–1692. doi: 10.1023/a:1016444808000. [DOI] [PubMed] [Google Scholar]
- 29.Luo X, Feng M, Pan S, Wen Y, Zhang W, Wu C. Charge shielding effects on gene delivery of polyethylenimine/DNA complexes: PEGylation and phospholipid coating. J Mater Sci Mater Med. 2012;23(7):1685–1695. doi: 10.1007/s10856-012-4632-4. [DOI] [PubMed] [Google Scholar]
- 30.Goldman CK, Soroceanu L, Smith N, et al. In vitro and in vivo gene delivery mediated by a synthetic polycationic amino polymer. Nat Biotechnol. 1997;15(5):462–466. doi: 10.1038/nbt0597-462. [DOI] [PubMed] [Google Scholar]
- 31.Ogris M, Brunner S, Schuller S, Kircheis R, Wagner E. PEGylated DNA/transferrin-PEI complexes: reduced interaction with blood components, extended circulation in blood and potential for systemic gene delivery. Gene Ther. 1999;6(4):595–605. doi: 10.1038/sj.gt.3300900. [DOI] [PubMed] [Google Scholar]
- 32.Vader P, van der Aa LJ, Engbersen JF, Storm G, Schiffelers RM. Physicochemical and biological evaluation of siRNA polyplexes based on PEGylated poly(amido amine)s. Pharm Res. 2012;29(2):352–361. doi: 10.1007/s11095-011-0545-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erbacher P, Zou S, Bettinger T, Steffan AM, Remy JS. Chitosan-based vector/DNA complexes for gene delivery: biophysical characteristics and transfection ability. Pharm Res. 1998;15(9):1332–1339. doi: 10.1023/a:1011981000671. [DOI] [PubMed] [Google Scholar]
- 34.Saikumar P, Dong Z, Mikhailov V, Denton M, Weinberg JM, Venkatachalam MA. Apoptosis: definition, mechanisms, and relevance to disease. Am J Med. 1999;107(5):489–506. doi: 10.1016/s0002-9343(99)00259-4. [DOI] [PubMed] [Google Scholar]