New discoveries reveal crucial roles for the Dectin-2 family in many aspects of the immune response.
Keywords: C-type lectin-like receptor, carbohydrate recognition domain, ITAM, ITIM, pattern recognition receptor
Abstract
Myeloid and non-myeloid cells express members of the C-type lectin-like receptor (CTLR) family, which mediate crucial cellular functions during immunity and homeostasis. Of relevance here is the dendritic cell-associated C-type lectin-2 (Dectin-2) family of CTLRs, which includes blood dendritic cell antigen 2 (BDCA-2), dendritic cell immunoactivating receptor (DCAR), dendritic cell immunoreceptor (DCIR), Dectin-2, C-type lectin superfamily 8 (CLECSF8) and macrophage-inducible C-type lectin (Mincle). These CTLRs possess a single extracellular conserved C-type lectin-like domain and are capable of mediating intracellular signalling either directly, through integral signalling domains, or indirectly, by associating with signalling adaptor molecules. These receptors recognize a diverse range of endogenous and exogenous ligands, and can function as pattern recognition receptors for several classes of pathogens including fungi, bacteria and parasites, driving both innate and adaptive immunity. In this review, we summarize our knowledge of each of these receptors, highlighting the exciting discoveries that have been made in recent years.
Introduction
C-type lectin-like receptors (CTLRs) constitute a group of over 1000 receptors found in either membrane bound or soluble forms (1). These receptors are characterized by the presence of at least one carbohydrate recognition domain (CRD), or in the broader sense, a C-type lectin-like domain (CTLD), which are formed by disulphide bonds between highly conserved cysteine residues (1). Initially named ‘C-type’ due to their requirement for Ca2+ to bind carbohydrates, it was subsequently discovered that not all CTLRs are Ca2+ dependent and that these receptors can recognize a more diverse range of ligands. CTLRs are often also multivalent (able to bind different classes of ligands) and recognize both exogenous and endogenous ligands. Importantly, many of these receptors can mediate cellular responses by triggering intracellular signalling pathways either directly, through integral signalling domains, or indirectly, by associating with signalling adaptor molecules.
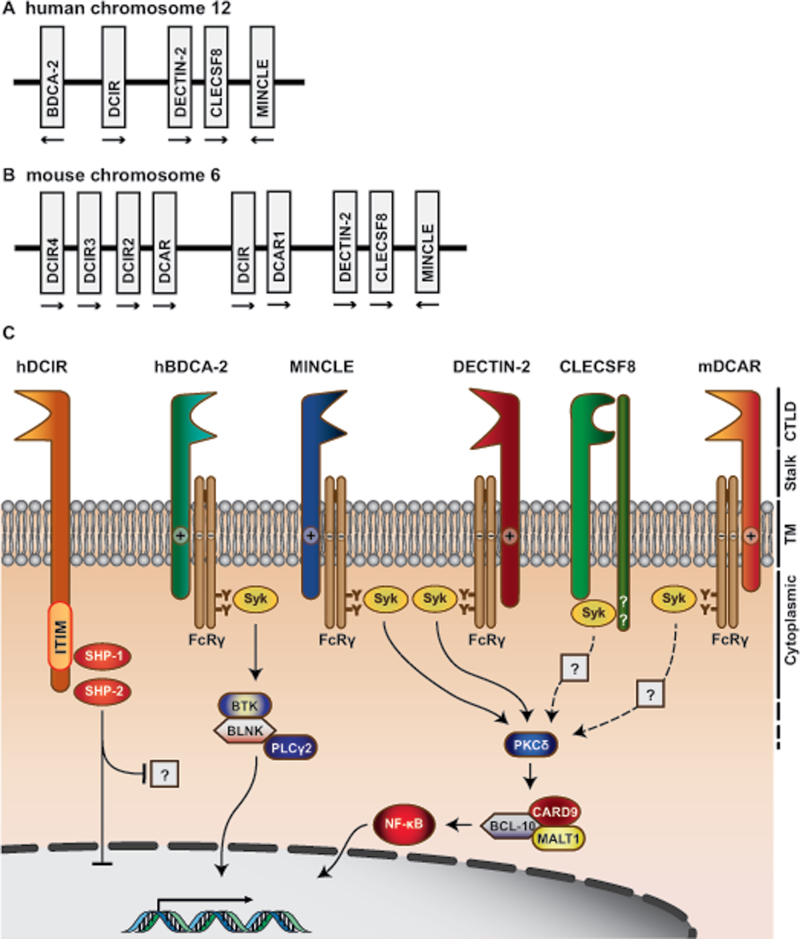
Here, we focus on the dendritic cell-associated C-type lectin-2 (Dectin-2) family of CTLRs, which comprises blood dendritic cell antigen 2 (BDCA-2), dendritic cell immunoactivating receptor (DCAR), dendritic cell immunoreceptor (DCIR), Dectin-2, C-type lectin superfamily 8 (CLECSF8) and macrophage-inducible C-type lectin (Mincle) (2) (Fig. 1). The genes encoding these receptors are grouped on the telomeric region of the NK gene cluster on mouse chromosome 6 and on human chromosome 12. These CTLRs are type II transmembrane receptors possessing a single extracellular conserved CTLD and all members of this family have short cytoplasmic tails and induce intracellular signalling indirectly, except for DCIR that has a longer cytoplasmic tail containing an integral inhibitory signalling motif. In this review, we provide an update on each of these CTLRs (2), focusing on the most recent and exciting discoveries relating to their expression, ligands, signalling and physiological roles.
Fig. 1.
The Dectin-2 cluster of C-type lectins. The Dectin-2 family of C-type lectin receptors are encoded at the telomeric end of the NK gene cluster on human chromosome 12 (A) and mouse chromosome 6 (B). (C) Schematic representation of signal transduction pathways induced by the various Dectin-2 family CTLRs. BLNK, B-cell linker protein; BTK, Bruton’s tyrosine kinase; TM, transmembrane.
Blood dendritic cell antigen 2
BDCA-2 is widely accepted as a highly specific marker for human plasmacytoid dendritic cells (pDCs), although a murine homologue has not been defined (3). Lacking signalling motifs in its cytoplasmic tail, human BDCA-2 (hBDCA-2) associates through its transmembrane domain with the immunoreceptor tyrosine-based activation motif (ITAM)-containing signalling adaptor, FcRγ, and can induce intracellular signal transduction in a B-cell receptor (BCR)-like fashion employing Syk and other downstream signalling molecules (4–6) (Fig. 1). Importantly, unlike many other ITAM-coupled receptors, signalling through BDCA-2 does not lead to activation of the nuclear factor (NF)-κB pathway. In fact, cross-linking of BDCA-2 on pDCs inhibits activation of the NF-κB pathway and the production of type I interferons and other cytokines, notably in response to TLR9 ligands (4–6) (Table 1). More recently, BDCA-2 signalling has also been shown to inhibit the pDC secretion of tumour necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) (7).
Table 1.
C-type lectin receptor ligands and signallinga
| Receptor | Alternative names | Ligands | Cellular effect upon triggering |
|---|---|---|---|
| hBDCA-2 | CLEC4C, CLECSF7, HECL, DLEC, CD303, CLECSF1, CLECSF11 | HIV-1 gp120; asialo-galactosyl-oligosaccharides with terminal β1–3- and β1–4-galactose; HCV E2 | ↓IFN; ↓TNF; ↓IL-6; ↑IL-10; ↑Ca2+ influx |
| mDCAR, mDCAR1 | CLEC4b1, CLEC4b2 | Unknown | ↓IL-10; ↑IL-12 |
| hDCIR, mDCIR1–4 | hCLEC4A, hCLECSF6, mCLEC4a1–4 | HIV-1 (gp120); HCV (E2); unknown | ↓IFN; ↓TNF; ↓IL-12; ↓Ca2+ influx; ↓Protein tyrosine phosphorylation; ↓DC expansion; cross-presentation |
| mDECTIN-2 | CLEC4n | α-mannans; M. tuberculosis; house dust mite; Fungi (e.g. Candida); CD4+ CD25+ T-cell ligand | ↑TNF; ↑IL-1; ↑IL-6; ↑IL-23; ↑Cysteinyl leukotrienes; ↑ROS; ↑NALP3 activation; ↑IL-4; ↑IL-10; ↑eicosanoids; phagocytosis |
| hCLECSF8 | CLEC4D, mMCL, CLEC6 | Unknown | ↑TNF; ↑ROS; phagocytosis |
| mMINCLE | CLECSF9, CLEC4e | Mycobacteria; Candida; Malassezia; Fonsecaea; SAP130 (dead cells) | ↑TNF; ↑MIP-2; ↑KC; ↑IL-10; ↑IL-6; ↑CXCL1/2; ↑NALP3 activation; respiratory burst |
aSee text for references.
Triggering of BDCA-2, like the BCR, induces phosphorylation of many cytoskeletal proteins, including actin, tubulin and the clathrin heavy chain, resulting in internalization of the receptor; a process that may also involve the EEE (late endosomal sorting) motif in the cytoplasmic tail of BDCA-2 (5, 6, 8). Targeting of BDCA-2 using antibodies was shown to lead to internalization of the antibody complexes and presentation to T cells, suggesting a possible role for this receptor in antigen capture and presentation (5). However, more recent evidence has demonstrated that BDCA-2 signalling actually inhibits antigen processing and presentation to T cells by pDCs (9, 10). BDCA-2 cross-linking also suppressed the expression of co-stimulatory molecules, including CD40 and CD86, on pDCs matured with the TLR9 ligand, CpG (9, 10). However, this did not occur during CD40 ligand-mediated pDC maturation, indicating specificity in the effects of BDCA-2 stimulation (9).
BDCA-2 contains an EPN motif in its CTLD, which would suggest that the receptor recognizes mannose-based ligands (8). However, such ligands have not been conclusively identified (11) and BDCA-2 was recently shown to recognize asialo-galactosyl-oligosaccharides with terminal β1–4- and β1–3-galactose residues (12). Indeed, through recognition of these carbohydrates, BDCA-2 was capable of binding certain CD14+ monocytes, monocyte-derived DCs, as well as several tumour cell lines (12). Importantly, co-culture experiments demonstrated that expression of these ligands correlated with the suppression of type I interferon secretion by pDCs (12). BDCA-2 has also been shown to bind the HIV-1 envelope glycoprotein gp120 (13) and very recently, the hepatitis C virus (HCV) glycoprotein E2 (14), both of which can inhibit interferon production by pDCs.
Dendritic cell immunoactivating receptor
Little is known about DCAR and DCAR1, both of which are not found in humans (15, 16). Murine DCAR (mDCAR) transcripts were detected in bone marrow-derived DCs, lung, spleen and at low levels in skin and lymph node tissue (15). In contrast, mDCAR1 expression (protein) was tissue dependent and restricted to CD8+ DCs in spleen and thymus, and to subpopulations of CD11b+ myeloid cell populations in spleen and bone marrow. However, DCAR1 was not detected in peripheral blood or lymph nodes (16).
Both receptors appear to be able to induce intracellular signalling, but only DCAR has been formally demonstrated to associate with the FcRγ chain, in part through association with an arginine residue in the transmembrane region of the CTLR (15, 16) (Fig. 1). Antibody-mediated targeting of model antigens to DCAR1 on primary cells resulted in endocytosis, trafficking to lysosomal compartments and efficient induction of humoral, CD8+ and CD4+ T-cell responses (16). Furthermore, DCAR1 signalling was able to modulate DC functions, enhancing IL-12 production and down-regulating IL-10 secretion (16) (Table 1). In summary, current data suggest that DCAR and DCAR1 may be activating receptors, but the ligands and physiological roles of these CTLRs remain unknown.
Dendritic cell immunoreceptor
Humans possess only a single gene encoding human DCIR (hDCIR), whereas mice encode four DCIR-like proteins (designated mDCIR1–4) (17, 18) (Fig. 1 and Table 1). The hDCIR is expressed by all CD14+ monocytes, CD15+ granulocytes, all DC subsets (including pDCs) and B cells in peripheral blood (18–21). Although originally not thought to be expressed on NK or T cells (18), recent evidence suggests that DCIR expression can be induced on CD4+ T cells following their infection with HIV and also by soluble factors released from HIV-infected cells (22). In mice, the expression of mDCIR1 shows similarity with the human receptor (23), whereas mDCIR2 appears to be a specific marker for CD8– DCs located in the red pulp and marginal zone of the mouse spleen (24). The expression patterns of the other murine receptors are still unclear, although mRNA analysis suggests that they are expressed on DCs and macrophages (17).
Unlike the other Dectin-2 family members, the DCIR proteins have long cytoplasmic tails, which, in hDCIR and mDCIR1 and mDCIR2, contain classical immunoreceptor tyrosine-based inhibitory signalling motifs (ITIMs) (17). Although the cytoplasmic domains of murine DCIR3 and DCIR4 lack consensus ITIMs, they possess conserved tyrosine residues, which is suggestive of an ability to transduce intracellular signals. The ITIMs of both hDCIR and mDCIR1 have been shown to mediate inhibitory signalling through activation of the phosphatases SHP-1 and SHP-2 (19, 23, 25, 26).
Activation of hDCIR on DCs or pDCs, e.g. by antibodies or HCV (see below) resulted in specific inhibition of TLR8-mediated IL-12 and TNF production, and TLR9-induced IFN-α production, respectively (14, 19, 20) (Table 1). In addition, targeting of this receptor with antibodies was found to cause clathrin-dependent receptor internalization, trafficking to lysosomal compartments, and antigen presentation (19, 20). Targeting of hDCIR was also found to efficiently drive antigen cross-presentation in DCs and activate CD8+ T cells (21). In mice, however, targeting of mDCIR2 was found to favour MHC class II presentation and CD4+ T-cell activation (24), as well as stimulation of natural FoxP3+ T regulatory cells (27). More recently, targeting of mDCIR2 was found to initiate extrafollicular B-cell responses to T-cell-dependent antigens (28).
The CTLD of DCIR possesses conserved features, including an EPS motif, suggesting that it is able to recognize carbohydrates (18). Although there is some evidence that this receptor can recognize fucose and mannose (11), definitive carbohydrate ligands have not been defined. However, DCIR has been identified as an attachment factor for HIV on DCs (29) and more recently for HCV (through glycoprotein E2) on pDCs (14). DCIR-mediated binding of HIV promoted infection of CD4+ T cells, and enhanced DCIR expression and HIV replication in these cells (22, 29); a process that required the neck region of DCIR and ITIM-mediated signal transduction from the receptor (26).
DCIR has also been implicated in autoimmunity. In mice, deficiency of mDCIR2 resulted in the spontaneous development of sialadenitis and enthesitis, and exacerbated responses to collagen-induced arthritis, effects that were attributed to unrestricted DC expansion (30). Moreover, in humans, polymorphisms of DCIR have been associated with susceptibility to rheumatoid arthritis and the receptor was found to be abundantly expressed in affected joints (31–33). However, the endogenous ligand(s) of DCIR involved in these functions are not yet defined.
Dendritic cell-associated C-type lectin-2
Dectin-2 was originally identified as a Langerhans cell-specific CTLR, but was subsequently shown to be expressed on a variety of myeloid cells, including tissue macrophages, neutrophils and several DC subsets (including pDCs) (34–38). Dectin-2 is also expressed at low levels on peripheral blood monocytes, although its expression on these cells can be greatly up-regulated by inflammatory stimuli (35). Human Dectin-2 has been detected on DCs and monocytes, and mRNA analysis suggests that it may also be expressed by lymphocytes (39, 40).
Like other activation CTLRs in this family, Dectin-2 has a short cytoplasmic tail and associates with the FcRγ. Interestingly, this interaction is not mediated by the transmembrane arginine residue of Dectin-2, but rather by a membrane-proximal part of its short cytoplasmic tail (41) (Fig. 1). Signalling from Dectin-2 is mediated through the Syk, PKCδ and CARD9–Bcl10–Malt1 (caspase recruitment domain 9–B-cell lymphoma 10–mucosa-associated lymphoid tissue lymphoma translocation gene 1) pathway, leading to the induction of several cytokines and chemokines (including TNF, IL-2, IL-10, IL-23, IL-1β, IL-6 and IL-12) (36, 41–44) (Table 1). More recently, Dectin-2 signalling has been shown to involve phospholipase Cγ2 and mitogen-activated protein kinases (MAPKs) (45) and to selectively activate the c-Rel NF-κB subunit, via Malt1, driving the production of Th17-polarizing cytokines (IL-23, IL-1β) (39). Triggering of Dectin-2 also induces the respiratory burst (45, 46), nucleotide-binding oligomerization domain-like receptor containing pyrin domains 3 (Nlrp3) inflammasome activation (46) and the production of several eicosanoids, some of which were found to promote Th2 immunity (47–49).
Dectin-2 contains a ‘classical’ CTLD containing a mannose-binding EPN motif, which binds structures with high mannose content (50), and much attention has been focused on its role as a pathogen recognition receptor (PRR). Indeed, Dectin-2 is capable of recognizing numerous pathogens, including Candida albicans, Saccharomyces cerevisiae, Mycobacterium tuberculosis, Paracoccidioides brasiliensis, Histoplasma capsulatum, Aspergillus fumigatus, non-encapsulated Cryptococcus neoformans, Microsporum audouinii, Trichophyton rubrum, Schistosoma mansoni and house dust mite allergens (41, 46, 47, 50).
The best-studied interaction is with C. albicans, an organism for which Dectin-2 function was found to be essential for protective host immunity (36, 43). Indeed, during infection with C. albicans, Dectin-2 was shown to drive host inflammatory cytokine responses and the development of adaptive immunity (particularly Th17 and Th1 responses), and mice lacking this receptor demonstrated greatly increased susceptibility (increased fungal burdens and rapid death). Notably, the functions of this CTLR appear to differ between the yeast and hyphal forms of C. albicans, possibly due to exposure of different ligands, suggesting that this PRR can distinguish between the different morphological forms of this pathogen (41–43). Interestingly, Dectin-2 has also been implicated in UV-induced immunosuppression through recognition of an endogenous ligand, but the identity of this molecule is unknown (51).
C-type lectin superfamily 8
CLECSF8 is another poorly studied CTLR in the Dectin-2 cluster and is found in both mice and humans (Fig. 1). It was originally isolated from mice as a macrophage-specific receptor (52), but recent human protein expression data have shown that it is more widely expressed on peripheral blood neutrophils, CD14+CD16– classical monocytes and weakly by several DC subsets (53). In contrast to other members of the Dectin-2 cluster, the CTLD of CLECSF8 lacks conserved amino acids normally associated with carbohydrate recognition, consistent with a failure to detect sugar ligands for this receptor using oligosaccharide microarrays (53).
Described previously as an endocytic receptor (54), recent studies have demonstrated that CLECSF8 can trigger intracellular signalling through the kinase Syk, inducing phagocytosis, the respiratory burst and pro-inflammatory cytokine (TNF) production (53) (Table 1). Interestingly, surface expression of CLECSF8 appeared to require a novel adaptor protein expressed in myeloid cells, as it did not associate with FcRγ, DNAX-activating protein of 10kDa (DAP10) or DAP12 (53). This observation was consistent with the lack of charged amino acid residues in the transmembrane and cytoplasmic domains of CLECSF8 (52) that are normally required for such associations. Unusually, the association with the unidentified adaptor appeared to require the CTLD of CLECSF8 (53).
CLECSF8–/– mice have been generated and although displaying slight, but not significant, differences in T- and B-cell phenotypes (2), these mice were found to have normal responses to necrotic cell death, sterile peritontis, the development of spontaneous autoimmune arthritis and experimental autoimmune uveoretinitis (53). Moreover, these mice had no defects in their ability to resist infections with C. albicans, Nippostrongylus brasiliensis, Staphylococcus aureus and Listeria monocytogenes (53). Thus, CLECSF8 appears to function as an activation receptor, but its ligands and physiological role remain to be defined.
Macrophage-inducible C-type lectin
As its name suggests, Mincle was discovered because it is strongly induced in macrophages by inflammatory stimuli, including LPS, TNF, IL-6 and IFN-γ (55). Found in both humans and rodents, Mincle is expressed on several cell types including monocytes, macrophages, neutrophils, myeloid DCs and some subsets of B cells (17, 56–60). T cells, pDCs and NK cells do not express the receptor (56). Interestingly, Mincle appears to have a reciprocal expression pattern on neutrophils and monocytes in individual humans, which has functional implications (see below) (56).
Like the other activating receptors in the Dectin-2 cluster, Mincle associates with the FcRγ to transduce intracellular signalling (58) (Fig. 1). This association is mediated by a positively charged arginine residue in the transmembrane domain of this CTLR (58). Triggering of Mincle–FcRγ induces intracellular signalling through Syk, PKCδ, CARD9–Bcl10–Malt1 and MAPK pathways resulting in the induction of several cytokines and chemokines, including TNF, macrophage inflammatory protein 2 (MIP-2; CXCL2), keratinocyte-derived chemokine (KC; CXCL1) and IL-6 (44, 58) (Table 1). Although Mincle-mediated cellular responses are MyD88 independent, recent evidence demonstrates that Mincle and the TLRs can collaborate to synergistically induce inflammatory cytokine production, the respiratory burst and up-regulation of complement receptor 3 (CR3) (59, 61).
Mincle possesses a classical CTLD domain containing an EPN motif and is capable of recognizing several types of carbohydrates, particularly those containing α-mannose (11, 62). Notably, fungal, mycobacterial and necrotic cell ligands for Mincle have been identified, implicating this receptor in anti-microbial immunity and homeostasis. Indeed, a cell wall component of C. albicans was one of the first ligands described, and Mincle has been shown to drive protective immunity (inflammatory cytokine responses, fungal killing and phagocytosis) to this pathogen (56, 63).
Interestingly, in human monocytes, expression of Mincle was required for inflammatory cytokine responses, but inversely correlated with fungal uptake and killing, whereas in neutrophils, Mincle expression was associated with promotion of the latter functions (56). Mincle also recognizes Fonsecaea pedrosoi, the causative agent of chromoblastomycosis (61) and recent data demonstrate that this infection stems from insufficient TLR co-stimulation of CTLR responses (61). Importantly, these infections could be resolved by stimulating protective inflammatory responses through the administration of exogenous TLR ligands (61). Mincle also recognizes Malassezia and was required for the induction of several cytokines, chemokines and inflammatory cell recruitment during infections in vivo (62).
In addition to fungi, Mincle recognizes trehalose dimycolate (TDM; also called mycobacterial cord factor), an immunostimulatory glycolipid component that is of considerable interest for its role in anti-mycobacterial immunity and as an adjuvant for driving cell-mediated immune responses (64, 65). Indeed, Mincle and the FcRγ chain were found to mediate all the responses induced by TDM and its synthetic analogue trehalose dibehenate, including the production of inflammatory cytokines, nitric oxide, NLRP3 inflammasome activation, granuloma formation, and Th1 and Th17 adjuvanticity (59, 60, 64–66).
In vitro studies have suggested that Mincle plays some role in driving these responses to intact mycobacteria (64, 65, 67), but the in vivo role of Mincle is contradictory, possibly because of the use of different mycobacteria. Experiments using e.g. Mycobacterium bovis BCG or M. tuberculosis Erdman, have suggested a protective role for this CTLR, as shown by altered inflammatory responses and increased bacterial burdens in the knockout animals (59, 68). In contrast, these parameters were unaltered in mice infected with M. tuberculosis H37Rv (67). Interestingly, Mincle deficiency did not affect M. tuberculosis-induced granuloma formation in either study (59, 67).
Mincle can also sense necrotic cell death, driving inflammatory responses (TNF and MIP-2) that result in neutrophil accumulation in the damaged tissues (58). Spliceosome-associated protein (SAP)130 was identified as the endogenous ligand that was recognized by Mincle following necrotic cell death (58). Interestingly, this interaction appeared to involve a binding site on Mincle that was different to that involved in its ability to recognize carbohydrates (58). The ability to recognize necrotic cells suggests that Mincle may be involved in autoimmunity, and although not been formally demonstrated in Mincle-deficient mice, a polymorphism in this receptor has recently been linked with protection against rheumatoid arthritis in men (69).
Conclusions
The last few years have provided exciting new insights into the functions of the ‘Dectin-2 cluster’ of CTLRs. These receptors recognize numerous pathogens and pathogen-derived products, trigger intracellular signalling cascades that have profound effects on cellular and immunological processes and play critical roles in anti-microbial immunity. Moreover, similar processes are triggered following the recognition of endogenous ligands, and these receptors have important functions in maintaining homeostasis and in the development of autoimmunity. Given the diverse and crucial roles of these CTLRs, it is perhaps surprising that we still know relatively little about their physiological functions. However, the burgeoning interest in this area will undoubtedly lead to a rapid increase in our knowledge in the coming years.
Funding
We thank the Wellcome Trust and Medical Research Council, UK for funding this research. B.K. is supported by a PhD Studentship from the University of Aberdeen, Institute of Medical Sciences.
References
- 1. Zelensky A. N., Gready J. E. 2005. The C-type lectin-like domain superfamily. FEBS J. 272: 6179 [DOI] [PubMed] [Google Scholar]
- 2. Graham L. M., Brown G. D. 2009. The Dectin-2 family of C-type lectins in immunity and homeostasis. Cytokine 48: 148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Dzionek A., Fuchs A., Schmidt P., et al. 2000. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J. Immunol. 165: 6037 [DOI] [PubMed] [Google Scholar]
- 4. Cao W., Zhang L., Rosen D. B., et al. 2007. BDCA2/Fc epsilon RI gamma complex signals through a novel BCR-like pathway in human plasmacytoid dendritic cells. PLoS Biol. 5: e248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dzionek A., Sohma Y., Nagafune J., et al. 2001. BDCA-2, a novel plasmacytoid dendritic cell-specific type II C-type lectin, mediates antigen capture and is a potent inhibitor of interferon alpha/beta induction. J. Exp. Med. 194: 1823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Röck J., Schneider E., Grün J. R., et al. 2007. CD303 (BDCA-2) signals in plasmacytoid dendritic cells via a BCR-like signalosome involving Syk, Slp65 and PLCgamma2. Eur. J. Immunol. 37: 3564 [DOI] [PubMed] [Google Scholar]
- 7. Riboldi E., Daniele R., Cassatella M. A., Sozzani S., Bosisio D. 2009. Engagement of BDCA-2 blocks TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. Immunobiology 214: 868 [DOI] [PubMed] [Google Scholar]
- 8. Fernandes M. J., Iscove N. N., Gingras G., Calabretta B. 2000. Identification and characterization of the gene for a novel C-type lectin (CLECSF7) that maps near the natural killer gene complex on human chromosome 12. Genomics 69: 263 [DOI] [PubMed] [Google Scholar]
- 9. Jähn P. S., Zänker K. S., Schmitz J., Dzionek A. 2010. BDCA-2 signaling inhibits TLR-9-agonist-induced plasmacytoid dendritic cell activation and antigen presentation. Cell Immunol. 265: 15 [DOI] [PubMed] [Google Scholar]
- 10. Wu P., Wu J., Liu S., et al. 2008. TLR9/TLR7-triggered downregulation of BDCA2 expression on human plasmacytoid dendritic cells from healthy individuals and lupus patients. Clin. Immunol. 129: 40 [DOI] [PubMed] [Google Scholar]
- 11. Lee R. T., Hsu T. L., Huang S. K., Hsieh S. L., Wong C. H., Lee Y. C. 2011. Survey of immune-related, mannose/fucose-binding C-type lectin receptors reveals widely divergent sugar-binding specificities. Glycobiology 21: 512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riboldi E., Daniele R., Parola C., et al. 2011. Human C-type lectin domain family 4, member C (CLEC4C/BDCA-2/CD303) is a receptor for asialo-galactosyl-oligosaccharides. J. Biol. Chem. 286: 35329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Martinelli E., Cicala C., Van Ryk D., et al. 2007. HIV-1 gp120 inhibits TLR9-mediated activation and IFN-α secretion in plasmacytoid dendritic cells. Proc. Natl Acad. Sci. U S A 104: 3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Florentin J., Aouar B., Dental C., et al. 2012. HCV glycoprotein E2 is a novel BDCA-2 ligand and acts as an inhibitor of IFN production by plasmacytoid dendritic cells. Blood 120: 4544 [DOI] [PubMed] [Google Scholar]
- 15. Kanazawa N., Tashiro K., Inaba K., Miyachi Y. 2003. Dendritic cell immunoactivating receptor, a novel C-type lectin immunoreceptor, acts as an activating receptor through association with Fc receptor gamma chain. J. Biol. Chem. 278: 32645 [DOI] [PubMed] [Google Scholar]
- 16. Kaden S. A., Kurig S., Vasters K., et al. 2009. Enhanced dendritic cell-induced immune responses mediated by the novel C-type lectin receptor mDCAR1. J. Immunol. 183: 5069 [DOI] [PubMed] [Google Scholar]
- 17. Flornes L. M., Bryceson Y. T., Spurkland A., Lorentzen J. C., Dissen E., Fossum S. 2004. Identification of lectin-like receptors expressed by antigen presenting cells and neutrophils and their mapping to a novel gene complex. Immunogenetics 56: 506 [DOI] [PubMed] [Google Scholar]
- 18. Bates E. E., Fournier N., Garcia E., et al. 1999. APCs express DCIR, a novel C-type lectin surface receptor containing an immunoreceptor tyrosine-based inhibitory motif. J. Immunol. 163: 1973 [PubMed] [Google Scholar]
- 19. Meyer-Wentrup F., Cambi A., Joosten B., et al. 2009. DCIR is endocytosed into human dendritic cells and inhibits TLR8-mediated cytokine production. J. Leukoc. Biol. 85: 518 [DOI] [PubMed] [Google Scholar]
- 20. Meyer-Wentrup F., Benitez-Ribas D., Tacken P. J., et al. 2008. Targeting DCIR on human plasmacytoid dendritic cells results in antigen presentation and inhibits IFN-alpha production. Blood 111: 4245 [DOI] [PubMed] [Google Scholar]
- 21. Klechevsky E., Flamar A. L., Cao Y., et al. 2010. Cross-priming CD8+ T cells by targeting antigens to human dendritic cells through DCIR. Blood 116: 1685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lambert A. A., Imbeault M., Gilbert C., Tremblay M. J. 2010. HIV-1 induces DCIR expression in CD4+ T cells. PLoS Pathogen. 6: e1001188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kanazawa N., Okazaki T., Nishimura H., Tashiro K., Inaba K., Miyachi Y. 2002. DCIR acts as an inhibitory receptor depending on its immunoreceptor tyrosine-based inhibitory motif. J. Invest. Dermatol. 118: 261 [DOI] [PubMed] [Google Scholar]
- 24. Dudziak D., Kamphorst A. O., Heidkamp G. F., et al. 2007. Differential antigen processing by dendritic cell subsets in vivo. Science 315: 107 [DOI] [PubMed] [Google Scholar]
- 25. Richard M., Thibault N., Veilleux P., Gareau-Pagé G., Beaulieu A. D. 2006. Granulocyte macrophage-colony stimulating factor reduces the affinity of SHP-2 for the ITIM of CLECSF6 in neutrophils: a new mechanism of action for SHP-2. Mol. Immunol. 43: 1716 [DOI] [PubMed] [Google Scholar]
- 26. Lambert A. A., Barabé F., Gilbert C., Tremblay M. J. 2011. DCIR-mediated enhancement of HIV-1 infection requires the ITIM-associated signal transduction pathway. Blood 117: 6589 [DOI] [PubMed] [Google Scholar]
- 27. Yamazaki S., Dudziak D., Heidkamp G. F., et al. 2008. CD8+ CD205+ splenic dendritic cells are specialized to induce Foxp3+ regulatory T cells. J. Immunol. 181: 6923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Chappell C. P., Draves K. E., Giltiay N. V., Clark E. A. 2012. Extrafollicular B cell activation by marginal zone dendritic cells drives T cell-dependent antibody responses. J. Exp. Med. 209: 1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lambert A. A., Gilbert C., Richard M., Beaulieu A. D., Tremblay M. J. 2008. The C-type lectin surface receptor DCIR acts as a new attachment factor for HIV-1 in dendritic cells and contributes to trans- and cis-infection pathways. Blood 112: 1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fujikado N., Saijo S., Yonezawa T., et al. 2008. DCIR deficiency causes development of autoimmune diseases in mice due to excess expansion of dendritic cells. Nat. Med. 14: 176 [DOI] [PubMed] [Google Scholar]
- 31. Guo J., Wu X., Too C. L., et al. 2012. A replication study confirms the association of dendritic cell immunoreceptor (DCIR) polymorphisms with ACPA - negative RA in a large Asian cohort. PLoS One 7: e41228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lorentzen J. C., Flornes L., Eklöw C., et al. 2007. Association of arthritis with a gene complex encoding C-type lectin-like receptors. Arthritis Rheum. 56: 2620 [DOI] [PubMed] [Google Scholar]
- 33. Eklöw C., Makrygiannakis D., Bäckdahl L., et al. 2008. Cellular distribution of the C-type II lectin dendritic cell immunoreceptor (DCIR) and its expression in the rheumatic joint: identification of a subpopulation of DCIR+ T cells. Ann. Rheum. Dis. 67: 1742 [DOI] [PubMed] [Google Scholar]
- 34. Ariizumi K., Shen G. L., Shikano S., et al. 2000. Cloning of a second dendritic cell-associated C-type lectin (dectin-2) and its alternatively spliced isoforms. J. Biol. Chem. 275: 11957 [DOI] [PubMed] [Google Scholar]
- 35. Taylor P. R., Reid D. M., Heinsbroek S. E., Brown G. D., Gordon S., Wong S. Y. 2005. Dectin-2 is predominantly myeloid restricted and exhibits unique activation-dependent expression on maturing inflammatory monocytes elicited in vivo. Eur. J. Immunol. 35: 2163 [DOI] [PubMed] [Google Scholar]
- 36. Robinson M. J., Osorio F., Rosas M., et al. 2009. Dectin-2 is a Syk-coupled pattern recognition receptor crucial for Th17 responses to fungal infection. J. Exp. Med. 206: 2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Seeds R. E., Gordon S., Miller J. L. 2009. Characterisation of myeloid receptor expression and interferon alpha/beta production in murine plasmacytoid dendritic cells by flow cytomtery. J. Immunol. Methods 350: 106 [DOI] [PubMed] [Google Scholar]
- 38. McDonald J. U., Rosas M., Brown G. D., Jones S. A., Taylor P. R. 2012. Differential dependencies of monocytes and neutrophils on dectin-1, dectin-2 and complement for the recognition of fungal particles in inflammation. PLoS One 7: e45781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gringhuis S. I., Wevers B. A., Kaptein T. M., et al. 2011. Selective C-Rel activation via Malt1 controls anti-fungal T(H)-17 immunity by dectin-1 and dectin-2. PLoS Pathogen. 7: e1001259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gavino A. C., Chung J. S., Sato K., Ariizumi K., Cruz P. D., Jr 2005. Identification and expression profiling of a human C-type lectin, structurally homologous to mouse dectin-2. Exp. Dermatol. 14: 281 [DOI] [PubMed] [Google Scholar]
- 41. Sato K., Yang X. L., Yudate T., et al. 2006. Dectin-2 is a pattern recognition receptor for fungi that couples with the Fc receptor gamma chain to induce innate immune responses. J. Biol. Chem. 281: 38854 [DOI] [PubMed] [Google Scholar]
- 42. Bi L., Gojestani S., Wu W., et al. 2010. CARD9 mediates dectin-2-induced IκBα kinase ubiquitination leading to activation of NF-κB in response to stimulation by the hyphal form of Candida albicans. J. Biol. Chem. 285: 25969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saijo S., Ikeda S., Yamabe K., et al. 2010. Dectin-2 recognition of alpha-mannans and induction of Th17 cell differentiation is essential for host defense against Candida albicans. Immunity 32: 681 [DOI] [PubMed] [Google Scholar]
- 44. Strasser D., Neumann K., Bergmann H., et al. 2012. Syk kinase-coupled C-type lectin receptors engage protein kinase C-σ to elicit Card9 adaptor-mediated innate immunity. Immunity 36: 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gorjestani S., Yu M., Tang B., Zhang D., Wang D., Lin X. 2011. Phospholipase Cγ2 (PLCγ2) is key component in Dectin-2 signaling pathway, mediating anti-fungal innate immune responses. J. Biol. Chem. 286: 43651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ritter M., Gross O., Kays S., et al. 2010. Schistosoma mansoni triggers Dectin-2, which activates the Nlrp3 inflammasome and alters adaptive immune responses. Proc. Natl Acad. Sci. USA 107: 20459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Barrett N. A., Maekawa A., Rahman O. M., Austen K. F., Kanaoka Y. 2009. Dectin-2 recognition of house dust mite triggers cysteinyl leukotriene generation by dendritic cells. J. Immunol. 182: 1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Suram S., Gangelhoff T. A., Taylor P. R., et al. 2010. Pathways regulating cytosolic phospholipase A2 activation and eicosanoid production in macrophages by Candida albicans. J. Biol. Chem. 285: 30676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Barrett N. A., Rahman O. M., Fernandez J. M., et al. 2011. Dectin-2 mediates Th2 immunity through the generation of cysteinyl leukotrienes. J. Exp. Med. 208: 593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McGreal E. P., Rosas M., Brown G. D., et al. 2006. The carbohydrate-recognition domain of Dectin-2 is a C-type lectin with specificity for high mannose. Glycobiology 16: 422 [DOI] [PubMed] [Google Scholar]
- 51. Aragane Y., Maeda A., Schwarz A., Tezuka T., Ariizumi K., Schwarz T. 2003. Involvement of dectin-2 in ultraviolet radiation-induced tolerance. J. Immunol. 171: 3801 [DOI] [PubMed] [Google Scholar]
- 52. Balch S. G., McKnight A. J., Seldin M. F., Gordon S. 1998. Cloning of a novel C-type lectin expressed by murine macrophages. J. Biol. Chem. 273: 18656 [DOI] [PubMed] [Google Scholar]
- 53. Graham L. M., Gupta V., Schafer G., et al. 2012. The C-type lectin receptor CLECSF8 (CLEC4D) is expressed by myeloid cells and triggers cellular activation through Syk kinase. J. Biol. Chem. 287: 25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Arce I., Martínez-Muñoz L., Roda-Navarro P., Fernández-Ruiz E. 2004. The human C-type lectin CLECSF8 is a novel monocyte/macrophage endocytic receptor. Eur. J. Immunol. 34: 210 [DOI] [PubMed] [Google Scholar]
- 55. Matsumoto M., Tanaka T., Kaisho T., et al. 1999. A novel LPS-inducible C-type lectin is a transcriptional target of NF-IL6 in macrophages. J. Immunol. 163: 5039 [PubMed] [Google Scholar]
- 56. Vijayan D., Radford K. J., Beckhouse A. G., Ashman R. B., Wells C. A. 2012. Mincle polarizes human monocyte and neutrophil responses to Candida albicans. Immunol. Cell Biol. 90: 889 [DOI] [PubMed] [Google Scholar]
- 57. Kawata K., Illarionov P., Yang G. X., et al. 2012. Mincle and human B cell function. J. Autoimmun. 39: 315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamasaki S., Ishikawa E., Sakuma M., Hara H., Ogata K., Saito T. 2008. Mincle is an ITAM-coupled activating receptor that senses damaged cells. Nat. Immunol. 9: 1179 [DOI] [PubMed] [Google Scholar]
- 59. Lee W. B., Kang J. S., Yan J. J., et al. 2012. Neutrophils promote mycobacterial trehalose dimycolate-induced lung inflammation via the Mincle pathway. PLoS Pathogen. 8: e1002614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schweneker K., Gorka O., Schweneker M., et al. 2012. The mycobacterial cord factor adjuvant analogue trehalose-6,6’-dibehenate (TDB) activates the Nlrp3 inflammasome. Immunobiology doi:10.1016/j.imbio.2012.07.029 [DOI] [PubMed] [Google Scholar]
- 61. da Gloria Sousa M., Reid D. M., Schweighoffer E., et al. 2011. Restoration of pattern recognition receptor costimulation to treat chromoblastomycosis, a chronic fungal infection of the skin. Cell Host Microbe 9: 436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Yamasaki S., Matsumoto M., Takeuchi O., et al. 2009. C-type lectin Mincle is an activating receptor for pathogenic fungus, Malassezia. Proc. Natl. Acad. Sci. U S A 106: 1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wells C. A., Salvage-Jones J. A., Li X., et al. 2008. The macrophage-inducible C-type lectin, mincle, is an essential component of the innate immune response to Candida albicans. J. Immunol. 180: 7404 [DOI] [PubMed] [Google Scholar]
- 64. Ishikawa E., Ishikawa T., Morita Y. S., et al. 2009. Direct recognition of the mycobacterial glycolipid, trehalose dimycolate, by C-type lectin Mincle. J. Exp. Med. 206: 2879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Schoenen H., Bodendorfer B., Hitchens K., et al. 2010. Cutting edge: Mincle is essential for recognition and adjuvanticity of the mycobacterial cord factor and its synthetic analog trehalose-dibehenate. J. Immunol. 184: 2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Werninghaus K., Babiak A., Gross O., et al. 2009. Adjuvanticity of a synthetic cord factor analogue for subunit Mycobacterium tuberculosis vaccination requires FcRγ-Syk-Card9-dependent innate immune activation. J. Exp. Med. 206: 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Heitmann L., Rani R., Dawson L., et al. 2012. TGF-β-responsive myeloid cells suppress type 2 immunity and emphysematous pathology after hookworm infection. Am. J. Pathol. 181: 897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Behler F., Steinwede K., Balboa L., et al. 2012. Role of Mincle in alveolar macrophage-dependent innate immunity against mycobacterial infections in mice. J. Immunol. 189: 3121 [DOI] [PubMed] [Google Scholar]
- 69. Wu X. Y., Guo J. P., Yin F. R., et al. 2012. Macrophage-inducible C-type lectin is associated with anti-cyclic citrullinated peptide antibodies-positive rheumatoid arthritis in men. Chin. Med. J. 125: 3115 [PubMed] [Google Scholar]