Abstract
Cell lines CΔ2+ and CΔ2− were developed from monocytes obtained from a 10-month-old, crossbred, female pig. These cells morphologically resembled macrophages, stained positively for α-naphthyl esterase and negatively for peroxidase. The cell lines were bactericidal and highly phagocytic. Both cell lines expressed the porcine cell-surface molecules MHCI, CD11b, CD14, CD16, CD172, and small amounts of CD2; however, only minimal amounts of CD163 were measured. The lines were negative for the mouse marker H2Kk, bovine CD2 control, and secondary antibody control. Additionally, cells tested negative for Bovine Viral Diarrhea Virus and Porcine Circovirus Type 2. Therefore, these cells resembled porcine macrophages based on morphology, cell-surface marker phenotype, and function and will be useful tools for studying porcine macrophage biology.
Keywords: Monocyte, Macrophage, Cytokine, Phagocytosis, Morphology, Cell surface molecule
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; CD, cluster of differentiation; E:T, effecter to target ratio; EDTA, ethylenediaminetetraacetic acid; FBS, fetal bovine serum; FCS, fetal calf serum; FITC, fluorescein isothiocyanate; GAPDH, glyceraldehydes 3-phosphate dehydrogenase; IACUC, Institutional Animal Care and Use Committee; IgG, immunoglobulin G; L-glut, L-glutamine; LPS, lipopolysaccharide; M-CSF, macrophage colony-stimulating factor; MHC, major histocompatibility complex; NK, natural killer; PBMC, peripheral blood mononuclear cells; PBS, phosphate-buffered saline; PCR, polymerase chain reaction; RPMI, Roswell Park Memorial Institute; TE, Trypsin-EDTA; USMARC, U.S. Meat Animal Research Center
1. Introduction
Macrophages are an important component of the innate immune response against pathogens. These cells are of myeloid origin and after circulating in the blood as monocytes, differentiate into tissue macrophages. In addition to protecting the host, macrophages also contribute to the infectious process by maintaining intracellular pathogens such as Porcine Reproductive and Respiratory Disease Syndrome Virus (PRRSV; [1]), Brucella [2], and Salmonella [3]. Monocytes make up only a small percentage of mononuclear cells in peripheral whole blood. In pigs, this value ranges from 0 to 0% [4]. Isolating sufficient numbers of these cells to perform in vitro experiments is time consuming and variation among animals in cell numbers and activity level is high. Although numerous human and murine monocytic/macrophage cell lines are publicly available, the same is not true for pigs. There are only three pig monocytic/macrophage cell lines (CRL-2843, -2844, and -2845), (ATCC “Cell Lines and Hybridomas” catalogue; https://www.atcc.org/ATCCAdvancedCatalogSearch/tabid/112/Default.aspx). All of these are virus transformed which can affect the function of the cells [5]. Other porcine cell lines of monocyte lineage have been described; however, these are not available in a public repository [6–9]. Therefore, there is a strong need for available, non-transformed, porcine monocyte-derived cell lines for agricultural research. These cells would allow for the completion of “proof of concept studies” and drug development work requiring macrophages without the time and expense (i.e., Institutional Animal Care and Use Committee [IACUC] approval and monitoring) of obtaining whole blood from experimental animals. We describe the development of porcine monocyte-derived cell lines with the characteristics of macrophages that will be deposited in a cell repository for public access.
2. Materials and methods
2.1. Culture of LM-929 cells for supernatant
LM-929 cells (ATCC CmCL 1.2) were used as the source of macrophage colony- stimulating factor (M-CSF; [5]). LM-929 cells were grown to confluency in tissue culture flasks in a Roswell Park Memorial Institute (RPMI) medium (Invitrogen, Carlsbad, CA) supplemented with 5% fetal bovine serum (FBS; HyClone), Antibiotic/Antimycotic (A/A; Invitrogen), and l-glutamine (l-glut; Invitrogen). Supernatants were stored at −80 °C, and then filter sterilized prior to use.
2.2. Isolation of porcine monocytes and generation of cell lines
Whole blood was obtained with IACUC approval in accordance with USDA animal care guidelines from a 10-week-old, mixed-breed, female pig housed at the U.S. Meat Animal Research Center (USMARC) swine facility. Approximately, 70 ml whole blood was obtained via jugular venapuncture, into 35-ml syringes containing 0.1 M EDTA. Peripheral blood mononuclear cells (PBMC) were isolated by density gradient centrifugation over Ficoll-Paque Plus (Amersham Pharmacia Biotech AB, Uppsala, Sweden), as previously described [10]. Purified PBMC were counted, cytocentrifuged, and stained to differentiate between monocytes and lymphocytes. Cells were resuspended at 1 × 106 monocytes/ml RPMI without serum and 11 ml were placed into 25-cm2 tissue culture flasks and allowed to adhere for 1 h at 37 °C in a humidified atmosphere containing 5% CO2. Medium was then replaced with RPMI containing 5% FBS, A/A, and l-glut (complete RPMI) to remove the lymphocyte population. After culturing under these conditions for 17 days, cells were cultured in medium containing 10% LM-929 supernatant as indicated by “+” in the cell line nomenclature. After 5 months in culture, a subculture of these cells was reintroduced to medium without LM-929 supernatant (CΔ2−). Culture medium was changed once per week until the cells formed a confluent monolayer stage. Cells were then passaged and replated or frozen.
2.3. Cell dispersal and freezing
Adherent cell monolayers were dispersed by treatment with Trypsin-EDTA (TE; Invitrogen; [11]). Cell preparations used for cell-surface phenotyping were dispersed using 0.2% EDTA without trypsin. 1–5 × 106 cells/vial/ml were prepared for storage in liquid nitrogen. They were suspended in freezing medium consisting of 10% dimethyl sulfoxide in FBS [12].
2.4. Karyotype analysis
Cell lines were subcultured 1:2 for karyotyping at passages 20–24. Briefly, cells were grown to confluence in 75-cm2 flasks, trypsinized and transferred to new flasks in culture medium containing 5-bromo-2′-deoxyuridine (BrdU) to a final concentration of 25 μg/ml (Sigma; [13]. After 20 h, medium was replaced with fresh culture medium lacking BrdU. Cultures were incubated for an additional 4 h, then medium was replaced with 0.075 M KCl. Mitotic cells were shaken from the flask into the hypotonic solution and incubated for 20 min, then fixed with multiple changes of a solution of 3:1 methanol: glacial acetic acid. Chromosomes were stained in 4% Giemsa (Life Technologies) or banded as described by Rønne [14], and karyotyped according to international convention [15]. Fluorescence in situ hybridization (FISH) to identify the sex chromosomes was conducted with bacterial artificial chromosome probes for KAL1 and CSF2RA genes as previously described [16].
2.5. Phenotypic and immunophenotypic analysis
Cytospin preparations of the cell lines were stained as per the manufacturer's directions using HEMA3 differential stain (Fisher Scientific Company, Kalamazoo, MI), the leukocyte peroxidase kit (Sigma-Aldrich, St. Louis, MO) and the α-naphthyl esterase kit (Sigma-Aldrich). Cells were stained for flow cytometric analysis of cell surface determinants essentially as described [17]. Primary antibodies against Mouse H-2Kk, CD172, CD16, CD11b (BD Pharmingen, San Jose, CA), MHC Class I, CD14, and bovine CD2 (VMRD, Inc., Pullman, WA), were added for a final concentration of 1:50. FITC-Streptavidin (KPL, Gaithersburg, MD) was added to cells stained with anti-mouse H-2Kk, and FITC-labeled anti-mouse IgG (KPL) was added to all other cells. Fixed samples were stored at 4 °C in the dark until assayed.
2.6. LPS stimulation of cell cultures
Cells were cultured in medium containing 1 μg/ml LPS [18]. To obtain RNA for the measure of cytokine expression, cells were cultured in either 25-cm2 tissue culture flasks or 24-well tissue culture plates for 0–48 h.
2.7. Nitrite production
A colorimetric assay (as described by [19]) was used to determine the amount of nitrite (NO2−) present in LPS-stimulated cell supernatants. A sodium nitrite (NaNO2) standard was assayed concurrently with the samples, and medium was used as a negative control. Quantity of NO2− present in the samples was determined by regression analysis.
2.8. Bactericidal assay
Colorimetric bactericidal assays using Escherichia. coli O157 and Staphylococcus aureus as targets were performed essentially as described by Stevens et al. [20]. Bacteria were opsonized by incubation at 37 °C with heat-inactivated bovine serum previously determined to have high antibody titers against E. coli O157. Non-opsonized bacteria were incubated in medium without serum. Cells (3 × 104) were placed into 96-well tissue culture plates with either opsonized on non-opsonized bacteria at an effecter to target ratio (E:T) of 1:100 for E. coli O157 and 1:10 for S. aureus. MTT (Sigma-Aldrich).
2.9. Phagocytosis assay
Phagocytosis was measured by the uptake of fluorescent microspheres as previously described, with some modifications [21]. Flow cytometric analysis to calculate microsphere uptake was performed on a Becton Dickinson FACSCalibur flow cytometer.
2.10. RNA isolation and cytokine expression
Total RNA was extracted from LPS-treated cells by acid guanidine phenol extraction [22]. First strand cDNA synthesis was performed on 1 μg total RNA using the SuperScript™ III Platinum® Two Step qRT-PCR Kit (Invitrogen) as per the manufacturer's instructions. Cytokine PCR was performed using a quantitative simultaneous multiplex real-time assay [23]. Three multiplexed reactions were run: Primer/Probe Set 1 assayed for the lymphokines IL-2, IL-4, and IFN-γ; Primer/Probe Set 2 assayed for the proinflammatory cytokines IL-1α, IL-6, and IL-10; and Primer/Probe Set 3 assayed for the housekeeping genes β-actin, GAPDH, and cyclophilin. Resulting values for cytokine Cts were normalized against the numerical average of the three housekeeping gene Cts.
2.11. Virus infection
Cells were tested by PCR for bovine viral diarrhea virus (BVDV) infection using the primer set F5′-CATGCCCATAGTAGGAC-3′ and R5′-CCATGTGCCATGTACAG-3′ for first round PCR amplification and cycle sequencing. This primer set amplifies sequences from the genomic 5′ untranslated region of type 1 and type 2 BVDV, but does not appear to amplify sequences from BVDV [24,25]. Additionally, aliquots of CΔ2+ and CΔ2− lysates were mixed 1:1 with Minimum Essential Medium (MEM, Invitrogen) and inoculated onto bovine turbinate (BT) cells that had been seeded into a 24-well plate. After 14 days of incubation at 37 °C, the BT cell lysates were tested by PCR for propagation of BVDV. Cell lines were tested for PCV2 by real-time PCR as described by Opriessnig et al. [26].
3. Results and discussion
We isolated monocytes from the peripheral blood of a crossbred pig in order to develop porcine monocyte-derived macrophage cell lines. The peripheral blood mononuclear cell population was isolated over a density gradient, and monocytes were obtained by removal of non-adherent cells from cultures. After two weeks in culture, the monocytes were adhered to the culture flasks and were considered monocyte-derived macrophages. At this time, LM929 supernatant was added to the cultures to provide a source of M-CSF to stimulate cell proliferation. Monolayers soon formed and cells morphologically resembled cultured macrophages (Fig. 1A). The addition of LPS to the medium caused the cells to develop the “fried egg” appearance of activated macrophages (Fig. 1B). This cell line was named CΔ2+, the “+” denoting the inclusion of LM929 supernatant in the culture medium. After several months in culture, the LM929 supernatant was removed from the medium of a subculture of the CΔ2+ cell line, and these cells continued to proliferate and this subculture was named CΔ2− to designate the absence of supernatant in the medium (Fig. 1A). The CΔ2− line responded to the addition of LPS to the medium similarly to the CΔ2+ cells (Fig. 1B). The cell lines were characterized both earlier and later than 10 passages, and were found to be stable.
Fig. 1.
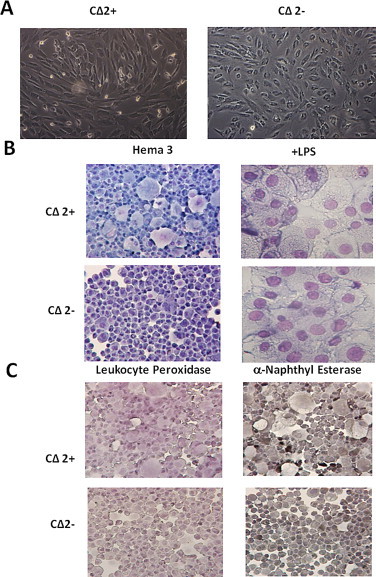
Histologic staining of cell lines. (A) Phase-contrast photomicrographs of CΔ2+ and CΔ2− cell lines. (B) Cytocentrifuged preparations of control and LPS-treated cell lines were fixed and stained with Hema 3 differential stain, and for (C) leukocyte peroxidase and α-naphthyl esterase activity as per the manufacturers’ directions.
Monocytes stain weakly for the enzyme myeloperoxidase found only in their lysozomal vacuoles, and staine diffusely for α-naphthyl-esterase [27]. Resident and resident-exudate macrophages have peroxidase-positive nuclear envelopes [27]. In the mouse, 95% of blood monocytes are positive for esterase activity as are 99% of resident peritoneal macrophages [27]. In contrast, only 60% of blood monocytes and 0% of resident peritoneal macrophages stain positively for peroxidase [27]. The CΔ2+ cell line stained strongly positively for α-naphthyl-esterase and diffusely for myeloperoxidase (Fig. 1C), in comparison, the CΔ2− cells stained diffusely for peroxidase, and not as strong as the CΔ2+ cell line for α-naphthyl-esterase (Fig. 1C). These results suggest that these cell lines are in the macrophage lineage beyond the monocyte stage since they had properties that were consistent with those described between monocyte and resident macrophage stages [27].
Cytogenetic analysis indicated that both cell lines are consistent with a diploid female pig karyotype of 38,XX. The CΔ2+ cell line contains a large metacentric chromosome derived from a reciprocal translocation of pig chromosomes SSC 8 and SSC 16 (Fig. 2A). The large derivative chromosome was observed in all metaphase nuclei examined and is stably maintained. In contrast, the translocated chromosome is not found in the CΔ2− cell line. This cell line appears to be near normal in the majority of cells, but some cells were observed to be aneuploidy, with chromosome numbers from 36 to 38. Sex chromosome identification was verified by FISH of probes for KAL1 and CSF2RA, both typically located in the pseudoautosomal region at the distal p-arm of the X chromosome (SSC X). Interestingly, a small rearrangement was observed in the CΔ2− cell line. In these cells, CSF2RA is translocated to from the tip of SSC X to a submetacentric chromosome tentatively identified as SSC 5 (supplemental figure). This region of SSC X remains intact in the CΔ2+ cells (not shown).
Fig. 2.
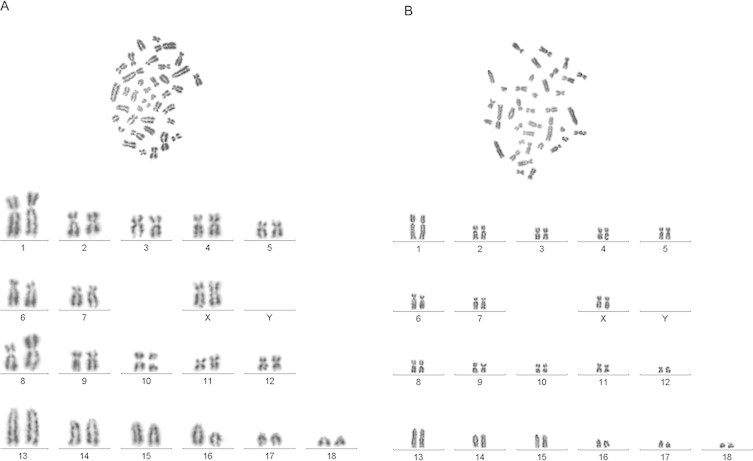
Karyotypes of cell lines. A) CΔ2+ and B) CΔ2− representative karyotypes are arranged according to standard nomenclature. Note derivative chromosomes SSC 8 and SSC 16 in A.
We stained the cell lines with a panel of antibodies against porcine cluster of differentiation (CD) markers normally found on cells of monocyte lineage, as well as several controls (Fig. 3, Table 1). Both cell lines were negative (≤2%) for murine H2Kk indicating that the lines were not contaminated by the murine LM-929 cells used as the source of M-CSF. They were also negative for the bovine CD2 marker. CΔ2− cells had a lower level of staining for CD2 (6%), CD11b (4%), CD14 (12%) and CD16 (5%) and MHCI, whereas in all cases CΔ2+ had a higher level of expression than the CΔ2− cells for these same cell surface markers (Table 1). In the presence of antibody, the low affinity Fcγ receptor, CD16, can facilitate phagocytosis and antibody-dependent cellular cytotoxicity (ADCC; [28]). The low levels of CD11b, CD14 and CD16 would be consistent with the hypothesis that CΔ2− cells were less differentiated than CΔ2+ cells [29–31]. Lastly, CD172, also known as SIRPα, is found on cells of monocyte/macrophage lineage [32] and was the highest expressed marker tested, and it too was expressed in greater numbers on the CΔ2+ cells (Table 1). CD2, normally found of the surface of T and NK cells and a sub-population of macrophages, was also present in low amounts on both CΔ2+ and CΔ2− cells (6 and 8%, respectively). Since we thought that CΔ2+ and CΔ2− might be useful tools for the study of PRRSV, we also examined the expression of CD163 (one of the described receptors for the virus; [33,34]). We found that CΔ2− cells had only 2% expression above background compared to 7% expression on CΔ2+ cells. It is not clear if this level of expression will allow for virus entry and replication. However, the cells may serve as a suitable host even if they have to be transfected with CD163 to improve the expression level since they can provide a suitable porcine macrophage environment necessary for virus growth.
Fig. 3.

Representative flow cytometry histogram. For each antibody, cells stained with isotype control were estimated by quantifying how many cells were localized under gate 1 (G1, Top Panel) compared to how many cells were localized under gate 2 (G2, Middle Panel). Overlay of the two histograms is shown for comparative purposes (Bottom Panel).
Table 1.
Cell surface staining/flow cytometry
| CΔ2+ | CΔ2- | |
|---|---|---|
| Antigen | % positive | % positive |
| Murine H2Kk | – | 2 |
| Bovine CD2 | – | – |
| CD2 | 8 | 6 |
| CD11b | 29 | 4 |
| CD14 | 14 | 12 |
| CD16 | 11 | 5 |
| MHCI | 16 | 5 |
| CD172 | 55 | 35 |
| CD163 | 7 | 2 |
Although both cell lines have undergone chromosomal rearrangements, they appear to be fairly stable cytogenetically. Each cell line is karyotypically distinct with two derivative chromosomes maintained in CΔ2+ cell line, and a rearrangement involving the X chromosome in the CΔ2− cells.
The expression of iNOS and production of nitrite/nitrate by porcine monocytes/macrophages are under debate [35]. We used the Griess reagent to measure nitrite production by the cell lines after exposure to LPS. In our hands, this assay reliably measures nitrite production by murine [36] and bovine monocytes/macrophages [37]. However, no nitrite production was measurable from either control or LPS-treated cell line supernatants (data not shown). The absence of a nitric oxide response could be because of the relative immature stages of both the CΔ2+ and CΔ2− cells. Alternatively, this may reflect the fact that porcine macrophages have –poor nos2 expression and nitric oxide responses and the cell lines parallel primary porcine monocyte/macrophages [38,39].
When we measured the bactericidal activity of the CΔ2+ and CΔ2− cells, we found that both cell lines were bactericidal against Gram− (E. coli) and Gram+ (S. aureus) organisms (Table 2). Differences were observed in the levels of killing between opsonized and non-opsonized bacteria were no statistically significant. This may be a result of the efficiency of direct bactericidal killing of bacteria, which did not leave room for significant enhancement with opsonization. Alternatively, the serum used for opsonization may have included some factors which interfered with the bactericidal activity of the cell lines.
Table 2.
Bactericidal activity of CD2+ and CD2− on E. coli and S. aureus
|
E. coli (1:100)* |
S. aureus (1:10)* |
|||
|---|---|---|---|---|
| Nonopsonized | Opsonized | Nonopsonized | Opsonized | |
| CΔ2+ | 62 ± 3 | 35 ± 16 | 56 ± 15 | 50 ± 20 |
| CΔ2− | 58 ± 23 | 36 ± 10 | 78 ± 24 | 53 ± 2 |
Values are expressed as % killed mean ± std of two experiments.
Effector to target cell ratio.
The efficient bactericidal activity of the CΔ2+ and CΔ2− cells was consistent with the observation that both cell lines were highly phagocytic. However, the CΔ2+ cells were more efficient phagocytes compared to the CΔ2− cells based on the speed that they phagocytosed latex beads (Fig. 4). Although 97% of the CΔ2+ cells ultimately phagocytosed beads by 18 h, compared to 85% for CΔ2−, at 3 h, over 75% of the CΔ2+ cells had phagocytosed beads compared to less that 25% of the CΔ2− cells. This difference in phagocytosis efficiency is consistent with the hypothesis that CΔ2− cells were less differentiated as CΔ2+ cells.
Fig. 4.
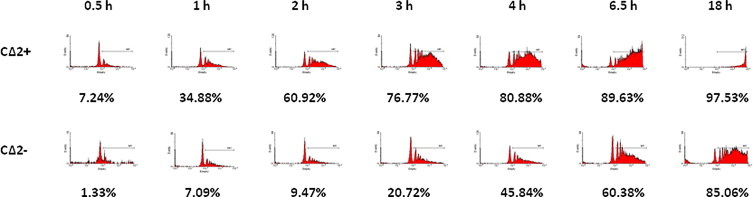
Phagocytosis of latex microspheres. Phagocytosis was measured by the uptake of fluorescent microspheres as described in Section 2. Data is presented as % cells ingesting microspheres at the indicated time-points.
To determine if the cell lines expressed cytokines normally attributed to cells of monocyte/macrophage lineage, we used sets of multiplexed assays for real-time PCR analysis. Cell lines were stimulated with LPS and compared to non-stimulated cultures over time ranging from 0 to 24 h (Fig. 5). Both cell lines expressed mRNA for the housekeeping genes tested, as well as the proinflammatory cytokines IL-1α and IL-6, but not for the cytokines IL-2, IL-4, and IFNγ, which are normally produced by cells of lymphocyte lineage. IL-1α was expressed in CΔ2− by 4 h with or without LPS treatment. However, it was only expressed by CΔ2+ at 24 and 48 h and not earlier time-points. IL-6 was measured in both LPS and non-treated CΔ2+ and CΔ2− lines, with CΔ2− expressing the most at all but the 48 h time-points.
Fig. 5.
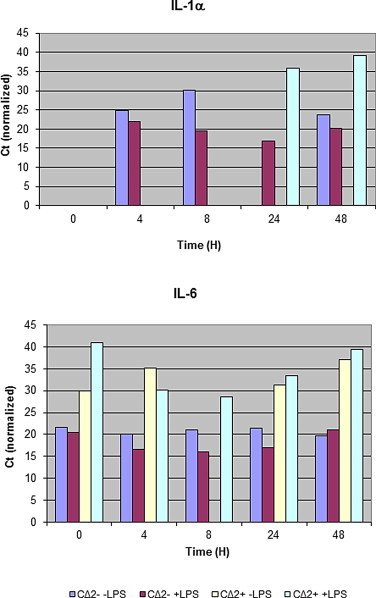
Cytokine expression measured by RT-qPCR and normalized against the average of three housekeeping genes.
Contamination of banked cell lines with Bovine Viral Diarrhea Virus (BVDV) through the use of contaminated FBS/FCS in culture medium is of great concern [40]. Therefore, we tested the cell lines for BVDV. Both cell lines were negative for BVDV, as determined by PCR analysis using a positive control 106 virions per ml (Fig. 6). Additionally, the cell lines were tested for the presence of the porcine respiratory pathogen PCV2 by real-time PCR. No endogenous virus was found at a minimum detection level of 20 copies per well in either line.
Fig. 6.
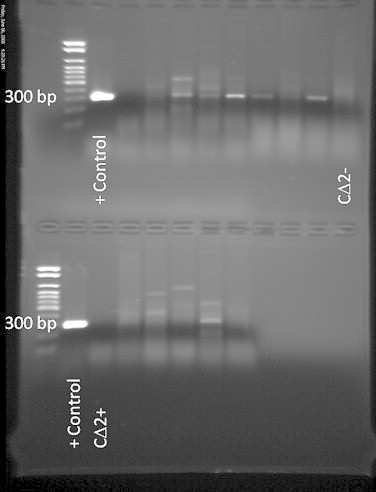
PCR test to measure contamination of cell lines with BVDV. Aliquots of CΔ2− and CΔ2+ lysates were mixed 1:1 with Minimum Essential Medium (MEM) and inoculated onto bovine turbinate (BT) cells that had been seeded into a 24-well plate. After 14 days of incubation at 37 °C, the BT cell lysates were tested by PCR for propagation of BVDV.
In conclusion, both porcine monocyte-derived macrophage cells CΔ2+ and CΔ2− closely mimic the morphology and activity of primary monocyte/macrophage cultures. Their relative ease of culture renders them useful tools for the in vitro study of porcine monocyte/macrophage biology.
Acknowledgements
The authors would like to thank Stacy Bierman, Tammy Sorensen, and Sarah Pohl for excellent technical assistance; the USMARC swine facility staff for assistance with blood collection; Dr. Jim Bono for assistance with real-time PCR analysis; and Joan Rosch, Sherry Kluver and Jan Watts for secretarial assistance; Dr. Terje Raudsepp for cytogenetic FISH analysis.
This project has been supported in part by USDA-ARS CRIS 5438-32000-029-00D; NASA Grant NNX08BA91G; American Heart Association Grant 0950036G; NIH Grants AI55052, AI052206, AI088070, RR16475, and RR17686; the Terry C. Johnson Center for Basic Cancer Research, and the Kansas Agriculture Experiment Station. This is Kansas Agriculture Experiment Station publication 12-161-J.
Names are necessary to report factually on available data; however, the USDA neither guarantees nor warrants the standard of the product, and the use of the name by USDA implies no approval of the product to the exclusion of others that may also be suitable. The USDA is an equal opportunity provider and employer.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-No Derivative Works License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Contributor Information
Carol G. Chitko-McKown, Email: carol.chitkomckown@ars.usda.gov, bluesurly@yahoo.com.
Stephen K. Chapes, Email: skcbiol@k-state.edu.
Laura C. Miller, Email: laura.miller@ars.usda.gov.
Penny K. Riggs, Email: riggs@tamu.edu.
M. Teresa Ortega, Email: mto5535@ksu.edu.
Benedict T. Green, Email: ben.green@ars.usda.gov.
Richard D. McKown, Email: parasite@windstream.net.
Supplementary material
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.rinim.2013.03.001.
Appendix A. Supplementary materials
Fluorescence in situ hybridization to CΔ2-1 cell line of KAL1 (green) and CSF2RA (red). The two red and green signals are together on the normal X chromosome, while the CSF2RA region (red) has been translocated from the second X chromosome SSC X to the smaller submetacentric chromosome.
References
- 1.Van Reeth K., Adair B. Macrophages and respiratory viruses. Pathologie Biologie. 1997;45:184–192. [PubMed] [Google Scholar]
- 2.Maria-Pilar, J.deB., Dudal, S., Dornand, J., Gross, A., 2005. Cellular bioterrorism: how Brucella corrupts macrophage physiology to promote invasion and proliferation. Clinical Immunology. 114, 227-238. [DOI] [PubMed]
- 3.Donné E., Pasmans F., Boyen F., Van Immerseel F., Adriaensen C., Hernalsteens J.-P., Ducatelle R., Haesebrouck F. Survival of Salmonella serovar Typhimurium inside porcine monocytes is associated with complement binding and suppression of the production of reactive oxygen species. Veterinary Microbiology. 2005;107:205–214. doi: 10.1016/j.vetmic.2005.01.027. [DOI] [PubMed] [Google Scholar]
- 4.The Merck Veterinary Manual (Seventh Edition). 1991. Fraser, C.M., Bergeron, J.A., Mays, A., Aiello, S.E. (Eds.), Merck & Co., Inc., Rahway, NJ.
- 5.Beharka A.A., Armstrong J.W., Chapes S.K. Macrophage cell lines derived from major histocompatibility complex II-negative mice. In Vitro Cellularand Developmental Biology. 1998;34:499–507. doi: 10.1007/s11626-998-0085-y. [DOI] [PubMed] [Google Scholar]
- 6.Wardley R.C., Lawman M.J., Hamilton F. The establishment of continuous macrophage cell lines from peripheral blood monocytes. Immunology. 1980;39:67–73. [PMC free article] [PubMed] [Google Scholar]
- 7.Kadoi K., Tsukise A., Shiba H., Ikeda K., Seki T., Ariga T. Establishment of a swine monocyte cell line. New Microbiologica. 2001;24:243–247. [PubMed] [Google Scholar]
- 8.Weingartl H.M., Sabara M., Pasick J., van Moorlehem E., Babiuk L. Continuous porcine cell line developed from alveolar macrophages: partial characterization and virus susceptibility. Journal of Virological Methods. 2002;104:203–216. doi: 10.1016/S0166-0934(02)00085-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee Y.J., Park C.-K., Nam E., Kim S.-H., Lee O.-S., Lee D.S., Lee C. Generation of a porcine alveolar macrophage cell line for the growth of porcine reproductive and respiratory syndrome virus. Journal of Virological Methods. 2010;163:410–415. doi: 10.1016/j.jviromet.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 10.Chitko-McKown C.G., Fox J.M., Miller L.C., Heaton M.P., Bono J.L., Keen J.E., Grosse W.M., Laegreid W.W. Gene expression profiling of bovine macrophages in response to Escherichia coli O157:H7 lipopolysaccharide. Developmental and Comparative Immunology. 2004;28:635–645. doi: 10.1016/j.dci.2003.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Helgason C.D. Culture of primary adherent cells and a continuously growing nonadherent cell line. In: Helgason C.D., Miller C.L., editors. Basic cell culture protocols. Humana Press; New Jersey: 2005. p. 8. [DOI] [PubMed] [Google Scholar]
- 12.Yokoyama W.M. Cryopreservation of cells. In: Coligan J.E., Kruisbeek A.M., Margulies D.H., Shevach E.M., Strober W., editors. Current protocols in immunology. John Wiley & Sons, Inc.; New Jersey: 1997. A.3G.1–A.3G.3. [Google Scholar]
- 13.Riggs P.K., Owens K.E., Rexroad C.E., III, Amaral M.E.J., Womack J.E. Development and initial characterization of a Bos taurus × B. gaurus interspecific hybrid backcross panel. Journal of Heredity. 1997;88:373–379. doi: 10.1093/oxfordjournals.jhered.a023121. [DOI] [PubMed] [Google Scholar]
- 14.Rønne M. Double synchronization of human lymphocyte cultures: selection for high-resolution banded metaphases in the first and second division. Cytogenetics and Cell Genetics. 1985;39:292–295. doi: 10.1159/000132160. [DOI] [PubMed] [Google Scholar]
- 15.Committee for the Standardized Karyotype of the domestic pig Standard karyotype of the domestic pig. Hereditas. 1988;109:151–157. doi: 10.1111/j.1601-5223.1988.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 16.Raudsepp T., Chowdhary B.P. The horse pseudoautosomal region (PAR): characterization and comparison with the human, chimp, and mouse PARs. Cytogenetic and Genome Research. 2008;121:102–109. doi: 10.1159/000125835. [DOI] [PubMed] [Google Scholar]
- 17.Potts B.E., Hart M.L., Snyder L.L., Boyle D., Mosier D.A., Chapes S.K. Differentiation of C2D macrophage cells after adoptive transfer. Clinical and Vaccine Immunology. 2008;15:243–252. doi: 10.1128/CVI.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laegreid W.W., Hoffman M., Keen J., Elder R., Kwang J. Development of a blocking enzyme-linked immunosorbant assay for detection of serum antibodies to O157 antigen of Escherichia coli. Clinical and Diagnostic Laboratory Immunology. 1998;5:242–246. doi: 10.1128/cdli.5.2.242-246.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuehr D.J., Gross S.S., Sakuma I., Levi R., Nathan C.F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity or endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. Journal of Experimental Medicine. 1989;169:1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stevens M.G., Kehrli M.E., Jr., Canning P.C. A colorimetric assay for quantitating bovine neutrophil bactericidal activity. Veterinary Immunology and Immunopathology. 1991;28:45–56. doi: 10.1016/0165-2427(91)90042-b. [DOI] [PubMed] [Google Scholar]
- 21.Potts B.E., Chapes S.K. Functions of C2D macrophage cells after adoptive transfer. Journal of Leukocyte Biology. 2008;83:602–609. doi: 10.1189/jlb.0607365. [DOI] [PubMed] [Google Scholar]
- 22.Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Analytical Biochemistry. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 23.Duvigneau J.C., Hartl R.T., Groiss S., Gemeiner M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. Journal of Immunological Methods. 2005;306:16–27. doi: 10.1016/j.jim.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 24.Bolin S.R., Ridpath J.F., Black J., Macy M., Roblin R.R. Survey of cell lines in the American Type Culture Collection for bovine viral diarrhea virus. Journal of Virological Methods. 1994;48:211–221. doi: 10.1016/0166-0934(94)90120-1. [DOI] [PubMed] [Google Scholar]
- 25.Ridpath J.F., Bolin S.R. Differentiation of types 1a, 1b and 2 bovine viral diarrhoea virus (BVDV) by PCR. Molecular and Cellular Probes. 1998;12:101–106. doi: 10.1006/mcpr.1998.0158. [DOI] [PubMed] [Google Scholar]
- 26.Opriessnig T., Yu S., Gallup J.M., Evans R.B., Fenaux M., Pallares F., Thacker E.L., Brockus C.W., Ackermann M.R., Thomas P., Meng X.J., Halbur P.G. Effect of vaccination with selective bacterins on conventional pigs infected with type 2 porcine circovirus. Veterinary Pathology. 2003;40:521–529. doi: 10.1354/vp.40-5-521. [DOI] [PubMed] [Google Scholar]
- 27.Van Furth R. Phagocytic cells: Development and distribution of mononuclear phagocytes in normal steady state and inflammation. In: Gallin J.I., Goldstein I.M., Snyderman R., editors. Inflammation. Raven Press; New York: 1988. pp. 281–296. [Google Scholar]
- 28.Ravetch J.V., Kinet J.-P. Fc receptors. Annual Review of Immunology. 1991;9:457–492. doi: 10.1146/annurev.iy.09.040191.002325. [DOI] [PubMed] [Google Scholar]
- 29.Fleit H.B., Kobasiuk C.D. The human monocyte-like cell line THP-1 expresses FcγRI and FcγRII. Journal of Leukocyte Biology. 1991;49:556–565. doi: 10.1002/jlb.49.6.556. [DOI] [PubMed] [Google Scholar]
- 30.Leenen P.J.M., Melis M., Slieker W.A.T., van Ewijk W. Murine macrophage precursor characterization. II. Monoclonal antibodies against macrophage precursor antigens. European Journal of Immunology. 1990;20:27–34. doi: 10.1002/eji.1830200105. [DOI] [PubMed] [Google Scholar]
- 31.Leenen P.J.M., de Bruijn M.F.T.R., Voerman J.S.A., Campbell P.A., van Ewijk W. Markers of mouse macrophage development detected by monoclonal antibodies. Journal of Immunological Methods. 1994;174:5–19. doi: 10.1016/0022-1759(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 32.Ezquerra A., Revilla C., Alvarez B., Pérez C., Alonso F., Domínguez J. Porcine myelomonocytic markers and cell populations. Developmental and Comparative Immunology. 2009;33:284–298. doi: 10.1016/j.dci.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 33.Calvert J.G., Slade D.E., Shields S.L., Jolie R., Mannan R.M., Ankenbauer R.G., Welch S.-K.W. CD163 expression confers susceptibility to porcine reproductive and respiratory syndrome viruses. Journal of Virology. 2007;81:7371–7379. doi: 10.1128/JVI.00513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Gorp H.W., Van Breedam W., Delputte P.L., Nauwynck H.J. Sialoadhesin and CD163 join forces during entry of the procine reproductive and respiratory syndrome virus. Journal of General Virology. 2008;89:2943–2953. doi: 10.1099/vir.0.2008/005009-0. [DOI] [PubMed] [Google Scholar]
- 35.Zelnickova P., Matiasovic J., Pavlova B., Kudlackova H., Kovaru F., Faldyna M. Quantitative nitric oxide production by rat, bovine and porcine macrophages. Nitric Oxide. 2008;19:36–41. doi: 10.1016/j.niox.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Fleming S.D., Iandolo J.J., Chapes S.K. Murine macrophage activation by staphylococcal exotoxins. Infection and Immunity. 1991;59:4049–4055. doi: 10.1128/iai.59.11.4049-4055.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chitko-McKown C.G., Reddy D.N., Chapes S.K., McKown R.D., Blecha F. Immunological characterization of pulmonary intravascular macrophages. Regulatory Immunology. 1992;4:236–244. [PubMed] [Google Scholar]
- 38.Pampusch M.S., Bennaars A.M., Harsch S., Murtaugh M.P. Inducible nitric oxide synthase expression in porcine immune cells. Veterinary Immunology and Immunopathology. 1998;61:279–289. doi: 10.1016/s0165-2427(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 39.Kapetanovic R., Fairbairn L., Beraldi D., Sester D.P., Archibald A.L., Tuggle C.K., Hume D.A. Pig bone marrow-derived macrophages resemble human macrophages in their response to bacterial lipopolysaccharide. Journal of Immunology. 2012;188:3382–3394. doi: 10.4049/jimmunol.1102649. [DOI] [PubMed] [Google Scholar]
- 40.Cobo F., Stacey G.N., Hunt C., Cabrera C., Nieto A., Montes R., Cortés J.L., Catalina P., Barnie A., Concha A. Microbiological control in stem cell banks: approaches to standardization. Applied Microbiology and Biotechnology. 2005;68:456–466. doi: 10.1007/s00253-005-0062-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fluorescence in situ hybridization to CΔ2-1 cell line of KAL1 (green) and CSF2RA (red). The two red and green signals are together on the normal X chromosome, while the CSF2RA region (red) has been translocated from the second X chromosome SSC X to the smaller submetacentric chromosome.


