Abstract
Objectives
The main reason for restoration failure is secondary caries caused by biofilm acids. Replacing the failed restorations accounts for 50–70% of all operative work. The objectives of this study were to incorporate a new quaternary ammonium monomer (dimethylaminododecyl methacrylate, DMADDM) and nanoparticles of silver (NAg) into a primer and an adhesive, and to investigate their effects on antibacterial and dentin bonding properties.
Methods
Scotchbond Multi-Purpose (SBMP) served as control. DMADDM was synthesized and incorporated with NAg into primer/adhesive. A dental plaque microcosm biofilm model with human saliva was used to investigate metabolic activity, colony-forming units (CFU), and lactic acid. Dentin shear bond strengths were measured.
Results
Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) of the new DMADDM were orders of magnitude lower than those of a previous quaternary ammonium dimethacrylate (QADM). Uncured primer with DMADDM had much larger inhibition zones than QADM (p<0.05). Cured primer/adhesive with DMADDM-NAg greatly reduced biofilm metabolic activity (p<0.05). Combining DMADDM with NAg in primer/adhesive resulted in less CFU than DMADDM alone (p<0.05). Lactic acid production by biofilms was reduced by 20-fold via DMADDM-NAg, compared to control. Incorporation of DMADDM and NAg into primer/adhesive did not adversely affect dentin bond strength.
Conclusions
A new antibacterial monomer DMADDM was synthesized and incorporated into primer/adhesive for the first time. The bonding agents are promising to combat residual bacteria in tooth cavity and invading bacteria at tooth-restoration margins to inhibit caries. DMADDM and NAg are promising for use into a wide range of dental adhesive systems and restoratives.
Keywords: Antibacterial dental adhesive, dentin bond strength, silver nanoparticles, quaternary ammonium methacrylate, human saliva microcosm biofilm, caries inhibition
1. Introduction
Half of all dental restorations fail within 10 years, mainly due to secondary caries and fracture.1–5 Replacing the failed restorations accounts for 50–70% of all restorations performed.6–8 This is costly, considering that the annual cost for tooth cavity restorations in the U.S. was $46 billion in 2005.9 Furthermore, the need is rapidly increasing with the baby boomers entering into retirement, and with increases in life expectancy and in tooth retention in seniors.10 Composites are the principal material for cavity restorations.4,5,11,12 Advances in polymers and fillers have significantly enhanced the composite properties.13–18 However, one major drawback is that composites tend to accumulate more biofilms/plaques in vivo than other restorative materials.19,20 Biofilms with the exposure to fermentable carbohydrates are responsible for tooth caries.21,22 Hence, efforts were made to develop antibacterial resins, and novel quaternary ammonium methacrylates (QAMs) were synthesized.23–30 Previous studies made 12-methacryloyloxydodecyl-pyridinium bromide (MDPB) and other antibacterial monomers to hinder bacteria and biofilm growth.23–30
Besides composites, it is also important to develop antibacterial adhesives, because composite restorations are bonded to the tooth structure via adhesives.31–35 Efforts have been made in previous studies to increase the dentin bond strength and determine the mechanisms of tooth-restoration adhesion.36–40 It is beneficial for the adhesive to be antibacterial to reduce biofilm acids and caries at the margins.23,25,41 Besides residual bacteria in the prepared tooth cavity, marginal leakage would allow bacteria to invade the tooth-restoration interface. Therefore, antibacterial adhesives are being developed to help inhibit the residual as well as the invading bacteria.42,43 Previous studies showed that MDPB-containing adhesives inhibited Streptococcus mutans (S. mutans) growth.24,44 Another study developed an antibacterial adhesive containing methacryloxyl ethyl cetyl dimethyl ammonium chloride (DMAE-CB).25 Besides the adhesive, it is also useful for the primer to be antibacterial, because the primer directly contacts the tooth structure. A primer containing MDPB was reported to possess strong antibacterial functions.44,45 In another study, chlorhexidine particles were mixed into a primer to obtain antibacterial properties.46 Recently, a quaternary ammonium dimethacrylate (QADM) was synthesized and incorporated into a nanocomposite and a primer.28,47,48 In our preliminary study, a new quaternary ammonium monomer, dimethylaminododecyl methacrylate (DMADDM), was synthesized. DMADDM was much more strongly antibacterial than QADM as shown in preliminary results. However, DMADDM has not been tested in primer and adhesive.
The objectives of this study were to incorporate the new DMADDM into primer and adhesive, and to investigate the effects on antibacterial and dentin bonding properties for the first time. It was hypothesized that: (1) The new DMADDM will possess much stronger antibacterial potency than the previously-synthesized QADM in minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) tests; (2) primer and adhesive containing DMADDM will inhibit microcosm biofilm growth, metabolic activity, and lactic acid production; (3) Combining DMADDM with nanoparticles of silver (NAg) in primer and adhesive will further increase the anti-biofilm potency, without compromising dentin bond strength, compared to commercial non-antibacterial primer and adhesive control.
2. Materials and methods
2.1. Developing new antibacterial monomers
Two new antibacterial monomers were synthesized: dimethylaminohexane methacrylate (DMAHM) with an alkyl chain length of 6, and dimethylaminododecyl methacrylate (DMADDM) with an alkyl chain length of 12. A modified Menschutkin reaction method was employed, which used a tertiary amine group to react with an organo-halide, following previous studies.28,47,48 To synthesize DMAHM, 2-bromoethyl methacrylate (BEMA) served as the organo halide, and N,N-dimethylaminohexane (DMAH) served as the tertiary amine. Ten mmol of DMAH (Tokyo Chemical Industry, Tokyo, Japan), 10 mmol of BEMA (Monomer-Polymer and Dajac Labs, Trevose, PA), and 3 g of ethanol were added to a 20 mL scintillation vial with a magnetic stir bar. The vial was capped and stirred at 70 °C for 24 h. After the reaction was complete, the ethanol solvent was removed via evaporation, yielding DMAHM as a clear, colorless, and viscous liquid. To synthesize the second new monomer DMADDM, BEMA was the organo halide, and 1-(dimethylamino)docecane (DMAD) was the tertiary amine. Ten mmol of DMAD (Tokyo Chemical Industry) and 10 mmol of BEMA were added in a 20 mL vial, while otherwise following the same procedures as for DMAHM. Fourier transform infrared (FTIR) spectroscopy (Nicolet 6700, Thermo Scientific, Waltham, MA) spectra of the starting materials and the products were collected between two KBr windows in the 4000 to 400 cm−1 region. 1H NMR spectra (GSX 270, JEOL) were taken in deuterated chloroform at a concentration of about 3%.28 The reactions and products of DMAHM and DMADDM were all verified in preliminary studies.
2.2. Minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC)
The use of S. mutans (ATCC 700610, American Type Culture, Manassas, VA) was approved by the University of Maryland. S. mutans is a cariogenic, aerotolerant anaerobic bacterium and the primary causative agent of dental caries.22 MIC and MBC were determined via serial microdilution assays.45,49 Unpolymerized DMAHM or DMADDM monomer was dissolved in brain heart infusion (BHI) broth (BD, Franklin Lakes, NJ) to a concentration of 200 mg/mL. From these starting solutions, serial two fold dilutions were made into 1 mL volumes of BHI broth. Fifteen μL of stock S. mutans was added to 15 mL of BHI broth with 0.2% sucrose and incubated at 37 °C with 5% CO2. Overnight cultures of S. mutans were adjusted to 2×106 CFU/mL with BHI, and 50 μL of inoculum was added to each well of a 96-well plate containing 50 μL of a series of antibacterial monomer dilution broths. BHI with 1×106 CFU/mL bacteria suspension without antibacterial agent served as negative control. Chlorhexidine diacetate (CHX) (Sigma, St. Louis, MO) served as positive control. The previously-synthesized QADM28,47 served as an antibacterial monomer control. The wells were read for turbidity after incubation at 37 °C in 5% CO2 for 48 h, referenced by the negative and positive control wells. MIC was defined as the endpoint (the well with the lowest antibacterial agent concentration) where no turbidity could be detected with respect to the controls.49 An aliquot of 50 μL from each well without turbidity was inoculated on BHI agar plates. After incubation at 37 °C in 5% CO2 for 48 h, the MBC value was defined as the lowest concentration of antibacterial agent that produced no colonies on the plate. The tests were performed in triplicate.49
2.3. Fabrication of antibacterial primer and adhesive
Scotchbond Multi-Purpose (3M, St. Paul, MN), referred to as “SBMP”, was used as the parent bonding system to test the effect of incorporation of antibacterial agents. According to the manufacturer, SBMP etchant contains 37% phosphoric acid. SBMP primer contains 35–45% 2-Hydroxyethylmethacrylate (HEMA), 10–20% copolymer of acrylic and itaconic acids, and 40–50% water. SBMP adhesive contains 60–70% BisGMA and 30–40% HEMA.
DMAHM was mixed with SBMP primer at a DMAHM/(primer + DMAHM) mass fraction of 5%. The 5% was selected following a previous study.45 Similarly, 5% of DMAHM was incorporated into the SBMP adhesive. The second new monomer, DMADDM, was also incorporated into the SBMP primer and adhesive at 5% mass fraction.
Another antibacterial agent NAg was also incorporated into the primer and adhesive. Silver 2-ethylhexanoate powder (Strem, New Buryport, MA) was dissolved in 2-(tert-butylamino)ethyl methacrylate (TBAEMA, Sigma) at 0.1 g of silver salt per 0.9 g of TBAEMA.47,50 TBAEMA was used because it improves the solubility by forming Ag-N coordination bonds with Ag ions, thereby facilitating the Ag salt to dissolve in the resin solution. TBAEMA was selected since it contains reactive methacrylate groups and therefore can be chemically incorporated into a dental resin upon photo-polymerization.50 This method produced NAg with a mean particle size of 2.7 nm that were well dispersed in the resin matrix.47,50 The Ag solution was mixed with SBMP primer at a silver 2-ethylhexanoate/(primer + silver 2-ethylhexanoate) mass fraction of 0.1%, following a previous study.51 The same 0.1% was used in the SBMP adhesive to formulate the antibacterial adhesive.
2.4. Dental plaque microcosm model
The dental plaque microcosm model was approved by the University of Maryland. Saliva was collected from a healthy adult donor having natural dentition without active caries or periopathology, and without the use of antibiotics within the past three months.48,52 The donor did not brush teeth for 24 h and abstained from food/drink intake for at least 2 h prior to donating saliva. Stimulated saliva was collected during parafilm chewing and kept on ice. Saliva was diluted in sterile glycerol to a saliva concentration of 70 % and stored at −80 °C.48
2.5. Agar disk-diffusion test of uncured antibacterial primers
Agar disk diffusion test (ADT) was used to examine the antibacterial effect of uncured primers. Five primers were tested: SBMP control primer (referred to as “P”); P + 5% QADM; P + 5% DMAHM; P + 5% DMADDM; P + 5% DMADDM + 0.1% NAg.
Three types of culture media were used: (A) Tryptic Soy Blood Agar culture plates to determine total microorganisms; (B) mitis salivarius agar (MSA) culture plates, containing 15% sucrose, to determine total streptococci; (C) MSA agar culture plates plus 0.2 units of bacitracin per mL to determine mutans streptococci. The saliva-glycerol stock was added to a growth medium containing mucin (at a concentration of 2.5 g/L), bacteriological peptone (2.0 g/L), tryptone (2.0 g/L), yeast extract (1.0 g/L), NaCl (0.35 g/L), KCl (0.2 g/L), CaCl2 (0.2 g/L), and cysteine hydrochloride (0.1 g/L), at pH of 7.52 The inoculum was incubated at 37 °C in 5% CO2 for 24 h. After 24 h, 0.4 mL of bacteria suspension was swabbed across an agar plate with a diameter of 90 mm. A sterile paper disk with a diameter of 6 mm and a thickness of 1.5 mm was impregnated with 20 μL of a primer. The primer-impregnated paper disk was placed on an agar plate with bacteria, and incubated in 5% CO2 at 37 °C for 48 h. Bacteria inhibition zone size = (Outer diameter of inhibition zone − paper disk diameter)/2.45
2.6. Specimen fabrication and biofilm culture
The MIC, MBC and ADT results showed that DMADDM was much more strongly antibacterial than DMAHM and QADM. Therefore, DMADDM was selected for incorporation into SBMP primer and adhesive for the subsequent experiments.
Six bonding agents were used in biofilm tests:
SBMP control primer P and adhesive A (referred to as “SBMP P & A control”)
P+5% DMADDM, with unmodified adhesive A (referred to as “P+DMADDM, A control”)
P+5% DMADDM+0.1% NAg, unmodified A (“P+DMADDM+NAg, A control”)
A+5% DMADDM, with biofilm on adhesive without primer (“A+DMADDM, no P”)
A+5% DMADDM+0.1% NAg, biofilm on adhesive, no primer (“A+DMADDM+NAg, no P”)
5% DMADDM and 0.1% NAg were added to both A and P (“A&P+DMADDM+NAg”)
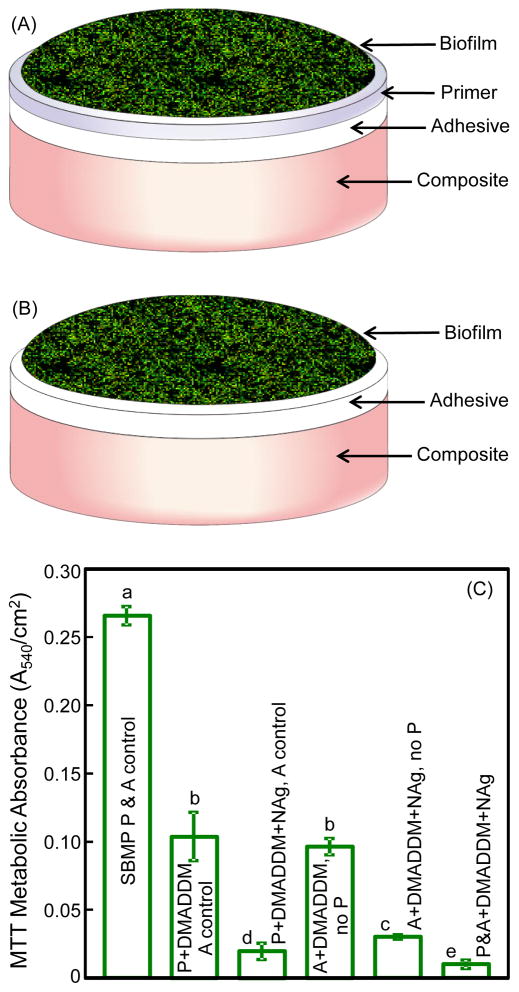
The purpose of i–iii was to investigate the new DMADDM and its combination with NAg in cured primer on antibacterial properties (schematic in Fig. 3A). The purpose of iv and v was to examine the antibacterial effect of adhesive, with biofilms on adhesive without primer (schematic in Fig. 3B). The purpose of vi was to determine the effect of both primer and adhesive being antibacterial (Fig. 3A), instead of using antibacterial primer alone (iii), or antibacterial adhesive alone (v).
Fig. 3.
Biofilm experiments and MTT metabolic activity: (A) Schematic of biofilm on the cured disk of primer covering the adhesive and composite, (B) biofilm on the cured disk of adhesive covering the composite, and (C) MTT metabolic activity. Biofilms were grown for 2 days using a microcosm model. In (C), groups 1–3, and 6, were tested following schematic A. Groups 4 and 5 were tested following schematic B without a primer layer, to investigate the antibacterial activity of the adhesive. Each values is mean ± sd (n = 6). Values with dissimilar letters are significantly different from each other (p < 0.05).
Layered disk specimens for biofilm experiments were fabricated following previous studies.25,44 A polyethylene disk mold (inner diameter = 9 mm, thickness = 2 mm) was situated on a glass slide. For groups i, ii, iii and vi, a primer was first applied into the mold to cover the glass slide. After drying with a stream of air, an adhesive was applied and cured for 20 s (Optilux VCL 401, Demetron Kerr, Danbury, CT). Then, a composite (TPH, Caulk/Dentsply, Milford, DE) was placed on the adhesive to fill the disk mold and was light-cured for 1 min. For groups iv and v, each adhesive was applied into the mold to cover the glass slide. Then, a composite (TPH) was placed onto the adhesive to fill the disk mold and light-cured for 1 min. The disks were immersed in sterile water and agitated for 1 h to remove any uncured monomer, following a previous study.44 The disks were then dried and sterilized with ethylene oxide (Anprolene AN 74i, Andersen, Haw River, NC).
The saliva-glycerol stock was added, with 1:50 final dilution, into the growth medium as inoculum, as described above. A layered disk was placed in a well of 24-well plates, and 1.5 mL of inoculum was added and incubated in 5% CO2 at 37 °C for 8 h. The disks were then transferred to new 24-well plates filled with fresh medium and incubated. After 16 h, the disks were transferred to new 24-well plates with fresh medium and incubated for 24 h. Microcosm biofilms were formed on the disks with this 48 h incubation, as shown previously.48,51
2.7. MTT assay of biofilm metabolic activity
A MTT (3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide) assay was used to examine the metabolic activity of biofilms.47 MTT is a colorimetric assay that measures the enzymatic reduction of MTT, a yellow tetrazole, to formazan. Disks with 2-day biofilms were transferred to a new 24-well plate, and 1 mL of MTT dye (0.5 mg/mL MTT in PBS) was added to each well and incubated at 37 °C in 5% CO2 for 1 h. During this process, metabolically active bacteria reduced the MTT to purple formazan. After 1 h, the disks were transferred to a new 24-well plate, 1 mL of dimethyl sulfoxide (DMSO) was added to solubilize the formazan crystals, and the plate was incubated for 20 min at room temperature in the dark. After mixing via pipetting, 200 μL of the DMSO solution from each well was transferred to a 96-well plate, and the absorbance at 540 nm was measured via a microplate reader (SpectraMax M5, Molecular Devices, Sunnyvale, CA). A higher absorbance is related to a higher formazan concentration, which indicates a higher metabolic activity in the biofilm on the disk.
2.8. Live/dead bacterial staining assay for biofilms
The disks with 2-day biofilms were washed with phosphate buffered saline (PBS) and stained using the BacLight live/dead kit (Molecular Probes, Eugene, OR). Live bacteria were stained with Syto 9 to produce a green fluorescence, and bacteria with compromised membranes were stained with propidium iodide to produce a red fluorescence. The stained disks were examined using a confocal laser scanning microscopy (CLSM 510, Carl Zeiss, Thornwood, NY).
2.9. Colony-forming units (CFU) and lactic acid production
Disk with 2-day biofilms were rinsed with cysteine peptone water (CPW) to remove loose bacteria. The disks were transferred to 24-well plates containing buffered peptone water (BPW) plus 0.2% sucrose, and incubated in 5% CO2 at 37 °C for 3 h to allow the biofilms to produce acid.47,48 The BPW solutions were stored for lactate analysis. Disks with biofilms were transferred into tubes with 2 mL CPW, and the biofilms were harvested by sonication and vortexing (Fisher, Pittsburgh, PA). Three types of agar plates were used to measure the CFU counts to assess the microorganism viability.48 First, tryptic soy blood agar culture plates were used to determine total microorganisms. Second, (MSA) culture plates containing 15% sucrose were used to determine total streptococci.53 Third, MSA agar culture plates plus 0.2 units of bacitracin per mL was used to determine mutans streptococci.
The lactate concentrations in BPW solutions were determined using an enzymatic (lactate dehydrogenase) method.47,48 The microplate reader was used to measure the absorbance at 340 nm. Standard curves were prepared using a lactic acid standard (Supelco, Bellefonte, PA).47,48
2.10. Dentin shear bond strength
The use of extracted human teeth was approved by the University of Maryland. Caries-free molars were cleaned and stored in 0.01% thymol solution. Flat mid-coronal dentin surfaces were prepared by cutting off the tips of crowns with a diamond saw (Isomet, Buehler, Lake Bluff, IL). Each tooth was embedded in a poly-carbonate holder (Bosworth, Skokie, IL) and ground perpendicular to the longitudinal axis on 320-grit silicon carbide paper until the occlusal enamel was removed. The dentin surface was etched with 37% phosphoric acid gel for 15 s and rinsed with water for 15 s.54 A primer was applied with a brush-tipped applicator and rubbed in for 15 s. The solvent was removed with a stream of air for 5 s. An adhesive was applied and light-cured for 10 s (Optilux). A stainless-steel iris, having a central opening with a diameter of 4 mm and a thickness of 1.5 mm, was held against the adhesive-treated dentin surface. The opening was filled with a composite (TPH) and light-cured for 60 s.54 The bonded specimens were stored in water at 37 °C for 24 h. A chisel with a Universal Testing Machine (MTS, Eden Prairie, MN) was aligned to be parallel to the composite-dentin interface.48,54 The load was applied at a rate of 0.5 mm/min until the bond failed. Dentin shear bond strength, SD, was calculated as: SD = 4P/(πd2), where P is the load at failure, and d is the diameter of the composite.48,54
2.11. Statistical analyses
One-way analysis of variance (ANOVA) was performed to detect the significant effects of the variables. Tukey’s multiple comparison test was used to compare the data at a p value of 0.05.
3. Results
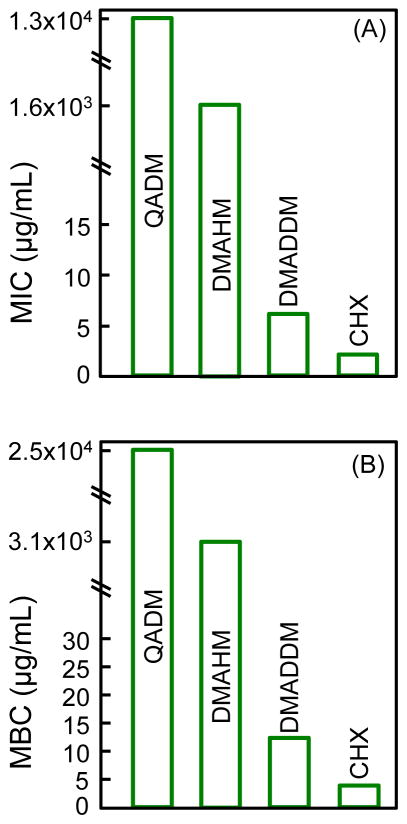
The MIC and MBC results are plotted in Fig. 1. A low concentration needed to inhibit the bacteria indicates a high potency for the antibacterial agent. The new DMAHM was more strongly antibacterial than the previously-synthesized QADM, requiring a lower concentration, by an order of magnitude, to achieve the same bacteria-inhibitory effect. The new DMADDM was even more potent than DMAHM. The MIC and MBC of DMADDM was three orders of magnitude lower than those of DMAHM, and four orders of magnitude lower than QADM. The MIC and MBC of DMADDM approached those of CHX control.
Fig. 1.
Minimum inhibitory concentration (MIC), and minimum bactericidal concentration (MBC) against S. mutans. A lower MIC and MBC indicate a stronger antibacterial potency for the antibacterial agent. CHX had the lowest MIC and MBC. Regarding the two new monomers, DMADDM (dimethylaminododecyl methacrylate) with an alkyl chain length of 12 was much more potent than DMAHM (dimethylhexylamine methacrylate) with a chain length of 6. The tests were performed in triplicate (n = 3).
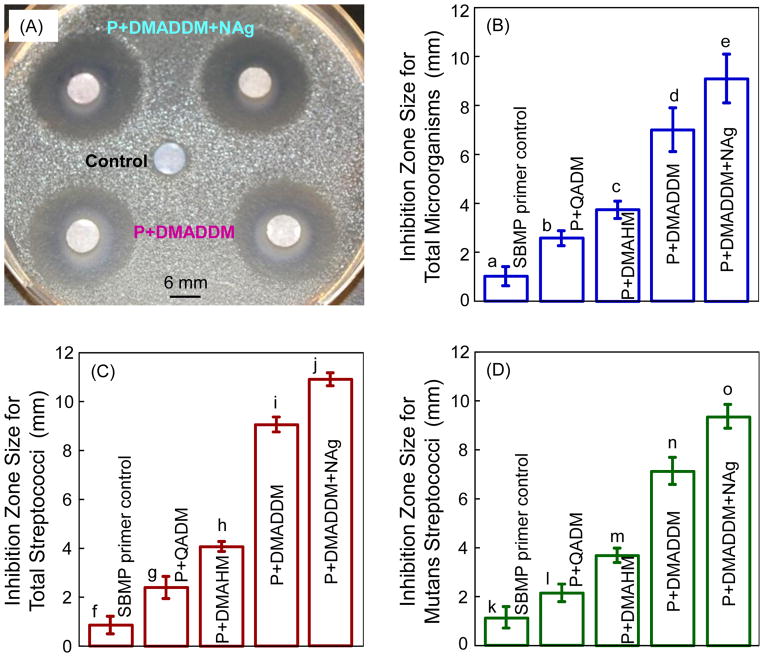
The ADT results for uncured primers are shown in Fig. 2. In (A), the commercial control primer had a minimal inhibition zone, as expected. Incorporation of QADM, DMAHM and DMADDM significantly increased the inhibition zone size. In (B–D), the primer with DMADDM had significantly larger inhibition zones than the primers with DMAHM and QADM (p < 0.05). Incorporating 0.1% NAg into the primer with DMADDM further increased the inhibition zone size, which was about 10-fold those of the SBMP control primer (p < 0.05).
Fig. 2.
Antibacterial activity of uncurd primers against human saliva microcosm bacteria in agar disk diffusion test (ADT). In the examples shown in (A), the top two paper disks were impregnated with P+DMADDM+NAg. The middle disk was impregnated with control primer. The two lower disks were impregnated with P+DMADDM. Primer with the new DMADDM and NAg had strong antibacterial effects against (B) total microorganisms, (C) total streptococci, and (D) mutans streptococci. Each value is mean ± sd (n = 6). In each plot, values with dissimilar letters are different from each other (p < 0.05).
The biofilm setup schematic and metabolic activity are shown in Fig. 3. In (A), the biofilm was adherent on the cured primer covering the adhesive and the composite. In (B), the biofilm was on the adhesive covering the composite without a primer, for the purpose of testing the antibacterial properties of the adhesive. In (C), the first three groups followed the setup in (A), groups 4 and 5 followed the setup in (B), and the last group also followed (A). The MTT results (mean ± sd; n = 6) in (C) showed that biofilms on SBMP had a high metabolic activity. Incorporation of DMADDM and NAg into the primer greatly reduced the metabolic activity (p < 0.05). Similarly, incorporating DMADDM and NAg into the adhesive also reduced the metabolic activity. In the last group, with both primer and adhesive being antibacterial, the lowest metabolic activity was achieved. A&P+DMADDM+NAg yielded a biofilm metabolic activity that was about 20-fold lower than that of commercial bonding agent control.
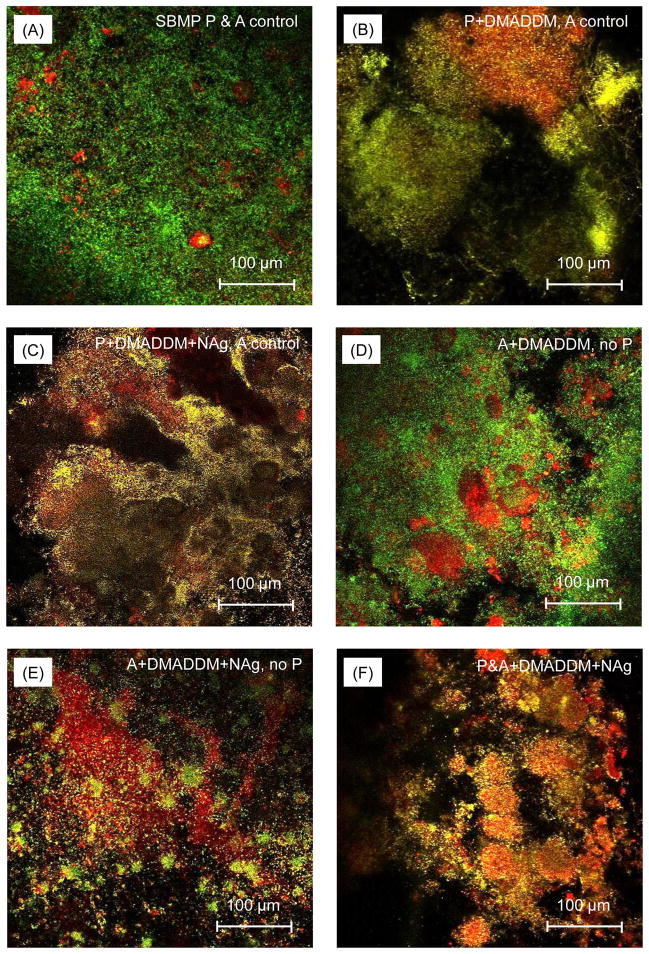
Fig. 4 shows representative confocal images of live/dead staining of biofilms adherent on the disks for the six groups. Biofilms on control disks had primarily live bacteria (A). In contrast, substantial increases in dead bacteria occurred when DMADDM (B), or DMADDM and NAg (C), were incorporated into primer. Similarly, the adhesive containing DMADDM (D), or DMADDM and NAg (E), had more dead bacteria. In (F), when both primer and adhesive contained DMADDM + NAg, the biofilms consisted of primarily dead bacteria.
Fig. 4.
Confocal laser scanning microscopy (CLSM) images of biofilms on the cured disks of the six groups. Each group name is indicated in the image. Live bacteria were stained green, and dead bacteria were stained red. Live and dead bacteria in close proximity to each other yielded yellow and orange colors. Incorporation of DMADDM and NAg into the primer and adhesive provided strong antibacterial effects.
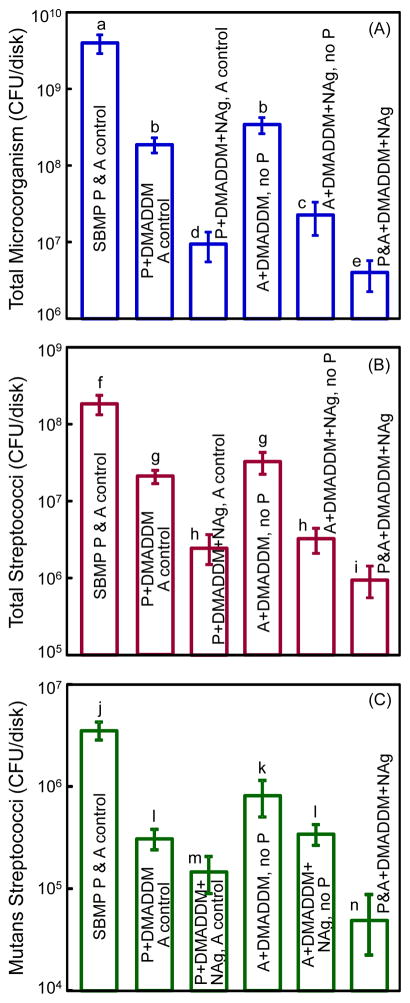
Fig. 5 plots the CFU for: (A) total microorganisms, (B) total streptococci, and (C) mutans streptococci. The values are shown in a log scale. Incorporation of DMADDM into primer greatly reduced all three CFU counts, compared to control group (p < 0.05). Combining DMADDM with NAg in the primer significantly decreased the CFU than that using DMADDM alone (p < 0.05). The same trend was observed when DMADDM and NAg were incorporated into the adhesive.
Fig. 5.
Biofilm CFU on the cured disks of the six groups for: (A) Total microorganisms, (B) total streptococci, and (C) mutans streptococci. Note the log scale for the y-axis. The new DMADDM greatly reduced the CFU. Adding DMADDM and NAg together in primer or adhesive was more potent than using DMADDM alone. Each values is mean ± sd (n = 6). Values with dissimilar letters are significantly different from each other (p < 0.05).
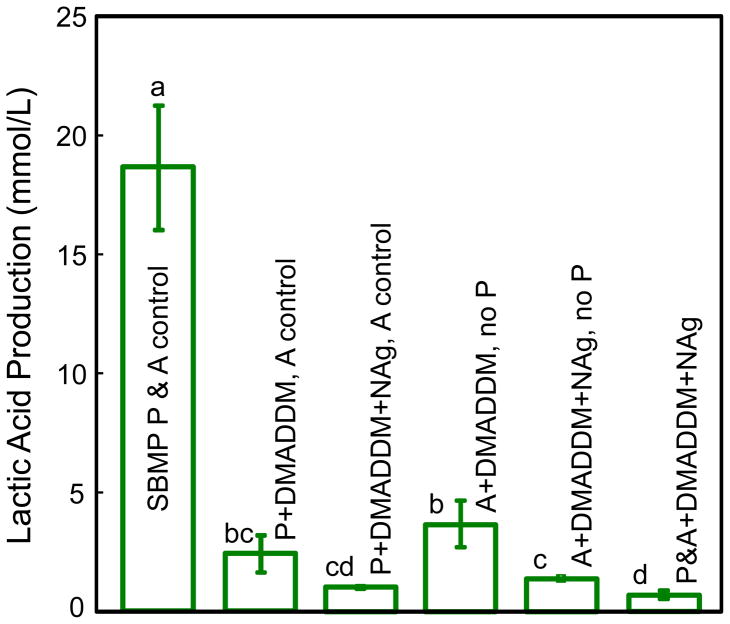
Lactic acid production by biofilms is plotted in Fig. 6. Biofilms on control primer produced the most acid. Adding 5% of DMADDM in either primer or adhesive greatly reduced acid production, comparing to the control (p < 0.05). Using DMADDM+NAg had a stronger acid-inhibiting effect than using DMADDM alone (p < 0.05). Adding DMADDM+NAg in both primer and adhesive further reduced lactic acid (p < 0.05). The lactic acid production by biofilms on P&A+DMADDM+NAg was approximately 1/20 of that on the commercial bonding agent.
Fig. 6.
Lactic acid production by microcosm biofilms adherent on the cured specimens for the six groups. Bars 1–3 are for biofilms on primer, with the adhesive being the unmodified SBMP adhesive. Bars 4 and 5 are for biofilms on the adhesive without a primer. Bar 6 is for primer and adhesive both being antibacterial. Each value is mean ± sd (n = 6). Values with dissimilar letters are significantly different from each other (p < 0.05).
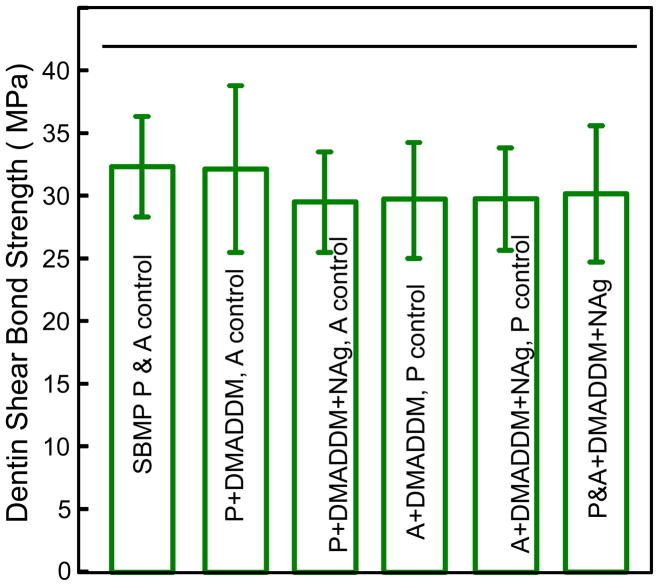
Fig. 7 plots the dentin shear bond results (mean ± sd; n = 10). The six groups had shear bond strengths that were not significantly different from each other (p > 0.1). This indicates that incorporation of the new quaternary ammonium monomer DMADDM and NAg into primer and adhesive to obtain antibacterial activity did not adversely affect the dentin shear bond strength.
Fig. 7.
Dentin shear bond strength. Ten molars were used for each group, for a total of sixty teeth. Each value is mean ± sd (n = 10). Horizontal line indicates that the bond strength was not compromised by adding DMADDM and NAg into primer/adhesive (p > 0.1).
4. Discussion
The present study synthesized new antibacterial monomers DMAHM and DMADDM, and they both possessed much stronger antibacterial potency than a previously-reported QADM,28,47 manifested in MIC, MBC, and ADT results. In particular, DMADDM with a carbon chain length of 12 was much more strongly antibacterial than DMAHM with a chain length of 6. Quaternary ammonium salts can cause bacteria lysis by binding to cell membrane to cause cytoplasmic leakage.55 When the negatively-charged bacterial cell contacts the positive charge of quaternary amine N+, the electric balance is disturbed and the bacterium could explode under its own osmotic pressure.41 The long cationic polymers can penetrate bacterial cells to disrupt the membrane, like a needle bursting a balloon.56,57 Hence, the carbon chain length needs to be long enough to penetrate the cell membrane.56,57 However, there has been little report on the effect of carbon chain length in dental monomers on antibacterial effects. A literature search revealed only one report on the effect of chain length on antibacterial dental materials: a recent study on glass ionomers showed that increasing the chain length significantly increased the antibacterial potency.26 In the present study, increasing the chain length from 6 to 12 reduced the MIC and MBC by three orders of magnitude. Further study is needed to increase the chain length beyond 12 to investigate if the antibacterial potency can be further increased. It needs to be determined whether there exists an optimal chain length above which the antibacterial activity plateaus or even decrease, and if physical properties such as viscosity and degrees of conversion of dental resins containing these new QAMs will be affected.
DMADDM-containing primer and adhesive were prepared in the present study for the first time. The results showed that the new antibacterial bonding agents had strong antibacterial functions, without adversely affecting the dentin bond strength. Antibacterial primer and adhesive are important, because recurrent caries at the tooth-restoration margins is the main reason for failure.6–8 Caries is a dietary carbohydrate-modified bacterial infectious disease caused by the secretion of acids from biofilms.21,22 After removal of carious tissue, there are often residual bacteria present in the prepared tooth cavity.25,43 With the increased interest in less removal of tooth structure and in minimal intervention dentistry,58 more carious tissues with active bacteria are expected to be left in the tooth cavity. Dentin primer has direct contact with the tooth structure and flows into dentinal tubules. Therefore, the antibacterial primer of the present study, in the un-cured state, could be useful in killing the residual bacteria in the prepared tooth cavity. Indeed, the bacterial inhibition zone size was greatly increased when DMADDM and NAg were incorporated into the un-cured primer.
Besides the residual bacteria, there are also new bacteria to invade the tooth-composite margins during service. This is because it is often difficult to obtain a complete sealing of the tooth-restoration interface. The interface could deteriorate due to polymerization shrinkage stresses as well as cyclic fatigue and wear actions. As a result, microgaps are often present at the tooth-restoration interfaces.59,60 These microgaps could harbor biofilms, and their acid production could cause demineralization at the margin to further degrade the interface. The microgaps were observed between the adhesive resin and the primed dentin, or between the adhesive resin and the hybrid layer.59,60 Hence a large portion of the marginal gap would be surrounded by adhesive, and the invading bacteria would come into contact with the adhesive surface.43 Therefore, it is beneficial to render the adhesive antibacterial. In the present study, both the cured primer and adhesive with DMADDM and NAg had strong anti-biofilm properties. When DMAHM and NAg were added to the primer and adhesive, the biofilm metabolic activity, CFU and lactic acid production were all greatly reduced, compared to the un-modified commercial bonding agent. Therefore, the new DMADDM and NAg containing primer and adhesive are promising to inhibit biofilm growth and secondary caries at the margins.
The incorporation of QAMs in resins is meritorious, because the antibacterial agent is copolymerized with the resin by forming a covalent bonding with the polymer network.23–30 Therefore, the antibacterial agent is immobilized in resin and not released or lost over time, thus providing a durable antibacterial capability.23,24 In addition, Ag is another important antibacterial agent.61,62 Ag ions could interact with and inactivate the vital enzymes of bacteria, thus causing the bacterial DNA to lose its replication ability, leading to cell death.62 Ag is useful because it has a low toxicity and good biocompatibility with human cells,61 and yet can provide a lasting antibacterial activity due to the sustained silver ion release,63 while having less bacterial resistance than antibiotics.64 The NAg in the primer and adhesive of the present study have two additional merits. First, due to the small size of NAg (measured to be 2.7 nm in a previous study47,50) and the high surface area, a low level of NAg of 0.1% in the resin in this study achieved potent antibacterial effects. At such a low mass fraction, there was not noticeable change in the color compared to the control primer and adhesive, and there was no decrease in dentin bond strength. Second, Ag salt was dissolved in TBAEMA which was mixed with primer/adhesive and photo-polymerized, thereby forming the NAg in the resin in situ. The resulting NAg were dispersed in the resin without agglomeration.47,50 Hence, there was no need to mix pre-fabricated Ag nanoparticles with resin, which could cause agglomerates. In the present study, the incorporation of the new DMADDM into primer and adhesive greatly reduced the microcosm biofilm viability and lactic acid production. Comparing the commercial control with the primer/adhesive containing dual DMADDM and NAg, the bacteria inhibition zone was increased by 10-fold, MTT was reduced by 20-fold, CFU counts were reduced by 2–3 orders of magnitude, and lactic acid was decreased by 20-fold. While previous studies usually employed a single antibacterial agent,23–26 combining two antibacterial agents (DMADDM and NAg) appeared to be an effective approach to further enhancing the antibacterial activity.
The novel DMADDM and NAg containing primer and adhesive are expected to be useful in a wide range of dental restorations. In particular, root caries is a growing problem in older adults as the incidence of lesions increases due to root exposure.10,65 Root caries is prevalent among people over the age of 60, with nearly 70% of them having one or more decayed or filled root lesions.65,66 The treatment of root caries remains a challenge, and the occurrence of secondary caries is frequent,1,6,7 while the need for treatment is increasing rapidly with the aging population.10,65 Seniors are a high caries risk group.10 Roots are susceptible to acid dissolution, and the reduced saliva flow in seniors reduces the buffering and acid neutralization capacity via saliva in the oral environment. Therefore, antibacterial restorations are especially needed and beneficial for seniors to combat biofilm growth, acid production, and recurrent caries. Further studies should investigate the effects of primer and adhesive with DMADDM and NAg for biofilm/caries inhibition in root cavities and other restorations, and the use of new monomer DMADDM and NAg in cements, sealants and composites.
5. Conclusions
The present study synthesized new antibacterial monomers DMAHM and DMADDM, and investigated their incorporation into primer and adhesive for the first time. DMAHM and DMADDM were much more strongly antibacterial than the previous QADM in MIC, MBC and ADT tests. Furthermore, DMADDM with a chain length of 12 was far more potent than DMAHM with a chain length of 6. Therefore, DMADDM was incorporated into primer and adhesive, in addition to NAg. The primer and adhesive containing DMADDM inhibited microcosm biofilm growth, metabolic activity, and lactic acid production. Dual addition of DMADDM and NAg further enhanced the anti-biofilm potency, resulting in orders of magnitude reductions in biofilm activity and lactic acid production which causes tooth decay. No adverse effect was found in dentin shear bond strength compared to commercial non-antibacterial control. The new adhesive and primer are promising to combat residual bacteria in tooth cavity and invading bacteria at the margins to inhibit secondary caries. The new monomer DMADDM and the combination with NAg are promising for applications in a wide range of bonding agents, cements, sealants and composites.
Acknowledgments
We thank Drs. Joseph M. Antonucci, Nancy J. Lin and Sheng Lin-Gibson of the National Institute of Standards and Technology, and Ashraf F. Fouad of the University of Maryland School of Dentistry for discussions and experimental assistance. We thank Dr. Huaibing Liu at Caulk/Dentsply (Milford, DE) for donating the TPH composite. This study was supported by NIH R01DE17974 (HX), National Natural Science Foundation of China grant 81100745 (LC), and a seed fund (HX) from the University of Maryland School of Dentistry.
References
- 1.Mjor IA, Toffeneti F. Secondary caries: a literature review with caries reports. Quintessence International. 2000;31:165–179. [PubMed] [Google Scholar]
- 2.Sakaguchi RL. Review of the current status and challenges for dental posterior restorative composites: clinical, chemistry, and physical behavior considerations. Dental Materials. 2005;21:3–6. doi: 10.1016/j.dental.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 3.Sarrett DC. Clinical challenges and the relevance of materials testing for posterior composite restorations. Dental Materials. 2005;21:9–20. doi: 10.1016/j.dental.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 4.Ferracane JL. Resin composite - State of the art. Dental Materials. 2011;27:29–38. doi: 10.1016/j.dental.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 5.Demarco FF, Correa MB, Cenci MS, Moraes RR, Opdam NJM. Longevity of posterior composite restorations: Not only a matter of materials. Dental Materials. 2012;28:87–101. doi: 10.1016/j.dental.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Deligeorgi V, Mjor IA, Wilson NH. An overview of reasons for the placement and replacement of restorations. Primary Dental Care. 2001;8:5–11. doi: 10.1308/135576101771799335. [DOI] [PubMed] [Google Scholar]
- 7.Frost PM. An audit on the placement and replacement of restorations in a general dental practice. Primary Dental Care. 2002;9:31–36. doi: 10.1308/135576102322547548. [DOI] [PubMed] [Google Scholar]
- 8.Dental Resin Composites and Caries. Mar 5, 2009. National Institute of Dental and Craniofacial Research (NIDCR) announcement # 13-DE-102. [Google Scholar]
- 9.Beazoglou T, Eklund S, Heffley D, Meiers J, Brown LJ, Bailit H. Economic impact of regulating the use of amalgam restorations. Public Health Report. 2007;122:657–663. doi: 10.1177/003335490712200513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saunders RH, Meyerowitz C. Dental caries in older adults. Dental Clinics of North America. 2005;49:293–308. doi: 10.1016/j.cden.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 11.Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dental Materials. 2006;22:211–222. doi: 10.1016/j.dental.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Drummond JL. Degradation, fatigue, and failure of resin dental composite materials. Journal of Dental Research. 2008;87:710–719. doi: 10.1177/154405910808700802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayne SC, Thompson JY, Swift EJ, Stamatiades P, Wilkerson M. A characterization of first-generation flowable composites. Journal of the American Dental Association. 1998;129:567–577. doi: 10.14219/jada.archive.1998.0274. [DOI] [PubMed] [Google Scholar]
- 14.Ruddell DE, Maloney MM, Thompson JY. Effect of novel filler particles on the mechanical and wear properties of dental composites. Dental Materials. 2002;18:72–80. doi: 10.1016/s0109-5641(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 15.Samuel SP, Li S, Mukherjee I, Guo Y, Patel AC, Baran GR, Wei Y. Mechanical properties of experimental dental composites containing a combination of mesoporous and nonporous spherical silica as fillers. Dental Materials. 2009;25:296–301. doi: 10.1016/j.dental.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 16.Pashley DH, Tay FR, Imazato S. How to increase the durability of resin-dentin bonds. Compendium of Continuing Education in Dentistry. 2011;32:60–64. 66. [PubMed] [Google Scholar]
- 17.Guo G, Fan Y, Zhang JF, Hagan JL, Xu X. Novel dental composites reinforced with zirconia–silica ceramic nanofibers. Dental Materials. 2012;28:360–368. doi: 10.1016/j.dental.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Satterthwaite JD, Maisuria A, Vogel K, Watts DC. Effect of resin-composite filler particle size and shape on shrinkage-stress. Dental Materials. 2012;28:609–614. doi: 10.1016/j.dental.2012.01.007. [DOI] [PubMed] [Google Scholar]
- 19.Zalkind MM, Keisar O, Ever-Hadani P, Grinberg R, Sela MN. Accumulation of Streptococcus mutans on light-cured composites and amalgam: An in vitro study. Journal of Esthetic Dentistry. 1998;10:187–190. doi: 10.1111/j.1708-8240.1998.tb00356.x. [DOI] [PubMed] [Google Scholar]
- 20.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. Journal of Dentistry. 2007;35:201–206. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Featherstone JDB. The science and practice of caries prevention. Journal of the American Dental Association. 2000;131:887–899. doi: 10.14219/jada.archive.2000.0307. [DOI] [PubMed] [Google Scholar]
- 22.Deng DM, ten Cate JM. Demineralization of dentin by Streptococcus mutans biofilms grown in the constant depth film fermentor. Caries Research. 2004;38:54–61. doi: 10.1159/000073921. [DOI] [PubMed] [Google Scholar]
- 23.Imazato S. Review: Antibacterial properties of resin composites and dentin bonding systems. Dental Materials. 2003;19:449–457. doi: 10.1016/s0109-5641(02)00102-1. [DOI] [PubMed] [Google Scholar]
- 24.Imazato S. Bioactive restorative materials with antibacterial effects: new dimension of innovation in restorative dentistry. Dental Materials Journal. 2009;28:11–19. doi: 10.4012/dmj.28.11. [DOI] [PubMed] [Google Scholar]
- 25.Li F, Chen J, Chai Z, Zhang L, Xiao Y, Fang M, Ma S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. Journal of Dentistry. 2009;37:289–296. doi: 10.1016/j.jdent.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 26.Xie D, Weng Y, Guo X, Zhao J, Gregory RL, Zheng C. Preparation and evaluation of a novel glass-ionomer cement with antibacterial functions. Dental Materials. 2011;27:487–496. doi: 10.1016/j.dental.2011.02.006. [DOI] [PubMed] [Google Scholar]
- 27.Tezvergil-Mutluay A, Agee KA, Uchiyama T, Imazato S, Mutluay MM, Cadenaro M, Breschi L, Nishitani Y, Tay FR, Pashley DH. The inhibitory effects of quaternary ammonium methacrylates on soluble and matrix-bound MMPs. Journal of Dental Research. 2011;90:535–540. doi: 10.1177/0022034510389472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Antonucci JM, Zeiger DN, Tang K, Lin-Gibson S, Fowler BO, Lin NJ. Synthesis and characterization of dimethacrylates containing quaternary ammonium functionalities for dental applications. Dental Materials. 2012;28:219–228. doi: 10.1016/j.dental.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu X, Wang Y, Liao S, Wen ZT, Fan Y. Synthesis and characterization of antibacterial dental monomers and composites. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2012;100:1511–1162. doi: 10.1002/jbm.b.32683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weng Y, Howard L, Guo X, Chong VJ, Gregory RL, Xie D. A novel antibacterial resin composite for improved dental restoratives. Journal of Materials Science: Materials in Medicine. 2012;23:1553–1561. doi: 10.1007/s10856-012-4629-z. [DOI] [PubMed] [Google Scholar]
- 31.Spencer P, Wang Y. Adhesive phase separation at the dentin interface under wet bonding conditions. Journal of Biomedical Materials Research. 2002;62:447–456. doi: 10.1002/jbm.10364. [DOI] [PubMed] [Google Scholar]
- 32.Ikemura K, Tay FR, Endo T, Pashley DH. A review of chemical-approach and ultramorphological studies on the development of fluoride-releasing dental adhesives comprising new pre-reacted glass ionomer (PRG) fillers. Dental Materials Journal. 2008;27:315–329. doi: 10.4012/dmj.27.315. [DOI] [PubMed] [Google Scholar]
- 33.Park J, Ye Q, Topp E, Misra A, Kieweg SL, Spencer P. Water sorption and dynamic mechanical properties of dentin adhesives with a urethane-based multifunctional methacrylate monomer. Dental Materials. 2009;25:1569–1575. doi: 10.1016/j.dental.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ritter AV, Swift EJ, Jr, Heymann HO, Sturdevant JR, Wilder AD., Jr An eight-year clinical evaluation of filled and unfilled one-bottle dental adhesives. Journal of the American Dental Association. 2009;140:28–37. doi: 10.14219/jada.archive.2009.0015. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Godoy F, Kramer N, Feilzer AJ, Frankenberger R. Long-term degradation of enamel and dentin bonds: 6-year results in vitro vs. in vivo. Dental Materials. 2010;26:1113–1118. doi: 10.1016/j.dental.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Shinohara MS, De Goes MF, Schneider LF, Ferracane JL, Pereira PN, Hipolito VD, Nikaido T. Fluoride-containing adhesive: Durability on dentin bonding. Dental Materials. 2009;25:1383–1391. doi: 10.1016/j.dental.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Park J, Eslick J, Ye Q, Misra A, Spencer P. The influence of chemical structure on the properties in methacrylate-based dentin adhesives. Dental Materials. 2011;27:1086–1093. doi: 10.1016/j.dental.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pashley DH, Tay FR, Breschi L, Tjaderhane L, Carvalho RM, Carrilho M, Tezvergil-Mutluay A. State of the art etch-and-rinse adhesives. Dental Materials. 2011;27:1–16. doi: 10.1016/j.dental.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roeder L, Pereira RNR, Yamamoto T, Ilie N, Armstrong S, Ferracane J. Spotlight on bond strength testing-Unraveling the complexities. Dental Materials. 2011;27:1197–1203. doi: 10.1016/j.dental.2011.08.396. [DOI] [PubMed] [Google Scholar]
- 40.Van Meerbeek B, Yoshihara K, Yoshida Y, Mine A, De Munck J. State of the art of self-etch adhesives. Dental Materials. 2011;27:17–28. doi: 10.1016/j.dental.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 41.Namba N, Yoshida Y, Nagaoka N, Takashima S, Matsuura-Yoshimoto K, Maeda H, Van Meerbeek B, Suzuki K, Takashida S. Antibacterial effect of bactericide immobilized in resin matrix. Dental Materials. 2009;25:424–430. doi: 10.1016/j.dental.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 42.Imazato S, Tay FR, Kaneshiro AV, Takahashi Y, Ebisu S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dental Materials. 2007;23:170–176. doi: 10.1016/j.dental.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 43.Imazato S, Kinomoto Y, Tarumi H, Ebisu S, Tay FR. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dental Materials. 2003;19:313–319. doi: 10.1016/s0109-5641(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 44.Imazato S, Ehara A, Torii M, Ebisu S. Antibacterial activity of dentine primer containing MDPB after curing. Journal of Dentistry. 1998;26:267–271. doi: 10.1016/s0300-5712(97)00013-4. [DOI] [PubMed] [Google Scholar]
- 45.Imazato S, Kuramoto A, Takahashi Y, Ebisu S, Peters MC. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dental Materials. 2006;22:527–532. doi: 10.1016/j.dental.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 46.Hiraishi N, Yiu CK, King NM, Tay FR. Effect of chlorhexidine incorporation into a self-etching primer on dentine bond strength of a luting cement. Journal of Dentistry. 2010;38:496–502. doi: 10.1016/j.jdent.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Cheng L, Weir MD, Xu HHK, Antonucci JM, Kraigsley AM, Lin NJ, Lin-Gibson S, Zhou XD. Antibacterial amorphous calcium phosphate nanocomposite with quaternary ammonium salt and silver nanoparticles. Dental Materials. 2012;28:561–572. doi: 10.1016/j.dental.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheng L, Zhang K, Melo MAS, Weir MD, Zhou XD, Xu HHK. Anti-biofilm dentin primer with quaternary ammonium and silver nanoparticles. Journal of Dental Research. 2012;91:598–604. doi: 10.1177/0022034512444128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang L, Xiao YH, Xing XD, Li F, Ma S, Qi LL, Chen JH. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Archives of Oral Biology. 2011;56:367–373. doi: 10.1016/j.archoralbio.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 50.Cheng YJ, Zeiger DN, Howarter JA, Zhang X, Lin NJ, Antonucci JM, Lin-Gibson S. In situ formation of silver nanoparticles in photocrosslinking polymers. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;97:124–131. doi: 10.1002/jbm.b.31793. [DOI] [PubMed] [Google Scholar]
- 51.Zhang K, Melo MAS, Cheng L, Weir MD, Bai YX, Xu HHK. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dental Materials. 2012;28:842–852. doi: 10.1016/j.dental.2012.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.McBain AJ. In vitro biofilm models: an overview. Advances in Applied Microbiology. 2009;69:99–132. doi: 10.1016/S0065-2164(09)69004-3. [DOI] [PubMed] [Google Scholar]
- 53.Lima JP, Sampaio de Melo MA, Borges FM, Teixeira AH, Steiner-Oliveira C, Nobre Dos Santos M, Rodrigues LK, Zanin IC. Evaluation of the antimicrobial effect of photodynamic antimicrobial therapy in an in situ model of dentine caries. European Journal of Oral Sciences. 2009;117:568–574. doi: 10.1111/j.1600-0722.2009.00662.x. [DOI] [PubMed] [Google Scholar]
- 54.Antonucci JM, O’Donnell JN, Schumacher GE, Skrtic D. Amorphous calcium phosphate composites and their effect on composite-adhesive-dentin bonding. Journal of Adhesion Science and Technology. 2009;23:1133–1147. doi: 10.1163/156856109x432767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 56.Tiller JC, Liao CJ, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:5981–5. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Murata H, Koepsel RR, Matyjaszewski K, Russell AJ. Permanent, non-leaching antibacterial surfaces -2: How high density cationic surfaces kill bacterial cells. Biomaterials. 2007;28:4870–9. doi: 10.1016/j.biomaterials.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 58.Tyas MJ, Anusavice KJ, Frencken JE, Mount GJ. Minimal intervention dentistry - a review FDI commission project 1–97. International Dental Journal. 2000;50:1–12. doi: 10.1111/j.1875-595x.2000.tb00540.x. [DOI] [PubMed] [Google Scholar]
- 59.Perdigao J, Lambrechts P, Van Meerbeek B, Braem M, Yildiz E, Yucel T, Vanherle G. The interaction of adhesive systems with human dentin. American Journal of Dentistry. 1996;9:167–173. [PubMed] [Google Scholar]
- 60.Loguercio AD, Reis A, Bortoli G, Patzlaft R, Kenshima S, Rodrigues Filho LE, de Accorinte ML, van Dijken JW. Influence of adhesive systems on interfacial dentin gap formation in vitro. Operative Dentistry. 2006;31:431–441. doi: 10.2341/05-53. [DOI] [PubMed] [Google Scholar]
- 61.Slenters TV, Hauser-Gerspach I, Daniels AU, Fromm KM. Silver coordination compounds as light-stable, nano-structured and anti-bacterial coatings for dental implant and restorative materials. Journal of Materials Chemistry. 2008;18:5359–5362. [Google Scholar]
- 62.Rai M, Yada A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnology Advances. 2009;27:76–83. doi: 10.1016/j.biotechadv.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 63.Damm C, Munsted H, Rosch A. Long-term antimicrobial polyamide 6/silver nanocomposites. Journal of Materials Science. 2007;42:6067–6073. [Google Scholar]
- 64.Percival SL, Bowler PG, Russell D. Bacterial resistance to silver in wound care. Journal of Hospital Infection. 2005;60:1–7. doi: 10.1016/j.jhin.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Imazato S, Ikebe K, Nokubi T, Ebisu S, Walls AWG. Prevalence of root caries in a selected population of older adults in Japan. Journal of Oral Rehabilitation. 2006;33:137–143. doi: 10.1111/j.1365-2842.2006.01547.x. [DOI] [PubMed] [Google Scholar]
- 66.Galan D, Lynch E. Epidemiology of root caries. Gerodontology. 1993;10:59–71. doi: 10.1111/j.1741-2358.1993.tb00084.x. [DOI] [PubMed] [Google Scholar]