Abstract
We used fMRI to investigate the neural processes engaged as individuals down- and up-regulated the emotions associated with negative autobiographical memories (AMs) using cognitive reappraisal strategies. Our analyses examined neural activity during 3 separate phases, as participants: (a) viewed a reappraisal instruction (i.e., Decrease, Increase, Maintain), (b) searched for an AM referenced by a self-generated cue, and (c) elaborated upon the details of the AM being held in mind. Decreasing emotional intensity primarily engaged activity in regions previously implicated in cognitive control (e.g., dorsal and ventral lateral prefrontal cortex), emotion generation and processing (e.g., amygdala, insula), and visual imagery (e.g., precuneus) as participants searched for and retrieved events. In contrast, increasing emotional intensity engaged similar regions during the instruction phase (i.e., before a memory cue was presented) and again as individuals later elaborated upon the details of the events they had recalled. These findings confirm that reappraisal can modulate neural activity during the recall of personally-relevant events, though the time course of this modulation appears to depend on whether individuals are attempting to down- or up-regulate their emotions.
External stimuli in our environments, such as an argument with a loved one or an anxiety-provoking public speaking engagement, often elicit emotional reactions that we desire to regulate (Gross & Thompson, 2007), and indeed much of the extant emotion regulation literature focuses on attempts to change which emotions are experienced, or the intensity of emotions evoked, in response to external information like images or film clips (see Ochsner & Gross, 2008; 2005, for reviews). However, internally-generated cognitions also can produce emotional reactions. For example, recalling a past fight or nerve-wracking presentation can re-invoke in the here and now the emotions that were present at the time of an event’s occurrence (e.g., Westermann, Kordelia, Stahl, & Hesse, 1996). Just as with external information, we are not merely passive recipients of our internally-generated emotional experiences. The present study sought to determine the neural correlates of emotion regulation in response to negative autobiographical memories (AMs). Because cognitive reappraisal, or the reinterpretation of emotional information in such a way as to decrease or increase its emotional impact, is one of the most studied and most effective means for emotion regulation (reviewed by Gross & Thompson, 2007), we focused specifically on how this strategy influences AM.
Although the link between AM recall and emotion regulation is relatively understudied, there is both behavioral and neural evidence that the two processes are interrelated. Individuals report recalling their AMs in everyday life in such a way as to regulate their emotions (e.g., recalling a successful exam performance to quell anxiety about an upcoming exam; Bluck et al., 2005). The reciprocal relation is also true: Regulatory goals can influence which AMs are most likely to be recalled (Josephson, Singer, & Salovey, 1996). In addition, when individuals are instructed to recall a particular event (e.g., high school graduation), regulatory goals can influence which details and appraisals are reported about those AMs (Holland, Tamir, & Kensinger, 2010). The relation between AM and emotion regulation is perhaps most evident in the clinical literature, which illustrates that a failure to effectively regulate emotional responses regarding past negative events (e.g., by ruminating on rather than reappraising negative emotions) is associated with affective disorders like depression (reviewed by Gotlib & Joormann, 2010). Taken together, evidence for the relation between emotion regulation and AM is in line with Conway’s (2005) assertion that the constructive nature of AM retrieval leaves it malleable and open to modulation by personally-relevant goals.
In addition to their behavioral links, both AM recall and cognitive reappraisal in response to emotional images or film clips engage neural networks with substantial overlap. There are commonalities in prefrontal cortex (PFC) activity, likely due to the similar cognitive demands of AM recall and cognitive reappraisal. For example, both tasks rely on dorsolateral PFC [associated with maintaining and manipulating emotional information in working memory during reappraisal (reviewed by Ochsner & Gross, 2008) and manipulating the products of retrieval in working memory during AM recall (reviewed by Cabeza & St. Jacques, 2007)], ventrolateral PFC [associated with selecting appropriate reappraisals during regulation (Denny, Silvers, & Ochsner, in press) and controlled retrieval processes, including appropriate cue specifications, during the AM access period (Cabeza & St. Jacques, 2007)], and medial PFC [associated with self-referential processing during both regulation (Ochsner & Gross, 2008) and recall (Cabeza & St. Jacques, 2007)]. Cognitive reappraisal is further associated with increased activity in dorsal anterior cingulate cortex (dACC), a region associated with conflict monitoring (e.g., between competing affective responses; Denny et al., in press) and correlated with reappraisal success (Ochsner et al., 2002), as well as orbitofrontal PFC (OFC), thought to support the selection of situationally appropriate emotions (reviewed by Denny et al., in press).
Both reappraisal and recall processes also engage regions associated with emotional processing, such as the amygdala and the insula. Recalling emotional AMs also has been associated with amygdala and other medial temporal lobe (MTL) activity (Markowitsch et al., 2000; Svoboda et al., 2006; Cabeza & St. Jacques, 2007), particularly during the initial search for and accessing of events, suggesting that the amygdala may guide the recall of emotionally salient events (Daselaar et al., 2008). Further, amygdala activity during AM recall has been linked to activity in the PFC and hippocampus (Greenberg et al., 2005), and the emotional intensity of events is positively correlated with activity in the PFC and amygdala (Botzung et al., 2010), as well as the hippocampus (Addis et al., 2004).
Much of the emotion regulation literature focuses on the control of “hot” emotion processing areas via “cold” cognitive regions in the PFC (see Ochsner & Gross, 2005; 2008, for review). However, cognitive reappraisal also modulates posterior activity in regions associated with visuospatial processing and attention, such as the parietal lobe, visual cortex, cuneus, and precuneus (Ochsner et al., 2004; Urry et al., 2006; Phan et al., 2005; Goldin et al., 2008). Interestingly, activity in similar regions—particularly the visual cortex, precuneus, and posterior cingulate/retrosplenial cortex—are activated by the visual imagery that is a defining feature of AM (e.g., Cabeza & St. Jacques, 2007), and this activity has been positively correlated with behavioral ratings of emotional intensity associated with AMs (Botzung et al., 2010). Though their role in reappraisal is not often elaborated upon, one possibility is that decreasing or increasing mental imagery is a useful strategy for decreasing or increasing emotional intensity, respectively (see Ochsner et al., 2004, for similar discussion).
To date, only a handful of studies have addressed how regions belonging to the AM neural network might be modulated as emotion regulation is occurring during recall. Two studies have focused on a more automatic form of regulation that can occur during mood-incongruent recall, revealing in healthy adults that being instructed to recall a positive AM following a negative mood induction recruited OFC and ACC (Cooney et al., 2007), with a similar pattern evident in ACC and lateral PFC in healthy adolescents (Joormann et al., 2012). Two other studies have compared the more effortful cognitive reappraisal with rumination during the recall of negative AMs. Ruminating on angry AMs led to enhanced connectivity between inferior frontal gyrus and amygdala (Fabiansson et al., 2012), and focusing on sad AMs led to increased activity in anterior cingulate cortex and medial PFC (Kross et al., 2009). In both studies, self-reported negative affect was lower for reappraisal than rumination conditions. However, reappraisal did not lead to any greater connectivity during the recall of angry AMs (Fabiansson et al., 2012), and it only led to increased activity in a region of left PFC that also was activated during rumination on sad AMs (Kross et al., 2009).
Taken together, the Kross et al. (2009) and Fabiansson et al. (2012) experiments suggest that regulatory strategies can modulate core regions of the AM network, but that rumination may do so to a greater extent than reappraisal (see Kross et al., 2009). However, the designs of these studies leave open a number of questions. One limitation of this work from a memory research standpoint is that participants were asked to apply multiple regulatory strategies to a limited number of AMs (9 in Kross et al., 2009; 1 in Fabiansson et al., 2012) over the course of the scan. It is unclear what the long-term effects of regulating the emotion related to an AM might be. For example, it is possible that being asked to ruminate upon the negative emotions associated with a memory might influence the details that are later constructed about that event and/or the amount of emotion experienced during recall. If this is indeed the case, then being asked to perform such a strategy first might fundamentally change the experience of later trying to reappraise the emotion associated with that same memory.
A second open question is how the up-regulation of negative emotions might modulate AM recall. Kross et al. (2009) and Fabiansson et al. (2012) focused on the down-regulation of negative emotions, but reappraisal can also be used to increase negative emotions when feeling negative is deemed functional (e.g., recalling negative AMs in preparation for playing an aggressive game; Tamir, Mitchell, & Gross, 2008). Although PFC regions are recruited during both the down- and up-regulation of responses to negative images, the consequence of that activation varies, resulting in a decrease or increase, respectively, of amygdala activity (Ochsner et al., 2004).
To address these first two questions, we modified a cognitive reappraisal task traditionally used with emotional images or film clips for use with AMs. We scanned participants as they decreased, increased, or maintained the emotions associated with negative AMs that had been reported at a pre-scan session. Each memory cue appeared with only one regulatory instruction to prevent initial reappraisal attempts from influencing subsequent attempts. We predicted that attempts to down- and up-regulate (vs. maintain) negative emotions during AM recall would be associated with cognitive control regions in PFC and dACC that have previously been associated with image-based reappraisal (e.g., Ochsner et al., 2004). We also predicted that activity in several regions would be modulated according to the direction of regulation (e.g., less activity during down-regulation and greater activity during up-regulation), including emotion processing regions (e.g., amygdala, insula, medial PFC), medial temporal lobe regions shown to be sensitive to emotional intensity (e.g., hippocampus; Botzung et al., 2010), and visual imagery regions (e.g., visual cortex, precuneus).
A third exploratory question that the present study addressed concerns the timing of reappraisal during AM recall. The constructive nature of AM recall is relatively lengthy and can be subdivided into at least two phases, including the initial search for and retrieval of AM details as a memory is being accessed (referred to here as the “memory onset” phase), followed by an elaboration phase during which time the searched-for memory is held in mind and its details expounded upon (e.g., Daselaar et al., 2008; Addis et al., 2007). At this point, it is unclear during which phase of AM recall cognitive reappraisal might occur, and whether up-regulating and down-regulating emotional responses occur over the same time course. Kross et al. (2009) and Fabiansson et al. (2012) provided regulation instructions only after the AMs had been recalled and were being held in mind; such designs might illustrate how regulation strategies influence the elaboration phase of AM, but leave open the question of how having regulatory goals in mind prior to recall influences the AM retrieval network during the initial memory onset and later memory elaboration phases. In the present experiment we compared activity during three phases: an instruction phase, when participants were presented only with the reappraisal instructions (i.e., “Decrease,” “Increase,” or “Maintain”) for the subsequent memory cue (see Herwig et al., 2007 for a similar design); a memory onset phase, when the memory cue first was presented; and a memory elaboration phase, demarcated by participants’ response that they had an AM in mind (see similar distinctions between memory onset and elaboration in Daselaar et al., 2008; Addis et al., 2007).
Methods
Participants
Twenty-eight young adults (16 female, range = 18-28 years, M = 21.89 years, SD = 3.25 years) participated in this study. Two participants were dropped from subsequent analyses for failing to make button box responses during the scan, 1 participant was excluded for failing to complete the pre-scan appointment, 1 participant was excluded for failing to complete the scan appointment, 1 participant was excluded for excessive motion, and 1 participant was excluded due to scanner malfunction. The final sample included 22 participants (13 female; M = 22.27, SD = 3.56) who had no history of psychiatric, neurological, or learning disorders, nor any history or current use of psychiatric medication. Informed consent was obtained from all participants in accordance with the Boston College Institutional Review Board.
Pre-Scan Stimulus Collection Session
Approximately 7-14 days before the scan session (M = 6.73 days, SD = 2.00 days, range = 4-13 days), participants completed a stimulus collection session. Each participant generated 90 specific AMs (i.e., events that lasted no longer than a day and were unique to time and place). Sixty of the AMs were required to be negative in valence, and the remaining 30 were neutral. For each event, participants were instructed to create a title that was just a few words but specific enough that if they were to see that title in the scanner they would know which event it was referencing. In addition to the title, participants wrote a brief sentence describing each event and rated the AMs on a 7-point scale for how emotionally intense, negative, positive, and vivid they were. Finally, participants provided their approximate age in years at the time of each event’s occurrence. The AM portion of the study took approximately 1.5 to 2 hours.
Scan Session
AM stimuli
From the AMs generated during the pre-scan stimulus collection session, 15 negative events were assigned to each of three emotion regulation conditions (Decrease, Increase, and Maintain) and 15 neutral events were assigned to a Maintain condition. The behavioral characteristics of the events assigned to each condition are presented in Table 1. For each participant, the negative events assigned to each emotion regulation condition were matched on each of the behavioral ratings that participants made (intensity, negativity, positivity, vividness, and age). The neutral and negative events were matched on vividness and age.
Table 1.
Behavioral characteristics of negative and neutral AMs from the pre-scan session that were assigned to the scan session reappraisal or maintain conditions. Standard deviations are presented in parentheses.
| Pre-Scan Behavioral Ratings | |||||
|---|---|---|---|---|---|
|
| |||||
| Intensity | Negative | Positive | Vividness | Age | |
| Decrease Neg | 4.72 (0.75) | 5.55 (0.58) | 1.27 (0.27) | 5.35 (0.77) | 4.56 (2.45) |
| Increase Neg | 4.64 (0.74) | 5.54 (0.65) | 1.28 (0.33) | 5.29 (0.77) | 4.52 (2.50) |
| Maintain Neg | 4.70 (0.74) | 5.57 (0.64) | 1.28 (0.27) | 5.38 (0.80) | 4.56 (2.52) |
| Maintain Neutral | 2.40 (1.05) | 1.43 (0.26) | 3.92 (1.38) | 5.13 (0.85) | 3.66 (2.76) |
Emotion regulation task
Immediately before being scanned, participants received instructions for the emotion regulation task that they performed in the scanner. Participants were instructed that when they saw either “Decrease” or “Increase” prompts, they should attempt to re-interpret (i.e., cognitively reappraise) the subsequent event cue in such a way as to feel a weaker or stronger emotional reaction than normal to the memory, respectively. Example cognitive reappraisal strategies were given for both decrease and increase instructions. For instance, participants were instructed that if they had a negative event cue about a friend forgetting their birthday, they might decrease their emotional reaction to that memory by focusing on how they still had a great time celebrating their birthday even though that friend forgot. If they had to increase their emotional reaction to that event, they might focus on the feelings of sadness or disappointment they had when they realized that friend forgot. For both the maintain negative and maintain neutral conditions, participants were instructed to recall the events without trying to alter their feelings toward them. Participants practiced each possible instruction with two example events (not used during the scan).
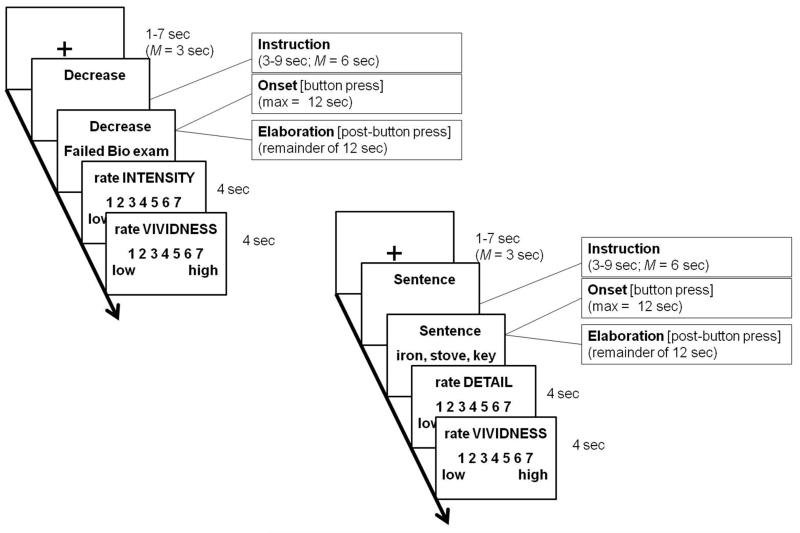
The 60 regulation trials (15 each of Decrease, Increase, Maintain Negative, and Maintain Neutral) were pseudo-randomized such that no instruction appeared more than twice in a row; the trials were divided among 4 functional scanning runs. The overall design for the emotion regulation task is summarized in the left panel of Fig. 1. Each trial began with a fixation cross that lasted an average of 3 sec (jittered between 1 and 7 sec). The fixation period was followed by an instruction phase, during which time a regulation prompt (Decrease, Increase, or Maintain) appeared on the screen for an average of 6 sec (jittered between 3 and 9 sec). The onset of this prompt was modeled as the start of the Instuction phase. A memory title created during the pre-scan session then appeared on the line below the instruction for 12 sec. The onset of this prompt was modeled as the start of the Memory Onset phase. Participants were instructed to make a button press when they felt they had the fully formed event memory in mind. Following the button press, participants were instructed to continue thinking about and elaborating on the details of the memory in accordance with the regulation instructions for that trial for the remainder of the 12 sec. This button press indicated the start of the Memory Elaboration phase. Finally, each trial ended with two 7-point rating scales that asked participants to rate Emotional Intensity and Vividness. Each rating scale appeared for a maximum of 4 sec.
Figure 1.
(Left) Example trial for an autobiographical memory task in which individuals decreased, increased, or maintained the emotional intensity associated with negative events or maintained the emotional intensity associated with neutral events. Note that the trial types were pseudo-randomized such that no instruction appeared more than twice in a row. (Right) Example trial for a sentence baseline task in which participants generated a sentence with the format “X is smaller than Y is smaller than Z” for different groups of 3 concrete nouns and then elaborated upon the appearance and functions of the objects. Sentence baseline trials were interspersed with the autobiographical memory task.
Sentence control task
Sixteen trials of a sentence control task adapted from Addis et al. (2009) were divided among the 4 functional scanning runs and randomly interspersed with the regulation trials. The sentence control task included sets of 3 concrete, highly imageable and familiar nouns (selected from the Clark and Paivio extended norms; Clark & Paivio, 2004).
The overall design of the sentence control task trials mirrored that of the regulation task and is summarized in the right panel of Fig. 1. Each trial began with a fixation cross in the center of the screen for an average of 3 sec (jittered between 1 and 7 sec). The sentence instruction phase, during which time the word “Sentence” appeared on the screen for an average of 6 sec (jittered between 3 and 9 sec), followed. A set of three nouns then appeared on the screen on the line underneath the “Sentence” instruction for 12 sec. Participants were instructed to put the items in physical-size order and place them in a sentence with the structure “X is smaller than Y is smaller than Z.” Once participants had sub-vocalized the sentence, they made a button press that demarcated the end of the onset phase. The sentence onset phase controls for the memory search and integration processes in the memory onset phase. They were then instructed to think about and elaborate on the appearance and functions of the three objects for the remainder of the 12 sec trial; this period between the button press and the end of the trial will be referred to as the elaboration phase. Because the sentence elaboration phase involved visuospatial processing, it controls for the elaboration of visuospatial details during the AM task. The sentence trials ended with two rating scales lasting a maximum of 4 sec each: (a) Detail [1 (low) – 7 (high)], and (b) Vividness [1 (low) – 7 (high)].
Post-scan task
Following the scanning portion of the study, after an approximately 0.5 hour delay, participants were given a spreadsheet that included the 60 event titles, 1-sentence descriptions, and ages of the AMs they recalled in the scanner. They were asked to make several ratings about each event: (a) how emotionally intense they felt about the event [1 (not at all) – 7 (very)], (b) how negative they felt about the event [1 (not at all) – 7 (very)], (c) how positive they felt about the event [1 (not at all) – 7 (very)], and (d) how vivid their recall of the event was [1 (not at all) – 7 (vivid)]. The AMs were presented in the spreadsheet in the same order that participants saw them while in the scanner, and the task was self-paced.
Scanning parameters
Images were acquired on a 3 Tesla Siemens Tim Trio MRI scanner using a 32-channel head coil. Stimuli were presented using MacStim presentation software. All words, instructions, and rating scales used in the experiment appeared in white text (Arial 36-point font) on a black background. Stimuli were projected onto a screen located at the back of the magnet bore, and participants viewed the stimuli using a mirror attached to the head coil.
T1-weighted localizer images and a T1-weighted inversion recovery echo planar image required for auto-alignment were collected. Anatomic data were collected with a multi-echo multi-planar rapidly acquired gradient-echo (MEMPRAGE) sequence (TR = 2200 ms; TE = 1.64, 3.5, 5.46, 7.22 ms; flip angle = 7°; field of view = 256 × 256 mm; slice thickness = 1 mm, no gap; 1 × 1 × 1 mm resolution). Functional images were collected using a T2*-weighted echo-planar imaging (EPI) sequence with the following parameters: TR = 3000 ms, TE = 30 ms, FOV = 216 mm, flip angle = 85°. Forty-seven interleaved coronal-oblique slices aligned perpendicular to the long axis of the hippocampus were collected in a 3 mm3 matrix (slice thickness = 3 mm).
Preprocessing and data analysis were conducted in SPM8 (Wellcome Department of Cognitive Neurology, London). Preprocessing steps were as follows: (1) slice timing correction, (2) motion correction using a six parameter, rigid body transformation algorithm, (3) normalization to the Montreal Neurological Institute (MNI) template (resampling at 3 mm isotropic voxels), and (4) spatial smoothing using a 3 mm full-width half maximum isotropic Gaussian kernel.
Imaging data analysis
The memory recall phase was divided into two separate events—memory onset and elaboration—based on self-paced RTs to recall each memory. The RTs were random and highly variable (see Table 2), thereby effectively jittering the beginning of the elaboration phase (see Addis, Wong, & Schacter, 2007; Steinvorth, Corkin, & Halgren, 2006, for similar design and discussion). For each individual, the following events were modeled and analyzed using the general linear model approach on a voxel-by-voxel basis: (a) Decrease Instruction, (b) Increase Instruction, (c) Maintain Negative Instruction, (d) Maintain Neutral Instruction, (e) Sentence Instruction, (f) Decrease Onset, (g) Increase Onset, (h) Maintain Negative Onset, (i) Maintain Neutral Onset, (j) Sentence Onset, (k) Decrease Elaboration, (l) Increase Elaboration, (m) Maintain Negative Elaboration, (n) Maintain Neutral Elaboration, and (o) Sentence Elaboration. Contrasts between the various trial types were computed as described below, and the resulting contrast images were entered into second-level, random-effects analyses that used a statistical threshold of p < .001, uncorrected, and a 5-voxel threshold extent. Because we had a priori hypotheses about how reappraisal instructions would modulate activity in the amygdala (following e.g., Ochsner et al., 2004), we applied a small volume correction using an anatomically-defined mask of the bilateral amygdala from the MARINA toolbox (Walter, Blecker, Kirsch, Sammer, Schienle, et al., 2003). Regions of the amygdala resulting from this small volume correction are noted in the relevant Tables.
Table 2.
Behavioral characteristics of negative and neutral AMs that participants were instructed to decrease, increase, or maintain during the scan session. Standard deviations are presented in parentheses.
| Scan Behavioral Ratings | |||
|---|---|---|---|
|
| |||
| Trial Type | RT (sec) | Intensity | Vividness |
| Decrease Neg | 3.63 (1.46) | 3.69 (0.92) | 4.75 (0.81) |
| Increase Neg | 3.44 (1.43) | 5.19 (0.58) | 5.34 (0.73) |
| Maintain Neg | 3.46 (1.47) | 4.65 (0.79) | 4.96 (0.75) |
| Maintain Neutral | 3.04 (1.13) | 2.79 (1.09) | 4.74 (0.77) |
Results
Scan Behavioral Results
The behavioral results from the scan session are summarized in Table 2.
Reaction time
A within-subjects ANOVA examining the effect of emotion regulation instruction on RT to access an AM revealed a main effect of instruction, F(3, 63) = 7.98, p < .001, partial-η2 = .28, with significantly faster RT for the maintain neutral condition than any of the negative AM regulation conditions, ps < .01, as well as a trend for longer RTs in the decrease condition than AMs in the increase condition, p < .10. Because these RT differences would mean different lengths of the onset phase depending on condition, we included RT as a parametric regressor for the onset phase on a trial-by-trial basis in the first-level analysis for each participant.
Memory Qualities
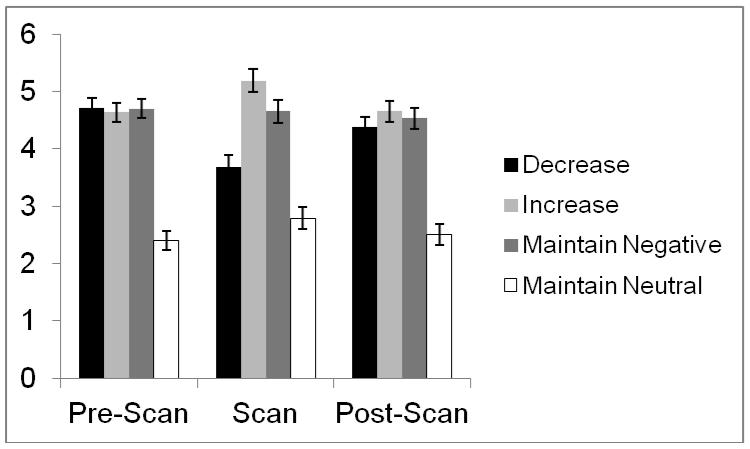
As expected, there was a main effect of instruction on emotional intensity, F(3, 63) = 56.80, p < .001, partial-η2 = .73 (Fig. 2). Post-hoc comparisons confirmed that neutral AMs were rated as lower in intensity than any of the negative AM conditions, ps < .001, and intensity for negative AMs was lowest in the decrease condition, higher in the maintain condition, and highest in the increase condition, all ps < .001.
Figure 2.
Average emotional intensity ratings (on a 1-7 scale) for negative and neutral AMs before the scan (i.e., when no regulation instructions were given), during the scan (when regulation instructions were given), and approximately 0.5 hours after the scan (in the absence of any regulation instructions).
Consistent with predictions, regulation instruction also influenced vividness ratings, F(3, 63) = 7.61, p < .001, partial-η2 = .27. Post-hoc comparisons confirmed that negative events in the increase condition were rated as more vivid than any other memories (ps < .006); negative events in the maintain condition were rated as significantly more vivid than those in the decrease condition (p = .003) and trended toward being more vivid than neutral events (p < .10). The vividness of negative memories in the decrease condition was equivalent to that of neutral memories (p = .97).
Imaging Results
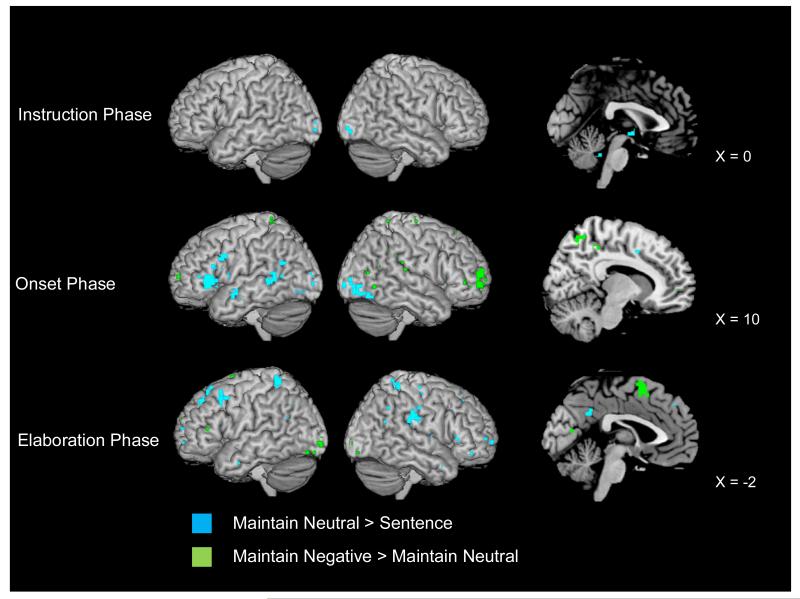
Identifying the AM Retrieval Network
We first compared the neural activity during the neutral maintain condition to the activity during the sentence control task to establish that regions previously associated with AM recall were present in our task (see Fig. 3). Consistent with prior research on the AM retrieval network (see Cabeza & St. Jacques, 2007; Svoboda et al., 2006, for reviews), the onset phase of AM (i.e., in the time between the appearance of the memory cue and the button press indicating that a fully formed memory was in mind; see middle panel of Fig. 3) was associated with activity in several left-lateralized regions of ventrolateral (BAs 44 and 47) PFC regions, left lateral temporal lobe regions (BAs 21/22), left posterior cingulate (BA 30), bilateral occipital lobe (BA 18), and bilateral cuneus (BAs 17/18). During memory elaboration (see bottom panel of Fig. 3) activation was present in right-lateralized regions of ventrolateral PFC (BA 47) and frontal pole (BA 10), as well as throughout the medial PFC (BAs 8, 9, and 10). Activity was also evident throughout the bilateral temporal (BAs 21 and 22) and parietal (BAs 5 and 40) lobes, and in left posterior cingulate (BA 23/31), left fusiform gyrus, and left precuneus (BA 31).
Figure 3.
Neural activity for the maintain neutral > sentence and maintain negative > maintain neutral contrasts during the instruction, onset, and elaboration phases. Right-most column shows saggital cutaways for each phase. Activity is significant at p < .001 and a 5-voxel threshold extent.
Retrieving Negative vs. Neutral AMs
Replicating previous findings demonstrating greater right-lateralized activity during emotional AM recall (see Cabeza & St. Jacques, 2007, for review), at memory onset, negative AM retrieval recruited more right ventrolateral PFC (BA 44) and bilateral frontal pole (BA 10) as well as right lateral temporal regions (BAs 37/19, 39, 41, and 42/22) and bilateral precuneus (BA 7) than did neutral AM retrieval (see middle panel of Fig. 3).
Unlike the onset phase, which recruited primarily right-lateralized activity during negative AM recall, elaborating upon negative AMs disproportionately engaged mostly left-lateralized regions (see bottom panel of Fig. 3), including in dorsal (BAs 45/46) and ventral (BA 47) lateral PFC and lateral temporal lobe areas. Negative AM elaboration also engaged medial PFC (BA 6) and a number of visuospatial processing regions [e.g., bilateral cuneus (BAs 17, 18, and 31), left fusiform (BA 37) and right lingual (BA 18) gyri, bilateral inferior (BAs 18 and 19) and left middle (BAs 19 and 37) occipital gyri]).
Emotion Regulation During Autobiographical Memory Recall
Having generally replicated prior findings with regard to the AM retrieval network and its modulation by emotion (reviewed by Cabeza & St. Jacques; Svoboda et al., 2006), we next analyzed the neural activity present as individuals down- and up-regulated (vs. maintained) the negative emotions associated with their AMs. For each phase we will first discuss the results for the decrease and increase conditions compared to the maintain condition, and then discuss the results for the direct comparisons between the decrease and increase trials.
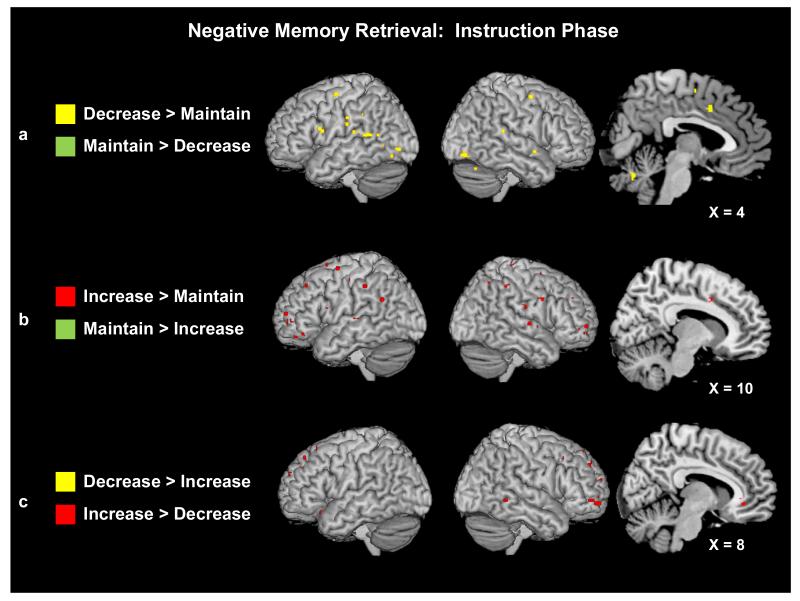
Regulation During Recall: Instruction Phase
Reappraising vs. Maintaining Emotions
We explored whether there was any anticipatory activity evident as individuals prepared to increase or decrease negative AMs versus to maintain negative AMs, or vice-versa. The overall pattern revealed that being instructed to reappraise negative emotions engaged more regions than being instructed to maintain emotions, with the increase condition revealing the most extensive activity. More specifically, during the decrease (vs. maintain) instruction condition, regions of left dorsolateral PFC (BA 47) and right anterior cingulate (BA 24) previously implicated in the reappraisal of negative images (Ochsner et al., 2004) were activated, as was right medial PFC (BA 6), and bilateral temporal (BAs 22/21) and occipital (BAs 19/18) cortices (see Fig. 4a). Although a conjunction analysis between the decrease > maintain and increase > maintain contrasts during the Instruction phase did not reveal any statistically significant overlap in clusters of activations, right anterior cingulate (BA 32/24), bilateral temporal (BAs 42/22/39/41) and right occipital (BAs 31/18/19) lobe activity was also present in the increase (vs. maintain) instruction contrast. This contrast also revealed more extensive activity in regions throughout the PFC (see Fig. 4b). Areas of right dorsolateral PFC (BA 9), left medial PFC (BA 8) and bilateral frontal pole (BA 10) were all engaged more by the increase than maintain instruction. Few regions were more active during the maintain instruction phase; only a single cluster of left posterior cingulate (BA 31) and two clusters of left caudate nucleus were engaged to a greater extent in the maintain instruction than the decrease instruction phase, and only a region of left cerebellum was engaged to a greater extent than in the increase instruction phase.
Figure 4.
(a) Neural activity for the decrease and maintain trials during the negative AM instruction phase. (b) Neural activity for the increase and maintain trials during the negative AM instruction phase. (c) Neural activity for the decrease and increase trials during the negative AM instruction phase. Plotted activity is significant at p < .001 and a 5-voxel threshold extent.
Decreasing vs. Increasing Emotions
A direct contrast of the decrease and increase instruction phases confirmed that activity was more widespread during increase than decrease instructions (see Fig. 4c; Table 3). Most striking were a number of regions in the PFC, including left ventrolateral (BA 47), dorsolateral (BA 9), and orbitofrontal (BA 11) areas; and right-lateralized frontal pole (BA 10). Regions previously associated with emotion processing and regulation, such as right insula (BA 13) and bilateral anterior cingulate gyrus (BAs 32/24, 23, and 25) were also engaged more during the “Increase” than “Decrease” instructions, as were posterior areas important for visuospatial processing and representation [right cuneus (BA 31), left precuneus (BA 31/7), left posterior cingulate gyrus (BAs 31/24/23) and inferior parietal lobe (BA 40)].
Table 3.
Group activations for the increase > decrease instruction contrast.
| Lobe/Region | BA | Hemisphere | Talairach | t | k | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Increase > Decrease Instruction | |||||||
|
| |||||||
| Frontal | |||||||
| Inferior frontal gyrus | 47 | L | −32 | 23 | −15 | 4.68 | 8 |
| 47 | L | −26 | 27 | −6 | 4.60 | 7 | |
| 47 | L | −30 | 33 | −8 | 4.20 | 9 | |
| Middle frontal gyrus | 10 | R | 34 | 60 | −10 | 4.51 | 31 |
| Medial frontal gyrus | 10 | R | 6 | 61 | 14 | 3.77 | 6 |
| Superior frontal gyrus | 11 | L | −24 | 50 | −11 | 4.35 | 13 |
| 8 | L | −12 | 46 | 31 | 3.95 | 5 | |
| 9 | R | 18 | 52 | 32 | 4.20 | 9 | |
| Parietal | |||||||
| Inferior parietal lobe | 40 | L | −28 | −45 | 28 | 4.86 | 7 |
| Precuneus | 31/7 | L | −20 | −47 | 36 | 5.06 | 10 |
| Occipital | |||||||
| Cuneus | 31 | R | 26 | −73 | 7 | 4.66 | 7 |
| Limbic | |||||||
| Insula | 13 | R | 32 | 20 | 16 | 4.17 | 8 |
| Cingulate gyrus | 31 | L | −24 | −33 | 40 | 5.93 | 13 |
| 24/23 | L | −20 | −20 | 27 | 5.38 | 14 | |
| 32/24 | L | −24 | 25 | 25 | 4.22 | 5 | |
| 24/23 | R | 22 | 7 | 27 | 4.92 | 5 | |
| 25 | R | 4 | 6 | −5 | 4.51 | 6 | |
| 32 | R | 8 | 35 | −7 | 4.44 | 6 | |
| Other | |||||||
| Basal ganglia | R | 22 | −7 | 22 | 4.68 | 7 | |
| L | −28 | 9 | 16 | 4.13 | 6 | ||
| L | −24 | 22 | 15 | 3.76 | 5 | ||
By contrast, when examining the decrease > increase contrast, no regions survived our statistical threshold of p < .001 and a 5-voxel cluster extent (but see Table 4 for regions that arose at a reduced threshold of p < .005).
Table 4.
Group activations for the decrease > increase instruction contrast. Note that activity in reported regions was significant only at a more liberal threshold of p < .005 and a 5-voxel cluster extent.
| Lobe/Region | BA | Hemisphere | Talairach | t | k | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| x | y | z | |||||
| Decrease > Increase Instruction | |||||||
|
Regions reported are significant at p < .005, k ≥ 5 voxels
| |||||||
| Medial temporal | |||||||
| Parahippocampal gyrus | L | −16 | −41 | −3 | 3.84 | 7 | |
| Hippocampus | L | −28 | −22 | −11 | 2.95 | 5 | |
| Other temporal | |||||||
| Fusiform gyrus | L | −30 | −36 | −12 | 3.84 | 5 | |
| Limbic | |||||||
| Cingulate gyrus | L | −20 | −54 | 14 | 3.26 | 6 | |
| Occipital | |||||||
| Middle occipital gyrus | 37/19 | R | 38 | −74 | −8 | 3.31 | 6 |
| Other | |||||||
| Cerebellum | R | 16 | −59 | −7 | 3.70 | 6 | |
Regulation During Recall: Onset Phase
Reappraising vs. Maintaining Negative Emotions
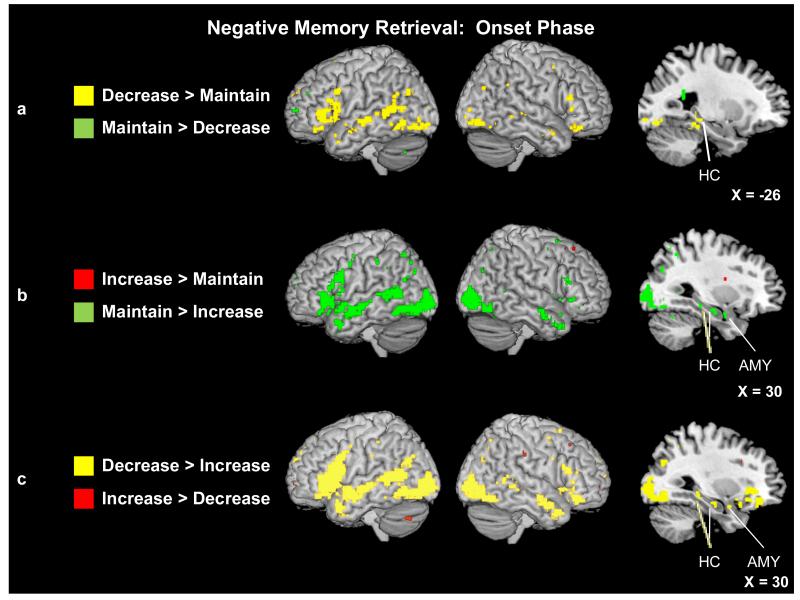
There was a relative paucity of regions showing statistically greater activity for the increase than maintain conditions during the onset phase, with only a small cluster of basal ganglia revealed (see Fig. 5b). By contrast, and in line with our expectations based on the results of image-based reappraisal studies (e.g., Ochsner et al., 2004), when comparing activity in the decrease and maintain conditions (Fig. 5a), regions of the bilateral dorsal (BA 46) and ventral (BAs 11, 44, and 47) PFC, and of the left frontal pole (BA 10), were engaged more by the decrease condition, as were bilateral regions of temporal cortex (BAs 21, 22, 38 and 39), left hippocampus, and bilateral posterior cingulate cortex (BAs 30 and 23). Contrary to expectations, emotion processing regions [left medial PFC (BA 6) and left insula (BA 13)] and visuospatial [left cuneus (BAs 18 and 30), right precuneus (BA 31), right angular (BA 39) and bilateral occipital (BAs 18 and 19) gyri], previously associated with enhanced emotional intensity during cognitive reappraisal (e.g., Ochsner et al., 2004) and AM (Botzung et al., 2010) tasks, were more active as individuals accessed negative events that they were instructed to down-regulate vs. maintain.
Figure 5.
(a) Neural activity for the decrease and maintain trials during negative AM onset. Saggital slice shows a region of left hippocampus (Tal: x = −26, y = −35, z = 0) that was more active during the decrease than maintain trials. (b) Neural activity for the increase and maintain trials during negative AM onset. Saggital slice shows a region of right amygdala (Tal: x = 30, y = −3, z = −17) as well as two regions of right hippocampus (Tal: x = 28, y = −14, z = −14; Tal: x = 30, y = −29, z = −7) that were more active during the maintain than increase trials. (c) Neural activity for the decrease and increase trials during negative AM onset. Saggital slice shows a region of right amygdala (Tal: x = 30, y = 1, z = −17) as well as two regions of right hippocampus (Tal: x = 26, y = −33, z = −8; Tal: x = 32, y = −16, z = −14) that were more active during the decrease than increase trials. Activity is significant at p < .001 and a 5-voxel threshold extent.
There was very little enhancement of activity when maintaining rather than decreasing negative AMs during the onset period (Fig. 5a). Only a small cluster in the left frontal pole (BA 10) as well as two clusters in the left caudate nucleus were more active during the maintain negative than decrease onset conditions. By contrast, and contrary to our expectations, there was greater activity evident for the maintain negative > increase contrast in a number of regions during the onset phase (Fig. 5b), including throughout bilateral PFC [dorsal (BAs 9 and 46), ventral (BA 47), and orbitofrontal (BA 11) regions], bilateral posterior cingulate gyrus (BAs 23, 30 and 31), and right-lateralized medial [hippocampus, parahippocampal and fusiform gyri, amygdala] and bilateral temporal lobes (BAs 21, 22, 38 and 39). In addition to greater amygdala activity, the maintain negative condition also engaged other emotion processing regions bilaterally, including insula (BA 13), and medial PFC (BAs 6 and 10) to a greater extent than the increase negative condition during the memory onset phase. Finally, a number of posterior regions were also revealed in this contrast, including sensory processing regions in both the occipital [bilateral precuneus (BAs 7, 19 and 31), left lingual gyrus (BA 19), bilateral occipital gyri (BAs 18 and 19)] and inferior (left BA 40) and superior parietal (bilateral BA 7) lobes.
Decreasing vs. Increasing Negative Emotions
A direct contrast of the decrease and increase conditions during the onset phase confirmed that the decrease condition engaged more activity (Table 5; Fig. 5c); a whole-brain contrast of decrease > increase revealed reappraisal and retrieval-related regions throughout the PFC [right dorsal (BAs 6, 9, and 46) and bilateral ventral lateral (BAs 11/47) PFC and left frontal pole (BA 10)] and retrieval-related regions in bilateral posterior cingulate gyrus (BAs 24 and 31), primarily right-lateralized medial [hippocampus, fusiform and parahippocampal gyri] and bilateral temporal (BAs 21, 22 and 39) lobes. Contrary to our hypotheses (but in line with the above-reported results contrasting the reappraisal and maintain negative onset conditions), the decrease condition also was associated with greater activity in primarily left-lateralized emotional processing regions [medial PFC (BAs 6, 9 and 11) and amygdala] during the onset phase. Accessing memories in the decrease (vs. increase) condition was further associated with increased activity in visual processing regions [right precuneus (BAs 7 and 31), bilateral cuneus (BAs 18 and 23), and bilateral occipital gyri (BAs 18 and 19)]. On the other hand, activity revealed by the increase > decrease contrast was limited to relatively small clusters in left insula (BA 13), and right anterior (BA 24) and left posterior (BA 23) cingulate gyrus (Table 6).
Table 5.
Group activations for the decrease > increase onset contrast. Regions that were present in the Condition × Phase Interaction examining the onset and elaboration phases are noted in the last column with the distance between the peak evident in the t-test and the interaction.
| Lobe/Region | BA | H | Talairach | t | k | Present in Condition × Phase Interaction |
||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| Decrease > Increase Onset | ||||||||
|
| ||||||||
| Frontal | ||||||||
| Inferior frontal gyrus | 47 | R | 42 | 34 | −12 | 6.34 | 535 | 10 mm |
| 13 | R | 32 | 11 | −11 | 5.08 | 6 | 7 mm | |
| 46 | R | 50 | 26 | 15 | 4.97 | 92 | 2 mm | |
| 45 | R | 51 | 27 | 4 | 4.88 | 9 | ||
| Medial frontal gyrus | 6 | L | −2 | 16 | 47 | 6.41 | 623 | 8 mm |
| 6 | L | −6 | 46 | 34 | 4.61 | 22 | ||
| 9 | L | −8 | 42 | 18 | 4.47 | 19 | ||
| 11 | L | −6 | 52 | −13 | 5.47 | 106 | 4 mm | |
| 10 | R | 6 | 53 | 10 | 4.03 | 6 | ||
| Middle frontal gyrus | 6/9 | L | −46 | 0 | 39 | 4.21 | 6 | |
| Superior frontal gyrus | 10 | L | −8 | 61 | 19 | 5.68 | 118 | 3 mm |
| 9 | R | 4 | 52 | 29 | 4.92 | 25 | ||
| Precentral gyrus | 44 | L | −46 | 10 | 7 | 7.91 | 1698 | 6 mm |
| Medial temporal | ||||||||
| Hippocampus | R | 32 | −16 | −14 | 6.62 | 30 | 3 mm | |
| R | 26 | −33 | −8 | 6.09 | 46 | 6 mm | ||
| Parahippocampal gyrus | L | −22 | −37 | −7 | 9.92 | 3448 | ||
| 30/19 | R | 22 | −39 | −3 | 4.68 | 10 | ||
| 30 | R | 18 | −45 | 1 | 3.97 | 7 | ||
| Other temporal | ||||||||
| Superior temporal gyrus | 22 | L | −48 | −25 | 0 | 9.23 | 1307 | |
| Middle temporal gyrus | 39 | L | −50 | −67 | 27 | 4.96 | 94 | |
| 21 | R | 55 | −6 | −13 | 6.16 | 314 | 8 mm | |
| 21 | R | 65 | −41 | 0 | 4.71 | 20 | ||
| 22 | R | 46 | −35 | 4 | 4.67 | 56 | 8 mm | |
| 21/22 | R | 61 | −48 | 10 | 4.41 | 21 | ||
| Fusiform gyrus | 37 | R | 42 | −51 | −8 | 4.10 | 5 | |
| Parietal | ||||||||
| Superior parietal lobe | 7 | R | 28 | −61 | 53 | 3.92 | 6 | |
| Precuneus | 7 | R | 28 | −52 | 49 | 3.76 | 6 | |
| 31 | R | 14 | −65 | 20 | 4.15 | 11 | ||
| 31 | R | 10 | −71 | 22 | 3.74 | 6 | ||
| Occipital | ||||||||
| Inferior occipital gyrus | 18 | R | 36 | −80 | −6 | 7.40 | 1311 | |
| Middle occipital gyrus | 19 | L | −26 | −73 | 20 | 4.16 | 5 | |
| Superior occipital gyrus | 19 | L | −32 | −70 | 31 | 5.61 | 23 | |
| 19 | R | 32 | −68 | 29 | 4.80 | 96 | 6 mm | |
| Cuneus | 23 | L | −6 | −71 | 13 | 4.64 | 63 | |
| 18 | L | −12 | −83 | 13 | 3.80 | 5 | ||
| 18 | R | 10 | −73 | 17 | 3.89 | 11 | ||
| Limbic | ||||||||
| Amygdala | L | −30 | −4 | −12 | 6.18 | 2* | ||
| L | −28 | 1 | −17 | 4.70 | 4* | |||
| L | −18 | −6 | −13 | 4.26 | 3* | |||
| R | 30 | 1 | −17 | 5.16 | 7* | |||
| R | 30 | 1 | −17 | 5.16 | 7 | |||
| Cingulate gyrus | 31 | L | −2 | −29 | 38 | 7.00 | 92 | 9 mm |
| 24 | L | −4 | −4 | 28 | 3.97 | 5 | ||
| 24 | R | 2 | 3 | 27 | 6.71 | 71 | ||
| 31 | R | 8 | −35 | 29 | 4.77 | 36 | ||
| Other | ||||||||
| Basal ganglia | L | −6 | 0 | −2 | 5.13 | 7 | ||
| L | −18 | −6 | −6 | 5.10 | 17 | |||
| Cerebellum | L | −4 | −60 | −36 | 4.69 | 6 | ||
| L | −26 | −68 | −39 | 4.80 | 5 | |||
| L | −28 | −56 | −22 | 4.93 | 9 | |||
| R | 4 | −54 | −26 | 7.60 | 23 | 3 mm | ||
| R | 6 | −72 | −10 | 4.91 | 12 | |||
| Hypothalamus | -- | 0 | −10 | −10 | 4.17 | 6 | ||
Note: Regions revealed by a small volume correction applied to an anatomical mask of the bilateral amygdala.
Table 6.
Group activations for the increase > decrease onset contrast. Regions that were present in the Condition × Phase Interaction examining the onset and elaboration phases are noted in the last column with the distance between the peak evident in the t-test and the interaction.
| Lobe/Region | BA | H | Talairach | t | k | Present in Condition × Phase Interaction |
||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| Increase > Decrease Onset | ||||||||
|
| ||||||||
| Limbic | ||||||||
| Insula | 13 | L | −28 | −6 | 26 | 4.24 | 13 | |
| Cingulate gyrus | 23 | L | −10 | −36 | 17 | 6.36 | 5 | |
| 24 | R | 24 | 15 | 27 | 4.25 | 8 | ||
| Parietal | ||||||||
| Postcentral gyrus | 3/2/1 | R | 57 | −24 | 34 | 4.24 | 8 | |
| Other | ||||||||
| Basal ganglia | L | −22 | 18 | 16 | 6.83 | 104 | 6 mm | |
| L | −18 | −11 | 23 | 5.39 | 18 | |||
| R | 20 | 14 | 16 | 4.59 | 8 | |||
| R | 16 | −7 | 19 | 4.48 | 11 | |||
| Cerebellum | L | −32 | −68 | −30 | 4.78 | 38 | ||
| Thalamus | L | −6 | −3 | 15 | 3.96 | 8 | ||
| R | 14 | −3 | 13 | 3.93 | 10 | |||
Regulation During Recall: Elaboration Phase
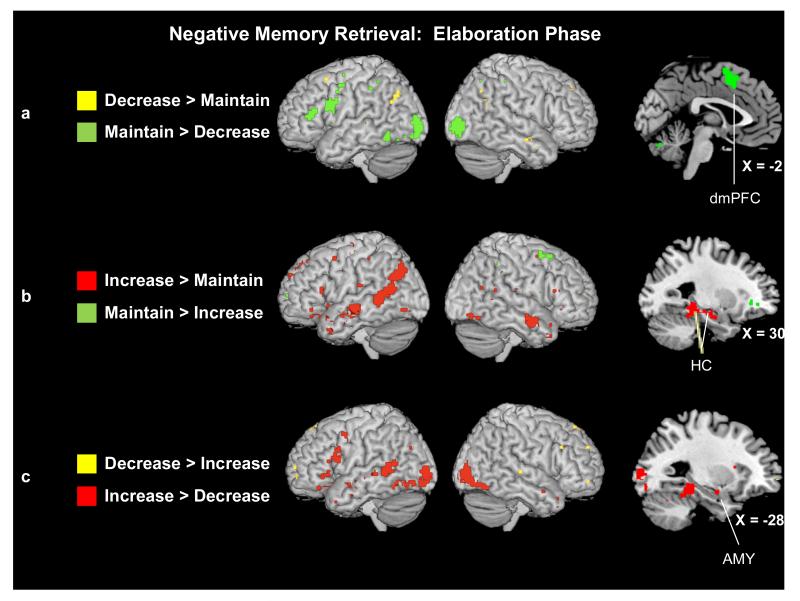
Reappraising vs. Maintaining Negative Emotions
Contrasts comparing the reappraisal conditions to the maintain conditions as individuals elaborated upon the details of their negative AMs revealed a pattern opposite to that observed during the memory onset phase. Despite revealing greater activity during the onset phase, the decrease > maintain contrast in the elaboration phase revealed only two regions in the PFC [left middle (BA 8) and right superior (BA 9) frontal gyri], one in right middle temporal gyrus (BA 21), and one in left angular gyrus (BA 39) that were more active when decreasing than maintaining the negative emotions associated with AMs. However, the reverse contrast (maintain negative vs. decrease negative) revealed greater activity in regions previously associated with memory retrieval [left-lateralized ventrolateral (BA 44) PFC, bilateral middle frontal gyrus (BA 6), and left inferior PFC (BA 46) and temporal (BA 37) cortices], emotion processing [left medial PFC (BA 6)], and the processing and representation of visual information [bilateral middle occipital gyrus (BAs 18 and 31), right cuneus (BA 31), bilateral inferior parietal lobe (BA 40), and left fusiform gyrus (BA 37)].
The contrast comparing neural activity as individuals elaborated upon negative AMs in the increase (vs. maintain) was more in line with our expectations about how up-regulation might modulate neural activity during recall (see Fig. 6b). For example, the increase condition engaged significantly greater activity in regions known to support both memory retrieval and cognitive reappraisal [primarily left-lateralized ventrolateral PFC (BAs 11, 44/45 and 47) and left frontal pole (BA 10)], as well as regions more specific to memory retrieval [right hippocampus and bilateral superior (BAs 38 and 39) and middle (BAs 21 and 22) temporal gyri]. In addition, as hypothesized, increasing (vs. maintaining) the negative emotions associated with AMs engaged regions associated with emotional processing [left medial PFC (BAs 6, 8, and 9) and left insula (BA 13)] and visual processing [left precuneus (BAs 7 and 19), left lingual (BA 18) and bilateral occipital (BAs 19 and 39) gyri, and posterior cingulate gyrus (BA 24)]. In contrast, maintaining negative emotions during the elaboration phase engaged fewer regions to a greater extent than increasing, primarily in the left medial (BA 6) and bilateral middle frontal gyrus (BAs 6, 10, and 11), right inferior parietal lobe (BA 40), and bilateral anterior cingulate gyrus (BAs 24 and 32).
Figure 6.
(a) Neural activity for the decrease and maintain trials during negative AM elaboration. Saggital slice shows a region of left dorsomedial PFC (Tal: x = −2, y = 8, z = 49) that was more active during the maintain than decrease trials. (b) Neural activity for the increase and maintain trials during negative AM elaboration. Saggital slice shows two regions of right hippocampus (Tal: x = 30, y = −14, z = −16; Tal: x = 30, y = −37, z = 0) that were more active during the increase than maintain trials. (c) Neural activity for the decrease and increase trials during negative AM elaboration. Saggital slice shows a region of left amygdala (Tal: x = −28, y = −6, z = −13) that was more active during the decrease than increase trials. Activity is significant at p < .001 and a 5-voxel threshold extent.
Decreasing vs. Increasing Negative Emotions
Directly comparing the decrease > increase conditions as individuals elaborated on the details of their negative AMs (see Fig. 6c) confirmed that the increase condition recruited the greatest neural activity during the elaboration phase. Few regions were more engaged during decrease than increase trials; these regions included small clusters of bilateral superior frontal gyrus (BAs 9 and 10) and right superior temporal gyrus (BA 22) (see Table 7).
Table 7.
Group activations for the decrease > increase elaboration contrast. Regions that were present in the Condition × Phase Interaction examining the onset and elaboration phases are noted in the last column with the distance between the peak evident in the t-test and the interaction.
| Lobe/Region | BA | H | Talairach | t | k | Present in Condition × Phase Interaction |
||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| Decrease > Increase Elaboration | ||||||||
|
| ||||||||
| Frontal | ||||||||
| Superior frontal gyrus | 10 | L | −32 | 58 | −6 | 4.48 | 6 | |
| 9 | R | 20 | 50 | 25 | 3.73 | 6 | ||
| Temporal | ||||||||
| Superior temporal gyrus | 22 | R | 65 | −25 | 5 | 4.48 | 6 | |
| Other | ||||||||
| Basal ganglia | R | 16 | 1 | 20 | 5.04 | 20 | 3 mm | |
The reverse contrast, however, revealed that several regions that were more active as individuals elaborated on their negative AMs that had appeared with increase (vs. decrease) regulation instructions (see Fig. 6c; Table 8). These regions included primarily left-lateralized dorsal (BA 45) and ventrolateral (BAs 11, 44 and 47) PFC. In addition, several regions of the medial temporal lobes [right hippocampus, left parahippocampal (BA 34), left fusiform gyri, left amygdala], lateral temporal cortices [bilateral middle (BAs 21, 39/19) and left superior (BA 22 and 38) temporal gyri] and posterior cingulate (BA 31 and 30/23) were engaged more by the increase than decrease trials during the elaboration phase. The same was true of visual processing regions [bilateral precuneus (BA 31), left cuneus (BA 18), and bilateral middle occipital gyrus (BA 19)].
Table 8.
Group activations for the increase > decrease elaboration contrast. Regions that were present in the Condition × Phase Interaction examining the onset and elaboration phases are noted in the last column with the distance between the peak evident in the t-test and the interaction.
| Lobe/Region | BA | H | Talairach | t | k | Present in Condition × Phase Interaction |
||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| x | y | z | ||||||
| Increase > Decrease Elaboration | ||||||||
| Frontal | ||||||||
| Inferior frontal gyrus | 45/44 | L | −38 | 11 | 22 | 6.78 | 148 | |
| 45 | L | −53 | 22 | 14 | 3.87 | 6 | ||
| 47 | L | −34 | 30 | −12 | 4.83 | 12 | ||
| 47 | L | −42 | 27 | −3 | 4.29 | 22 | ||
| 47 | L | −38 | 21 | −13 | 3.90 | 5 | ||
| 47 | R | 28 | 13 | −17 | 4.54 | 6 | 2 mm | |
| 47 | R | 36 | 31 | −2 | 3.95 | 8 | 4 mm | |
| Medial frontal gyrus | 6/32 | L | −4 | 10 | 47 | 6.27 | 256 | 3 mm |
| 11 | L | −10 | 48 | −12 | 4.55 | 16 | 2 mm | |
| Middle frontal gyrus | 6 | L | −44 | 6 | 44 | 4.42 | 20 | |
| Superior frontal gyrus | 8 | L | −6 | 20 | 52 | 4.45 | 14 | |
| Medial temporal | ||||||||
| Hippocampus | R | 26 | −12 | −15 | 4.93 | 20 | 9 mm | |
| R | 28 | −33 | 0 | 4.32 | 6 | 2 mm | ||
| Parahippocampal gyrus | 34 | L | −16 | −10 | −15 | 4.70 | 15 | |
| Other temporal | ||||||||
| Middle temporal gyrus | L | −53 | −31 | 2 | 4.84 | 28 | ||
| 21 | L | −50 | −10 | −10 | 4.34 | 23 | ||
| 21 | L | −53 | −20 | −2 | 4.03 | 8 | ||
| 21 | R | 53 | 1 | −17 | 4.41 | 5 | 7 mm | |
| 39/19 | R | 32 | −67 | 20 | 4.33 | 6 | ||
| Superior temporal gyrus | 22 | L | −50 | −46 | 13 | 6.24 | 266 | 6 mm |
| 38 | L | −50 | −2 | −10 | 4.23 | 6 | 8 mm | |
| Fusiform gyrus | L | −46 | −37 | −12 | 7.25 | 682 | ||
| Parietal | ||||||||
| Precuneus | 31 | L | −10 | −45 | 35 | 4.54 | 12 | 2 mm |
| 31 | R | 2 | −65 | 27 | 4.65 | 15 | ||
| Occipital | ||||||||
| Middle occipital gyrus | 19 | L | −32 | −91 | 6 | 6.16 | 381 | |
| 19 | R | 38 | −72 | −6 | 6.28 | 671 | ||
| Cuneus | 18 | L | −8 | −79 | 17 | 3.99 | 9 | |
| Limbic | ||||||||
| Amygdala | L | −28 | −6 | −13 | 5.00 | 19 | ||
| Cingulate gyrus | 31 | L | −2 | −67 | 13 | 4.39 | 14 | |
| 31 | L | −4 | −37 | 35 | 4.53 | 27 | 0 mm | |
| 30/23 | L | −14 | −54 | 8 | 8.66 | 810 | 10 mm | |
| Other | ||||||||
| Cerebellum | R | 2 | −54 | −28 | 5.60 | 13 | 0 mm | |
| R | 2 | −18 | 25 | 3.91 | 9 | |||
| Thalamus | L | −2 | −15 | 4 | 4.63 | 20 | 4 mm | |
Condition by Phase Interaction
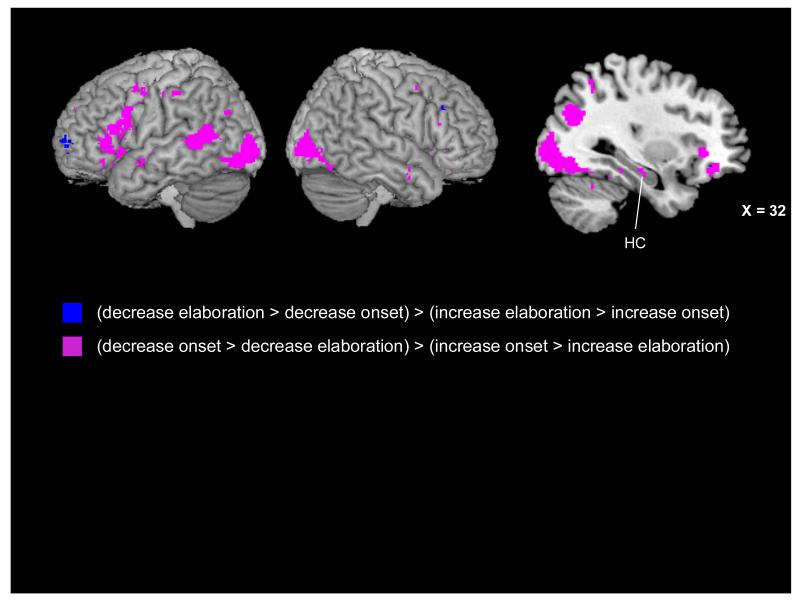
The results of our t-test analyses for each phase suggested that down- and up-regulation might engage neural regions over a different time course, with down-regulation engaging the greatest neural activity during the memory onset phase and up-regulation during the memory elaboration phase. We further examined this possibility by submitting the first-level reappraisal > maintain negative contrast images to a second-level 2 (Reappraisal Condition: Decrease, Increase) × 3 (Phase: Instruction, Onset, Elaboration) repeated-measures analysis of variance (ANOVA). Two follow-up interaction contrasts investigated the interaction of condition and the onset and elaboration phases given that these were the phases in which condition-based patterns differed based on the t-test analyses (see Fig. 7). Each contrast used an inclusive mask from the 2 × 3 ANOVA (mask p < .001) and a statistical threshold of p < .001 and a 5-voxel threshold extent. The first contrast examined which regions were more active for the decrease condition in the memory onset phase and increase condition in the memory elaboration phase [i.e., (decrease onset > decrease elaboration) > (increase onset > increase elaboration)]. Several of the regions revealed by this contrast were similar to the regions revealed by the t-test analyses and are noted in Tables 5 and 7. Consistent with our t-test analyses, this interaction contrast revealed a widespread set of regions, including left-lateralized medial PFC (BAs 6, 9, and 11) and bilateral ventrolateral PFC (BAs 44/45 and 47). This interaction contrast also revealed regions throughout the bilateral lateral temporal lobes (BAs 20, 21, 22) and right medial temporal lobe (hippocampus, parahippocampal gyrus). Also evident were regions of left precuneus (BA 31), right middle occipital gyrus (BA 19), and bilateral posterior cingulate cortex (BAs 23 and 30).
Figure 7.
Neural activity present in the interaction contrasts inclusively masked with the Condition (decrease, increase) × Phase (instruction, onset, elaboration) interaction results. Regions in blue were more active for the decrease condition during the elaboration phase and the increase condition in the memory onset phase. Regions in purple were more active for the decrease condition during the memory onset phase and the increase condition in the elaboration phase, including a region of right hippocampus (Tal: x = 32, y = −18, z = −13) evident on the saggital slice.
The second interaction contrast was the reverse of the first contrast and examined which regions were more active for the decrease condition during the elaboration phase and the increase condition in the memory onset phase [i.e., (decrease elaboration > decrease onset) > (increase elaboration > increase onset)]. Consistent with the t-test analyses, this interaction contrast revealed little activity: a region of left frontal pole (BA 10) and left caudate (see Tables 6 and 8).
Post-Scan Behavioral Results
We also examined the effect of regulation condition on the behavioral ratings made about AMs following the scan. A within-subjects ANOVA revealed a significant effect of instruction on post-scan emotional intensity ratings, F(3, 60) = 60.95, p < .001, partial-η2 = .75 (Table 9). As would be expected, post-hoc pairwise comparisons confirmed that neutral AMs continued to be rated as lower in intensity than all of the negative AM regulation conditions, ps < .001. In addition, pairwise comparisons revealed that negative AMs that had been in the decrease condition during the scan continued to be rated as lower in emotional intensity than those in the increase condition, p = .003.
Table 9.
Post-scan mean behavioral characteristics of negative and neutral AMs from that had appeared with decrease, increase, or maintain instructions during the scan. Standard deviations are presented in parentheses.
| Post-Scan Behavioral Ratings | ||||
|---|---|---|---|---|
|
| ||||
| Trial Type | Intensity | Negative | Positive | Vividness |
| Decrease Neg | 4.38 (0.73) | 4.79 (0.81) | 1.79 (0.88) | 5.12 (0.77) |
| Increase Neg | 4.65 (0.76) | 5.28 (0.75) | 1.55 (0.75) | 5.17 (0.67) |
| Maintain Neg | 4.53 (0.75) | 5.10 (0.62) | 1.54 (0.54) | 5.20 (0.66) |
| Maintain Neutral | 2.51 (0.84) | 1.34 (0.23) | 3.95 (1.39) | 4.88 (0.75) |
There was also a significant main effect of instruction on post-scan negative emotion ratings, F(3, 60) = 333.31, p < .001, partial-η2 = .94 (Table 9). As with intensity ratings, post-hoc pairwise comparisons confirmed that neutral memories were rated as significantly less negative than negative AMs from each of the regulation conditions, ps < .001. More interestingly, participants rated the negativity of their negative AMs in line with what would be predicted based on instruction condition: Negative AMs that had been in the decrease condition during the scan were rated as less negative than negative AMs from both the increase and maintain conditions, ps < .009, and negative AMs that had been in the increase condition during the scan were rated as more negative than AMs from both the decrease and maintain conditions, ps ≤ .05.
A similar pattern was also present for positive emotion ratings, including an overall main effect of instruction, F(3, 63) = 77.39, p < .001, partial-η2 = .80 (Table 9). Pairwise comparisons confirmed that neutral memories were rated higher in positive emotion than negative AMs from any regulation condition, ps < .001. Negative AMs that had appeared in the decrease condition during the scan were rated as higher in positive emotion than those negative AMs from both the increase and maintain conditions, ps < .04, though AMs from the increase and maintain conditions did not differ from one another in positive ratings, p = .91.
Finally, there was a main effect of instruction for the post-scan vividness ratings, F(3, 60) = 4.54, p = .01, partial-η2 = .19 (Table 9). Pairwise comparisons revealed that this main effect was driven by neutral memories being rated as less vivid than negative AMs from the increase and maintain conditions, ps < .02; there was also a trend for negative AMs from the decrease condition to be rated as less vivid than the neutral AMs, p = .10.
Discussion
We used a novel cognitive reappraisal paradigm adapted from the emotion regulation literature for use with AMs and asked participants to decrease, increase, or maintain the emotions associated with negative events from their personal pasts while undergoing an fMRI scan. Emotional intensity ratings about the events made during the scan confirmed that participants were reappraising in the instructed direction, as AMs that had appeared with the increase instruction were rated as the most intense, followed by AMs that had appeared with the maintain instruction, and then by AMs that had appeared with the decrease instruction. By scanning individuals as they prepared to reappraise or maintain their emotions, accessed events associated with personal cues, and then elaborated upon the AMs they had recalled, the present experiment revealed that a different time course of activation was associated with the down-versus the up-regulation of AMs.
Down-Regulation of Negative Emotions
The down-regulation of negative emotions during AM recall recruited the greatest neural activity during the memory onset phase (i.e., the time between the presentation of a memory cue and a button press indicating that the fully formed memory was in mind). As would be expected based on previous reappraisal studies with emotional images (Ochsner & Gross, 2005; 2008), decreasing emotional responses was associated with increased activity in cognitive control regions throughout the PFC when compared to maintaining emotional responses. Areas of activation were revealed in dorsolateral PFC regions associated with maintaining and manipulating information in working memory (e.g., Curtis & D’Esposito, 2003); ventrolateral PFC regions associated with autobiographical memory retrieval (Cabeza & St. Jacques, 2007) and the selection of context-appropriate reappraisals (reviewed by Denny et al., in press; see also Badre, Poldrack, Pare-Blagoev, Insler, & Wagner, 2005); and medial PFC regions associated with self-referential processing (Kelly et al., 2002), with the flexible assignment of affective value (D’Argembeau, Xue, Lu, Van der Linden, & Bechara, 2008), and with the extinction of conditioned emotional responses (particularly ventromedial PFC; Phelps, Delgado, Nearing, & LeDoux, 2004; Delgado, Nearing, LeDoux, & Phelps, 2008).
Although this PFC activity follows in line with other cognitive reappraisal tasks (reviewed by Ochsner & Gross, 2008; 2005) that have used a maintenance/view condition as a baseline, the disproportionate PFC activity during the memory onset phase for decreasing versus increasing the intensity of AMs was contrary to our hypotheses. Also contrary to expectations, there was disproportionate activation in the MTL (e.g., hippocampus, parahippocampal gyrus) and emotion processing regions, including the insula and amygdala, during down-regulation as compared to up-regulation of negative AMs.
One possible explanation for this pattern of findings for the down-regulation of negative emotions during AM recall may be gleaned from the process model of emotion regulation, which posits that the reappraisal of negative emotions may be especially sensitive to timing effects (Sheppes & Gross, 2011). The model presumes that emotions unfold over time and that down-regulating negative emotions is most effective at early time points before intensity increases. For instance, being instructed to down-regulate emotions about a sad film early during the film is an effective way to reduce negative affect, but reappraisal instructions given later during the film are ineffective, presumably as emotional intensity has passed a “point of no return” (Sheppes & Meiran, 2007).
The timing hypothesis put forth by the process model (Sheppes & Gross, 2011) may apply to down-regulation of AM intensity; just as decreasing negative emotion is most effective at early time points during a film clip, it may also be most effective at early time points during AM recall (i.e., as a memory is initially being accessed) and thus down-regulation of AM may be associated with earlier retrieval-related activity than up-regulation. Indeed, in striking contrast to the memory onset phase, the memory elaboration phase revealed the greatest neural activity for the maintain and increase trials, consistent with the hypothesis that much of the work in down-regulating negative emotions occurred early on in AM recall. This finding dovetails nicely with previous work suggesting that reappraisal (when compared to rumination) did not lead to greater neural activity (Kross et al., 2009) or connectivity ((Fabiansson et al., 2012). These studies presented regulation instructions only after AMs were being held in mind (and presumably after emotional appraisal had already occurred), which would correspond with our memory elaboration phase, perhaps explaining why they found few regions associated with reappraisal.
Up-Regulation of Negative Emotions
Whereas decreasing emotions was primarily associated with increased neural activity during the AM onset phase, increasing emotions instead was related to increased activity in both the instruction and elaboration phases. Although both down- and up-regulation engaged cognitive control regions in the PFC during the instruction phase, up-regulation led to the most extensive PFC engagement in comparison to the maintain instruction phase. Directly comparing the increase and decrease conditions revealed widespread activity in regions previously implicated in the up-regulation of negative images (Ochsner et al., 2004), including in cognitive control regions in the PFC and anterior cingulate, visuospatial regions (e.g., cuneus, precuneus), as well as in the insula. This pattern of activity is consistent with prior research demonstrating that PFC, ACC, insula, and amygdala activity is elevated when participants are expecting the presentation of unpleasant stimuli (e.g., Herwig et al., 2007). In addition, this anticipatory activity is in line with behavioral reports from a post-scan debriefing questionnaire. Whereas participants reported trying to “relax” and “let go of negative emotions” when viewing the decrease instructions, they reported “tensing up” and preparing to “engage with,” “relive,” and “recall specific details about” the subsequent negative event.
Despite this widespread anticipatory activity during the instruction phase, there was a relative paucity of activity during the memory onset phase when the increase condition was compared to either the decrease or maintain conditions. As mentioned in the earlier discussion of down-regulation, part of this difference may relate to when, in the timecourse of memory retrieval, reappraisal-related activity is most effective in altering the intensity of an emotional response: Activity during the onset phase may be more effective for down-regulation than for up-regulation. But another possibility is that the anticipatory activity in emotion and visuospatial regions during the instruction phase enabled participants to more efficiently access the emotional and sensory details about negative events, leading to relatively less neural activity compared to negative AMs that had not been preceded by such anticipatory activity. In other words, adapting a particular mindset to increase negative emotions before recall even occurs may facilitate retrieval of negative AMs.
Neural activity during the time when individuals were elaborating upon their AMs was consistent with our predictions based on prior cognitive reappraisal (Ochsner & Gross, 2005; 2008) and emotional AM (e.g., Botzung et al., 2010) experiments. In particular, regions of medial PFC, MTL (including hippocampus, parahippocampal gyrus, and amygdala), posterior visuospatial regions, and emotion processing regions (including insula) were all more engaged by the increase than decrease or maintain conditions during memory elaboration. These results are in accordance with prior research on the up-regulation of emotions during the presentation of negative images (Ochsner et al., 2004) and on the recall of emotionally intense AMs (Botzung et al., 2010). In addition, the increased activity in visuospatial regions corresponds with the higher vividness ratings for the increase condition; such a relation between vividness ratings and posterior activity was previously found to be specific to the AM elaboration phase (Daselaar et al., 2008).
Interestingly, the increased activity in visuospatial regions and higher vividness ratings may fit with prior findings of increased activity in these regions during the up-regulation of negative images (e.g., Ochsner et al., 2004). One proposed strategy for increasing emotional intensity in response to an emotional image is to imagine oneself as a central figure in the scene and to enhance the subjective feeling of experiencing the sights and sounds associated with that scene (Ochsner et al., 2004); a similar strategy of increasing or decreasing recall of specific sensory details may be a useful regulatory strategy during autobiographical recall. During AM recall, the elaboration phase has specifically been associated with the recall and elaboration of vivid sensory details (Daselaar et al., 2008). A reasonable hypothesis, then, might be that recalling and expounding upon vivid sensory details during the elaboration phase is a useful strategy for successfully up-regulating emotional intensity.
Future Directions
An important next step in the research on emotion regulation and AM is testing how these two processes are linked on a behavioral and neural level in clinical populations, such as individuals with depression. Emotion dysregulation, and pervasive maintenance of negative affect, are considered defining features of depression, possibly because poor executive functioning leads to deficits in the ability to use cognitively demanding strategies like reappraisal (Gotlib & Joormann, 2010). In line with this hypothesis, participants with major depressive disorder exhibit both increased bilateral PFC (Johnstone et al., 2007) and increased amygdala and insula activity (Beauregard, Paquette, & Levesque, 2006) during cognitive reappraisal tasks using negative images, perhaps reflecting ineffective compensatory attempts at emotion regulation (see Denny et al., in press, for similar discussion). Examining neural activity as depressed individuals attempt to down-regulate negative AMs might shed further light on their regulatory deficits: For example, depressed individuals may engage PFC and insula/amygdala while accessing negative AMs (as our healthy participants did), but continue to sustain these activations when elaborating upon the details of their memories. The pervasive sad mood associated with depression might also lead to anticipatory neural responses similar to what we found during the increase instruction phase. In turn, depressed individuals may have greater success in accessing negative and vivid emotional details about events, leading to the perpetuation of their sad mood and depression.
In addition to depression, these questions are also directly relevant to individuals with post-traumatic stress disorder (PTSD). A recent fMRI experiment revealed that individuals with PTSD (vs. controls) engaged the amygdala/hippocampus to a greater extent during a negative AM search phase and the vmPFC to a greater extent during both the search and elaboration phases (St. Jacques, Botzung, Miles, & Rubin, 2011). The authors suggest that this increased recruitment of amygdala and vmPFC, and a greater functional coupling between these regions, might reflect an up-regulation of negative emotional intensity during recall (St. Jacques et al., 2011). Because PTSD can be characterized by either an undermodulation (i.e., in the case of hyperarousal) or overmodulation (i.e., in the case of a dissociative subtype of PTSD) of emotional intensity (Lanius et al., 2010), an interesting next step would be to differentiate between individuals with these two subtypes of PTSD as they complete an instructed reappraisal task. We might expect individuals with the hyperarousal subtype of PTSD to over-recruit emotional appraisal regions like the amygdala, insula, and mPFC, whereas individuals with the dissociative subtype might under-recruit these same regions while over-recruiting cognitive control regions like lateral PFC and dACC.
The post-scan behavioral ratings suggest a possible long-term influence of regulation instructions on memory characteristics. During the post-scan session, participants were given the titles of each event they recalled in the scanner, but were not given any reminder of which reappraisal instruction had appeared with which AM. Events that had previously appeared with the decrease instruction continued to be rated as significantly lower in intensity than events that had appeared with the increase instruction, suggesting that there may have been an effect of reappraisal on AM recall that lasted at least across the 0.5 hour delay between the scan and post-scan ratings. An important question for future research to examine is how long-lasting reappraisal effects are; this question is of interest from a basic science perspective with regard to the malleability of memory and may have relevance to clinical populations undergoing cognitive behavioral therapy aimed at reducing the emotional intensity associated with cognitions (e.g., Gotlib & Joormann, 2010).
There are also a number of open questions regarding the types of emotional events that individuals are asked to reappraise. For example, it remains to be seen whether the valence of the event being reappraised would lead to differences in which neural regions are engaged during recall. One possibility is that down- and up-regulation modulate neural activity in similar regions over the same timecourse regardless of the valence of the information that is being regulated. However, recalling positive events has sometimes been associated with greater engagement of medial OFC and MTL compared to negative events (Markowitsch, Vanderckhove, Lanfermann, & Russ, 2003; Piefke, Weiss, Zilles, Markowitsch, & Fink, 2003; but see St. Jacques et al., 2011), leaving open the possibility that the reappraisal of positive events may modulate activity in these regions to a greater extent than the reappraisal of negative events.
Conclusion
In sum, the present experiment modified a cognitive reappraisal task for use with AMs, and asked individuals to decrease, increase, or maintain the emotions associated with negative events while undergoing an fMRI scan. Our results revealed that down- and up-regulation were differentiated by the time course over which they recruited neural activity: Down-regulation primarily engaged regions during the memory onset phase, whereas up-regulation engaged regions during the instruction and memory elaboration phases. More broadly, this study suggests that invoking goals prior to the retrieval of AMs can influence the behavioral (e.g., emotional intensity and vividness ratings) and neural correlates associated with recall, in line with Conway’s (2005) proposal of the goal-directed constructive nature of AM. Indeed, the ability to flexibly reappraise emotional details may be a critical function of memory and have important implications for the development and treatment of clinical disorders.
Acknowledgments
This research was supported by NIH grant MH080833 to EAK and a National Defense Science and Engineering Graduate Fellowship to ACH. We thank Tammy Moran and Ross Mair at the Harvard Center for Brain Science for their assistance with MR data collection and Daniel Schacter, Scott Slotnick, Ehri Ryu, and Donna Rose Addis for helpful discussion. Portions of this research were included in a Ph.D. dissertation by ACH.
References
- Addis DR, Moscovitch M, Crawley AP, McAndrews MP. Recollective qualities modulate hippocampal activation during autobiographical memory retrieval. Hippocampus. 2004;14:752–762. doi: 10.1002/hipo.10215. [DOI] [PubMed] [Google Scholar]
- Addis DR, Pan L, Vu MA, Laiser N, Schacter DL. Constructive episodic simulation of the future and the past: Distinct subsystems of a core brain network mediate imagining and remembering. Neuropsychologia. 2009;47:2222–2238. doi: 10.1016/j.neuropsychologia.2008.10.026. [DOI] [PubMed] [Google Scholar]
- Badre D, Poldrack RA, Pare-Blagoev EJ, Insler RZ, Wagner AD. Dissociable controlled retrieval and generalized selection mechanisms in ventrolateral prefrontal cortex. Neuron. 2005;47:907–918. doi: 10.1016/j.neuron.2005.07.023. [DOI] [PubMed] [Google Scholar]
- Beauregard M, Paquette V, Levesque J. Dysfunction in the neural circuitry of emotional self-regulation in major depressive disorder. Neuroreport. 2006;17:843–6. doi: 10.1097/01.wnr.0000220132.32091.9f. [DOI] [PubMed] [Google Scholar]
- Bluck S, Alea N, Habermas T, Rubin DC. A tale of three functions: The self-reported uses of autobiographical memory. Social Cognition. 2005;23:91–117. [Google Scholar]
- Botzung A, Rubin DC, Miles A, Cabeza R, LaBar KS. Mental hoop diaries: Emotional memories of a college basketball game in rival fans. The Journal of Neuroscience. 2010;30:2130–2137. doi: 10.1523/JNEUROSCI.2481-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Jacques P. Functional neuroimaging of autobiographical memory. Trends in Cognitive Sciences. 2007;11:219–227. doi: 10.1016/j.tics.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Clark JM, Paivio A. Extensions of the Paivio, Yuille, and Madigan (1968) norms. Behavior Research Methods, Instruments, & Computers. 2004;36:371–383. doi: 10.3758/bf03195584. [DOI] [PubMed] [Google Scholar]
- Conway MA. Memory and the self. Journal of Memory and Language. 2005;53:594–628. [Google Scholar]
- Cooney RE, Joormann J, Atlas JY, Eugène F, Gotlib IH. Remembering the good times: neural correlates of affect regulation. Neuroreport. 2007;18:1771–1774. doi: 10.1097/WNR.0b013e3282f16db4. [DOI] [PubMed] [Google Scholar]
- Curtis CE, D’Esposito M. Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences. 2003;7:415–423. doi: 10.1016/s1364-6613(03)00197-9. [DOI] [PubMed] [Google Scholar]
- D’Argembeau A, Xue G, Lu Z, Van der Linden M, Bechara A. Neural correlates of envisioning emotional events in the near and far future. NeuroImage. 2008;40:398–407. doi: 10.1016/j.neuroimage.2007.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daselaar SM, Rice HJ, Greenberg DL, Cabeza R, LaBar KS, Rubin DC. The spatiotemporal dynamics of autobiographical memory: Neural correlates of recall, emotional intensity, and reliving. Cerebral Cortex. 2008;18:217–229. doi: 10.1093/cercor/bhm048. [DOI] [PubMed] [Google Scholar]
- Delgado MR, Nearing KI, LeDoux JE, Phelps EA. Neural circuitry underlying the regulation of conditioned fear and its relation to extinction. Neuron. 2008;59:829–838. doi: 10.1016/j.neuron.2008.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny B, Silvers J, Ochsner K. How we heal what we don’t want to feel: The functional neural architecture of emotion regulation. In: Kring AM, Sloan DM, editors. Emotion regulation and psychopathology: A transdiagnostic approach to etiology and treatment. Guilford Press; New York: pp. 59–87. in press. [Google Scholar]
- Fabiansson EC, Denson TF, Moulds ML, Grisham JR, Schira MM. Don’t look back in anger: Neural correlates of reappraisal, analytical rumination, and angry rumination during recall of an anger-inducing autobiographical memory. NeuroImage. 2012;59:2974–2981. doi: 10.1016/j.neuroimage.2011.09.078. [DOI] [PubMed] [Google Scholar]
- Goldin PR, McRae K, Ramel W, Gross JJ. The neural bases of emotion regulation: Reappraisal and suppression of negative emotion. Biological Psychiatry. 2008;63:577–586. doi: 10.1016/j.biopsych.2007.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Joormann J. Cognition and depression: Current status and future directions. Annual Review in Clinical Psychology. 2010;6:285–312. doi: 10.1146/annurev.clinpsy.121208.131305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg DL, Rice HJ, Cooper JJ, Cabeza R, Rubin DC, LaBar KS. Coactivation of the amygdala, hippocampus, and inferior frontal gyrus during autobiographical memory retrieval. Neuropsychologia. 2005;43:659–674. doi: 10.1016/j.neuropsychologia.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Thompson RA. Emotion regulation: Conceptual foundations. In: Gross JJ, editor. Handbook of emotion regulation. Guilford Press; New York: 2007. pp. 3–24. [Google Scholar]
- Herwig U, Baumgartner T, Kaffenberger T, Bruhl A, Kottlow M, et al. Modulation of anticipatory emotion and perception processing by cognitive control. NeuroImage. 2007;37:652–662. doi: 10.1016/j.neuroimage.2007.05.023. [DOI] [PubMed] [Google Scholar]
- Holland AC, Tamir M, Kensinger EA. The effect of regulation goals on emotional event specific knowledge. Memory. 2010;18:504–521. doi: 10.1080/09658211.2010.481628. [DOI] [PubMed] [Google Scholar]
- Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: Counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. The Journal of Neuroscience. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Cooney RE, Henry ML, Gotlib IH. Neural correlates of automatic mood regulation in girls at high risk for depression. Journal of Abnormal Psychology. 2012;121:61–72. doi: 10.1037/a0025294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson BR, Singer JA, Salovey P. Mood regulation and memory: Repairing sad moods with happy memories. Cognition and Emotion. 1996;10:437–444. [Google Scholar]
- Kelley WM, Macrae CN, Wyland CL, Caglar S, Inati S, Heatherton TF. Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience. 2002;14:785–794. doi: 10.1162/08989290260138672. [DOI] [PubMed] [Google Scholar]
- Kross E, Davidson M, Weber J, Ochsner K. Coping with emotions past: The neural bases of regulating affect associated with negative autobiographical memories. Biological Psychiatry. 2009;65:361–366. doi: 10.1016/j.biopsych.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitsch HJ, Thiel A, Reinkemeier M, Kessler J, Koyuncu A, Heiss WD. Right amygdalar and temporofrontal activation during autobiographic, but not during fictitious memory retrieval. Behavioral Neurology. 2000;12:181–190. doi: 10.1155/2000/303651. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ochsner K, Gross JJ. Cognitive emotion regulation: Insights from social cognitive and affective neuroscience. Current Directions in Psychological Science. 2008;17:153–158. doi: 10.1111/j.1467-8721.2008.00566.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: an FMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Ray RD, Cooper JC, Robertson ER, Chopra S, Gabrieli JDE, Gross JJ. For better or for worse: Neural systems supporting the cognitive down- and up-regulation of negative emotion. NeuroImage. 2004;23:483–499. doi: 10.1016/j.neuroimage.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Phan KL, Fitzgerald DA, Nathan PJ, Moore GJ, Uhde TW, Tancer ME. Neural substrates for voluntary suppression of negative affect: a functional magnetic resonance imaging study. Biological Psychiatry. 2005;57:210–219. doi: 10.1016/j.biopsych.2004.10.030. [DOI] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Gross JJ. Is timing everything? Temporal considerations in emotion regulation. Personality and Social Psychology Review. 2011;15:319–331. doi: 10.1177/1088868310395778. [DOI] [PubMed] [Google Scholar]
- Sheppes G, Meiran N. Better late than never? On the dynamics of online regulation of sadness using distraction and cognitive reappraisal. Personality and Social Psychology Bulletin. 2007;33:1518–1532. doi: 10.1177/0146167207305537. [DOI] [PubMed] [Google Scholar]
- Svoboda E, McKinnon MC, Levine B. The functional neuroanatomy of autobiographical memory: A meta-analysis. Neuropsychologia. 2006;44:2189–2208. doi: 10.1016/j.neuropsychologia.2006.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamir M, Mitchell C, Gross JJ. Hedonic and instrumental motives in anger regulation. Psychological Science. 2008;19:324–328. doi: 10.1111/j.1467-9280.2008.02088.x. [DOI] [PubMed] [Google Scholar]
- Urry HL, van Reekum CM, Johnstone T, Kalin NH, Thurow ME, et al. Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. The Journal of Neuroscience. 2006;26:4415–4425. doi: 10.1523/JNEUROSCI.3215-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walter B, Blecker C, Kirsch P, Sammer G, Schienle A, Stark R, Vaitl D. MARINA: An easy to use tool for the creation of MAsks for Region of INterest Analyses; Presented at the 9th International Conference on Functional Mapping of the Human Brain; New York, NY. 2003; Jun 19-22, 2003. abstract. [Google Scholar]
- Westermann R, Kordelia S, Stahl G, Hesse FW. Relative effectiveness and validity of mood induction procedures: a meta-analysis. European Journal of Social Psychology. 1996;26:557–580. [Google Scholar]