Abstract
Background
The diagnosis of lumbar intraforaminal and extraforaminal stenosis (lumbar foraminal stenosis) is sometimes difficult. However, sensory nerve action potential (SNAP) decreases in amplitude when the lesion is at or distal to the dorsal root ganglion. Therefore, the amplitude of SNAP with lumbar foraminal stenosis should be decreased. In this cohort study, the usefulness of SNAP for the preoperative diagnosis of L5/S foraminal stenosis was assessed.
Methods
In 63 patients undergoing unilateral L5 radiculopathy, bilateral SNAPs were recorded for the superficial peroneal nerve (L5 origin). The patients were divided into two groups according to the results of imaging examinations. Group A (37 patients) included patients whose lesion was located only at the intraspinal canal. In group B (26 patients), the lesion was located only at the intra- or extraforaminal area. All patients received surgery and the symptoms were diminished. The ratios of the amplitudes of SNAPs on the affected side to that on the unaffected side were compared between groups A and B.
Results
SNAPs could not be elicited bilaterally in four patients. The amplitude ratio for group B (median 0.42, max 1.17, min 0) was significantly lower than that in group A (median 0.85, max 1.43, min 0) (p < 0.001 by Mann–Whitney U test). Using a cut-off value of 0.5 for the amplitude ratio, the sensitivity for the diagnosis of lumbar foraminal stenosis was 91.3 % with a specificity of 85.7 %.
Conclusions
Measurement of SNAP could be useful to diagnose a unilateral L5/S foraminal stenosis.
Keywords: Electrodiagnosis, Sensory nerve action potential, L5 radiculopathy, Intraforaminal and extraforaminal root entrapment
Introduction
Anatomically, a lumbar vertebra consists of the intraspinal canal, the intraforaminal zone and the extraforaminal zone [1]. The intraspinal canal is the area surrounded by bilateral pedicles, and the intraforaminal zone is between the medial and lateral borders of the pedicle. The space outside the lateral border of the pedicle is the extraforaminal zone. The cauda equina and root are usually compressed in the intraspinal canal, but it has become clear from many studies that the spinal nerve is potentially compressed by different kinds of factors in the intraforaminal and extraforaminal regions [1–8]. The clinical features of intraforaminal and extraforaminal lumbar stenosis (lumbar foraminal stenosis) result in neurological symptoms such as severe pain of the lower extremities and/or neurogenic intermittent claudication because the dorsal root ganglion (DRG) is compressed. Although various imaging studies have been utilized conventionally for the diagnosis of this pathological condition, the precise diagnosis is sometimes difficult and has been termed the “hidden zone” by Macnab [9].
A total or partial laminectomy is selected to release the compression of neural tissue at the intraspinal canal. However, it is necessary to decompress outside the spinal canal for treating lumbar foraminal stenoses. In other words, spine surgeons must choose the appropriate operative approach method according to the site of neural compression after correct preoperative diagnosis. In addition, because the frequency of stenosis at intraforaminal and extraforaminal regions is reported to be 8 % among patients with lumbar spinal canal stenoses [1], everyone should pay attention to this pathology before performing an operation of lumbar radiculopathy. There is a risk of a ‘failed back’ syndrome because of inadequate surgery if an accurate preoperative diagnosis is not made in such patients. There is a need for an objective functional diagnostic tool to supplement the imaging examination.
On the other hand, during electrophysiological examinations there is a decrease in amplitude of the sensory nerve action potential (SNAP) resulting from nerve damage at or distal to the DRG. In general, the preserved DRG and distal nerve with the intraspinal canal stenosis provide a normal wave form of SNAP recording. Alternatively, in patients with lumbar foraminal stenosis the SNAP is not elicited or the amplitude of SNAP decreases because the compression of neural tissue at or distal to the DRG is followed by distal axonal degeneration. Therefore, an application of the SNAP technique can contribute to the preoperative diagnosis of lumbar foraminal stenosis. The aim of this cohort study was to clarify the significance and limitations of this method.
Patients and methods
There were 114 patients with lumbar spinal canal stenoses who underwent decompression surgery for unilateral L5 radiculopathy from December 2002 to March 2007. According to the imaging examinations including myelograms, computer tomography (CT)-based myelograms, magnetic resonance imaging (MRI), and selective nerve root infiltration, the lesion site of 63 patients was located either at the intraspinal canal or outside the canal. Remaining 51 patients had both intraspinal canal and outside the canal lesion. This study enrolled these 63 patients in whom the imaging diagnosis of the lesion site was performed by the agreement of 7 specialists for a spine surgery. They were divided into two groups. Group A (37 patients, 21 men and 16 women with a mean age of 62.2 years) included patients whose lesion was located only at the intraspinal canal. In group B (26 patients, 18 men and 8 women with a mean age of 65.9 years), the lesion existed only in the intra- or extraforaminal area. The surgical approaches were decided upon imaging examinations and the lesion sites were confirmed at surgery on all patients. The patients in group A had intraspinal canal decompressions and those in group B received extra canal decompressions. All patients had unilateral shin pain and/or neurological intermittent claudication due to L5 radiculopathy. Preoperative Japanese Orthopaedic Association score for low back pain (JOA score, Table 1) was 13.9 ± 3.0 in group A and 13.0 ± 5.4 in group B. No significant differences in the preoperative JOA scores were observed between the two groups (p = 0.40).
Table 1.
Japanese Orthopaedic Association scoring system for low back pain
| Criteria of the JOA scoring system | Score |
|---|---|
| Subjective symptoms (9 points) | |
| Low back pain | |
| None | 3 |
| Occasionally mild | 2 |
| Always mild or occasionally severe | 1 |
| Always severe | 0 |
| Leg pain and/or numbness | |
| None | 3 |
| Occasionally mild | 2 |
| Always mild or occasionally severe | 1 |
| Always severe | 0 |
| Walking ability | |
| Normal | 3 |
| Able to walk more than 500 m, pain, numbness, and muscle weakness | 2 |
| Unable to walk 500 m due to pain, numbness, and muscle weakness | 1 |
| Unable to walk 100 m due to pain, numbness, and muscle weakness | 0 |
| Objective findings (6 points) | |
| Straight leg raising test (including tight hamstring) | |
| Normal | 2 |
| 30°–70° | 1 |
| <30° | 0 |
| Sensory function | |
| Normal | 2 |
| Mild sensory disturbance | 1 |
| Marked sensory disturbance | 0 |
| Motor function (MMT) | |
| Normal | 2 |
| Slight muscle weakness (grade 4) | 1 |
| Marked muscle weakness (grade 0–3) | 0 |
| Restriction of ADLs (14 points) | |
| Turning over while lying down | 0–2 |
| Standing | 0–2 |
| Washing face | 0–2 |
| Leaning forward | 0–2 |
| Sitting (1 h) | 0–2 |
| Lifting or holding | 0–2 |
| Walking | 0–2 |
| Bladder function (−6 points) | |
| Normal | 0 |
| Mild dysuria | −3 |
| Severe dysuria | −6 |
| Total score | 29 |
In restriction of ADLs, a score of 0 = severe restriction, a score of 1 = moderate restriction, and a score of 2 = no restriction
ADL activities of daily living, MMT manual muscle testing
Nerve conduction studies (NCS) including SNAP and needle electromyography (EMG) were routinely performed prior to surgery in the patients with lumbar spinal lesion. NCS and EMG showed no other peripheral neuropathy in this study. The SNAP was recorded in all patients from the bilateral superficial peroneal nerves (SPNs), supplied from the L5 spinal nerve, using electrodiagnostic equipment (Keypoint, Dantec, Skovlunde, Denmark). SPN-SNAP was recorded by the modified method of Jabre [10]. The active electrode was placed just medial to the lateral malleolus with the reference electrode placed 4 cm distally. Electrical stimulation was delivered at the anterior border of the fibula with supramaximal stimulation via electrodes situated 12 cm proximal to the recording electrode. Electrical shocks using 0.2 ms duration of constant current were applied for a mean of 50 times per patient (Fig. 1). The ratios of the amplitude of SPN-SNAP on the affected side to that on unaffected side were compared between groups A and B and evaluated statistically using the non-parametric Mann–Whitney U test.
Fig. 1.
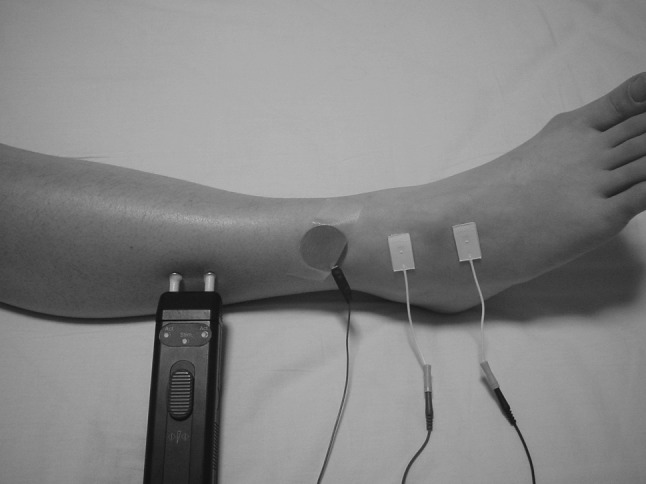
The method to obtain SPN-SNAP. The active electrode was placed medial to the lateral malleolus with the reference electrode placed 4 cm distally. Electrical stimulation was delivered at anterior border of the fibula with supramaximal stimulation via electrodes placed 12 cm proximal to the recording electrode
Results
After the operation, the radicular symptoms were diminished in all patients. JOA score at the final follow-up was 22.5 ± 3.2 in group A and 22.1 ± 5.4 in group B. No significant differences in the postoperative JOA scores were observed between the two groups (p = 0.79).
Bilateral SPN-SNAP was not recorded in one patient of group A and three of group B. In one patient of group A, SPN-SNAP could not be elicited on the unaffected side. These five patients without a response were excluded so as not to bias the overall mean ratio. Therefore, a post-hoc analysis was performed. The amplitude ratio for group B (median 0.42, max 1.17, min 0) was significantly lower than that in group A (median 0.85, max 1.43, min 0) (p < 0.001; Tables 2, 3).
Table 2.
The amplitude of SPN-SNAP in intraspinal canal lesion (group A)
| Case no. | Amplitude affected side (μV) | Amplitude unaffected side (μV) | Ratio of amplitude affected side/unaffected side |
|---|---|---|---|
| 1 | 3.3 | 0 | N/A |
| 2 | 0 | 0 | N/A |
| 3 | 0 | 1.0 | 0 |
| 4 | 0.8 | 3.9 | 0.21 |
| 5 | 1.6 | 7.1 | 0.23 |
| 6 | 21 | 52 | 0.40 |
| 7 | 2.9 | 6.8 | 0.43 |
| 8 | 2.4 | 3.9 | 0.62 |
| 9 | 4.7 | 7.5 | 0.63 |
| 10 | 3.3 | 5.0 | 0.66 |
| 11 | 3.2 | 4.6 | 0.70 |
| 12 | 8.0 | 11 | 0.73 |
| 13 | 4.2 | 5.5 | 0.76 |
| 14 | 6.5 | 8.2 | 0.79 |
| 15 | 1.9 | 2.3 | 0.83 |
| 16 | 1.9 | 2.2 | 0.86 |
| 17 | 2.6 | 2.8 | 0.93 |
| 18 | 1.7 | 1.8 | 0.94 |
| 19 | 11 | 11 | 1.00 |
| 20 | 4.8 | 4.7 | 1.02 |
| 21 | 4.4 | 4.0 | 1.10 |
| 22 | 3.9 | 3.5 | 1.11 |
| 23 | 8.3 | 7.3 | 1.14 |
| 24 | 7.9 | 6.8 | 1.16 |
| 25 | 19 | 16 | 1.19 |
| 26 | 6.2 | 5.2 | 1.19 |
| 27 | 7.7 | 5.9 | 1.31 |
| 28 | 11.7 | 8.2 | 1.43 |
| 29 | 2.6 | 1.8 | 1.44 |
| 30 | 9 | 6.2 | 1.45 |
| 31 | 6.1 | 3.8 | 1.61 |
| 32 | 2.9 | 1.7 | 1.71 |
| 33 | 4.1 | 2.4 | 1.71 |
| 34 | 6.5 | 3.8 | 1.71 |
| 35 | 6.1 | 3.2 | 1.91 |
| 36 | 5.7 | 2.2 | 2.59 |
| 37 | 5.0 | 1.9 | 2.63 |
| Median | 0.85 |
Table 3.
The amplitude of SPN-SNAP in intra- or extraforaminal lesion (group B)
| Case no. | Amplitude affected side (μV) | Amplitude unaffected side (μV) | Ratio of amplitude affected side/unaffected side |
|---|---|---|---|
| 38 | 0 | 0 | N/A |
| 39 | 0 | 0 | N/A |
| 40 | 0 | 0 | N/A |
| 41 | 0 | 5.0 | 0 |
| 42 | 0 | 1.4 | 0 |
| 43 | 0 | 2.7 | 0 |
| 44 | 0.6 | 6.2 | 0.10 |
| 45 | 0.3 | 1.9 | 0.16 |
| 46 | 2.7 | 14 | 0.19 |
| 47 | 0.5 | 2.0 | 0.25 |
| 48 | 1.0 | 2.9 | 0.34 |
| 49 | 8.7 | 25 | 0.35 |
| 50 | 2.4 | 6.2 | 0.39 |
| 51 | 1.4 | 3.6 | 0.39 |
| 52 | 3.2 | 7.7 | 0.42 |
| 53 | 3.4 | 8.0 | 0.43 |
| 54 | 0.3 | 0.7 | 0.43 |
| 55 | 3.6 | 8.3 | 0.43 |
| 56 | 3.0 | 6.9 | 0.43 |
| 57 | 3.9 | 8.6 | 0.45 |
| 58 | 1.9 | 3.9 | 0.49 |
| 59 | 2.1 | 4.3 | 0.49 |
| 60 | 2.5 | 4.9 | 0.51 |
| 61 | 2.3 | 4.2 | 0.55 |
| 62 | 4.7 | 5.6 | 0.84 |
| 63 | 6.8 | 5.8 | 1.17 |
| Median | 0.42 |
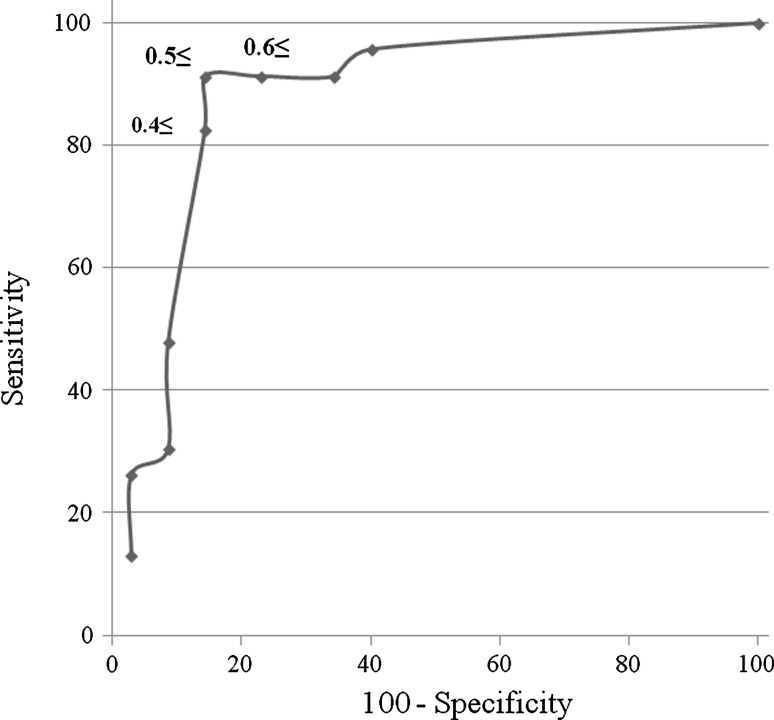
A receiver operating characteristic curve (ROC curve) was acquired applying the amplitude ratio of SPN-SNAP in group A and group B to obtain the appropriate cut-off value to determine whether the lesion is intra-, extraforamen, or intraspinal canal.
When amplitude ratio was 0.4 or above 0.4 and less than 0.5 (≥0.4 to <0.5), sensitivity was 82.6 % and specificity was 85.7 %. If amplitude ratio was ≥0.5 to <0.6, sensitivity was 91.3 % and specificity was 85.7 %. Furthermore, the ratio of ≥0.6 to <0.7 showed 91.3 % sensitivity and 77.1 % specificity (Fig. 2). These results suggested that the best cut-off value was 0.5 for deciding the lesion site. When cut-off value was 0.5, the likelihood ratio of a positive test was 6.38, and the likelihood ratio of a negative test was 0.10 for the diagnosis of lumbar foraminal stenosis using SPN-SNAP.
Fig. 2.
Receiver operating characteristic curve (ROC curve) for obtaining of the optimal amplitude ratio for cut-off value. The amplitude ratio of 0.5 showed 91.3 % of sensitivity and 85.7 % of specificity. This ratio is optimal for cut-off value
Case reports
Case 17
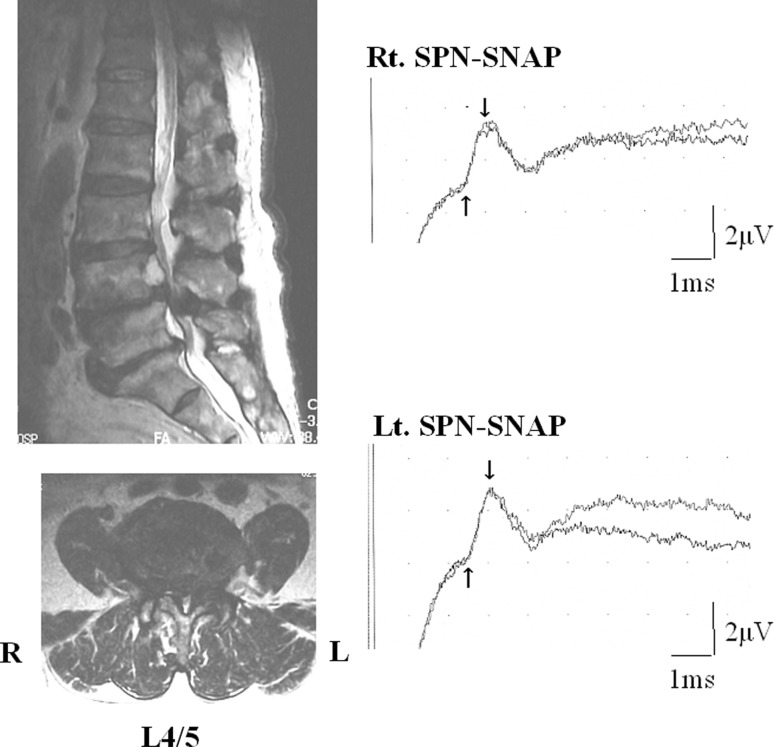
A 65-year-old man complained of right lateral shin pain, intermittent claudication, and the disturbance of dorsiflexion of the right ankle. He was thought to have right L5 radiculopathy. Severe lumbar spinal canal stenosis in L4/5 was shown by MRI. The right side SPN-SNAP amplitude was 2.6 μV and left side amplitude was 2.8 μV. The ratio of the amplitudes on affected side to that on unaffected side was 0.93. The operation revealed an intraspinal canal stenosis. The patient’s symptoms improved after L4/5 partial laminectomy (Fig. 3).
Fig. 3.
Case 17. A 65-year-old man with intraspinal canal stenosis at L4/5 level. MRI showed severe intraspinal canal stenosis at L4/5. Amplitudes of SPN-SNAP did not show laterality
Case 41
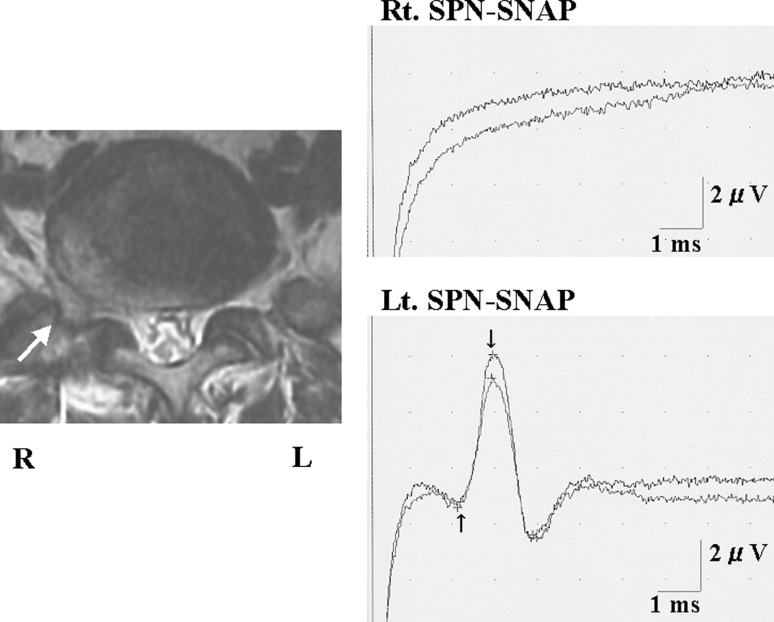
A 69-year-old man suffered from right lateral shin pain and intermittent claudication. Lateral disc herniation was shown to be present on the right side in L5/S by MRI. The SPN-SNAP showed a normal wave pattern on the unaffected left side and the amplitude was 5.0 μV; however, the potential could not be elicited on the affected right side. The amplitude ratio was 0 (Fig. 4). In this patient, the right L5 spinal nerve was affected by lateral disc herniation when we performed L5/S right side lateral fenestration, and the nerve compression was removed by resection of the herniation. After surgery the patient’s symptoms disappeared completely.
Fig. 4.
Case 41. A 69-year-old man with lateral lumbar disc herniation in the right side at L5/S level. MRI revealed right side lateral disc herniation at L5/S level. The unaffected side (left side) of SPN-SNAP was normal pattern, but the affected side (right side) potential was not possible to be recorded
Case 59
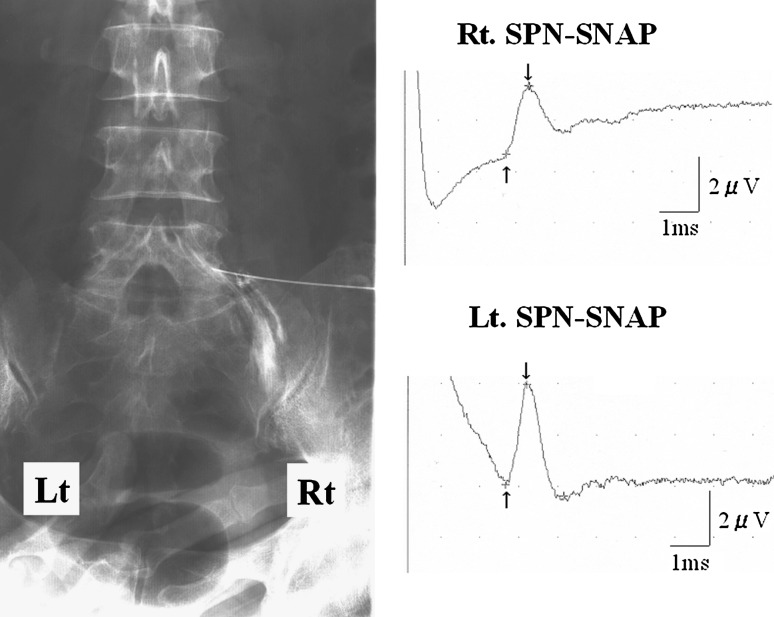
A 63-year-old woman complained of extremely severe right lateral shin pain at rest even after a discectomy at L4/5. No apparent compression of neural tissue was detected by MRI. A slight compression of the spinal nerve was seen by right L5 selective nerve root infiltration. The amplitude of SPN-SNAP on the affected side was 2.1 μV and that on the unaffected side was 4.3 μV. The amplitude ratio was 0.49, which indicated the possibility of lumbar intraforaminal or extraforaminal stenosis (Fig. 5). Scar tissue was found at the L4/5 level intraoperatively, but there was no compression of the spinal nerve root. On the other hand, the DRG was compressed horizontally by osteophytes of the L5 and S1 vertebral bodies and the L5 pedicle at the lateral margin of the intraforaminal region. The patient’s lower extremity pain disappeared completely after decompression surgery.
Fig. 5.
Case 59. A 63-year-old woman with lumbar foraminal stenosis of right side at L5/S level. Right L5 selective nerve root infiltration showed slight compression of the nerve at right foraminal zone. Amplitude of SPN-SNAP of affected side decreased to about 50 % of unaffected side, which indicated the possibility of lumbar foraminal stenosis
Discussion
The pathomechanism of intraforaminal and extraforaminal nerve root entrapment is multifactorial. Kunogi and Hasue [1] classified intraforaminal nerve root involvements into five types. Lateral lumbar herniation, ‘far-out’ syndrome [8], lumbosacral ligament [6, 7], and osteophytes of the L5–S1 vertebral bodies [3] were reported as causes of extraforaminal spinal nerve entrapment. Kunogi and Hasue [1] reported the frequency of intraforaminal and extraforaminal stenosis among the patients with lumbar spinal canal stenoses to be 8 %. This high prevalence is because the L5 spinal nerve is easily compressed outside the spinal canal [6–8].
The diagnosis of entrapment has been mainly based on imaging examinations. Ducker [11] reported that an extruded disc herniation showed a lower signal intensity than the original disc, using MRI. In addition, half coronal MRI views [12] and parasagittal images [13] have been used. Hashiguchi and Taguchi [14] measured the branching angle of the root between the root and the dural tube using selective nerve root infiltration, and reported that it was very likely that root compression occurred in the foramen when the branching angles were more than 60°. Kunogi et al. [13] reported a high sensitivity for parasagittal MRI images in diagnosing lumbar intraforaminal stenoses. However, he recommended that selective nerve root infiltrations, CT scans, and other imaging studies should be added for elderly patients and for those with spondylolisthesis or multiple level compressions, because MRI showed false positive results in such cases. Furthermore, the diagnosis becomes more difficult when spinal degeneration develops in elderly patients, even when utilizing a variety of imaging modalities. To supplement the limitations of imaging examinations, it is necessary to use functional diagnosis such as a neurophysiological study.
An abnormal SNAP value occurs in patients with neural damage at or distal to the DRG. It is thought that abnormality of the SNAP occurs in cases with lumbar intraforaminal or extraforaminal stenosis because the lesion site is at the DRG or is more peripheral [15]. In general, abnormality of the SNAP does not occur during typical radiculopathy when the root is compressed at an intraspinal canal [16, 17]. In the present study, the ratio of the SNAP of the affected side to the unaffected side in lumbar foraminal stenosis group was significantly lower than that seen in patients with an intraspinal canal stenosis. When the cut-off value of the ratio is 0.5, the sensitivity of diagnosis is 91.3 % and the specificity is 85.7 %. This cut-off value supports Levin’s SPN-SNAP criteria [18]. The measurement of the SPN-SNAP was useful for the diagnosis of unilateral L5/S intraforaminal and extraforaminal stenoses. Levin [18] reported intraspinal canal lesion cases with more than 50 % reduction of SNAP amplitude to the opposite side. He concluded that loss of the SPN-SNAP did not exclude intraspinal canal lesions because between 13 and 38.9 % of DRGs are located in the intraspinal canal [19–22]. Ho et al. [23] also performed a similar report. However, in our series, we confirmed that the DRGs were not located at an intraspinal canal by imaging examinations and/or operative findings. Additional NCS and needle EMG examinations also excluded accompanying peripheral neuropathy in our patients. Therefore in our study, abnormal SPN-SNAP amplitude of less than 50 % of opposite side indicates the presence of a lumbar foraminal stenosis.
In our series, bilateral SPN-SNAPs were not able to be derived in four cases (6 %) of the total group. According to Levin [18], up to 6 % of normal limbs might have an unrecordable response to SPN-SNAP in normal individuals less than 60 years of age. SNAPs tend not to be elicited in elderly patients, which is a limitation of this method.
Conflict of interest
No funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1.Kunogi J, Hasue M. Diagnosis and operative treatment of intraforaminal and extraforaminal nerve root compression. Spine. 1991;16:1312–1320. doi: 10.1097/00007632-199111000-00012. [DOI] [PubMed] [Google Scholar]
- 2.Briggs CA, Chandraraj S. Variations in the lumbosacral ligament and associated changes in the lumbosacral region resulting in compression of the fifth dorsal root ganglion and spinal nerve. Clin Anat. 1995;8:339–346. doi: 10.1002/ca.980080506. [DOI] [PubMed] [Google Scholar]
- 3.Matsumoto M, Chiba K, Nojiri K, et al. Extraforaminal entrapment of the fifth lumbar spinal nerve by osteophytes of the lumbosacral spine—anatomical study and a report of four cases. Spine. 2002;27:E169–E173. doi: 10.1097/00007632-200203150-00020. [DOI] [PubMed] [Google Scholar]
- 4.Muller A, Reulen HJ. A paramedian tangential approach to lumbosacral extraforaminal disc herniations. Neurosurgery. 1998;43:854–862. doi: 10.1097/00006123-199810000-00077. [DOI] [PubMed] [Google Scholar]
- 5.Nathan H, Weizenbluth M, Halperin N. The lumbosacral ligament (LSL) with special emphasis on the “lumbosacral tunnel” and the entrapment of the 5th lumbar nerve. Int Orthop. 1982;6:197–202. doi: 10.1007/BF00267730. [DOI] [PubMed] [Google Scholar]
- 6.Olsewski JM, Simmons EH, Kallen FC, Mendel FC. Evidence from cadavers suggestive of entrapment of fifth lumbar spinal nerves by lumbosacral ligaments. Spine. 1991;16:336–347. doi: 10.1097/00007632-199103000-00014. [DOI] [PubMed] [Google Scholar]
- 7.Transfeldt EE, Robertson D, Bradford DS. Ligaments of the lumbosacral spine and their role in possible extraforaminal spinal nerve entrapment and tethering. J Spinal Disord. 1993;6:507–512. doi: 10.1097/00002517-199306060-00006. [DOI] [PubMed] [Google Scholar]
- 8.Wiltse LL, Guyer RD, Spencer CW. Alar transverse process impingement of the L5 spinal nerve: the far-out syndrome. Spine. 1984;9:31–41. doi: 10.1097/00007632-198401000-00008. [DOI] [PubMed] [Google Scholar]
- 9.Macnab I. Negative disc exploration. An analysis of the causes of nerve-root involvement in sixty-eight patients. J Bone Joint Surg. 1971;53-A:891–903. [PubMed] [Google Scholar]
- 10.Jabre JF. The superficial peroneal sensory nerve revisited. Arch Neurol. 1981;38:666–667. doi: 10.1001/archneur.1981.00510100094019. [DOI] [PubMed] [Google Scholar]
- 11.Ducker TB. Extreme lateral lumbar disc herniation. J Spinal Disord. 1989;2:131–132. doi: 10.1097/00002517-198906000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hashimoto M, Watanabe O, Hirano H. Extraforaminal stenosis in the lumbosacral spine. Efficacy of MR imaging in the coronal plane. Acta Radiol. 1996;37:610–613. doi: 10.3109/02841859609177684. [DOI] [PubMed] [Google Scholar]
- 13.Kunogi J, Hasue M, Hamanaka K. Usefulness of MRI and its limitation in the diagnosis of intra-and extraforaminal nerve root compression. Rinshouseikeigeka. 1992;27:503–511. [Google Scholar]
- 14.Hashiguchi T, Taguchi T. Evaluation of lumbar radiculopathy. J West Jpn Res Soc Spine. 1995;21:113–117. [Google Scholar]
- 15.Tani T, Yamamoto H, Kida K. Does the sensory nerve action potential remain intact in lumbosacral radiculopathy? Chubu seikeigekasaigaigeka zasshi. 1989;32:1379–1381. [Google Scholar]
- 16.Fisher MA. Electrophysiology of radiculopathies. Clin Neurophysiol. 2002;113:317–335. doi: 10.1016/S1388-2457(02)00018-4. [DOI] [PubMed] [Google Scholar]
- 17.Wilbourn AJ, Aminoff MJ. AAEM minimonograph 32: the electrodiagnostic examination in patients with radiculopathies. Muscle Nerve. 1998;21:1612–1631. doi: 10.1002/(SICI)1097-4598(199812)21:12<1612::AID-MUS2>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 18.Levin KH. L5 radiculopathy with reduced superficial peroneal sensory responses: intraspinal and extraspinal causes. Muscle Nerve. 1998;21:3–7. doi: 10.1002/(SICI)1097-4598(199801)21:1<3::AID-MUS1>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 19.Hamanishi C, Tanaka S. Dorsal root ganglia in the lumbosacral region observed from the axial views of MRI. Spine. 1993;18:1753–1756. doi: 10.1097/00007632-199310000-00006. [DOI] [PubMed] [Google Scholar]
- 20.Hasue M, Kunogi J, Konno S. Classification by position of dorsal root ganglia in the lumbosacral region. Spine. 1989;14:1261–1264. doi: 10.1097/00007632-198911000-00021. [DOI] [PubMed] [Google Scholar]
- 21.Kikuchi S, Sato K, Konno S. Anatomic and radiographic study of dorsal root ganglia. Spine. 1994;19:6–11. doi: 10.1097/00007632-199401000-00002. [DOI] [PubMed] [Google Scholar]
- 22.Sato K, Kikuchi S. An anatomic study of foraminal nerve lesions in the lumbar spine. Spine. 1993;18:2246–2251. doi: 10.1097/00007632-199311000-00017. [DOI] [PubMed] [Google Scholar]
- 23.Ho YH, Yan SH, Lin YT. Sensory nerve conduction studies of the superficial peroneal nerve in L5 radiculopathy. Acta Neurologica Taiwanica. 2004;13:114–119. [PubMed] [Google Scholar]