Abstract
Purpose
To report morphological patterns of osteoporotic vertebral compression fractures (OVCFs) presenting for surgery. To describe surgical options based on fracture pattern. To evaluate clinical and radiological outcome.
Methods
Forty consecutively operated OVCFs nonunion patients were retrospectively studied. We define four patterns of OVCFs that needed surgical intervention. Group1 mini open vertebroplasty (N = 10) no neurologic deficits and kyphotic deformity, but with intravertebral instability and significant radiological spinal canal compromise. Group2 with neurologic deficits (N = 24) (2A)—transpedicular decompression (TPD) with instrumentation (N = 14). Fracture morphology similar to (1) and localized kyphosis <30° (2B)—pedicle subtraction osteotomy (PSO) with instrumentation (N = 10). Fracture morphology similar to (1) and local kyphosis >30°. Group3 posterolateral decompression with interbody reconstruction (N = 06) endplate(s) destroyed, with instability at discovertebral junction, with neurologic deficit. Average follow-up was 34 months. VAS, ODI and Cobb angle were recorded at 3, 6, 12 months and yearly.
Results
There was significant improvement in the clinical (VAS and ODI) scores and radiologic outcome in each group at last follow-up. 30 patients out of 40, had neurologic deficits (Frankel’s grade C = 16, Frankel’s grade D = 14). The motor power gradually improved to Frankel’s grade E. Average duration of surgery was 97 min. Average blood loss was 610 ml.
Conclusion
Different surgical techniques were used to suit different fracture patterns, with good clinical and radiological results. This could be a step forward in devising an algorithm to surgical treatment of OVCF nonunions.
Keywords: Osteoporotic vertebral compression fracture, Nonunion, Surgery, Neurologic deficit
Introduction
The incidence of osteoporotic vertebral compression fracture (OVCF) is steadily rising [1] with an increase in the longevity of the population. About one-third of these fail to unite and these patients present with painful nonunions with or without neurologic deficits [2–5].
Though a large percentage of these can be treated with cement augmentation techniques like vertebroplasty (VP) and kyphoplasty alone, a subgroup of patients with neurologic deficit are not suitable for it [6]. They often have normal neurology in the supine position, when the spine is off-loaded. The collapse of nonunion on standing results in ‘dynamic’ spinal cord compression [7] and neurologic deficit in them.
In this study, we describe the various patterns of un-united osteoporotic vertebral compression fractures (VCFs), different surgical treatments options and analyse their clinical as well as radiological results.
Materials and methods
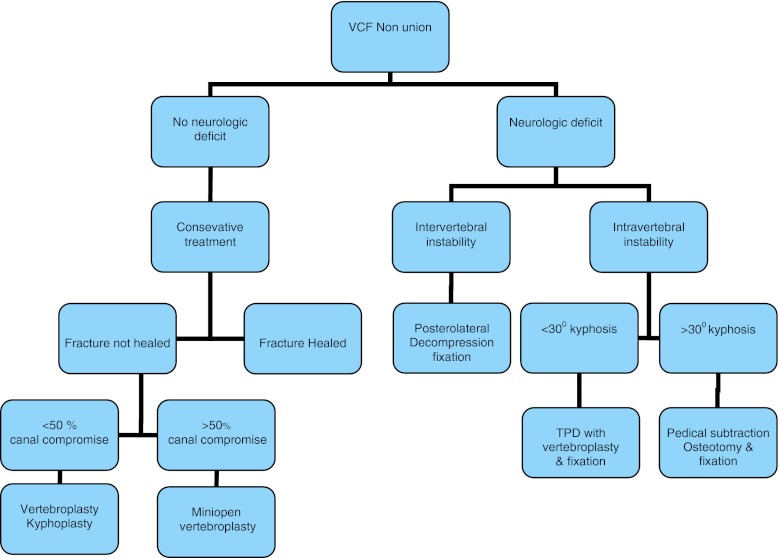
There were 134 consecutive OVCF patients, between 2006 and 2011. Of these 17 fractures healed with conservative treatment and 77 patients underwent vertebroplasty. This is a retrospective study of 40 patients, who had symptomatic un-united VCFs, with fracture geometry unsuitable for cement augmentation due to spinal canal compression by fracture fragments. These patients underwent surgery based on a protocol as shown in the flow chart (Fig. 1). The technique used was individualized depending on fracture geometry.
Fig. 1.
Algorithm to surgical treatment of un-united osteoporotic VCFs
Patients were broadly classified into those with neurologic deficits and those without. All patients without neurological deficit were given fair duration of conservative care for 3 months. Those who remained disabled despite an adequate conservative treatment were planned for surgery. Patients with neurological deficit were planned for surgery on day one. Thus, persistent dysfunction after a reasonable trial of conservative treatment and neurological deficit at the time of presentation formed the inclusion criteria for this study.
Dynamic roentgenographs to prove nonunion and magnetic resonance imaging (MRI) scans to look for spinal cord compression and define the local anatomy, were done. Preoperative computed tomography (CT) scans were done in cases where better bony definition was required and as a prerequisite when intra-op VP was anticipated.
Patients were classified into four groups, based on neurological status and fracture pattern as follows:
Group 1 No neurological deficit: in patients, who had un-united OVCFs, with intact endplate(s) and no kyphotic deformity, but had axial instability of intravertebral type and significant radiological spinal canal compromise [more than 50 % thecal sac compression or complete obliteration of the cerebrospinal fluid (CSF) column anterior to the cord on mid-sagittal MRI], mini open VP was performed (Fig. 2).
Group 2 With neurological deficit: patients with neurological deficit were treated by posterior spinal surgery and the technique depended on the fracture pattern, as below.
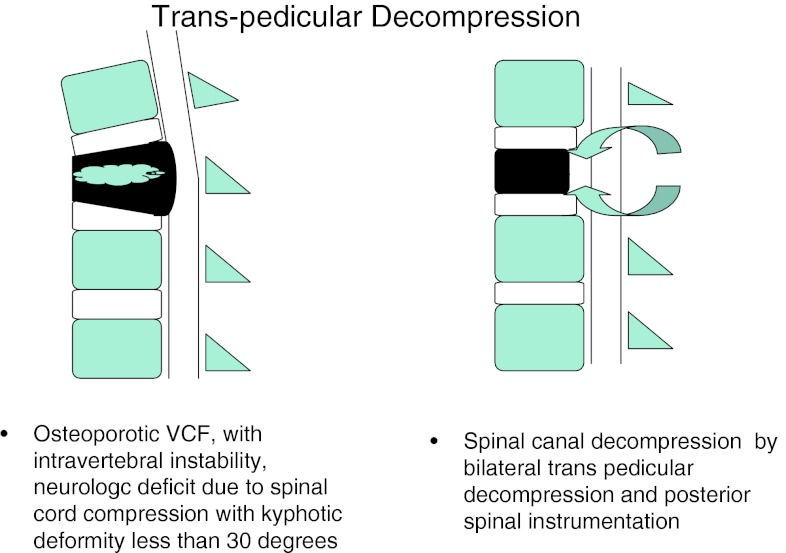
A. Patients with un-united OVCFs similar to (1) with kyphotic deformity less than 30° underwent TPD with instrumentation (Fig. 3).
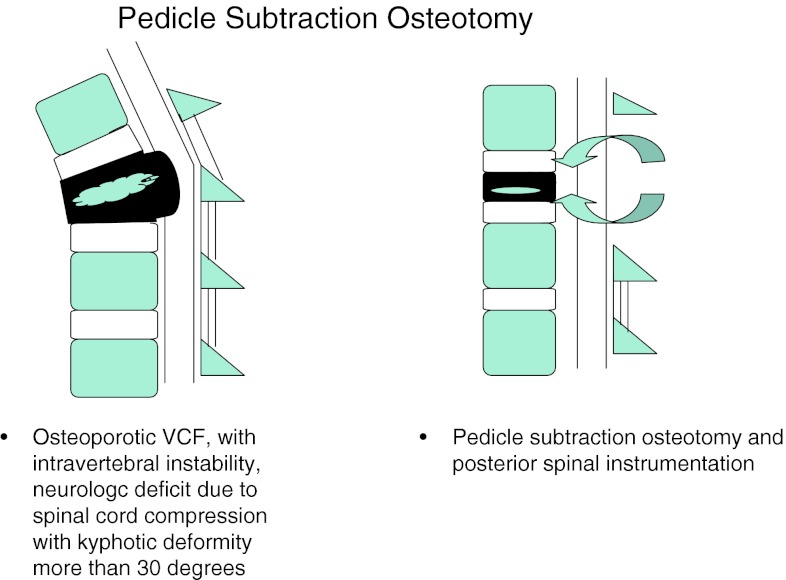
B. Patients with un-united OVCFs similar to (1) with kyphotic deformity more than 30° underwent PSO with instrumentation (Fig. 4).
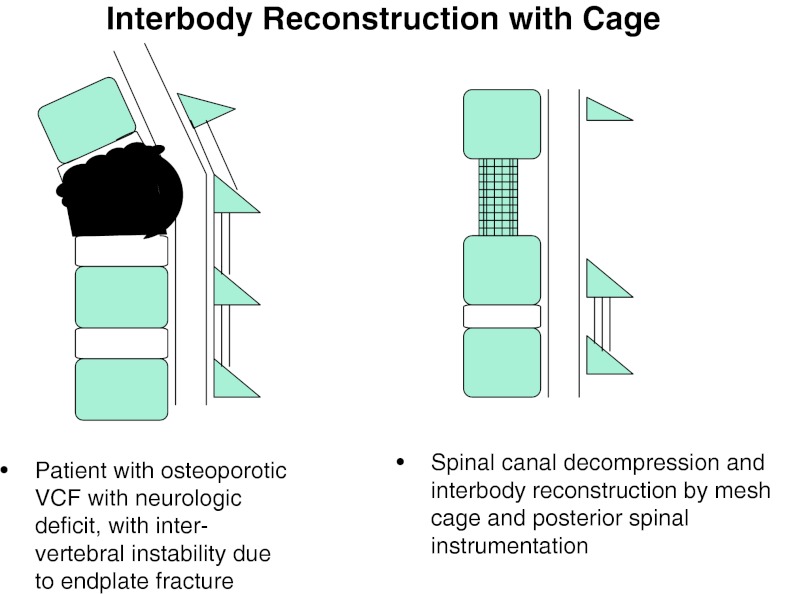
Group 3 Patients with un-united OVCFs, with endplate(s) destroyed/involved in the fracture, with significant instability and kyphotic deformity at disc–vertebral junction leading to neurologic deficit, underwent wide posterolateral decompression and IBR using mesh cage with bone graft and instrumentation (Fig. 5).
Fig. 2.
Mini open VP
Fig. 3.
Transpedicular decompression
Fig. 4.
Pedicle subtraction osteotomy
Fig. 5.
Posterolateral decompression and interbody cage reconstruction
All these surgeries were performed by same team. The demographic, clinical and radiological data were obtained retrospectively from the patient’s case sheets and subsequently during follow-up. Student’s paired t test was used for statistical analysis (Version SPSS 15.0). The outcome measures included
Demographic data
- Clinical data
- VAS scores
- ODI scores
- Neurological results (Frankel’s grading)
Radiologic data (segmental kyphosis by Cobb angle).
Results
There were 26 females and 14 males, with an average age of 64 years (45–85 years). The average follow-up period was 34 months (12–62 months). All these patients had OVCFs due trivial injury. In 36 (90 %) patients it was due to trivial fall and in 4 (10 %) due to bout of sneezing. In this series, 37 (92.5 %) patients had fracture at thoracolumbar junction, at lumbar spine in 2 (5 %) and one patient had fracture in thoracic spine (2.5 %). Overall there was a significant improvement in the clinical [preop mean VAS = 7.5 and mean ODI = 64.6 % to a mean VAS = 3 and mean ODI = 30.8 % at last follow-up (p value 0.001)] and radiological results [mean preop kyphosis of 40° (30°–50°) to a mean kyphosis of 9.3° (5°–15°) at last follow-up (p value of 0.001)]. Individually the results are as shown in the bar diagram A, B and Tables 1, 2.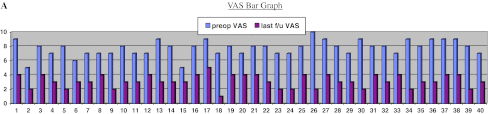
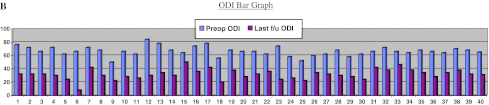
Group 1 This group consisted of 10 female patients. The mean age was 71.3 years (58–89 years), with a mean follow-up of 38 months (21–60 months). There was significant improvement in VAS and ODI scores from preop VAS of 7.7–3.3 and preop ODI of 64.8–29.4 % (p value 0.001) at the last follow-up.
Group 2A This group consisted of 14 patients (04 males, 10 females). The mean age was 64.2 years (45–70), with a mean follow-up of 42.2 months (21–62 months). There was significant improvement in the VAS and ODI scores from preop VAS of 7.35–3.21 and preop ODI of 68.7–31.57 % (p value 0.001) at the last follow-up.
Group 2B This group consisted of 10 patients (seven males, three females). The mean age was 67.3 years (48–85), with a mean follow-up of 25.4 months (12–38 months). There was significant improvement in the VAS and ODI scores from preop VAS of 8.2–2.8 and preop ODI of 65.4–31.4 % (p value 0.001) at last follow-up. The average preop kyphotic deformity of 41.5° in PSO group improved to 12° (range 35°–50° preop to 10°–15°)(p value 0.001) at last follow-up.
Group 3 There were six (four males, two females) patients in this group. The mean age was 69 years (48–77 years), with a mean follow-up of 28.5 months (12–53 months). There was significant improvement in the VAS and ODI scores from preop VAS of 8.1–3.1 (p value 0.002) and preop ODI of 64.3–37 % (p value 0.001) at last follow-up. Average preop kyphotic deformity of 37.50 improved to 6° (range 35°–40° preop to 5°–10° postop) (p value 0.001) at last follow-up.
Out of 40, 16 (40 %) patients had significant neurologic deficits of Frankel’s grade C and 14 (35 %) patients had Frankel’s grade D. At last follow-up, the neurologic deficit gradually improved to Frankel’s grade E.
Table 1.
Mean preop VAS and mean VAS at final followup of each individual surgical group
| Surgery type | Preop VAS | VAS at last follow-up |
|---|---|---|
| Mini open VP | 77/10 = 7.7 | 33/10 = 3.3 |
| TPD | 103/14 = 7.35 | 45/14 = 3.21 |
| PSO | 82/10 = 8.2 | 28/10 = 2.8 |
| IBR with cage | 49/6 = 8.1 | 19/6 = 3.1 |
Table 2.
Mean preop ODI and mean ODI at final followup of each individual surgical group
| Surgery type | Preop ODI | ODI at last follow-up |
|---|---|---|
| Mini open VP | 648/10 = 64.8 | 294/10 = 29.4 |
| TPD | 962/14 = 68.7 | 442/14 = 31.57 |
| PSO | 654/10 = 65.4 | 314/10 = 31.4 |
| IBR with cage | 386/6 = 64.3 | 222/6 = 37 |
Complications
Minor complications Persistent back pain, thigh pain and leg pain was seen in two patients each. Asymptomatic cage sinkage and suboptimal screw placement was seen in one patient each.
Major complications Two patients had pedicle screw back out at 1 year. One patient had asymptomatic sublaminar wire cut out and implant prominence. Wound dehiscence with superficial infection was seen two patients. These complications were not specific to any particular group.
Discussion
Majority of OVCF heal or remain asymptomatic, with conservative treatment [8]. About 10–30 % of them fail to do so and present with painful nonunions, kyphotic deformity and neurologic deficit [9]. About 60–100 % of painful nonunions become painless following VP [10, 11].
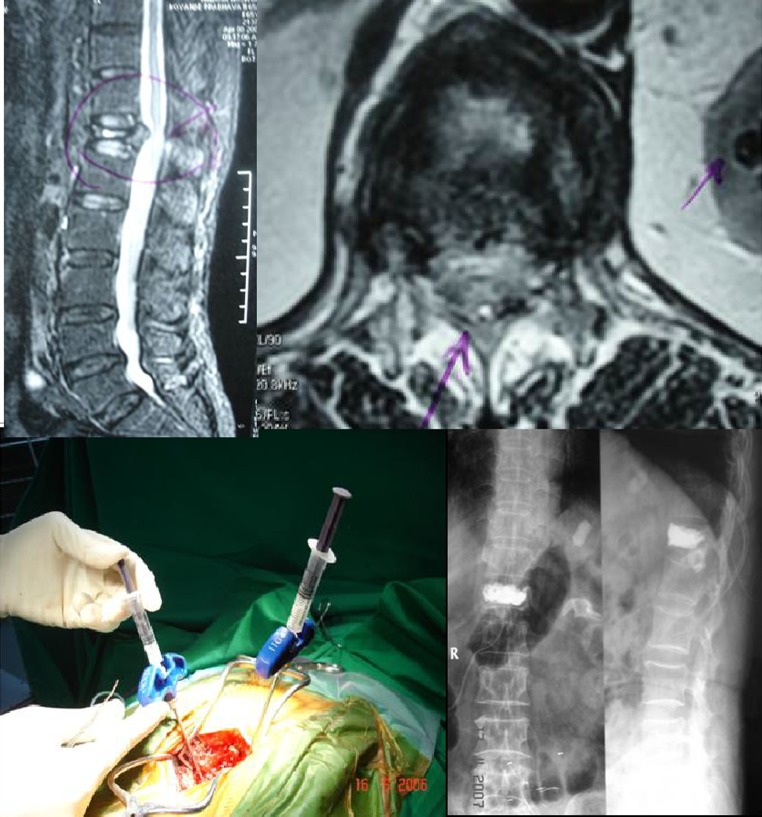
Group 1 patients present with difficult combinations of VCF nonunions, spinal instability and neurogenic pain due to retropulsed bone fragment(s). In these patients choosing between an open surgery or conservative treatment becomes a clinical dilemma. Symptomatic spinal canal compromise has been cited as a contraindication for VP due to fear of cement extravasation and further neurologic compression. Traditional procedures advocate spinal decompression and fusion with instrumentation. However, these surgeries are fraught with complications like difficult instrumentation (due to osteoporosis), debilitated state with high medical co-morbidities and high risk of open surgeries in them. The nonoperative treatment may lead to ongoing neurogenic pain, dysfunction and progressive deformity. With retropulsed bone fragment in situ, neurologic deficit can occur insidiously. The apprehension about VP or an open surgical treatment or conservative treatment is highly justifiable if done alone. Hence, a less extensive and minimally invasive surgical procedure, which not only allows spinal decompression and removal of retropulsed bone fragment but also a safer cement augmentation, is preferred. Hemilaminectomy allows enough access to the retropulsed bone fragment so as to remove it and preserve much of the posterior tension band structures (without destabilizing it). The VP performed with direct visualization of the posterior vertebral body wall through hemilaminectomy window makes it a safer procedure. In this study, we have described minimal and adequate spinal decompression (hemilaminectomy) followed by vertebroplasty as mini open VP. The small case series of Singh et al. [12], shows good results following limited decompression and open VP/KP, at a short follow-up of 44 weeks. Boszczyk et al. [13] showed similar results with interlaminar decompression and thecal sac mobilization, followed by VP/KP. In contrast to above, our series has 10 patients, with OVCF nonunion and significant spinal canal stenosis at thoracolumbar junction (cord level), who showed significantly improved clinical outcome at a longer follow-up of 38 months (Fig. 6).
Fig. 6.
Preop MRI, intraop photo and postop X-ray of mini open vertebroplasty
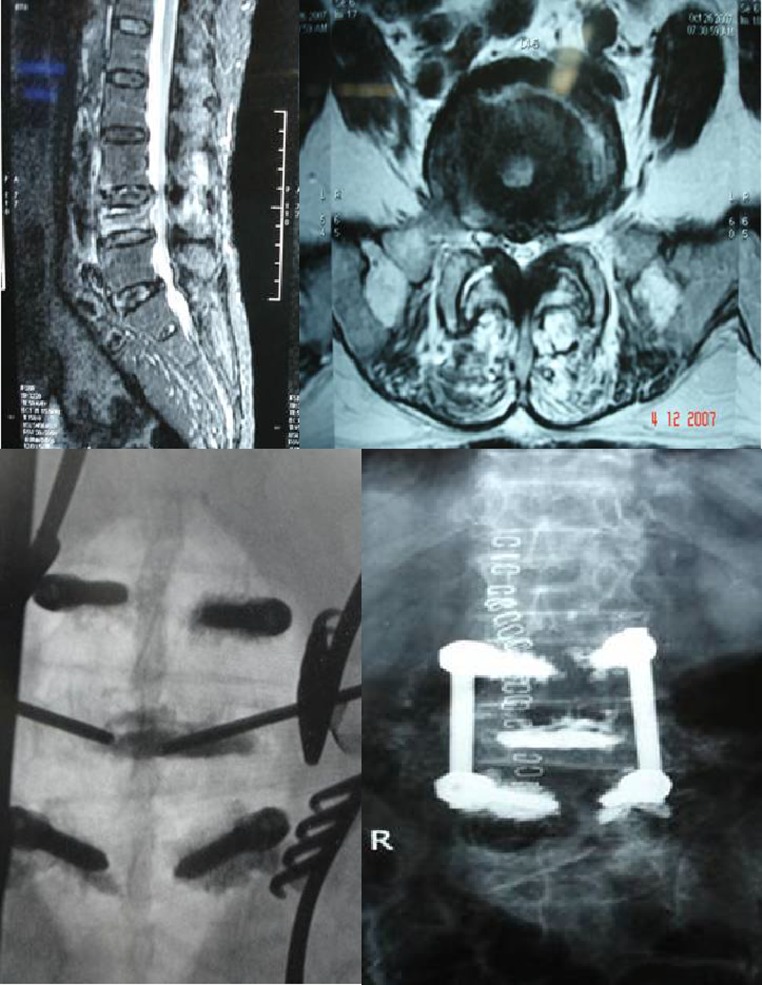
In Group 2A, the surgical treatment is logical due to ongoing neurologic injury. In these patients, surgery is aimed at decompressing the spinal canal and compression anterior to the cord. Transpedicular route appears to be a gateway to the structures anterior to the spinal cord, allows limited decompression, anterior and middle column reconstruction and posterior instrumentation [14]. Direct visualization of the posterior vertebral wall after TPD may allow a safer cement augmentation, thus obviating the need for extensive spinal reconstruction. The anterior and (or) middle column reconstruction is done depending on presence of intravertebral cavity or cleft. In presence of cavity, acrylic cement filling of the cavity was done. In presence of the cleft, closure of the cleft was done by applying compression over instrumentation. We describe this procedure as “cement for metal”. In this study, we had 14 patients who underwent TPD with anterior and middle reconstruction and posterior instrumentation. All these patients had significantly improved clinical and radiological results at last the follow-up (Fig. 7).
Fig. 7.
Preop MRI, IITV image and post op X-ray of transpedicular decompression and instrumentation
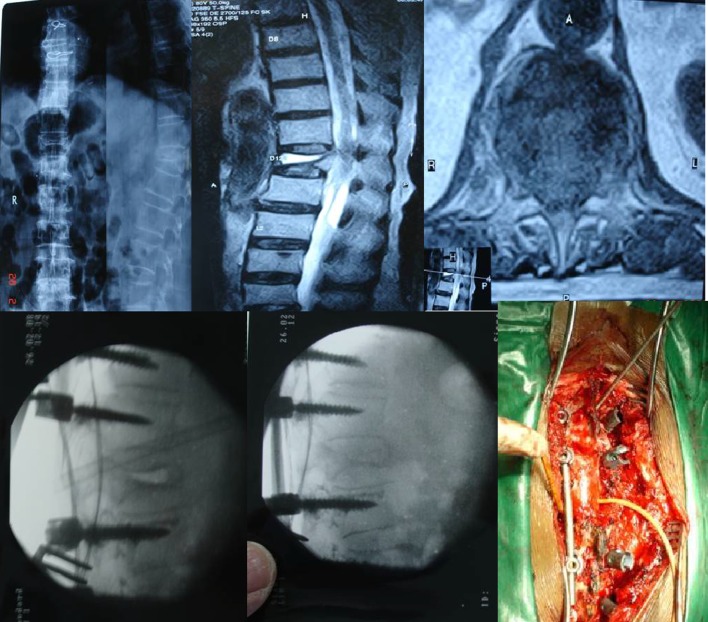
In group 2B, the neurologic deficit and significant kyphotic deformity (more than 30°) become an indication for surgery. The posterior spinal shortening makes the cephalad and caudal endplates parallel, axial and ventro-inferior dislodgement vectors neutral, thus correcting the significant kyphotic deformity. The PSO achieves better kyphosis correction by less operative time, less blood loss and lesser morbidity through a posterior approach. In this study, we had 10 patients who underwent PSO and spinal instrumentation. All these patients had significant improvement in clinical and radiological results at the last follow-up (Fig. 8).
Fig. 8.
Preop X-ray, MRI, intraop IITV image and photo of pedicle subtraction osteotomy with instrumentation
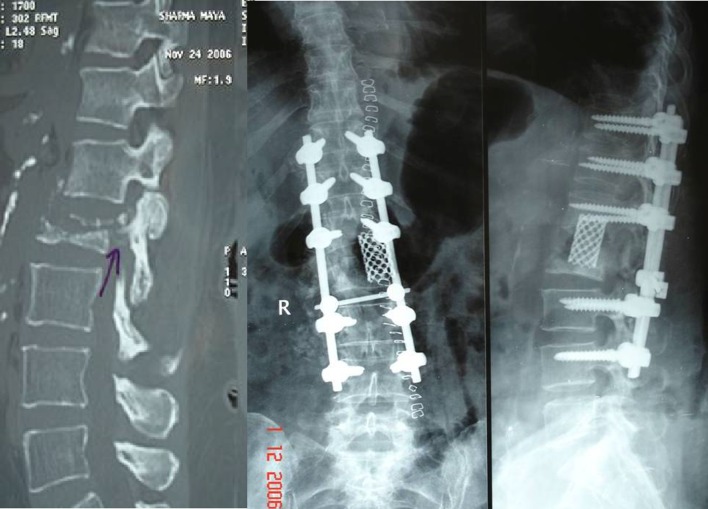
Group 3 patients had OVCFs, with neurologic deficits and kyphotic deformity. In these patients, the endplate(s) were part of the fracture/involved in the fracture, leading to significant translational instability at disc–vertebral or intervertebral junction. On radiographs, the patients had fractured endplates, with or without adjacent vertebral body fracture. The term intervertebral or discovertebral instability had the following background. The retrospective analysis of our VCF patients with involvement of superior or inferior endplates had suboptimal results following VP. In long follow-up, they presented with complications like bony collapse around the cement, cement extrusion and recurrent fracture of the previously augmented vertebra, leading to instability at disc–vertebral or intervertebral junction. This led us to introduce a new concept called intervertebral/discovertebral instability or collapse.
In Group 3 patients, VP is contraindicated due to presence of neurologic deficit. The high probability of cement leakage anteriorly/laterally into soft tissues, superiorly or inferiorly into adjacent disc space and posteriorly into spinal canal, makes VP a relative contraindication even in absence of neurologic deficit. The presence of neurologic deficit makes the spinal canal decompression must, which includes removal of fractured endplate fragments and adjacent disc. The huge intervertebral gap following spinal decompression and kyphotic deformity at the fracture site makes the anterior and middle column reconstruction mandatory. This can be better done by a wide posterolateral decompression and interbody reconstruction using a metallic mesh cage incorporated with autologous bone graft. The entire surgery is done from posterior approach, as this is less morbid and also allows instrumentation [15]. We had six patients, who underwent a wide posterolateral approach to fractured disc–vertebral junction. The fractured endplate, vertebral body bone fragments and disc, lying anterior and lateral to the cord were removed. This was followed by interbody reconstruction using metal mesh cage filled with autologous bone graft and posterior spinal instrumentation. All these patients showed significant improvement in clinical and radiological outcome at last follow-up (Fig. 9).
Fig. 9.
Preop MRI, CT scan and postop X-ray of posterolateral decompression with interbody reconstruction and instrumentation
The strength of pedicle screw fixation can be significantly increased after augmentation [16]. The laminar hook and pedicle screw combination increases strength by 49.2 % [17]. The spinal loop rectangle and sublaminar wires is a good alternative to instrumenting osteoporotic spine, especially when anterior column reconstruction is not planned. Sublaminar wires give good extraosseous hold and allow controlled collapse, without causing angular deformity. Being cheap, easily available and requiring less technological backup, it has advantage of wide applicability across all social strata. It can also be used as a fall back option in pedicle screw backouts. In this study, cement-augmented pedicle screws were used in three patients, supralaminar hooks in two patients and spinal loop rectangle with sublaminar wiring in six patients to augment the instrumentation. Among these, two patients underwent stabilization with spinal loop rectangle and sublaminar wiring as a bailout procedure for pedicle screw backout.
Conclusion
Our approach to these fractures included various most common clinical and radiological scenarios like significant radiological spinal canal compromise, neurologic deficit, different types of instability and kyphotic deformity. The positive clinical and radiologic outcome at a long follow-up in this study, could be a step forward in devising an algorithm that we propose, while treating OVCF nonunions.
Conflict of interest
No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript.
References
- 1. Assessment of fracture risk and its applications to screen for postmenopausal osteoporosis (1994) Report of WHO study group Geneva (WHO Technical Report Series, No 843) [PubMed]
- 2.Hasegawa K, Homma T, Uchiyama S. Osteosynthesis without instrumentation for vertebral pseudarthrosis in the osteoporotic spine. J Bone Joint Surg (Br) 1997;79:452–456. doi: 10.1302/0301-620X.79B3.7457. [DOI] [PubMed] [Google Scholar]
- 3.Shikata J, Yamamuro T, Iida H. Surgical treatment for paraplegia resulting from vertebral fractures in senile osteoporosis. Spine. 1990;15:485–489. doi: 10.1097/00007632-199006000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Kaneda K, Asano S, Hashimoto T. The treatment of osteoporotic post-traumatic vertebral collapse using the Kaneda device and bioactive ceramic vertebral prosthesis. Spine. 1992;17:S295–S303. doi: 10.1097/00007632-199208001-00015. [DOI] [PubMed] [Google Scholar]
- 5.Baba H, Maezawa Y, Kamitani K. Osteoporotic vertebral collapse with late neurological complications. Paraplegia. 1995;33:281–289. doi: 10.1038/sc.1995.64. [DOI] [PubMed] [Google Scholar]
- 6.Blattert, Thomas R 1, Josten, Christoph 2 (2011) affiliated society meeting Abstracts, Spine Supplement 1, October
- 7.Ataka H, Tanno T, Yamazaki M. Posterior instrumented fusion without neural decompression for incomplete neurological deficits following vertebral collapse in the osteoporotic thoracolumbar spine. Eur Spine J. 2009;18(1):69–76. doi: 10.1007/s00586-008-0821-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roush RN, Karen MSN, FNP- BC. Prevention and treatment of osteoporosis in postmenopausal women: a review. Am J nurs. 2011;111(8):26–35. doi: 10.1097/01.NAJ.0000403358.44058.f7. [DOI] [PubMed] [Google Scholar]
- 9.Cooper C, Atkinson EJ, O’Fallon WM, Melton LJ., 3rd Incidence of clinically diagnosed vertebral fractures. A population based study in Rochester, Minnesota, 1985–1989. J Bone and Miner Res. 1992;7:221–227. doi: 10.1002/jbmr.5650070214. [DOI] [PubMed] [Google Scholar]
- 10.Gangi A, Kastler BA, Dietmann JL. Percutaneous vertebroplasty guided by a combination of CT and fluoroscopy. AJNR. 1994;15:83–86. [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen ME, Evans AJ, Mathis JM. Percutaneous polymethylmethacrylate vertebroplasty in the treatment of osteoporotic vertebral compression fracture, technical aspects. AJNR. 1997;18:1897–1904. [PMC free article] [PubMed] [Google Scholar]
- 12.Singh K, Heller JG, Samartzis D. Open vertebral cement augmentation combined with lumbar decompression for the operative management of thoracolumbar stenosis secondary osteoporotic burst fractures. J Spinal Disord Tech. 2005;18:413–419. doi: 10.1097/01.bsd.0000173840.59099.06. [DOI] [PubMed] [Google Scholar]
- 13.Boszczyk BM, Bierschneider M, Schmid K, Grillhösl A, Robert B, Jaksche H. Microsurgical interlaminary vertebro- and kyphoplasty for severe osteoporotic fractures. J Neurosurg. 2004;100(1 Suppl Spine):32–37. doi: 10.3171/spi.2004.100.1.0032. [DOI] [PubMed] [Google Scholar]
- 14.Suk S, Kim JH, Lee SM. Anterior-posterior surgery versus posterior closing wedge osteotomy in post traumatic kyphosis with neurologic compromised osteoporotic spine. Spine. 2003;28:2170–2175. doi: 10.1097/01.BRS.0000090889.45158.5A. [DOI] [PubMed] [Google Scholar]
- 15.Bhojaraj SY, Dandavate AV, Ramakantan R. Preoperative embolisation, transpedicular decompression and posterior stabilization for metastatic disease of the thoracic spine causing paraplegia. Paraplegia. 1992;30:292–299. doi: 10.1038/sc.1992.72. [DOI] [PubMed] [Google Scholar]
- 16.Chan MC, Lin CL, Chen TH. Poly methyl methacrylate augmentation of pedicle screw for osteoporotic spinal surgery a novel technique. Spine. 2008;33:E317–E324. doi: 10.1097/BRS.0b013e31816f6c73. [DOI] [PubMed] [Google Scholar]
- 17.Hasegawa K, Takahashi HE, Uchiyama S. An experimental study of a combination method using a pedicle screw and laminar hook for the osteoporotic spine. Spine. 1997;22:958–963. doi: 10.1097/00007632-199705010-00004. [DOI] [PubMed] [Google Scholar]