Abstract
Purpose
To investigate the frequency of tandem lumbar and cervical intervertebral disc degeneration in asymptomatic subjects.
Methods
We evaluated magnetic resonance imaging (MRI) results from 94 volunteers (48 men and 46 women; mean age 48 years) for age-related intervertebral disc degeneration in the lumbar and cervical spine.
Results
MRI indicated degenerative changes in the lumbar spine in 79 subjects (84 %), with decreased disc signal intensity in 74.5 %, posterior disc protrusion in 78.7 %, anterior compression of the dura in 81.9 %, disc space narrowing in 21.3 %, and spinal canal stenosis in 12.8 %. These findings were more common in older subjects at caudal levels. MRI showed degenerative changes in both the lumbar and cervical spine in 78.7 % of the volunteers.
Conclusions
Degenerative findings in both the lumbar and cervical spine, suggesting tandem disc degeneration, was common in asymptomatic subjects. These results provide normative data for evaluating patients with degenerative lumbar and cervical disc diseases.
Keywords: MRI, Disc degeneration, Lumbar spine, Cervical spine, Asymptomatic subjects
Introduction
Ageing affects intervertebral discs throughout the entire spine, as shown by epidemiologic, cadaveric, and radiologic studies [1–9]. Age-related degenerative changes have been extensively studied in lumbar and cervical spine radiographs and magnetic resonance imaging (MRI) scans from asymptomatic subjects, and data from these studies are used as referable norms for diagnosing and treating patients with spinal disorders. In the first MRI study of degenerative lumbar spine changes in asymptomatic, healthy volunteers, Boden et al. [1] found degenerative changes in about one-third of the subjects. Jensen et al. [2] also found herniated lumbar discs in 28 % of the MR images from 98 healthy subjects.
In cervical spine radiographs from 200 asymptomatic subjects aged 60–65 years, Gore et al. [3] found degenerative changes at one or more intervertebral levels in 95 % of the males and 70 % of the females. Boden et al. [4], using cervical spine MRI from asymptomatic subjects, found degenerative changes in 14 % of those under 40 years old and 28 % of those aged 40 years or older. In a previous study of cervical spine MRI from 497 asymptomatic volunteers, we found that degenerative changes in cervical intervertebral discs were more frequent in older populations [5]. In a 10-year follow-up study of 223 of the original cohort of 497 volunteers, we found that degenerative changes had progressed in 84.7 % of the subjects [10].
Tandem degenerative lumbar and cervical spine changes have been noted in clinical, anatomical, and genetic studies [11–16]. After evaluating lumbar and cervical spine MR images from 174 monozygotic and 154 dizygotic twins, Sambrook et al. [15] reported that a heritability of intervertebral disc degeneration was 74 % at the lumbar spine and 73 % at the cervical spine, and concluded that genetic factors had a greater influence than environmental factors in these cases. Master et al. [14] found degenerative changes in both the lumbar and cervical spine in 80 % of 234 cadaveric specimens studied, with more severe degeneration in the lumbar spine.
To date, most studies using healthy subjects have focused on either the lumbar or the cervical spine, rather than on tandem age-related lumbar and cervical disc degeneration. A better understanding of how disc degeneration in the lumbar spine relates to that in the cervical spine in healthy subjects may provide valuable control data when treating patients with tandem degenerative lumbar and cervical spinal disorders. In this study, therefore, we investigated factors associated with age-related changes in the lumbar spine, as well as the frequency of tandem lumbar and cervical intervertebral disc degeneration in asymptomatic subjects.
Materials and methods
This study was approved by each participating facility’s institutional review board. This was a follow-up to a study in which 497 originally asymptomatic volunteers underwent MRI of the cervical spine between 1993 and 1996 [5]; of these, 223 underwent MRI again 10 years later [10, 16–18]. In the present study, 129 of the original participants gave written consent after the details of the study protocol were explained and underwent follow-up MRI of both the lumbar and cervical spine. Participants completed a questionnaire about any clinical symptoms related to the cervical spine (such as neck or back pain) and about their daily habits, including smoking (smoking daily for more than 10 years), sports (regular participation in a sports activity at least once a week), and occupation. After completing the questionnaire, 35 subjects who reported neck, shoulder, or back pain were excluded, and 94 subjects without neck or back pain (48 men, 46 women; mean age 48.0 ± 13.4 years) were included in the final analysis.
MRI protocol
The protocol for cervical spine MRI in this study was as previously reported [10, 16, 17]. For lumbar spine MRI, T2-weighted sagittal images were obtained with a 1.5 T superconducting imager (Signa Excite HD 1.5 T, General Electronic, WI, USA) using a fast-recovery fast spin-echo protocol as follows: repetition time (TR)/echo time (TE) 2,500/105; echo train length, 20; thickness of slice, 4 mm; slice gap, 1 mm; field of view (FOV), 30 cm; matrix size, 320 × 224; number of excitations (NEX), 2. Due to time and cost constraints, no other images were taken for the lumbar area.
MRI evaluation
Lumbar (L1–2 to L5–S1) and cervical (C2–3 to C7–T1) spine MR images were evaluated for five indicators of intervertebral disc degeneration: (1) Decrease in signal intensity of intervertebral disc (decreased disc signal intensity), (2) posterior disc protrusion in the sagittal images, (3) anterior compression of the dura, (4) disc space narrowing, and (5) spinal canal stenosis.
Images were assessed as previously described for the cervical spine [10, 16, 17]; the lumbar spine was assessed using the same system with some modifications. Each type of MR finding was assigned a rating scale of two or four grades (Table 1). The magnitude of posterior disc protrusion and anterior dural sac compression was assessed by spinal cord compression in the cervical spine, and by dural sac compression in the lumbar spine. The presence of spinal canal stenosis in the lumbar spine was determined by simultaneous anterior and posterior compression of the dura, and in the cervical spine by grade 2 anterior compression of the dura along with posterior dural compression.
Table 1.
Grading system of MRI
| Decrease in signal intensity of intervertebral disc | 0: As bright as or slightly less bright than cerebrospinal fluid |
| 1: Markedly darker than cerebrospinal fluid | |
| 2: No signal | |
| Posterior disc protrusion | 0: No protrusion |
| 1: Disc material protruding beyond the posterior margin of the vertebral body without cord compression (less than one-third of dural sac) | |
| 2: Beyond vertebral body with cord compression (more than one-third of dural sac) | |
| Anterior compression of dura and spinal cord | 0: No compression |
| 1: Compression on dural sac only (slight dural compression) | |
| 2: Compression on less than one-third of spinal cord (dural sac) | |
| 3: Compression on more than one-third and less than two-third of spinal cord (dural tube) | |
| 4: Compression on more than two-third of spinal cord (dural sac) | |
| Disc space narrowing | 0: 100–75 % of height of upper healthy disc |
| 1: 75–50 % of height of upper healthy disc | |
| 2: Less than 50 % of height of upper healthy disc |
Parentheses indicate modifications for the evaluation of the lumbar spine which lacks in the spinal cord
An experienced neuroradiologist conducted a blinded reading of all the lumbar spine MR images. As image grading in previous studies, conducted by the same neuroradiologist and using the same grading system in the cervical and thoracic spine [16, 17], had good inter-observer reliability, we did not investigate inter-observer reliability in the present study.
MRI degenerative findings and associated factors
Using logistic regression analysis, we investigated potential associations between degenerative MRI findings in the lumbar spine and the following factors: age (less than 40 years or 40 or older), gender, smoking habits, sports activities (none, or one or more times per week), body mass index (BMI) (less than 25.0 or 25.0 and above), and the presence of degenerative MRI findings in the cervical spine. Most participants were white-collar workers, so occupation was not included among potential factors.
Statistical analyses
Statistical analysis was conducted by McNemar’s test and by logistic regression analysis. A p value <0.05 was considered statistically significant. All statistical analyses were performed using IBM SPSS Statistics 20 for Windows (IBM Japan Inc., Tokyo).
Results
Degenerative changes in the lumbar spine
Seventy-nine subjects (84 %) had degenerative disc changes at one or more intervertebral levels in the lumbar spine, including decreased disc signal intensity in 74.5 % of the subjects, posterior disc protrusion in 78.7 %, anterior compression of the dura in 81.9 %, disc space narrowing in 21.3 %, and spinal canal stenosis in 12.8 % (Table 2). The average number of intervertebral discs with degenerative changes per subject was as follows: decreased disc signal intensity, 1.9 discs; posterior disc protrusion, 1.6; anterior compression of the dura, 1.7; disc space narrowing, 0.4; and spinal canal stenosis, 0.2 (Table 3). The percentage of degenerative changes increased at more caudal levels (Table 2) and with age for all types of degenerative MRI findings (Table 4).
Table 2.
Number of the discs with positive MRI findings at each intervertebral level
| L1–2 | L2–3 | L3–4 | L4–5 | L5–S1 | Totala | |
|---|---|---|---|---|---|---|
| Decrease in signal intensity | 19 (20.2) | 26 (27.7) | 31 (33.0) | 46 (48.9) | 55 (58.5) | 70 (74.5) |
| Posterior disc protrusion | 6 (6.4) | 21 (22.3) | 33 (35.1) | 46 (48.9) | 49 (52.1) | 74 (78.7) |
| Anterior compression of dura | 7 (7.4) | 23 (24.5) | 35 (37.2) | 51 (54.3) | 48 (51.1) | 77 (81.9) |
| Disc space narrowing | 2 (2.1) | 3 (3.2) | 5 (5.3) | 11 (11.7) | 13 (13.8) | 20 (21.3) |
| Spinal canal stenosis | 2 (2.1) | 2 (2.1) | 4 (4.2) | 6 (6.3) | 4 (4.2) | 12 (12.8) |
The numbers in the parentheses indicate percentages
aNumber of the subjects with a positive MR finding at one or more levels
Table 3.
Number of patients with multilevel involvement of disc degeneration
| Number of intervertebral discs with positive findings | ||||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| Decrease in signal intensity | 24 (25.5) | 26 (27.7) | 17 (18.1) | 7 (7.4) | 4 (4.3) | 16 (17.0) |
| Posterior disc protrusion | 20 (21.3) | 31 (22.0) | 19 (20.2) | 12 (12.8) | 10 (10.6) | 2 (2.1) |
| Anterior compression of dura | 17 (18.1) | 32 (34.0) | 20 (21.3) | 12 (12.8) | 9 (9.6) | 4 (4.3) |
| Disc space narrowing | 74 (78.7) | 10 (10.6) | 8 (8.5) | 0 (0) | 2 (2.1) | 0 (0) |
| Spinal canal stenosis | 82 (87.2) | 19 (9.6) | 2 (2.1) | 0 (0) | 0 (0) | 1 (1.1) |
Table 4.
Degenerative changes in each age group (in years)
| 20–29 | 30–39 | 40–49 | 50–59 | 60–69 | Over 70 | |
|---|---|---|---|---|---|---|
| No. of Subjects | 6 | 22 | 27 | 19 | 12 | 8 |
| Decrease in signal intensity | 2 (33.3) | 12 (54.5) | 22 (81.5) | 17 (89.5) | 10 (83.3) | 7 (87.5) |
| Posterior disc protrusion | 3 (50.0) | 13 (59.1) | 21 (77.8) | 19 (100) | 11 (91.7) | 7 (87.5) |
| Anterior compression of dura | 3 (50.0) | 13 (59.1) | 23 (85.2) | 19 (100) | 12 (100) | 7 (87.5) |
| Disc space narrowing | 0 (0) | 2 (9.1) | 5 (18.5) | 6 (31.6) | 3 (25.0) | 4 (50.0) |
| Spinal canal stenosis | 0 (0) | 1 (4.5) | 1 (3.7) | 4 (21.1) | 4 (33.3) | 2 (25.0) |
| Any one of the four findings | 3 (50.0) | 14 (63.6) | 24 (88.9) | 19 (100) | 12 (100) | 7 (87.5) |
Comparison of the lumbar and cervical spine
The percentage of subjects with positive degenerative MRI findings at one or more intervertebral discs did not differ significantly between the lumbar and cervical spine (Table 5). Positive degenerative MRI findings in both the lumbar and cervical spine were observed in 78.7 % of the patients, as follows: decreased disc signal intensity in 64.9 %, posterior disc protrusion in 66.0 %, anterior compression of the dura in 68.1 %, disc space narrowing in 10.6 %, and spinal canal stenosis in 4.3 % (Fig. 1). Thus, subjects with degenerative changes in the lumbar spine were likely to have those changes in the cervical spine as well.
Table 5.
Percentage of subjects with positive MRI findings at one or more intervertebral discs comparison between lumbar and cervical spines
| Lumbar spine | Cervical spine | p value* | Tandem positive findingsa | |
|---|---|---|---|---|
| Decrease in signal intensity | 70 (74.5 %) | 76 (80.9 %) | 0.31 | 61 (64.9 %) |
| Posterior disc protrusion | 74 (78.7) | 72 (76.6) | 0.83 | 62 (66.0) |
| Anterior compression of dura | 77 (81.9) | 76 (80.9) | 1.0 | 64 (68.1) |
| Disc space narrowing | 20 (21.3) | 32 (34.0) | 0.05 | 10 (10.6) |
| Spinal canal stenosis | 12 (12.8) | 13 (13.8) | 1.0 | 4 (4.3) |
| Any one of the five findings | 79 (84.0) | 85 (90.4) | 0.21 | 74 (78.7) |
* McNemar test
aPercentage of subjects with positive MRI findings both in the lumbar and cervical spines
Fig. 1.
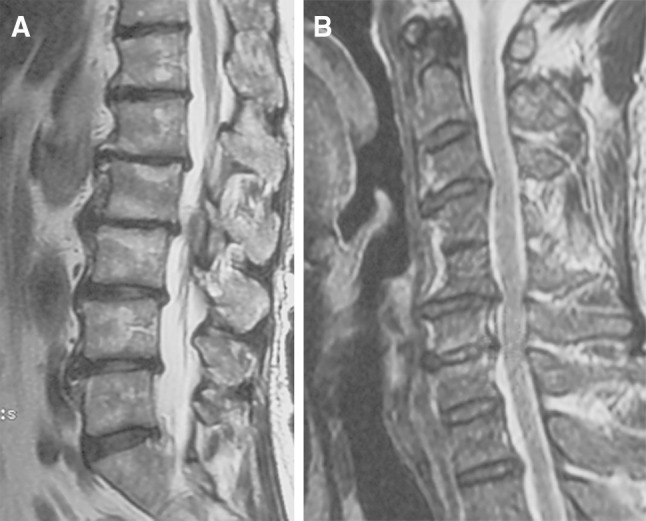
MRI of a 59-year-old asymptomatic patient demonstrating tandem degenerative changes both in lumbar and cervical spines. He had spinal canal stenosis both at L2–3 and C5–6. a T2 sagittal image of lumbar spine. b T2 sagittal image of cervical spine
Factors associated with degenerative MRI findings in the lumbar spine
A decrease in disc signal intensity in the lumbar spine was significantly associated with an increase in age [odds ratio (OR) 4.2; 95 % confidence interval (CI) 1.2–14.9; p = 0.024] and with the presence of decreased disc signal intensity in the cervical spine (OR 4.9; 95 % CI 1.4–17.1; p = 0.012). Posterior disc protrusion in the lumbar spine was significantly associated with increased age (OR 7.9; 95 % CI 2.0–32.2; p = 0.004) and with the presence of posterior disc protrusion in the cervical spine (OR 9.0; 95 % CI 2.3–35.8; p = 0.002). Anterior compression of the dura was significantly associated with increased age (OR 8.8; 95 % CI 2.2–35.4; p = 0.002) and with female gender (OR 0.16; 95 % CI 0.03–0.82; p = 0.027). We did not detect any factors significantly associated with disc space narrowing or with spinal canal stenosis (Table 6).
Table 6.
Relationships between degenerative MRI findings of lumbar spine and factors
| No. of patients | Decrease in signal intensity | Posterior disc protrusion | Anterior compression of dura | Disc space narrowing | Spinal canal stenosis | |
|---|---|---|---|---|---|---|
| Age | ||||||
| <40 | 28 | 14 (50.0) | 16 (57.1) | 16 (57.1) | 2 (7.1) | 1 (3.6) |
| ≥40 | 66 | 56 (84.8)* | 58 (87.9)* | 61 (92.4)* | 18 (27.3) | 11 (16.7) |
| Gender | ||||||
| Male | 46 | 40 (87.0) | 40 (87.0) | 30 (70.8)* | 9 (19.6) | 5 (10.9) |
| Female | 48 | 30 (62.5) | 34 (70.8) | 43 (93.5) | 11 (22.9) | 7 (14.6) |
| Smoking | ||||||
| Smoker | 21 | 13 (61.9) | 16 (76.2) | 16 (76.2) | 5 (23.8) | 2 (9.5) |
| Non-smoker | 72 | 57 (78.1) | 58 (79.5) | 61 (83.6) | 15 (20.5) | 10 (13.7) |
| Sports | ||||||
| Regularly | 16 | 9 (56.2) | 12 (75.0) | 12 (75.0) | 1 (6.2) | 2 (12.5) |
| None | 78 | 61 (78.2) | 62 (79.5) | 65 (83.3) | 19 (24.4) | 10 (12.8) |
| BMI | ||||||
| ≥25.0 | 25 | 15 (64.0) | 18 (72.0) | 20 (80.0) | 5 (20) | 2 (8.0) |
| <25.0 | 64 | 49 (76.6) | 5 (79.7) | 52 (81.2) | 13 (20.3) | 9 (14.1) |
| MRI findings in cervical spinea | ||||||
| Positive | 61/76 (80.3)* | 62/72 (86.1)* | 64/77 (83.1) | 10/20 (50.0) | 2/6 (33.3) | |
| Negative | 9/18 (50.0) | 12/22 (54.5) | 12/17 (70.6) | 22/74 (29.7) | 10/88 (11.4) | |
The numbers in the parentheses indicate percentages
* p < 0.05 (Logistic regression analysis)
aMRI finding in the cervical spine corresponding to that in the lumbar spine in the rows
Discussion
This study demonstrated the frequency of positive degenerative MRI findings in the lumbar spine: 84 % of asymptomatic subjects with a mean age of 48 years had positive MRI findings of disc degeneration at one or more intervertebral levels in the lumbar spine. There were more degenerative MRI findings in the lumbar spine in older subjects and at rostral levels such as L4–5 and L5–S1. These results correspond to the results of previous studies, both with asymptomatic and symptomatic subjects [1, 2].
Decreased disc signal intensity and posterior disc protrusion were significantly associated with increased age and with the presence of corresponding MRI findings in the cervical spine. Anterior compression of the dura was significantly associated with age and with female gender.
Although not statistically significant, anterior compression of the dura, disc space narrowing, and spinal canal stenosis in the lumbar spine were observed more frequently in those subjects with corresponding MRI findings in the cervical spine. In 78.7 % of the subjects, at least one type of degenerative MRI finding was observed in both the lumbar and cervical spine. These results suggest that disc degeneration occurs concurrently in the lumbar and cervical spine in asymptomatic subjects. In our previous studies, we found a significant association between thoracic and cervical disc degeneration in asymptomatic subjects [16]. Thus, the physiological ageing process involves the whole spine, and where disc degeneration is found in one part of the spine, other parts of the spine should be examined as well.
At the same time, the pathological significance of degenerative MRI findings should be evaluated carefully in patients with lumbar and/or cervical spinal disorders, since concurrent asymptomatic disc degeneration in both lumbar and cervical intervertebral discs is not uncommon on MRI. Tsutsumimoto et al. [19] recently reported a natural history of asymptomatic lumbar spinal canal stenosis in patients treated surgically for cervical myelopathy. Asymptomatic lumbar spinal canal stenosis was present in 32 % of the patients, and 89.6 % of the patients were free from leg symptoms for 3 years after surgery. In the present study, tandem canal stenosis was identified in only 4.3 % of the subjects; this is a lower percentage than that reported by Tsutsumimoto. This may be in part because our study included only asymptomatic, healthy and relatively younger subjects free from neck and back pain.
We did not find a significant association between lumbar disc degeneration and any parameters other than age and cervical disc degeneration, although several studies have reported that high BMI and smoking promote disc degeneration [20].
This study has several limitations. First, only T2 sagittal images were obtained for the lumbar spine due to limitations of imaging time and costs. Although T2 sagittal images provide fundamental information for assessing lumbar disc degeneration, the lack of T2 axial images might limit the diagnostic accuracy of the MR images in detecting degenerative changes. Especially, diagnosis of spinal canal stenosis could have been made better on axial images than on sagittal images. Second, our study population had some bias in terms of age distribution and occupation and, therefore, may not precisely represent the general population. Nonetheless, this is the first study to compare degenerative changes in the lumbar spine with those in the cervical spine in asymptomatic subjects. The results can be used as a control for evaluating MRI findings in patients with degenerative lumbar diseases and in those with tandem lumbar and cervical lesions.
In conclusion, MR images from asymptomatic subjects frequently showed degenerative changes in the lumbar spine, and these changes were significantly associated with degeneration in the cervical spine, suggesting that disc degeneration occurs in tandem in the lumbar and cervical spine.
Acknowledgments
This study was supported by a grant from the General Insurance Association of Japan. We express our cordial thanks to Mr. Toshio Watanabe at the Central Radiotechnology Department of Keio University Hospital, for his cooperation with this study.
Conflict of interest
None.
References
- 1.Boden SD, Davis DO, Dina TS, et al. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg. 1990;72(3):403–408. [PubMed] [Google Scholar]
- 2.Jensen MC, Brant-Zawadzki MN, Obuchowski N, et al. Magnetic resonance imaging of the lumbar spine in people without back pain. N Engl J Med. 1994;331:69–73. doi: 10.1056/NEJM199407143310201. [DOI] [PubMed] [Google Scholar]
- 3.Gore DR, Sepic SB, Gardner GM. Roentgenographic findings of the cervical spine in asymptomatic people. Spine. 1986;11:521–524. doi: 10.1097/00007632-198607000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Boden SD, McCowin PR, Davis DO, et al. Abnormal magnetic-resonance scans of the cervical spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:1178–1184. [PubMed] [Google Scholar]
- 5.Matsumoto M, Fujimura Y, Suzuki N, et al. MRI of cervical intervertebral discs in asymptomatic subjects. J Bone Joint Surg Br. 1998;80:19–24. doi: 10.1302/0301-620X.80B1.7929. [DOI] [PubMed] [Google Scholar]
- 6.Lehto IJ, Tertti MO, Komu ME, et al. Age-related MRI changes at 0.1 T in cervical discs in asymptomatic subjects. Neuroradiology. 1994;36:49–53. doi: 10.1007/BF00599196. [DOI] [PubMed] [Google Scholar]
- 7.Yasuma T, Koh S, Okamura T, et al. Histological changes in aging lumbar intervertebral discs. Their role in protrusions and prolapses. J Bone Joint Surg Am. 1990;72:220–229. [PubMed] [Google Scholar]
- 8.Leboeuf-Yde C, Nielsen J, Kyvik KO, et al. Pain in the lumbar, thoracic or cervical regions: do age and gender matter? A population-based study of 34,902 Danish twins 20–71 years of age. BMC Musculoskelet Disord. 2009;20(10):39. doi: 10.1186/1471-2474-10-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mio F, Hirose Y, Chiba K, et al. A functional polymorphism in COL11A1, which encodes the a1 chain of type XI collagen, is associated with susceptibility to lumbar disc herniation. Am J Hum Gen. 2007;81:1271–1277. doi: 10.1086/522377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Okada E, Matsumoto M, Ichihara D, et al. Aging of the cervical spine in healthy volunteers: a 10-year longitudinal magnetic resonance imaging study. Spine. 2009;34:706–712. doi: 10.1097/BRS.0b013e31819c2003. [DOI] [PubMed] [Google Scholar]
- 11.Dagi TF, Tarkington MA, Leech JJ. Tandem lumbar and cervical spinal stenosis. Natural history, prognostic indices, and results after surgical decompression. J Neurosurg. 1987;66:842–849. doi: 10.3171/jns.1987.66.6.0842. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs B, Ghelman B, Marchisello P. Coexistence of cervical and lumbar disc disease. Spine. 1990;15:1261–1264. doi: 10.1097/00007632-199012000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Aydogan M, Ozturk C, Mirzanli C, et al. Treatment approach in tandem (concurrent) cervical and lumbar spinal stenosis. Acta Orthop Belg. 2007;3:234–237. [PubMed] [Google Scholar]
- 14.Master DL, Eubanks JD, Ahn NU. Prevalence of concurrent lumbar and cervical arthrosis: an anatomic study of cadaveric specimens. Spine. 2009;34:E272–E275. doi: 10.1097/BRS.0b013e318195d10b. [DOI] [PubMed] [Google Scholar]
- 15.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Ichihara D, Okada E, et al. Age-related changes in thoracic intervertebral discs in asymptomatic subjects. Spine. 2010;35:1359–1364. doi: 10.1097/BRS.0b013e3181fab802. [DOI] [PubMed] [Google Scholar]
- 17.Matsumoto M, Okada E, Ichihara D, et al. Anterior cervical decompression and fusion accelerates adjacent segment degeneration: comparison with asymptomatic volunteers in 10-year MRI follow-up study. Spine. 2010;35:36–43. doi: 10.1097/BRS.0b013e3181b8a80d. [DOI] [PubMed] [Google Scholar]
- 18.Okada E, Matsumoto M, Fujiwara H, Toyama Y. Disc degeneration of cervical spine on MRI in patients with lumbar disc herniation: comparison study with asymptomatic volunteers. Eur Spine J. 2011;20:585–591. doi: 10.1007/s00586-010-1644-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsutsumimoto T, Shimogata M, Yui M, et al. The natural history of asymptomatic lumbar canal stenosis in patients undergoing surgery for cervical myelopathy. Bone Joint Surg Br. 2012;94:378–384. doi: 10.1302/0301-620X.94B3.27867. [DOI] [PubMed] [Google Scholar]
- 20.Battié MC, Videman T, Gill K, et al. Smoking and lumbar intervertebral disc degeneration: an MRI study of identical twins. Spine. 1991;16:1015–1021. doi: 10.1097/00007632-199109000-00001. [DOI] [PubMed] [Google Scholar]


